Introduction
Breast cancer is one of the leading causes of
cancer-related deaths in women. Breast cancer is a group of
heterogeneous diseases with various responses to chemotherapy
(1). Cancer stem cells (CSCs) are
the tumor-initiating cells that are believed to be responsible for
breast cancer recurrence, metastasis, and drug resistance (2,3). CSCs
express specific biomarkers such as CD44, aldehyde dehydrogenase
1A1 (ALDH1A1), and β-catenin (4–6).
Expression of these CSC markers has prognostic values for the
recurrence, metastasis, and therapeutic resistance in various
tumors such as lung, hepatocellular, and breast cancer (7–9).
ALDH1, a member of a family of
NAD(P)+-dependent enzymes that detoxify a wide variety
of aldehydes, have been found to be associated with poor prognosis
in breast cancer (10). ALDH1A1 has
been extensively studied, and is found to be associated with tumor
progression and poor prognosis of breast cancer patients (11,12).
ALDH1A1 plays an important role in the proliferation of breast CSCs
and resistance to chemotherapy, particularly cyclophosphamide
(13,14). However, the signaling pathways that
regulate the expression of ALDH1A1 in breast cancer remain
unclear.
Recently, Condello et al have reported that
β-catenin upregulates the expression of ALDH1A1, and
β-catenin-regulated ALDH1A1 contributes to the maintenance of
ovarian cancer spheroids (15).
β-catenin is located in the intracellular side of the cytoplasmic
membrane, and plays an important role in cell-to-cell adhesion by
linking the cytoskeleton and cadherin (16). It is well known that β-catenin is
involved in the Wnt signaling pathway that plays an active role in
CSCs and carcinogenesis of various tumors such as breast cancer
(17,18) and ovarian cancer (19). Activation of Wnt signaling leads to
translocation of β-catenin from the membrane to the cytoplasm and
nucleus, where it promotes transcription of many genes that are
associated with cancer growth, invasion, progression, and invasion
(20). However, it remains unclear
whether β-catenin regulates the expression of ALDH1A1 in breast
cancer patients.
In the present study, we investigated the expression
of ALDH1A1 and β-catenin in 276 breast cancer patients. We studied
the association between the expression of ALDH1A1 and β-catenin in
breast cancer patients, and analyzed the correlation of their
combined expression with clinicopathological features,
chemotherapeutic responses, and prognosis of breast cancer
patients.
Materials and methods
Patients and tissue samples
The Medical Ethics Committee of China Medical
University approved this study. Due to the retrospective nature of
the study, the Medical Ethics Committee waived the need of written
informed consents by the patients.
This study included human breast tissues from 276
female patients with breast cancer, who underwent surgery at the
First Affiliated Hospital of China Medical University between 2004
and 2008. Breast cancer was diagnosed based on pathological
staining. The patient age, menopausal status, tumor type, tumor
size, and lymph node metastasis were retrospectively obtained from
medical records. Of the 276 breast cancer patients, 220 had
invasive ductal carcinoma, 30 had invasive lobular carcinoma, and
26 had other types of tumors including mucinous, medullary, and
cribriform carcinoma. The histological grade of the cancer was
determined according to the World Health Organization grading
system. The stage of the cancer was evaluated according to the TNM
staging system.
No patients underwent radiation therapy and
chemotherapy before surgery. After surgery, 165 patients received
adjuvant chemotherapy, and the remaining 11 patients did not
receive chemotherapy. All chemotherapy regimens contained
cyclophosphamide, including CMF (cyclophosphamide + methotrexate +
fluorouracil, n=65), AC (adriamycin + cyclophosphamide, n=32), CEF
(cyclophosphamide + epimbicin + fluorouracil, n=32), CE
(cyclophosphamide + epimbicin, n=20), and CAF (cyclophosphamide +
adriamycin + fluorouracil, n=18).
Tissue microarray (TMA)
Paraffinized donor blocks were stained with
hematoxylin and eosin, and representative breast cancer samples
were selected by reviewing the hematoxylin and eosin-stained
blocks. Tissue cores with a diameter of 1.5 mm were extracted from
each donor block, and precisely arrayed into a new paraffin
recipient block with a maximum of 200 cores, using the Organization
Microarrayer (Pathology Devices, USA).
Immunohistochemistry
Immunohistochemistry was performed as previously
described (21). Briefly, sections
(4-µm thick) were obtained from formalin-fixed and
paraffin-embedded TMA recipient blocks, and mounted on
poly-L-lysine-coated glass slides. Sections were deparaffinized
with xylene, rehydrated in a graded alcohol series, and heated in a
microwave oven to retrieve antigen. Sections were incubated with
primary antibodies against ALDH1A1 (mouse anti-human monoclonal
antibodies, 1:100 dilution; Abcam, UK) and β-catenin (mouse
anti-human monoclonal antibodies, 1:200 dilution; Cell Signaling
Technology, USA) overnight at 4°C, followed by incubation with
biotinylated secondary antibodies for 30 min at 37°C. Sections were
then incubated with streptavidin horseradish peroxidase for
additional 30 min (LSAB kit; Dako, Glostrup, Denmark), and stained
with 3,3-diaminobenzidine (DAB). Sections were counterstained with
hematoxylin, dehydrated, and mounted. Negative control sections
were treated in the same conditions, except no primary
antibodies.
Evaluation of immunohistochemistry
The immunostaining was examined under a light
microscope, and was evaluated by two pathologists blinded to the
experimental conditions. The immunoreactivity intensity was scored
as follows: 0 for negative staining, 1 for weak positive staining,
2 for moderate positive staining, and 3 for strong positive
staining. The percentage of stained cells was scored 0–100%. The
final immunoreactive score was calculated by multiplying the
intensity score with the score for the percentage of positively
stained cells, ranging from 0 to 300%. The scores were assigned by
using 5% increments (0, 5, 10, and 300%) as previously reported
(22,23). The final immunoreactive scores were
used to determine the cut-off value for discriminating tumors with
the high expression of ALDH1A1 or β-catenin from those with low
expression, using receiver operating characteristic (ROC) curves.
To discriminate tumors with the cytoplasmic expression of β-catenin
from tumor with the membrane expression of β-catenin, the
percentage of β-catenin membrane expression for each sample was
used to determine the cut-off value, using ROC curves. The
percentage of β-catenin membrane expression was calculated as
follows: β-catenin membrane expression % = The number of cells with
membrane expression/(the number of cells with membrane expression +
the number of cells with cytoplasmic expression) x 100%. The
sensitivity and specificity for the survival status (alive or dead)
of breast cancer patients was plotted to generate ROC curves.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). For data with normal distribution,
Student's t-test was used to compare the difference in the means.
For data with unequal variance, Wilcoxon rank sum tests or
Kruskal-Wallis tests were used to compare the difference. The
association between the expression of ALDH1A1 and β-catenin and
clinicopathological characteristics of breast cancer patients was
evaluated using the Pearson's Chi-square test or Fisher's exact
probability test. The Pearson's rank correlation analysis was
applied to assess the association between the expression of ALDH1A1
and β-catenin. The Kaplan-Meier curves were plotted to demonstrate
the survival difference, and the survival probabilities were
assessed by a log-rank test. To assess the association of potential
confounding variables with the prognosis, overall survival (OS), or
disease-free survival (DFS), univariate and multivariate Cox
proportional hazards regression models were used. The OS was
defined as the time between the day of diagnosis and the
disease-related death, or last known follow-up. The DFS was
calculated as the time between the day of diagnosis and the
occurrence of local recurrence or distant metastasis. Probability
values ≤0.05 were considered statistically significant.
Results
Clinicopathological characteristics
Table I summarizes
clinicopathological characteristics of 276 breast cancer patients.
