Introduction
Breast cancer is one of the most common cancers and
the leading cause of cancer death in females, accounting for 23% of
the total cancer cases and 14% of the cancer deaths, and the
incidence is still increasing (1).
The major dilemma is that many women suffering from breast cancer
end up with metastatic breast cancer (MBC) and recurrence, even
though they all received surgery, adjuvant chemotherapy or/and
radiotherapy (2). At present, with
better understanding of breast cancer progression with molecular
markers such as Her-2, VEGF and EGFR, the treatment of breast
cancer has entered a new era of molecular targeted therapy, from
which more and more patients could benefit (3). Due to heterogeneity of breast tumor,
the current treatments are invalid. Exploring new gene-related
breast cancer and further clarifing the mechanism of these genes is
essential to overcome breast adenocarcinoma metastasis and
recurrence.
RSK is a serine-threonine kinase and belongs to the
p90 ribosomal S6 kinase family (the 90,000 ribosomal S6 kinase
RPS6KA), which is an important downstream effector of Ras-MAPKs
signaling cascade (4–6). RSK consists of four isoforms of RSK1,
RSK2, RSK3, and RSK4 (7), RSKs play
a crucial role in the stimulation of cellular proliferation and
survival via phosphorylation and regulation of the transcription
factors, kinases, cyclin-dependent kinase inhibitor,
p27Kip1, the tumor suppressor, tuberin, and the
pro-apoptotic protein, Bad (8–10). An
important role for RSKs is suggeted in the regulation of the actin
cytoskeleton for cellular migration (11). RSK4 is an outlier and functionally
distinct, showing low expression compared with other RSKs, and
overexpression of RSK4 may restrict cell growth (12). Several studies have reported that
high expression of RSK4 in breast cancer cells is anti-oncogenic,
anti-invasive, and anti-metastatic (13), and RSK4 also exhibits a tumor
suppressor effect in colon and renal carcinomas (14).
RNA interference (RNAi) is a post-transcriptional
process by which double-stranded RNA triggers the degradation
sequence-specifically. The double-stranded RNA is processed into
short, 21- to 22-nucleotide double-stranded RNAs in the cell
(15). The use of the RNAi is a
powerful tool to block target gene expression has greatly
facilitated the understanding of gene function (16).
In the present study, we used Lenti-RSK4-siRNA
vectors interfering with expression of RSK4 gene in MCF-7 cells,
and investigated its effects on cell proliferation and invasion. In
addition, we explored the changes of Ki-67, cyclin D1, CXCR4 and
E-cadherin in xenograft tumors.
Materials and methods
Construction and transfection of shRNAs
for RSK4
shRNA targeting human RSK4 gene and a non-targeting
RNA were synthesized by GeneChem Technology Co., Ltd. (Shanghai,
China). The RNAi sequences that target RSK4 were
GATTATCCAAAGAGGTTCT, confirming that there was no homology with
other gene by gene database retrieval. Human breast carcinoma MCF-7
cells (Shanghai Institute of Cell Biology) were transfected by
lentiviral vector of RSK4-shRNA routinely cultured in 5%
CO2 at 37°C in Dulbecco's modified Eagle's medium (DMEM;
HyClone, Logan, UT, USA) containing 5 µg/ml polybrene in
6-well plates. The green fluorescent protein GFP expression was
observed by fluorescence microscopy (Olympus, Tokyo, Japan) 48 h
after transfection, and cells were collected at 72 h after
transfection for in vivo experiments.
RNA extraction and qRT-PCR
Total RNA extraction from different groups used
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to
manufacturer's protocol. The RNA concentration was measured by
ultraviolet (UV) light at 260 nm, and the integrity of the
extracted total RNA was detected by 1% agarose gel electrophoresis.
Quantitative real-time PCR was performed using SYBR®
Premix Ex Taq™ II (Tli RNaseH Plus) according to the manufacturer's
instructions. Primers for RSK4, Ki-67, cyclin D1, E-cadherin, CXCR4
and GAPDH were as follows: RSK4 forward, 5′-ATATGGACCCACATCAGCGG-3′
and reverse, 5′-AGCAGCTACAGGCTCTAGGA-3′ (191 bp); Ki-67 forward,
5′-AGAGAGTGTCTATCAGCCGA-3′ and reverse, 5′-CATTGACCTTTGAGGACCAT-3′
(157 bp); cyclin D1 forward, 5′-AGGAACAGAAGTGCGAGGAGG-3′ and
reverse, 5′-GATGGAGTTGTCGGTGTAGATG-3′ (192 bp); E-cadherin forward,
5′-GGTGCTCTTCCAGGAACCTC-3′ and reverse, 5′-GGAAACTCTCTCGGTCCAGC-3′
(136 bp); CXCR4 forward, 5′-ACCACAGTCATCCTCATCCTG-3′ and reverse,
5′-TCTCAAACTCACACCCTTGCT-3′ (128 bp); and GAPDH forward,
5′-AAGAAGGTGGTGAAGCAGGC-3′ and reverse,
5′-ACCACCCTGTTGCTGTGZAGCC-3′ (200 bp). The reaction conditions for
the real-time PCR was 95°C for 30 sec followed by 40 cycles of 95°C
for 5 sec and 60°C for 30 sec. All reactions were performed in
triplicate. Results were analyzed by calculating the Ct values for
target gene and GAPDH using the following formula: −ΔΔCt = average
ΔCt of control group −ΔCt of the treated group, and the relative
expression of target gene in each group was calculated using the
2−ΔΔCt method.
