Introduction
Prostate cancer is the most common malignancy and
the second leading cause of cancer-related deaths in many Western
countries. It accounts for 27%/233,000 of incident cases in the US
alone with a total of 29,480 predicted deaths, according to cancer
statistics reported by the American Cancer Society in 2014
(1). The incidence of prostate
cancer in Asian population, including the Chinese, has been
increasing in recent years, although still lower than that of
Western countries (2,3). The incidence of prostate cancer is
known to increase with advancing age, and it inevitably becomes an
increasingly greater problem as life expectancy is globally
improving.
Apoptosis is an important process in a wide variety
of biological systems, including cell development and maintenance
of tissue homeostasis, and is well documented to play an essential
role as a protective mechanism against carcinogenesis (4). In prostate cancer, a fine balance
between cell proliferation and apoptosis is lost contributing to
the increased cellular mass and tumor progression (5). Intervention using cancer
chemopreventive compounds has shown a promising opportunity for
preventing or slowing the progression of this downloaded disease
(6). Increasing attention has been
focused on the utilization of naturally occurring botanicals or
dietary substances for prostate cancer therapy (7–9). In
this regard, for prostate cancer chemoprevention at the present
time, there is considerable emphasis in identifying novel
botanicals that selectively induce apoptosis and growth arrest of
prostate cancer cells without producing cytotoxic effects on normal
cells.
Acetylbritannilactone (ABL) is a sesquiterpene
lactone abundant in Inula Britannica L, a traditional
Chinese medicinal herb (Xuan Fu Hua). It has been reported to have
chemopreventive properties by inducing cell apoptosis in breast and
ovarian cancers (10,11). We have recently synthesized a
derivative compound of ABL,
5-(5-(ethylperoxy)pentan-2-yl)-6-methyl-3-methylene-2-oxo-2,3,3a,4,7,7a-hexahydrobenzofuran-4-yl2-(6-methoxynaphthalen-2-yl)
propanoate (ABL-N; Fig. 1A). Our
previous study indicated that ABL-N treatment causes a significant
inhibition of tumor growth in vivo and ABL-N induces
apoptosis in breast cancer cells through the activation of caspases
and JNK signaling pathways, suggested that ABL-N may be a potential
drug for breast cancer prevention and intervention (12). However, the anti-tumor activity and
the molecular targets of ABL in prostate cancer cells have not been
determined. In the present study, we investigated the
antiproliferative and pro-apoptotic effects of ABL-N, as well as
the expression of apoptosis-related proteins in ABL-induced growth
suppression of human prostate cancer cells and the xenograft mouse
model.
Materials and methods
Cell lines
The human prostate cancer cells PC3, DU145 and
LNcap, were purchased from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and were routinely cultured in RPMI-1640
supplemented with 10% fetal bovine serum (FBS) (both from
Invitrogen, Carlsbad, CA, USA), 4 mmol/l glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin. The normal epithelial
prostate cells PrEC were obtained from Clonetics-BioWhittaker, Inc.
(Walkersville, MD, USA), were cultured in prostate epithelial basal
medium with PrEgM BulletKit (both from Clonetics). All cells were
incubated at 37°C and 5% CO2 in a humid environment and
subcultured twice a week.
ABL-N preparation
ABL-N was prepared as described in our previous
study (12). The purity and
chemical structure of ABL-N were certified by melting point,
elemental analysis and spectral studies. The purified ABL-N was
dissolved in ethanol using ultrasonication. The effects of ABL-N on
our experiments were compared with those of ethanol at a final
concentration of 0.5% as vehicle.
MTT assay
The PC3, DU145, LNcap and PrEC cells were seeded
into 96-well plates at a density of 4,000/well and were cultured
for 24 h. Cells were then treated with ABL-N of different
concentrations (0, 2.5, 5, 10, 15, 20, 25, 30, 35 and 40
µM). After 24 h of incubation, the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reagent, 5 mg/ml in phosphate-buffered saline (PBS) was added to
each well (20 µl/well) followed by incubation for 4 h at
37°C, and then the plate was centrifuged at 1,000 rpm for 5 min at
4°C. After careful removal of the medium, formazan crystals were
dissolved in 0.1 ml buffered dimethylsulfoxide (DMSO; Sigma), and
the absorbance was read on a microplate reader at the wavelength of
570 nm. Absorbance values were normalized to the values obtained
from the vehicle-treated cells. Taking into account the more
aggressive and highly metastatic nature of prostate cancer, PC3
cells were selected as a model system to conduct further
experiments.
Wound migration assay
Confluent PC3 cells grown in 10 cm2
dishes were wounded using sterile pipette tips, washed twice with
1X PBS, and grown in RPMI-1640 medium (Invitrogen) supplemented
with 10% FBS and various concentrations of ABL-N for 24 h. Then PC3
cells were photographed under a phase-contrast microscope
(magnification, ×10).
Apoptosis assays
Apoptosis of PC3 cells was firstly determined using
terminal deoxynucleotidyl transferase-mediated nick-end labeling
(TUNEL) kit (DeadEnd Fluorometric TUNEL System; Promega, Madison,
WI, USA) after cultivation with different concentrations of ABL-N
for 24 h. Cells were fixed in 4% paraformaldehyde in PBS for 25 min
at 4°C. After permeabilized in 0.1% Triton X-100 in PBS for 5 min
on ice, the samples were incubated in TUNEL reaction mixture for 1
h at 37°C in a dark and humidified atmosphere.
For nuclear staining, the fixed cells were placed on
slides and stained with 1 mg/ml DAPI for 15 min. After three
washes, images were captured immediately using a digital camera
attached to the fluorescence microscope.
For quantification of apoptosis by flow cytometry,
PC3 cells were grown at a density of 70–80% confluency and treated
with different concentrations of ABL-N for 24 h. The cells were
trypsinized, washed with PBS and were processed for labeling with
Annexin V and propidium iodide (PI) using an Annexin V-FLUOS
staining kit (Roche Diagnostic Corporation) according to the
manufacturer's protocol. The labeled cells were analyzed by flow
cytometry (Becton-Dickinson, San Jose, CA, USA).