The average age of breast cancer patients was 50.1±7.9 years
(range, 31–82 years). Of the 276 patients, the age, menopausal
status, tumor size, tumor type, histological grade, TNM stage, and
lymph node metastasis were recorded in 276, 266, 229, 276, 267,
197, and 266 patients, respectively. The majority of these patients
had a tumor with invasive ductal carcinoma (79.7%), and <2 cm in
size (62.0%). Of the 267 patients with known histological grade,
136 (50.9%) patients had grade I breast cancer, and 131 (49.1%)
patients had grade II–II breast cancer. The stage of the cancer was
evaluated in 197 patients, including 46 patients with stage I
breast cancer, 122 patients with stage II breast cancer, and 29
patients with stage III breast cancer. Lymph node metastasis
occurred in 91 (34.2%) of 266 patients.
 | Table IClinicopathological characteristics
of breast cancer patients. |
Table I
Clinicopathological characteristics
of breast cancer patients.
| Cases
|
|---|
| N | % |
|---|
| Age (years) | 276 | |
| ≤50 | 149 | 53.9 |
| >50 | 127 | 46.1 |
| Menopausal
status | 266 | |
| Pre-menopause | 150 | 56.4 |
|
Post-menopause | 116 | 43.6 |
| Tumor size
(cm) | 229 | |
| ≤2.0 | 142 | 62.0 |
| >2.0 | 87 | 38.0 |
| Tumor type | 276 | |
| Ductal | 220 | 79.7 |
| Lobular | 30 | 10.9 |
| Others | 26 | 9.4 |
| Histological
grade | 267 | |
| I | 136 | 50.9 |
| II–III | 131 | 49.1 |
| TNM stage | 198 | |
| I | 46 | 23.4 |
| II | 122 | 61.9 |
| III | 29 | 14.7 |
| Lymph node
metastasis | 265 | |
| No | 175 | 65.8 |
| Yes | 91 | 34.2 |
Follow-up information was available for 168 breast
cancer patients. During the follow-up period of 9–118 months,
relapses occurred in 62 cases and cancer-associated deaths were
found in 48 cases. The 5-years survival rate was 84.5%. The mean OS
and DFS were 98.5 and 91.5 months, respectively.
Expression of ALDH1A1 and β-catenin
We studied the expression of ALDH1A1 and β-catenin
in 276 samples from breast cancer patients and 80 control samples
from patients with benign hyperplasia, using immunohistochemistry
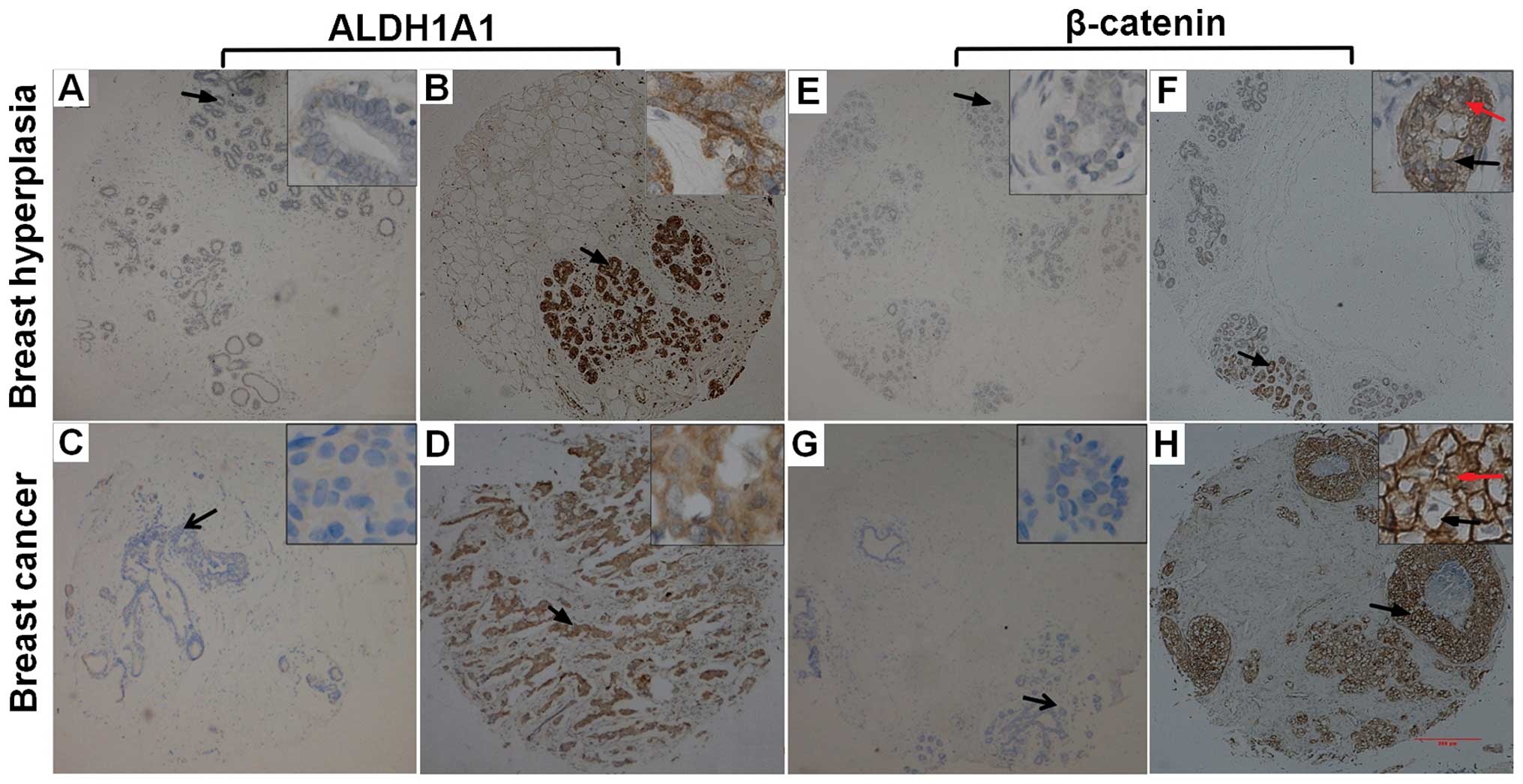
(Fig. 1). ALDH1A1-immunopositive
staining was mainly observed in the cytoplasm in both breast cancer
and control samples. ALDH1A1-immunopositive staining was observed
in 149 (54%) of 276 breast cancer samples and 25 (31.3%) of 80
control samples. ALDH1A1 immunoreactivity occurred significantly
more frequently in breast cancer samples than in control samples
(P<0.001). In contrast, β-catenin immunoreactivity was observed
in the membrane and cytoplasm in both breast cancer samples and
control samples. β-catenin immunoreactivity was observed in 246
(89.1%) of 276 breast cancer samples and 72 (90.0%) of 80 control
samples. There was no significant difference of
β-catenin-immunopositive staining between breast cancer samples and
control samples (P= 0.824).