Western blot analysis
Cells from different groups were homogenized and
lysed in protein extraction reagent (Beyotime, Shanghai, China).
The homogenate was then centrifuged at 12,000 rpm and supernatant
was collected as total cell lysate and stored at −20°C. The total
proteins were applied to a 10% polyacrylamide gel (Sangon Biotech,
Shanghai, China), then electrophoresed, and transferred to
polyvinylidene difluoride membranes (Sangon Biotech). The membranes
were washed in blocking buffer Tween-20 (TBST; 3X, 5 min each
time), incubated with 5% non-fat milk at room temperature overnight
and then the membranes were incubated overnight at 4°C with mouse
anti-human RSK4 (1:1,000; Santa Cruz Biotechnology, USA) and
β-actin (1:1,000; Boster, Wuhan, China) monoclonal antibodies in
blocking buffer. After being washed three times with TBST (3X, 5
min each time), the membranes were treated with secondary
anti-mouse antibodies (1:5,000; Boster). Densitometric analysis of
western blotting signals was carried out using image-analyzing
software. The protein levels were normalized to β-actin. RSK4
signal values divided by those of the corresponding β-actin signals
(RSK4/β-actin) were used for statistical analysis.
MTT assay
The cells transfected with lentivirus RSK4-shRNA,
lentivirus negative-shRNA and MCF-7 were harvested, and cell
suspension concentration was adjusted to a density of
1×105 cells/ml, plated into a 96-well plate
(2×103 cells/well). Cell proliferation was assessed at
1, 2, 3, 4 and 5 day, following the instructions of the MTT
proliferation assay kit (Sigma, USA). The experiment was performed
three times.
Transwell migration assay
Transwell plates (24-well) with 8.0 µm pore
size were coated with Matrigel, which was diluted 1:4 in DMEM, and
allowed to gel at 37°C. Lower chambers were loaded with DMEM
containing 10% FBS. Cell suspensions (5×104 cells/well)
were added to the upper chambers, and allowed to invade for 48 h at
37°C in a CO2 incubator. The cells in the upper chamber
were removed with a swab, fixed in 4% paraformaldehyde for 30 min.
Invasive cells were stained with crystal violet for 30 min and
washed in PBS for 10 min. Invasive cells were counted from five
random microscopic fields of each membrane, and the average of
cells was calculated. All the experiments in each group were
performed in triplicate.
Cell cycle assay
The cells transfected with lentivirus RSK4-shRNA,
lentivirus negative-shRNA and MCF-7 were collected, and cell
suspension concentration was adjusted to a density of
1×106 cells/ml. The cells were washed with PBS and fixed
in 70% ethanol at 4°C overnight. Then cells were centrifuged and
washed with PBS to remove ethyl alcohol, and incubated with 50
µg/ml Ribonuclease A at 37°C for 30 min. The cells were
added with 10 µg/ml PI and incubated at 37°C for 30 min.
Finally, cell cycle distribution were analyzed with flow cytometer
(Coulter Epics XL; Beckman Coulter, USA).
In vivo study of breast adenocarcinoma
xenograft tumor model in nude mice
Animal experiments were approved by the Animal
Center Animal Care and use Committee of guangxi Medical university.
Five-week-old female BALB/c nude mice (weight, 16–20 g) were
purchased from guangxi Medical university Experimental Animal
Center. Nude mice were fed and housed in laminar flow cabinets,
pathogen-free animal facility, at a constant temperature (25–28°C)
and humidity. Mice were randomly divided into three groups with 13
mice/group: lentivirus RSK4-shRNA (RSK4-shRNA), lentivirus
negative-shRNA (negative) and MCF-7 control (blank) group. The
RSK4-shRNA group of nude mice were subcutaneously injected with
MCF-7 cells (5×106) carrying lentivirus RSK4-shRNA while
the negative control group of nude mice were subcutaneously
injected with MCF-7 cells (5×106) carrying lentivirus
negative-shRNA, and the blank control group of nude mice were
subcutaneously injected with MCF-7 cells (5×106). After
35 days, the mice were sacrificed, and the tumors and lungs were
harvested. Tumor volume was calculated as: Volume (mm3)
= width2 (mm2) × length (mm)/2. Consecutive
sections were made of every tissue block of the lungs and subjected
to H&E histostaining. The incidence of lung metastasis was
evaluated independently by two pathologists. The tumors were
collected for quantitative real-time PCR. These experiments were
repeated three times to confirm the results.
Statistical analysis
All statistical analysis was performed by using SPSS
16.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance
(ANOVA), followed by the LSD post hoc test was used to investigate
differences between the three groups. Values are expressed as mean
± standard deviation (SD), and a p-value <0.05 was considered
statistically significant.
