Introduction
Human glioblastoma is one of the most common and
malignant tumors, that originates from glial cells (1). Glioblastoma is characterized by rapid
invasion and distant migration (2).
Current therapeutic strategies for glioblastoma include surgery,
radiation therapy and chemotherapy. However, the median survival of
glioblastoma patients is extremely poor (3).
Mitogen-activated protein kinase (MAPK) signaling
regulates diverse cellular functions, such as cell proliferation,
the cell cycle, cell survival, angiogenesis and cell migration
(4). The MAPK cascade is initiated
by Ras activation which is regulated by receptor tyrosine kinases
at the cell surface. Ras activation recruits C-Raf to the cell
membrane, processing the phosphorylation of multiple co-factors
(5). Downstream of the C-Raf
proteins are MEK1 and MEK2, a dual serine/threo-nine and tyrosine
kinase (6). MEK further transmits
signals to extracellular regulated serine/threonine kinases (ERK),
comprised of ERK1 and ERK2 (7). The
cellular functions of ERK play diverse roles in the regulation of
cell proliferation, survival, mitosis and migration (8).
The cytoskeleton plays pivotal roles in various
signaling pathways related to cellular adhesion, cytokinesis, cell
survival and transcriptional processes (9). Particularly, reorganization of the
actin cytoskeleton is essential for the migration and metastasis of
malignant tumors. Actin reorganization is regulated by Rac, the
Wiskott-Aldrich syndrome protein (WASP) family and actin-related
protein (Arp) (10). Rac is a
Ras-related GTP binding protein that controls assembly and
disassembly of actin cytoskeleton by transducing extracellular
chemoattractive signals to downstream effectors such as WASP family
verprolin-homologous protein-2 (WAVE-2) and Arp-2. WAVE-2 activates
the Arp2/3 complex through their binding and consequently the
Arp2/3 complex locates to actin filament branches and crosslinks
into a branching network (11). In
addition, they play a functional role in lamelli-podium formation
that is related to the invasion and metastasis of malignant tumors
(12,13).
Metastasis is a mechanism by which new tumors form
in distant tissues from a primary tumor. Cell migration and
invasion are required for cancer metastasis. Invasion is induced by
matrix metalloproteinases (MMPs) that induce degradation of the
extracellular matrix (ECM) and basement membrane (14,15).
Thus, inhibition of MMPs is considered to be a therapeutic strategy
for tumor metastasis.
Sargassum (Sargassaceae, Fucales) is a genus
of brown seaweed that is found in all the oceans. Previous research
on Sargassum spp. extracts have been reported to exhibit
anticancer, antibacterial, antifungal, antiviral,
anti-inflammatory, anticoagulant, antioxidant, hepatoprotective and
neuroprotective activities (16).
However, the pharmacological effect of extracts from Sargassum
serratifolium (S. serratifolium) has not been thoroughly
studied in glioblastoma. Therefore, the present study aimed to
investigate the anticancer effects of an extract from S.
serratifolium on U87MG glioblastoma cells.
Materials and methods
Preparation of the hexane extracts
S. serratifolium was collected from the coast
of Busan, Korea, in April 2013. Taxonomic identification was
confirmed by an algal taxonomist (C.K. Choi) at the Department of
Ecological Engineering, Pukyong National University, Korea. The
seaweed was rinsed in tap water to remove salt and air-dried under
sunlight for 3 days. The dried powder (1.5 kg) of S.
serratifolium underwent extraction three times with 96% (v/v)
ethanol (EtOH) for 3 h at 70°C. The combined extracts were
concentrated under reduced pressure to obtain the EtOH extract. For
further fractionation of the EtOH extract, the extract was
resuspended in water:EtOH (9:1) and partitioned successively with
n-hexane and ethyl acetate. The n-hexane extract,
which showed the highest cell cytotoxic activities against U87MG
cells, was maintained at −20°C and was used for the present
study.
Cell culture
Human glioblastoma cancer U87MG, human cervical
cancer HeLa, human gastric cancer MKN-28 and human liver cancer
SK-HEP 1 cells as well as human embryonic kidney HEK-293 cells,
were obtained from the American Tissue Culture Collection (ATCC;
Manassas, VA, USA). U87MG and SK-HEP 1 cells were incubated with
minimum essential medium (MEM), MKN-28 cells were incubated with
RPMI-1640 medium, and HeLa and HEK-293 cells were incubated in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 1% penicillin-streptomycin (PAA
Laboratories GmbH, Pasching, Austria). The cells were cultured in a
5% CO2 incubator (binder) at 37°C in a humidified
atmosphere. The culture was sub-cultured every 3 to 4 days and
routinely checked under an inverted microscope for any
contamination.
Cell cytotoxicity and morphology
The effects of the extracts of Sargassum
serratifolium on cell viability were evaluated by WST-1 assay,
based on the reduction of the number of metabolically active cells,
and the results are expressed as a percentage of the control. Cells
were seeded in 96-well micro-plates at a density of
1×104 cells/well and were cultured for 24 h. After 24 h,
the cells were treated with the extracts at various concentrations
and incubated for 12 and 24 h. Then, the media was replaced and
2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulforphenyl)-2H-tetrazolium,
monosodium salt (WST-1 solution) was added to each well at 10
µl, and the cells were incubated at 37°C for 3 h. Finally
the optical density (OD) was measured with an ELISA reader
(Molecular Devices, Silicon Valley, CA, USA) at 460 nm. The
cytotoxic activity of the extract was expressed as an
IC50 value, which is the concentration of the extract
that caused 50% cell death. The extract with an IC50
value ≤16 µg/ml was considered active. Dimethylsulfoxide
(DMSO) was used to dilute the extracts and the final concentration
of DMSO in each well was not in excess of 0.5% (v/v). No adverse
effect due to the presence of DMSO was observed.