Selection of the cut-off value for the
expression of ALDH1A1 and β-catenin
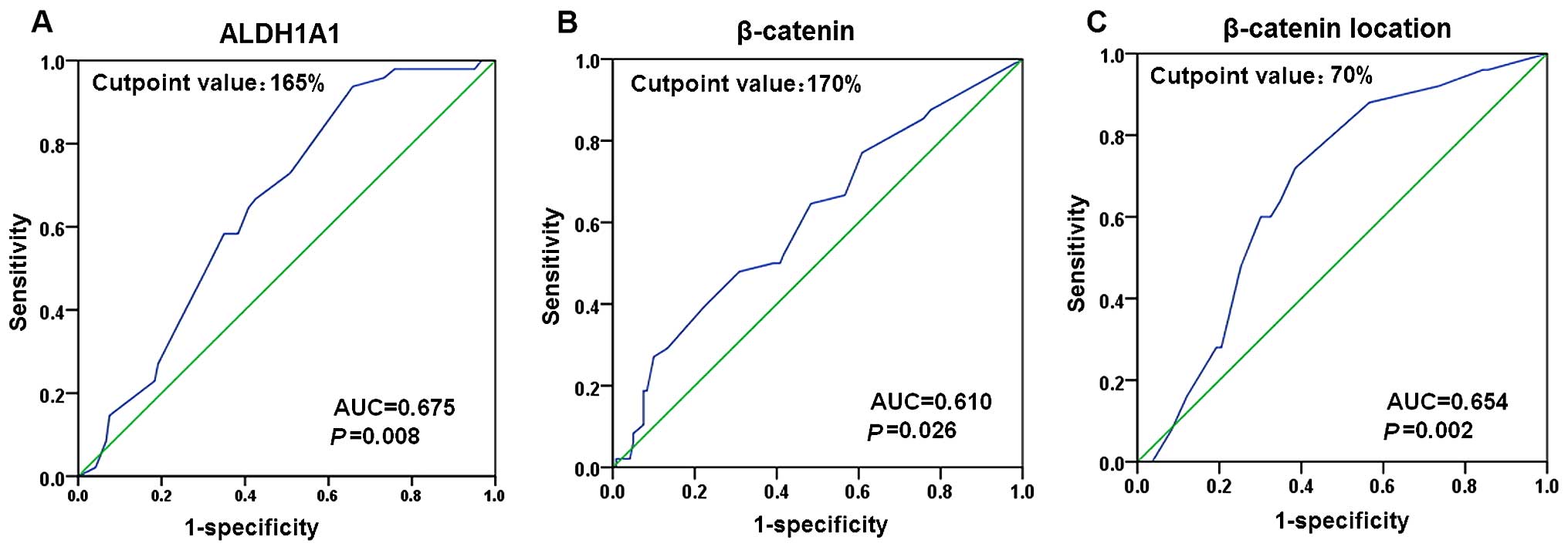
ROC curve analysis was performed to determine an
optimal cut-off score for the expression of ALDH1A1 and β-catenin
in breast cancer samples. Based on the survival status, a cut-off
score of 165 and 170% were selected for the expression of ALDH1A1
and β-catenin, respectively (Fig. 2A
and B). Tumors with immunohistological scores ≥165 and <165%
were defined as tumors with 'high' [ALDH1A1(high)] and 'low'
[ALDH1A1(low)] expression of ALDH1A1, respectively. Tumors with
immunohistological scores ≥170 and <170% were defined as tumors
with 'high' [β-catenin(high)] and 'low' [β-catenin(low)] expression
of β-catenin, respectively. In total 149 tumors (54.0%) exhibited
ALDH1A1(high) expression, and 127 (46.0%) tumors showed
ALDH1A1(low) expression; 196 (71.0%) tumors exhibited
β-catenin(high) expression, and 80 (29.0%) tumors showed
β-catenin(low) expression.
We further performed ROC analysis to determine an
optimal cut-off score for discriminating the membrane expression
from the cytoplasmic expression of β-catenin in breast cancer with
β-catenin(high) expression. Based on the survival status, a cut-off
score of 70% was selected (Fig.
2C). Tumors with the membrane expression ≥70 and <70% were
defined as tumors with β-catenin(m), and β-catenin (c) expression,
respectively. Of the 196 β-catenin(high) breast cancers, 109
(55.6%) had β-catenin(m) expression, and 87 (44.4%) had
β-catenin(c) expression.
Association between the expression of
ALDH1A1 and β-catenin
Pearson's rank correlation analysis was used to
analyze the association between the expression of ALDH1A1 and
β-catenin in breast cancer. There was no significant correlation
between the expression of ALDH1A1 and β-catenin in the breast
cancer patients (n=276). We then examined the subcellular
expression levels of β-catenin in breast cancer with
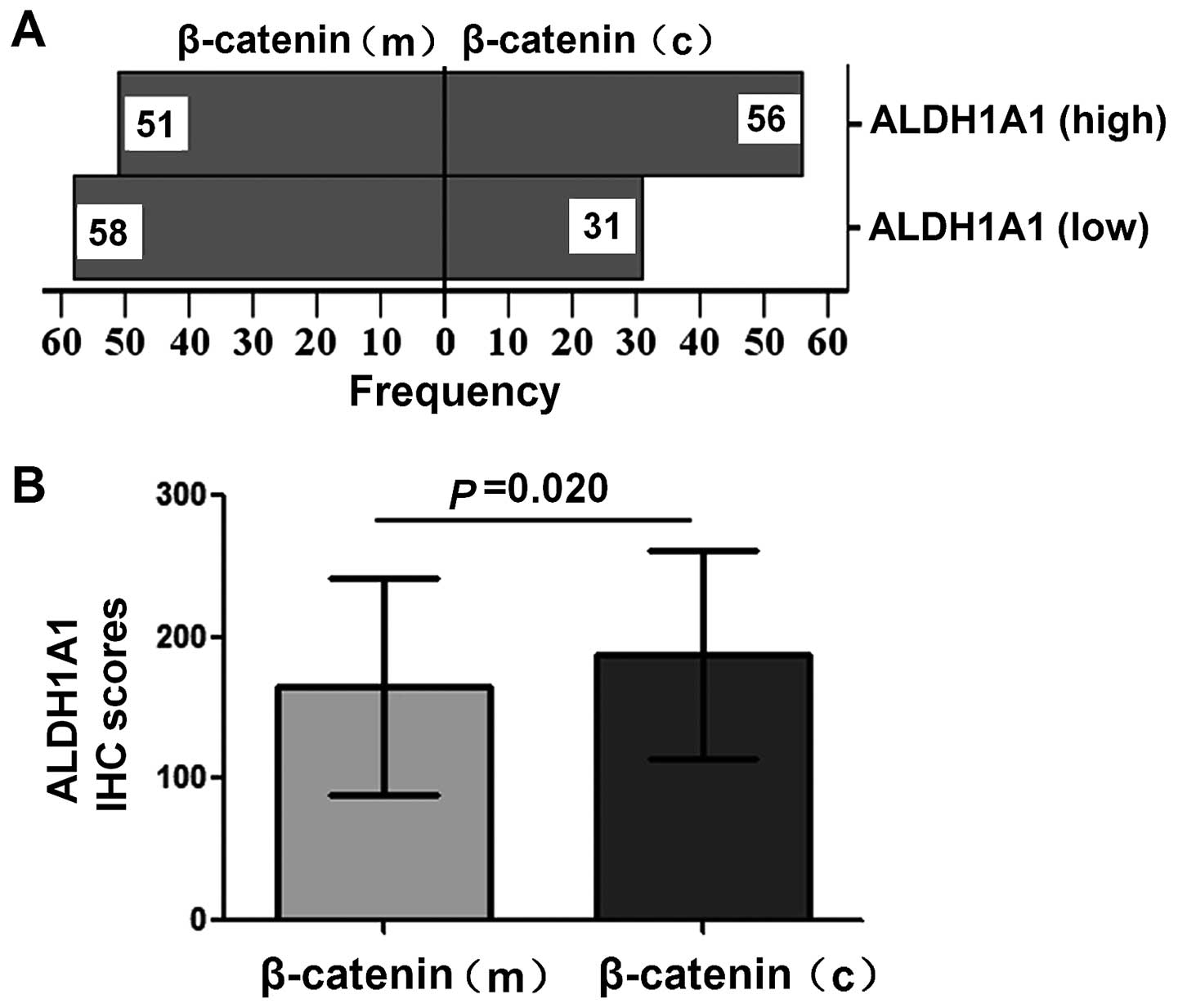
β-catenin(high) expression. In breast cancer with ALDH1A1(low)
expression, β-catenin(m) expression was observed in 58 (65.2%) of
89 cases, and β-catenin(c) expression was found in 31 (34.8%) of 89
cases. In breast cancer with ALDH1A1(high) expression, β-catenin(m)
expression was observed in 51 (47.7%) of 107 cases, and
β-catenin(c) expression was found in 56 (52.3%) of 107 cases.
