Introduction
Salivary adenoid cystic carcinoma (SACC) is one of
the most virulent salivary gland cancers and accounts for ~30% of
all salivary gland malignancies (1). Generally, SACC has a lengthy clinical
course with potential local infiltration, hematogenous distant
metastases, and poor response to classical chemotherapeutic
approaches (2). A prominent
hallmark of SACC is perineural invasion (PNI), which is the process
of cancer cell invasion in, around and through the nerves. PNI of
SACC is the key factor responsible for the incomplete surgical
resection and the striking characteristic of SACC that
distinguishes it from other salivary gland malignancies (3). The mechanisms involved in the PNI of
SACC are still ambiguous although it has been investigated over a
long period of time. Thus, it is crucial to establish a PNI model
in vitro to mimic the perineural invasion process of SACC
for further research on its molecular mechanisms.
Schwann cells (SCs) constitute the main cells of
peripheral nerves, which are involved in the maintenance of axons
and crucial for neuronal survival. When the peripheral nerve is
injured or invaded by tumor cells, SCs exert essential function for
maintaining the health of axons and the survival of neurons by
producing a variety of neurotrophins (4). One of the most important neurotrophins
is brain-derived neurotrophic factor (BDNF). BDNF acts on certain
neurons of the central and the peripheral nervous systems, helping
to maintain the survival of existing neurons and to encourage the
growth and differentiation of new neurons and synapses (5). However, recent studies have revealed
that BDNF and its receptor tropomyosin-related kinase B (TrkB) are
also involved in the malignant progression of various tumors such
as head and neck squamous cell carcinoma, hepatocellular carcinoma,
and colorectal cancer (6,7). Our recent study for the first time
found that BDNF and TrkB were highly expressed in SACC, and the
elevated expression levels of BDNF and TrkB were significantly
associated with PNI in SACC (8).
Therefore, the molecular mechanisms of the BDNF/TrkB axis in the
PNI of SACC require further study.
An increasing number of studies suggest that
epithelial-mesenchymal transition (EMT), mediated by key
transcription factors and induced by the local microenvironment, is
a key biological process in epithelial tumor invasion and
metastasis (9). During this
process, tumor cells acquire increased migration and invasion
abilities, and this provides a likely mechanism by which epithelial
tumor cells leave primary sites and establish metastases. Recent
research found that EMT is an important process involved in the PNI
process in some neurotrophic cancers (8,10,11).
In addition, accumulating evidence suggests that the expression of
Schwann cell biomarkers is obviously increased in neurotrophic
cancers (10,12). Some scholars hypothesized that
Schwann-like cell differentiation might be also involved in the PNI
process of neurotrophic cancers (13).
Despite recognition of the PNI phenomenon in
neurotrophic cancers, little progress has been made in the
understanding of the molecular mechanisms of PNI in SACC. The
present study was designed to investigate whether SCs could promote
the process of EMT and the Schwann-like differentiation in the
process of PNI in SACC cells via the BDNF/TrkB axis.
Materials and methods
Cell lines and cell culture
The human adenoid cystic carcinoma cell lines
SACC-83 and SACC-LM were obtained from Peking University School of
Stomatology (Beijing, China). The human mucoepidermoid carcinoma
cell line MEC-1 was kindly provided by the Department of Oral
Biology, the Fourth Military Medical University (Xi'an, China).
Tumor cells were maintained in RPMI-1640 medium with 10% fetal
bovine serum (FBS) in a 5% CO2 humidified atmosphere at
37°C. The primary culture and identification of SCs were carried
out as in a previous study (14).
SCs were isolated from the sciatic nerves of neonatal SD rats,
which were obtained from the Laboratory Animal Center of the Fourth
Military Medical University. The harvested cells, suspended in
RPMI-1640 medium with 10% FBS, were plated onto dishes pre-coated
with poly-L-lysine (PLL) and purified by means of a differential
attachment technique.
Establishment of a co-culture system
between tumor cells and SCs
A modified protocol of Transwell cultures was used,
based on previous studies (15,16). A
Transwell® (24 mm) with a 0.4-µm pore polyester
membrane insert (Corning, Inc., Corning, NY, USA) was used to
establish the co-culture system. SCs
(1×105/cm2) were seeded in the upper chamber,
pre-coated with PLL, while 5×104/cm2 tumor
cells were seeded in the lower chamber. Then the tumor cells were
co-cultured with SCs in serum-free RPMI-1640 for 72 h. Solely
cultured tumor cells or SCs were set as the negative controls.
Enzyme-linked immunosorbent assay (ELISA)
analysis
ELISA assay was performed using the rat BDNF
Quantikine™ ELISA kit (R&D Systems, Minneapolis, MN, USA). BDNF
secretions from the medium of the solely cultured tumor cells, SCs
and tumor cells co-cultured with SCs were measured after 72 h of
culturing. The procedures recommended by the manufacturer were
followed.
Photography and laser confocal
imaging
After being maintained in serum-free RPMI-1640
medium for 72 h, SACC-83 cells in each group were photographed
using a phase-contrast photomicroscope (Olympus, Center Valley, PA,
USA). To visualize the cytoskeleton of the SACC-83 cells, cells
were fixed in 4% paraformaldehyde, stained with phalloidin
(Sigma-Aldrich, St. Louis, MO, USA) and counterstained with
4,6-diamidino-2-phenylindole (DAPI) (Invitrogen Inc., Carlsbad, CA,
USA). Then the cytoskeleton of the SACC-83 cells was photographed
by a FluoView laser scanning confocal microscope (Olympus, Tokyo,
Japan).
Quantitative RT-PCR analysis
Total RNA was isolated using Takara MiniBEST
Universal RNA Extraction kit (Takara Bio, Inc., Otsu, Japan).
Reverse transcription was completed by utilization of
PrimeScript™RT Master Mix (Takara Bio, Inc.). PCR amplification of
the cDNA template was carried out using SYBR® Premix Ex
Taq™II (Takara Bio, Inc.) on CFX96™ Real-Time PCR detection
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin
functioned as the housekeeping gene. The relative expression level
of the genes was calculated using the ΔΔCt method. Primers of the
detected genes are listed in Table
I.
 | Table IPrimers used for real-time PCR
analysis. |
Table I
Primers used for real-time PCR
analysis.