Results
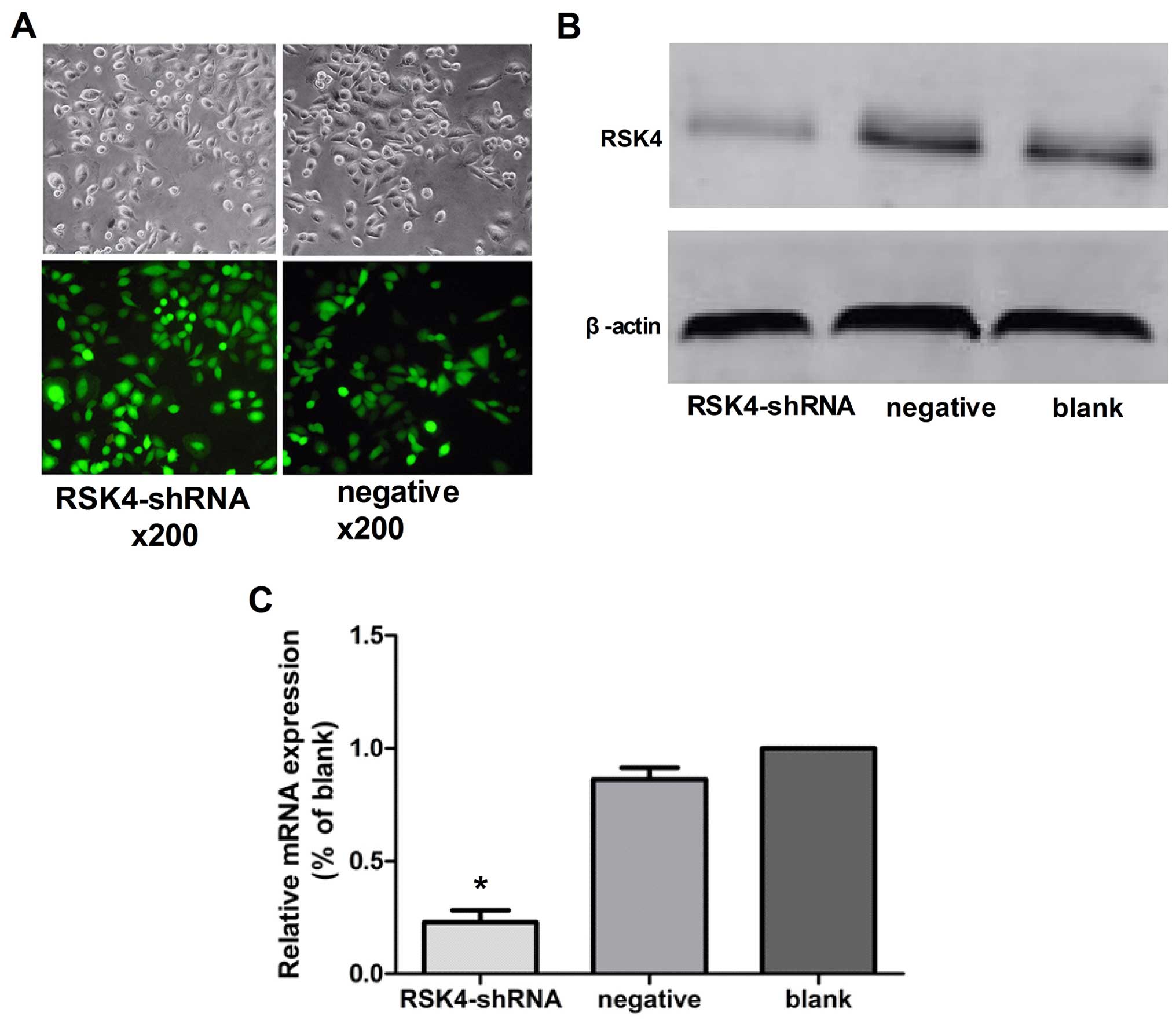
Transfection efficiency
MCF-7 cells transfected with Lenti-RSK4-shRNA
targeting human RSK4 gene or a non-targeting negative control shRNA
exhibiting green fluorescence under a fluorescence microscope were
considered to be successfully transfected. Under the lentiviral
vector MOI=10 conditions, the expression of GFP stability increased
after 72 h, and the lentiviral infection rate was high (Fig. 1A).
Effects of lentivirus-mediated RSK4 RNAi
on RSK4 mRNA expression by real-time PCR
In the lentivirus RSK4-shRNA, negative control, and
blank control groups, the relative expression levels of RSK4 mRNA
were ~0.22±0.06, 0.86±0.05 and 1.000±0.00, respectively.
Furthermore, the relative expression levels of RSK4 mRNA in the
lentivirus RSK4-shRNA group was significantly lower than levels in
the negative (P<0.05) and blank control groups (P<0.05),
although there was no statistical significance between negative and
blank control groups (P=0.35, Fig.
1C).
Effects of lentivirus-mediated RSK4 RNAi
on RSK4 expression by western blot assays
In the lentivirus RSK4-shRNA, negative control, and
blank control groups, the relative expression levels of RSK4
protein were ~0.16±0.03, 0.57±0.05 and 0.49±0.04, respectively. The
results showed that levels of RSK4 protein in the RSK4-shRNA group
was significantly lower than the blank levels (P<0.05) and
negative (P<0.05) control groups, although there was no
statistical significance between negative and blank control groups
(P=0.62, Fig. 1B).
