Introduction
The cellular and molecular mechanisms of tumor
angiogenesis and its prospects for anti-angiogenic cancer therapy
are major issues for both cancer biology and targeted cancer
therapy. Multiple mechanisms of tumor neovascularization have been
established, yet considerable controversy persists (1).
Among these mechanisms, angiogenesis and
vasculogenesis are widely accepted. In the first mechanism,
vascular endothelial cells of the host sprout from preexisting
vasculature to form new vessels; in the second mechanism, bone
marrow-derived endothelial progenitor cells are recruited to form
new blood vessels (2). However, the
contribution of these two mechanisms to the process of tumor
neovascularization remains unclear.
Recent research has indicated that the tumor
neovascularization process is more complex than previously assumed
(3). Vasculogenic mimicry (VM) was
first found in human melanoma by Maniotis et al in 1999
(4). Unlike the angiogenesis and
vasculogenesis mechanisms, VM facilitates tumor cells to form
functional blood vessels in the absence of endothelial cells as has
been witnessed in numerous solid tumors such as breast, prostate,
ovarian and lung cancer, synovial sarcoma, rhabdomyosarcoma,
pheochromocytoma and glioma (5).
Meanwhile, the effect of tumor stem/progenitor cells
on tumor neovascularization is attracting increased attention. Our
research group (6,7), Ricci-Vitiani et al (8) and Wang et al (9) reported that glioma stem cells directly
participate in the formation of tumor vessels by
transdifferentiating or differentiating into endothelial cells. In
addition, there is increasing evidence that mesenchymal stem cells
(MSCs) have the ability to migrate to tumor sites and exert
stimulatory or inhibitory effects on tumor angiogenesis through
direct and/or indirect interaction with tumor cells (10).
The main divergence is whether tumor vascular cells
are transformed from tumor cells or supplied by the host vascular
cells. In the present study, human glioma stem/progenitor SU3 cells
were transfected with red fluorescent protein (SU3-RFP cells), and
then co-cultured with bone marrow-derived mesenchymal stem cells
(BMSCs)-GFP, and fused cells co-expressing RFP and green
fluorescent protein (GFP) were identified and detected for their
tube formation ability in vitro and biological
characteristics in vivo; meanwhile, SU3-RFP cells were
inoculated into the brains of GFP nude mice. In the xenograft
tumors, de novo tumor vessels that originated from the cell
fusion of tumor cells and host BMSCs were detected.
Materials and methods
Cells and animals
The human glioma stem/progenitor cell line SU3 was
previously established in our laboratory (11). According to the published methods by
which we established SU1 and SU2 (12), SU3 was obtained from a surgical
specimen of an adult male patient diagnosed with glioblastoma
multiforme. SU3 cells expressed CD133 and nestin consistent with
the characteristics of glioma stem cells (11). SU3 cells were transfected with the
RFP gene using a lentiviral-mediated gene transfection kit
(GeneChem, Shanghai, China). Under a fluorescence microscope (Zeiss
Axio Observer A1; Carl Zeiss, Germany), nearly 100% of the tumor
cells expressed RFP (13). SU3
cells with stable RFP expression were isolated using flow cytometry
(FACSCanto II; BD Biosciences, USA) and were amplified.
NC-C57BL/6J-GFP nude mice (6–8 weeks of age) with
whole-body expression of the GFP gene were prepared by our research
group (14). Foxn1nu
mice were purchased from the Model Animal Research Center of
Nanjing University (Nanjing, China). The mice were housed under
specific pathogen-free conditions with a 12-h light/dark cycle and
controlled temperature at the Laboratory Animal Center of Soochow
University.
BMSC isolation and culture
Isolation
NC-C57BL/6J-GFP nude mice (6–8 weeks of age) were
sacrificed by CO2 asphyxiation. After being immersed in
75% alcohol for 5–10 min, the mice were placed on a sterile culture
dish. Both femurs and tibiae were dissected out, and bone marrow
plugs were extracted by flushing the bone marrow cavity with
phosphate-buffered saline (PBS) containing penicillin and
streptomycin.
Culture
Bone marrow-derived cells were cultured in complete
medium containing Dulbecco's modified Eagle's medium (DMEM; Gibco,
USA) and 10% fetal bovine serum (FBS; HyClone, USA). The cells were
plated on a 24-well plate (3×106 cells/well) and were
incubated at 37°C in a humidified atmosphere with 5%
CO2. After 3–4 days, the adherent cells attained
confluency, and the non-adherent cells were discarded. At this
point, the cells were considered to be at stage 0 (P0). The
confluent cells were detached with Accutase (Innovative Cell
Technologies, San Diego, CA, USA) and passaged. The culture medium
was replaced every 3 days.
Biological characteristics of the
BMSCs
Phenotypic analysis by flow
cytometry
BMSCs were harvested with Accutase and washed twice
with PBS, and then 5×105 cells were suspended in 10
µl of PBS for binding with each specific antibody. BMSCs
were then incubated in the dark for 30 min at room temperature with
antibodies against CD45, CD11b, CD44 and CD105 (Abcam, Cambridge,
UK). Flow cytometry was performed on the FACSCanto II (BD
Biosciences).
Mesenchymal differentiation
Adipogenesis differentiation
BMSCs were seeded at a density of 5×104
cells/ml in a 6-well plate, cell differentiation was induced with
adipogenic differentiation medium which contained 10% FBS, 1
µM dexamethasone (Sigma, USA), 500 µM
1-methyl-3-isobutyl xanthine (Sigma), 60 µM indomethacin
(Sigma) and 5 µM insulin (Sigma). The medium was replaced
every 3 days. After 2 weeks, adipocytes were visualized by
Oil-O-Red staining for fatty drops.
Osteogenesis differentiation
BMSCs were incubated in DMEM/F12 supplemented with
10% FBS, 0.1 µM dexamethasone, 10 mM β-glycerophosphate
(Sigma), 50 µM ascorbic acid-2-phosphate (Sigma). On day 21,
cells were performed Alizarin Red S staining to detect
osteogenesis.
Chondrogenic differentiation
BMSCs were cultured in high-glucose DMEM
(Invitrogen) supplemented with 10% FBS, 100 nM dexamethasone, 1 mM
sodium pyruvate (Invitrogen), 10 µM insulin, 50 mg/l
ascorbate 2-phosphate, and 10 ng/ml transforming growth factor-β
(Sigma). On day 28, cells were cultured on the coverslide and fixed
with paraformaldehyde, then incubated with a rabbit polyclonal
anti-mouse collagen II antibody (Abcam, ab34712, 1:200). The
secondary antibody used was a peroxidase-conjugated anti-rabbit IgG
(Vector Labs, MP7401). Positive reaction was defined as blue using
DAB Peroxidase Substrate Kit (Blue Color, Boster, AR1025, Wuhan,
China).
Colony forming unit assay
Colony forming cell assay was based on the method of
Liu et al (15), BMSCs at P5
were seeded into 6-well plates at 10–100 cells/well in duplicates.
Culture media were changed every 3 days. On day 10, the cells were
stained with 0.25% crystal violet (Santa Cruz, Dallas, TX, USA) for
10 min and then observed under an inverted fluorescence microscope
(Zeiss Axio Observer A1).
Co-culture of BMSCs and SU3-RFP
cells
The SU3-RFP cells were added to the BMSCs at a ratio
of 1:15. Half of the culture medium was renewed every 3 days, and
the cells were passaged once they reached 80–90% confluency.
