Introduction
Esophageal cancer is the sixth leading cause of
cancer-related death worldwide and one of the most difficult
malignant tumors to treat and cure (1). Squamous cell carcinoma is responsible
for 95% of all esophageal cancers worldwide (2,3).
Although advances have been made in the therapy with multimodal
treatment strategies, including neoadjuvant chemotherapy or
radiochemotherapy, esophageal cancer still remains one of the most
deadly human malignancies. In addition, neoadjuvant chemotherapy
and radiochemotherapy also bring many complications (4–6).
Therefore, it is urgently necessary to study the pathogenesis of
esophageal cancer and explore new treatment approaches.
The ability to evade immune surveillance is a well
accepted feature of malignant tumors. Recently, manipulation of
costimulatory signaling has been implicated as a potential immune
escape mechanism in human cancer (7–9).
Costimulatory signaling plays a key role in the initiation and
termination of immune responses by regulation of T-cell activation
(10).
Programmed death-1 (PD-1) is a costimulatory
molecule that provides an inhibitory signal in T-cell activation.
PD-1 belongs to the CD28 family (11,12).
PD-1 is expressed on T cells, B cells and myeloid cells. Programmed
death-ligands (PD-Ls) are ligands for PD-1, including PD-L1 and
PD-L2, which are cell-surface glycoprotein belonging to the B7
family (13–16). Previous studies have shown that
PD-1/PD-Ls interaction inhibits the function of T cell (14,16).
Recently, aberrant PD-L1 and PD-L2 expression by
cancer cells has been reported in many human malignancies (17–19). A
series of clinical trials concerning the systemic administration of
therapeutic antibodies for blocking PD-1 or PD-L1 have shown a
promising clinical effect in various tumors (20,21).
However, further studies on the PD-1/PD-Ls pathway in esophageal
squamous cell carcinoma (ESCC) are required. There is no previous
study on the expression of PD-L1, PD-L2 and PD-1 simultaneously in
ESCC tissues. The association between their expression and ESCC
prognosis is still controversial, and the mechanism of the
PD-1/PD-Ls pathway in ESCC is not clear. Our research is likely to
provide important evidence to delineate the cellular immune
deficiency mechanism in ESCC and a potential strategy for
immunotherapy against ESCC.
Materials and methods
Tissue samples
We examined 106 patients with esophageal cancer who
underwent surgery at Department of Surgery, the Affiliated Cancer
Hospital of Zhengzhou University, between January 2008 and December
2009. The patients had undergone primary surgical resection with
curative intent without preoperative chemotherapy or radiotherapy.
Seventy-six patients were male and 30 were female. The median age
of the patients was 59 years, with a range of 38 to 80 years. Tumor
stage was defined according to the pathological tumor node
metastasis (pTNM system) classifcation proposed by the
International Union against Cancer (UICC/AJCC, 7th edition) [stage
I (n=17), stage II (n=61), stage III (n=23) and stage IV (n=5)].
The median follow-up time for all patients was 55 months.
Postoperative pathohistologic analysis indicated that all tumors in
this study were ESCC. We also obtained 30 cases of paracancerous
tissue (>5 cm away from the cancer margin) as control.
Immunohistochemistry (IHC)
The IHC streptavidin-peroxidase staining method was
performed on 5 µm-thick formalin-fixed and paraffin-embedded
tissue sections. The sections were deparaffinized in xylene,
rehydrated in gradient ethanol solutions. The antigen retrieval was
conducted in 0.01 mol/l citrate (pH 6.0). Slides were incubated
overnight with rabbit anti-human PD-L1 polyclonal antibody (1:40;
ab58810; Abcam, Cambridge, MA, USA), rabbit anti-human PD-L2
polyclonal antibody (1:60; AB21968a; Sangon, Shanghai, China),
mouse anti-human PD-1 monoclonal antibody (1:50; ab52587; Abcam)
and phosphate-buffered saline as blank control. Incubation of the
biotinylated secondary antibody with horseradish peroxidase and 3,
3′-diaminobenzidine chromogen (all from ZSGB-Bio, Beijing, China)
was performed sequentially. Next, the slides were counterstained
with hematoxylin and then covered with neutral balsam.