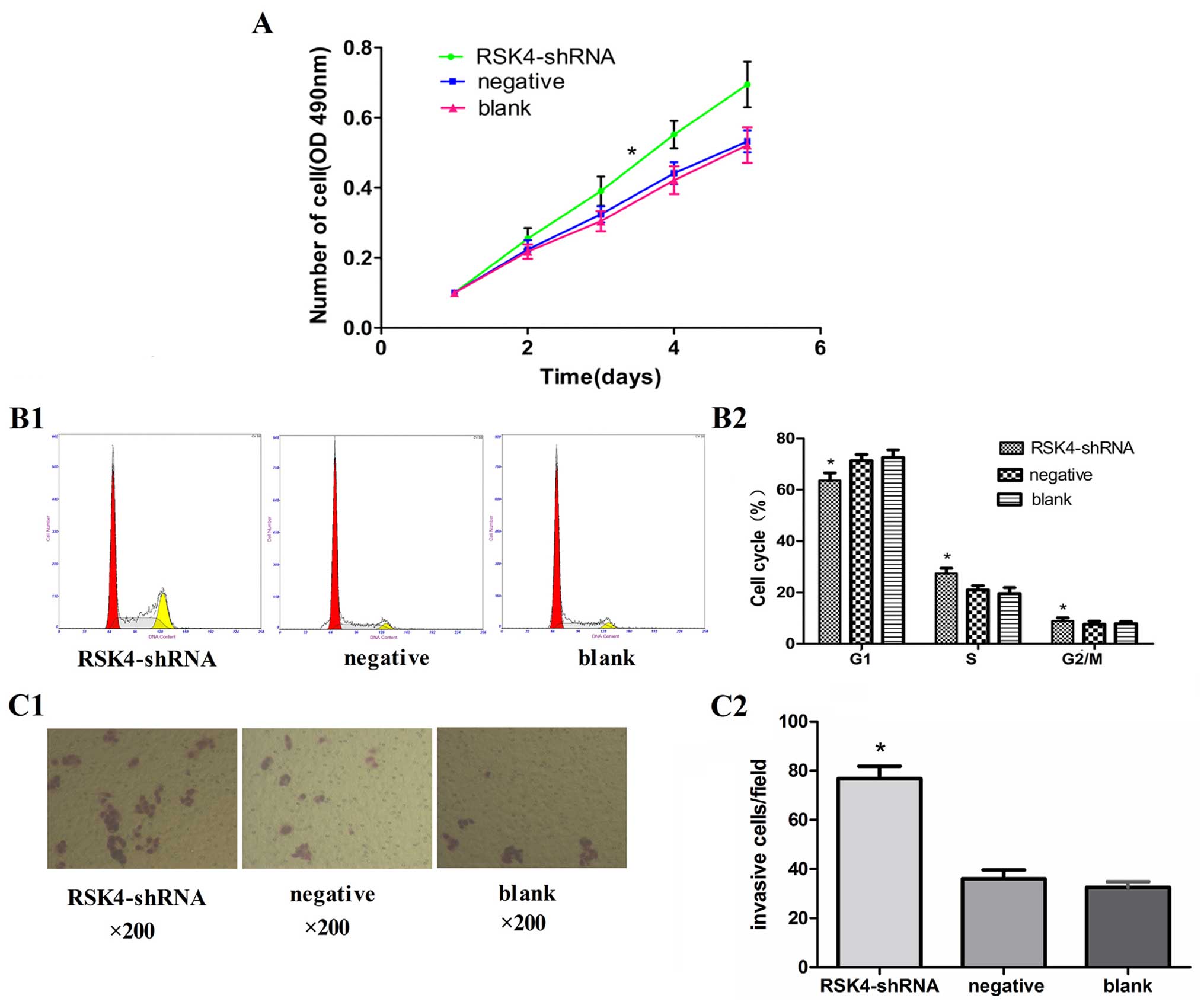
Effect of RSK4 knockdown on the
proliferation ability of MCF-7 cells
The MTT assay showed that cell proliferation was
significantly promoted in the RSK4-shRNA group as compared to that
in the negative and blank control group (P<0.05, Fig. 2A)
Effects of RSK4 knockdown on the cell
cycle of MCF-7 cells
The number of cells in the G0/G1 phase in the
RSK4-shRNA group (63.6±2.8%) was significantly less (P<0.05)
than that of the negative (71.3±2.7%) and blank control groups
(72.6±3.0%), while the number of cells in S phases in the
RSK4-shRNA group (27.4±1.8%) was significantly more (P<0.05)
than that of the negative (21.0±1.7%) and blank control groups
(19.5±2.5%). However, no significant difference (P>0.05) in the
number of cells in the G2/M phase was found in the RSK4-shRNA group
(8.9±1.9%), negative (7.6±1.6%) and blank control groups (7.8±1.7%)
(Fig. 2B1 and B2).
Effects of RSK4 knockdown on the
invasiveness of MCF-7 cells
Transwell cell migration assays showed the capacity
of MCF-7 cell enhancement, the number of cells in the RSK4-shRNA
group migrated through the Matrigel barriers was more than that of
the negative (76.8±5.7 vs. 36.1±3.4, P<0.05) and blank control
groups (76.8±5.7 vs. 32.5±2.0, P<0.05). No significant
difference was observed between the negative group and blank
control groups (36.1±3.4 vs. 32.5±2.0, P>0.05) (Fig. 2C).
