Introduction
Cervical cancer, a gynecological malignancy, is both
the fourth most common cause of cancer and the fourth most common
cause of cancer-related mortality among women worldwide (1). There are ~528,000 new cases diagnosed
every year worldwide, and approximately a third are Chinese
(2). Radiotherapy and chemotherapy
are the two main effective treatments for advanced cervical cancer.
Yet, more than one-third of patients develop recurrence or
metastatic disease, despite the availability of modern advanced
technology. Since the pathogenesis of cervical cancer remains
unclear, appropriate cervical cancer treatments are still difficult
to achieve, particularly individual control strategies.
Receptor activator of nuclear factor κB ligand
(RANKL, also called TNFSF11, OPGL and TRANCE) is a member of the
tumor necrosis factor (TNF) superfamily. After binding to its
cognate receptor RANK (also called TNFSF11A, TRANCE-R and CD265),
they are essential regulators of osteoclast differentiation and
thereby fundamental regulators of bone physiology, bone remodeling
(3,4), mammary gland development during
pregnancy (5,6), establishment of the thymic
microenvironment (7) and bone
metastasis of cancer (8,9). The co-expression of RANKL and RANK has
been observed in diverse types of malignant human tumors, and was
found to correlate with metastasis and poor patient survival
(10). Moreover, RANKL promotes the
migration and invasion of several types of human tumor cells
expressing its receptor RANK (11–14).
However, the role of the RANKL-RANK axis in modulating the
behaviors of cervical cancer cells is mostly unknown.
The chemokines, a family of small cytokines or
signaling proteins secreted by cells, regulate the trafficking of
leukocytes to sites of inflammation and recirculation in secondary
lymphatics by interaction with chemokine receptors (15,16).
In addition, chemokines regulate multiple behaviors of tumor cells,
including growth migration, invasion and angiogenesis (17,18).
Our previous study showed that chemokine CXCL8 (also known as IL-8)
induced by hypoxia stimulated the proliferation of cervical cancer
cells (19). In addition, Secchiero
et al showed that the RANKL/RANK system may contribute to
the pathogenesis of B chronic lymphocytic leukemia (B-CLL) by
upregulating IL-8 (20). However,
the expression and possible role of RANKL/RANK in cervical cancer
cells and the relationship with IL-8 remain elusive.
Therefore, the present study aimed to explore
whether cervical cancer cells co-express RANKL and RANK, and
whether the RANKL/RANK system regulates the proliferation and
apoptosis of cervical cancer cells by modulating the IL-8 level
in vitro.
Materials and methods
Tissue collection
Written informed consent was obtained from all
patients before sampling. All tissue samples were solely obtained
for research purposes and obtained with informed consent in
accordance with the requirements of the Research Ethics Committee
of the Obstetrics and Gynecology Hospital of Fudan University.
Samples from 12 patients in International Federation of Gynecology
and Obstetrics (FIGO) stages of cervical cancer were obtained from
women 31–58 years of age. All the samples were histologically
confirmed according to established criteria, and squamous cell
carcinoma was diagnosed in all patients.
Immunohistochemistry (IHC)
Immunohistological staining was performed as
previously described (19,21). Paraffin sections (5 µm) of
tissues from cervical cancer (n=12) were dehydrated in graded
ethanol, and incubated with hydrogen peroxide in 1% bovine serum
albumin (BSA)/TBS to block endogenous peroxidase. The cervical
cancer samples were then incubated with the mouse anti-human RANKL
(25 µg/ml) or the RANK (25 µg/ml) (both from R&D
Systems, USA) antibody or mouse IgG isotype antibody overnight at
4°C in a humid chamber. After washing three times with TBS, the
sections were overlaid with peroxidase-conjugated anti-mouse IgG
antibody (Golden Bridge International, Inc., Beijing, China), and
the reaction was developed with 3,3′-diamino-benzidine (DAB) and
counterstained with hematoxylin. The experiments were repeated five
times.
Cell culture
Cervical epidermoid carcinoma HeLa and SiHa cells
were purchased from the Chinese Center for Type Culture Collection
(CCTCC). HeLa and SiHa cells were grown in Dulbecco's modified
Eagle's medium (DMEM)/F-12 medium supplemented with 5% fetal bovine
serum (FBS) (both from HyClone, Logan, UT, USA).
BrdU cell proliferation and apoptosis
assays
HeLa and SiHa cells were seeded at a density of
5×103 cells/well into 96-well flat-bottom microplates
(for BrdU cell proliferation assay) or 2×105 cells/well
into 12-well flat-bottom microplates (for apoptosis assay), and
were subsequently starved with DMEM/F-12 medium containing 1% FBS
for 12 h before treatment, and were then stimulated with rhRANKL
protein (1, 10 or 100 ng/ml), anti-RANKL neutralizing antibody
(α-RANKL; 0.1, 1 or 10 µg/ml) or recombinant human
osteoprotegrin protein (rhOPG) (1, 10 or 100 ng/ml) (all from
R&D Systems) for 24 or 48 h. In addition, vehicle was added to
various wells as a negative control. Then the abilities of the HeLa
and SiHa cells to proliferate and undergo apoptosis were detected
with BrdU cell proliferation assay (Millipore, Darmstadt, Germany)
and Annexin V-FITC apoptosis assay (Invitrogen, USA) according to
the manufacturer's instructions, respectively. Each experiment was
performed in six parallel wells and repeated three times.
Enzyme-linked immunosorbent assay (ELISA)
for sRANKL and IL-8 determination
HeLa (2×105 cells/well) and SiHa cells
(2×105 cells/well) were seeded into 24-well plates and
cultured for 48 h. Then culture supernatants were harvested,
centrifuged to remove cellular debris, and then stored at −80°C
until being assayed by ELISA. In addition, the secretion of RANKL
by the supernatants was detected using a human RANKL ELISA kit
(BioVendor GmbH, Kassel, Germany) according to the manufacturer's
instructions.
In addition, HeLa and SiHa cells (2×105
cells/well) were treated with α-RANKL (10 µg/ml) for 48 h
with vehicle as a control. Then, the IL-8 level in the supernatant
from the HeLa and SiHa cells was analyzed by ELISA (Shanghai ExCell
Biology, Inc., Shanghai, China) according to standard
procedures.
Flow cytometry (FCM)
HeLa (2×105 cells/well) and SiHa cells
(2×105 cells/well) were cultured for 48 h, and then
digested with 0.25% trypsin only for 30–50 sec, blown-off gently
and washed with phosphate-buffered saline (PBS). After blocking
with 10% FBS, the recovered cells were mixed with phycoerythrin
(PE)-conjugated RANKL (BioLegend, San Diego, CA, USA) or RANK (PE;
R&D Systems) antibody in darkness for 30 min at room
temperature. As a negative control, an isotope control (BioLegend)
was used. After incubation, the cells were washed and immediately
analyzed by a flow cytometer (FACSCalibur; BD, USA) and CellQuest
software (Becton-Dickinson, USA). The statistical analysis was
conducted using isotype-matched controls as references. The
experiments were repeated three times.
HeLa and SiHa cells were incubated with α-RANKL (10
µg/ml) or rhOPG (100 ng/ml) for 48 h, with vehicle as a
control. Then the expression levels of Ki-67 (PE; BioLegend),
B-cell lymphoma 2 (Bcl-2) [fluorescein isothiocyanate (FITC); BD
Biosciences, San Jose, CA, USA), Fas allophycocyanin (APC), Fas
ligand (FasL) (PE) (both from BioLegend), CXCR1 (PE) and CXCR2 (PE)
(both from R&D Systems) in the HeLa and SiHa cells were
analyzed by FCM, respectively.