| mRNA | Size (bp) | Primer sequence |
|---|
| BDNF | 100 | F:
5′-GCCCTGTATCAACCCAGAAA-3′ |
| | R:
5′-AATGCCAACTCCACATAGCC-3′ |
| TrkB | 100 | F:
5′-GGGACACCACGAACAGAAGT-3′ |
| | R:
5′-GACGCAATCACCACCACAG-3′ |
| E-cadherin | 104 | F:
5′-GGTCTCTCTCACCACCTCCA-3′ |
| | R:
5′-CCTCGGACACTTCCACTCTC-3′ |
| N-cadherin | 129 | F:
5′-ATTTGAGGGCACATGCAGTAG-3′ |
| | R:
5′-GAACTGTCCCATTCCAAACCT-3′ |
| Vimentin | 111 | F:
5′-GGAAGAGAACTTTGCCGTTG-3′ |
| | R:
5′-TGGTATTCACGAAGGTGACG-3′ |
| S100A4 | 150 | F:
5′-GTACTCGGGCAAAGAGGGTG-3′ |
| | R:
5′-TTGTCCCTGTTGCTGTCCAA-3′ |
| GFAP | 128 | F:
5′-ACCTGCAGATTCGAGGGGG-3′ |
| | R:
5′-CGGCGGCGTTCCATTTACAA-3′ |
| CD133 | 187 | F:
5′-CATACCTAGGTCCCCGTCCG-3′ |
| | R:
5′-ATTTATGACCCGGCTTCTGGG-3′ |
| β-actin | 205 | F:
5′-TGACGTGGACATCCGCAAAG-3′ |
| | R:
5′-CTGGAAGGTGGACAGCGAGG-3′ |
Western blot analysis
Proteins extracted from each sample were separated
on 8% SDS-PAGE and transferred to polyvinylidene difluoride
membranes (Millipore, Billerica, MA, USA). Membranes were blocked
with non-fat dry milk in Tris-buffered saline containing 0.1%
Tween-20 (TBST) for 2 h at room temperature. Immunoblotting was
performed using specific primary and secondary antibodies
conjugated to horseradish peroxidase respectively. Primary rabbit
polyclonal antibody for BDNF [Cell Signaling Technology Inc., (CST)
Danvers, MA, USA, 1:1,000], TrkB (CST, 1:1,000), E-cadherin (CST,
1:1,000), N-cadherin (CST, 1:1,000), vimentin (CST, 1:1,000),
S100A4 (CST, 1:1,000) and GFAP (CST, 1:1,000) were used. Bands were
scanned using Chemidoc™ XRS+ with Image Lab™ software (Bio-Rad
Laboratories, Inc.) and quantification was carried out using
Quantity One 4.4.0 software.
Scratch wound healing assay
The SACC-83 cells of each group were plated in the
lower chamber of 24-well Transwell plates. When cells reached 80%
confluency, the individual wells were wounded by scratching with a
pipette tip and incubated with medium containing no FBS for 24 h.
The cells were fixed in methanol and photographed to measure the
wound distance.
Transwell perineural invasion assay
For the Transwell perineural invasion assays,
3×104 SACC-83 cells in 200 µl serum-free
RPMI-1640 medium were seeded onto the Matrigel-covered inserts (for
migration, 8 µm; Corning) in 24-well plates. The lower
chamber was seeded with 5×104 SCs to simulate the
perineural surrounding environment. The control groups consisted of
seeded SACC-83 cells solely or treated with 100 nM K252a. After 24
h of incubation, no invaded tumor cells were removed, and the
invaded cells were fixed in 95% ethanol and stained with
methylrosanilinium chloride solution. Quantification was performed
by counting the invaded cells in five independent fields under a
magnification of x400.
Immunofluorescence staining
SACC-83 cells in each group were fixed with 4%
paraformaldehyde and permeabilized with 0.2% Triton X-100. The
samples were incubated with a primary rabbit polyclonal antibody
for S100A4 (CST, 1:100) or GFAP (CST, 1:100) at 4°C overnight,
following by a secondary Alexa 750-conjugated goat anti-rabbit IgG
(1:1,000) or Alexa 488-conjugated goat anti-rabbit IgG (1:1,000)
(both from Abcam, Cambridge, MA, USA). The nuclei were
counter-stained with DAPI, and protein expression levels of S100A4
and GFAP were evaluated by fluorescence intensity under
fluorescence microscopy (Carl Zeiss Microimaging Japan, Tokyo,
Japan).
Patients and specimens
The present study was approved by the Medical
Research Ethics Committee of the Fourth Military Medical
University. After informed consent, formalin-fixed and
paraffin-embedded samples from 187 primary SACC patients who had
not undergone chemoradiation therapy prior to surgery between 2005
and 2012 were obtained from our Affiliated Hospital tissue
archives. In addition, 20 normal salivary glands were included in
the present study.
Immunohistochemical staining
A total of 187 formalin-fixed paraffin-embedded SACC
specimens and 20 normal salivary glands were sectioned (4-µm
thickness) for use. Immunohistochemical staining was performed as
described in our previous study (8). Polyclonal rabbit anti-human BDNF
(1:100) (Abcam), TrkB (CST, 1:100), E-cadherin (CST, 1:400), and
S100A4 (CST, 1:500) were used for the primary antibodies and
peroxidase-conjugated anti-rabbit antibody was used for the
secondary antibody. Omitting the primary antibodies was set as a
negative control.
All sections were evaluated in a blinded manner by
two independent pathologists. The intensity of immunostaining
(weak, 1; intense, 2) and the percentage of positive tumor cells
(0–5%, 0; 6–50%, 1; >50%, 2) were assessed in 5 high power
fields (magnification, ×400) at least. The scores of intensity and
percentage were multiplied to give a final score, and each SACC
specimen was assessed for immunoreactivity, as negative expression:
−, score 0; low expression: +, score 1 or 2; high expression: ++,
score 4.
Statistical analysis
All in vitro experiments were performed in
triplicate. The t-test and the one-way ANOVA tests were performed
to compare the results. The relationship between the expression of
TrkB, E-cadherin, S100A4 and clinical PNI was performed by
Spearman's rank correlation coefficient test. The correlation
between the expression of TrkB and the expression of E-cadherin or
S100A4 was evaluated by Spearman's rank correlation coefficient
test. SPSS 17.0 software package (USA) was used to perform
statistical analysis. P<0.05 was set the level of statistical
significance.