Evaluation of IHC
IHC results for all examined costimulatory molecules
were evaluated by scanning each slide under low-power magnification
(×40) to identify regions containing positive immunoreactivity.
Immunostaining was further evaluated at high-power magnification
(×200). Tumor samples were examined by two observers in a blinded
manner. Expression of PD-L1, PD-L2 and PD-1 was evaluated as
staining on the cell membrane and cytoplasm. PD-L1 and PD-L2
staining-positive cases were determined by staining intensity and
the positive cell percentage according to the methods previously
published (22,23). The staining intensity grading: 0
point (no staining), 1 point (faint yellow), 2 points (clay-bank),
3 points (sepia). The percentage of the tumor cell population
staining was scored as follows: 1 point (<10%), 2 points
(10–50%), 3 points (>50%). The positive cases were determined
according to the two items multiplied by products: positive (>3
points), negative (≤3 points). The mean count of PD-1+
TILs (tumor-infiltrating lymphocytes) of 106 cases was used as
threshold and the cases were divided into high PD-1+
TILs group and low PD-1+ TIL group according to the
threshold.
Cells and cultures
The Ec109 cells were cultured in RPMI-1640
(Biological Industries, Kibbutz Beit. Haemek, Israel) supplemented
with 10% fetal bovine serum (FBS), (Biological Industries), 2
mmol/l glutamine, 100 U/ml penicillin, and 100 µg/ml
streptomycin at 37°C in 5% CO2. The lymphocytes were
provided by the Biological Treatment Center of the Second
Affiliated Hospital, Zhengzhou University. Cells were plated into
culture fasks with the medium at the concentration of
2×106/ml and placed at 37°C in 5% CO2. The
lymphocytes were sampled and counted every 2–3 days maintained at
1–4×106/ml by supplementing culture medium or
subculture. The cells were harvested between day 10 and day 17.
Lymphocyte culture medium was similar with cancer cell medium
above, except added with 1,000 U/ml interleukin (IL)-2 (KEXIN,
Beijing, China).
Magnetic activated cell sorting
(MACS)
The CD8+ T cells were generated using
miniMACS system. The lymphocytes were labeled with anti-CD8
microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). The
isolation was carried out according to the manufacturer's
instructions. The purity of CD8+ T cells was measured by
fow cytometry (FCM). Then CD8+ T cells were cultured in
lymphocyte culture medium.
Transfection
The PD-L1 and PD-L2 cDNA were digested with
KpnI/XhoI and constructed into pcDNA3.1 expression
vector by Sangon Biotech (Shanghai, China). Ec109 cells were
cultured in a 6-well plate (1×106/well). When the cells
were 70% confluent, they were used for transfection with
pcDNA3.1/PD-L1, pcDNA3.1/PD-L2 or pcDNA3.1, respectively. The
complex of DNA-Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA)
was prepared according to the manufacturer's instructions and was
added to the culture wells. The culture plate was shaken gently so
that the complex of DNA-Lipofectamine 2000 distributed well. Ec109
cells were cultured for another 4 h, and then culture medium was
replaced by fresh medium. The transfected Ec109 cells (Ec109/PD-L1,
Ec109/PD-L2 and Ec109/mock) were cultured for 48 h. Then G418 (400
µg/ml) was used to select the stable transfection
clones.
FCM
FCM was performed by standard method. The data were
acquired by using a FACSCanto cytometer (Becton-Dickinson, Franklin
Lakes, NJ, USA) and analyzed by CellQuest Pro software. Monoclonal
antibody used to measure the purity of CD8+ T cells
before and afer MACS included mouse anti-human-CD8-PE, -CD3-PerCP
(both from Miltenyi Biotec). The following monoclonal antibodies
were used to measure PD-1 expression of CD8+ T cells and
PD-L1, PD-L2 expression of Ec109 cells before and after
transfection: mouse anti-human -PD-L1-APC (Biolegend, San Diego,
CA, USA), -PD-L2-Fitc and -PD-1-PE (both from Miltenyi Biotec). IgG
isotype controls were used in FCM.
Real-time quantitative PCR (qRT-PCR)
Ec109 cells before and after transfection in
logarithmic growth phase were collected. The total RNA was
extracted using the RNA extraction kit spin column method (Qiagen,
Dusseldorf, Germany) according to the manufacturer's instructions.