Briefly, we removed and discarded the culture medium and rinsed the
cell layer with Ca++/Mg++-free Dulbecco's
PBS. We then added 2.0–3.0 ml of Accutase to the dishes. After 2
min, we added 2.0–3.0 ml of complete culture medium and aspirated
the cells by gentle pipetting. Finally, we centrifuged the mixture
for 5 min (1,000 rps, 179 × g) and subcultivated the cells at a
ratio of 1:2. Cell growth was observed under an inverted
fluorescence microscope. RFP+/GRP+ cells were
detected by flow cytometry and sorted.
In vitro tube formation assay of the
fused cells
To detect the tube formation ability of the fused
cells, 100 µl of Matrigel (BD Biosciences, San Jose, CA,
USA) was poured onto a 24-well dish and placed in a CO2
incubator (Jouan, France; with 5% CO2 at 37°C). Fused
cells (RFP+/GFP+ cells) in DMEM containing
10% FBS supplemented with 5 ng/ml basic fibroblast growth factor
(bFGF) and 10 ng/ml epidermal growth factor (EGF) (both from
Gibco), were seeded onto each well at a density of 2×104
cells/well. The cells were periodically observed under an inverted
microscope (Zeiss Axio Observer A1).
In vivo experiments
To investigate the ability of the fused cell to form
endothelial vessels in vivo, 1×105 sorted
RFP+/GFP+ cells suspended in 20 µl PBS
were injected into the right caudate nucleus of Foxn1nu
mice with the assistance of a stereotaxic apparatus. After 3–4
weeks, the mice were sacrificed and the xenograft tumors were
harvested, and continuously sectioned at a thickness of 5
µm. The sections were either stained with hematoxylin and
eosin (H&E) or observed under a fluorescence microscope.
To establish the dual-color orthotopic model of
transplantable xenograft glioma, 1×105 SU3-RFP cells
were injected into the right caudate nucleus of NC-C57BL/6J-GFP
nude mice using a 20-µl Hamilton syringe with the assistance
of a stereotaxic apparatus. All of the procedures were carried out
under general anesthesia by intraperitoneal injection of 10%
chloral hydrate (200 mg/kg). After 3–4 weeks, the tumor xenografts
were sampled and cut into two pieces. One-half of the fresh tumor
tissue was cut into 1 mm3 pieces and pressed on slides
for fluorescence microscopy. The other tumor was embedded in the
Optimal Cutting Temperature medium, frozen in liquid nitrogen. The
frozen samples were continuously sectioned at a thickness of 5
µm. Nuclei were stained with DAPI, then either observed
under a fluorescence microscope or performed immunohistochemical or
H&E staining.
Immunocytochemical/immunohistochemical
staining
Immunocytochemical staining of CD105 was performed
on SU3-RFP cells, BMSCs and fused cells, while immunohistochemical
staining of CD105 and CD31 was performed on the tissue sections.
Briefly, 5×103 cells were placed on a slide on a 24-well
plate, and the slide was taken out when covered by 80–90% of the
cell population. Meanwhile, a frozen section of the xenograft tumor
was made. Primary antibodies used were rabbit polyclonal antibody
to CD105/endoglin (ab107595, dilution 1:250) and rabbit polyclonal
antibody to CD31 (ab28364, dilution 1:50) (both from Abcam). After
incubation with the primary antibodies at 4°C overnight, the slides
were incubated with peroxidase-conjugated anti-rabbit IgG (MP7401)
and stained with diaminobenzidine (DAB) chromogen solution
(SK-4105) (both from Vector Laboratories), and then counterstained
with hematoxylin.
Statement of ethics
The present study received approval from the ethics
Committee of the Second Affiliated hospital of Soochow University.
The animal experiments were approved by the Medical Review Board of
Soochow University, and all procedures were conducted in accordance
with the Chinese laws governing animal care.
Statistical analysis
Data are expressed as mean ± SEM. Statistical
significance was determined by the Student's t-test. A value of
P<0.05 was considered to indicate a statistically significant
result.
Results
BMSC immunophenotype analysis by flow
cytometry
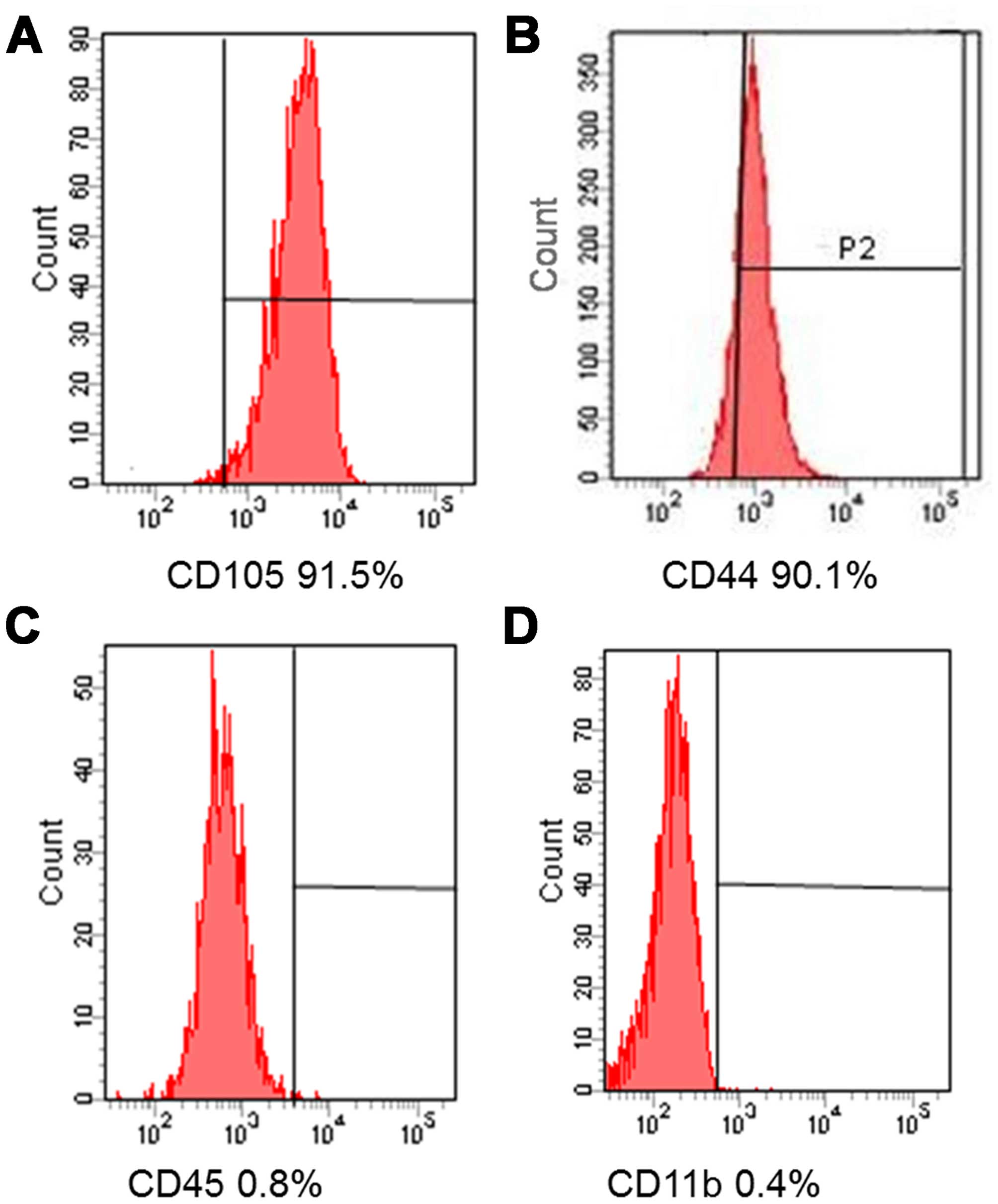
We investigated the immunophenotypes of the BMSCs
(passages 1, 3 and 5) using immunofluorescence flow cytometry. As
shown in Fig. 1, BMSCs expressed
high levels of CD105 and CD44, but very low levels of CD45 and
CD11b.