Protein extraction and western blot
analysis
U87MG cells were treated with Sargassum
serratifolium. The treated cells were washed with ice-cold 1X
phosphate-buffered saline (PBS) and collected in lysis buffer [(50
mM Tris-Cl (pH 7.5), 150 mM NaCl, 1 mM DTT, 0.5% NP-40, 1% Triton
X-100, 1% deoxycholate, 0.1% SDS] and a proteinase inhibitor
cocktail [PMSF, EDTA, aptotinin, leupeptin, prostatin A (Intron
Biotechnology, Gyeonggi, Korea)] on ice. After incubation on ice
for 30 min, the insoluble materials were removed by centrifugation
at 14,000 rpm for 20 min. Protein contents of the cell lysates were
determined by a protein quantification kit (Commasie Brilliant Blue
solution®) (Dojindo Molecular Technologies, Rockville,
MD, USA) with bovine serum albumin (BSA) as a standard. The
absorbance was determined at 595 nm. An aliquot from each sample
was boiled for 5 min and then resolved by 12% SDS-polyacrylamide
gel electrophoresis (SDS-PAGE). Then, the proteins were
electrotransferred to a nitrocellulose membrane (Pall Life
Sciences, Pensacola, FL, USA) and blocked in PBST buffer (135
µM NaCl, 2.7 mM KCl, 4.3 mM NaPO4, 1.4 mM
KH2PO4 and 0.5% Tween-20) containing 5%
non-fat milk (w/v). After blocking, the membranes were then
incubated with primary antibodies against GAPDH, integrin β1,
Rac1/2/3, Ras, PI3K p110α, WAVE-2, Arp-2, MMP-2, MMP-9,
phospho-C-RafSer338, phospho-MEK
1/2Ser217/221, phospho-ERK 1/2Thr202/Tyr204
overnight at 4°C. The membranes were next incubated with
HRP-conjugated secondary antibodies (Cell Signaling Technology) for
60 min. All membranes were visualized using West Save Gold ECL
(AbFrontier) and exposed to Hyperfilm (GE Healthcare). GAPDH was
used as a loading control.
Wound healing assay
U87MG cells (5.0×105 cells/well) were
seeded in the chamber of an Ibidi culture insert (Ibidi GmbH,
Martinsried, Germany) consisting of two reservoirs separated by a
500 µm well and incubated at 37°C in an atmosphere of 5%
CO2 for 24 h. After incubation, the inserts were gently
removed, and the cells were cultured with medium to facilitate cell
migration. Cell migration was recorded by phase contrast microscopy
over a time course of 2h following treatment with the S.
serratifolium extract. On-line based Wimasis image analysis was
used to carry out quantitative analysis of the cell migration.
Cell invasion assay
The invasion of the tumor cells was assessed in
Matrigel-coated Τranswell chambers with a 6.5-mm
polyvinyl/pyrolidone-free polycarbonate filter with 8-µm
pore size (Corning Life Sciences) as previously described (17). The U87MG cells (5×104
cells/well and test compounds at different concentrations were
suspended in 100 µl of serum-free medium and placed in the
upper Τranswell chamber and incubation was carried out for 24 h at
37°C. Then, the cells on the upper surface of the filter were
completely wiped away with a cotton swab, and the cells on the
lower surface were lysed with 4% formaldehyde and stained with
crystal violet. After staining, cells on the lower surface were
lysed with 2% SDS for 1 h and the lysate was measured using a
microplate reader at 570 nm.
Immunofluorescence
Cultured U87MG cells on a cover glass-bottom dish
were incubated for 30 min with 10 µg/ml HES. For this, the
cells were pre-treated with 1 µg/ml DAPI for 20 min at 37°C,
then fixed with 4% formaldehyde (Junsei Chemical Co., Ltd., Japan)
for 15 min at 25°C and blocked for 1 h in a blocking solution,
including 5% mouse and rabbit normal serum (Santa Cruz
Biotechnology, Inc.) with 0.3% Triton X-100 (Sigma-Aldrich, St.
Louis, Mo, USA). Fixed and blocked cells were incubated with the
primary antibodies (β-actin,
phospho-ERK1/2Thr202/Tyr204) for 3 h and washed three
times with PBS buffer. After washing, the cells were treated with
0.1 µg/ml of anti-mouse IgG (H+L), F(ab′)2
fragment (Alexa Fluor® 555-conjugated) and anti-rabbit
IgG (H+L), F(ab′)2 fragment (Alexa Fluor®
488-conjugated) for 1 h. Stained cells were mounted on a slide with
ProLong Gold antifade reagent (Invitrogen, Grand Island, NY, USA)
and fluorescence was determined under a Carl Zeiss LSM 710 confocal
laser scanning microscope.
Statistical analysis
Data are presented as the mean ± standard deviation
for the indicated number of separate experiments. The mean of the
control was compared with the mean of each individual treatment
group by one-way ANOVA followed by Tukey's test using the
statistical software SigmaPlot v.12.3 s(Systat Software, Inc., San
Jose, CA, USA), and a statistically significant difference was set
at p<0.001.