Caspase activity assay
The activities of caspase-2, -3, -6 and -8 were
separately assayed using the respective Caspase-Glo assays
(Promega) according to the manufacturer's protocol. Briefly, cells
were solubilized with lysis buffer for fluorometric assay. After
incubation at 37°C for 1 h, the caspase-2, -3, -6 and -8 activities
were monitored by measuring the fluorescence at 460 nm. Each sample
was measured in triplicates.
Western blot analysis
PC3 cells were treated with ABL-N, harvested and
lysed. Equal amounts of cell extracts were separated using SDS-PAGE
and transferred to a PVDF membrane. The protein was visualized
using the enhanced chemiluminescence kit (Amersham Biosciences) as
previously described (13).
Tumor xenograft experiments
The 4-week-old athymic male nude mice (BALB/c) were
purchased from the Vital River Laboratory Animal Technology Co.,
Ltd. [certificate no. SCXK (Jing) 2007–0001]. An aliquot of
1×106 PC3 cells suspended in 50% Matrigel were implanted
subcutaneously into both flanks of each BALB/c mouse. Six days
after tumor cell inoculation, small tumors were identified. Animals
were randomly divided into experiment and control groups (n=6),
ABL-N (15 mg/kg body weight) or an equal volume of the vehicle was
intra-peritoneally injected, respectively. Tumor growth was
assessed every other day by caliper measurement and tumor volume
was estimated by the formula width2 x length x 1/2. At
the time of sacrifice, tumors were excised and a portion fixed in
10% buffered formalin for 24 h for immunohistochemical studies. The
animal study was approved by the Ethics Committee for animal
research of Hebei Medical University. All the animals were bred and
maintained in the Specific Pathogen Free Animal Care Facility. The
National Institutes of Health guidelines for the care and use of
laboratory animals were followed in all animal procedures.
Immunohistochemistry
Portions of the dissected mouse tumors were
immediately fixed in 10% neutral buffered formalin for 24 h at room
temperature after harvesting, and were then placed in 70% ethanol.
Formalin-fixed tissues were embedded in paraffin, sectioned at 5
µm and incubated with the specific antibodies against
Stat5b, KLF5, ICAM-1, Bcl-2 and Bax (Santa Cruz Biotechnology) for
1 h at room temperature, followed by biotinylated secondary
antibodies for 30 min at room temperature. Sections were
counterstained with hematoxylin. The specimens were viewed with an
Olympus BX51 microscope. Staining intensities were determined by
measurement of the integrated optical density (IOD) with light
microscopy using a computer-based Image-Pro Morphometric system.
Measurements were conducted by two independent observers in a
double-blind manner.
Statistical analysis
Data are expressed as the means ± SE. ANOVA and the
paired or unpaired t-test was performed for statistical analysis as
appropriate. p<0.05 was considered to indicate a statistically
significant result.
Results
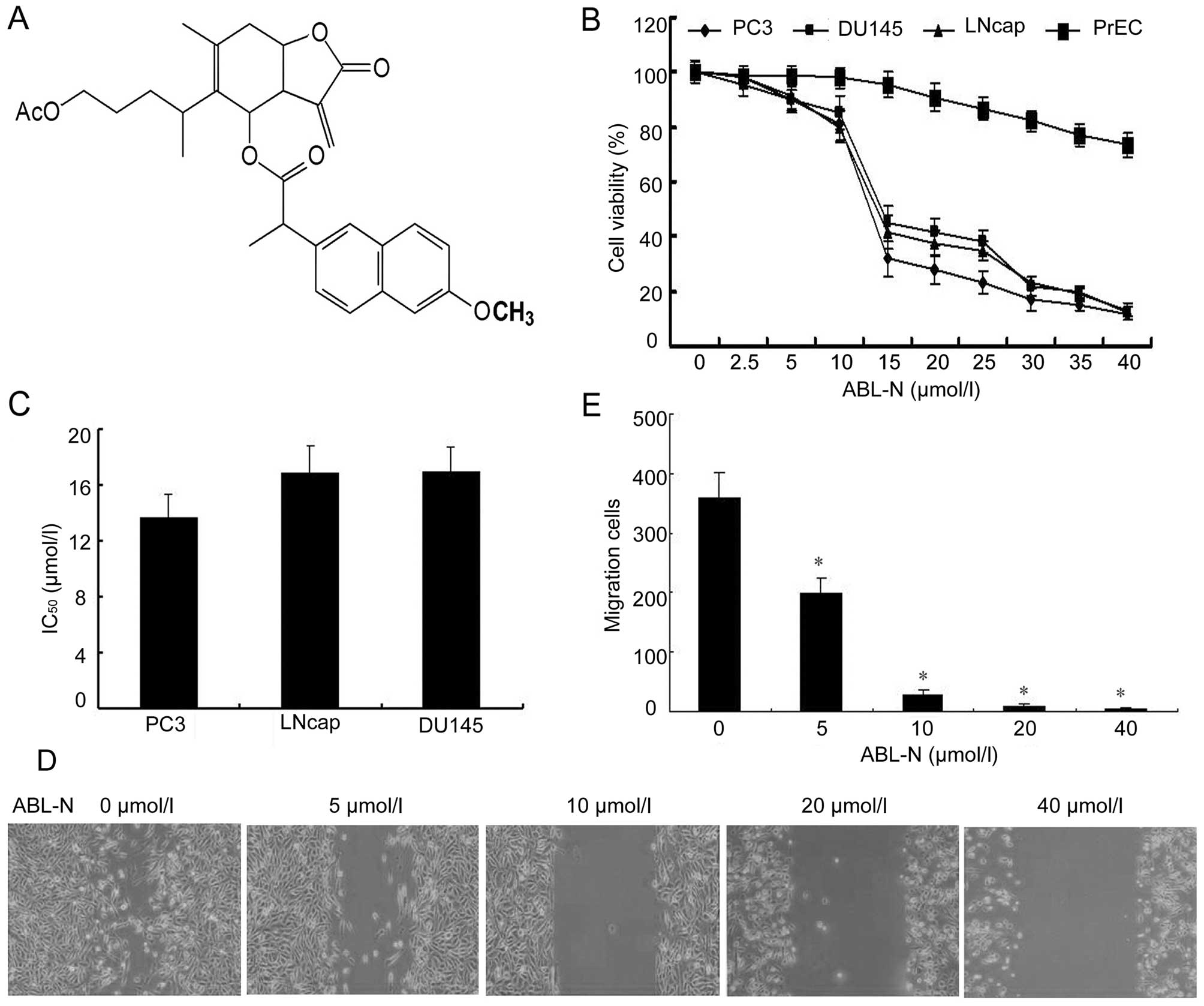
ABL-N inhibits the cell viability of PC3,
DU145 and LNCap cells
MTT assay showed that ABL-N suppressed the cell
viability of human prostate cancer cells (PC3, DU145 and LNcap) in
a dose-dependent manner (Fig. 1B),
with similar IC50 values obtained 24 h after ABL-N
treatment (Fig. 1C). However, the
survival of normal human prostate epithelial PrEC cells was
minimally affected by ABL-N treatment, even at high concentrations
(40 µmol/l) that were highly cytotoxic to the prostate
cancer cells (Fig. 1B).