Results
The expression of BDNF and TrkB in the
interaction between SCs and tumor cells
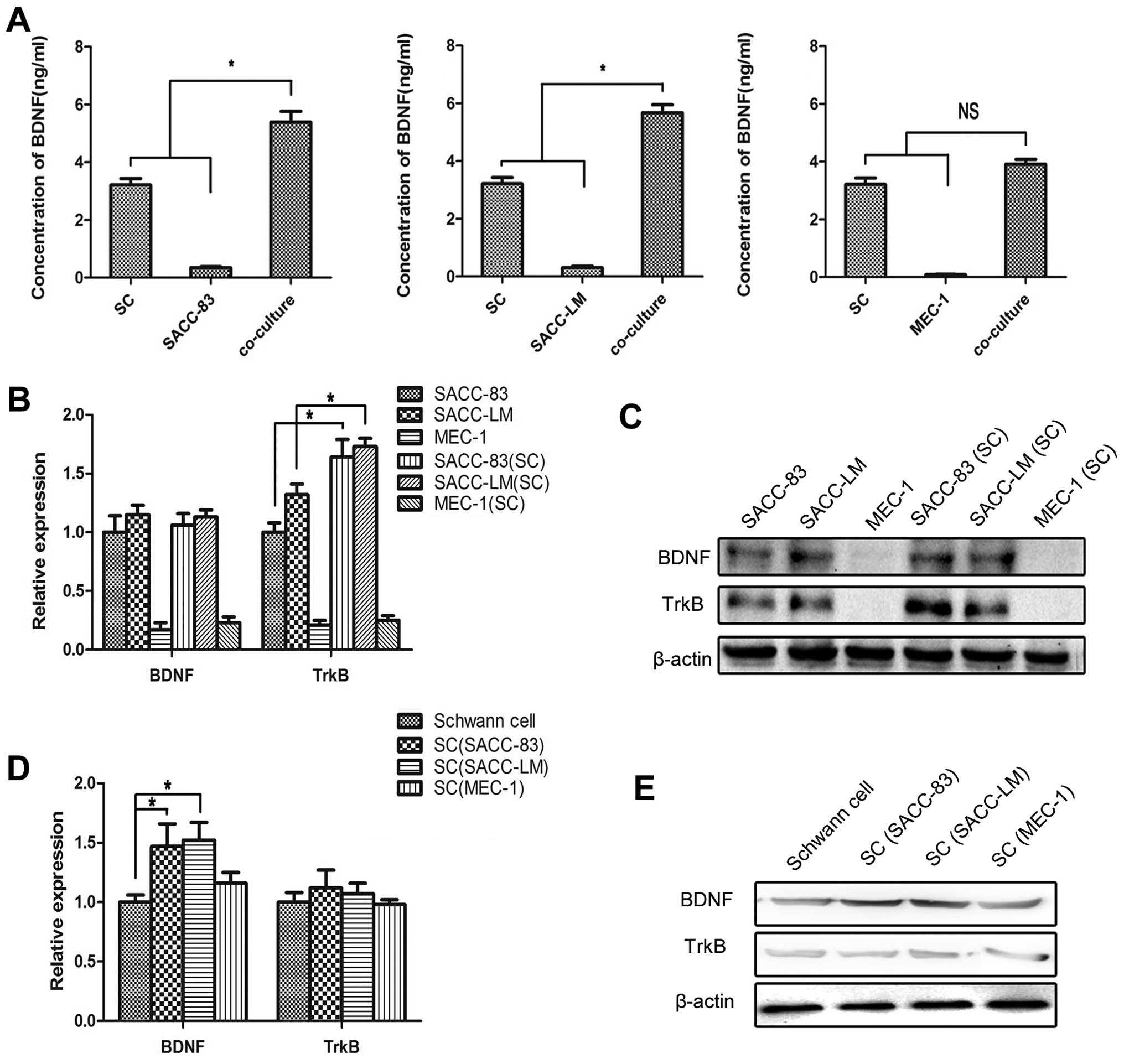
The co-culture models vividly mimicked the crosstalk
between tumor cells and SCs in the PNI process. ELISA analysis was
carried out to detect the concentration of BDNF in the medium. BDNF
was mainly produced by SCs and the concentration of BDNF in the
medium of the co-cultured SACC cell lines with SCs was
significantly higher than the sum of their solely cultured groups
(P<0.05) (Fig. 1A). The
concentration of BDNF in the medium of co-cultured MEC-1 cells with
SCs exhibited no obvious changes (P>0.05).
The solely cultured or co-cultured tumor cells were
collected and analyzed by quantitative RT-PCR and western blot
assays. The expression of BDNF in the SACC cell lines exhibited no
significant changes before and after co-culturing with the SCs
(P>0.05), while the expression of TrkB was markedly elevated in
the co-cultured groups compared with the solely cultured SACC-83 or
SACC-LM cells (P<0.05) (Fig. 1B and
C). The expression levels of both BDNF and TrkB in the MEC-1
cell lines were low and exhibited no obvious changes before and
after co-culturing with the SCs (P>0.05).
The solely cultured and co-cultured SCs were also
tested by quantitative RT-PCR and western blot assays. The
expression of BDNF in the SCs co-cultured with SACC-83 or SACC-LM
cells was significantly increased compared with the solely cultured
SCs (P<0.05), while the expression of BDNF in the SCs
co-cultured with the MEC-1 cells exhibited no significant changes
(P>0.05) (Fig. 1D and E). The
expression of TrkB in the SCs exhibited no significantly changes
before and after co-culturing with these tumor cells
(P>0.05).
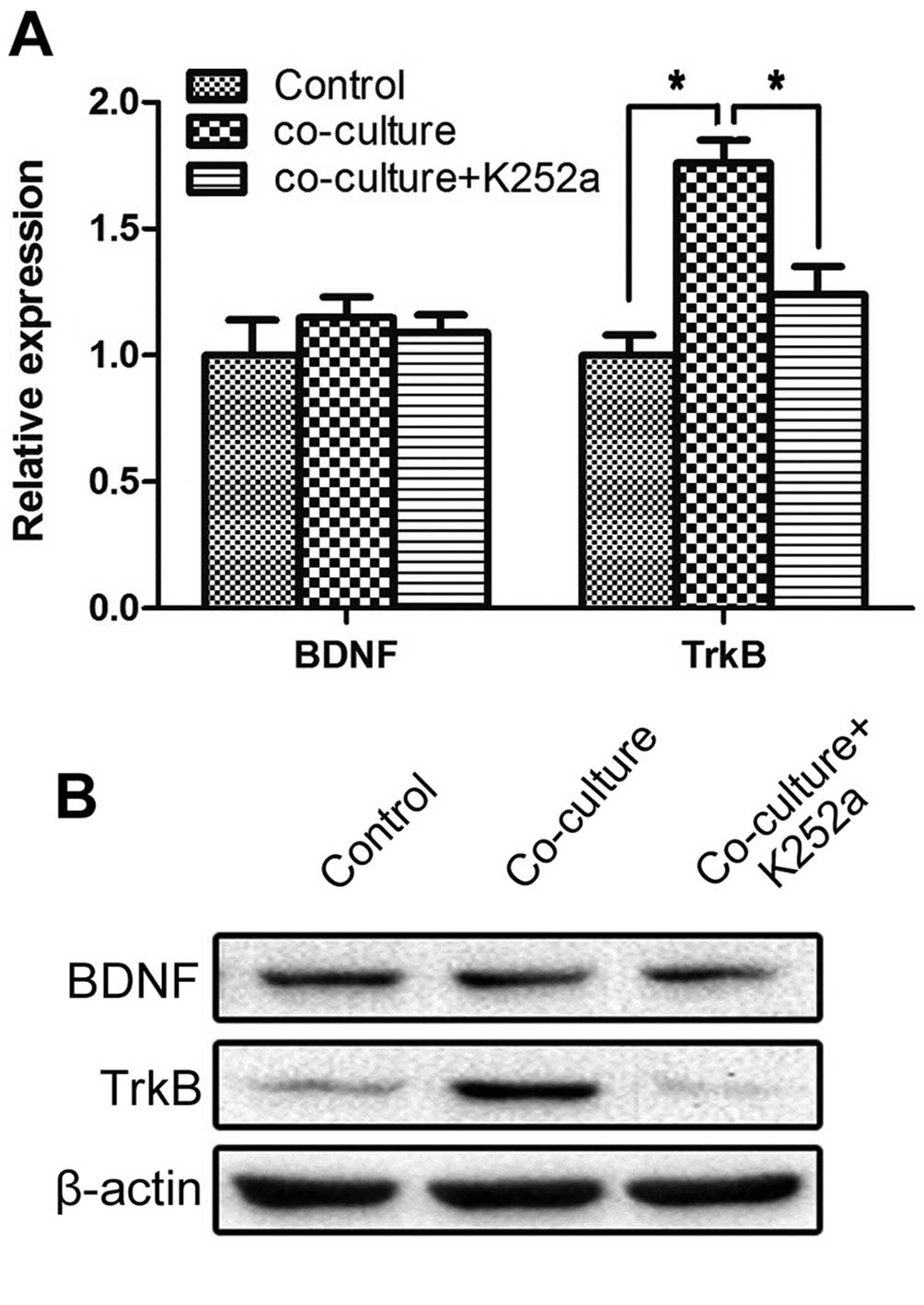
K252a interrupts the interaction between
SCs and SACC-83 cells
To explore the effects of SCs on SACC cells, the