Moreover, in order to investigate whether IL-8 is
involved in the regulation of HeLa and SiHa cells mediated by
RANKL, we treated HeLa and SiHa cells with rhIL-8 (100 ng/ml;
R&D Systems), α-RANKL (10 µg/ml) or rhIL-8 plus α-RANKL
for 48 h. Then the expression of Ki-67, Bcl-2, Fas and FasL, and
cell proliferation and apoptosis were assessed as previously
described, respectively.
Statistical analysis
All values are shown as the mean ± SEM. The data
were analyzed with GraphPad Prism version 5 by t-test or one-way
ANOVA. Differences were considered to be statistically significant
at P<0.05.
Results
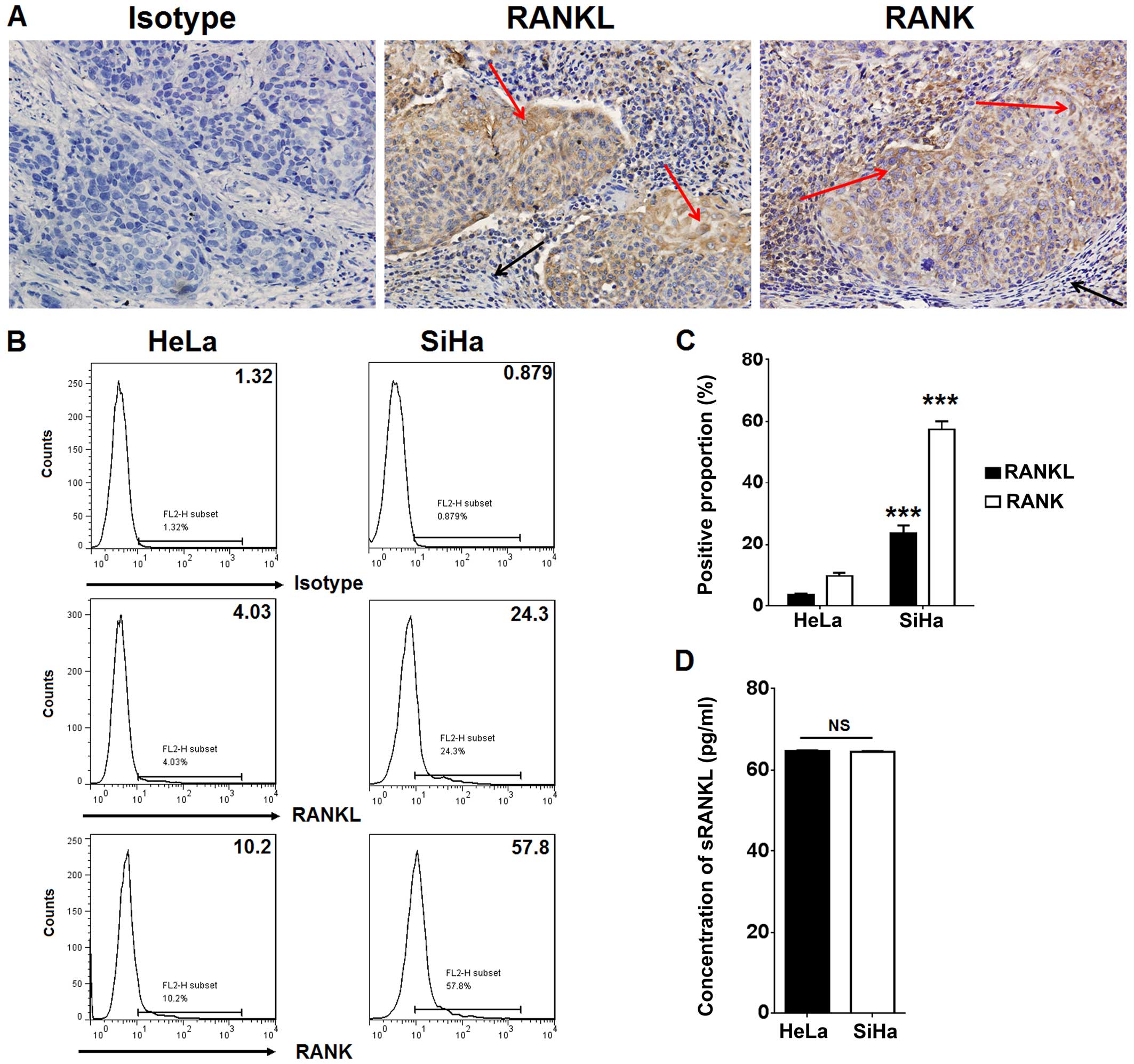
Cervical cancer cells highly co-express
RANKL and RANK
To analyze whether cervical cancer cells express
RANKL and RANK, IHC was used to evaluate the expression of RANKL
and RANK in cervical cancer tissues. As shown, compared to
paracancerous cells, the cervical cancer tissues exhibited strong
positive staining for RANKL and RANK (Fig. 1A). RANKL and RANK were located in
the cell membrane and cytoplasm (Fig.
1A). Further analysis by FCM showed that HeLa and SiHa cells
co-expressed member RANKL (mRANKL) and its receptor RANK,
particularly in the SiHa cells. The percentage of RANKL and
RANK-positive SiHa cells was more than 6-fold higher than that in
the HeLa cells (P<0.001) (Fig. 1B
and C). However, the result of ELISA showed that the secretion
of sRANKL from the HeLa and SiHa cells was at an equivalent level
(65 pg/ml) (P>0.05) (Fig. 1D).
These data suggest that a high level of RANKL/RANK expression in
cervical cancer cells may play a regulatory role in the biological
behavior of cervical cancer cells.
RANKL enhances the proliferation and
restricts apoptosis in the HeLa and SiHa cells
In order to investigate the effect of RANKL/RANK
signaling in growth regulation, we treated HeLa and SiHa cells with
rhRANKL (1, 10 or 100 ng/ml), α-RANKL (0.1, 1 or 10 µg/ml)
or rhOPG (1, 10 or 100 ng/ml) at different concentrations. We found
that rhRANKL treatment did not alter the proliferation of the HeLa
and SiHa cells (P>0.05) (Fig. 2A and
B). However, we observed that blocking RANKL/RANK interaction
with α-RANKL for 24 or 48 h led to a decrease in HeLa and SiHa cell
proliferation, particularly at a concentration of 10 µg/ml
(P<0.05, P<0.01 or P<0.001) (Fig. 2A and B). In addition, blocking RANKL
with rhOPG had a similar effect when compared to the α-RANKL group,
but could not influence the proliferation of the HeLa cells at 24 h
(P>0.05) (Fig. 2A). The
differential effect of RANKL and RANK on HeLa cells may be related
to the different RANKL/RANK expression in these two cells.