Results
Effects of S. serratifolium extracts on
cell proliferation
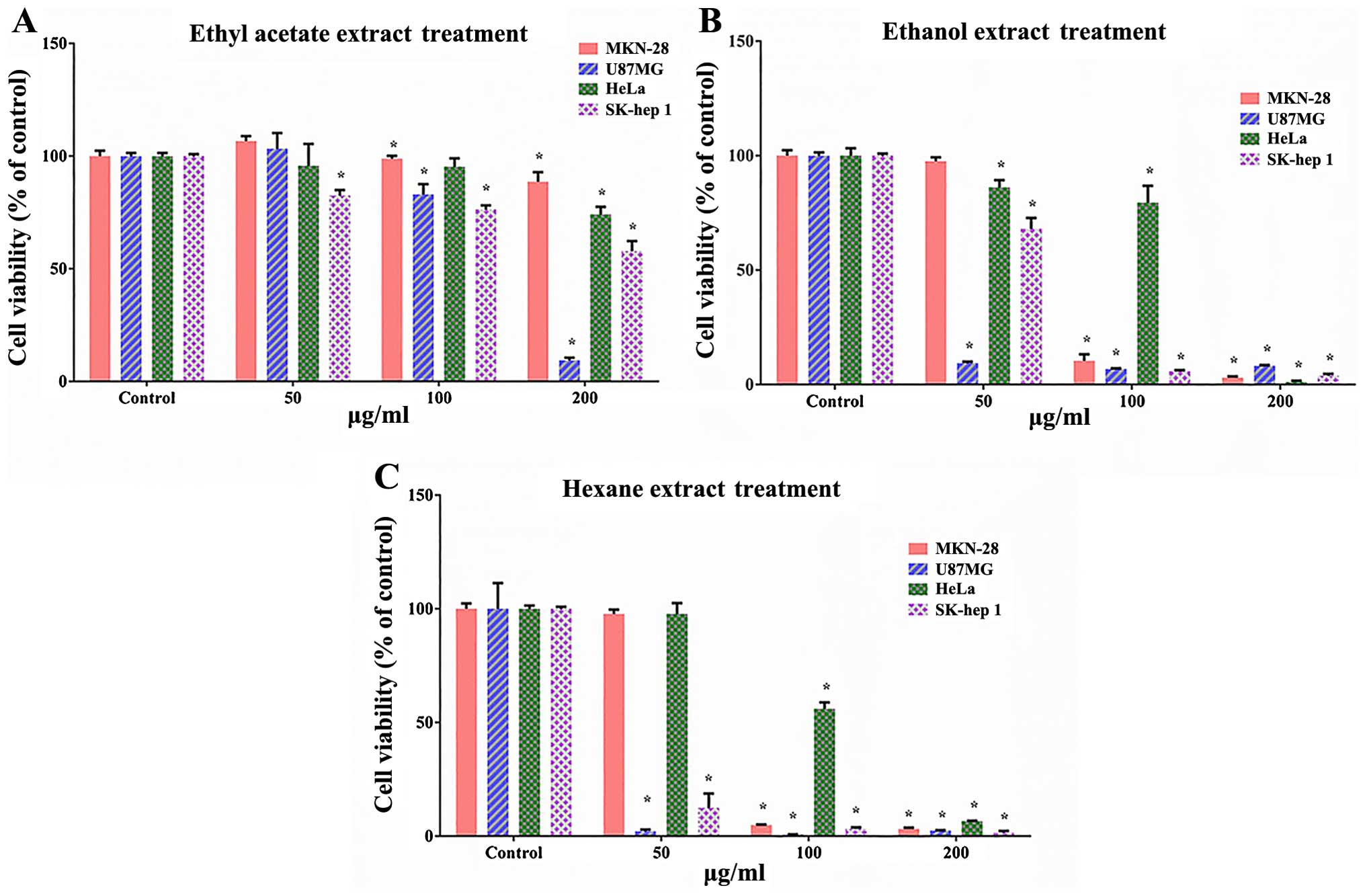
To investigate the antiproliferative effects of
three types of extracts, hexane, ethanol and ethyl acetate, from
S. serratifolium, we performed WST-1 assay on human
glioblastoma cancer U87MG, human cervical cancer HeLa, human
gastric cancer MKN-28, human liver cancer SK-HEP 1 and noncancerous
HEK293 cells following treatments with the extracts. As shown in
Fig. 1, each extract had a
cytotoxic effect on the human cancer cell lines. Among these
extracts, the hexane extract from S. serratifolium (HES)