Finally, 50 µl RNA was collected and RNA purity (D260/D280)
was 1.8–2.0, tested using an ultraviolet spectrophotometer
(SMA4000; Merinton, Beijing, China). Subsequently, reverse
transcription was conducted according to the reverse transcription
kit recommendations (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). In total, 20 µl cDNA was obtained and stored at −20°C
until use. The following primers synthesized by Sangon Biotech Co.,
Ltd., Shanghai, China were used for cDNA amplifcation system:
PD-L1: forward, 5′-GCATGGAGAGGAAGACCTGA-3′ and reverse,
5′-TTGTAGTCGGCACCACCATA-3′; PD-L2: forward,
5′-CAGCAATGTGACCCTGGAAT-3′ and reverse 5′-GGACTTGAGGTATGTGGAACG-3′;
β-actin as control. qRT-PCR was performed using the SYBR Green PCR
kit (Qiagen) according to the manufacturer's instructions. Samples
were denatured for 15 min at 95°C, followed by 40 cycles including
denaturation at 95°C for 15 sec, annealing at 52°C for 30 sec and
extension at 72°C for 34 sec, then by continuous fuorescence
measurement during heating from 60°C to 90°C (0.1°C/s). The data
was normalized to the β-actin expression of Ec109 cells and
analyzed using the ABI 7500 Fast system (Applied Biosystems, Foster
City, CA, USA). ΔCT = CT (target gene) - CT (β-actin).
Co-culture
To delineate the role of PD-Ls in tumor-T-cell
interactions in ESCC, co-culture experiments were carried out by
simulating the tumor microenvironment. The following monoclonal
antibodies were used to block PD-L1, PD-L2 and PD-1: mouse
anti-human-PD-L1, mouse anti-human-PD-L2 (Biolegend), rabbit
anti-human-PD-1 (Miltenyi Biotec). The experiments were divided
into 6 groups for research on the PD-L1 signal: group (A)
CD8+ T cells + Ec109/PD-L1 cells + IgG antibody; group
(B) CD8+ T cells + Ec109/PD-L1 cells + PD-L1 antibody;
group (C) CD8+ T cells + Ec109/PD-L1 cells + PD-1
antibody; group (D) CD8+ T cells + Ec109/mock cells +
IgG antibody; group (E) CD8+ T cells + Ec109/mock cells
+ PD-L1 antibody; group (F) CD8+ T cells + Ec109/mock
cells + PD-1 antibody. Another 6 groups for research on the PD-L2
signal: group (A) CD8+ T cells + Ec109/PD-L2 cells + IgG
antibody; group (B) CD8+ T cells + Ec109/PD-L2 cells +
PD-L2 antibody; group (C) CD8+ T cells + Ec09/PD-L2
cells + PD-1 antibody; group (D) CD8+ T cells +
Ec109/mock cells + IgG antibody; group (E) CD8+ T cells
+ Ec109/mock cells + PD-L2 antibody; group (F) CD8+ T
cells + Ec109/mock cells + PD-1 antibody. Each group was repeated
at least five times.
CCK-8 cell proliferation assay
According to the above groups, CD8+ T
cells (5×104/well) were co-cultured in 96-well plates
with mitomycin (15 µg/ml) treated Ec109 cells
(1×104/well) at a ratio of 5:1. Cell co-cultures were
maintained in complete media with recombinant human IL-2 (1,000
U/ml) and the antibodies (10 µg/ml). The proliferation of
CD8+ T cells was estimated by CCK-8 (Beyotime, Jiangsu,
China). After co-cultured for 48 h, 20 µl CCK-8 was added to
each well. The absorbance of each well was measured with a
microplate reader at 450 nm.
Apoptosis
According to the above groups, CD8+ T
cells (5×104/well) were co-cultured in 96-well plates
with Ec109 cells (1×104/well) at a ratio of 5:1. Cell
co-cultures were maintained in complete media with recombinant
human IL-2 (1,000 U/ml) and the antibodies (10 µg/ml). The
apoptosis of CD8+ T cells was estimated by Annexin
V-FITC/PI (Miltenyi Biotec) following its manufacturer's
instructions after co-cultured for 48 h.