ABL-N inhibits the cell migration of PC3
cells
According to the experimental results of MTT, the
final concentrations of 0, 5, 10, 20 and 40 µmol/l were used
for ABL-N treatment in the further experiments. In the
wound-healing assay, ABL-N treatment significantly decreased wound
healing of PC3 cells in a dose-dependent manner when compared with
the control cells (Fig. 1D and
E).
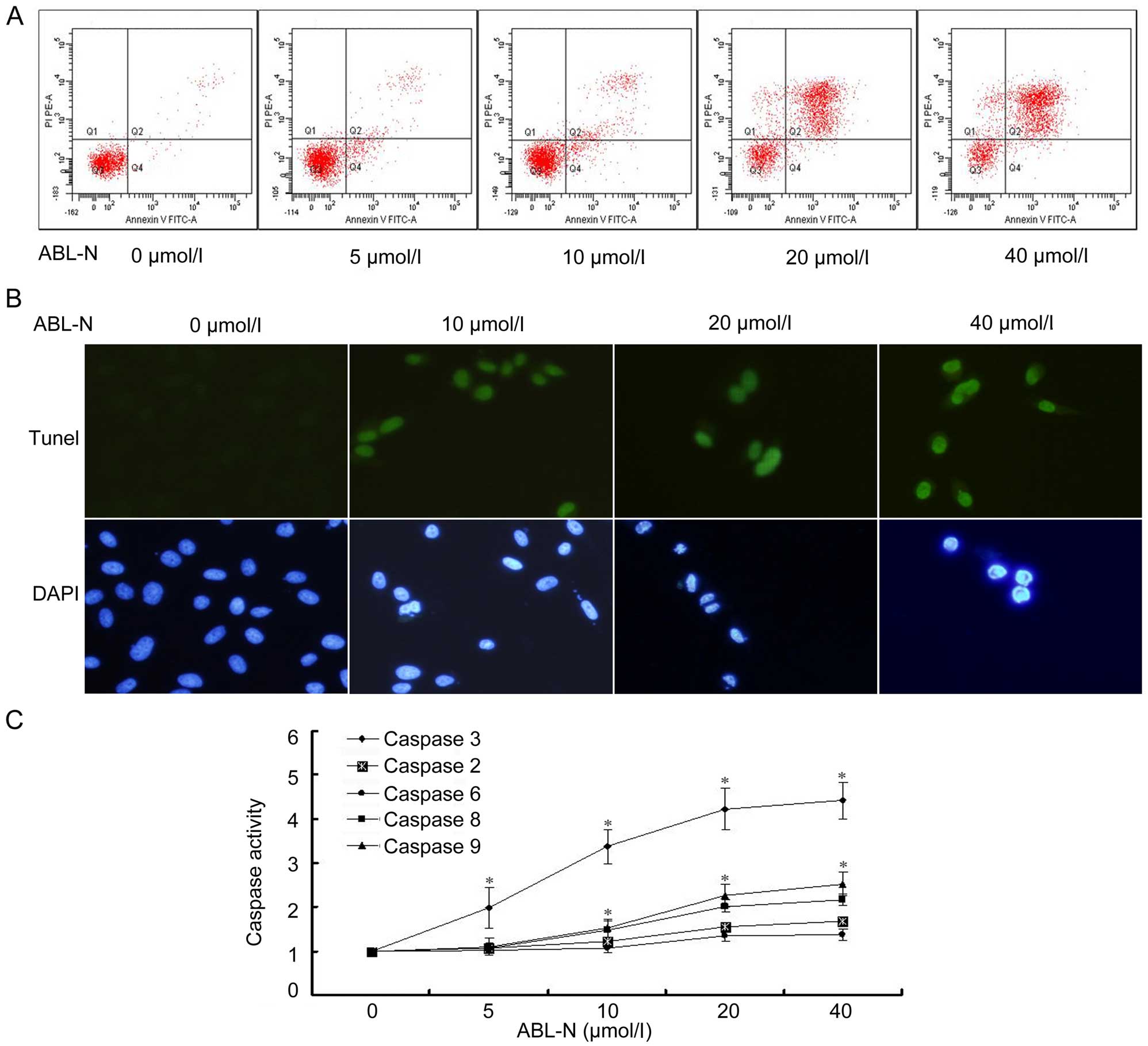
ABL-N induces apoptosis of PC3 cells
To investigate whether ABL-N reduced cell viability
involving the induction of cell apoptosis, we conducted flow
cytometric analysis and TUNEL assay of ABL-N-treated PC3 cells. As
shown in Fig. 2, in the cells
treated with ABL-N, the percentage of cells stained positive for
Annexin V and negative for PI was significantly increased after
ABL-N treatment, even at the lower concentrations. The number of
Annexin V and PI-positive cells was significantly increased at
higher concentrations of ABL-N (20 and 40 µmol/l, Fig. 2A). Cells were further stained for
TUNEL and DAPI to assess the effects of ABL-N on apoptosis, results
showed enhanced apoptosis of PC3 cells after exposure to ABL-N, and
the condensed and fragmented nuclei increased with ABL-N treatment
(Fig. 2B).