β-catenin(c) expression occurred significantly more frequently in
ALDH1A1(high) breast cancer than in ALDH1A1(low) breast cancer
(Chi-square test, P= 0.014; Fig.
3A). Furthermore, the expression level of ALDH1A was
significantly higher in β-catenin(c) breast cancer than in
β-catenin(m) breast cancer (P=0.020, Fig. 3B).
Association of the expression of ALDH1A1
and β-catenin with clinicopathological characteristics of breast
cancer patients
Table II summarizes
the association between the expression of ALDH1A1 and β-catenin and
the clinicopathological characteristics of breast cancer patients.
The age, menopausal status, tumor type, tumor size, histological
grade, and TNM stage were not significantly associated with the
expression of ALDH1A1. ALDH1A1(high) expression was associated with
lymph node metastasis (P=0.029). The age, menopausal status, tumor
size, tumor type, and TNM stage were not significantly associated
with the subcellular expression of β-catenin. β-catenin(c)
expression was associated with grade II–III tumors (P=0.014) and
lymph node metastasis (P=0.002).
 | Table IIAssociation of β-catenin localization
and ALDH1A1 expression with clinicopathological features of breast
cancer. |
Table II
Association of β-catenin localization
and ALDH1A1 expression with clinicopathological features of breast
cancer.
| ALDH1A1
| β-catenin
localization
|
|---|
| High (n/%) | Low (n/%) | P-value | β-catenin (c)
(n/%) | β-catenin (m)
(n/%) | P-value |
|---|
| Age at diagnosis
(years) | | | 0.690 | | | 0.364 |
| ≤50 | 80/55.2 | 65/44.8 | | 42/36.2 | 61/63.8 | |
| >50 | 67/52.8 | 60/47.2 | | 43/47.3 | 48/52.7 | |
| Menopausal
state | | | 0.736 | | | 0.908 |
| Pre-menopause | 82/54.7 | 68/45.3 | | 47/43.5 | 61/56.5 | |
|
Post-menopause | 61/52.6 | 55/47.4 | | 35/42.7 | 47/57.3 | |
| Tumor size
(cm) | | | 0.729 | | | 0.622 |
| ≤2.0 | 75/52.8 | 67/47.2 | | 45/35.5 | 58/64.5 | |
| >2.0 | 48/55.2 | 39/44.8 | | 30/47.6 | 33/52.4 | |
| Tumor type | | | 0.065 | | | 0.102 |
| Ductal | 109/49.5 | 111/50.5 | | 76/48.1 | 82/51.9 | |
| Lobular | 20/66.7 | 10/33.3 | | 6/30.0 | 14/70.0 | |
| Others | 18/78.2 | 5/21.8 | | 5/27.8 | 13/72.2 | |
| Histological
grade | | | 0.876 | | | 0.014 |
| I | 75/55.1 | 61/44.9 | | 35/36.1 | 62/63.9 | |
| II–III | 71/54.2 | 60/45.8 | | 49/53.8 | 42/46.2 | |
| TNM stage | | | 0.267 | | | 0.139 |
| I–II | 87/51.5 | 82/48.5 | | 48/40.0 | 72/60.0 | |
| III | 17/63.0 | 10/37.0 | | 9/60.0 | 6/40.0 | |
| Lymph node
metastasis | | | 0.029 | | | 0.002 |
| No | 85/48.6 | 90/51.4 | | 48/36.5 | 84/63.5 | |
| Yes | 57/62.6 | 34/37.4 | | 36/60.0 | 24/40.0 | |
Table III
summarizes the association of combined expression of ALDH1A1 and
β-catenin with lymph node metastasis. Compared with tumors with
ALDH1A1(low) and β-catenin(m) expression and ALDH1A1(low) and
β-catenin(c) expression, tumors with ALDH1A1(high) and β-catenin(c)
expression was associated with lymph node metastasis (P<0.05,
Table III). Compared with tumors
with ALDH1A1(high) and β-catenin(m) expression, tumors with
ALDH1A1(high) and β-catenin(c) expression exhibited a tendency
toward more lymph node metastasis (P=0.076, Table III).
 | Table IIIAssociation of the combination of
β-catenin location and ALDH1A1 with lymph node metastasis in breast
cancer. |
Table III
Association of the combination of
β-catenin location and ALDH1A1 with lymph node metastasis in breast
cancer.
| Features | Node metastasis
| P-valueb
|
|---|
| No (%)a | Yes (%)a | | | |
|---|
| ALDH1A1(low) and
β-catenin(m) | 47 (82.5) | 10 (17.5) | 0.003 | | |
| ALDH1A1(low) and
β-catenin(c) | 37 (72.5) | 14 (27.5) | 0.216c | | |
| ALDH1A1(high) and
β-catenin(m) | 21 (70.0) | 9 (30.0) | 0.181c | 0.806d | |
| ALDH1A1(high) and
β-catenin(c) | 27 (50.0) | 27 (50.0) |
<0.001c |
0.018d | 0.076e |
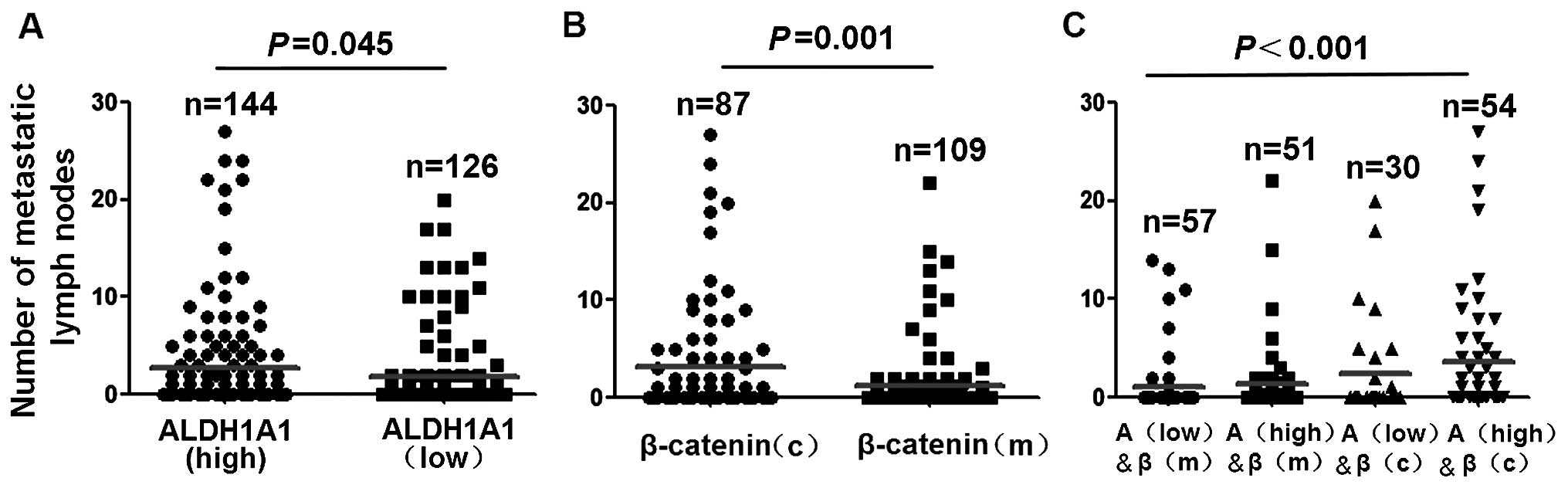
We then examined the effect of ALDH1A1 and β-catenin
expression on the number of metastatic lymph nodes in breast
cancer. The number of metastatic lymph nodes was significantly
higher in patients with ALDH1A1(high) expression compared with
patients with ALDH1A1(low) expression (P=0.045, Fig. 4A). The number of metastatic lymph
nodes was significantly higher in patients with β-catenin(c)
expression compared with patients with β-catenin(m) expression
(P=0.001, Fig. 4B). Patients with
ALDH1A1(high) and β-catenin(c) expression had significantly more
metastatic lymph nodes compares with tumors with other combined
expression of ALDH1A and β-catenin (P<0.001, Fig. 4C).