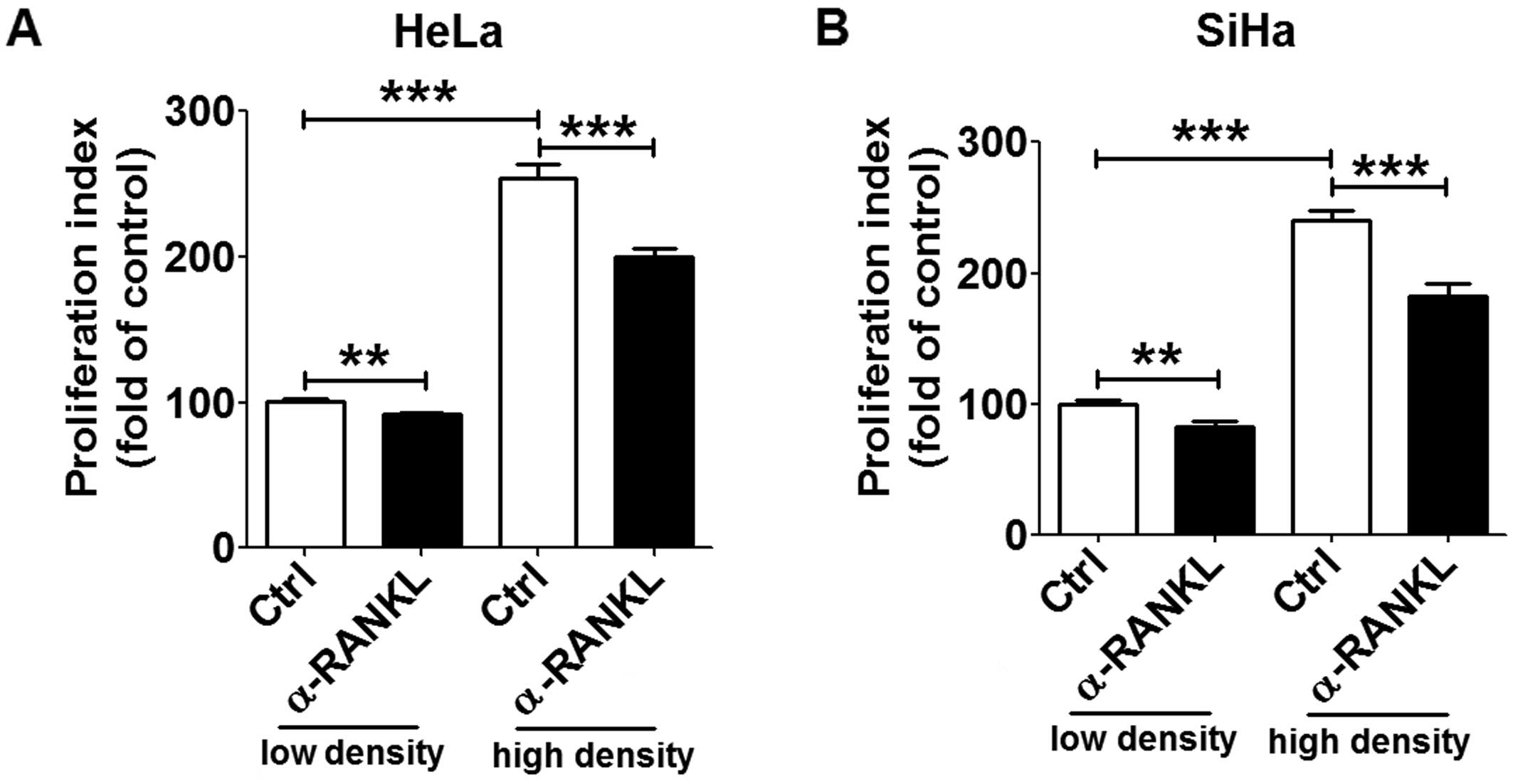
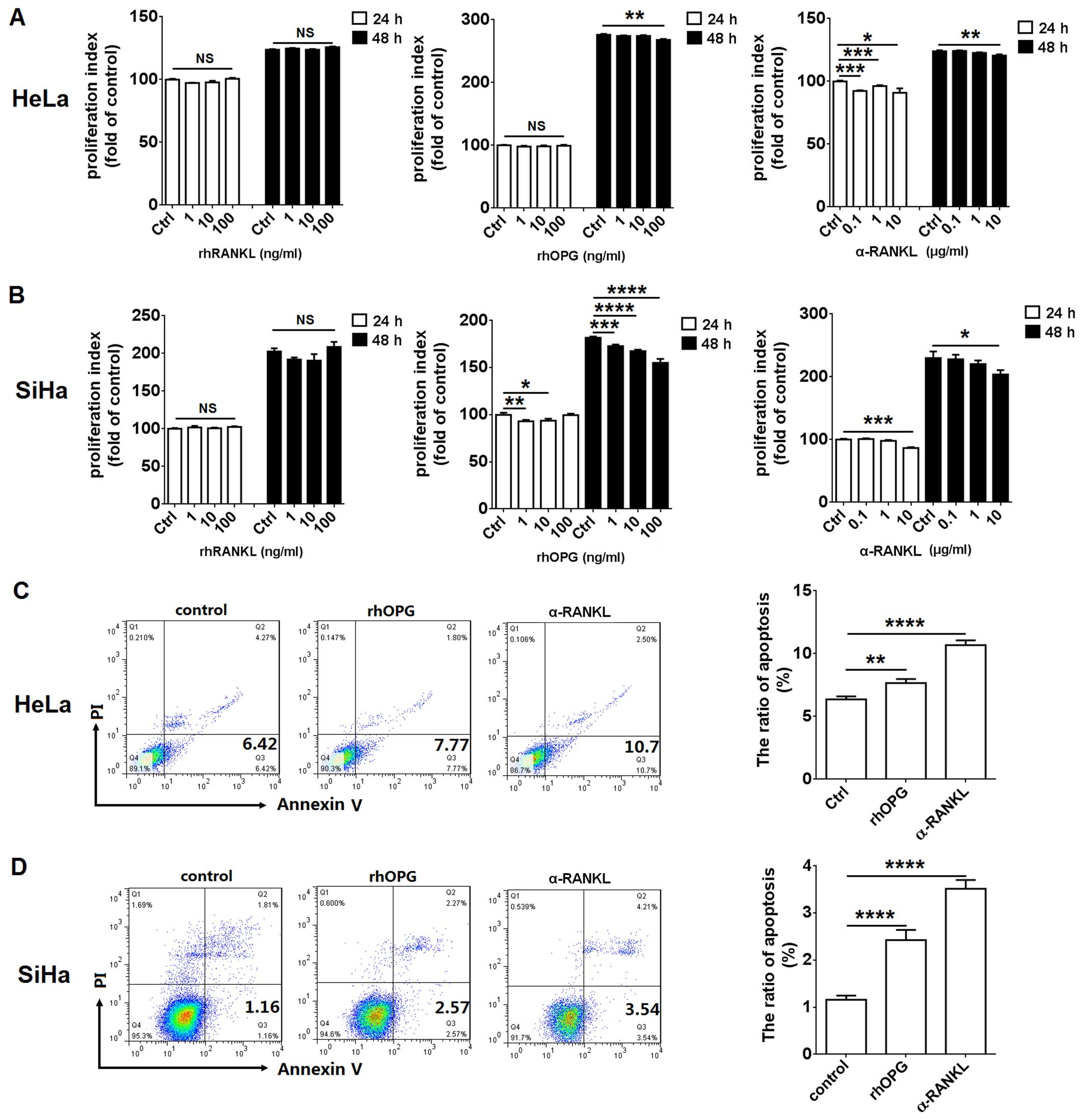 | Figure 2RANKL enhances proliferation and
restricts apoptosis in HeLa and SiHa cells. (A) HeLa and (B) SiHa
cells were incubated with rhRANKL (1, 10 or 100 ng/ml), α-RANKL
(0.1, 1 or 10 µg/ml) or rhOPG (1, 10 or 100 ng/ml) for 24 h
(white columns) or 48 h (black columns), and then cell
proliferation in HeLa and SiHa cells was detected by BrdU
proliferation assay. In addition, (C) HeLa and (D) SiHa cells were
incubated with α-RANKL (10 µg/ml) or rhOPG (100 ng/ml) for
48 h, and the apoptosis in Hela and SiHa cells was analyzed by
apoptosis assay. rhRANKL, recombinant human RANKL protein; α-RANKL,
anti-human RANKL neutralizing antibody; rhOPG, recombinant human
OPG protein. The data are expressed as the mean ± SEM.