Analysis of cytokine secretion
According to the above groups, CD8+ T
cells (5×104/well) were co-cultured in 96-well plates
with Ec109 cells (1×104/well) at a ratio of 5:1. Cell
co-cultures were maintained in complete media with recombinant
human IL-2 (1,000 U/ml) and the antibodies (10 µg/ml). The
interferon (IFN)-γ was estimated by human IFN-γ pre-coating ELISA
kit (Dakewe, Beijing, China) following its manufacturer's
instructions after co-cultured for 48 h.
Statistical analysis
SPSS 17.0 software was used for statistical
analysis. The significance of the difference between PD-Ls
expression and several clinical and pathologic variables was
assessed by the Chi-square test. The Kaplan-Meier method was used
to estimate the probability of survival. Quantitative values were
expressed as mean ± standard deviation or median and range. The
t-test and one-way analysis of variance were used to analyze the
differences between groups. All statistical tests were conducted as
two-sided, and P<0.05 was considered to indicate a statistically
significant difference.
Results
The expression of PD-L1, PD-L2 in ESCC
and the correlation with clinicopathological parameters
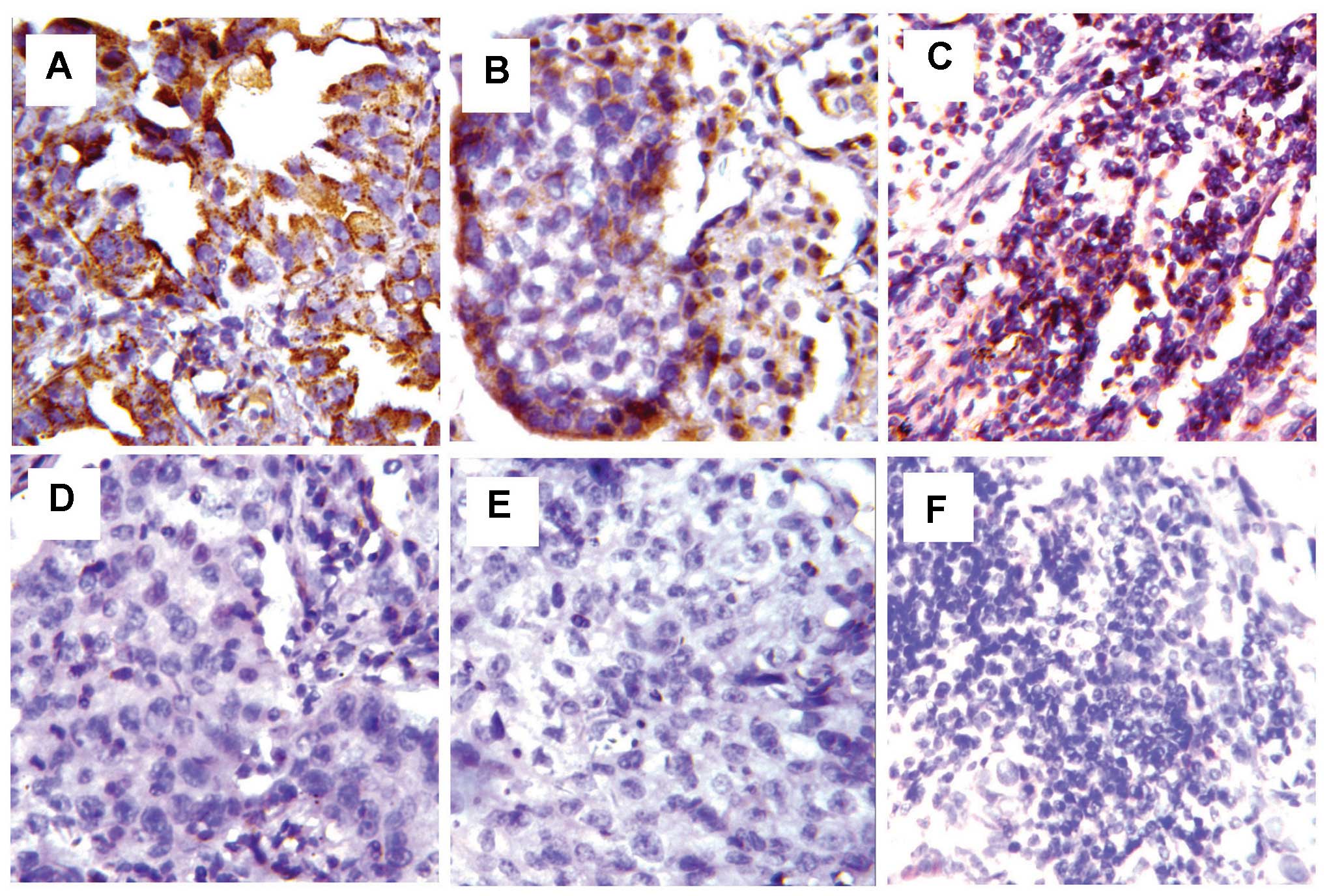
Expression of PD-L1, PD-L2 and PD-1 was evaluated as
staining on the cell membrane and cytoplasm (Fig. 1). The expression of PD-L1 and PD-L2
was detected in tumor cells. The positive expression of PD-L1 and
PD-L2 in ESCC tissues was 46.2 and 42.5%, respectively. But no
immunoreactivity was found in surrounding normal esophageal
tissues. We examined the relationship between PD-L1 and PD-L2
expression and various clinical pathological parameters. However,
there was no significant relationship between either PD-L1 or PD-L2
expression with gender, age, tumor location, tumor grade or
pathologic stage (Table I).
 | Table IThe correlation between PD-L1, PD-L2