co-culture model of SACC-83 cells with SCs was chosen for further
in vitro studies. To explore the function of the BDNF/TrkB
axis on SACC cells, TrkB inhibitor K252a (100 nM, Sigma-Aldrich)
was added into the co-culture system. Quantitative RT-PCR and
western blot analysis were used to investigate the expression of
BDNF and TrkB in the solely cultured SACC-83 cells, co-cultured
SACC-83 cells and co-cultured SACC-83 cells treated with K252a. The
gene and protein expression of TrkB in the SACC-83 cells
co-cultured with SCs was increased significantly compared with the
solely cultured SACC-83 cells (P<0.05), while 100 nM K252a
markedly blocked these effects (P<0.05). The expression of BDNF
in each group exhibited no significant change (P>0.05) (Fig. 2A and B).
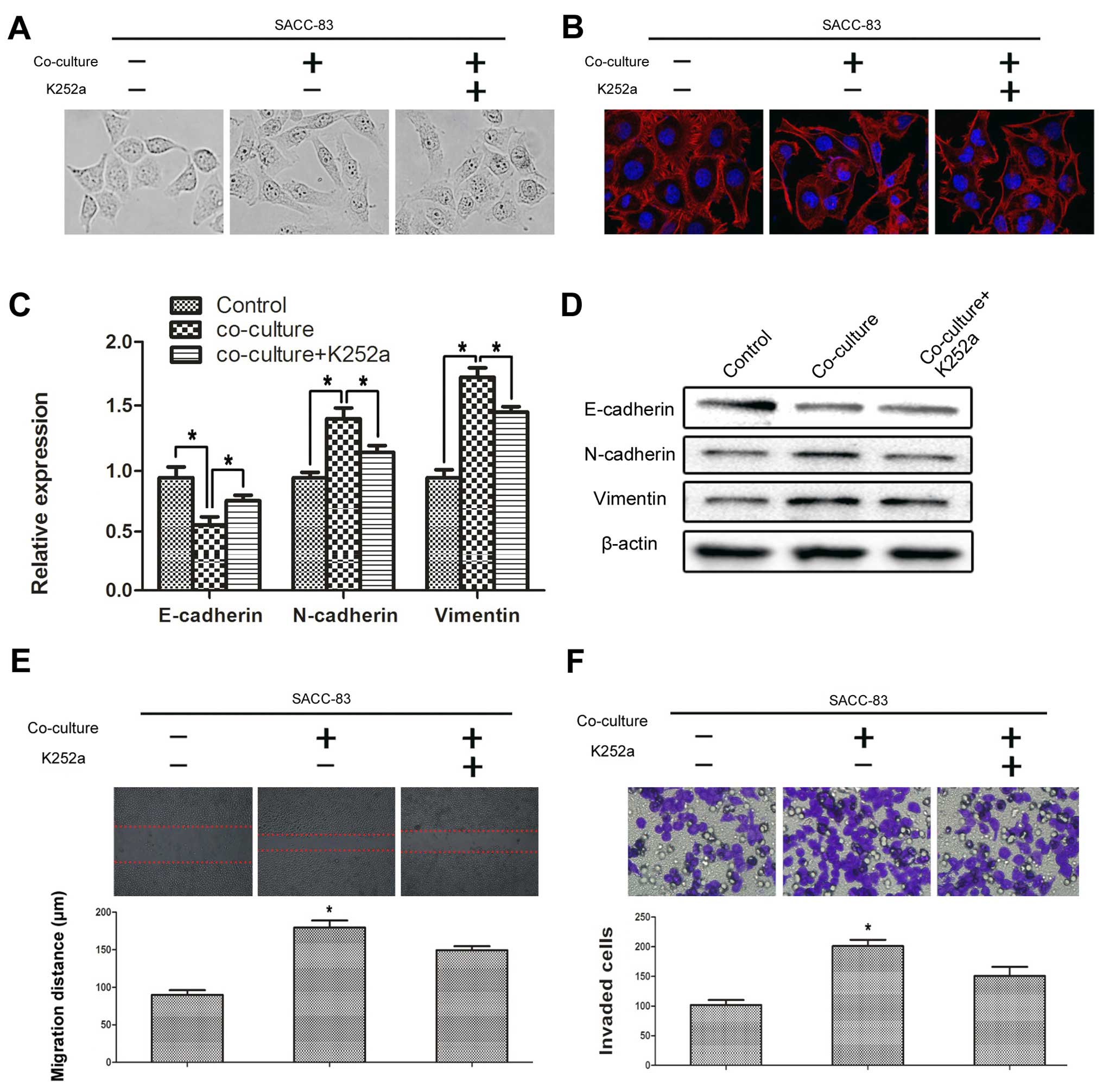
SCs promote the EMT progression of SACC
cells via the BDNF/TrkB axis
Typical characteristics involved in the EMT process
include cytoskeletal changes, increased motility and changes in a
series of biomarkers such as E-cadherin, N-cadherin and vimentin.
After co-culturing with SCs for 72 h, the SACC-83 cells were
visualized using phase contrast microscopy and the cytoskeleton was
photographed by laser scanning confocal microscope. The SACC-83
cells co-cultured with SCs exhibited obvious morphological changes
compared with the solely cultured SACC-83 cells (Fig. 3A and B). The morphology of the
SACC-83 cells co-cultured with SCs changed to a spindle-shape and a
polygon-shape and the intercellular junction decreased. The ratio
of spindle-shaped to polygon-shaped cells significantly decreased
following treatment with K252a. Co-culturing with SCs significantly
repressed the expression of E-cadherin (P<0.05), but promoted
the expression of N-cadherin and vimentin in the SACC-83 cells
(P<0.05) (Fig. 3C and D); and
these effects were signifi-cantly blocked by K252a (P<0.05).
