Introduction
Lung cancer is a major cause of morbidity and
mortality worldwide and the most common cause of cancer-related
death (1). Non-small cell lung
cancer (NSCLC) accounts for ~85% of all lung cancers. Although
surgical and chemotherapeutic treatments have made great
contributions in lung cancer, these methods may induce serious
long-term adverse effects. Various natural herbal products have
gained increasing attention due to their potential anticancer
effects against NSCLC (2,3).
Phloretin (Ph)
(2′,4′,6′-trihydroxy-3-(4-hydroxyphenyl)-propiophenone) is a
natural polyphenolic compound existing in apples, pears and other
plants of the rosaceae family and has been found to have
anti-inflammatory and immunosuppressive effects on both lymphoid-
and myeloid-derived cell lines (4).
Ph has also been shown to have antitumor activities by inducing
apoptosis in human leukemia cells, bladder cancer and human colon
cancer cells (5–7), and inhibiting the growth, invasiveness
and migration of human liver cancer cells (8). However, little is known about its
effects on human lung cancer cells.
In the present study, we investigated the possible
anticancer effects of Ph on A549 lung adenocarcinoma cells in
vitro and in vivo, and discussed the underlying
molecular mechanisms. We demonstrated that Ph could inhibit A549
cell proliferation by inducing apoptosis, and that upregulation of
JNK, ERK, Bax and P38 MAPK by Ph was associated with the
downregulation of Bcl-2 and NF-κB, and the activation of caspase-3
and -9, and P53, suggesting that Ph may be a useful plant product
for the treatment of lung cancer.
Materials and methods
Chemicals and reagents
Ph,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and dimethylsulfoxide (DMSO) were purchased from Sigma Chemical Co.
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were obtained from Life Technologies. FITC-Annexin V/PI
apoptosis detection kit was purchased from BD Biosciences. Ph stock
solution was prepared into 50, 100 and 200 µM concentrations
in DMSO and stored at -20°C. The final concentration of DMSO for
all treatments was consistently <0.1%. Specific inhibitors for
JNK1/2 (SP600125), ERK1/2 (U0126) or P38 (SB202190) were purchased
from Calbiochem. The following antibodies were used: JNK1, p-JNK1/2
(Cell Signaling), P53, cleaved caspase-3, caspase-9, NF-κB, MMP-9
(Santa Cruz), P38, p-P38, ERK1/2, p-ERK1/2, Bcl-2, Bax and PARP
(Bioworld Technology), and GAPDH (Sigma).
Cell culture
Human NSCLC A549, H1299 and bronchial epithelial
cells (Beas-2b) were from the Institute of Biochemistry and Cell
Biology (Shanghai Institutes for Biological Sciences, CAS). Cells
were maintained in DMEM supplemented with 10% FBS in a humidified
incubator under 5% CO2 at 37°C.
In vitro cytotoxicity assay
MTT was performed as previously described (9). Cells were cultured into a 96-well
plate (1×104/well), stimulated with different
concentrations (0, 25, 50, 100 and 200 µM) of Ph in culture
medium when the cells were 80–90% confluent. After 48 h Ph of
stimulation, the medium was removed and 100 µl MTT was added
to each well (0.5 mg/ml final concentration) for further incubation
for 4 h. Then the medium was removed, and 100 µl DMSO was
added to dissolve the solid formazan for 15 min. The absorbance of
each well was read at 570 nm using a microplate reader (Thermo
Fisher).
Fluorescence observation of cell
death
A549 cells (6×103 cells/well) on 96-well
plates were incubated with phosphate-buffered saline (PBS) control
and Ph (50, 100 and 200 µM) for 24 h, then treated with
Hoechst 33342 (10 mg/ml) for another 1 h, stained with propidium
iodide (PI; 100 mg/ml) for 15 min (10), washed with PBS three times and
observed on Operetta high content analysis system
(Perkin-Elmer).
Annexin V/PI double staining
To detect apoptosis in A549 cells after exposure to
Ph, the FITC-Annexin V apoptosis detection kit was used to quantify
the number of cells in different stages of cell death. Briefly,
A549 cells seeded into 6-well plates, and treated with PBS control
and Ph (50, 100 and 200 µM) for 48 h. Then, 1×105
cells were re-suspended in 100 µl 1X binding buffer. After
addition of FITC-Annexin V and PI (5 µl each), the cell
suspension was gently vortexed and incubated for 20 min at room
temperature in the dark. After addition of 400 µl 1X binding
buffer to each tube, cells were analyzed by flow cytometry (BD
Calibur).
Cell cycle analysis
To determine the effect of Ph on the cell cycle,
A549 cells were seeded into 6-well plates, treated with PBS control
and Ph (50, 100 and 200 µM) for 48 h, fixed with 70% ethanol
at 4°C for 30 min, and then incubated for another 30 min in the
dark, at room temperature with PI buffer [50 mg/ml containing
ribonuclease A (50 ng/ml)]. Cell cycle distribution was analyzed
for 10,000 collected cells with Aria II flow cytometer (BD
Biosciences).
Transwell migration assay
The effect of Ph on migration of A549 cells was
further analyzed using Transwell chambers with 8-mm porous membrane
(Corning, Corning, NY, USA). Cells were treated with PBS control
and different concentrations (10, 20 and 40 µM) of Ph for 24
h, and then loaded into the migration chamber at 1×105.
Medium containing 10% FBS was placed in the lower chambers. After
allowing cell migration for 6 h, cells were removed from the upper
side of the membrane; migratory cells on the lower side of the
membrane were fixed with 4% paraformaldehyde for 20 min, and then
washed with PBS three times before being stained with crystal
violet for another 10 min (11).
The number of migratory cells was counted by fluorescence
microscopy (magnification, ×100).
Western blotting
A549 cells were seeded into 6-well plates and
incubated with PBS control and different concentrations (50, 100
and 200 µM) of Ph for 48 h, lysed in RIPA buffer (50 mM
Tris-HCl, pH 7.2, 150 mM NaCl, 1% NP40, 0.1% SDS, 0.5% DOC, 1 mM
PMSF, 25 mM MgCl2, supplemented with a phosphatase
inhibitor cocktail) and finally subjected to immunoblotting
analysis with indicated antibodies. GAPDH were diluted to 1:2,000,
P53, cleaved caspase-3 and -9, NF-κB, MMP-9 were diluted to 1:200;
and JNK1, p-JNK1/2, P38, p-P38, ERK1/2, p-ERK1/2, Bcl-2, Bax and
PARP were diluted to 1:1,000.