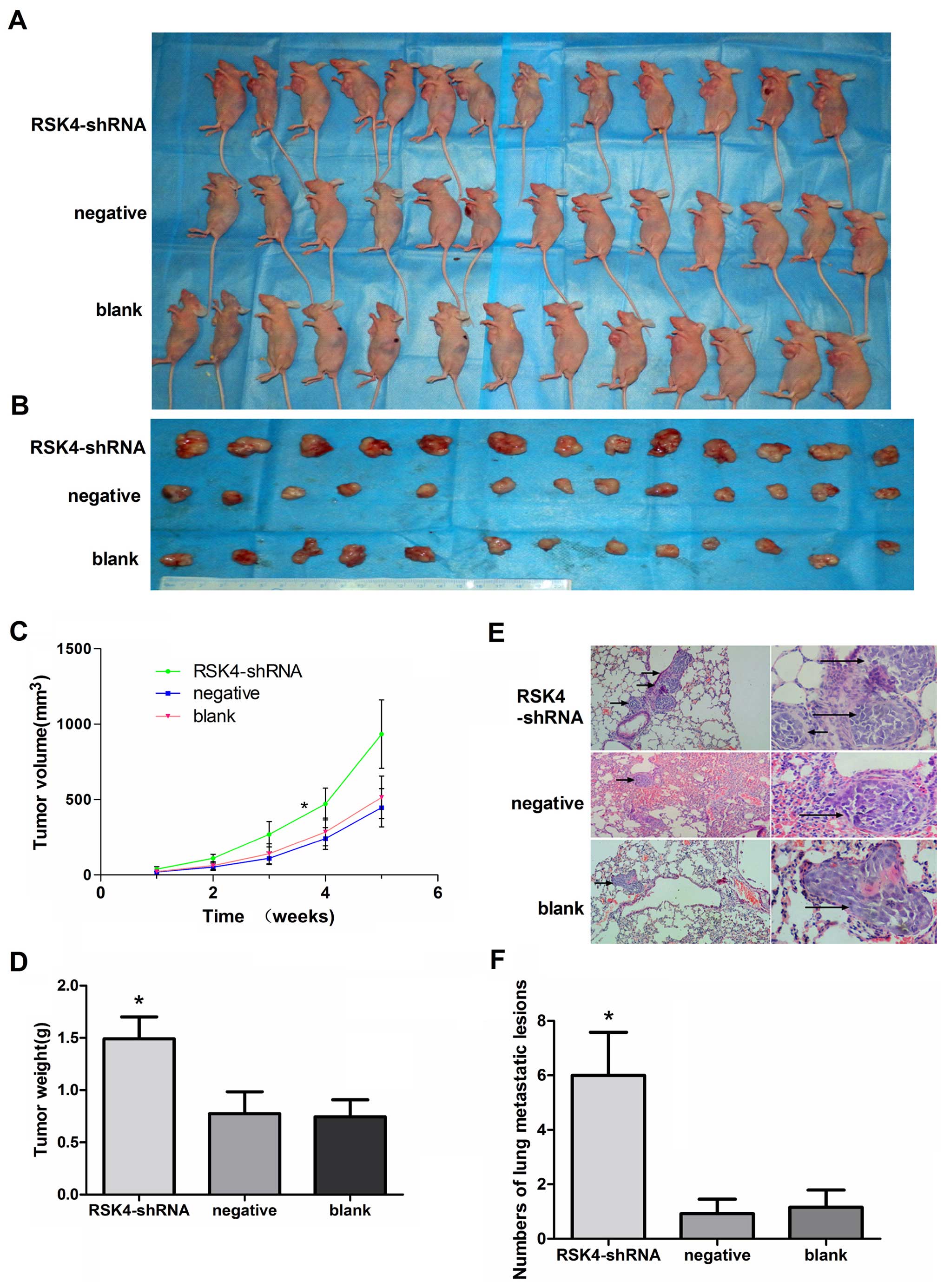
In vivo studies of breast adenocarcinoma
xenograft tumor model in nude mice
After 35 days, the tumor tissues were harvested and
weighed. The xenografts of the RSK4-shRNA group (933.49±227.51
mm3) had a substantially greater tumors as compared to
negative (445.43±126.45 mm3, P<0.05) or blank groups
(513.68±141.37 mm3, P<0.05) (Fig. 3A–C). The mean tumor weight derived
from the RSK4-shRNA group (1.49±0.25 g) was significantly higher
compared to the weights of tumors from the negative (0.77±0.22 g,
P<0.05) and blank control groups (0.74±0.18 g, P<0.05)
(Fig. 3D). The number of lung
metastatic lesions in the RSK4-shRNA group was greatly increased
compared with the respective negative (P<0.05) and blank control
groups (P<0.05, Fig. 3E and
F).