*P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 (one-way
ANOVA). NS, no statistical difference. |
Subsequently, the results of the apoptosis assay
showed that incubation with α-RANKL (10 µg/ml) or rhOPG (100
ng/ml) led to a significant increase in apoptosis in the HeLa
(P<0.01 or P<0.0001) (Fig.
2C) and SiHa (P<0.0001) (Fig.
2D) cells.
In addition, as shown in Fig. 3, the ability of cell proliferation
at a low cell density was lower than that at a high cell density
(P<0.001). The decrease in cell proliferation induced by
blocking RANKL/RANK with α-RANKL was more apparent in the group
with high cell density. These results indicate that RANKL/RANK
interaction may promote the proliferation of cervical cancer cells
by strengthening the dialogue between cervical cancer cells.
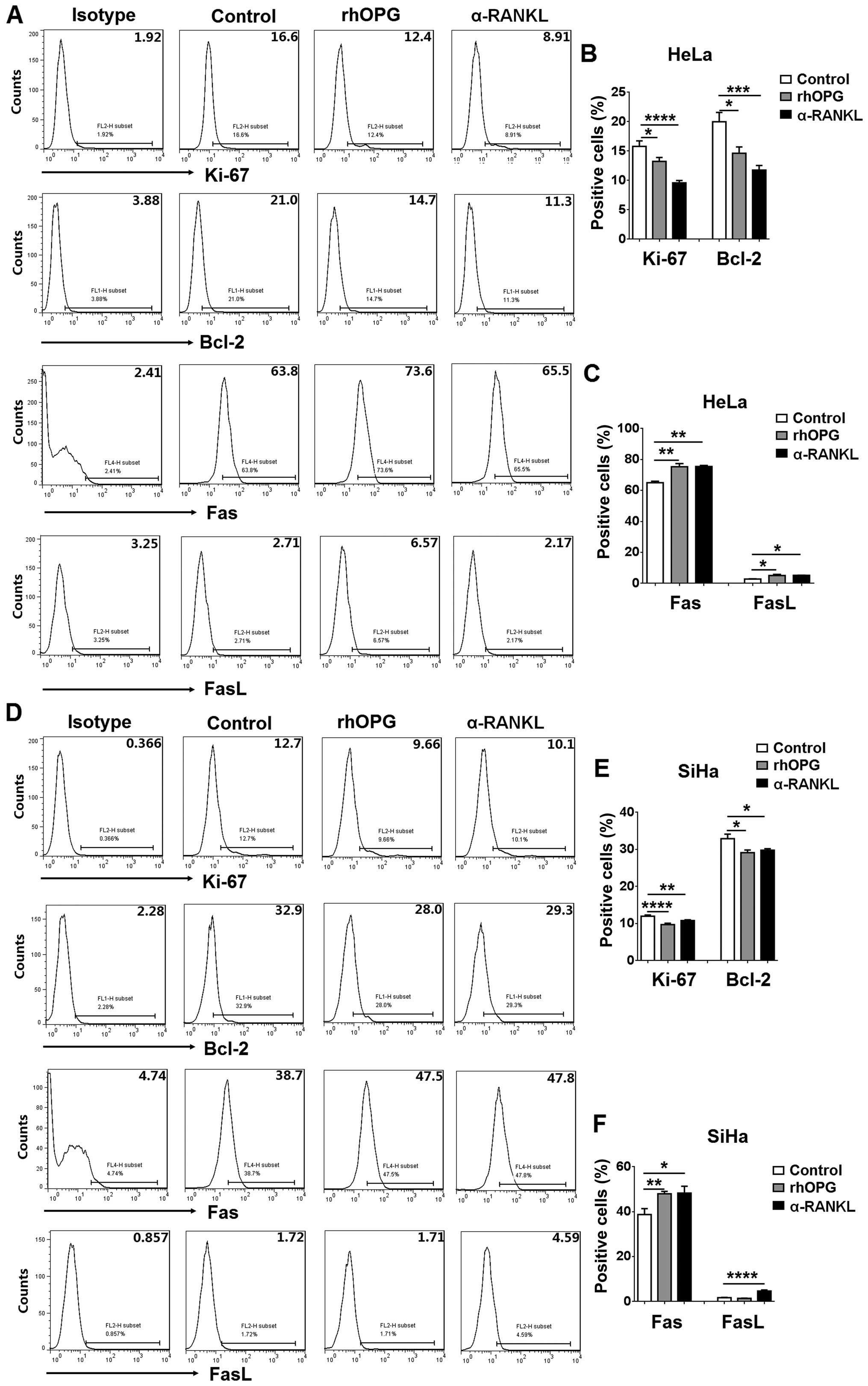
RANKL regulates Ki-67, Bcl-2, Fas and
FasL expression in HeLa and SiHa cells
Next, FCM was performed to investigate whether RANKL
regulates proliferation- and apoptosis-related molecules in
cervical cancer cells. As shown in Fig.
4, both α-RANKL and rhOPG markedly downregulated Ki-67 and
Bcl-2 expression (P<0.05, P<0.01 or P<0.0001) (Fig. 4A and B), and upregulated Fas and
FasL expression in the HeLa cells (P<0.05 or P<0.01)
(Fig. 4A and C). Meanwhile, we
found that stimulation with α-RANKL or rhOPG resulted in decreases
in Ki-67 and Bcl-2 expression and increases in Fas and FasL
expression in the SiHa cells (P<0.05, P<0.01 or P<0.0001)
(Fig. 4D–F). Collectively, these
data indicate that the regulation of expression of these
proliferation- and apoptosis-related molecules may be involved in
the effect of RANKL on the proliferation and apoptosis in cervical
cancer cells.