In vivo antitumor effect
Female nude mice (BK Biotech) aged 5 weeks were
used. A549 cells (5×106) were suspended in Matrigel (BD
Biosciences) and injected subcutaneously (s.c.) into the mice. All
animal procedures were performed following the protocol approved by
the Institutional Animal Care Committee of Shanghai Institute of
Biochemistry and Cell Biology (Shanghai, China). Mice bearing
evident tumors were randomly divided into PBS control group,
low-dose (10 mg/kg) Ph group, and high-dose (20 mg/kg) Ph group. Ph
was dissolved in PBS for intraperitoneal (i.p.) administration to
the mice every two days for three weeks. Animals were euthanized
with carbon dioxide. Tumor masses were isolated and tumor weight
was measured as previously described (12).
Statistical analysis
Results are expressed as mean ± SD (range) or
percentage. The difference between two groups was analyzed using
the Student's t-test. Statistical analyses were performed using the
one-way analysis of variance (ANOVA) followed by Tukey's post hoc
test when more than three groups were analyzed. A P-value <0.05
was considered to indicate a statistically significant result. The
differentiation of amount of protein expressions were calculated
using Image Lab version 4.0 software (Bio-Rad Laboratories, Inc.).
All calculations were performed using GraphPad Prism software
(GraphPad Software, San Diego, CA, USA).
Results
Cytotoxic effects of Ph-treated A549 and
H1299 cells
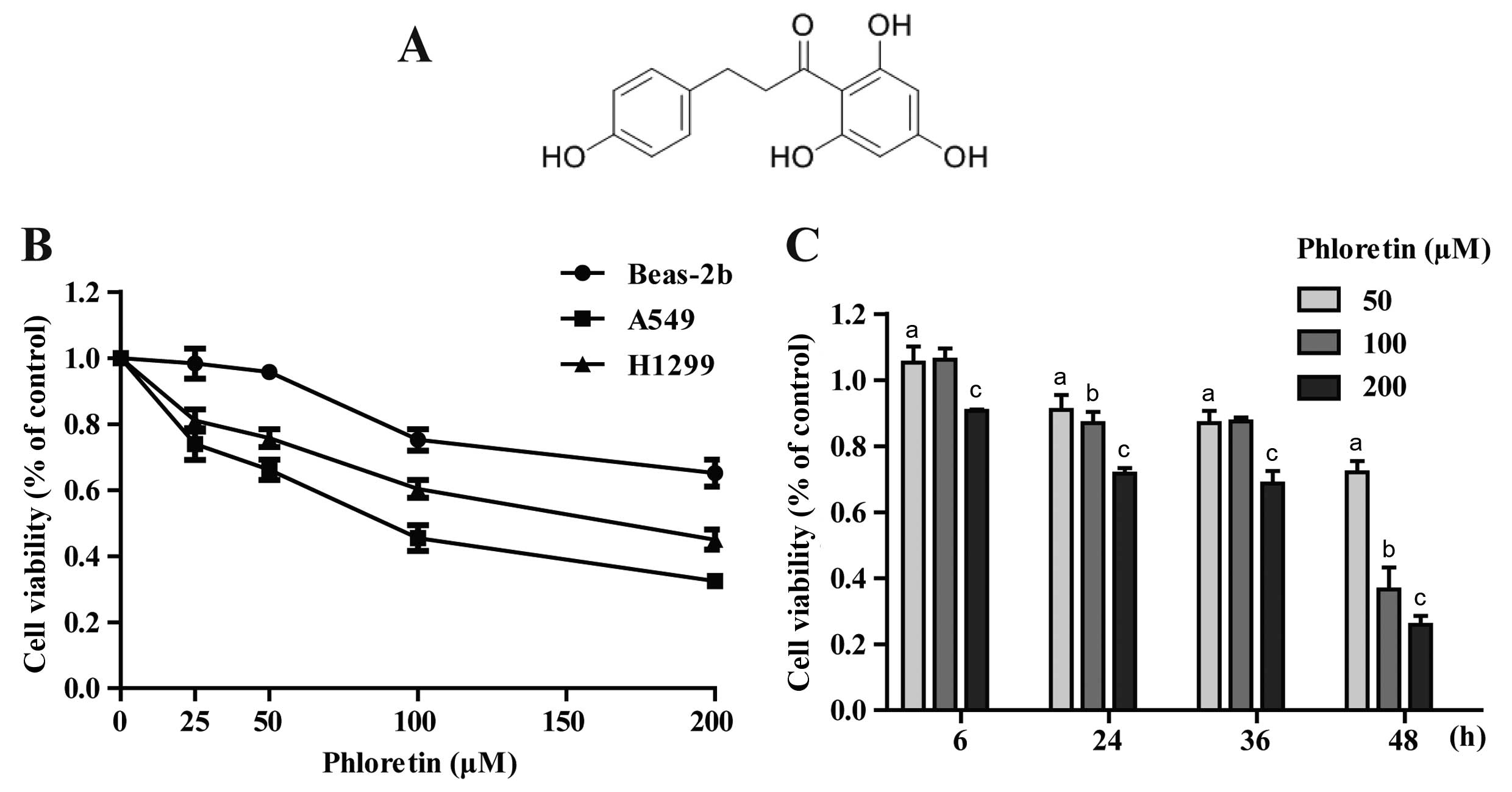
The chemical structure of Ph is shown in Fig. 1A (13). The cytotoxicity comparison of Ph on
A549, H1299 and Beas-2b cells was evaluated. After cells were
treated with different concentrations (0, 25, 50, 100 and 200
µM) of Ph for 48 h, Ph exhibited a moderate effect on normal
human Beas-2b cells, and Ph had more cytotoxic effects on A549 than
H1299 cells (Fig. 1B). Furthermore,
A549 cancer cells were dose- and time-dependently observed when the
cells were treated with Ph for 6, 24, 36 and 48 h (Fig. 1C).