ABL-N induces the activities of caspases
in PC3 cells
To test whether caspases were involved in the
ABL-N-induced apoptosis, the activities of caspase-2, -3, -6 and -8
were colorimetrically assayed. As shown in Fig. 2C, caspase-3 activity was
significantly increased after ABL-N treatment and was
dose-dependent. The activities of caspase-8 and -9 were also
enhanced to some extent, while this effect was less significant
than that of the caspase-3. By contrast, caspase-2 and -6
activities were not significantly influenced by ABL-N treatment,
even at the high concentration of 40 µmol/l (Fig. 2C).
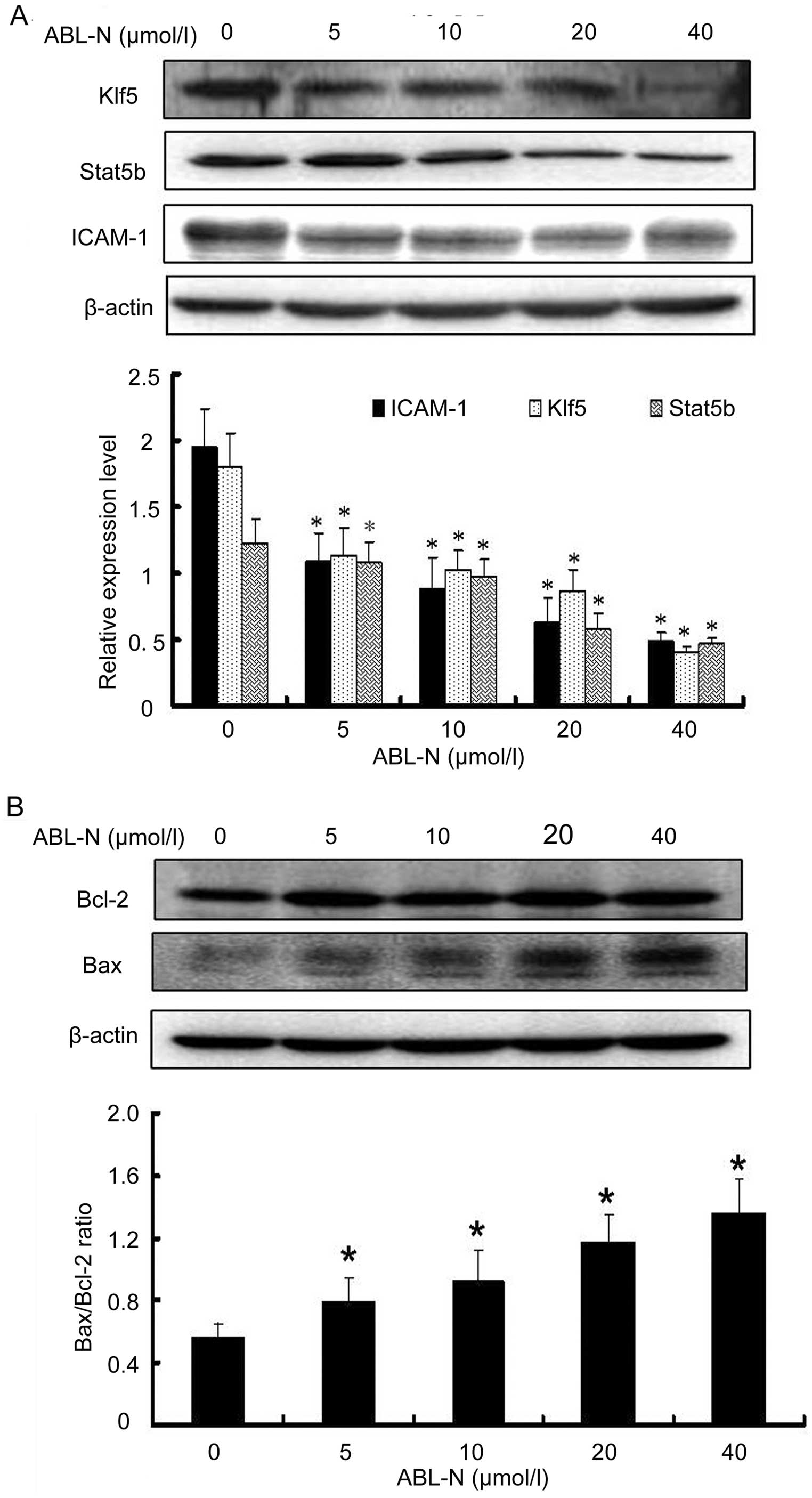
ABL-N inhibits cancer-related proteins in
PC3 cells
Western blotting was performed to investigate the
expression of cancer-related proteins Stat5b and Klf5 and
pro-angiogenic factor ICAM-1 in ABL-N-treated PC3 cells. As shown
in Fig. 3A, ABL-N treatment
significantly decreased the expression of Stat5b, KLF5 and ICAM-1,
and this effect was enhanced with the increasing concentration of
ABL-N (p<0.05).
ABL-N treatment results in an elevated
Bax/Bcl-2 ratio in PC3 cells
The ratio of Bax/Bcl2 is often considered as a
decisive factor in cell apoptosis or survival. As shown in Fig. 3B, the exposure of human PC3 cells to
ABL-N caused a marked increase in Bax protein expression, while the
levels of Bcl-2 protein were not obviously affected after ABL-N
treatment. This resulted in a substantial increase in Bax/Bcl-2
ratio, which favors apoptosis and collectively forms a molecular
basis for the apoptotic action of ABL-N (Fig. 3B).