In addition, we investigated the effects of SCs on
the motility of SACC-83 cells by a scratch wound healing and
Transwell perineural invasion assays. Co-culturing with SCs
significantly promoted the motility of the SACC-83 cells
(P<0.05) (Fig. 3E and F). In
contrast, inhibition of TrkB by K252a significantly impeded the
motility of the SACC-83 cells even under the co-culture condition
(P<0.05).
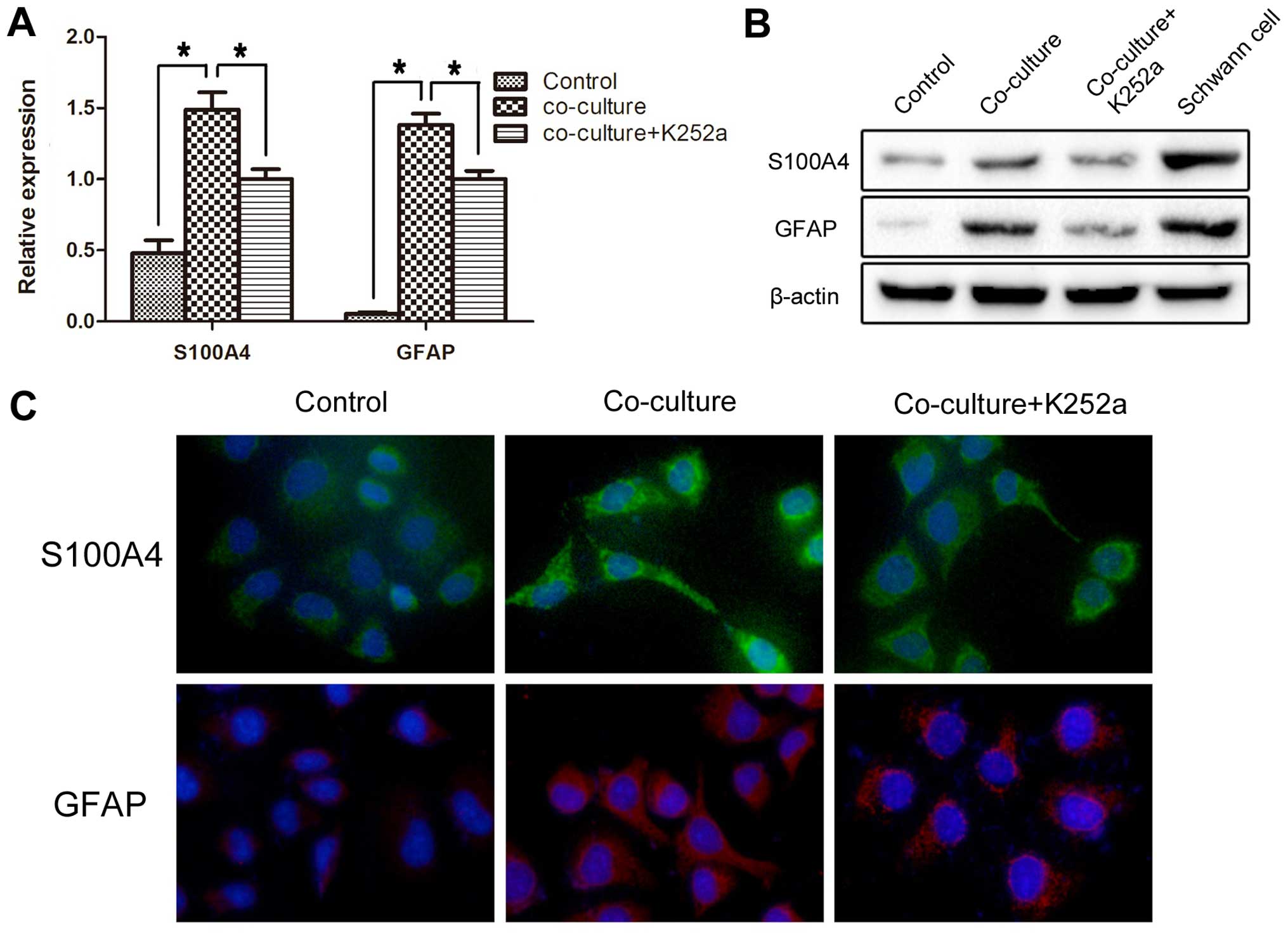
SCs promote the Schwann-like
differentiation of SACC cells via the BDNF/TrkB axis
SACC cells co-cultured with SCs were assessed for
expression of SC markers: S100A4 and GFAP. The gene and protein
expression of S100A4 and GFAP in the co-cultured SACC-83 cells was
markedly increased compared with the solely cultured SACC-83 cells
(P<0.05) (Fig. 4A and B); these
effects were significantly blocked by K252a (P<0.05). We also
performed immunofluorescence staining to compare the changes in the
expression of S100A4 and GFAP in the SACC-83 cells of each group.
The number and fluorescence intensity of the S100A4- and GFAP-
positive SACC-83 cells were significantly increased after
co-culture with the SCs (P<0.05), while treatment with K252a
significantly blocked this conversion (P<0.05) (Fig. 4C).