exhibited the most effective inhibition of cell proliferation. HES
particularly decreased the cell viability of the U87MG cells to a
higher degree than that observed in the other cancer cell lines.
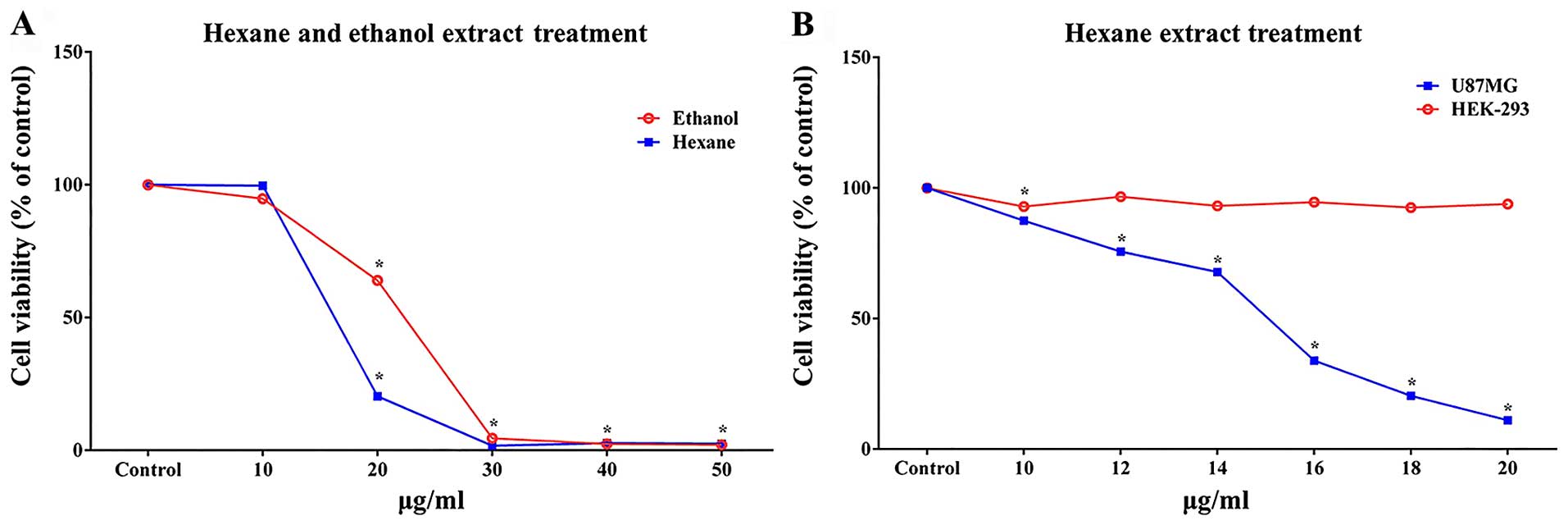
The IC50 value of HES was observed between 14 and 16
µg/ml HES (Fig. 2). In
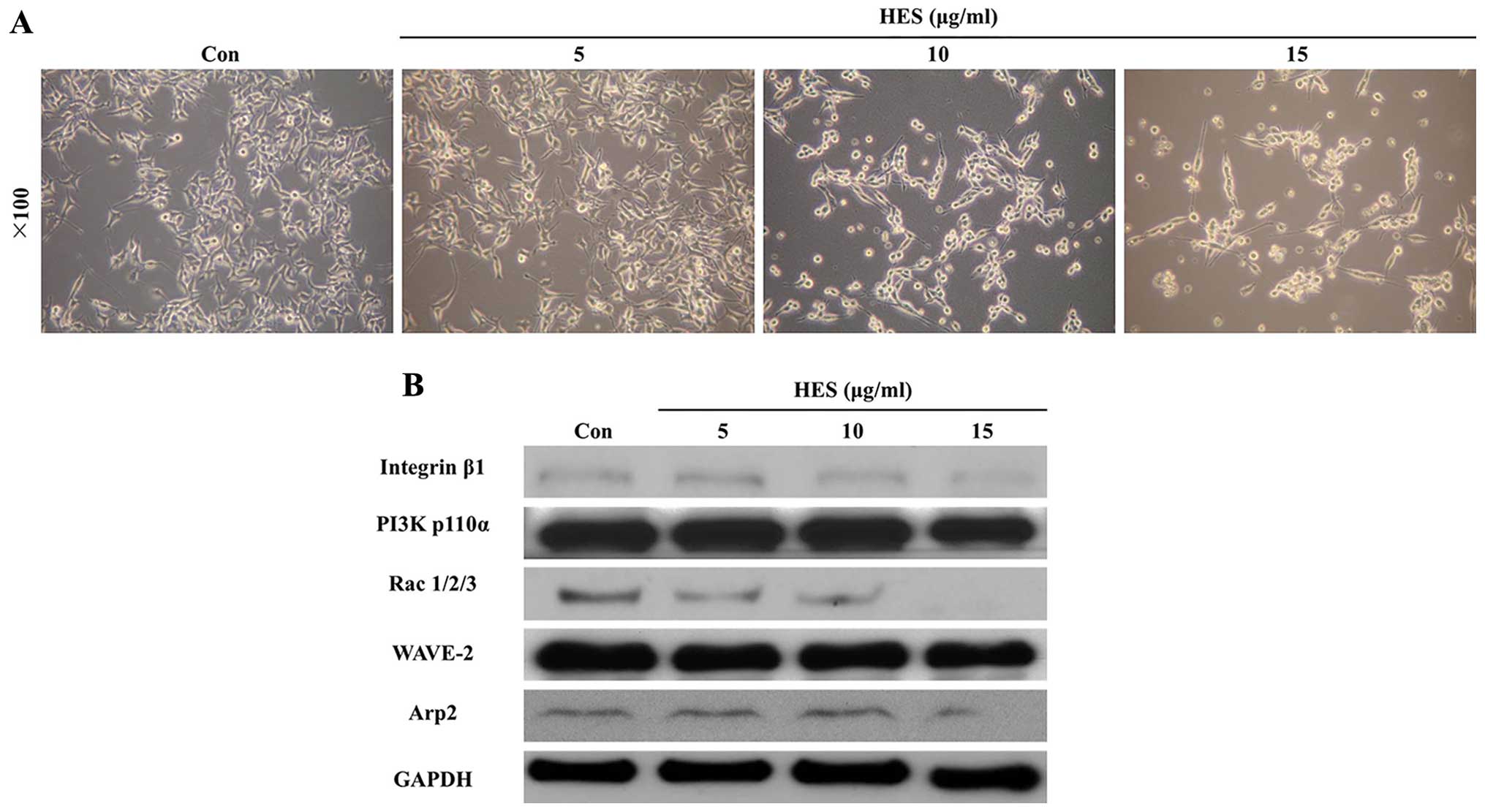
addition, morphological cell changes were observed in a
dose-dependent manner (Fig. 4A).
However, there was no inhibitory effect on cell proliferation of
the noncancerous HEK293 cells, indicating that HES particularly has
antiproliferation effects on U87MG cells (Fig. 2B).