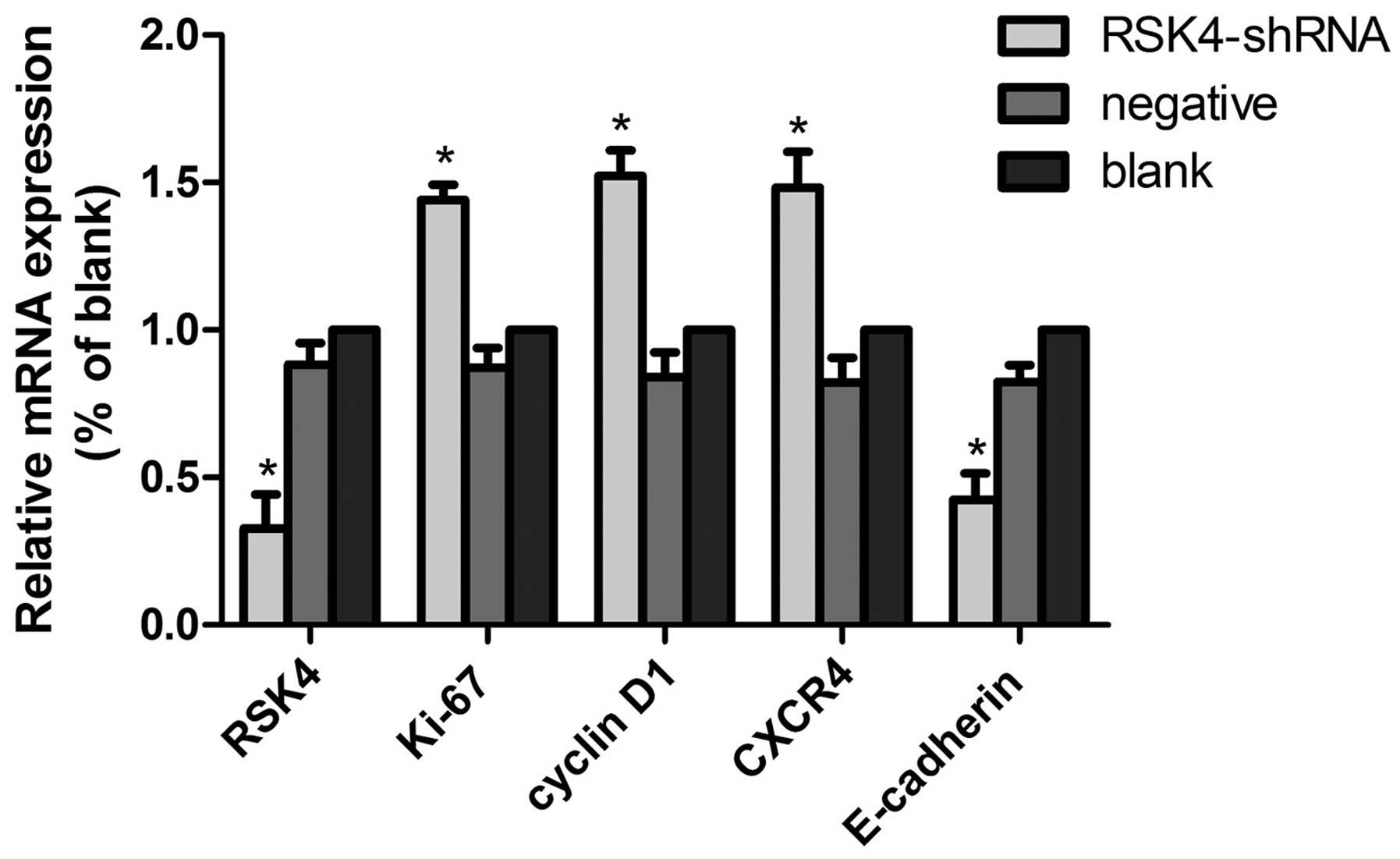
Effects of lentivirus-mediated RSK4 RNAi
on RSK4 mRNA, Ki-67 mRNA, cyclin D1 mRNA,CXCR4 mRNA and E-cadherin
mRNA expression of tumor xenografts by real-time PCR
In the RSK4-shRNA, negative control, and blank
control groups, the relative expression levels of RSK4 mRNA were
~0.32±0.10, 0.88±0.08 and 1.000±0.00, the E-cadherin mRNA were
~0.42±0.11, 0.82±0.06 and 1.000±0.00, the CXCR4 mRNA were
~1.48±0.15, 0.82±0.08 and 1.000±0.00, the Ki-67 mRNA were
~1.44±0.07, 0.87±0.07, and 1.000±0.00, the cyclin D1 mRNA were
~1.52±0.09, 0.84±0.08 and 1.000±0.00, respectively. The relative
expression levels of RSK4 mRNA and E-cadherin mRNA in the
lentivirus RSK4-shRNA group was significantly lower than the levels
in the negative (both P<0.05) and blank (both P<0.05) control
groups, while the levels of CXCR4 mRNA, Ki-67 mRNA, cyclin D1 mRNA
in the lentivirus RSK4-shRNA group were significantly higher than
levels that in the blank (both P<0.05) and negative (both
P<0.05) control groups (Fig.
4).
Discussion
Breast cancer is one of the most common cancers and
the leading cause of cancer death among females worldwide, and
invasion and metastasis is an important prognostic factor in
patients with breast cancer, and as a serious threat to women's
health. Therefore, further clarifing the mechanism of the
development of breast cancer and exploring new therapeutic targets
are essential.