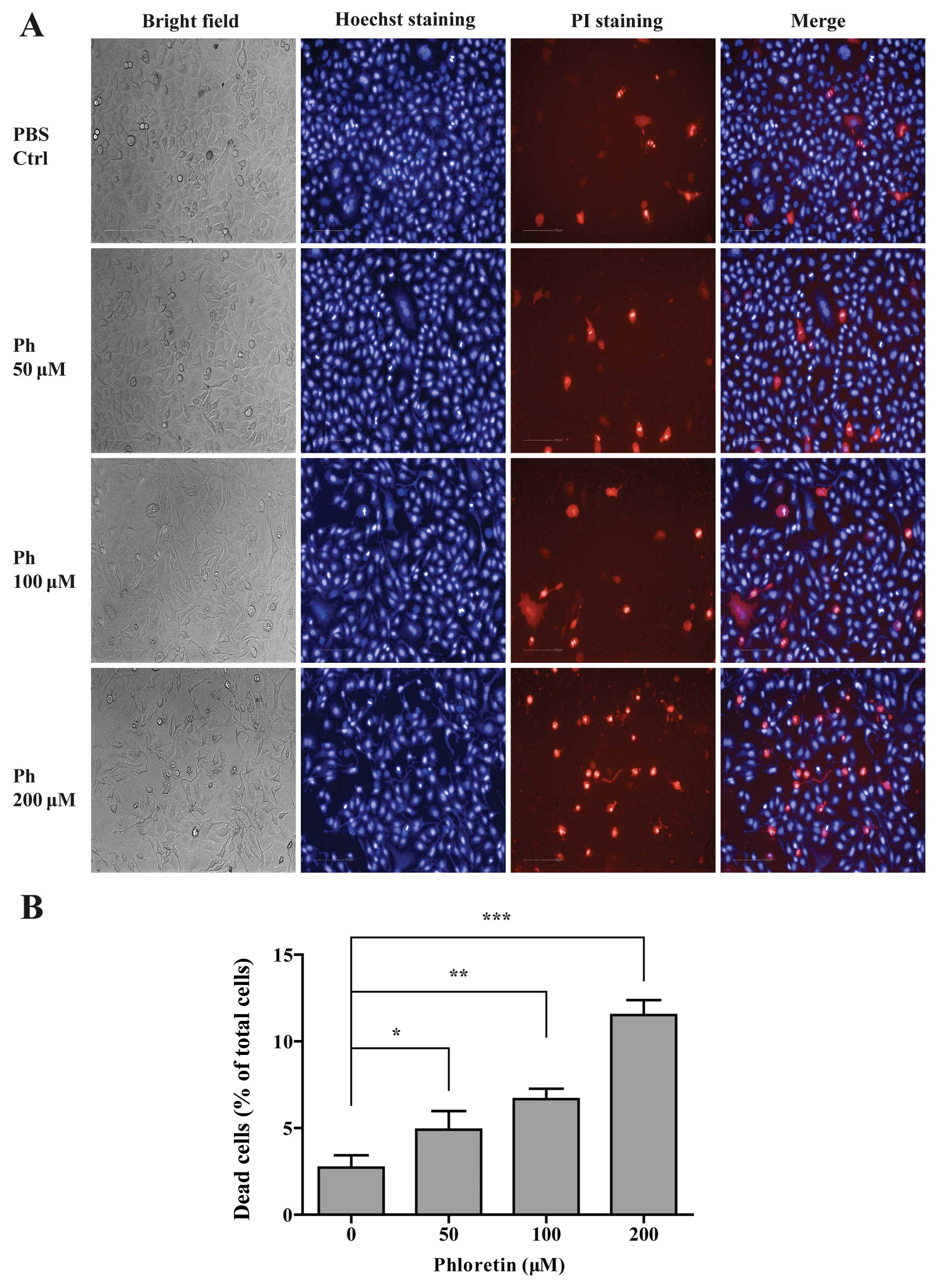
Ph-induced cell death in A549 cells
To further compare the cytotoxicity between
different concentrations of Ph on A549 cells, cell death assay was
performed on Operetta high content analysis system. A549 cells were
incubated with PBS control and different concentrations (50, 100
and 200 µM) of Ph for 24 h, and then double stained with
Hoechst 33342 (blue indicates the nucleus) and PI (red indicates
dead cells). As shown in Fig. 2, Ph
significantly increased the cell death rate in a dose-dependent
manner, and A549 cells became more curved and thinner with the
concentration of Ph increasing.
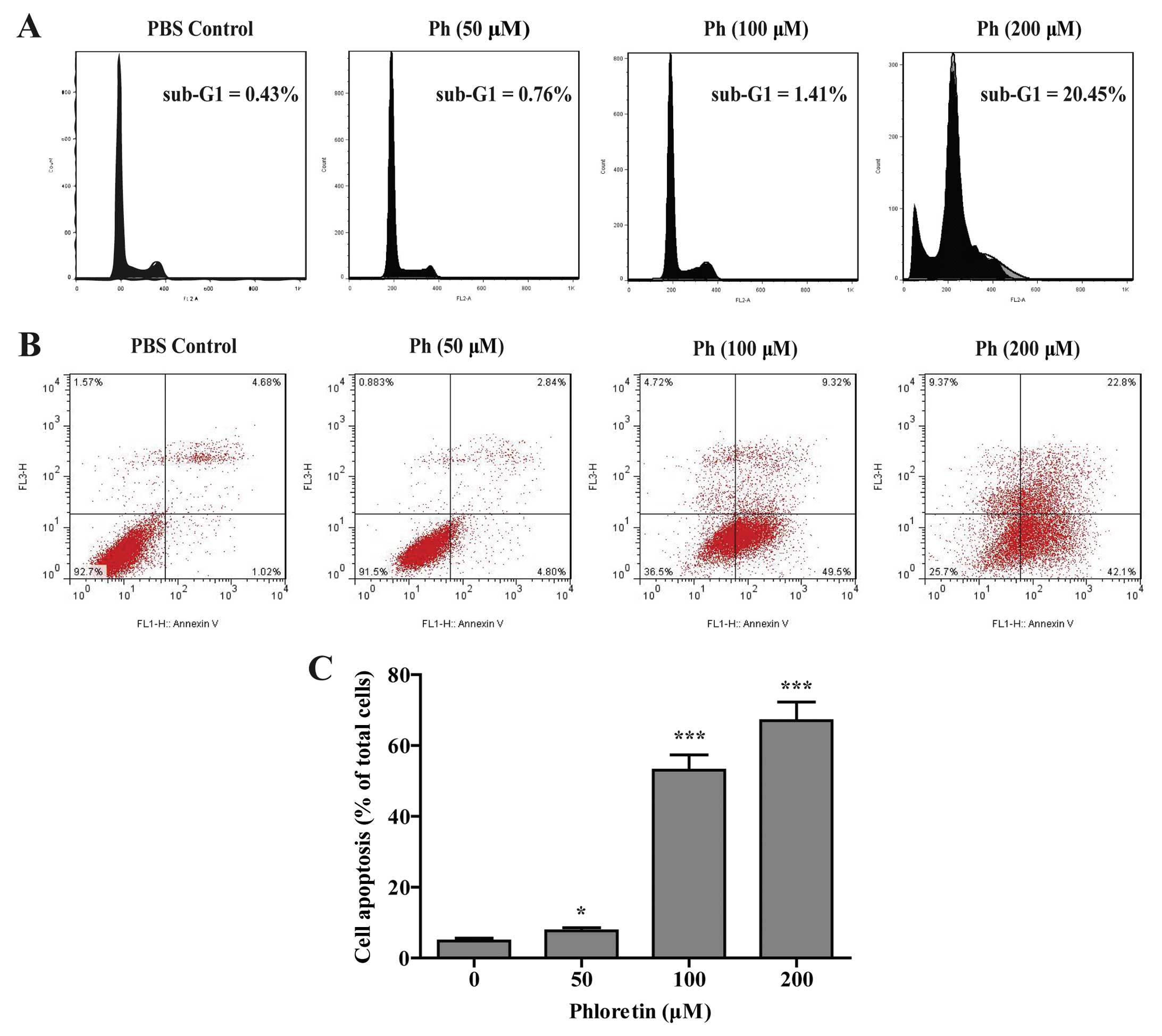
Ph-induced cell apoptosis in A549
cells
To determine whether the inhibitory effect of Ph on
cell viability was associated with the induction of cell apoptosis,
A549 cells were treated with different concentrations (0, 50, 100
and 200 µM) of Ph for 24 h. As shown in Fig. 3A, in PBS control group, the
percentage of cells in the sub-G1 fraction was low (0.43%), and a
significant proportion of cells (20.45%) went into sub-G1 phase
when treated with Ph at 200 µM. Apoptotic cells with a lower
DNA content should fall into similar sub-G1 region in cell cycle on
flow cytometric analysis (10).