Expression of TrkB, E-cadherin and S100A4
in the SACC specimens
The expression of TrkB, E-cadherin and S100A4 in
SACC and normal salivary gland specimens was evaluated by
immunohistochemistry. TrkB and S100A4 were mainly expressed in the
cytoplasm of the tumor cells, while E-cadherin was mainly expressed
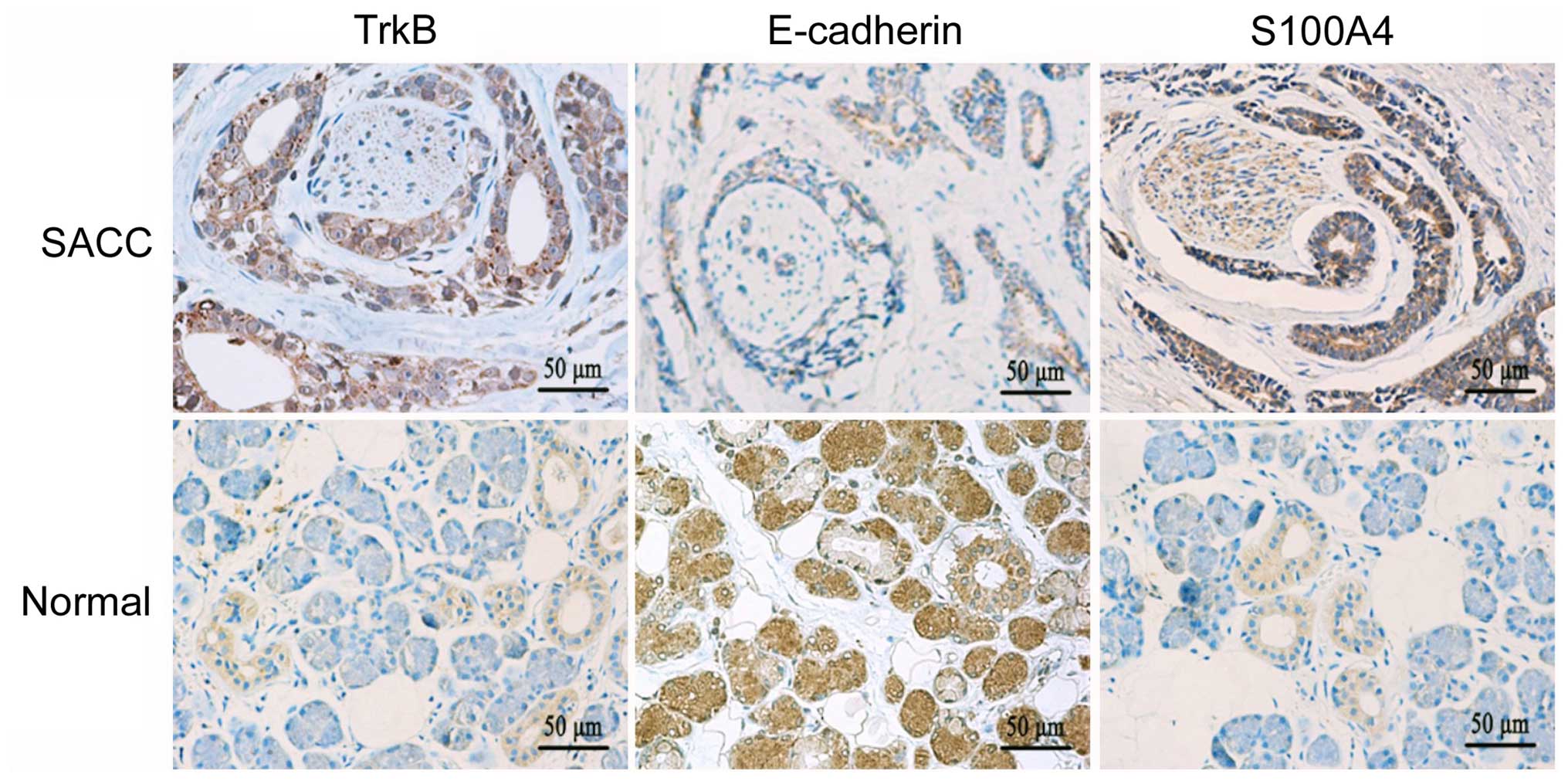
in the cell membrane and cytoplasm of the tumor cells (Fig. 5). TrkB and S100A4 were highly
expressed in the SACC tissues, while they were only detected in
some tuber cells and nervous tissues in the normal salivary glands.
We also found that the staining intensity of TrkB and S100A4 around
the peripheral nerve was obviously enhanced in the SACC specimens.
Contrary to the expression of TrkB and S100A4, the expression of
E-cadherin exhibited an opposite trend in the SACC and normal
salivary gland. In the SACC tissues, the elevated expression levels
of TrkB (92.0%, 172/187) and S100A4 (80.7%, 151/187) were
significantly higher than the levels in the normal salivary gland
tissues (15.0%, 3/20, P<0.01; 20.0%, 4/20, P<0.01,
respectively). The expression of E-cadherin in the SACC tissues
(47.6%, 89/187) was significantly lower than that in the normal
salivary gland tissues (100%, 20/20, P<0.01).
Correlation between the expression of
TrkB, E-cadherin and S100A4 and clinical PNI
As summarized in Table
II, the expression levels of TrkB and S100A4 in the SACC
tissues were both significantly associated with PNI (P<0.05),
while E-cadherin was significantly inversely associated with PNI
(P<0.05). Additionally, we assessed the correlation between the
expression of TrkB and the expression of E-cadherin and S100A4 in
the SACC specimens. As shown in Table
III, the TrkB expression was significantly inversely associated
with the E-cadherin expression (P<0.05) while significantly
positively associated with S100A4 expression (P<0.05).
 | Table IIRelationship between clinical PNI and
the expression of TrkB, E-cadherin and S100A4 in SACC. |
Table II
Relationship between clinical PNI and
the expression of TrkB, E-cadherin and S100A4 in SACC.
| PNI | n | TrkB expression
| P-value | E-cadherin
expression
| P-value | S100A4 expression
| P-value |
|---|
| − | + | ++ | − | + | ++ | − | + | ++ |
|---|
| − | 105 | 11 | 54 | 40 | 0.002a | 45 | 35 | 25 | 0.003a | 25 | 48 | 32 | 0.001a |
| + | 82 | 4 | 28 | 50 | | 53 | 18 | 11 | | 11 | 26 | 45 | |
 | Table IIICorrelation between the expression of
TrkB and the expression of E-cadherin and S100A4 in SACC. |
Table III
Correlation between the expression of
TrkB and the expression of E-cadherin and S100A4 in SACC.
| TrkB | n | E-cadherin
expression
| rs | P-value | S100A4 expression
| rs | P-value |
|---|
| − | + | ++ | − | + | ++ |
|---|
| − | 15 | 3 | 4 | 8 | −0.251 | 0.001a | 3 | 7 | 5 | 0.246 | 0.001a |
| + | 82 | 40 | 23 | 19 | | | 20 | 38 | 22 | | |
| ++ | 90 | 55 | 26 | 9 | | | 12 | 28 | 50 | | |
Discussion
PNI is a striking characteristic of SACC that is
responsible for incomplete surgical resection, locoregional
recurrence and distant metastasis (17). PNI also has been regarded as an
independent indicator of aggressive behavior and poor prognosis in
several neurotrophic cancers, most notably prostate and pancreatic
cancer (18,19). The pathogenesis of PNI involves
complex signaling between tumor cells and the nerves, and research
in this area is still largely in its infancy. To investigate the
likely mechanism of PNI in SACC, we hypothesized that the crosstalk
between SACC cells and SCs in the PNI process plays a pivotal role.
Thus, in this study, we established a co-culture model of SACC
cells and SCs by a Transwell system to mimic the tumor-nerve cell
interaction in the process of PNI.
BDNF, a member of the neurotrophin family, plays an
important role in the maintenance of axons and survival of neurons
when the peripheral nerve is injured (5). Yet in recent studies, increasing
evidence has revealed that both BDNF and its receptor TrkB are
overexpressed in a variety of malignances, including head and neck
squamous cell carcinoma (21),
breast cancer (6), colorectal
cancer (7), hepatocellular cancer
(22) and gastric cancer (23). Overexpression of these two markers
in malignant tumors is consistently associated with a more
aggressive behavior and poor prognosis (6,7,21–23).