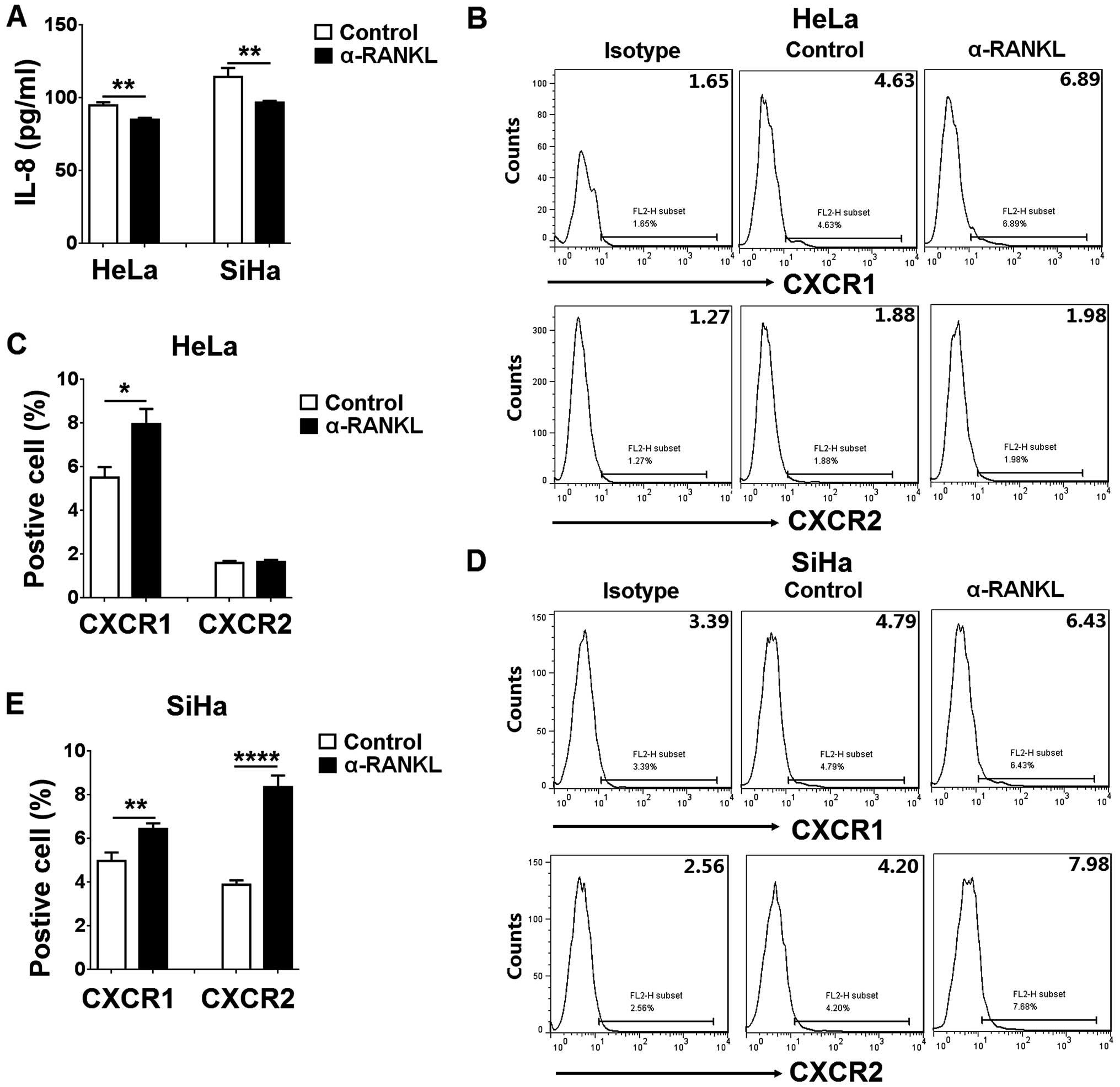
IL-8 secretion and its receptor
expression in HeLa and SiHa cells are regulated by RANKL
To know the potential effect of RANKL on IL-8 and
its receptors (CXCR1 and CXCR2) in cervical cancer cells in
vitro, we detected IL-8 secretion, and CXCR1 and CXCR2
expression in the HeLa and SiHa cells after treatment with α-RANKL.
As observed by ELISA, α-RANKL led to a decrease in IL-8 secretion
from the HeLa and SiHa cells (P<0.01) (Fig. 5A). In contrast, treatment with
α-RANKL gave rise to an increase in CXCR1 expression in the HeLa
cells (P<0.05) (Fig. 5B and C).
However, the CXCR2 level exhibited no changed (P>0.05) (Fig. 5B and C). The difference was that
α-RANKL treatment led to an increase in CXCR1 and CXCR2 expression
in the SiHa cells (P<0.01 or P<0.0001) (Fig. 5D and E). These opposite effects
should be a style of negative feedback.
RANKL/RANK axis promotes the growth of
HeLa and SiHa cells possibly by stimulating IL-8
Taking into account the important role of IL-8
(19) in the regulation of cervical
cancer cell growth, we cultured HeLa and SiHa cells with rhIL-8,
α-RANKL or rhIL-8 plus α-RANKL, and found that rhIL-8 alone
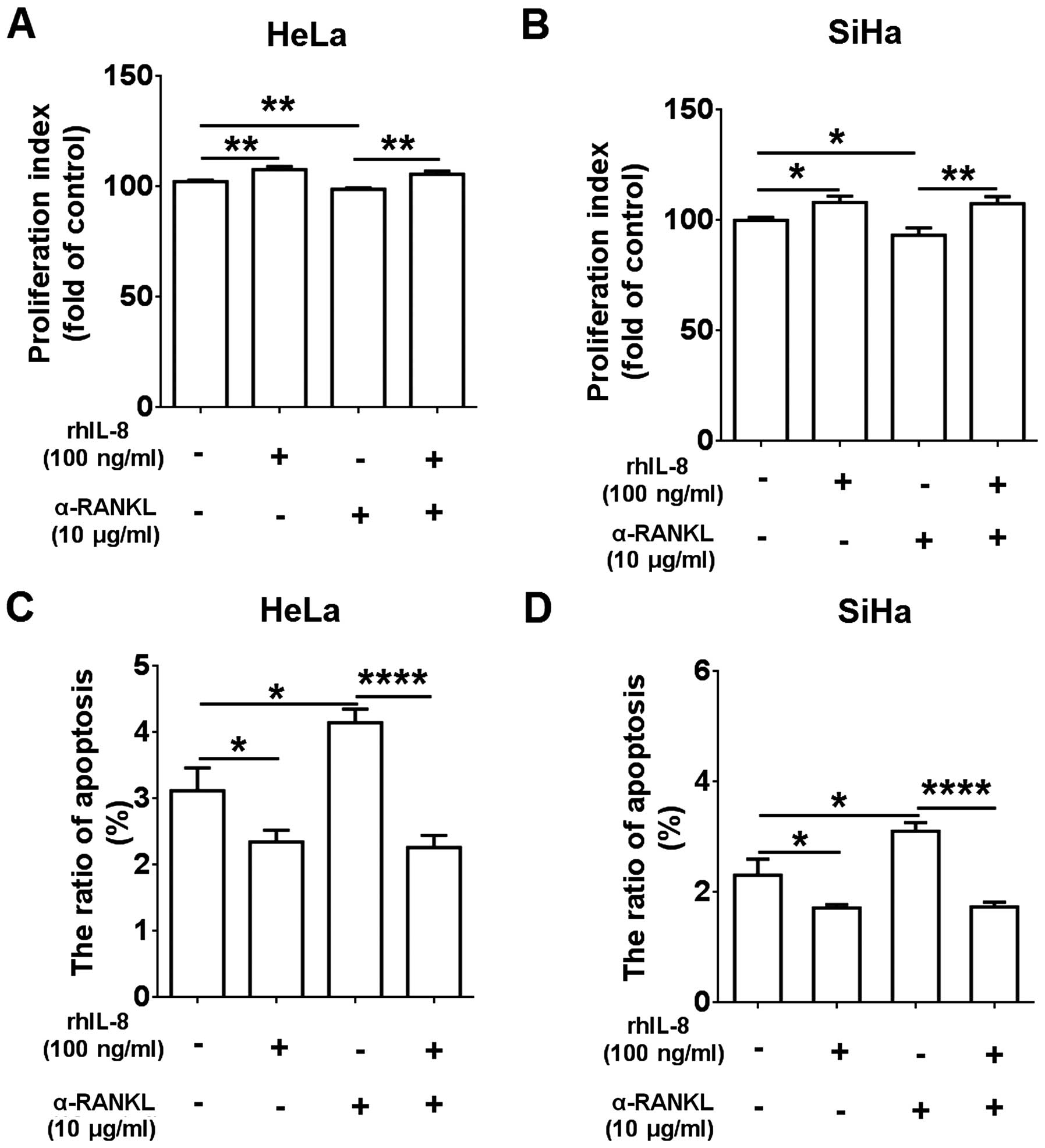
promoted cell proliferation (P<0.05 or P<0.01) (Fig. 6A and B) and inhibited the apoptosis
(P<0.05) (Fig. 6C and D) of HeLa
and SiHa cells, and α-RANKL alone had an opposite effect (P<0.05
or P<0.01) (Fig. 6A–D).