Cell cycle analysis by flow cytometry showed a dose-dependent
increased accumulation of cell population in sub-G1 phase. Annexin
V and PI double staining displayed an increased percentage of
apoptotic cells and dead cells after Ph treatment for 24 h
(Fig. 3B and C). These results
suggest that the strong effect of Ph on A549 cells may be due to
induction of more apoptosis with the increased concentration.
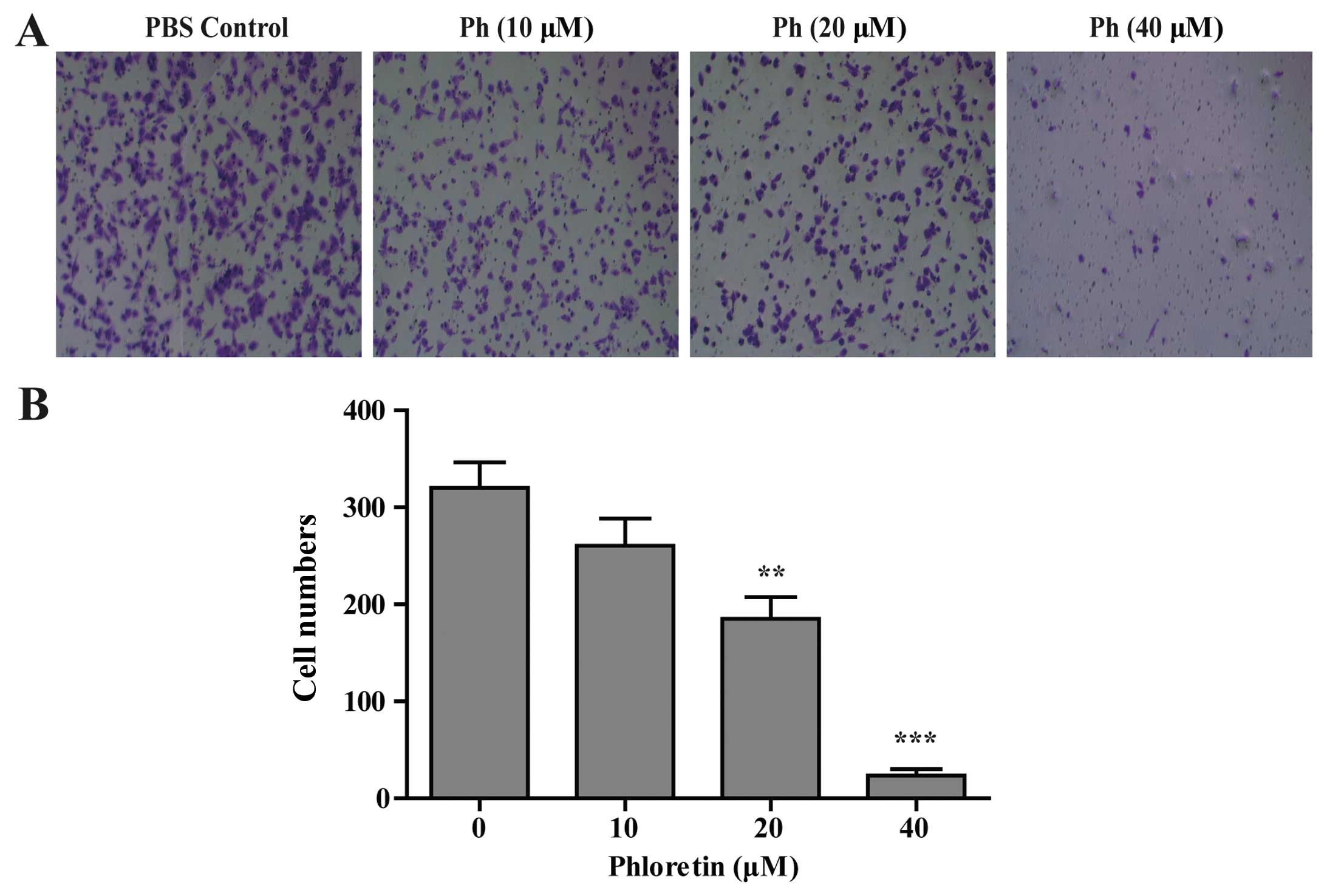
Effect of Ph on A549 cell migration
The potential function of Ph on A549 tumor cell
migration was characterized by Transwell migration assay. Cells
were treated with indicated concentrations of Ph for 24 h, and
loaded into the migration chamber at 1×105. The results
showed that Ph treatment slowed down the migration of A549 cells in
a concentration-dependent manner. As shown in Fig. 4, 40 µM Ph markedly inhibited
the migration of A549 cells.
Ph induces activation of caspase-3 and -9
in A549 cells
To further investigate whether caspase activation
was involved in Ph-induced apoptosis, activation of caspase-3 and
-9 and PARP was detected. As shown in Fig. 5A, exposure of A549 cells to Ph (0,
50, 100 and 200 µM) for 24 h increased the number of cleaved
fragments of caspase-3 and -9 in a dose-dependent manner. It is
known that PARP is a characteristic marker of apoptosis (14), the abundance of the cleaved form of
PARP was increased compared to the control. Ph treatment at 200
µM for 24 h increased the expression level of cleaved
caspase-3 and -9 and cleavage form of PARP by 2.6-, 1.3- and
1.5-fold compared to the control, respectively. Ph decreased Bcl-2
and NF-κB, and increased the expression level of P53 and Bax in a
dose-dependent manner. As shown in Fig.
5B, compared to the control, Ph treatment at 200 µM for
24 h significantly increased the expression level of P53 and Bax by
1.8- and 2.3-fold, respectively, and decreased the expression level
of Bcl-2 and NF-κB by 34 and 32%, respectively. These data suggest
that caspase-3, and -9, PARP, Bcl-2, Bax, NF-κB and P53 were
involved in Ph-induced apoptosis. MMPs can degrade the basement
membrane and play main roles in promotion of cancer invasion and
metastasis (15). As anticipated,
we also found that the expression level of MMP-9 was decreased in
Ph-treated A549 cells by 22%, which is consistent with previous
data from the migration assay (Fig.