Biological characteristics of the
BMSCs
In this experiment, BMSCs were collected from the
bone marrow of hybrid generations of NC nude and C57BL/6J-GFP mice.
According to the specific experimental requirements, several
passages were performed in vitro, and the biological
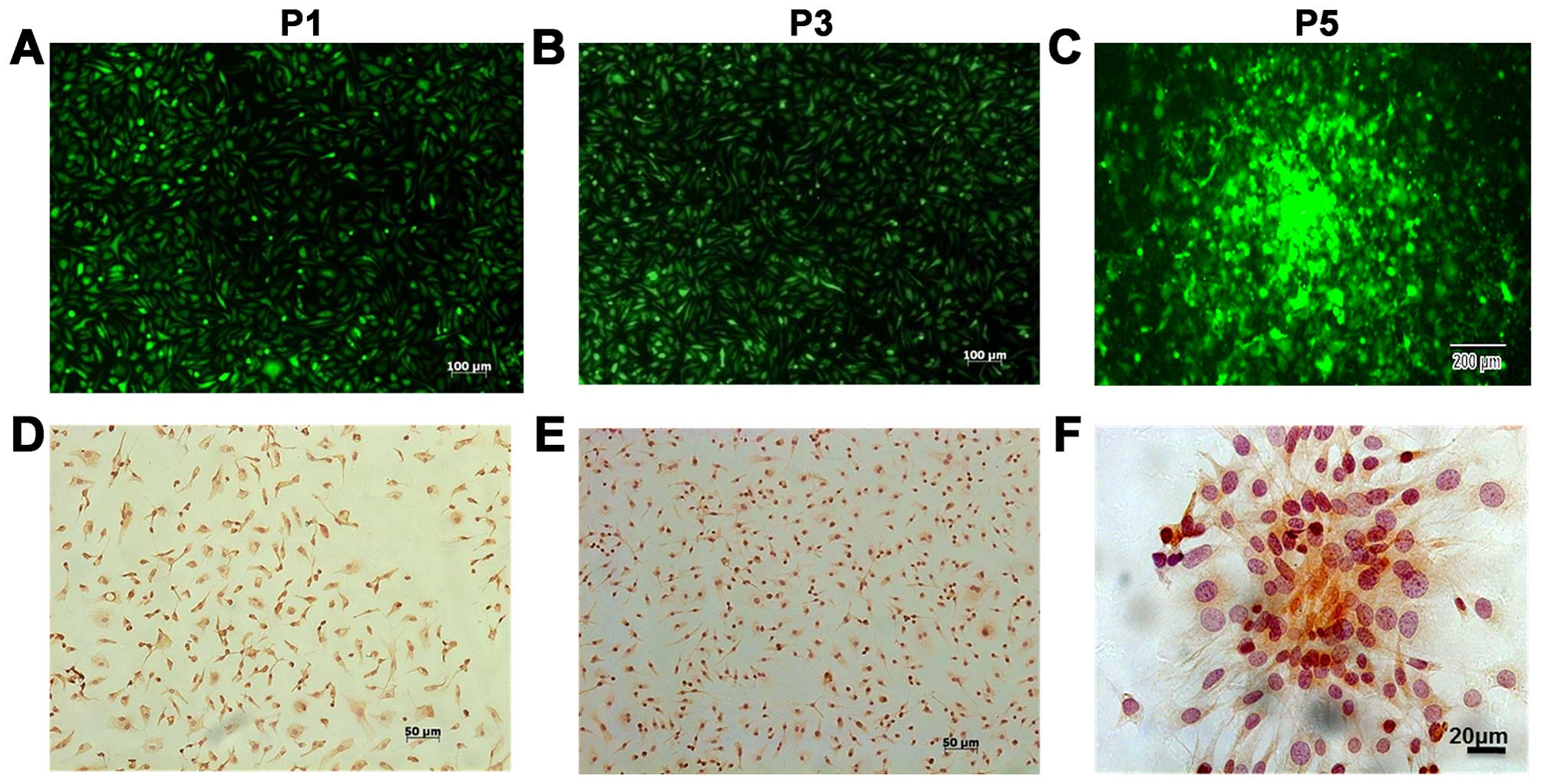
characteristics of the cells were investigated. The results
indicated that the 1st to 5th generation of BMSCs expressed GFP
protein (Fig. 2A–C) and had the
capacity to form cell colonies (Fig.
2C). Immunocytochemical staining of BMSCs at P1, P3 and P5
indicated that most of these cells expressed CD105 (Fig. 2D–F). To investigate the ability of
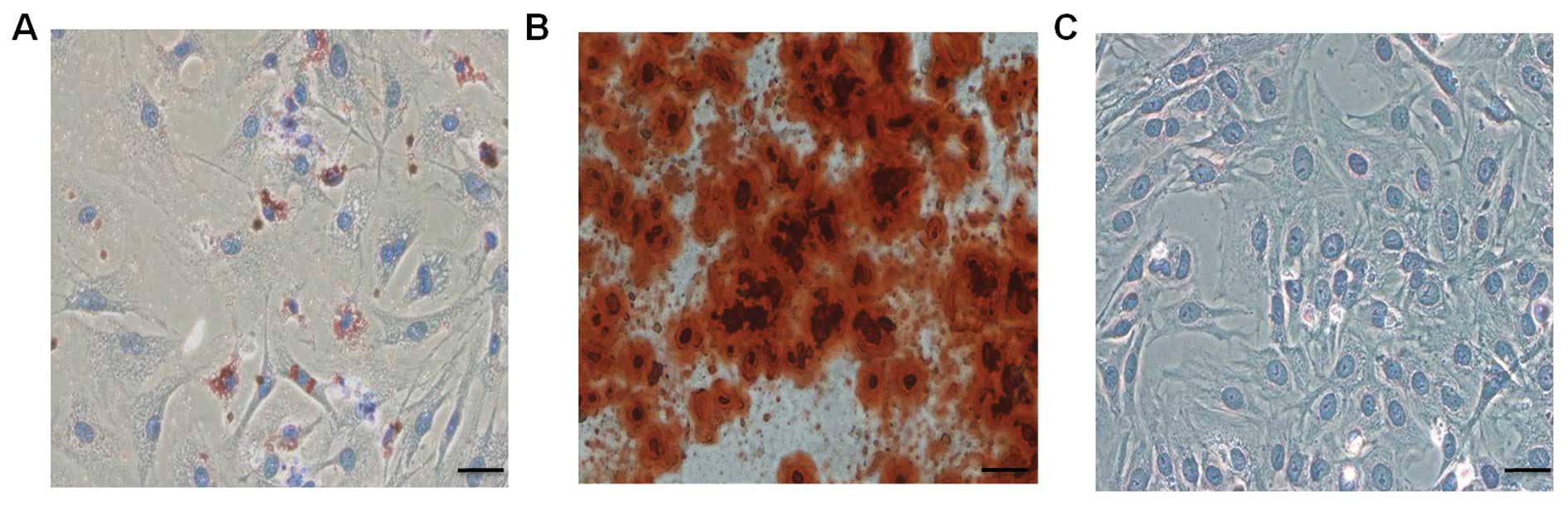
the BMSCs to differentiate into osteoblasts, adipocytes, and
chondroblasts in vitro, we induced cells of passages 1, 3,
and 5 under different culture conditions. We found that all the
samples from various donors kept their multipotent differentiation
potential (Fig. 3).