Furthermore, our previous studies demonstrated that overexpression
of BDNF/TrkB is significantly correlated with clinical stage,
perineural or vascular invasion, distant metastasis, and poor
prognosis of SACC (8). In the
present study, we mimicked the crosstalk between SACC cells and SCs
in the PNI process, and found that the co-cultured SCs with SACC
cells secreted more BDNF. Meanwhile, the expression of TrkB in SACC
cells was significantly increased in the co-culture condition with
SCs. We also treated the co-cultured SACC-83 cells with the TrkB
inhibitior and found that 100 nM K252a significantly decreased the
TrkB expression in the SACC-83 cells. Our data from the
immunohistochemical staining also indicated that BDNF and TrkB were
significantly overexpressed in the SACC specimens when compared
with the normal salivary gland tissues. Interestingly, the staining
intensity of BDNF/TrkB around the peripheral nerve in the SACC
tissues was much stronger. These results suggest a potential role
of the BDNF/TrkB axis in the PNI progression of SACC.
EMT is a process characterized by loss of cell
polarity and intercellular adhesion molecules and acquisition of a
fibroblast-like morphology with cytoskeleton reorganization
(9). Increasing evidence suggests
that EMT plays a crucial role in the acquisition of invasive and
metastatic potential in a number of cancers, such as head and neck
squamous cell carcinoma (21),
breast cancer (24), pancreatic
cancer (25) and lung cancer
(26). Recent studies found that
the BDNF/TrkB axis is involved in the EMT process in various
cancers including SACC (8,21,27).
Although the correlation between EMT and PNI has been rarely
reported, it is plausible to infer that EMT plays an important role
in the PNI process since it can confer tumor cells with increased
migration and invasion abilities (8,10,11).
In the present study, we hypothesized that SCs might promote the
progression of PNI through the EMT process via the BDNF/TrkB
axis.
Our data demonstrated that the phenotype of SACC-83
cells co-cultured with SCs changed from an epithelial morphology to
a mesenchymal morphology accompanied by the conversion of EMT
hallmarks (downregulation of E-cadherin and upregulation of
N-cadherin and vimentin) and increased motility. This was in accord
with the results from the immunohistochemistry of the SACC
specimens that revealed that the expression of E-cadherin in SACC
around the peripheral nerve was much lower than that in the normal
salivary glands. Our Transwell PNI assay demonstrated that the
co-cultured SCs significantly promoted the in vitro PNI
ability of SACC-83 cells. In contrast, treatment with K252a
markedly blocked this phenomenon in the SACC-83 cells. These
results indicated that the interreaction of SCs and SACC-83 cells
mediated the PNI process by inducing the EMT of SACC-83 cells via
the BDNF/TrkB axis.
Cumulating evidence demonstrates that Schwann-like
cell differentiation may be one of the likely PNI molecular
mechanisms in neurotropic cancers (12,13,28–30).
Reed and Leonard firstly reported that the specific differentiation
toward 'neuroma-like' qualities in melanoma may be relative to the
PNI characteristics of melanoma cells (31). Additionally, Iwamoto et al
demonstrated that perineural spread in desmoplastic melanomas was
analogous to that of neurotropism in Schwann cells (32). Furthermore, Sun et al
reported that myoepithelial cells differenting into Schwann-like
cells may be one of the mechanisms of PNI occurring in SACC
(12). In the present study, we
identified that the expression levels of SC markers S100A4, and
GFAP were significantly upregulated in SACC cells when co-cultured
with SCs, while inhibition of TrkB by K252a significantly blocked
this conversion. This was in accordance with our
immunohistochemistry results that the staining intensity of S100A4
around the peripheral nerve in SACC specimens was much stronger
than that in the normal salivary glands. These results suggest that
SCs might induce SACC-83 cells to differentiate into Schwann-like
cells via the BDNF/TrkB axis in the PNI process.
We also analyzed the relationship between the
expression of TrkB, E-cadherin, S100A4 and clinical PNI in the SACC
specimens. We found that the elevated expression of TrkB and S100A4
and decreased expression of E-cadherin were significantly
associated with the clinical PNI process. Moreover, the TrkB
expression was significantly directly associated with the S100A4
expression and significantly negatively association with the
E-cadherin expression. Once more, these data confirmed that the
BDNF/TrkB axis is implicated in the EMT process and Schwann-like
cell differentiation in the development of PNI in SACC. However, in
our in vitro cell co-culture experiments, we found that
inhibition of the BDNF/TrkB axis by K252a could not block the EMT
process and Schwann-like differentiation induced by SCs completely.
Thus we inferred that there must be other signaling pathways
implicated in the interaction between SACC cells and SCs. Thus, the
molecular mechanisms of PNI in SACC require further
investigation.
Taken together, the present study indicates that the
SACC cell-SC crosstalk mediated by the BDNF/TrkB axis promotes the
PNI process via inducing EMT and the Schwann-like cell
differentiation of SACC cells, which might be a likely PNI
mechanism of SACC. Targeting the interaction between SACC cells and
SCs by inhibition of BDNF/TrkB signaling may be a potential
strategy for anti-PNI therapy in SACC.
Acknowledgments
We thank Dr Yuan Liu and Dr Tao Liang for their
excellent technical assistance. The present study was supported by
the National Natural Science Foundation of China (grant no.
81302352 to X.Y. and grant no. 81372901 to D.L.).
References
|
1
|
Tian Z, Li L, Wang L, Hu Y and Li J:
Salivary gland neoplasms in oral and maxillofacial regions: A
23-year retrospective study of 6982 cases in an eastern Chinese
population. Int J Oral Maxillofac Surg. 39:235–242. 2010.
View Article : Google Scholar
|
|
2
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck - An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amit M, Binenbaum Y, Trejo-Leider L,
Sharma K, Ramer N, Ramer I, Agbetoba A, Miles B, Yang X, Lei D, et
al: International collaborative validation of intraneural invasion
as a prognostic marker in adenoid cystic carcinoma of the head and
neck. Head Neck. 37:1038–1045. 2015. View Article : Google Scholar
|
|
4
|
Jessen KR, Mirsky R and Lloyd AC: Schwann
cells: Development and role in nerve repair. Cold Spring Harb
Perspect Biol. 7:a0204872015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benarroch EE: Brain-derived neurotrophic
factor: Regulation, effects, and potential clinical relevance.