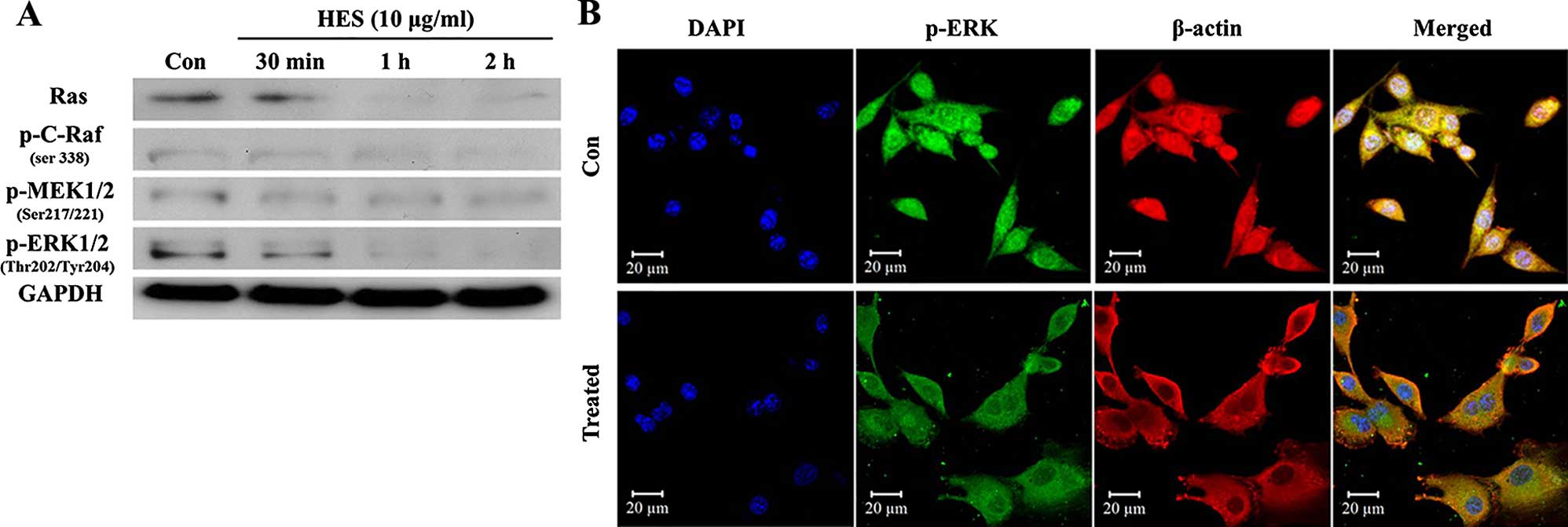
Antiproliferative effect of HES is
mediated through the MAPK/ERK pathway
The MAPK/ERK cascade is closely related to
activation of transcription factors and leads to cell
proliferation. In the present study, we investigated the effect of
HES on the MAPK/ERK pathway using western blot analysis. As shown
in Fig. 3A, the expression of Ras
and phosphorylation of C-Raf, MEK and ERK were downregulated by HES
in a time-dependent manner. Furthermore, the expression level of
phosphorylated ERK and translocation to the nucleus were also
decreased by HES (Fig. 3B). Thus,
our results indicated that HES suppressed cell proliferation via
regulation of the MAPK/ERK pathway in U87MG cells.
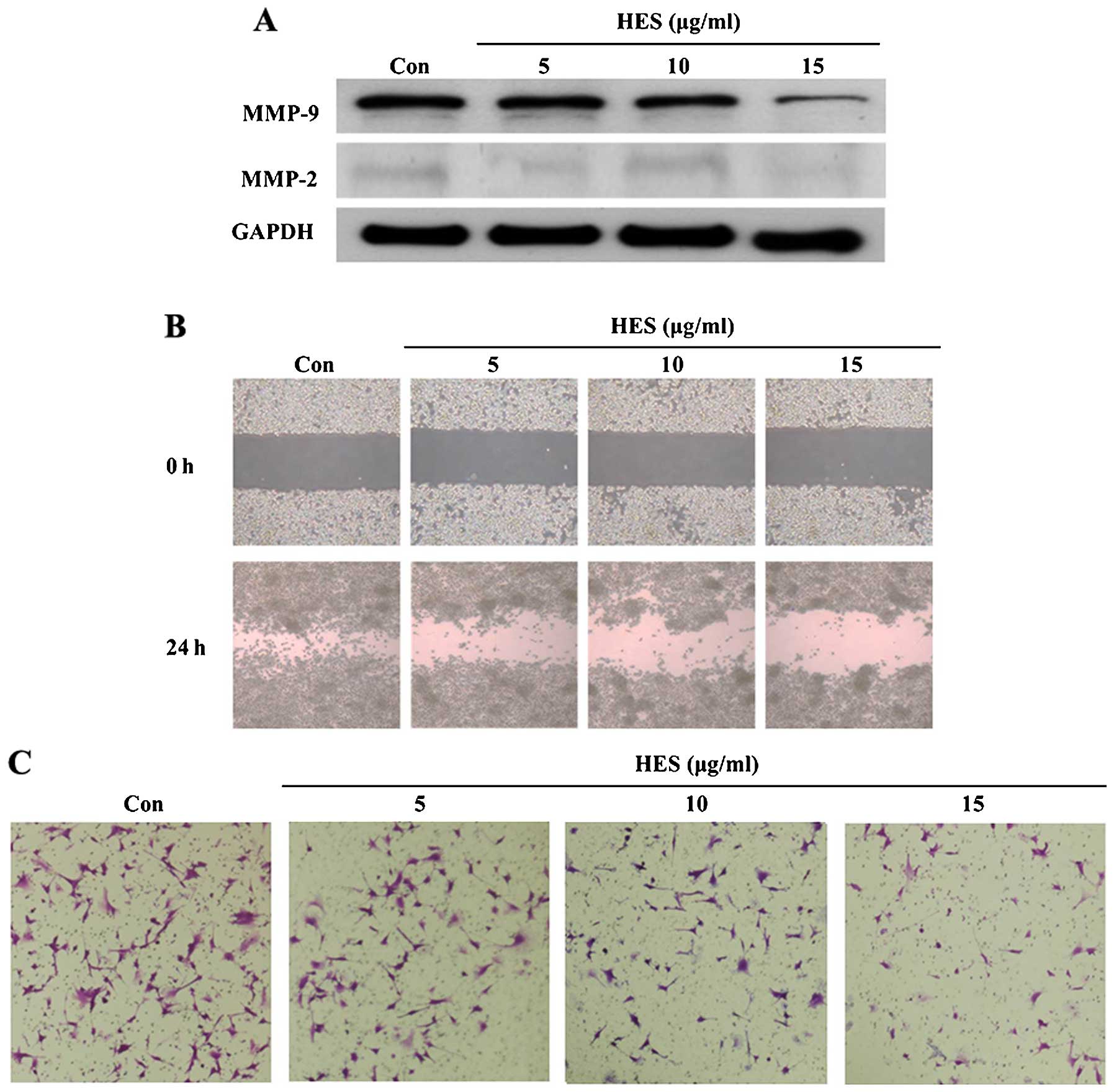
Effect of HES on the degradation of the
cytoskeleton
As HES induced morphological cell change (Fig. 4A), we hypothesized that the
expression of cytoskeleton-related proteins may be affected by HES.