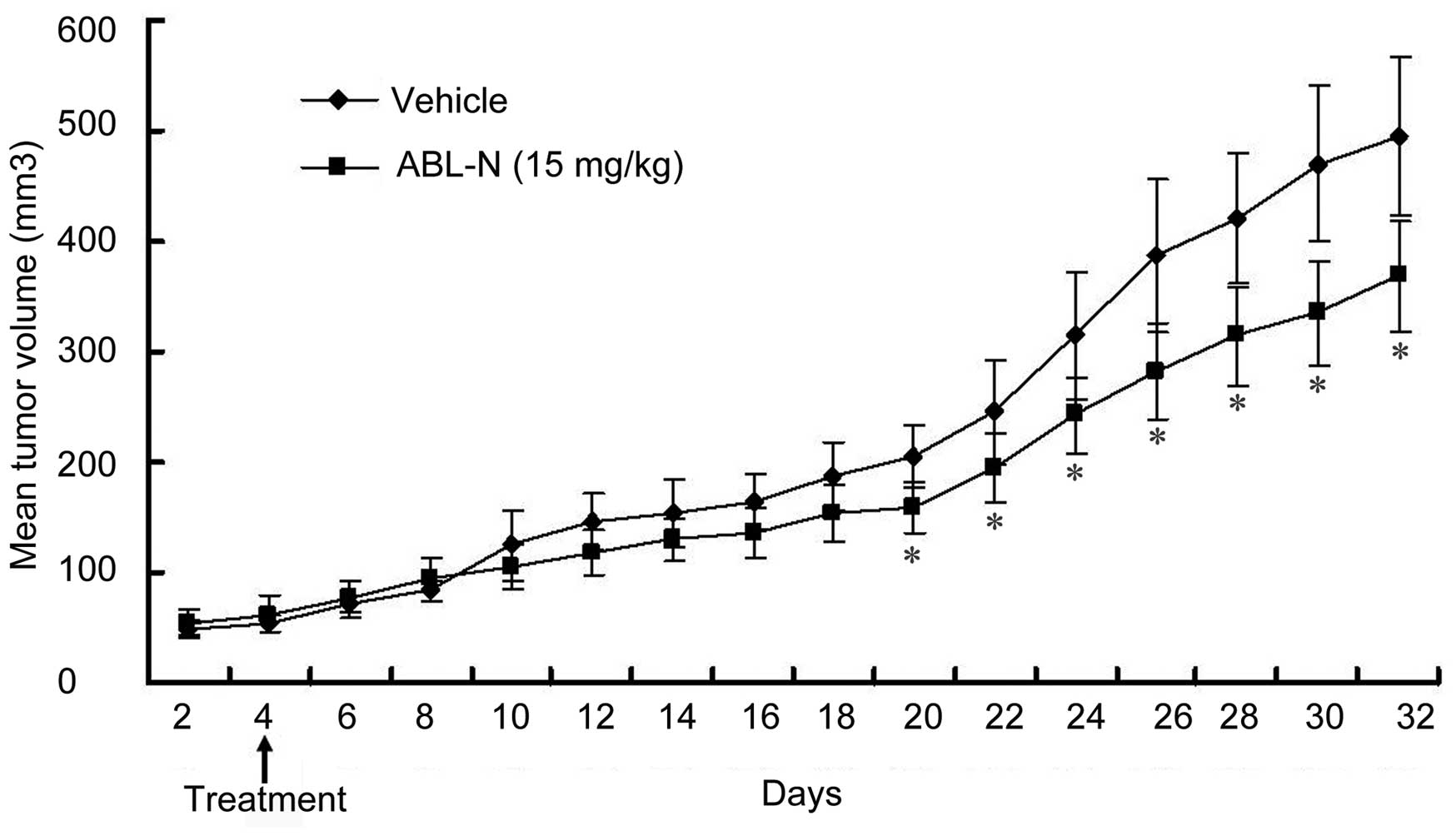
ABL-N inhibits the growth of prostate
cancer in vivo
The xenograft models of PC3 cells were established
in BALB/c mice to investigate the effect of ABL-N on tumor growth
in vivo. As shown in Fig. 4,
treatment with ABL-N (15 mg/kg) significantly suppressed the growth
of tumor cells when compared with the vehicle-treated control group
(p<0.05). No toxicity was observed and treated mice showed no
weight loss, decreased activity or anorexia (data not shown). These
data indicated beneficial therapeutic effect of ABL-N in the
xenograft prostate cancer mouse model.
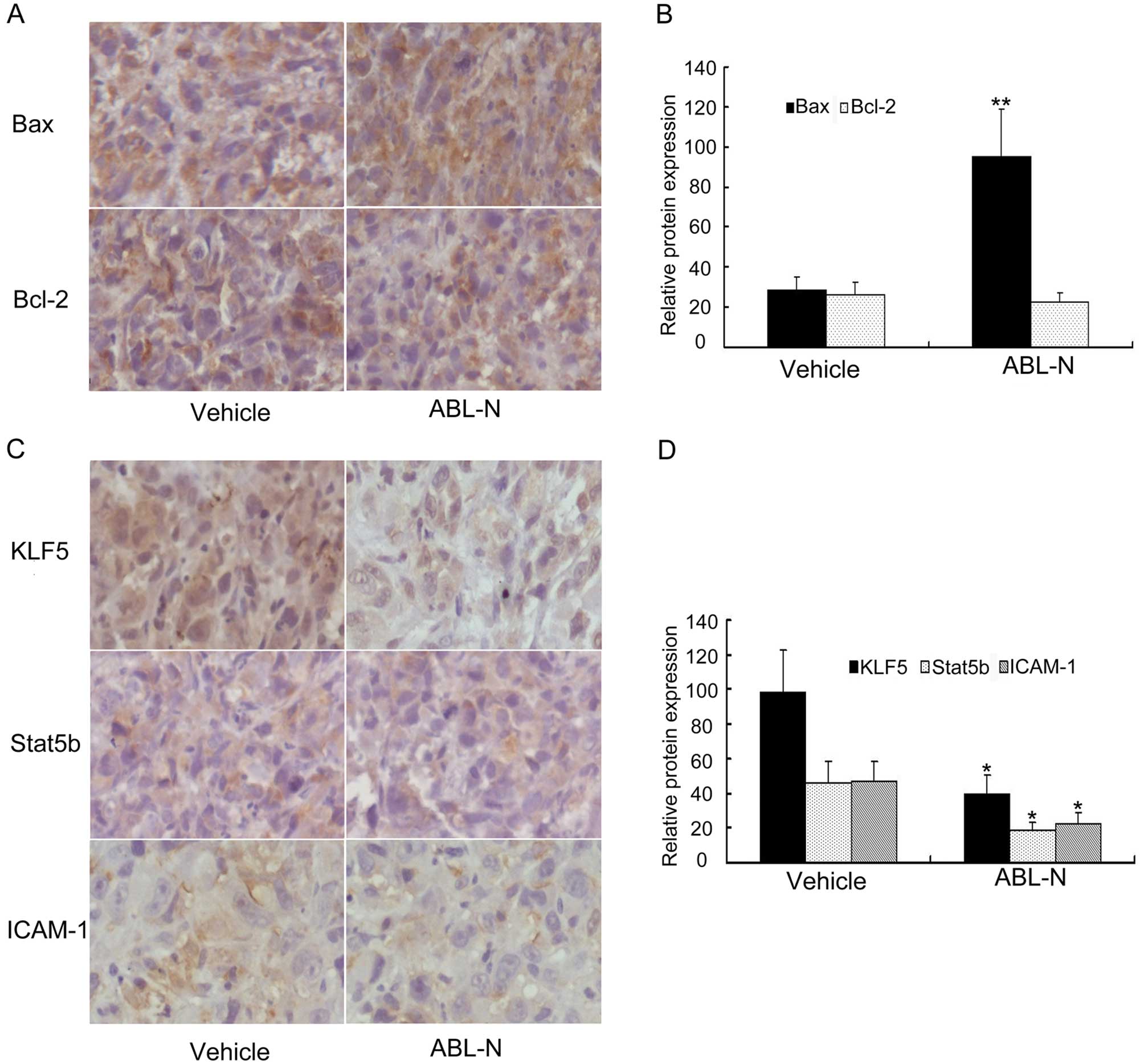
ABL-N modulates Bcl-2/Bax and inhibits
KLF5, Stat5 and ICAM-1 in the PC3 xenograft mouse model
ABL-N treatment was found to inhibit or decrease the
tumorigenic potential of PC3 cells in vivo, and we further
determined the effect of ABL-N administration on the expression
levels of Bax and Bcl-2, as well as KLF5, Stat5b and ICAM-1 in
tumors excised from PC3 xenograft mice. In accordance with the
results obtained in vitro, immunohistochemical analysis
showed that Bax expression was significantly enhanced in tumor
tissues of animals treated with ABL-N, and the level of Bcl-2
protein was not significantly altered after ABL-N treatment
(p<0.01, Fig. 5A and B).
Moreover, tumor sections from ABL-N- administered mice exhibited
significantly decreased protein expression of KLF5, Stat5b and
ICAM-1 when compared with vehicle-treated ones (p<0.05, Fig. 5C and D).
Discussion
Recently, the apoptosis signaling systems have been
shown to provide promising targets for development of novel
anticancer agents (14). Several
plant-derived bioactive agents, such as delphinidin, baicalein,
gambogic acid and green tea, are known as chemopreventive agents
and have been reported to induce apoptosis in a number of
experimental models of carcinogenesis (15). Induction of apoptosis is therefore
considered as a possible therapeutic mechanism of these
chemopreventive agents. ABL, which has been shown to be potently
antitumorigenic, has pro-apoptotic features in numerous carcinoma
cell types (11,16). We recently obtained a highly active
derivative ABL-N, which has shown exceptional antiproliferative
activity in human breast cancer cells (12). The aim of the present study was to
investigate the possible role of ABL-N-induced apoptosis in human
prostate cancer cells and delineate the potential mechanism.
Our results showed that ABL-N inhibited the cell
viability of human prostate cancer cell lines LNCaP, DU145 and PC3
at low concentration, and after increased treatment with 20