4A).
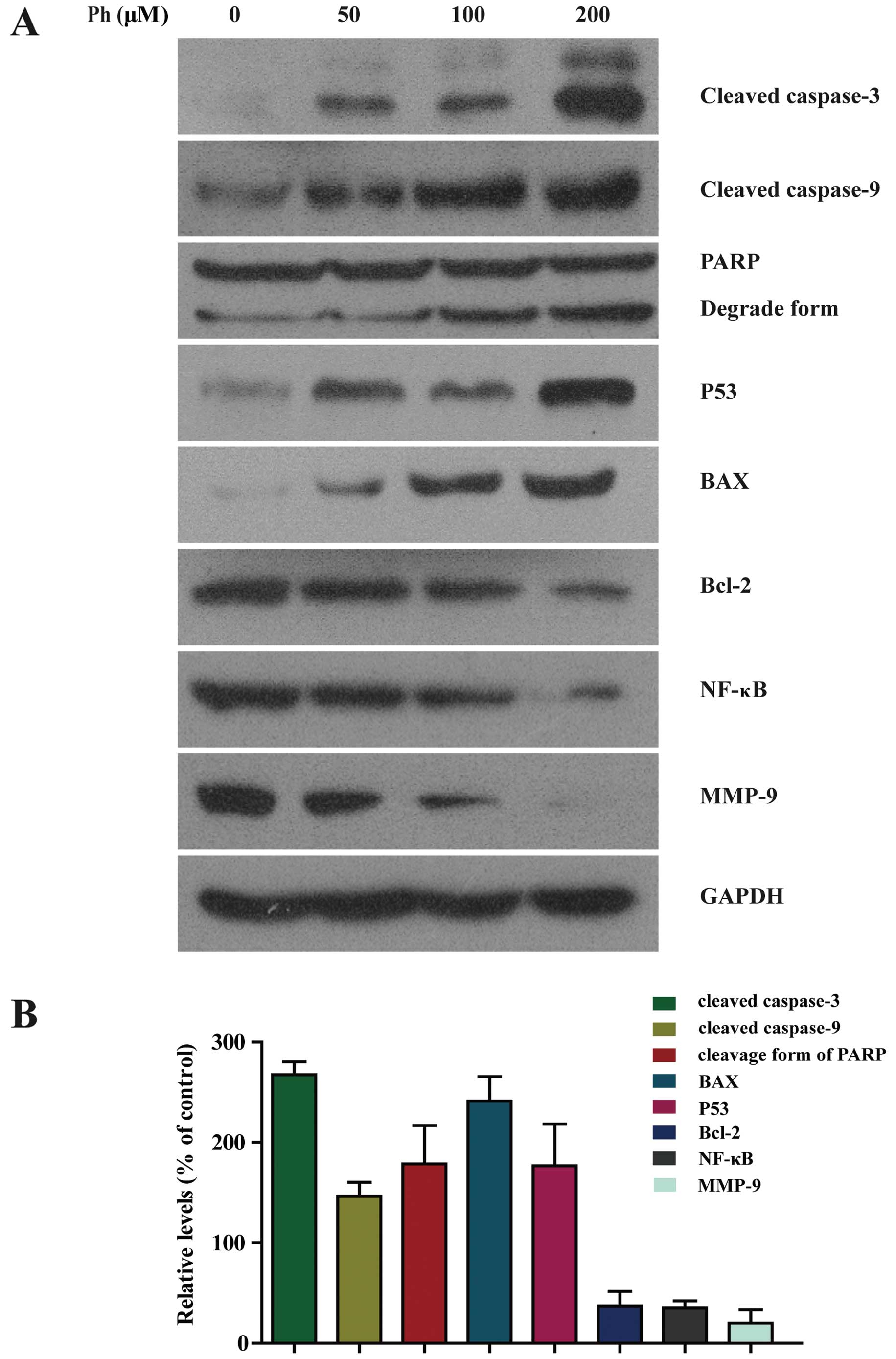 | Figure 5Activation of caspase-3 and -9, PARP,
BAX and P53 was increased in Ph-treated A549 cells. (A) A549 cells
were treated with 50, 100 and 200 µM Ph for 24 h and
subjected to western blotting with an antibody against cleaved
caspase-3 and -9, PARP, BAX, P53, Bcl-2, NF-κB and MMP-9 antibody.
(B) Quantitative results of cleaved caspase-3 and -9, PARP, BAX,
P53, Bcl-2, NF-κB and MMP-9 protein levels after 200 µM
Ph-treated for 24 h, which were adjusted to GAPDH protein level and
expressed as multiples of induction beyond each respective control.
Data are expressed as mean ± SD (n=3). |
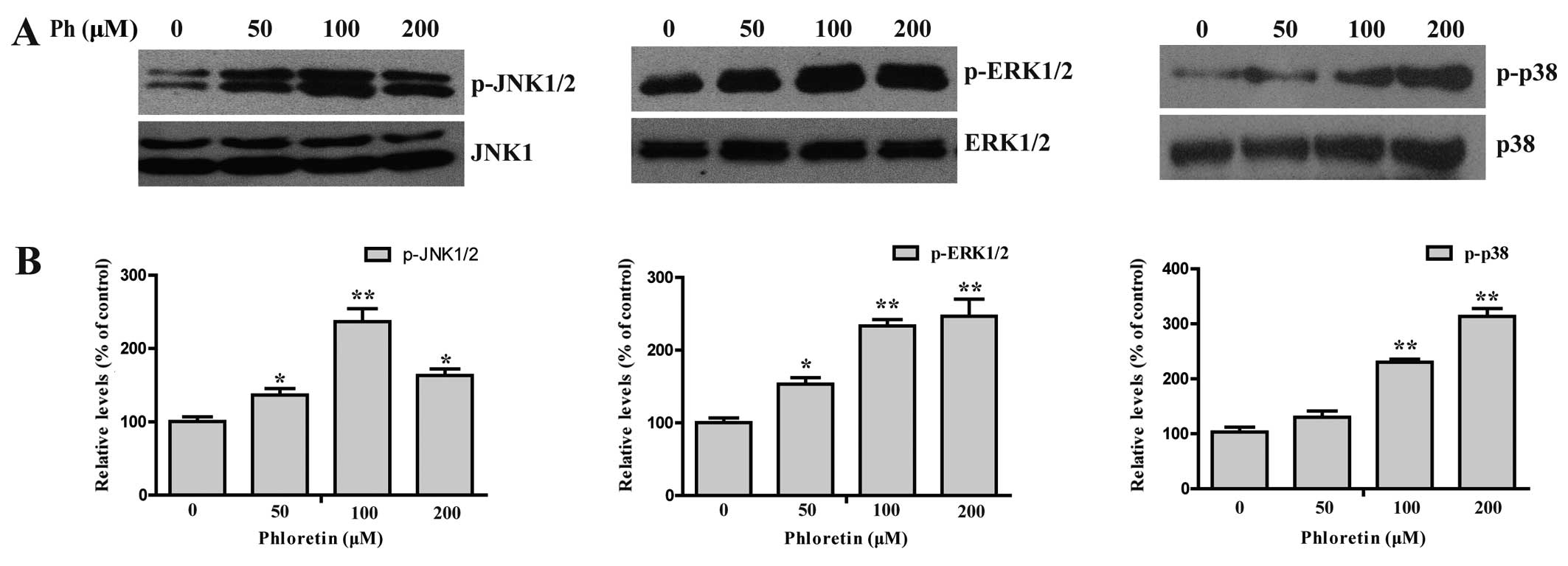
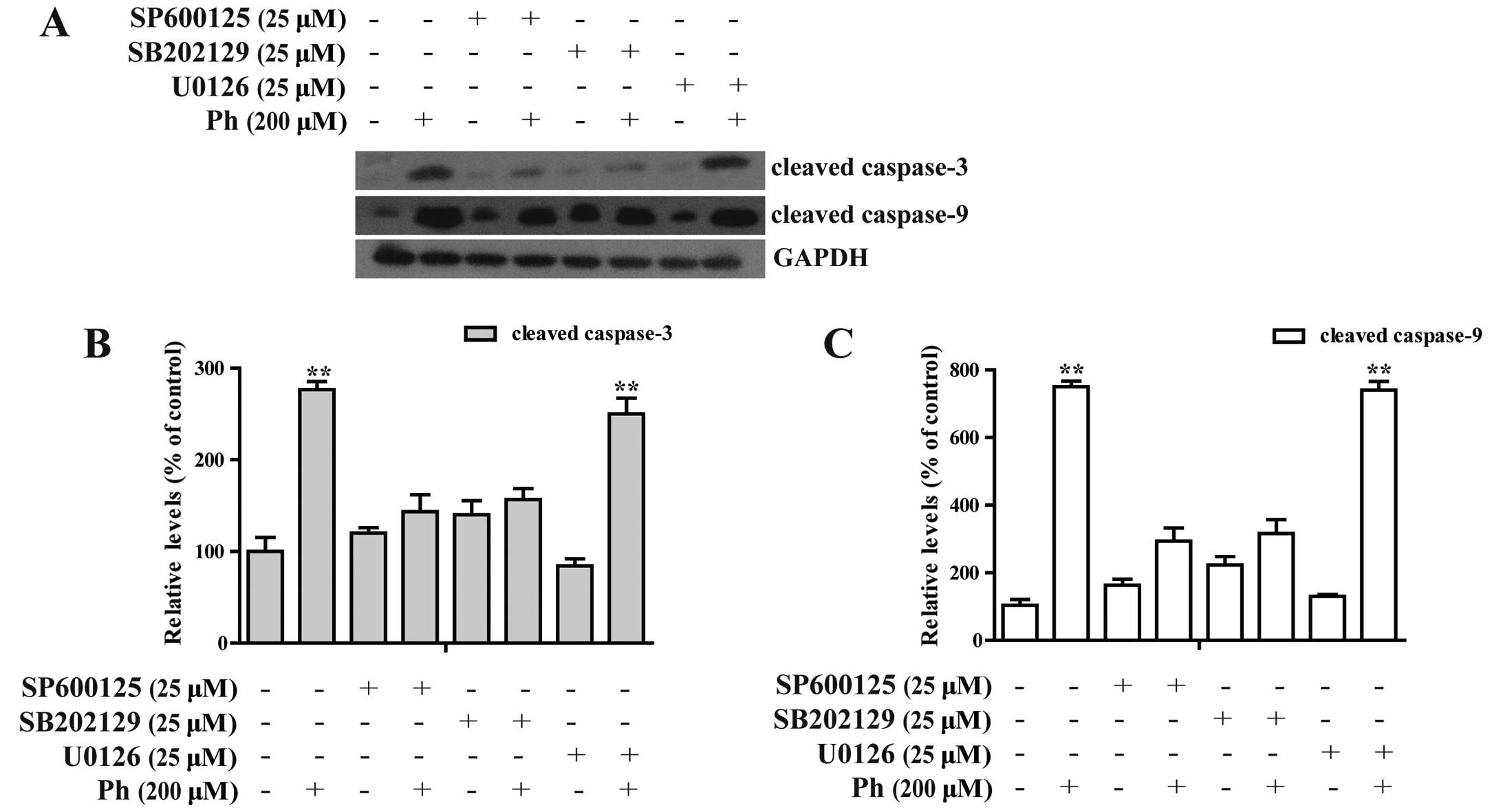
Ph-induced apoptosis is involved in the
regulation of P38 MAPK and JNK signaling pathways in A549
cells
MAPK signaling pathway plays an important role in
the action of chemotherapeutic drugs in the regulation of apoptosis
(16–18). To see whether MAPKs were involved in
Ph-induced apoptosis, we first examined the activation status of
JNK, ERK and P38 by western blotting with antibodies specific to
the phosphorylated forms of these kinases. As shown in Fig. 6, treatment of cells with (0, 50, 100
and 200 µM) Ph increased the phosphorylated form of JNK, ERK
and P38 in a dose-dependent manner, with the total protein levels
remaining steady, indicating the activation of JNK, ERK and P38 in
A549 cells. In contrast, A549 cells were pretreated with 25
µM SP600125 (a JNK inhibitor), U0126 (an ERK inhibitor) or
SB202190 (a P38 inhibitor) for 45 min, treated with 200 µM
Ph for another 24 h, and then cleaved caspase-3 and -9 were
analyzed by western blotting. SP600125 and SB202190 treatment
significantly attenuated Ph-induced caspase-3 and -9 activation, as
shown in Fig. 7. These findings
suggest that activation of JNK1/2 and P38 MAPK may play a crucial
upstream role in Ph-mediated caspase activation in A549 cells.