Thus, we measured protein expression associated with actin dynamic
signaling using western blot analysis. As shown in Fig. 4B, actin dynamic signaling, including
integrin β1, PI3K p110α, Rac, WAVE-2 and Arp-2, was decreased by
HES in a dose-dependent manner. Therefore, these results showed
that HES induced unstabilization of the cytoskeleton and affected
cell survival through inactivation of actin dynamic signaling in
U87MG cells.
HES inhibits the invasion and migration
of U87MG cells
One of the most representative characteristics of
glioblastoma is its invasive ability, diffusing into intact brain
regions, which hinders elimination of the cancer by surgery
(18). Thus, we investigated the
inhibitory effects of HES on the metastatic ability of the U87MG
cells. As shown in Fig. 5A, protein
expression of MMP-2 and -9 was inhibited by HES in a dose-dependent
manner. Additionally, wound healing assay results showed that the
entire wound area in the HES-treated group was markedly decreased
in comparison with the control group (Fig. 5B). In the Τranswell invasion assay,
the number of invasive cells was also decreased in a dose-dependent
manner (Fig. 5C). Hence, our
findings indicated that HES inhibited cell invasion and migration
of U87MG cells via negative regulation of MMP-2 and -9
expression.
Discussion
The present study is the first to demonstrate the
anticancer potential of extracts from S. serratifolium.
Among these extracts, a hexane extract from S. serratifolium
(HES) sensitively induced inhibition of cell proliferation and
morphological change in U87MG cells (Figs. 2B and 4A).
In glioblastoma, the Ras/Raf/MEK/ERK pathway is
required for cell proliferation (18). Recently research has shown that
various agents inhibiting this signaling pathway, such as
sorafenib, an inhibitor of Raf, are able to inhibit proliferation
and induce apoptosis in glioblastoma (20). This cascade signaling pathway is
initiated by Ras activation, interacting with C-Raf and
phosphorylating at serine (Ser) 338, tyrosine (Tyr) 341, threonine
(Thr) 491 and Ser497 of C-Raf (21). Phosphorylated C-Raf directly
activates MEK1 and MEK2, Tyr and Thr/Ser dual-specificity kinases,
through phosphorylation at Ser217 and 221 (22). These kinases subsequently
phosphorylate Thr202 and Tyr204 residues in p44/42 MAPK, ERK1/2,
leading to phosphorylation of p90 ribosomal S6 kinase (p90RSK)
which phosphorylates cAMP response element-binding protein (CREB)
(23). ERK and CREB kinase
phosphorylate transcription factors translocating to the nucleus
for gene transcription (24). Our
results demonstrated that the antiproliferative effects of HES were
exerted by suppression of MAPK/ERK cascade signaling (Fig. 3).
The cytoskeleton is essential for morphological
change, growth, survival, cell motility and cancer metastasis
(9,25). Hence, regulation of the cell
cytoskeleton and morphological changes may be used to treat
metastatic tumors (26). Actin
dynamic signaling is one of the crucial signaling pathways for
regulation of the cell cytoskeleton (27). This signaling is initiated by
integrin β1 which is located in the extracellular membrane and
converts extracellular stimuli into intracellular signals (28). Integrin β1 regulates expression of
phosphoinositide 3-kinase (PI3K) p110α and Rac (29,30).
Rac, Rho-family of small G proteins, regulates the cytoskeleton
through activation of WAVE-2 leading to activation of Arp-2,
consequently facilitating morphological change (31–33).
This change regulates cytoskeletal stability, cell structure, cell
motility and cell death (34,35).
Morphological change in the U87MG cells was observed following
treatment with HES (Fig. 4A).
Western blot analysis results also revealed that HES suppressed
reorganization of the cytoskeleton through regulation of actin
dynamic signaling, including integrin β1, PI3K p110α, Rac1, WAVE-2
and Arp-2, resulting in inhibition of cell survival, growth and
motility (Fig. 4B). Arp-2 and
WAVE-2 are closely related to lamellipodium formation which
regulates cell motility and invasion (36). In addition, degradation of the ECM
by matrix metalloproteinases (MMPs) is required for the migration
and invasion of metastatic cancer cells. MMPs, zinc-dependent
endopeptidases, promote invasion and metastasis of cancer cells
(37,38). Among the members of the MMP family,
MMP-2 and -9 are crucial for degradation of ECM (39). Therefore, western blot analysis,
Τranswell invasion and wound healing assays were performed to
demonstrate the effects of HES on cell migration and invasion of
the U87MG cells. As expected, the expression levels of MMP-2 and -9
were decreased in the glioblastoma cells following treatment with
HES in a dose-dependent manner (Fig.
5A). Likewise, HES showed inhibitory effects on cell migration
and invasion of the U87MG cells (Fig.
5B and C).