All of the above results indicated that the BMSCs in
this experiment had the following three major characteristics: i)
they consisted of a major proportion of CD105+ cells;
ii) they exhibited stable biological characteristics at passages
1–5; and iii) 100% of the BMSCs expressed GFP. Therefore, the BMSCs
were suitable for co-culture with tumor cells and were used to
study the effect of host BMSCs on tumor neovascularization based on
fluorescence tracing.
Harvesting
RFP+/GFP+ cells from the co-culture of
SU3-RFP cells and BMSCs
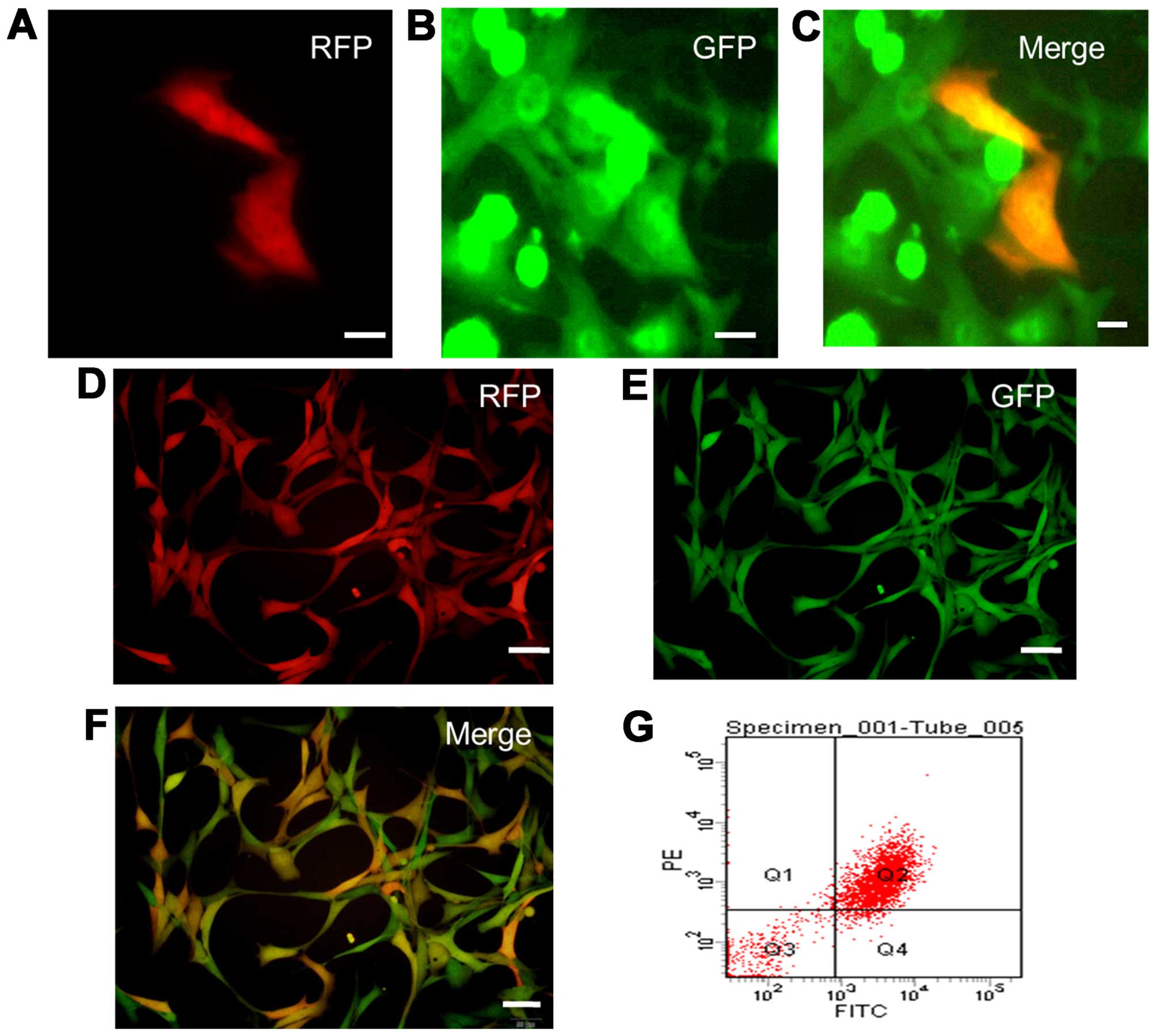
SU3-RFP cells were added to the BMSCs at the ratio
of 1:15 and were then co-cultured. On day 4, we were able to detect
a small proportion of GFP and RFP double-positive cells under an
inverted fluorescence microscope (Fig.
4A–C), which were identified as RFP+/GFP+
fused cells. With the extension of culture and passage, the
proportion of fused cells increased. Detection by flow cytometry
showed that the proportion of yellow
RFP+/GFP+ cells increased to 73.8% in the 5th
generation (Fig. 4D–G). The
immunocytochemical staining showed that most of the fused cells
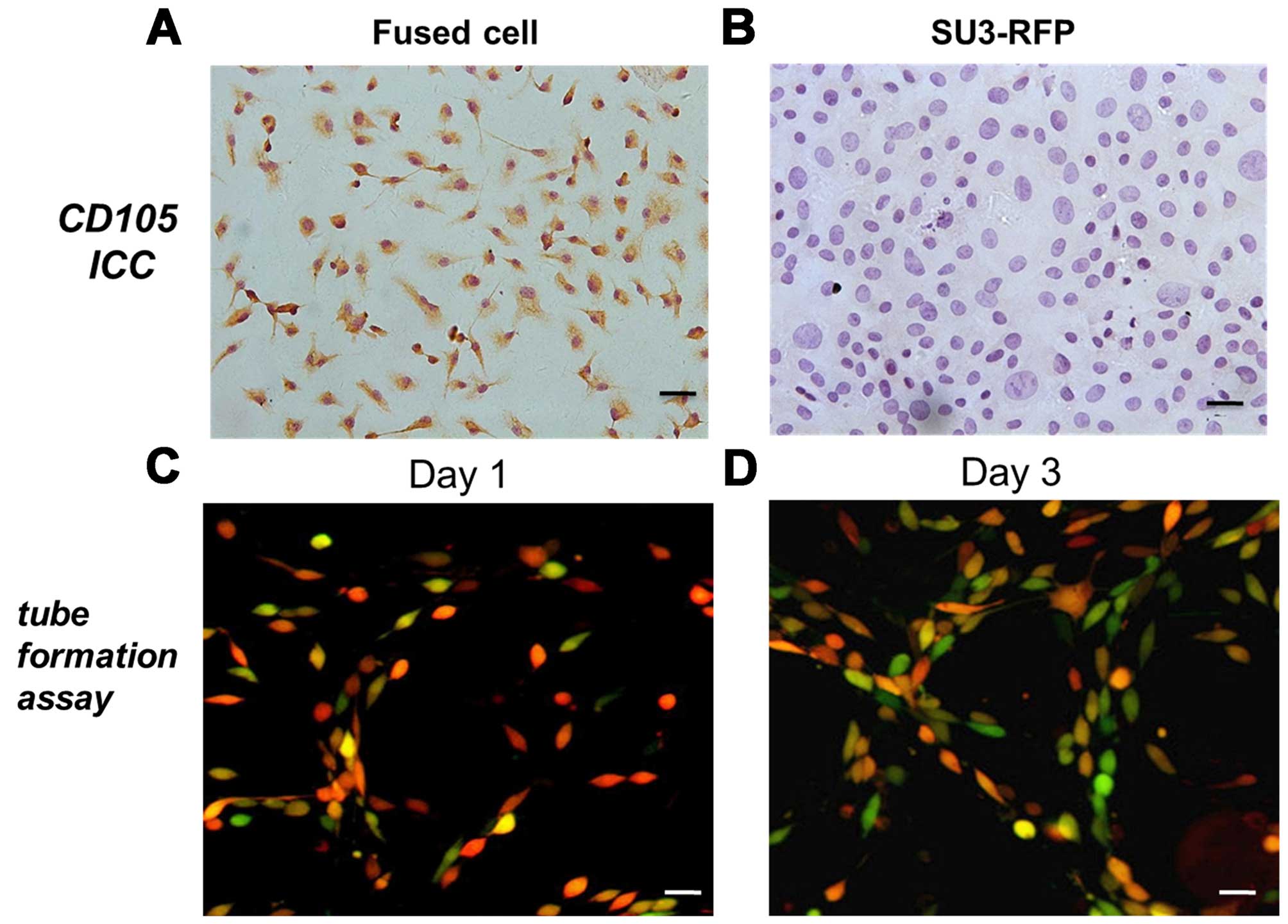
expressed CD105 (Fig. 5A), whereas,
SU3-RFP cells were found to be negative for CD105 (Fig. 5B).