Following treatment with rhIL-8 plus α-RANKL, the proliferation and
apoptosis capacities in the HeLa and SiHa cells were similar to the
group treated with rhIL-8 alone, suggesting that treatment with
rhIL-8 reversed the inhibitory effect on cell proliferation and the
stimulatory effect on cell apoptosis induced by α-RANKL (Fig. 6A–D).
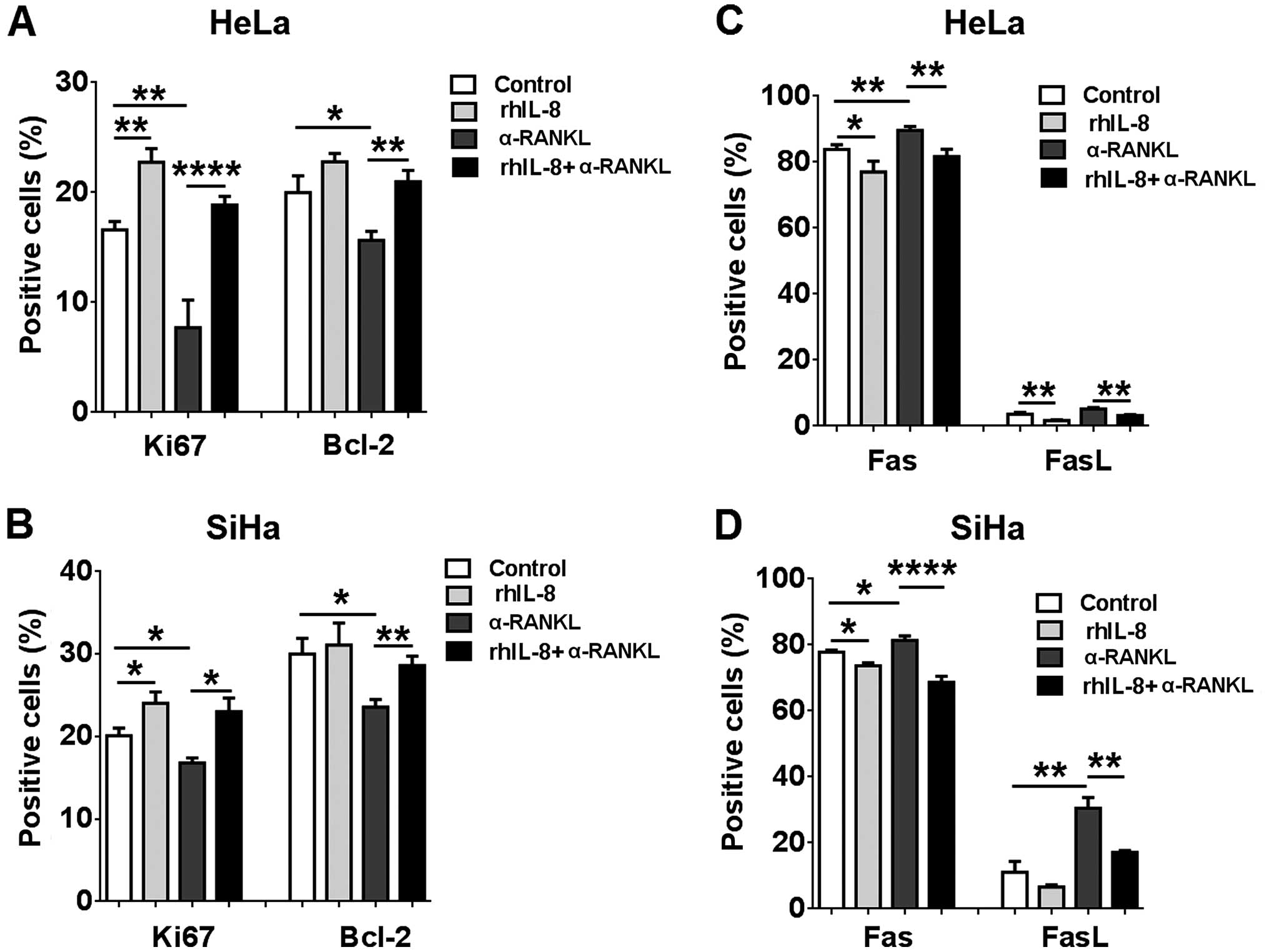
We next found that rhIL-8 increased Ki-67 and Bcl-2
expression, and decreased Fas and FasL expression in the HeLa and
SiHa cells (P<0.05 or P<0.01) (Fig. 7A–D). Similarly, it also abrogated
the regulatory effect on the levels of these molecules mediated by
α-RANKL (Fig. 7A–D). Therefore,
these findings provide evidence that RANKL may stimulate cervical
cancer cell growth by IL-8 production.
Discussion
Numerous studies have estimated that the RANKL/RANK
system mediates important osteoclast-dependent pathological
processes in metastatic disease to bone (22). Our previous study also showed that
RANKL was involved in the regulation of biological behaviors of
decidual stromal cells (23). In
the present study, IHC analysis showed that cervical cancer cells
had high levels of RANKL and RANK compared to paracancerous
cells.
Various factors have been described including
hormones and cytokines, such as progesterone, parathyroid
hormone-related protein (PTHrP), vitamin D3, prostaglandin E2
(PGE2), IL-1β, IL-6 and TNFα that affect the expression of RANKL
and RANK (9). Inflammation is an
essential element in tumorigenesis. Abundant evidence supports the
preposition that various types of cancers are triggered by
infection and chronic inflammatory disease (24,25).
In this process, interleukins play a role in cervical
carcinogenesis as autocrine and/or paracrine stimuli, such as
IL-1β, IL-6 and TNF-α (26).
Hypoxia is a common feature in the solid tumor microenvironment,
and is caused by the tumor outgrowing the existing vasculature.
Moreover, hypoxia upregulates RANK and RANKL expression and
increases RANKL-induced cell migration via the PI3K/AKT-hypoxia
inducible factor-1α (HIF-1α) pathway (27). Therefore, these inflammatory factors
and local hypoxia may contribute to a high level of RANKL/RANK in
cervical cancer cells, and this concept needs further research.
Subsequently, we observed the role of the RANKL/RANK
system in the regulation of the biological behaviors of cervical
cancer cells, and found that blocking RANKL/RANK inter-action by
α-RANKL or rhOPG could significantly decrease the proliferation and
promote the apoptosis of HeLa and SiHa cells. Yet, treatment with
rhRANKL showed no obvious regulatory effect. This difference
suggests that the stimulatory effect of RANKL on the growth of
cervical cancer cells may mainly be dependent on mRANKL/RANK
interaction between cervical cancer cells. Further analysis showed
that both α-RANKL and rhOPG markedly downregulated Ki-67 and Bcl-2
expression and upregulated Fas and FasL expression in the HeLa and
SiHa cells, suggesting that the regulation of these proliferation-
and apoptosis-related molecules may be involved in the regulatory
process of the RANKL/RANKL axis in cervical cancer cell growth.
RANKL binds and activates the receptor RANK, and
then induces the NF-κB, MAPK and PI3K/AKT pathways to control
several physiological and pathological processes (28). These signaling pathways (for
example, MAPK/ERK1/2, p38, JNK, AKT and NF-κB) were also proven to
be involved in the regulation of cervical cancer cell proliferation
and apoptosis (29–31). Therefore, the influence of RANKL on
cervical cancer cells may also be achieved by activation of these
signaling pathways.