Several types of cells were cultured under serum
conditions, RSK4 expression was low, but in serum-starved medium,
the RSK4 showed high expression, suggesting that RSK4 can restrict
cell growth (12). It has been
reported that the mouse RSK4 have the ability to inhibit the
transcriptional activation of receptor tyrosine kinase (RTK)
signaling and extracellular signal-regulated kinase (ERK) signaling
on the proliferation of cells (17). Berns et al showed that RSK4
allowed the p53-dependent growth arrest induced by
p21cip1 phosphorylation, thus, the knockdown of RSK4
prevented p53-dependent proliferation arrest (18). The above results suggest that RSK4
can inhibit cell proliferation. Thakur et al (19) revealed higher expression of RSK4 was
found in normal mammary ductals, and low expression or no
expression of RSK4 in benign breast lesions (papilloma). RSK4 is
likely to have a role in the development or progression of human
breast cancer. This is supported by the finding from Thakur et
al study (13). In their study,
the overexpressing exogenous RKS4 gene was transfected into the
invasive breast cancer cell line MDA-MB-231, and compared with its
parental cells, the high expression of RSK4 in transfected
MDA-MB-231 showed significantly decreased capacity of cell
proliferation, migration, invasion, tumorigenesis and metastasis.
It suggested that RSK4 may be a tumor suppressor gene in breast
cancer, inhibiting cell proliferation, tumorigenesis and metastasis
of breast cancer cells, and that downregulation of RSK4 may promote
tumorigenesis and metastasis of breast cancer cells.
RNAi is a highly conserved gene silencing mechanism
that introduce double-stranded RNA (dsRNA) to trigger the
elimination of the target gene (20). RNAi has been widely used as a
powerful tool for gene function analysis and ablating specific
genes for therapeutic purpose (21). It had been reported that lentivirus
vector-mediated expression of RNAi display effective and stable
gene silencing (22). Compared with
other methods, lentiviral vectors may be feasible.
We proposed a hypothesis that downregulation of RSK4
expression in the MCF-7 cell line would affect breast
adenocarcinoma tumorigenesis and metastasis. In order to prove this
hypothesis, we used lentivirus-mediated siRNA silencing to knock
down RSK4 expression in the breast cancer cell line MCF-7,
investigated the effect of the RSK4 gene on the cell proliferation
and invasion in vitro, and established a model of xenografts
in vivo. In the present study, for the first time we
investigated the association between RSK4 expression and human
breast adenocarcinoma cell proliferation, migration and metastasis
status.
We found that knockdown of RSK4 gene promoted the
proliferation and invasion of MCF-7 cells in vitro, and the
tumor growth was aggressive in RSK4 shRNA transfected tumors while
the growth of negative shRNA-infected xenografts and blank control
graft slowed down in the mouse model. Furthermore, knockdown of
RSK4 dramatically promoted tumorigenicity and increased the number
of lung metastatic lesions. It indicate that RSK4 is implicated in
the proliferation, invasion and metastasis of MCF-7 cells, and may
be manipulated therapeutically to delay the migration and
metastasis of MCF-7 cells. We also found that E-cadherin mRNA were
downregulated, whereas Ki-67 mRNA, cyclin D1 mRNA, CXCR4 mRNA
levels were increased in the xenograft tumor in nude mice when RSK4
was knocked down. Several studies have showed that cyclin D1
(23–26), Ki-67 (27,28),
E-cadherin (29,30), and CXCR4 (31) play important roles in the
development of breast carcinoma. It indicated that
lentivirus-mediated RNAi knockdown of RSK4 promoted tumor cell
proliferation and migration, and the molecular mechanism may
associate with changes of Ki-67, E-cadherin, CXCR4 and cyclin
D1.
In conclusion, the present study indicated that RSK4
was a significant tumor suppressor gene contributing to breast
tumor development. Furthermore, blocking the RSK4 caused genetic
instability, and may lead to upregulation of CXCR4, Ki-67, cyclin
D1 gene, and downregulation of E-cadherin gene promoting the
proliferation and migration of breast cancer cells.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30960427).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dent R, Valentini A, Hanna W, Rawlinson E,
Rakovitch E, Sun P and Narod SA: Factors associated with breast
cancer mortality after local recurrence. Curr Oncol. 21:e418–e425.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fischgräbe J and Wülfing P: Targeted
therapies in breast cancer: Established drugs and recent
developments. Curr Clin Pharmacol. 3:85–98. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frödin M and Gammeltoft S: Role and
regulation of 90 kDa ribosomal S6 kinase (RSK) in signal
transduction. Mol Cell Endocrinol. 151:65–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anjum R and Blenis J: The RSK family of
kinases: Emerging roles in cellular signalling. Nat Rev Mol Cell
Biol. 9:747–758. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nebreda AR and Gavin AC: Perspectives:
Signal transduction. Cell survival demands some Rsk. Science.
286:1309–1310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yntema HG, van den Helm B, Kissing J, van
Duijnhoven G, Poppelaars F, Chelly J, Moraine C, Fryns JP, Hamel
BC, Heilbronner H, et al: A novel ribosomal S6-kinase (RSK4;
RPS6KA6) is commonly deleted in patients with complex X-linked
mental retardation. Genomics. 62:332–343. 1999. View Article : Google Scholar
|
|
8
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roux PP, Ballif BA, Anjum R, Gygi SP and
Blenis J: Tumor-promoting phorbol esters and activated Ras
inactivate the tuberous sclerosis tumor suppressor complex via p90
ribosomal S6 kinase. Proc Natl Acad Sci USA. 101:13489–13494. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X, Matsuda K, Bialek P, Jacquot S,
Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes
TM, et al: ATF4 is a substrate of RSK2 and an essential regulator
of osteoblast biology; implication for Coffin-Lowry syndrome. Cell.