Fused cells display angiogenic properties
in vitro
When cultured on Matrigel, the fused cells changed
from a cluster of cells to sparse links between cells (day 1)
(Fig. 5C) and gradually formed
continuous net-like structures (day 3) (Fig. 5D).
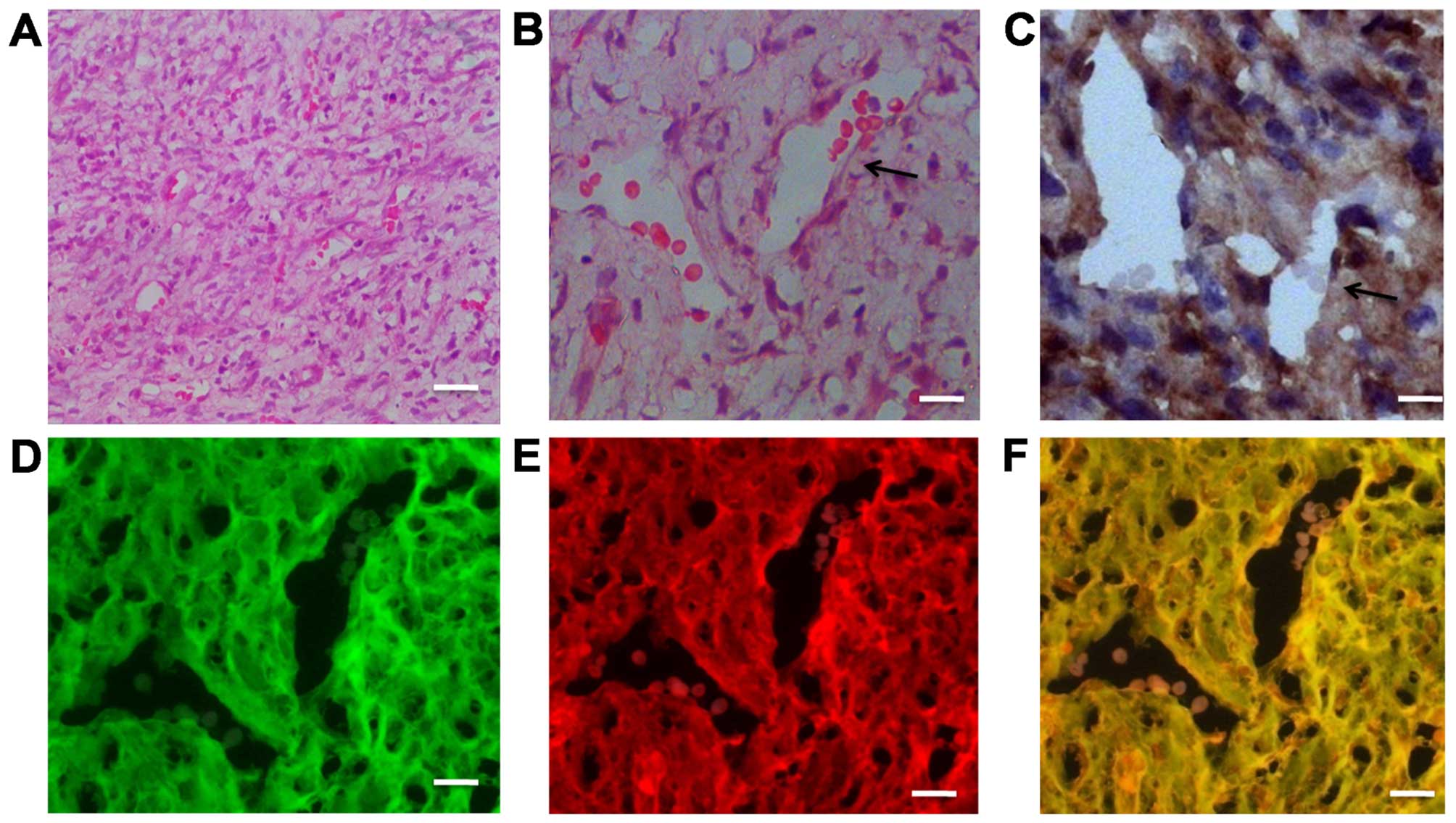
Fused cells generate newly formed tumor
vessels in vivo
To investigate the ability of fused cells to form
endothelial vessels in vivo, the
RFP+/GFP+ cells were sorted by FACS and
grafted into the Foxn1nu mice. After 3–4 weeks, highly
vascularized anaplastic tumors were detected; blood vessels were
abundant in the H&E-stained images of the grafted specimens
(Fig. 6A and B). A vessel
containing red blood cells is indicated by a black arrow in
Fig. 6B. Immunohistochemical
staining on the adjacent section of the same specimen indicated
that CD105+ cells were distributed in the transplanted
tumors, particularly on the not fully mature vascular walls
(Fig. 6C). Another adjacent section
was observed under a fluorescence microscope. We found that the
tumor cells co-expressed RFP and GFP; simultaneously fused cells
were also involved in vessel wall formation in the xenograft tumor
model (Fig. 6D–F).