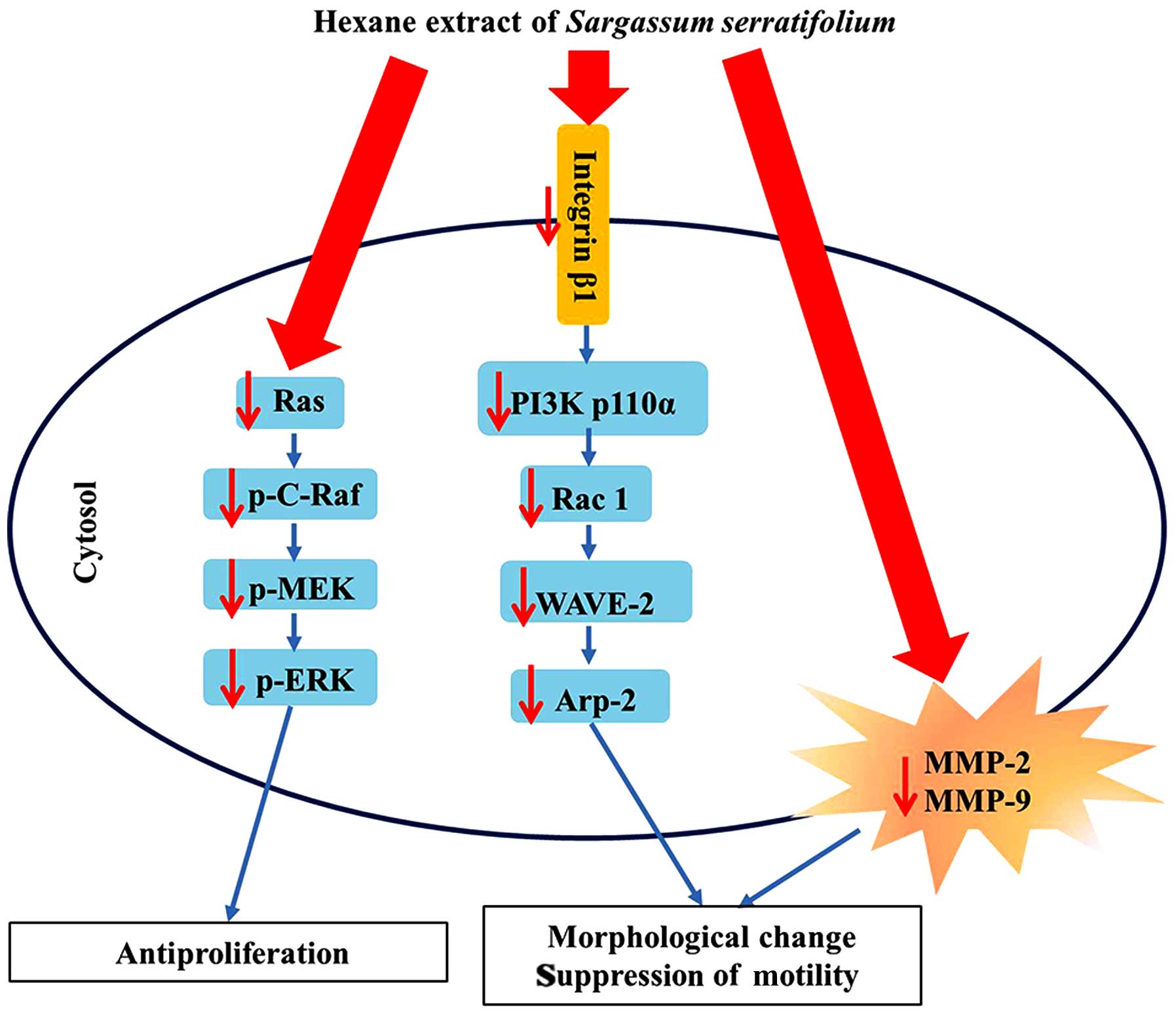
Taken together, the present study confirmed that HES
has anticancer potential through regulation of the MAPK/ERK and
actin dynamic pathways in human glioblastoma U87MG cells (Fig. 6). These findings demonstrated that
HES may be used as a candidate anticancer agent for the treatment
of human glioblastoma. To further analyze the detailed anticancer
mechanisms of extracts from Sargarssuim serratifolium, we
plan to use fractions of HES and investigate the effects of each
fraction on glioblastoma.
Acknowledgments
The present study was supported by a Research Grant
from KIOST-PKNU (Pukyong National University; 2015).
References
|
1
|
Black PM: Brain tumors. Part 1. N Engl J
Med. 324:1471–1476. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaboronok A, Isobe T, Yamamoto T, Sato E,
Takada K, Sakae T, Tsurushima H and Matsumura A: Proton beam
irradiation stimulates migration and invasion of human U87
malignant glioma cells. J Radiat Res. 55:283–287. 2014. View Article : Google Scholar
|
|
3
|
Papi A, Bartolini G, Ammar K, Guerra F,
Ferreri AM, Rocchi P and Orlandi M: Inhibitory effects of retinoic
acid and IIF on growth, migration and invasiveness in the U87MG
human glioblastoma cell line. Oncol Rep. 18:1015–1021.
2007.PubMed/NCBI
|
|
4
|
Liebmann C: Regulation of MAP kinase
activity by peptide receptor signalling pathway: Paradigms of
multiplicity. Cell Signal. 13:777–785. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernards A and Settleman J: GAP control:
Regulating the regulators of small GTPases. Trends Cell Biol.
14:377–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Blalock WL, Weinstein-Oppenheimer C, Chang
F, Hoyle PE, Wang XY, Algate PA, Franklin RA, Oberhaus SM, Steelman
LS and McCubrey JA: Signal transduction, cell cycle regulatory, and
anti-apoptotic pathways regulated by IL-3 in hematopoietic cells:
Possible sites for intervention with anti-neoplastic drugs.