µM or more of ABL-N, there was a pronounced accumulation of
apoptotic cells. By contrast, cell viability of normal human
prostate epithelial PrEC cells was not significantly influenced by
ABL-N even at high concentration, indicating that ABL-N selectively
induced apoptosis and arrested growth of prostate cancer cells
without producing cytotoxic effects on normal cells. Studies in our
laboratories have previously reported the death of different human
breast cancer cell lines induced by ABL-N, and this effect has been
linked to caspase-dependent apoptosis (12). Results of our TUNEL and DAPI assays
demonstrated that ABL-N induced apoptosis in PC3 cells. The number
of Annexin V and PI-positive cells increased with higher
concentrations of ABL-N, indicating an activation of the
pro-apoptotic pathway with consecutive apoptotic cell death. In the
quantification of apoptosis by flow cytometry experiments, the
number of the cells actively undergoing apoptosis during ABL-N
exposure was determined to investigate the sensitivity of prostate
cancer cells to ABL-N-induced apoptosis. After exposure to 40
µmol/l ABL-N, however, the late apoptotic/necrotic cells
predominated with high proportion of 65% compared with that of 15%
for early apoptotic cells, indicating the accelerated cell death
induced by ABL-N treatment.
Caspases are known as key mediators of apoptosis and
contributed to the apoptotic morphology through the cleavage of
various cellular substrates. Caspase-3 is an executioner caspase
that can be activated by a mitochondrial pathway involving
activation of caspase-9 due to release of cytochrome c to
the cytosol or a death receptor pathway involving caspase-8
(17). In the present study, the
results that caspase-3 activation enhanced markedly indicated that
caspase-3 plays a key role as an important executioner in
ABL-N-induced apoptosis in PC3 cell lines. Moreover, the results of
the present study indicated that ABL-N-induced apoptosis in PC-3
cells is probably mediated by both caspase-9 and caspase-8.
Consequently, we hypothesized that mitochondria or death
receptor-mediated activation of the caspases may be a potential
mechanism underlying ABL-N-induced apoptosis in prostate cancer
cells and further study was performed.
Bcl-2 family has been shown to play an important
regulatory role in apoptosis, either as activator (Bax) or as
inhibitor (Bcl-2) (14,18). Bax exerts pro-apoptotic activity by
translocation from the cytosol to the mitochondria, where it
induces cytochrome c release, while Bcl-2 exerts its
anti-apoptotic activity, at least in part by inhibiting the
translocation of Bax to the mitochondria (19,20).