expression and clinicopathological characteristics of ESCC
patients. |
Table I
The correlation between PD-L1, PD-L2
expression and clinicopathological characteristics of ESCC
patients.
|
Characteristics | Total (n) | PD-L1
| P-value | PD-L2
| P-value |
|---|
| Positive | Negative | Positive | Negative |
|---|
| Gender | | | | | | | |
| Male | 76 | 42 | 34 | 0.624 | 44 | 32 | 0.908 |
| Female | 30 | 15 | 15 | | 17 | 13 | |
| Age (years) | | | | | | | |
| ≥60 | 58 | 33 | 25 | 0.478 | 35 | 23 | 0.834 |
| <60 | 48 | 24 | 24 | | 28 | 20 | |
| Tumor location | | | | | | | |
| Proximal
third | 6 | 4 | 2 | 0.460 | 4 | 2 | 0.414 |
| Middle third | 83 | 42 | 41 | | 45 | 38 | |
| Distal third | 17 | 11 | 6 | | 12 | 5 | |
| Grading | | | | | | | |
| G1 | 22 | 10 | 12 | 0.513 | 11 | 11 | 0.152 |
| G2 | 47 | 28 | 19 | | 24 | 23 | |
| G3 | 37 | 19 | 18 | | 26 | 11 | |
| Pathologic
status | | | | | | | |
| Stage I | 17 | 10 | 7 | 0.744 | 9 | 8 | 0.364 |
| Stage II | 61 | 30 | 31 | | 32 | 29 | |
| Stage III | 23 | 14 | 9 | | 16 | 7 | |
| Stage IV | 5 | 3 | 2 | | 4 | 1 | |
The correlation between PD-1+
TILs with the expression of PD-L1 and PD-L2
PD-1 was predominantly expressed in the tumor
stromal lymphocytes. The count of PD-1+ TILs in the 106
ESCC cases (the range of the count of PD-1+ TILs in the
106 ESCC cases were 0–16; mean, 6.1) was significantly increased in
contrast to that in the normal tissues (0–7; mean, 2.59) (P=0.008).
The mean value of 6.1 was used as the threshold and, accordingly,
the 106 tumor cases were divided into PD-1+ TIL
high-density group (60 cases) and low-density group (46 cases). The
expression of PD-L1 and PD-L2 was found to inversely correlate with
PD-1+ TILs (P<0.05) (Table II).
 | Table IIThe correlation between
PD-1+ TILs with the expression of PD-L1 and PD-L2. |
Table II
The correlation between
PD-1+ TILs with the expression of PD-L1 and PD-L2.