117:387–398. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woo MS, Ohta Y, Rabinovitz I, Stossel TP
and Blenis J: Ribosomal S6 kinase (RSK) regulates phosphorylation
of filamin A on an important regulatory site. Mol Cell Biol.
24:3025–3035. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dümmler BA, Hauge C, Silber J, Yntema HG,
Kruse LS, Kofoed B, Hemmings BA, Alessi DR and Frödin M: Functional
characterization of human RSK4, a new 90-kDa ribosomal S6 kinase,
reveals constitutive activation in most cell types. J Biol Chem.
280:13304–13314. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thakur A, Sun Y, Bollig A, Wu J, Biliran
H, Banerjee S, Sarkar FH and Liao DJ: Anti-invasive and
antimetastatic activities of ribosomal protein S6 kinase 4 in
breast cancer cells. Clin Cancer Res. 14:4427–4436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
López-Vicente L, Armengol G, Pons B, Coch
L, Argelaguet E, Lleonart M, Hernández-Losa J, de Torres I and
Ramon y Cajal S: Regulation of replicative and stress-induced
senescence by RSK4, which is down-regulated in human tumors. Clin
Cancer Res. 15:4546–4553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elbashir SM, Lendeckel W and Tuschl T: RNA
interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev.
15:188–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sen GL and Blau HM: A brief history of
RNAi: The silence of the genes. FASEB J. 20:1293–1299. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Myers AP, Corson LB, Rossant J and Baker
JC: Characterization of mouse Rsk4 as an inhibitor of fibroblast
growth factor-RAS-extracellular signal-regulated kinase signaling.
Mol Cell Biol. 24:4255–4266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berns K, Hijmans EM, Mullenders J,
Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M,
Nijkamp W, Weigelt B, et al: A large-scale RNAi screen in human
cells identifies new components of the p53 pathway. Nature.
428:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thakur A, Rahman KW, Wu J, Bollig A,
Biliran H, Lin X, Nassar H, Grignon DJ, Sarkar FH and Liao JD:
Aberrant expression of X-linked genes RbAp46, Rsk4, and Cldn2 in
breast cancer. Mol Cancer Res. 5:171–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sharp PA: RNA interference - 2001. Genes
Dev. 15:485–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hannon GJ and Rossi JJ: Unlocking the
potential of the human genome with RNA interference. Nature.
431:371–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart SA, Dykxhoorn DM, Palliser D,
Mizuno H, Yu EY, An DS, Sabatini DM, Chen IS, Hahn WC, Sharp PA, et
al: Lentivirus-delivered stable gene silencing by RNAi in primary
cells. RNA. 9:493–501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McIntosh GG, Anderson JJ, Milton I,
Steward M, Parr AH, Thomas MD, Henry JA, Angus B, Lennard TW and
Horne CH: Determination of the prognostic value of cyclin D1
overexpression in breast cancer. Oncogene. 11:885–891.
1995.PubMed/NCBI
|
|
24
|
Vos CB, Ter Haar NT, Peterse JL,
Cornelisse CJ and van de Vijver MJ: Cyclin D1 gene amplification
and overexpression are present in ductal carcinoma in situ of the
breast. J Pathol. 187:279–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oyama T, Kashiwabara K, Yoshimoto K,
Arnold A and Koerner F: Frequent overexpression of the cyclin D1
oncogene in invasive lobular carcinoma of the breast. Cancer Res.
58:2876–2880. 1998.PubMed/NCBI
|
|
26
|
Bilalović N, Vranić S, Basić H, Tatarević
A and Selak I: Immunohistochemical evaluation of cyclin D1 in
breast cancer. Croat Med J. 46:382–388. 2005.
|
|
27
|
Niewiadomska H, Jeziorski A and Olborski
B: The expression of the proliferating antigen Ki67, PCNA and
products of suppressor gene p53 in primary invasive ductal breast
carcinoma. J Exp Clin Cancer Res. 17:503–510. 1998.
|
|
28
|
Zhou CJ, Zhang QH, Zhang TG, Sun SZ, Li H,
Wang Y and Liu ZY: Expression of ER, Ki-67 and cylinD1 in the
pre-cancerous breast of Chinese patients. Pathol Oncol Res.
15:153–158. 2009. View Article : Google Scholar
|
|
29
|
Shargh SA, Sakizli M, Khalaj V, Movafagh
A, Yazdi H, Hagigatjou E, Sayad A, Mansouri N, Mortazavi-Tabatabaei
SA and Khorram Khorshid HR: Downregulation of E-cadherin expression
in breast cancer by promoter hypermethylation and its relation with
progression and prognosis of tumor. Med Oncol. 31:2502014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siitonen SM, Kononen JT, Helin HJ, Rantala
IS, Holli KA and Isola JJ: Reduced E-cadherin expression is
associated with invasiveness and unfavorable prognosis in breast
cancer. Am J Clin Pathol. 105:394–402. 1996.PubMed/NCBI
|
|
31
|
Furusato B, Mohamed A, Uhlén M and Rhim
JS: CXCR4 and cancer. Pathol Int. 60:497–505. 2010. View Article : Google Scholar : PubMed/NCBI
|