The Bcl-2 and Bax protein ratio has been recognized as a key factor
in regulation of the apoptotic process (14,21).
They can activate or inhibit the release of downstream factors such
as cytochrome c which leads to the activation of caspase-3
and PARP in the execution of apoptosis (22). The results of the present study
indicated that ABL-N-induced apoptosis in human PC3 cells was
accompanied by upregulation of Bax, yet with no marked
downregulation effect on Bcl-2 protein expression.
Signal transducer and activator of transcription 5a
and 5b (Stat5a/b) is critical for the viability of human prostate
cancer cells, and it is activated in prostate cancer, yet not in
normal human prostate epithelium (23). The activation of Stat5a/b in primary
prostate cancer predicted early prostate cancer recurrence
(24). It has been reported that
active Stat5 promoted migration and invasion of prostate cancer
cells, induced re-arrangement of the microtubule network and
increased metastases formation of prostate cancer cells (25,26).
Pro-angiogenic factor ICAM-1 has been reported to provide a
structural and functional interface between epithelial cells and
the extracellular environment, and the expression of ICAM-1 in PC3
cells was correlated with increased metastatic potential of
prostate cancer cells (27).
Herein, we also showed that expression of ICAM-1 and Stat5b was
significantly decreased in highly metastatic PC-3 prostate cancer
cells treated with ABL-N.
KLF5 is a basic transcriptional factor that
functions in multiple cellular processes including cell
proliferation, differentiation and apoptosis, and it has both pro-
and anti-tumorigenic effects (28).
KLF5 is thought to be a tumor suppressor in prostate and breast
cancers (29,30), while it has also been shown to drive
proliferation in cultured cells and to be a prognostic factor for
the survival of patients with breast cancer (31). Studies that examine KLF5 expression
by stage reflected high KLF5 expression in early stages of cancer
progression and lower KLF5 expression in later stages (32,33).
Our results showed that ABL-N apparently decreased the protein
expression of KLF5 in a dose-dependent manner. Thus, KLF5 also
plays a promoting role in the process of prostate cancer
progression. Accordingly, we hypothesized that ABL-N antitumor
effect is possibly through inhibiting these factors and further
study was needed to demonstrate this effect.
We next used an experimental model of prostate
cancer to further evaluate the antitumor effect of ABL-N and the
potential mechanism. The findings of our in vivo study
confirmed the antitumor activity of ABL-N against the PC3 human
xeno-grafts, a widely accepted model of highly aggressive prostate
cancer. Administration of ABL-N for only 12 consecutive days
inhibited the growth of established PC3 tumors when compared with
that of the vehicle-treated ones. ABL-N treatment did not result in
significant changes in body weights and histologic data in nude
mice. Corroborating the results in vitro, our in vivo
study also demonstrated the upregulated expression of Bax and
increased Bax to Bcl-2 ratio, as well as the decreased expression
of cancer-related proteins KLF5, Stat5b and pro-angiogenic factor
ICAM-1. The present study provided clear experimental evidence that
ABL-N exerted therapeutic and preventive effects on prostate cancer
without notable toxicity, and it acts through suppression of KLF5,
ICAM-1 and Stat5b expression and upregulation of Bax/Bcl-2 ratio.
All the above results indicated that ABL-N may be a promising
candidate for cancer therapy, although further experimental studies
should be performed to demonstrate the therapeutic potential of
ABL-N in other types of cancer and the functional mechanism.
To the best of our knowledge, this is the first
study detailing apoptosis induction of ABL-N in prostate cancer
cell PC3 in vivo and in vitro. Our results indicated
that ABL-N inhibited proliferation of PC-3 cells at least partly by
causing apoptosis through suppressing the cancer-related protein
Stat5b, Klf5 and ICAM-1, and increasing Bax/Bcl-2 ratio indicating
that ABL-N may be developed as a potential anticancer agent against
human prostate cancer. The present study offers new therapeutic
perspective to prostate cancer therapy, and our data also support
further studies to explore the therapeutic potential of ABL-N in
other types of human cancer.
Acknowledgments
The authors express their thanks to Professor Jinkun
Wen and Professor Mei Han in the Institute of Basic Medicine, Hebei
Medical University for their assistance in the present study.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsuda T and Saika K: Comparison of time
trends in prostate cancer incidence (1973–2002) in Asia, from
cancer incidence in five continents, Vols IV–IX. Jpn J Clin Oncol.
39:468–469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng P, Gong Y, Bao P, Ke JZ, Xiang YM,
Zhang ML and Zheng Y: Estimates and prediction of prostate cancer
incidence, mortality and prevalence in China, 2008. Zhonghua Liu
Xing Bing Xue Za Zhi. 33:1056–1059. 2012.In Chinese.
|
|
4
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar
|
|
5
|
Bilbro J, Mart M and Kyprianou N:
Therapeutic value of quinazoline-based compounds in prostate
cancer. Anticancer Res. 33:4695–4700. 2013.PubMed/NCBI
|
|
6
|
Schmitz-Dräger BJ, Lümmen G, Bismarck E
and Fischer C: Prevention strategies for prostate cancer. Minerva
Urol Nefrol. 64:225–231. 2012.
|
|
7
|
Samarghandian S, Samini F and Taghavi M:
Antiproliferative and cytotoxic properties of honey in human
prostate cancer cell line (PC-3): Possible mechanism of cell growth
inhibition and apoptosis induction. Afr J Pharm Pharmacol. 8:9–15.
2014. View Article : Google Scholar
|
|
8
|
Hafeez BB, Siddiqui IA, Asim M, Malik A,
Afaq F, Adhami VM, Saleem M, Din M and Mukhtar H: A dietary
anthocyanidin delphinidin induces apoptosis of human prostate
cancer PC3 cells in vitro and in vivo: Involvement of nuclear
factor-kappaB signaling. Cancer Res. 68:8564–8572. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yun JM, Kweon MH, Kwon H, Hwang JK and
Mukhtar H: Induction of apoptosis and cell cycle arrest by a
chalcone panduratin A isolated from Kaempferia pandurata in
androgen-independent human prostate cancer cells PC3 and DU145.