| PD-1+
TILs | Total (n) | PD-L1
| P-value | PD-L2
| P-value |
|---|
| Positive | Negative | Negative | Positive |
|---|
| Low-density | 46 | 18 | 28 | 0.011 | 20 | 26 | 0.017 |
| High-density | 60 | 39 | 21 | | 41 | 19 | |
| Total (n) | 106 | 57 | 49 | | 61 | 45 | |
The correlation between PD-L1, PD-L2
expression and prognosis
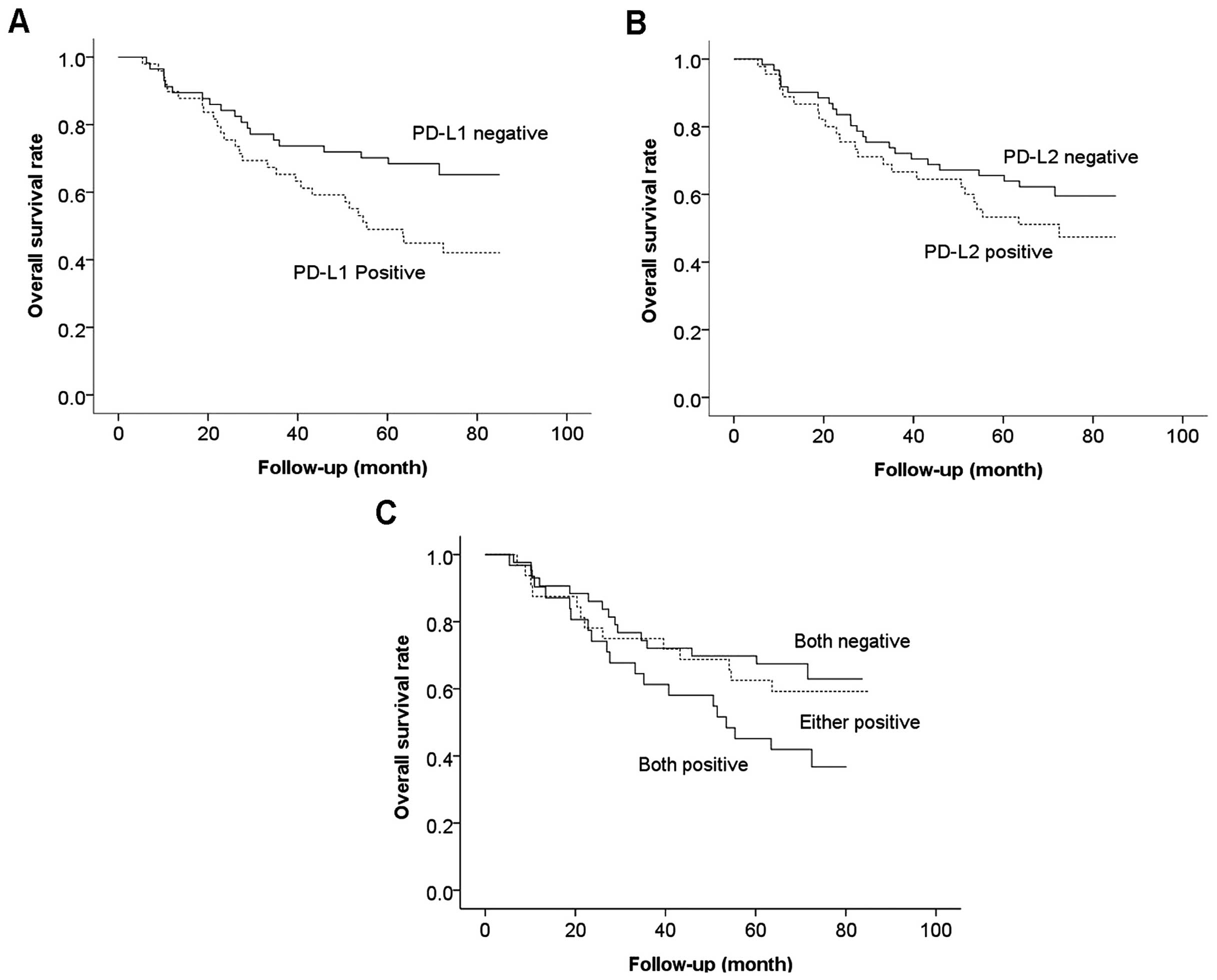
As shown in Fig. 2,
the overall survival of PD-L1 positive patients was significantly
worse than that of negative patients (P=0.027). However, the
overall survival of patients positive for PD-L2 tended to be worse
than that of negative patients but the difference was not
statistically significant (P=0.243). Furthermore, 31 patients had
tumors positive for both PD-L1 and PD-L2, 32 patients had tumors
positive for either PD-L1 or PD-L2 and 43 patients had tumors
negative for both PD-L1 and PD-L2. Overall survival of patients
with tumors positive for both PD-L1 and PD-L2 was significantly
worse than that with tumors negative for both (P<0.001). In
addition, overall survival of patients positive for either PD-L1 or
PD-L2 had a tendency to be better than that with both positive and
worse than that with both negative, although the differences were
not statistically significant (P= 0.094).