Association of the expression of ALDH1A1
and β-catenin with the survival of breast cancer patients
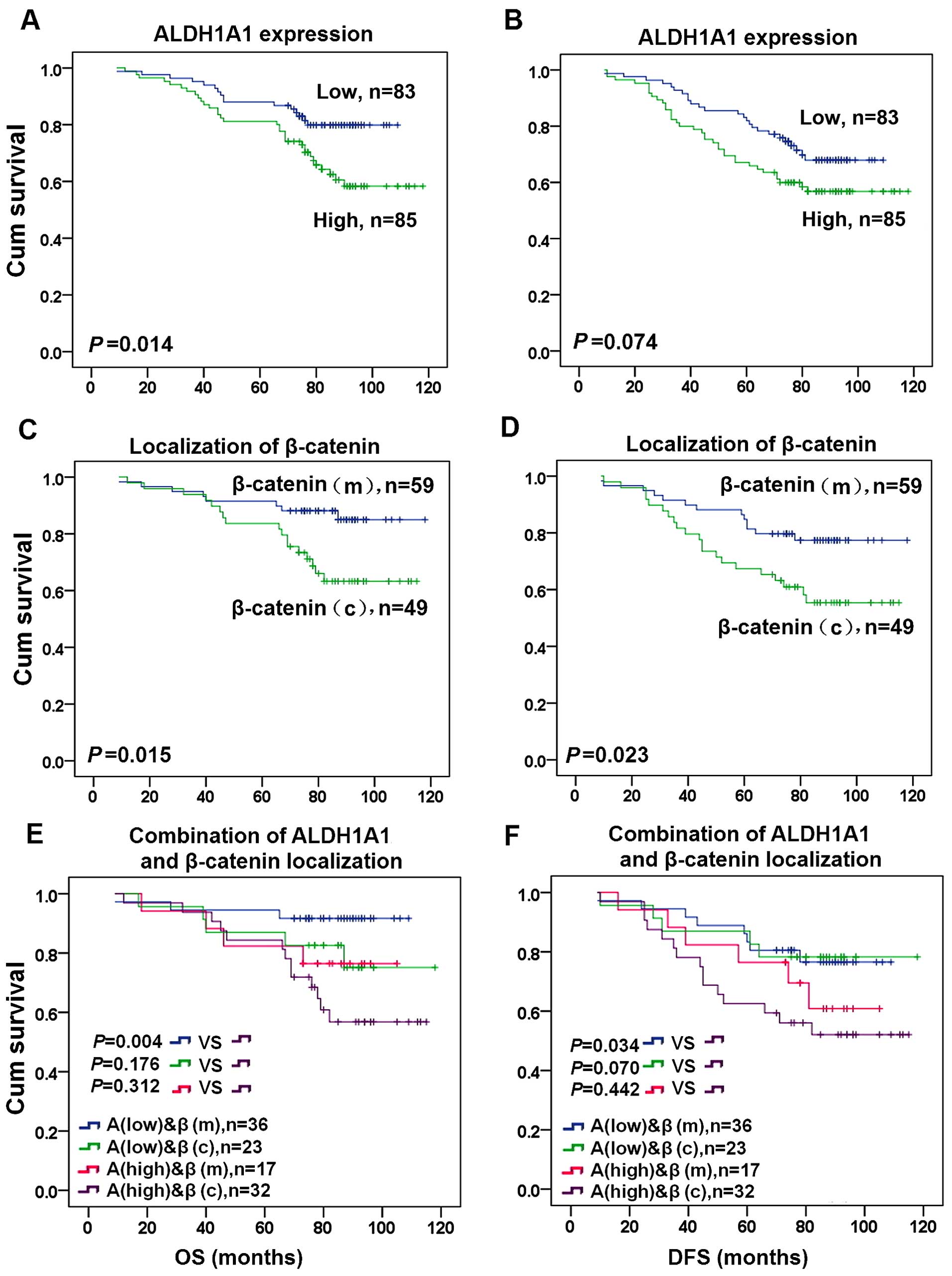
The Kaplan-Meier analysis and log-rank test were
used to evaluate the association of the expression level of ALDH1A1
and β-catenin with the OS or DFS in breast cancer patients.
ALDH1A1(high) expression was associated with significantly shorter
OS (P=0.014, Fig. 5A). Although
ALDH1A1(high) expression was associated with a tendency toward
shorter DFS in breast cancer patients, no significant difference
was found (P=0.074, Fig. 5B). In
addition, β-catenin(c) expression was associated with significantly
shorter OS (P=0.015, Fig. 5C) and
DFS (P=0.023, Fig. 5D). We further
examined the association of the combined expression of ALDH1A1 and
β-catenin with the OS or DFS in breast cancer patients. Compared
with ALDH1A1(low) and β-catenin(m) expression, ALDH1A1(high) and
β-catenin(c) expression was associated with shorter OS and DFS in
breast cancer patients (P<0.05, Fig.
5E and F).
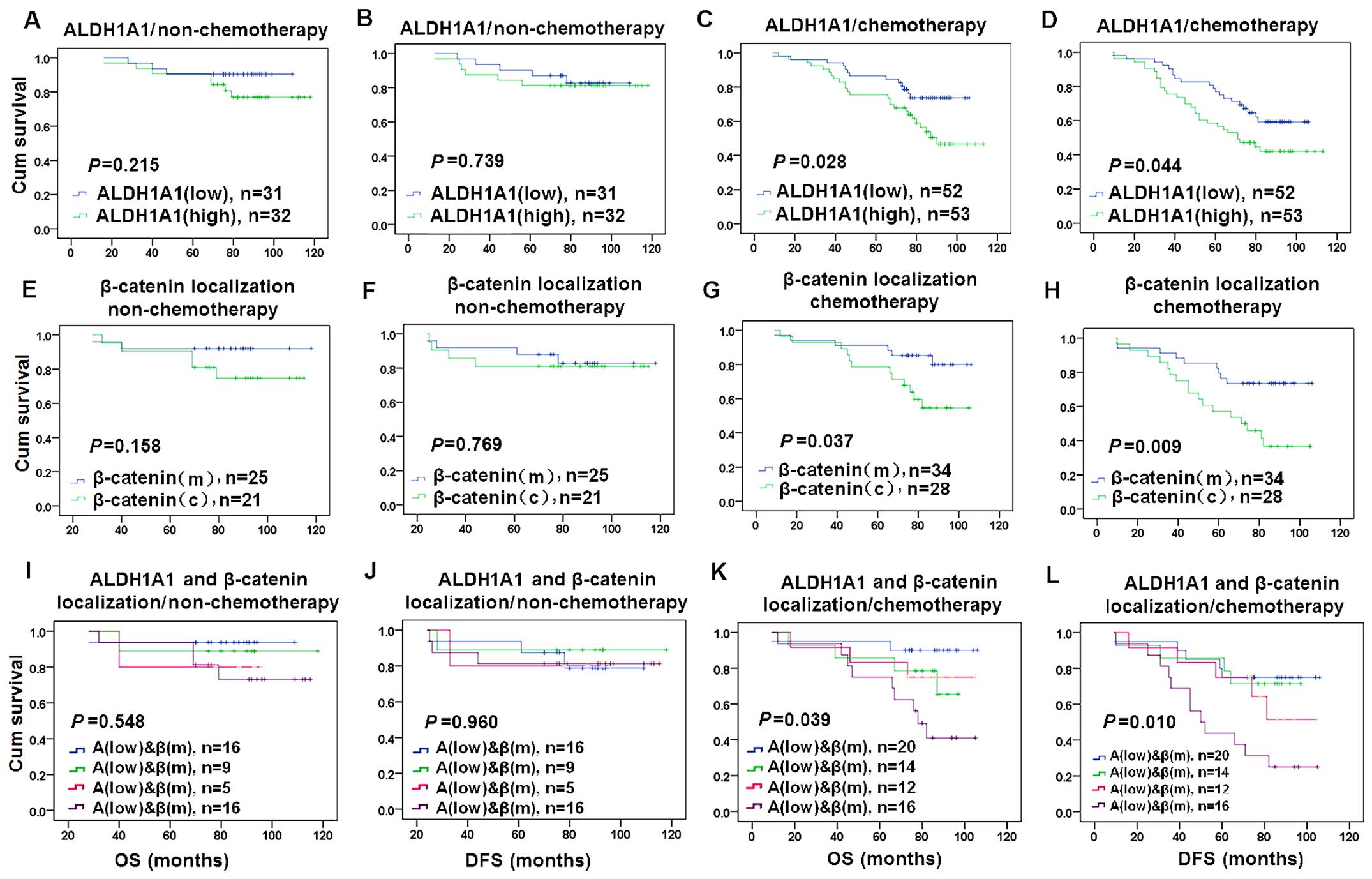
We examined the association of the expression of
ALDH1A1 and β-catenin with the therapeutic response in breast
cancer patients who received cyclophosphamide treatment. ALDH1A1
and β-catenin alone and their combined expression were not
significantly associated with the OS and DFS in breast cancer
patients with non-cyclophosphamide therapy (Fig. 6A, B, E, F, I and J). ALDH1A1(high)
expression was associated with shorter OS (P=0.004) and DFS
(P=0.002) in patients with cyclophosphamide treatment (Fig. 6C and D). β-catenin(c) expression was
associated with shorter OS (P=0.021) and DFS (P=0.003) in patients
with cyclophosphamide treatment (Fig.