Carcinogenesis. 27:1454–1464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang XM, Liu B, Liu YB, Wang JJ, Wen JK,
Li BH and Han M: Acetylbritannilactone suppresses growth via
upregulation of krüppel-like transcription factor 4 expression in
HT-29 colorectal cancer cells. Oncol Rep. 26:1181–1187.
2011.PubMed/NCBI
|
|
11
|
Rafi MM, Bai NS, Chi-Tang-Ho, Rosen RT,
White E, Perez D and Dipaola RS: A sesquiterpenelactone from Inula
britannica induces anti-tumor effects dependent on Bcl-2
phosphorylation. Anticancer Res. 25:313–318. 2005.PubMed/NCBI
|
|
12
|
Liu B, Han M, Sun RH, Wang JJ, Zhang YP,
Zhang DQ and Wen JK: ABL-N-induced apoptosis in human breast cancer
cells is partially mediated by c-Jun NH2-terminal kinase
activation. Breast Cancer Res. 12:R92010. View Article : Google Scholar
|
|
13
|
Dong L-H, Wen J-K, Liu G, McNutt MA, Miao
SB, Gao R, Zheng B, Zhang H and Han M: Blockade of the
Ras-extracellular signal-regulated kinase 1/2 pathway is involved
in smooth muscle 22 α-mediated suppression of vascular smooth
muscle cell proliferation and neointima hyperplasia. Arterioscler
Thromb Vasc Biol. 30:683–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao L and White E: Bcl-2 and the ICE
family of apoptotic regulators: Making a connection. Curr Opin
genet Dev. 7:52–58. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yi T, Yi Z, Cho SG, Luo J, Pandey MK,
Aggarwal BB and Liu M: Gambogic acid inhibits angiogenesis and
prostate tumor growth by suppressing vascular endothelial growth
factor receptor 2 signaling. Cancer Res. 68:1843–1850. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bai N, Lai CS, He K, Zhou Z, Zhang L, Quan
Z, Zhu N, Zheng QY, Pan MH and Ho CT: Sesquiterpene lactones from
Inula britannica and their cytotoxic and apoptotic effects on human
cancer cell lines. J Nat Prod. 69:531–535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf BB and Green DR: Suicidal tendencies:
Apoptotic cell death by caspase family proteinases. J Biol Chem.
274:20049–20052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
20
|
Wolter KG, Hsu YT, Smith CL, Nechushtan A,
Xi XG and Youle RJ: Movement of Bax from the cytosol to
mitochondria during apoptosis. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar
|
|
21
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tafani M, Schneider TG, Pastorino JG and
Farber JL: Cytochrome c-dependent activation of caspase-3 by tumor
necrosis factor requires induction of the mitochondrial
permeability transition. Am J Pathol. 156:2111–2121. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahonen TJ, Xie J, LeBaron MJ, Zhu J, Nurmi
M, Alanen K, Rui H and Nevalainen MT: Inhibition of transcription
factor Stat5 induces cell death of human prostate cancer cells. J
Biol Chem. 278:27287–27292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Zhang Y, Glass A, Zellweger T, Gehan
E, Bubendorf L, Gelmann EP and Nevalainen MT: Activation of signal
transducer and activator of transcription-5 in prostate cancer
predicts early recurrence. Clin Cancer Res. 11:5863–5868. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu L, Vogiatzi P, Puhr M, Dagvadorj A,
Lutz J, Ryder A, Addya S, Fortina P, Cooper C, Leiby B, et al:
Stat5 promotes metastatic behavior of human prostate cancer cells
in vitro and in vivo. Endocr Relat Cancer. 17:481–493. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu L, Dagvadorj A, Lutz J, Leiby B,
Bonuccelli G, Lisanti MP, Addya S, Fortina P, Dasgupta A, Hyslop T,
et al: Transcription factor Stat3 stimulates metastatic behavior of
human prostate cancer cells in vivo, whereas Stat5b has a
preferential role in the promotion of prostate cancer cell
viability and tumor growth. Am J Pathol. 176:1959–1972. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo NC, Chung HC, Chung HC, Park JO, Rha
SY, Kim JH, Roh JK, Min JS, Kim BS and Noh SH: Synchronous
elevation of soluble intercellular adhesion molecule-1 (ICAM-1) and
vascular cell adhesion molecule-1 (VCAM-1) correlates with gastric
cancer progression. Yonsei Med J. 39:27–36. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Zhang B, Wu Q, Ci X, Zhao R, Zhang
Z, Xia S, Su D, Chen J, Ma G, et al: Interruption of KLF5
acetylation converts its function from tumor suppressor to tumor
promoter in prostate cancer cells. Int J Cancer. 136:536–546.
2015.
|
|
29
|
Chen C, Bhalala HV, Vessella RL and Dong
JT: KLF5 is frequently deleted and down-regulated but rarely
mutated in prostate cancer. Prostate. 55:81–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen C, Sun X, Ran Q, Wilkinson KD, Murphy
TJ, Simons JW and Dong JT: Ubiquitin-proteasome degradation of KLF5
transcription factor in cancer and untransformed epithelial cells.
Oncogene. 24:3319–3327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong D, Czerwenka K, Heinze G, Ryffel M,
Schuster E, Witt A, Leodolter S and Zeillinger R: Expression of
KLF5 is a prognostic factor for disease-free survival and overall
survival in patients with breast cancer. Clin Cancer Res.
12:2442–2448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kwak MK, Lee H-J, Hur K, Park J, Lee HS,
Kim WH, Lee KU, Choe KJ, Guilford P and Yang HK: Expression of
Krüppel-like factor 5 in human gastric carcinomas. J Cancer Res
Clin Oncol. 134:163–167. 2008. View Article : Google Scholar
|
|
33
|
McConnell BB, Bialkowska AB, Nandan MO,
Ghaleb AM, Gordon FJ and Yang VW: Haploinsufficiency of
Krüppel-like factor 5 rescues the tumor-initiating effect of the
ApcMin mutation in the intestine. Cancer Res.
69:4125–4133. 2009. View Article : Google Scholar : PubMed/NCBI
|