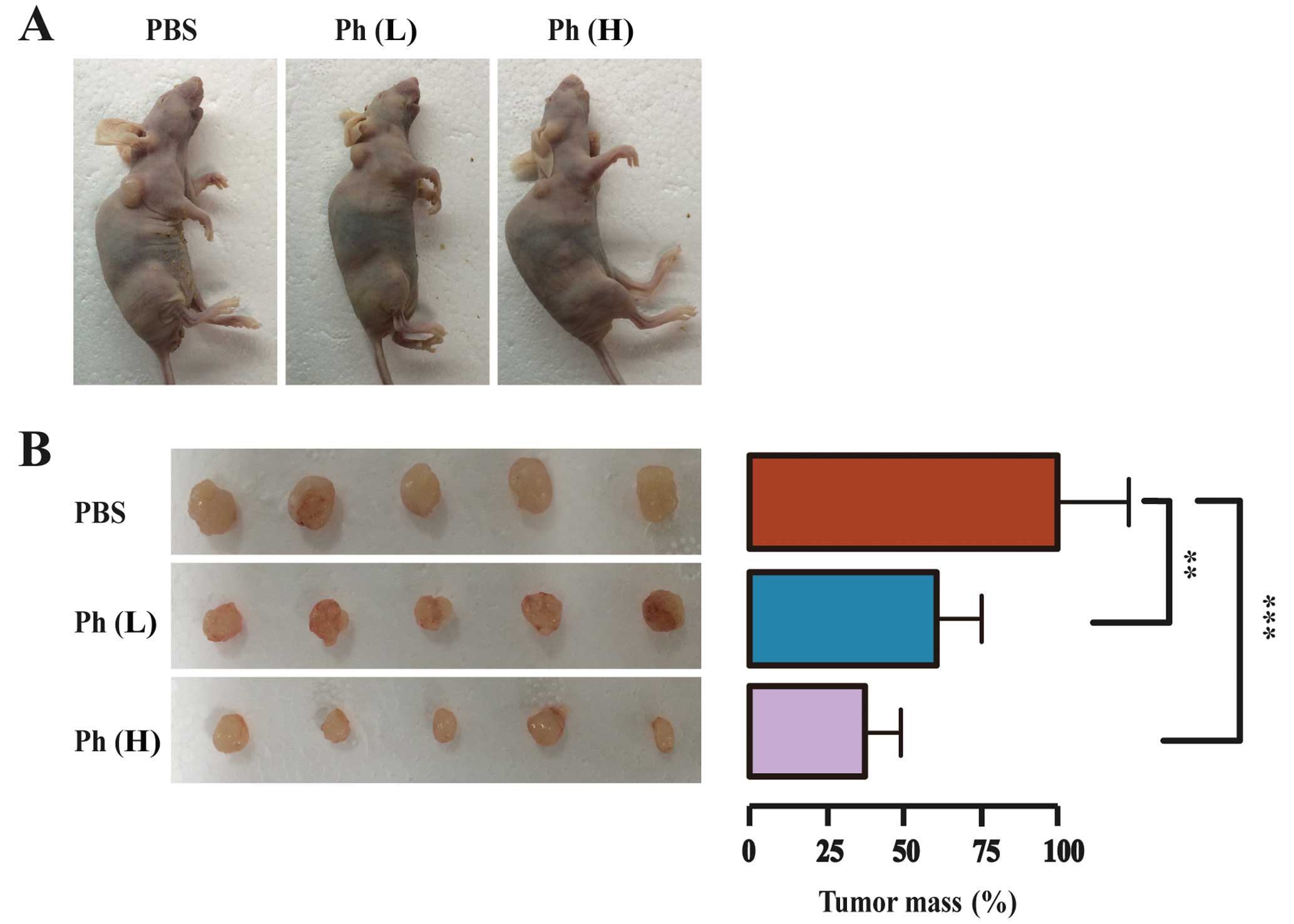
Antitumor activity of Ph on A549 lung
tumor xenografts
To further evaluate the tumor-suppressing effect of
Ph in vivo, a model for tumorigenicity of A549 cancer cells
in nude mice was established. A549 cells (5×106 cells)
were injected s.c. into the female nude mice aged 5 weeks and
weighing ~20 g. After three days, 15 mice bearing visible tumors
were equally randomized to a PBS control, a low-dose (10 mg/kg) Ph
group, and a high-dose (20 mg/kg) Ph group. Ph was dispersed in PBS
and administered i.p. every two days for three weeks. After three
weeks, mice were sacrificed and tumors were dissected and weighed.
Tumor images and mean tumor weight in each group are shown in
Fig. 8. As anticipated, the tumor
size was decreased significantly in both Ph groups, compared to
that in the control group. The mean tumor mass in low- and
high-dose Ph groups was ~61 and 38% of that in the control group
respectively, indicating that Ph had an inhibitory effect on lung
carcinoma xenograft growth in mice.
Discussion
Lung cancer is the most commonly diagnosed cancer
and one of the leading causes of cancer death in males, and was the
4th most commonly diagnosed cancer and the 2nd leading cause of
cancer-related death in females in 2008 worldwide. Lung cancer
accounted for 13% (1.6 million) of the total cases and 18% (1.4
million) of cancer deaths in 2008 (19,20).
How to enhance antitumor function and expand survival in lung
cancer patients has been an open question for decades. Apoptosis
(programmed cell death), is not only essential to the development
and maintenance of homeostasis during cell growth but plays an
important role in the prevention of tumor development (21,22).
Natural herbal products are currently studied for their antitumor
activities including apoptosis induction and antiproliferative
activities (23–25). However, their active components and
molecular mechanisms of action are not well understood. Ph is a
natural phenol existing in apples and a variety of vegetables
(26,27). Ph has been previously reported with
anticancer effects on breast and hepatocellular cancer and colon
cancer cell lines (5,12,28).
The present study for the first time demonstrated that Ph induced
apoptosis and inhibit migration of NSCLC A549 cells.
During the apoptotic process, pro-apoptotic Bcl-2
members such as BAX redistribute from the cytosol to mitochondria,
resulting in increased membrane permeability. Induction of BAX
results in a downstream program of mitochondrial dysfunction and
activation of caspases. Due to this event, the released
mitochondrial cytochrome participates in this process, leading to
caspase-9 activation and subsequent activation of caspase-3
(29), thus increasing the cleavage
form of PARP and inducing A549 cell apoptosis. It was found in the
present study that the expression of BAX and fractured PARP protein
was increased, the expression of Bcl-2 was decreased, and caspase-3
and -9 were activated in a dose-dependent manner after Ph
treatment. In addition, protein MMP-9 was inhibited after Ph
treatment, particularly in the 200 µM group. These findings
are consistent with the results of cell apoptosis assay and
migration assay in the previous experiments. These results proved
that Ph not only induced mitochondrial activation-mediated
apoptotic cell death but inhibited migration of A549 cells.
Previous studies have suggested that MAPKs can be
induced by various compounds and are involved in cell death in
NSCLC A549 cells (30,31). The MAPK family includes three kinase
members, including c-Jun NH2-terminal protein kinase/stress
activated protein kinases (JNK/SAPKs), P38 MAPK, and extracellular
signal-regulated kinase (ERK). Previous results tempted us to ask
whether the tumor-suppressing effect of Ph relied on the presence
of the P38 MAPK signaling system in A549 cells. To answer this
question, we further investigated activation of the MAPK family
proteins in Ph-treated A549 cells. The results showed that the
phosphorylation of ERK1/2, JNK1/2 and P38 MAPK was increased in
Ph-treatment A549 cells in a dose-dependent manner with the total
protein level remaining steady. However, treatment with JNK1/2
specific inhibitor (SP600125) or the P38 MAPK specific inhibitor
(SB202190) effectively inhibited activation of caspase-3 and
caspase-9 induced by Ph, whereas U0126 (an ERK1/2 inhibitor) showed
no effect on Ph-induced caspase activation. These findings suggest
that activation of JNK1/2 and P38 MAPK plays a critical role in
Ph-induced apoptosis in NSCLC A549 cells.