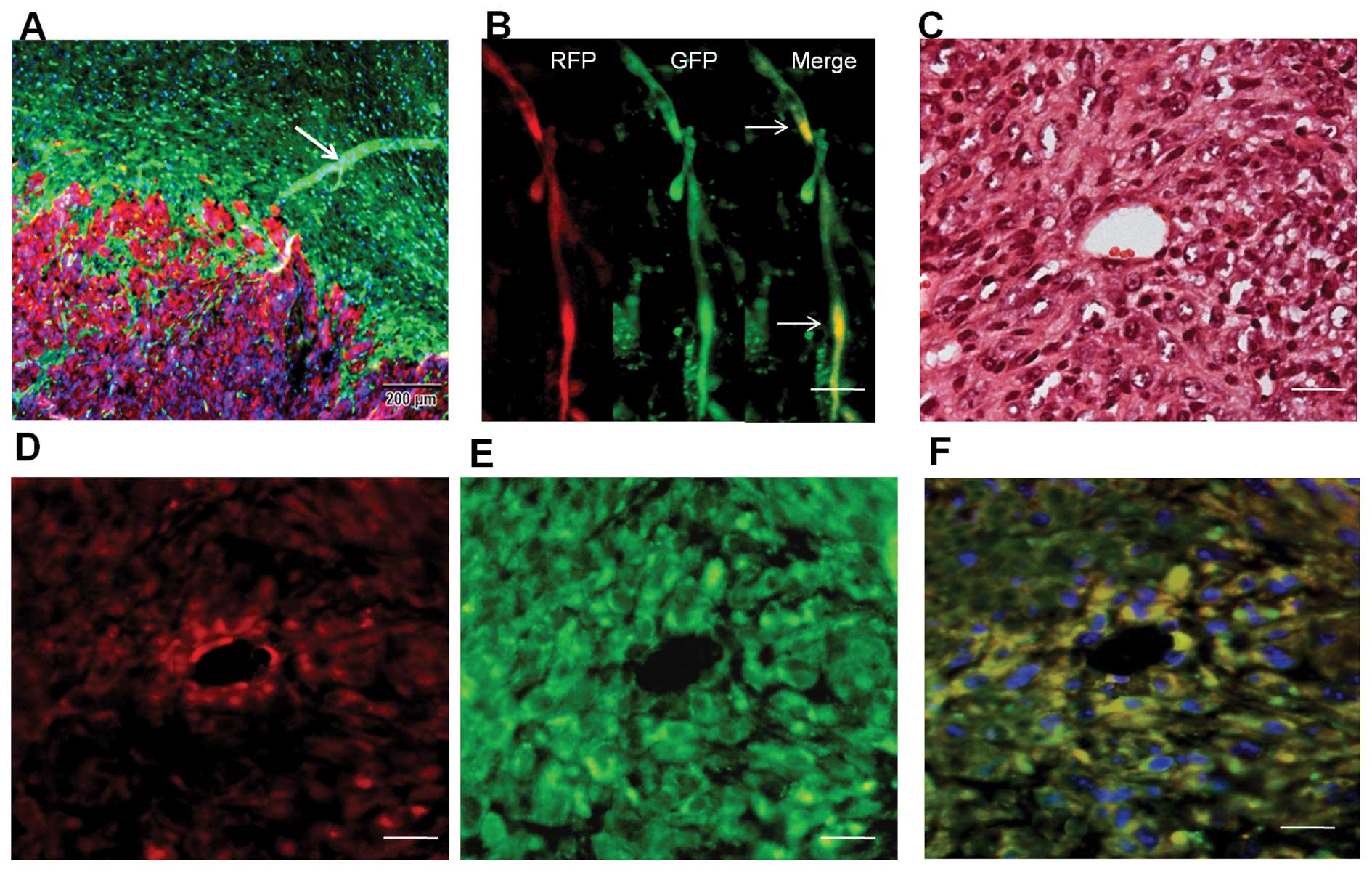
RFP+/GFP+ blood
vessels in dual-color transplanted tumor tissue
We transplanted SU3-RFP cells into the brains of
NC-C57BL/6J-GFP nude mice, and the xenograft tumors were examined
by fluorescence microscopy. Under fluorescence microscopy, tumor
(red) and peritumoral brain tissues (green) were easily
distinguished (Fig. 7A). A fraction
of tube-like structures co-expressing RFP and GFP were detected in
the preformed tissue slice (Fig.
7B), suggesting the occurrence of cell fusion in the tumors.
Transverse sections of the blood vessels were observed under
fluorescence microscopy. Merged images showed that these
RFP+/GFP+ blood vessels came from the host
vascular wall where the SU3-RFP cells localized and formed tumor
vessels by cell fusion since only SU3 cells successfully
transferred with the RFP gene would express red fluorescence, and
only the host-derived vessel cells would express green fluorescence
(Fig. 7F).
Whether tumor cells fused with host BMSCs was not
certain, since the host cells were GFP-expressing.
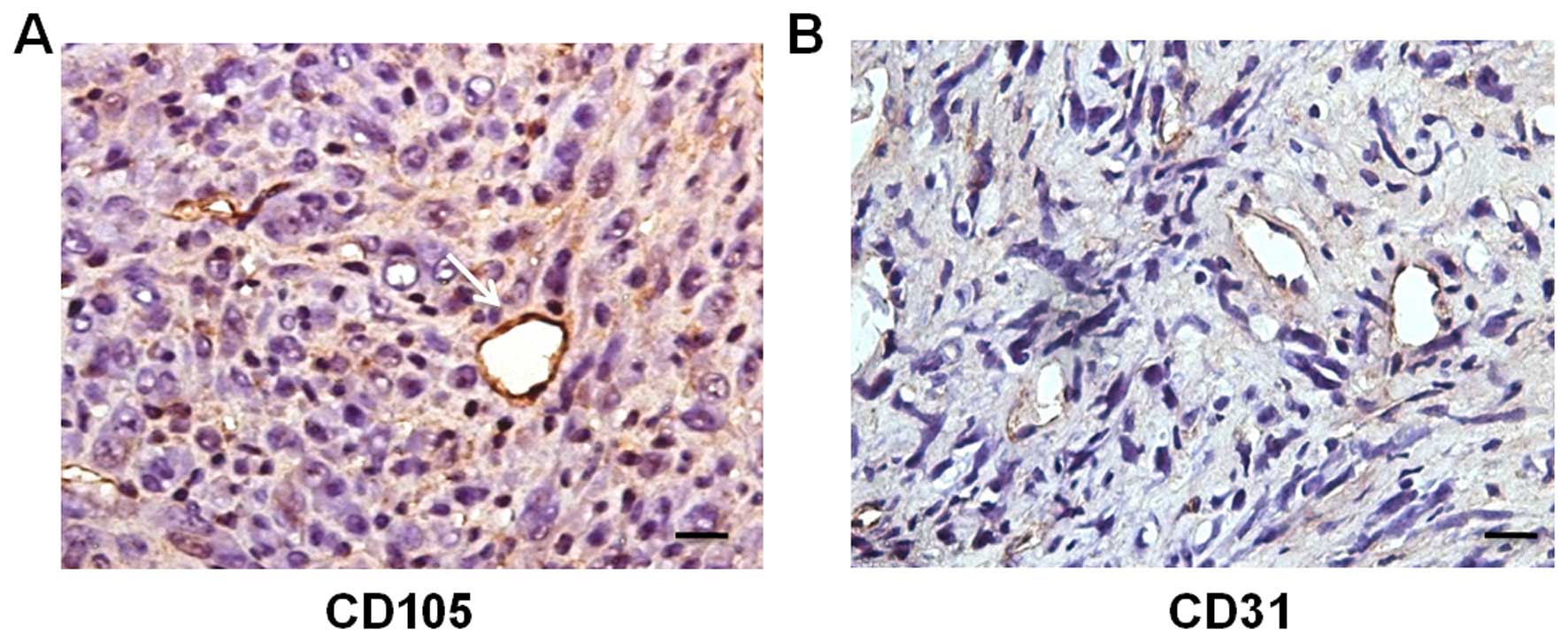
Immunohistochemical staining for CD105 on adjacent sections of the
RFP+/GFP+ vessels was positive (Fig. 8A), indicating that the
CD105+ vessels originated from the fusion of SU3-RFP
cells and host BMSCs. Meanwhile, most of the tumor vessels were
positive for CD31 (Fig. 8B).