Neurology. 84:1693–1704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Martin TA and Jiang WG: Biological
influence of brain-derived neurotrophic factor on breast cancer
cells. Int J Oncol. 41:1541–1546. 2012.PubMed/NCBI
|
|
7
|
Tanaka K, Okugawa Y, Toiyama Y, Inoue Y,
Saigusa S, Kawamura M, Araki T, Uchida K, Mohri Y and Kusunoki M:
Brain-derived neurotrophic factor (BDNF)-induced
tropo-myosin-related kinase B (Trk B) signaling is a potential
therapeutic target for peritoneal carcinomatosis arising from
colorectal cancer. PLoS One. 9:e964102014. View Article : Google Scholar
|
|
8
|
Jia S, Wang W, Hu Z, Shan C, Wang L, Wu B,
Yang Z, Yang X and Lei D: BDNF mediated TrkB activation contributes
to the EMT progression and the poor prognosis in human salivary
adenoid cystic carcinoma. Oral Oncol. 51:64–70. 2015. View Article : Google Scholar
|
|
9
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SJ, Choi SY, Kim WJ, Ji M, Lee TG, Son
BR, Yoon SM, Sung R, Lee EJ, Youn SJ, et al: Combined aberrant
expression of E-cadherin and S100A4, but not β-catenin is
associated with disease-free survival and overall survival in
colorectal cancer patients. Diagn Pathol. 8:992013. View Article : Google Scholar
|
|
11
|
Yang X, Jing D, Liu L, Shen Z, Ju J, Ma C
and Sun M: Downregulation of p53 promotes in vitro perineural
invasive activity of human salivary adenoid cystic carcinoma cells
through epithelial-mesenchymal transition-like changes. Oncol Rep.
33:1650–1656. 2015.PubMed/NCBI
|
|
12
|
Demir IE, Boldis A, Pfitzinger PL, Teller
S, Brunner E, Klose N, Kehl T, Maak M, Lesina M, Laschinger M, et
al: Investigation of Schwann cells at neoplastic cell sites before
the onset of cancer invasion. J Natl Cancer Inst. 106:1062014.
View Article : Google Scholar
|
|
13
|
Chen W, Dong S, Zhou J and Sun M:
Investigation of myoepi-thelial cell differentiation into
Schwann-like cells in salivary adenoid cystic carcinoma associated
with perineural invasion. Mol Med Rep. 6:755–759. 2012.PubMed/NCBI
|
|
14
|
Tao Y: Isolation and culture of Schwann
cells. Methods Mol Biol. 1018:93–104. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Zhang P, Ma Q, Kong L, Li Y, Liu B
and Lei D: EMMPRIN silencing inhibits proliferation and perineural
invasion of human salivary adenoid cystic carcinoma cells in vitro
and in vivo. Cancer Biol Ther. 13:85–91. 2012. View Article : Google Scholar
|
|
16
|
Yang X, Zhang P, Ma Q, Kong L, Li Y, Liu B
and Lei D: EMMPRIN contributes to the in vitro invasion of human
salivary adenoid cystic carcinoma cells. Oncol Rep. 27:1123–1127.
2012.
|
|
17
|
Dantas AN, de Morais EF, Macedo RA, Tinoco
JM and Morais ML: Clinical-pathological characteristics and
perineural invasion in adenoid cystic carcinoma: A systematic
review. Rev Bras Otorrinolaringol (Engl Ed). 8:329–335. 2015.
|
|
18
|
Bapat AA, Hostetter G, Von Hoff DD and Han
H: Perineural invasion and associated pain in pancreatic cancer.
Nat Rev Cancer. 11:695–707. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moreira DM, Fleshner NE and Freedland SJ:
Baseline perineural invasion is associated with shorter time to
progression in men with prostate cancer undergoing active
surveillance: Results from the REDEEM study. J Urol. May
16–2015.Epub ahead of print. View Article : Google Scholar
|
|
20
|
Takemura Y, Imai S, Kojima H, Katagi M,
Yamakawa I, Kasahara T, Urabe H, Terashima T, Yasuda H, Chan L, et
al: Brain-derived neurotrophic factor from bone marrow-derived
cells promotes post-injury repair of peripheral nerve. PLoS One.
7:e445922012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kupferman ME, Jiffar T, El-Naggar A,
Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D, et al:
TrkB induces EMT and has a key role in invasion of head and neck
squamous cell carcinoma. Oncogene. 29:2047–2059. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lam CT, Yang ZF, Lau CK, Tam KH, Fan ST
and Poon RT: Brain-derived neurotrophic factor promotes
tumorigenesis via induction of neovascularization: Implication in
hepatocellular carcinoma. Clin Cancer Res. 17:3123–3133. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanaka K, Shimura T, Kitajima T, Kondo S,
Ide S, Okugawa Y, Saigusa S, Toiyama Y, Inoue Y, Araki T, et al:
Tropomyosin-related receptor kinase B at the invasive front and
tumour cell dedifferentiation in gastric cancer. Br J Cancer.
110:2923–2934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bill R and Christofori G: The relevance of
EMT in breast cancer metastasis: Correlation or causality? FEBS
Lett. 589:1577–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang W, Gu W, Qiu R, Shen C, YaohaoWu EY,
Zhang J, Zhou J, Guo Y, Li Z, Deng J, et al: miRNA-101 suppresses
epithelial-to-mesenchymal transition by targeting HMGA2 in
pancreatic cancer cells. Anticancer Agents Med Chem. May
7–2015.Epub ahead of print.
|
|
26
|
Rudisch A, Dewhurst MR, Horga LG, Kramer
N, Harrer N, Dong M, van der Kuip H, Wernitznig A, Bernthaler A,
Dolznig H, et al: High EMT signature score of invasive non-small
cell lung cancer (NSCLC) cells correlates with NFκB driven
colony-stimulating factor 2 (CSF2/GM-CSF) secretion by neighboring
stromal fibroblasts. PLoS One. 10:e01242832015. View Article : Google Scholar
|
|
27
|
Ricci A, De Vitis C, Noto A, Fattore L,
Mariotta S, Cherubini E, Roscilli G, Liguori G, Scognamiglio G,
Rocco G, et al: TrkB is responsible for EMT transition in malignant
pleural effusions derived cultures from adenocarcinoma of the lung.
Cell Cycle. 12:1696–1703. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Izquierdo F, Suárez-Vilela D and Honrado
E: Perineurial cells in granular cell tumors and neoplasms with
perineural invasion: An immunohistochemical study. Am J
Dermatopathol. 34:800–809. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park K, Chen Z, MacDonald TY, Siddiqui J,
Ye H, Erbersdobler A, Shevchuk MM, Robinson BD, Sanda MG,
Chinnaiyan AM, et al: Prostate cancer with Paneth cell-like
neuroendocrine differentiation has recognizable histomorphology and
harbors AURKA gene amplification. Hum Pathol. 45:2136–2143. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kwon SY, Bae YK, Gu MJ, Choi JE, Kang SH,
Lee SJ, Kim A, Jung HR, Kang SH, Oh HK, et al: Neuroendocrine
differentiation correlates with hormone receptor expression and
decreased survival in patients with invasive breast carcinoma.
Histopathology. 64:647–659. 2014. View Article : Google Scholar
|
|
31
|
Reed RJ and Leonard DD: Neurotropic
melanoma. A variant of desmoplastic melanoma. Am J Surg Pathol.
3:301–311. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iwamoto S, Burrows RC, Agoff SN, Piepkorn
M, Bothwell M and Schmidt R: The p75 neurotrophin receptor,
relative to other Schwann cell and melanoma markers, is abundantly
expressed in spindled melanomas. Am J Dermatopathol. 23:288–294.
2001. View Article : Google Scholar : PubMed/NCBI
|