Secchiero et al reported that the RANKL/RANK
system may contribute to B-CLL pathogenesis by upregulating IL-8
(20). However, tumor-derived
interleukin-8 stimulates osteolysis independent of RANKL (32). These studies suggest that RANKL may
be an upstream molecule of IL-8. In the present study, we found
that RANKL stimulated IL-8 secretion from the HeLa and SiHa cells.
Owing to the important role of IL-8 in cervical cancer cells
(19), we next found that rhIL-8
upregulated Ki-67 and Bcl-2 expression, downregulated Fas and FasL
expression, enhanced the proliferation and repressed the apoptosis
in HeLa and SiHa cells. In addition, rhIL-8 reversed the effect of
α-RANKL on proliferation- and apoptosis-related molecules,
proliferation and apoptosis. These data indicate that the
RANKL/RANK system promotes cervical cancer cell growth by IL-8.
Taking into account the effect of hypoxia on IL-8 and our current
findings, it can be concluded that hypoxia may upregulate
RANKL/RANK expression and further stimulate IL-8 secretion
consequently promoting cervical cancer cell growth.
FCM analysis showed that RANKL decreased CXCR1 and
CXCR2 expression in the HeLa and SiHa cells. These results suggest
that RANKL, on the one hand, stimulates IL-8 production and
promotes cervical cancer cell growth, yet, on the other hand, forms
a negative feedback to control this effect through downregulation
of IL-8 receptors. The possible mechanism should be further
studied.
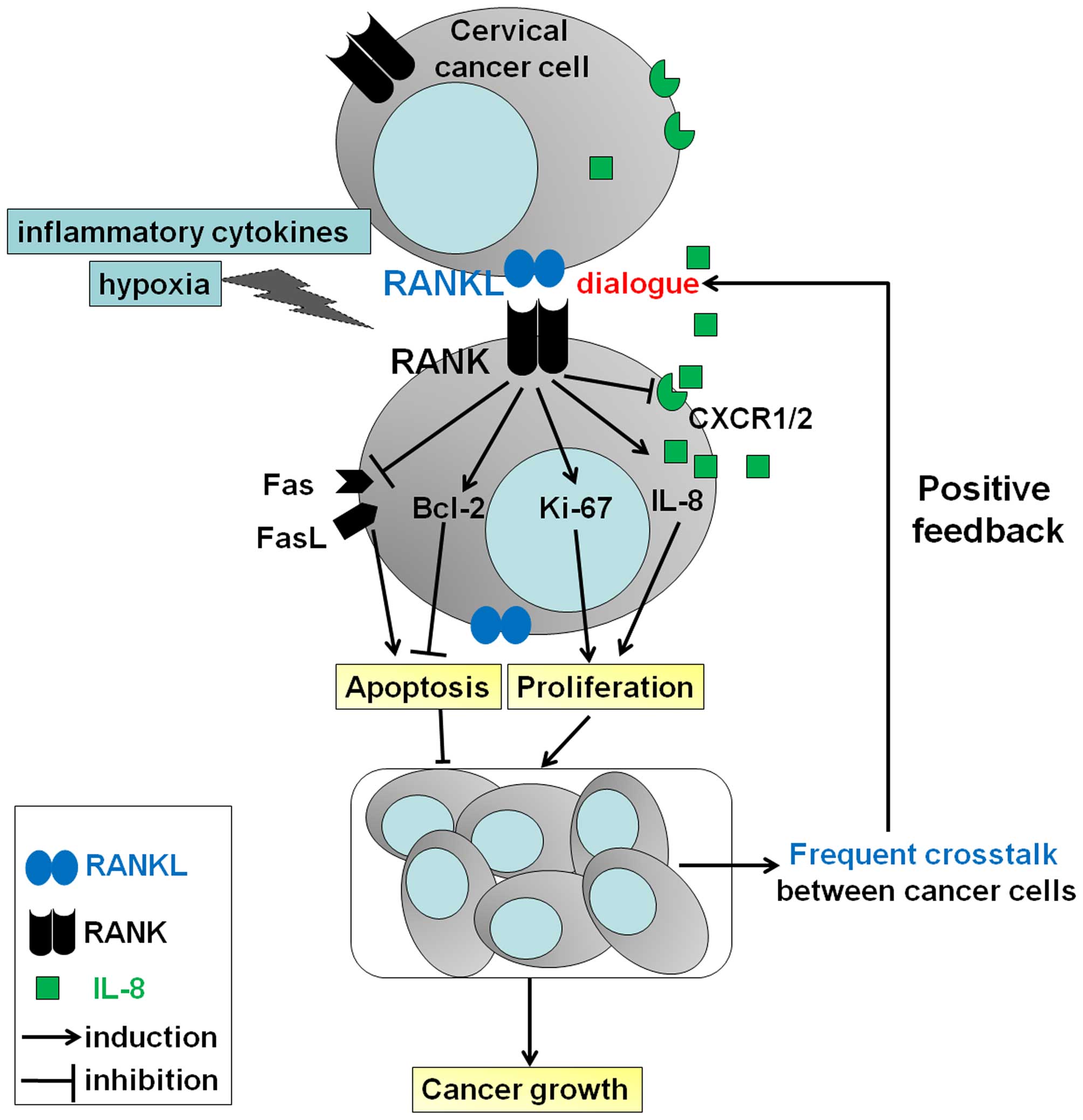
Based on our results and previous studies, as shown
in Fig. 8, it can be concluded that
a high level of mRANKL/RANK possibly induced by inflammatory
cytokines and or hypoxia, results in an increase in Ki-67 and
Bcl-2, and a decrease in apoptosis-related molecules Fas and FasL,
further promoting the growth of cervical cancer cells and
accelerating the development of cervical cancer by strengthening
the dialogue between cervical cancer cells and the stimulation of
IL-8 secretion. At the same time, RANKL restricts this effect by
downregulating IL-8 receptor expression. Accompanied by the rapid
growth of cervical cancer, the dialogue mediated by mRANKL/RANK
will be promoted between tumor cells, and the degree of hypoxia
will further increase. Hypoxia may amplify the stimulatory effect
of RANKL and IL-8 on the growth of cervical cancer cells. These
integral effects stimulate the development of cervical cancer. Our
data provide new insight into the mechanisms of the RANKL/RANK axis
in the pathogenesis of cervical cancer. Related research has shown
that administration of the RANKL inhibitor, denosumab (33) and antagonist, RANK-Fc (34) decreases bone metastases and delays
tumor progression. These findings have implications for future
therapeutic strategies targeting RANKL in cervical cancer,
particularly for patients with high expression of RANKL.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (NSFC) (81471513), the Training
Program for Young Talents of Shanghai Health System (XYQ2013104)
and the Program for Zhuoxue of Fudan University (all to M.-Q. Li),
the NSFC 81302260 (to F. Xie), and the Research Program for
Maternal and Child of Jiangsu Province (F201429) (to J.-J. Yu).
References
|
1
|
Stewart BW and Wild CP: World Cancer
Report 2014. World Health Organization. Chapter 5.12. IARC
Nonserial Publication; 2014
|
|
2
|
Fu Z, Chen D, Cheng H and Wang F:
Hypoxia-inducible factor-1α protects cervical carcinoma cells from
apoptosis induced by radiation via modulation of vascular
endothelial growth factor and p53 under hypoxia. Med Sci Monit.