Discussion
Cancer stem cells are a small fraction of cancer
cells that have the potential to drive tumorigenesis. Although
cancer stem cell models are controversial for some tumors,
glioblastoma stem cells have been reported to give rise to
endothelial cells (ECs) (8,9) and pericytes (16). However, some authors pointed out
that the discrepancies in this research may be caused by neglecting
the function of cell fusion; the cell-fusion-dependent event was
mistaken for cancer stem cell plasticity (17). Meanwhile, there is also growing
interest in the role of mesenchymal stem cells in tumor
angiogenesis. Bone marrow-derived mesenchymal stem cells (BMSCs)
are a population of non-hematopoietic stem cells in the bone marrow
microenvironment that have the ability to self-renew and
multilineage differentiation potential. There is accumulating
evidence indicating that BMSCs differentiate into endothelial-like
cells (18,19). However, the roles of the interaction
between BMSCs and tumor cells in the processes of tumor growth and
angiogenesis have not been well studied (20), and have currently generated strong
controversy. MSCs were even described as 'double-edged swords'
since they have stimulatory (21)
or inhibitory (22) effects on
tumor angiogenesis and progression. Recently, cells resembling MSCs
were able to be isolated from human glioma specimens (23) and glioma xenografts (24). Kim et al (24) found that these cells had similar
surface markers to MSCs and were located around vessels, yet they
failed to clarify the biological relationship between MSCs and
glioma stem cells, and showed weak evidence to prove the absence of
gliomagenesis characteristics. It cannot be excluded that BMSC may
be recruited and misused by tumor cells, which could be beneficial
for tumor angiogenesis, invasion, survival as a consequence of cell
fusion (25). To confirm this
hypothesis, we used fluorescence tracing in vivo and in
vitro to indicate interactions between RFP-labeled human tumor
cells and GFP-labeled murine BMSCs under direct vision.
In our research, we used short-term cultured mouse
BMSCs established in vitro. However, whether the biological
characteristics of these cells were suitable for such research is
uncertain. The present study indicated that BMSCs were suitable due
to their strong colony-forming capacity, stable CD105 and GFP
overexpression (Figs. 1 and
2).
Although there are no unique markers for BMSCs, it
is generally agreed that human BMSCs are positive for CD105, CD90
and CD73 and negative for CD34, CD45, CD14, CD11b, CD79a, CD19 or
HLA-DR surface molecules. In addition, they must be able to
differentiate to osteoblasts, adipocytes, and chondroblasts in
vitro, as BMSCs have multiple differentiation abilities
(26). Yet, these criteria apply
only to human MSCs, as for murine MSCs, surface antigen expression
is not universally well characterized (27).
In the present study, we found that BMSCs isolated
from bone marrow of NC-C57BL/6J-GFP nude mice expressed high levels
of CD105 and CD44, yet very low levels of CD45 and CD11b. CD44
glycoproteins are integral cell membrane components widely
distributed on various types of cells that function as adhesion
molecules of epithelial cells. CD105 (endoglin) is a type I
membrane glycoprotein located on cell surfaces and is part of the
TGF-β receptor complex. Recently, CD105 has been identified as a
unique marker of proliferating endothelial cells in vitro
and in vivo. It is preferentially expressed in the
angiogenic endothelium (28). Since
these markers are not specific for BMSCs, they are mainly
characterized by their ability to differentiate into multiple
mesenchymal lineages. In our study, BMSCs could differentiate into
osteoblasts, adipocytes, and chondroblasts in vitro.
Wang et al (9) indicated that CD105+ cells
were essentially absent in normal brain. In our research, we did
not detect the expression of CD105 in the SU3-RFP cells (Fig. 5B) or in normal murine brain tissues
(data not shown). However, we found that CD105 was expressed by
BMSCs at a high level. Thus, we confirmed that the
GFP+/CD105+ cells could trace murine BMSCs in
the present study.
In the co-culture of SU3-RFP cells and BMSCs, we
harvested RFP+/GFP+ cells (Fig. 4), and immunocytochemical staining of
these cells displayed CD105 expression, confirming the fusion event
of glioma stem cells and BMSCs.
In vivo, fused cells have the ability to
generate tumor vessels. In addition,
CD105+/RFP+/GFP+ vessels were
observed in the dual-color orthotopic xenograft glioma specimens
(Figs. 7 and 8), perticularly on intratumoral blood
vessels. We concluded that the green component in the
RFP+/GFP+ blood vessels was derived from the
CD105+ BMSCs of the GFP mice and hypothesized that this
transdifferentiation potential was acquired through cell
fusion.
Increasing evidence indicates that inappropriate
cell-cell fusion contributes to cancer initiation and progression
(29–31). It also contributes to the aneuploidy
and the aberrant gene expression patterns associated with malignant
cells. Moreover, some scholars believe that fusion of an adult stem
cell with a differentiated cell leads to the formation of a cancer
stem cell (29).
According to the assumption by Lu and Kang (32), cell fusions mainly take the
following forms: stem-differentiated, stem-stem and
differentiated-differentiated cells. The fusion between BMSCs and
SU3-RFP cells in this experiment was an example of stem-stem cell
fusion. In fact, the differentiation direction after cell fusion
should be given more attention. Quintana-Bustamante et al
(33) and Suzuki et al
(34) reported that fused cells
possess greater plasticity. In this experiment, the fused cells of
BMSCs and SU3-RFP also showed such features. The in vitro
test showed that the fused cells had splitting and differentiation
potentials; fused cells experienced sequential morphological
changes towards the shape of endothelial cells until formation of
vessel-like tubes on the Matrigel, while the in vivo test
found that the fused cells formed tumor blood vessels and tumor
cells. However, the entire process from the beginning of the fusion
between the BMSCs and SU3-RFP cells to the driving of
neovascularization in vivo was not observed. Rather, we only
speculated from the angiogenesis and vasculogenesis theory of
Carmeliet and Jain (3) that it
belonged to tumor-endothelial cell transdifferentiation.
Furthermore, we believed that this transdifferentiation potential
was acquired through the cell fusion process itself.
Cell fusion is a nuclear reprogramming technique
that involves two or more cell types to form a single identity.
However, its molecular mechanism and how fused cells contribute to
tumor neovascularization remains unclear. A general requirement for
cell fusion is that the two fusing membranes are in close contact,
which may be mediated by receptor-ligand interactions (25). Recently, Shinojima et al
(35) identified TGF-β as a tumor
factor that attracts BMSC homing to GSCs via TGF-β receptors on
BMSCs.
TGF-β promotes tumor development and
neovascularization by regulating cell proliferation,
differentiation and migration. As the TGF-β accessory receptor III,
CD105 promotes neovascularization by binding with TGF-β1 and TGF-β3
with high affinity as reported by Fonsatti et al (36) and Warrington et al (37).
The tumor neovascularization in this experiment was
realized by the fusion between tumor cells and BMSCs, a process
that may be regulated by TGF-β. A study on the regulatory
relationship between CD105 and the TGF-β family in the
neovascularization of glioma driven by cell fusion is in
progress.
Taken together, whether the mechanisms of
neovascularization and the origin of tumor endothelial cells are
related to the differentiation of tumor stem/progenitor cells or
the hyperplasia of host vascular endothelium remains poorly
defined. These two paradoxical theories may be unified in the
assumption proposed in the present study. That is, tumor cells and
host BMSCs become fused to promote tumor neovascularization. There
are intricate mechanisms in cell-to-cell as well as in cell-to-ECM
cross-talks, which finally guide stem cell fate and behavior. We
assumed that cell fusion gives the fused cells a particular
potential for proliferation, differentiation and
transdifferentiation to form tumor vessels to meet the increased
demand for blood supply under a condition of increased tumor cell
proliferation.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81272799,
81172400, 81071766 and 81172401), a project funded by the priority
Academic Program Development of Jiangsu Higher Education
Institutions, and the Scientific and Technological Development Plan
Project of Suzhou (no. SYSD2012090).