Leukemia. 13:1109–1166. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing J, Ginty DD and Greenberg ME:
Coupling of the RAS-MAPK pathway to gene activation by RSK2, a
growth factor-regulated CREB kinase. Science. 273:959–963. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roux PP, Ballif BA, Anjum R, Gygi SP and
Blenis J: Tumor-promoting phorbol esters and activated Ras
inactivate the tuberous sclerosis tumor suppressor complex via p90
ribosomal S6 kinase. Proc Natl Acad Sci USA. 101:13489–13494. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fletcher DA and Mullins RD: Cell mechanics
and the cytoskeleton. Nature. 463:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamazaki D, Kurisu S and Takenawa T:
Regulation of cancer cell motility through actin reorganization.
Cancer Sci. 96:379–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mullins RD: How WASP-family proteins and
the Arp2/3 complex convert intracellular signals into cytoskeletal
structures. Curr Opin Cell Biol. 12:91–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu C, Asokan SB, Berginski ME, Haynes EM,
Sharpless NE, Griffith JD, Gomez SM and Bear JE: Arp2/3 is critical
for lamellipodia and response to extracellular matrix cues but is
dispensable for chemotaxis. Cell. 148:973–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Machesky LM: Lamellipodia and filopodia in
metastasis and invasion. FEBS Lett. 582:2102–2111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curran S and Murray GI: Matrix
metalloproteinases in tumour invasion and metastasis. J Pathol.
189:300–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin HH, Chen JH, Chou FP and Wang CJ:
Protocatechuic acid inhibits cancer cell metastasis involving the
down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by
targeting RhoB activation. Br J Pharmacol. 162:237–254. 2011.
View Article : Google Scholar :
|
|
16
|
Liu L, Heinrich M, Myers S and Dworjanyn
SA: Towards a better understanding of medicinal uses of the brown
seaweed Sargassum in Traditional Chinese Medicine: A phytochemical
and pharmacological review. J Ethnopharmacol. 142:591–619. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang GJ, Yang CM, Chang YS, Amagaya S,
Wang HC, Hou WC, Huang SS and Hu ML: Hispolon suppresses SK-Hep1
human hepatoma cell metastasis by inhibiting matrix
metallo-proteinase-2/9 and urokinase-plasminogen activator through
the PI3K/akt and ERK signaling pathways. J Agric Food Chem.
58:9468–9475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke J, Butowski N and Chang S: Recent
advances in therapy for glioblastoma. Arch Neurol. 67:279–283.
2010.PubMed/NCBI
|
|
20
|
Carra E, Barbieri F, Marubbi D, Pattarozzi
A, Favoni RE, Florio T and Daga A: Sorafenib selectively depletes
human glioblastoma tumor-initiating cells from primary cultures.
Cell Cycle. 12:491–500. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chong H, Lee J and Guan KL: Positive and
negative regulation of Raf kinase activity and function by
phosphorylation. EMBO J. 20:3716–3727. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alessi DR, Saito Y, Campbell DG, Cohen P,
Sithanandam G, Rapp U, Ashworth A, Marshall CJ and Cowley S:
Identification of the sites in MAP kinase kinase-1 phosphorylated
by p74raf-1. EMBO J. 13:1610–1619. 1994.PubMed/NCBI
|
|
23
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bonni A, Brunet A, West AE, Datta SR,
Takasu MA and Greenberg ME: Cell survival promoted by the Ras-MAPK
signaling pathway by transcription-dependent and -independent
mechanisms. Science. 286:1358–1362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colomba A and Ridley AJ: Analyzing the
roles of Rho GTPases in cancer cell migration with a live cell
imaging 3D-morphology-based assay. Methods Mol Biol. 1120:327–337.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Najm P and El-Sibai M: Palladin regulation
of the actin structures needed for cancer invasion. Cell Adh Migr.
8:29–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Edwards KA, Demsky M, Montague RA,
Weymouth N and Kiehart DP: GFP-moesin illuminates actin
cytoskeleton dynamics in living tissue and demonstrates cell shape
changes during morphogenesis in Drosophila. Dev Biol. 191:103–117.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harburger DS and Calderwood DA: Integrin
signalling at a glance. J Cell Sci. 122:159–163. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyamoto S, Teramoto H, Coso OA, Gutkind
JS, Burbelo PD, Akiyama SK and Yamada KM: Integrin function:
Molecular hierarchies of cytoskeletal and signaling molecules. J
Cell Biol. 131:791–805. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Legate KR and Fässler R: Mechanisms that
regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell
Sci. 122:187–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kurisu S, Suetsugu S, Yamazaki D,
Yamaguchi H and Takenawa T: Rac-WAVE2 signaling is involved in the
invasive and metastatic phenotypes of murine melanoma cells.
Oncogene. 24:1309–1319. 2005. View Article : Google Scholar
|
|
33
|
Miki H, Suetsugu S and Takenawa T: WAVE, a
novel WASP-family protein involved in actin reorganization induced
by Rac. EMBO J. 17:6932–6941. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pollard TD and Cooper JA: Actin, a central
player in cell shape and movement. Science. 326:1208–1212. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang F, Liu DZ, Xu H, Li Y, Wang W, Liu BL
and Zhang LY: Thapsigargin induces apoptosis by impairing
cytoskeleton dynamics in human lung adenocarcinoma cells.
Scientific World Journal. 2014:6190502014.PubMed/NCBI
|
|
36
|
Small JV, Stradal T, Vignal E and Rottner
K: The lamellipodium: Where motility begins. Trends Cell Biol.
12:112–120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen JH, Lin HH, Chiang TA, Hsu JD, Ho HH,
Lee YC and Wang CJ: Gaseous nitrogen oxide promotes human lung
cancer cell line A549 migration, invasion, and metastasis via
iNoS-mediated MMP-2 production. Toxicol Sci. 106:364–375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Westermarck J and Kähäri VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEB J.
13:781–792. 1999.PubMed/NCBI
|
|
39
|
Parks WC and Shapiro SD: Matrix
metalloproteinases in lung biology. Respir Res. 2:10–19. 2001.
View Article : Google Scholar : PubMed/NCBI
|