6G and H). Combined ALDH1A1(high) and β-catenin(c) expression
was associated with shorter OS (P=0.020) and DFS (P=0.012) in
patients with cyclophosphamide treatment compared with other
combined expression of ALDH1A1 and β-catenin (Fig. 6K and L).
The univariate analysis identified that the
menopausal status, tumor size, histological stage, TNM stage, and
lymph node metastasis were significantly associated with the OS and
DFS in breast cancer patients (Table
IV). In addition, ALDH1A1(high) expression was significantly
associated with shorter OS of breast cancer patients. β-catenin(c)
expression was significantly associated with shorter OS and DFS of
breast cancer patients (Table IV).
Furthermore, multivariate Cox regression analysis found that
histological grade was an independent prognostic factor for OS and
DFS in breast cancer patients (P<0.05, Table V). Lymph node metastasis and
histological grade were independent prognostic factors for shorter
DFS in breast cancer patients (P<0.05, Table V).
 | Table IVUnivariate Cox regression analysis of
the association between clinicopathological data and OS and DFS in
breast cancer patients. |
Table IV
Univariate Cox regression analysis of
the association between clinicopathological data and OS and DFS in
breast cancer patients.
| Total no. | OS
| DFS
|
|---|
| Events n (%) | RR (95% CI) | P-value | Events n (%) | RR (95% CI) | P-value |
|---|
| Age (years) | | | | 0.126 | | | 0.116 |
| ≤50 | 87 | 21 (24.1) | 1 (reference) | | 27 (31.0) | 1 (reference) | |
| >50 | 79 | 27 (34.2) | 1.561
(0.883–2.763) | | 34 (43.0) | 1.500
(0.905–2.487) | |
| Menopausal
state | | | | 0.029 | | | 0.034 |
| Pre-menopause | 87 | 19 (21.8) | 1 (reference) | | 25 (28.7) | 1 (reference) | |
|
Post-menopause | 75 | 28 (37.3) | 1.911
(1.067–3.424) | | 33 (44.0) |
1.755(1.043–2.953) | |
| Tumor size
(cm) | | | |
<0.001 | | |
<0.001 |
| ≤2.0 | 76 | 11 (14.5) | 1 (reference) | | 19 (25.0) | 1 (reference) | |
| >2.0 | 60 | 33 (55.0) | 5.070
(2.557–10.054) | | 38 (63.3) |
3.546(2.036–6.173) | |
| Tumor type | | | | 0.541 | | | 0.387 |
| Ductal | 134 | 37 (27.6) | 1 (reference) | | 47 (35.1) | 1 (reference) | |
| Lobular | 17 | 6 (35.3) | 1.309
(0.552–3.102) | | 7 (41.2) | 1.178
(0.813–1.706) | |
| Histological
grade | | | |
<0.001 | | |
<0.001 |
| I | 79 | 6 (7.6) | 1 (reference) | | 13 (16.5) | 1 (reference) | |
| II–III | 84 | 42 (50.0) | 8.699
(3.687–20.524) | | 48 (57.1) | 4.673
(2.526–8.642) | |
| TNM stage | | | |
<0.001 | | |
<0.001 |
| I–II | 93 | 24 (25.8) | 1 (reference) | | 31 (33.3) | 1 (reference) | |
| III | 20 | 15 (75.0) | 5.039
(2.625–9.673) | | 16 (80.0) | 4.727
(2.554–8.749) | |
| Lymph node
metastasis | | | |
<0.001 | | |
<0.001 |
| No | 98 | 13 (13.3) | 1 (reference) | | 18 (18.4) | 1 (reference) | |
| Yes | 62 | 32 (51.6) | 4.997
(2.617–9.542) | | 40 (64.5) | 4.900
(2.800–8.574) | |
| ALDH1A1
expression | | | | 0.016 | | | 0.077 |
| Low | 83 | 16 (19.3) | 1 (reference) | | 25 (30.1) | 1 (reference) | |
| High | 85 | 32 (37.6) | 2.085
(1.144–3.800) | | 36 (42.4) | 1.585
(0.951–2.641) | |
| β-catenin
localization | | | | 0.019 | | | 0.027 |
| β-catenin(m) | 59 | 8 (13.6) | 1 (reference) | | 13 (22.0) | 1 (reference) | |
| β-catenin(c) | 49 | 17 (34.7) | 2.724
(1.175–6.316) | | 21 (42.9) | 2.182
(1.092–4.360) | |
 | Table VMultivariate Cox regression analysis
of the association between clinicopathological data and OS and DFS
in breast cancer patients. |
Table V
Multivariate Cox regression analysis
of the association between clinicopathological data and OS and DFS
in breast cancer patients.
| OS
| DFS
|
|---|
| RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Age (years)
(>50/≤50) | 1.510
(0.172–13.260) | 0.710 | 2.792
(0.506–15.416) | 0.239 |
| Menopausal state
(post/pre) | 0.658
(0.064–6.727) | 0.724 | 0.249
(0.037–1.694) | 0.155 |
| Tumor size (cm)
(>2.0/≤2.0) | 2.382
(0.733–7.743) | 0.149 | 1.647
(0.565–4.805) | 0.361 |
| Histological grade
(II–III/I) | 12.066
(2.488–58.518) | 0.002 | 3.005
(1.123–8.045) | 0.029 |
| TNM stage
(III/I–II) | 2.735
(0.929–8.049) | 0.068 | 2.049
(0.750–5.600) | 0.162 |
| Lymph node
metastasis (yes/no) | 3.851
(0.926–16.019) | 0.064 | 5.734
(1.600–20.556) | 0.007 |
| ALDH1A1 expression
(high/low) | 1.259
(0.395–4.006) | 0.697 | 0.654
(0.257–1.665) | 0.373 |
| β-catenin
localization (c/m) | 1.351
(0.446–4.089) | 0.594 | 0.935
(0.337–2.591) | 0.897 |
Discussion
In this study, we performed tissue microarray-based
immunohistochemistry to examine the expression of ALDH1A1 and
β-catenin in 276 breast cancer patients. We found that ALDH1A1
overexpression was associated with lymph node metastasis and
shorter OS in breast cancer patients. In addition, we found that
the β-catenin(c) expression was associated with lymph node
metastasis and shorter OS and DFS in breast cancer patients.