References
|
1
|
Plate KH, Scholz A and Dumont DJ: Tumor
angiogenesis and anti-angiogenic therapy in malignant gliomas
revisited. Acta Neuropathol. 124:763–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ping YF and Bian XW: Concise review:
Contribution of cancer stem cells to neovascularization. Stem
Cells. 29:888–894. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El Hallani S, Boisselier B, Peglion F,
Rousseau A, Colin C, Idbaih A, Marie Y, Mokhtari K, Thomas JL,
Eichmann A, et al: A new alternative mechanism in glioblastoma
vascularization: Tubular vasculogenic mimicry. Brain. 133:973–982.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong J, Zhao Y, Huang Q, Fei X, Diao Y,
Shen Y, Xiao H, Zhang T, Lan Q and Gu X: Glioma stem/progenitor
cells contribute to neovascularization via transdifferentiation.
Stem Cell Rev. 7:141–152. 2011. View Article : Google Scholar
|
|
7
|
Zhao Y, Dong J, Huang Q, Lou M, Wang A and
Lan Q: Endothelial cell transdifferentiation of human glioma stem
progenitor cells in vitro. Brain Res Bull. 82:308–312. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ricci-Vitiani L, Pallini R, Biffoni M,
Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G,
Larocca LM, et al: Tumour vascularization via endothelial
differentiation of glioblastoma stem-like cells. Nature.
468:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong IS, Lee HY and Kang KS: Mesenchymal
stem cells and cancer: Friends or enemies? Mutat Res. 768:98–106.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dong J, Zhang Q, Huang Q, Chen H, Shen Y,
Fei X, Zhang T, Diao Y, Wu Z, Qin Z, et al: Glioma stem cells
involved in tumor tissue remodeling in a xenograft model. J
Neurosurg. 113:249–260. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Q, Zhang QB, Dong J, Wu YY, Shen YT,
Zhao YD, Zhu YD, Diao Y, Wang AD and Lan Q: Glioma stem cells are
more aggressive in recurrent tumors with malignant progression than
in the primary tumor, and both can be maintained long-term in
vitro. BMC Cancer. 8:3042008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong J, Dai XL, Lu ZH, Fei XF, Chen H,
Zhang QB, Zhao YD, Wang ZM, Wang AD, Lan Q, et al: Incubation and
application of transgenic green fluorescent nude mice in
visualization studies on glioma tissue remodeling. Chin Med J.
125:4349–4354. 2012.PubMed/NCBI
|
|
14
|
Wu Z, Huang Q, Shao YX, Xue ZM, Dong J,
Diao Y, Wang AD and Lan Q: Transplantation of human glioma stem
cells in nude mice with green fluorescent protein expression.
Zhonghua Yi Xue Za Zhi. 88:2317–2320. 2008.In Chinese. PubMed/NCBI
|
|
15
|
Liu H, Toh WS, Lu K, MacAry PA, Kemeny DM
and Cao T: A subpopulation of mesenchymal stromal cells with high
osteogenic potential. J Cell Mol Med. 13:2436–2447. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng L, Huang Z, Zhou W, Wu Q, Donnola S,
Liu JK, Fang X, Sloan AE, Mao Y, Lathia JD, et al: Glioblastoma
stem cells generate vascular pericytes to support vessel function
and tumor growth. Cell. 153:139–152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu AY and Ouyang G: Tumor angiogenesis: A
new source of pericytes. Curr Biol. 23:R565–R568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva GV, Litovsky S, Assad JA, Sousa AL,
Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, et al:
Mesenchymal stem cells differentiate into an endothelial phenotype,
enhance vascular density, and improve heart function in a canine
chronic ischemia model. Circulation. 111:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janeczek Portalska K, Leferink A, Groen N,
Fernandes H, Moroni L, van Blitterswijk C and de Boer J:
Endothelial differentiation of mesenchymal stromal cells. PloS One.
7:e468422012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang WH, Chang MC, Tsai KS, Hung MC, Chen
HL and Hung SC: Mesenchymal stem cells promote growth and
angiogenesis of tumors in mice. Oncogene. 32:4343–4354. 2013.
View Article : Google Scholar
|
|
21
|
Beckermann BM, Kallifatidis G, Groth A,
Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner
W, Diehlmann A, et al: VEGF expression by mesenchymal stem cells
contributes to angiogenesis in pancreatic carcinoma. Br J Cancer.
99:622–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui
KM and Lam PY: Human bone marrow-derived mesenchymal stem cells
suppress human glioma growth through inhibition of angiogenesis.
Stem Cells. 31:146–155. 2013. View Article : Google Scholar
|
|
23
|
Kim SM, Kang SG, Park NR, Mok HS, Huh YM,
Lee SJ, Jeun SS, Hong YK, Park CK and Lang FF: Presence of glioma
stroma mesenchymal stem cells in a murine orthotopic glioma model.
Childs Nerv Syst. 27:911–922. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YG, Jeon S, Sin GY, Shim JK, Kim BK,
Shin HJ, Lee JH, Huh YM, Lee SJ, Kim EH, et al: Existence of glioma
stroma mesenchymal stemlike cells in Korean glioma specimens.
Childs Nerv Syst. 29:549–563. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schichor C, Albrecht V, Korte B, Buchner
A, Riesenberg R, Mysliwietz J, Paron I, Motaln H, Turnšek TL,
Jürchott K, et al: Mesenchymal stem cells and glioma cells form a
structural as well as a functional syncytium in vitro. Exp Neurol.
234:208–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop D and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tropel P, Noël D, Platet N, Legrand P,
Benabid AL and Berger F: Isolation and characterisation of
mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res.
295:395–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dallas NA, Samuel S, Xia L, Fan F, Gray
MJ, Lim SJ and Ellis LM: Endoglin (CD105): A marker of tumor
vasculature and potential target for therapy. Clin Cancer Res.
14:1931–1937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer
J and Terzis A: Opinion: The origin of the cancer stem cell:
Current controversies and new insights. Nat Rev Cancer. 5:899–904.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dittmar T, Nagler C, Schwitalla S, Reith
G, Niggemann B and Zänker KS: Recurrence cancer stem cells - made
by cell fusion? Med Hypotheses. 73:542–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu X and Kang Y: Cell fusion as a hidden
force in tumor progression. Cancer Res. 69:8536–8539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu X and Kang Y: Cell fusion hypothesis of
the cancer stem cell. Cell Fusion in health and Disease. Springer;
pp. 129–140. 2011, View Article : Google Scholar
|
|
33
|
Quintana-Bustamante O, Alvarez-Barrientos
A, Kofman AV, Fabregat I, Bueren JA, Theise ND and Segovia JC:
Hematopoietic mobilization in mice increases the presence of bone
marrow-derived hepatocytes via in vivo cell fusion. Hepatology.
43:108–116. 2006. View Article : Google Scholar
|
|
34
|
Suzuki K, Sun R, Origuchi M, Kanehira M,
Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S and Saijo Y:
Mesenchymal stromal cells promote tumor growth through the
enhancement of neovascularization. Mol Med. 17:579–587. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shinojima N, Hossain A, Takezaki T, Fueyo
J, Gumin J, Gao F, Nwajei F, Marini FC, Andreeff M, Kuratsu J, et
al: TGF-β mediates homing of bone marrow-derived human mesenchymal
stem cells to glioma stem cells. Cancer Res. 73:2333–2344. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fonsatti E, Nicolay HJ, Altomonte M, Covre
A and Maio M: Targeting cancer vasculature via endoglin/CD105: A
novel antibody-based diagnostic and therapeutic strategy in solid
tumours. Cardiovasc Res. 86:12–19. 2010. View Article : Google Scholar
|
|
37
|
Warrington K, Hillarby MC, Li C, Letarte M
and Kumar S: Functional role of CD105 in TGF-beta1 signalling in
murine and human endothelial cells. Anticancer Res. 25:1851–1864.
2005.PubMed/NCBI
|