Acknowledgments
This study was supported by the Key Program of the
Shanghai Committee of Science and Technology (no. 12JC1410901) and
the National Natural Science Funds of China (no. 81402449).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar
|
|
3
|
Boyer J and Liu RH: Apple phytochemicals
and their health benefits. Nutr J. 3:52004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fordham JB, Naqvi AR and Nares S:
Leukocyte production of inflammatory mediators is inhibited by the
antioxidants phloretin, silymarin, hesperetin, and resveratrol.
Mediators Inflamm. 2014:9387122014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu SP, Liu G, Wu XT, Chen FX, Liu JQ,
Zhou ZH, Zhang JF and Fei SJ: The effect of phloretin on human γδ T
cells killing colon cancer SW-1116 cells. Int Immunopharmacol.
15:6–14. 2013. View Article : Google Scholar
|
|
6
|
Devi MA and Das NP: In vitro effects of
natural plant polyphenols on the proliferation of normal and
abnormal human lymphocytes and their secretions of interleukin-2.
Cancer. 69:191–196. 1993.
|
|
7
|
Nelson JA and Falk RE: The efficacy of
phloridzin and phloretin on tumor cell growth. Anticancer Res.
13:2287–2292. 1993.PubMed/NCBI
|
|
8
|
Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH,
Chen JH, Wu CH and Ho YS: Apple polyphenol phloretin potentiates
the anticancer actions of paclitaxel through induction of apoptosis
in human hep G2 cells. Mol Carcinog. 48:420–431. 2009. View Article : Google Scholar
|
|
9
|
Zare Jahromi M, Ranjbarian P and Shiravi
S: Cytotoxicity evaluation of Iranian propolis and calcium
hydroxide on dental pulp fibroblasts. J Dent Res Dent Clin Dent
Prospects. 8:130–133. 2014.PubMed/NCBI
|
|
10
|
Shen J, Song G, An M, Li X, Wu N, Ruan K,
Hu J and Hu R: The use of hollow mesoporous silica nanospheres to
encapsulate bortezomib and improve efficacy for non-small cell lung
cancer therapy. Biomaterials. 35:316–326. 2014. View Article : Google Scholar
|
|
11
|
Zuo Y, Yang J, He J, Zhao Y and He Y: An
uncoordinated-5 homolog B receptor monoclonal antibody regulates
A375 melanoma cell migration. Monoclon Antib Immunodiagn
Immunother. 33:280–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H,
Wei PL, Lee CH, Liu RS and Lin SY: In vitro and in vivo study of
phloretin-induced apoptosis in human liver cancer cells involving
inhibition of type II glucose transporter. Int J Cancer.
124:2210–2219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shao X, Bai N, He K, Ho CT, Yang CS and
Sang S: Apple polyphenols, phloretin and phloridzin: New trapping
agents of reactive dicarbonyl species. Chem Res Toxicol.
21:2042–2050. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diefenbach J and Bürkle A: Introduction to
poly(ADP-ribose) metabolism. Cell Mol Life Sci. 62:721–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta 2005. 1755:37–69. 2005.
|
|
16
|
Dong ZH, Wang DC, Liu TT, Li FH, Liu RL,
Wei JW and Zhou CL: The roles of MAPKs in rabbit nucleus pulposus
cell apoptosis induced by high osmolality. Eur Rev Med Pharmacol
Sci. 18:2835–2845. 2014.PubMed/NCBI
|
|
17
|
Han R, Liang H, Qin ZH and Liu CY:
Crotoxin induces apoptosis and autophagy in human lung carcinoma
cells in vitro via activation of the P38 MAPK signaling pathway.
Acta Pharmacol Sin. 35:1323–1332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Palanivel K, Kanimozhi V and Kadalmani B:
Verrucarin A alters cell-cycle regulatory proteins and induces
apoptosis through reactive oxygen species-dependent P38 MAPK
activation in the human breast cancer cell line MCF-7. Tumour Biol.
35:10159–10167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woodle ES and Kulkarni S: Programmed cell
death. Transplantation. 66:681–691. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bobba A, Amadoro G, La Piana G, Calissano
P and Atlante A: Glycolytic enzyme upregulation and numbness of
mitochondrial activity characterize the early phase of apoptosis in
cerebellar granule cells. Apoptosis. 20:10–28. 2015. View Article : Google Scholar
|
|
23
|
Schwingel TE, Klein CP, Nicoletti NF, Dora
CL, Hadrich G, Bica CG, Lopes TG, da Silva VD and Morrone FB:
Effects of the compounds resveratrol, rutin, quercetin, and
quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and
neurotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol.
387:837–848. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong JY, Park SH, Min HY, Park HJ and Lee
SK: Anti-proliferative effects of evodiamine in human lung cancer
cells. J Cancer Prev. 19:7–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao B and Hu M: Gallic acid reduces cell
viability, proliferation, invasion and angiogenesis in human
cervical cancer cells. Oncol Lett. 6:1749–1755. 2013.
|
|
26
|
Wang L, Li ZW, Zhang W, Xu R, Gao F, Liu
YF and Li YJ: Synthesis, crystal structure, and biological
evaluation of a series of phloretin derivatives. Molecules.
19:16447–16457. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang WT, Huang WC and Liou CJ: Evaluation
of the anti-inflammatory effects of phloretin and phlorizin in
lipopolysaccharide-stimulated mouse macrophages. Food Chem.
134:972–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MS, Kwon JY, Kang NJ, Lee KW and Lee
HJ: Phloretin induces apoptosis in H-Ras MCF10A human breast tumor
cells through the activation of p53 via JNK and p38
mitogen-activated protein kinase signaling. Ann N Y Acad Sci.
1171:479–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gross A, Jockel J, Wei MC and Korsmeyer
SJ: Enforced dimerization of BAX results in its translocation,
mitochondrial dysfunction and apoptosis. EMBO J. 17:3878–3885.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park WH and Kim SH: MAPK inhibitors
augment gallic acid-induced A549 lung cancer cell death through the
enhancement of glutathione depletion. Oncol Rep. 30:513–519.
2013.PubMed/NCBI
|
|
31
|
Hsiao YC, Kuo WH, Chen PN, Chang HR, Lin
TH, Yang WE, Hsieh YS and Chu SC: Flavanone and 2′-OH flavanone
inhibit metastasis of lung cancer cells via down-regulation of
proteinase activities and MAPK pathway. Chem Biol Interact.
167:193–06. 2007. View Article : Google Scholar : PubMed/NCBI
|