Furthermore, combined expression of ALDH1A1(high) and β-catenin(c)
was associated with shorter OS and DFS in breast cancer patients,
especially in patients with cyclophosphamide treatment. Our
findings suggest that translocation of β-catenin from the membrane
to the cytoplasm may subsequently promote the expression of ALDH1A1
in breast cancer, and β-catenin-regulated ALDH1A1 may contribute to
poor outcome of breast cancer patients.
In the present study, we examined the association of
ALDH1A1 expression with clinical characteristics and outcomes of
breast cancer patients. We found that ALDH1A1(high) expression was
associated with poor clinical outcome of breast cancer patients in
agreement with previous studies showing that ALDH1A1 is associated
with poor prognosis of breast cancer patients (11,12).
In addition, we found that ALDH1A1(high) expression was associated
with lymph node metastasis, and patients with ALDH1A1(high)
expression had more metastatic lymph nodes than those with
ALDH1A1(low) expression. Consistent with our results, Khoury et
al found that ALDH1A1 expression correlated with lymph node
metastasis in breast cancer patients (24). Several studies have demonstrated
that ALDH1 expression is associated with lymph node metastasis and
poor survival in various tumors such as esophageal squamous cell
carcinoma, gastric cancer, and rectal cancer (25–27).
Our findings that ALDH1A1(high) was associated with lymph node
metastasis and poor clinical outcome suggests that ALDH1A1
expression may define breast cancer with strong metastatic
potential and be used for predicting disease outcome. Furthermore,
ALDH1A1 is known to inactivate cyclophosphamide, and thus
contribute to resistance to cyclophosphamide and poor clinical
outcome of breast cancer patient (14,24).
Moreover, we found that ALDH1A1(high) expression was associated
with shorter OS and DFS in patients with cyclophosphamide
treatment, but not in those with non-cyclophosphamide treatment.
Therefore, ALDH1A1 may be used as a biomarker to predict poor
clinical outcome after cyclophosphamide treatment.
The Wnt signaling pathway is known to be involved in
the development of breast cancer (28). During Wnt signaling activation,
β-catenin is translocated from the membrane to the cytoplasm and
the nucleus, where it promotes transcription of multiple
protumorigenic genes via interaction with LEF/TCF transcription
factors (20). The cytoplasmic
accumulation of β-catenin has been found to be associated with poor
clinical outcome of breast cancer patients (20,29–32).
In the present study, we found that the cytoplasmic expression of
β-catenin was associated with grade II–II tumors, lymph node
metastasis, and shorter OS and DFS in breast cancer patients. Our
study supports that the cytoplasmic expression of β-catenin can be
used as a prognostic marker for poor outcome of breast cancer
patients. Although the mechanisms by which β-catenin is
translocated from the membrane to the cytoplasm in breast cancer
remain largely unclear, Adenomatous polyposis coli (APC), a
scaffolding protein that regulate the cellular level of β-catenin
in the Wnt signaling pathway (33),
may be involved. It has been reported that the low expression of
APC is associated with overexpression of β-catenin in the cytoplasm
of breast cancer cells (34). In
breast cancer cell lines, APC truncation leads to an increase in
the cytoplasmic expression of β-catenin (35).
Although ALDH1A1 is known to be a CSC marker that is
associated with poor prognosis of many tumors including breast
cancer (10–12), little is known about the regulation
of ALDH1A1 expression. Recently, it has been reported that ALDH1A1
is a direct target of β-catenin in ovarian cancer spheroids
(15). In the present study, we
found that β-catenin(c) expression occurred more frequently in
ALDH1A1(high) breast cancer, and the expression level of ALDH1A was
higher in β-catenin(c) breast cancer, suggesting that β-catenin may
regulate the expression of ALDH1A1 in breast cancer. This idea is
supported by the finding that the ALDH activity was reduced by the
inhibition of Wnt/β-catenin signaling by a TCF4-dominant negative
construct in human breast cancer cells (36). Thus, β-catenin may regulate the
expression of ALDH1A1 via interaction with the TCF4 transcription
factor in breast cancer. In addition, we found that tumors with
ALDH1A1(high) and β-catenin(c) expression were associated with
lymph node metastasis and poor clinical outcome in breast cancer
patients. Consistent with our findings, Xu et al reported
that in patients with colorectal carcinoma, β-catenin was
preferentially expressed in tumors with high expression of ALDH1A1,
and were associated with high potential of metastasis and poor
outcome (37). Furthermore, we
found that ALDH1A1(high) and β-catenin(c) expression was associated
with shorter OS and DFS in patients with cyclophosphamide
treatment, suggesting that tumors with ALDH1A1(high) and
β-catenin(c) expression exhibited resistance to cyclophosphamide
treatment. ALDH1A1(high) and β-catenin(c) expression may be used
for predicting poor outcome of breast cancer patients.
In summary, we investigated the expression of
ALDH1A1 and β-catenin in breast cancer patients, and analyzed the
association of their expression alone and in combination with the
clinicopathological characteristics and prognosis of breast cancer
patients. We found that ALDH1A1(high) and β-catenin(c) expression
was associated with lymph node metastasis and worse clinical
outcome in breast cancer patients, especially those receiving
cyclophosphamide treatment. Combined expression of ALDH1A1(high)
and β-catenin(c) was associated with lymph node metastasis, poor
outcome, and resistance to cyclophosphamide treatment. Our study
suggests that β-catenin may regulate ALDH1A1 expression in a
subtype of breast cancer with ALDH1A1(high) and β-catenin(c)
expression. ALDH1A1(high) and β-catenin(c) expression may be used
as a biomarker for predicting poor prognosis in breast cancer
following cyclophosphamide treatment.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of the People's Republic of China (no.
81373427), Program for Liaoning Innovative Research Team in
University, LNIRT (LT2014016) and the Shenyang Science and
Technology Plan Project (F14-232-6-05).
References
|
1
|
Stebbing J and Ellis P: An overview of
drug development for metastatic breast cancer. Br J Nurs. 21(Suppl
4): S18–S22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
et al: Intrinsic resistance of tumorigenic breast cancer cells to
chemotherapy. J Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sampieri K and Fodde R: Cancer stem cells
and metastasis. Semin Cancer Biol. 22:187–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neumeister V, Agarwal S, Bordeaux J, Camp
RL and Rimm DL: In situ identification of putative cancer stem
cells by multiplexing ALDH1, CD44, and cytokeratin identifies
breast cancer patients with poor prognosis. Am J Pathol.