21:318–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lacey DL, Timms E, Tan HL, Kelley MJ,
Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S,
et al: Osteoprotegerin ligand is a cytokine that regulates
osteoclast differentiation and activation. Cell. 93:165–176. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anderson DM, Maraskovsky E, Billingsley
WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D
and Galibert L: A homologue of the TNF receptor and its ligand
enhance T-cell growth and dendritic-cell function. Nature.
390:175–179. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fata JE, Kong YY, Li J, Sasaki T,
Irie-Sasaki J, Moorehead RA, Elliott R, Scully S, Voura EB, Lacey
DL, et al: The osteoclast differentiation factor
osteoprotegerin-ligand is essential for mammary gland development.
Cell. 103:41–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schramek D, Leibbrandt A, Sigl V, Kenner
L, Pospisilik JA, Lee HJ, Hanada R, Joshi PA, Aliprantis A,
Glimcher L, et al: Osteoclast differentiation factor RANKL controls
development of progestin-driven mammary cancer. Nature. 468:98–102.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossi SW, Kim MY, Leibbrandt A, Parnell
SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger
JM, Jenkinson EJ, et al: RANK signals from
CD4+3− inducer cells regulate development of
Aire-expressing epithelial cells in the thymic medulla. J Exp Med.
204:1267–1272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jones DH, Nakashima T, Sanchez OH,
Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R,
Hojilla CV, et al: Regulation of cancer cell migration and bone
metastasis by RANKL. Nature. 440:692–696. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanada R, Hanada T, Sigl V, Schramek D and
Penninger JM: RANKL/RANK-beyond bones. J Mol Med (Berl).
89:647–656. 2011. View Article : Google Scholar
|
|
10
|
Cheng ML and Fong L: Effects of
RANKL-targeted therapy in immunity and cancer. Front Oncol.
3:3292014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palafox M, Ferrer I, Pellegrini P, Vila S,
Hernandez-Ortega S, Urruticoechea A, Climent F, Soler MT, Muñoz P,
Viñals F, et al: RANK induces epithelial-mesenchymal transition and
stemness in human mammary epithelial cells and promotes
tumorigenesis and metastasis. Cancer Res. 72:2879–2888. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu CJ, Lin TY, Kuo CC, Tsai CH, Lin MZ,
Hsu HC, Fong YC and Tang CH: Involvement of integrin up-regulation
in RANKL/RANK pathway of chondrosarcomas migration. J Cell Biochem.
111:138–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Armstrong AP, Miller RE, Jones JC, Zhang
J, Keller ET and Dougall WC: RANKL acts directly on RANK-expressing
prostate tumor cells and mediates migration and expression of tumor
metastasis genes. Prostate. 68:92–104. 2008. View Article : Google Scholar
|
|
14
|
Chen LM, Kuo CH, Lai TY, Lin YM, Su CC,
Hsu HH, Tsai FJ, Tsai CH, Huang CY and Tang CH: RANKL increases
migration of human lung cancer cells through intercellular adhesion
molecule-1 up-regulation. J Cell Biochem. 112:933–941. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murdoch C and Finn A: Chemokine receptors
and their role in inflammation and infectious diseases. Blood.
95:3032–3043. 2000.PubMed/NCBI
|
|
16
|
Mantovani A, Savino B, Locati M, Zammataro
L, Allavena P and Bonecchi R: The chemokine system in cancer
biology and therapy. Cytokine Growth Factor Rev. 21:27–39. 2010.
View Article : Google Scholar
|
|
17
|
Vicari AP and Caux C: Chemokines in
cancer. Cytokine Growth Factor Rev. 13:143–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nickel R, Beck LA, Stellato C and
Schleimer RP: Chemokines and allergic disease. J Allergy Clin
Immunol. 104:723–742. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu LB, Xie F, Chang KK, Li MQ, Meng YH,
Wang XH, Li H, Li DJ and Yu JJ: Hypoxia promotes the proliferation
of cervical carcinoma cells through stimulating the secretion of
IL-8. Int J Clin Exp Pathol. 7:575–583. 2014.PubMed/NCBI
|
|
20
|
Secchiero P, Corallini F, Barbarotto E,
Melloni E, di Iasio MG, Tiribelli M and Zauli G: Role of the
RANKL/RANK system in the induction of interleukin-8 (IL-8) in B
chronic lymphocytic leukemia (B-CLL) cells. J Cell Physiol.
207:158–164. 2006. View Article : Google Scholar
|
|
21
|
Xie F, Meng YH, Liu LB, Chang KK, Li H, Li
MQ and Li DJ: Cervical carcinoma cells stimulate the angiogenesis
through TSLP promoting growth and activation of vascular
endothelial cells. Am J Reprod Immunol. 70:69–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chu GC and Chung LW: RANK-mediated
signaling network and cancer metastasis. Cancer Metastasis Rev.
33:497–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng YH, Li H, Chen X, Liu LB, Shao J,
Chang KK, Du MR, Jin LP, Li MQ and Li DJ: RANKL promotes the growth
of decidual stromal cells in an autocrine manner via CCL2/CCR2
interaction in human early pregnancy. Placenta. 34:663–671. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deivendran S, Marzook KH and Radhakrishna
Pillai M: The role of inflammation in cervical cancer. Adv Exp Med
Biol. 816:377–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Husseinzadeh N and Davenport SM: Role of
toll-like receptors in cervical, endometrial and ovarian cancers: A
review. Gynecol Oncol. 135:359–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castrilli G, Tatone D, Diodoro MG, Rosini
S, Piantelli M and Musiani P: Interleukin 1alpha and interleukin 6
promote the in vitro growth of both normal and neoplastic human
cervical epithelial cells. Br J Cancer. 75:855–859. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang ZN, Zhang F, Tang P, Qi XW and Jiang
J: Hypoxia induces RANK and RANKL expression by activating HIF-1α
in breast cancer cells. Biochem Biophys Res Commun. 408:411–416.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada T, Nakashima T, Hiroshi N and
Penninger JM: RANKL-RANK signaling in osteoclastogenesis and bone
disease. Trends Mol Med. 12:17–25. 2006. View Article : Google Scholar
|
|
29
|
Wang L, Guo H, Yang L, Dong L, Lin C,
Zhang J, Lin P and Wang X: Morusin inhibits human cervical cancer
stem cell growth and migration through attenuation of NF-κB
activity and apoptosis induction. Mol Cell Biochem. 379:7–18. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SH, Kim SH, Kim YB, Jeon YT, Lee SC
and Song YS: Genistein inhibits cell growth by modulating various
mitogen-activated protein kinases and AKT in cervical cancer cells.
Ann NY Acad Sci. 1171:495–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rashmi R, DeSelm C, Helms C, Bowcock A,
Rogers BE, Rader JL, Grigsby PW and Schwarz JK: AKT inhibitors
promote cell death in cervical cancer through disruption of mTOR
signaling and glucose uptake. PLoS One. 9:e929482014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bendre MS, Margulies AG, Walser B, Akel
NS, Bhattacharrya S, Skinner RA, Swain F, Ramani V, Mohammad KS,
Wessner LL, et al: Tumor-derived interleukin-8 stimulates
osteolysis independent of the receptor activator of nuclear
factor-kappaB ligand pathway. Cancer Res. 65:11001–11009. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bouganim N and Clemons MJ: Bone-targeted
agents in the treatment of bone metastases: RANK outsider or new
kid on the block? Future Oncol. 7:381–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sordillo EM and Pearse RN: RANK-Fc: A
therapeutic antagonist for RANK-L in myeloma. Cancer. 97(Suppl):
S802–S812. 2003. View Article : Google Scholar
|