176:2131–2138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak
J, Xing L, Wang H, Liu Z, Su Y, Stass SA, et al: Aldehyde
dehydrogenase 1 is a tumor stem cell-associated marker in lung
cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marhaba R, Klingbeil P, Nuebel T,
Nazarenko I, Buechler MW and Zoeller M: CD44 and EpCAM:
Cancer-initiating cell markers. Curr Mol Med. 8:784–804. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nomura M, Fukuda T, Fujii K, Kawamura T,
Tojo H, Kihara M, Bando Y, Gazdar AF, Tsuboi M, Oshiro H, et al:
Preferential expression of potential markers for cancer stem cells
in large cell neuroendocrine carcinoma of the lung. An FFPE
proteomic study. J Clin Bioinforma. 1:232011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng D, Wang N, Hu J and Li W: Surface
markers of hepatocellular cancer stem cells and their clinical
potential. Neoplasma. 61:505–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Croker AK, Goodale D, Chu J, Postenka C,
Hedley BD, Hess DA and Allan AL: High aldehyde dehydrogenase and
expression of cancer stem cell markers selects for breast cancer
cells with enhanced malignant and metastatic ability. J Cell Mol
Med. 13:2236–2252. 2009. View Article : Google Scholar
|
|
10
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar
|
|
11
|
Liu Y, Lv DL, Duan JJ, Xu SL, Zhang JF,
Yang XJ, Zhang X, Cui YH, Bian XW and Yu SC: ALDH1A1 expression
correlates with clinicopathologic features and poor prognosis of
breast cancer patients: A systematic review and meta-analysis. BMC
Cancer. 14:4442014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu S, Xue W, Huang X, Yu X, Luo M, Huang
Y, Liu Y, Bi Z, Qiu X and Bai S: Distinct prognostic values of
ALDH1 isoenzymes in breast cancer. Tumour Biol. 36:2421–2426. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pandrangi SL, Chikati R, Chauhan PS, Kumar
CS, Banarji A and Saxena S: Effects of ellipticine on
ALDH1A1-expressing breast cancer stem cells - an in vitro and in
silico study. Tumour Biol. 35:723–737. 2014. View Article : Google Scholar
|
|
14
|
Sládek NE, Kollander R, Sreerama L and
Kiang DT: Cellular levels of aldehyde dehydrogenases (ALDH1A1 and
ALDH3A1) as predictors of therapeutic responses to
cyclophosphamide-based chemotherapy of breast cancer: A
retrospective study. Rational individualization of
oxazaphosphorine-based cancer chemotherapeutic regimens. Cancer
Chemother Pharmacol. 49:309–321. 2002. View Article : Google Scholar
|
|
15
|
Condello S, Morgan CA, Nagdas S, Cao L,
Turek J, Hurley TD and Matei D: β-catenin-regulated ALDH1A1 is a
target in ovarian cancer spheroids. Oncogene. 34:2297–2308. 2015.
View Article : Google Scholar
|
|
16
|
Jiang W and Hiscox S: β-catenin - cell
adhesion and beyond (Review). Int J Oncol. 11:635–641.
1997.PubMed/NCBI
|
|
17
|
Madjd Z, Gheytanchi E, Erfani E and
Asadi-Lari M: Application of stem cells in targeted therapy of
breast cancer: A systematic review. Asian Pac J Cancer Prev.
14:2789–2800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lindvall C, Bu W, Williams BO and Li Y:
Wnt signaling, stem cells, and the cellular origin of breast
cancer. Stem Cell Rev. 3:157–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arend RC, Londoño-Joshi AI, Straughn JM Jr
and Buchsbaum DJ: The Wnt/β-catenin pathway in ovarian cancer: A
review. Gynecol Oncol. 131:772–779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin SY, Xia W, Wang JC, Kwong KY, Spohn B,
Wen Y, Pestell RG and Hung MC: Beta-catenin, a novel prognostic
marker for breast cancer: Its roles in cyclin D1 expression and
cancer progression. Proc Natl Acad Sci USA. 97:4262–4266. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu Z, Xiao Q, Zhao L, Ren J, Bai X, Sun M,
Wu H, Liu X, Song Z, Yan Y, et al: DNA methyltransferase 1/3a
overexpression in sporadic breast cancer is associated with reduced
expression of estrogen receptor-alpha/breast cancer susceptibility
gene 1 and poor prognosis. Mol Carcinog. Jan 25–2014.Epub ahead of
print. View
Article : Google Scholar
|
|
22
|
Fang Y, Wei J, Cao J, Zhao H, Liao B, Qiu
S, Wang D, Luo J and Chen W: Protein expression of ZEB2 in renal
cell carcinoma and its prognostic significance in patient survival.
PLoS One. 8:e625582013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ,
Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, et al: Overexpression
of EIF5A2 promotes colorectal carcinoma cell aggressiveness by
upregulating MTA1 through C-myc to induce
epithelial-mesen-chymaltransition. Gut. 61:562–575. 2012.
View Article : Google Scholar
|
|
24
|
Khoury T, Ademuyiwa FO, Chandrasekhar R,
Jabbour M, Deleo A, Ferrone S, Wang Y and Wang X: Aldehyde
dehydrogenase 1A1 expression in breast cancer is associated with
stage, triple negativity, and outcome to neoadjuvant chemotherapy.
Mod Pathol. 25:388–397. 2012. View Article : Google Scholar :
|
|
25
|
Teng HW, Yang SH, Lin JK, Chen WS, Lin TC,
Jiang JK, Yen CC, Li AF, Chen PC, Lan YT, et al: CIP2A is a
predictor of poor prognosis in colon cancer. J Gastrointest Surg.
16:1037–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y,
Uraoka N, Anami K, Sentani K, Oue N and Yasui W: Expression of
cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and
lymph node metastasis of gastric cancer. Pathol Int. 62:112–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Avoranta ST, Korkeila EA, Ristamäki RH,
Syrjänen KJ, Carpén OM, Pyrhönen SO and Sundström JT: ALDH1
expression indicates chemotherapy resistance and poor outcome in
node-negative rectal cancer. Hum Pathol. 44:966–974. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Howe LR and Brown AM: Wnt signaling and
breast cancer. Cancer Biol Ther. 3:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
López-Knowles E, Zardawi SJ, McNeil CM,
Millar EK, Crea P, Musgrove EA, Sutherland RL and O'Toole SA:
Cytoplasmic localization of beta-catenin is a marker of poor
outcome in breast cancer patients. Cancer Epidemiol Biomarkers
Prev. 19:301–309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dolled-Filhart M, McCabe A, Giltnane J,
Cregger M, Camp RL and Rimm DL: Quantitative in situ analysis of
beta-catenin expression in breast cancer shows decreased expression
is associated with poor outcome. Cancer Res. 66:5487–5494. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geyer FC, Lacroix-Triki M, Savage K,
Arnedos M, Lambros MB, MacKay A, Natrajan R and Reis-Filho JS:
β-catenin pathway activation in breast cancer is associated with
triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol.
24:209–231. 2011. View Article : Google Scholar
|
|
32
|
Wang S, Li W, Lv S, Wang Y, Liu Z, Zhang
J, Liu T and Niu Y: Abnormal expression of Nek2 and β-catenin in
breast carcinoma: Clinicopathological correlations. Histopathology.
59:631–642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Barker N: The canonical Wnt/beta-catenin
signalling pathway. Methods Mol Biol. 468:5–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ozaki S, Ikeda S, Ishizaki Y, Kurihara T,
Tokumoto N, Iseki M, Arihiro K, Kataoka T, Okajima M and Asahara T:
Alterations and correlations of the components in the Wnt signaling
pathway and its target genes in breast cancer. Oncol Rep.
14:1437–1443. 2005.PubMed/NCBI
|
|
35
|
Schlosshauer PW, Brown SA, Eisinger K, Yan
Q, Guglielminetti ER, Parsons R, Ellenson LH and Kitajewski J: APC
truncation and increased beta-catenin levels in a human breast
cancer cell line. Carcinogenesis. 21:1453–1456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Debeb BG, Lacerda L, Xu W, Larson R,
Solley T, Atkinson R, Sulman EP, Ueno NT, Krishnamurthy S, Reuben
JM, et al: Histone deacetylase inhibitors stimulate
dedifferentiation of human breast cancer cells through
WNT/β-catenin signaling. Stem Cells. 30:2366–2377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu SL, Zeng DZ, Dong WG, Ding YQ, Rao J,
Duan JJ, Liu Q, Yang J, Zhan N, Liu Y, et al: Distinct patterns of
ALDH1A1 expression predict metastasis and poor outcome of
colorectal carcinoma. Int J Clin Exp Pathol. 7:2976–2986.
2014.PubMed/NCBI
|