The expression of PD-L1, PD-L2 and PD-1
on Ec109 and CD8+ T cells
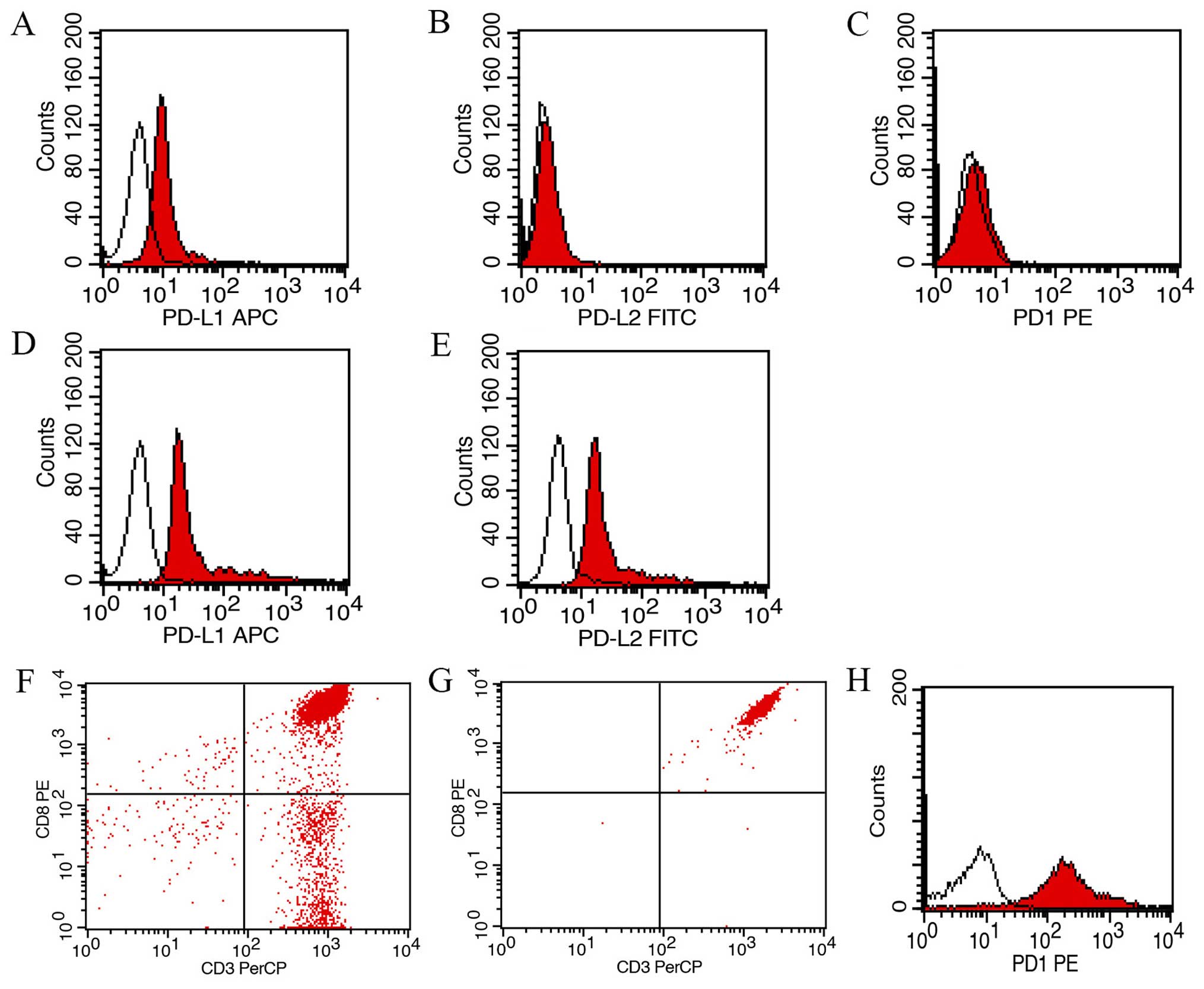
As shown in Fig. 3,
the expression of PD-L1 on Ec109 cell line was not high
(24.5±4.2%), and Ec109 cell line did not express PD-L2 and PD-1. We
subsequently examined the expression of PD-L1 and PD-L2 on Ec109
cells before and after transfection. Ec109/PD-L1 cells and
Ec109/PD-L2 cells were selected with high levels of PD-L1 (97.3)
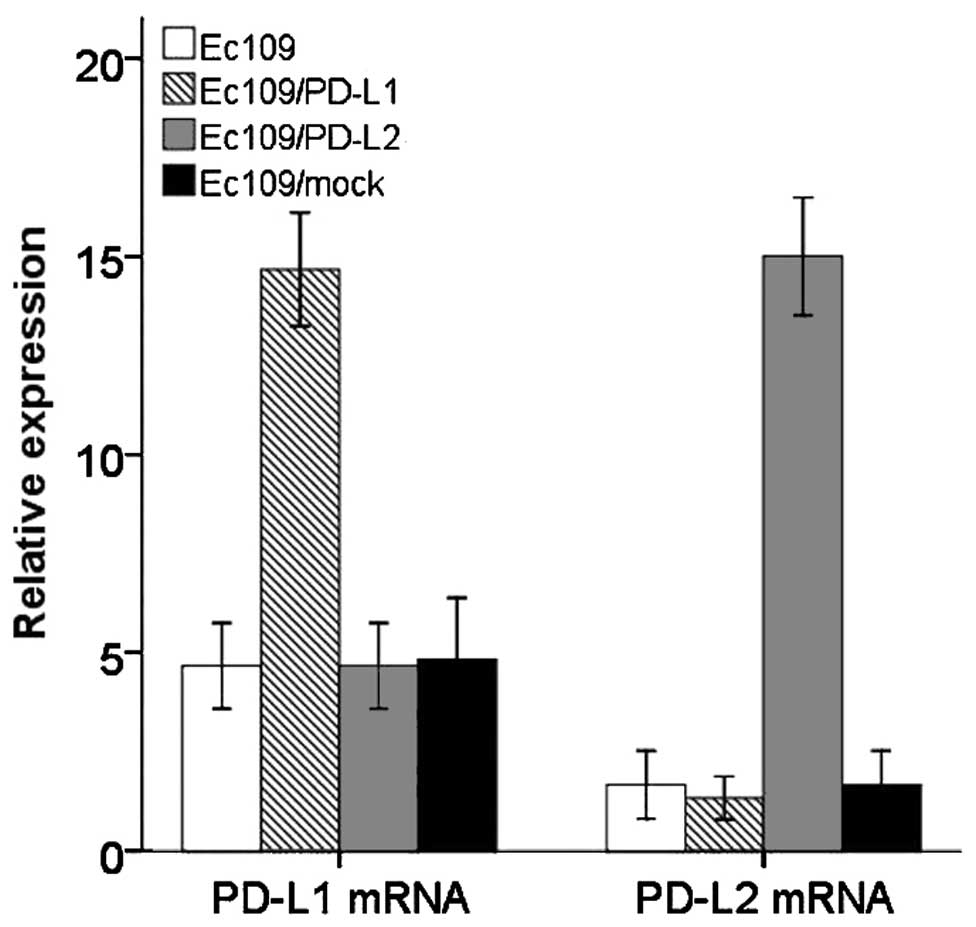
and PD-L2 (93.6%) for further study. Transcriptions of PD-L1 gene
and PD-L2 gene were identified by qRT-PCR indicated that the stable
transfected Ec109 cell line was successfully established (Fig. 4). The purity of CD8+ T
cells before and after separation by MACS was 75.2 and 99.7%,
respectively. In addition, the expression of PD-1 in
CD8+ T cells separated by MACS was 91.2% (Fig. 3).
Functional significance of PD-L1 and
PD-L2 for purified allogeneic CD8+ T cells
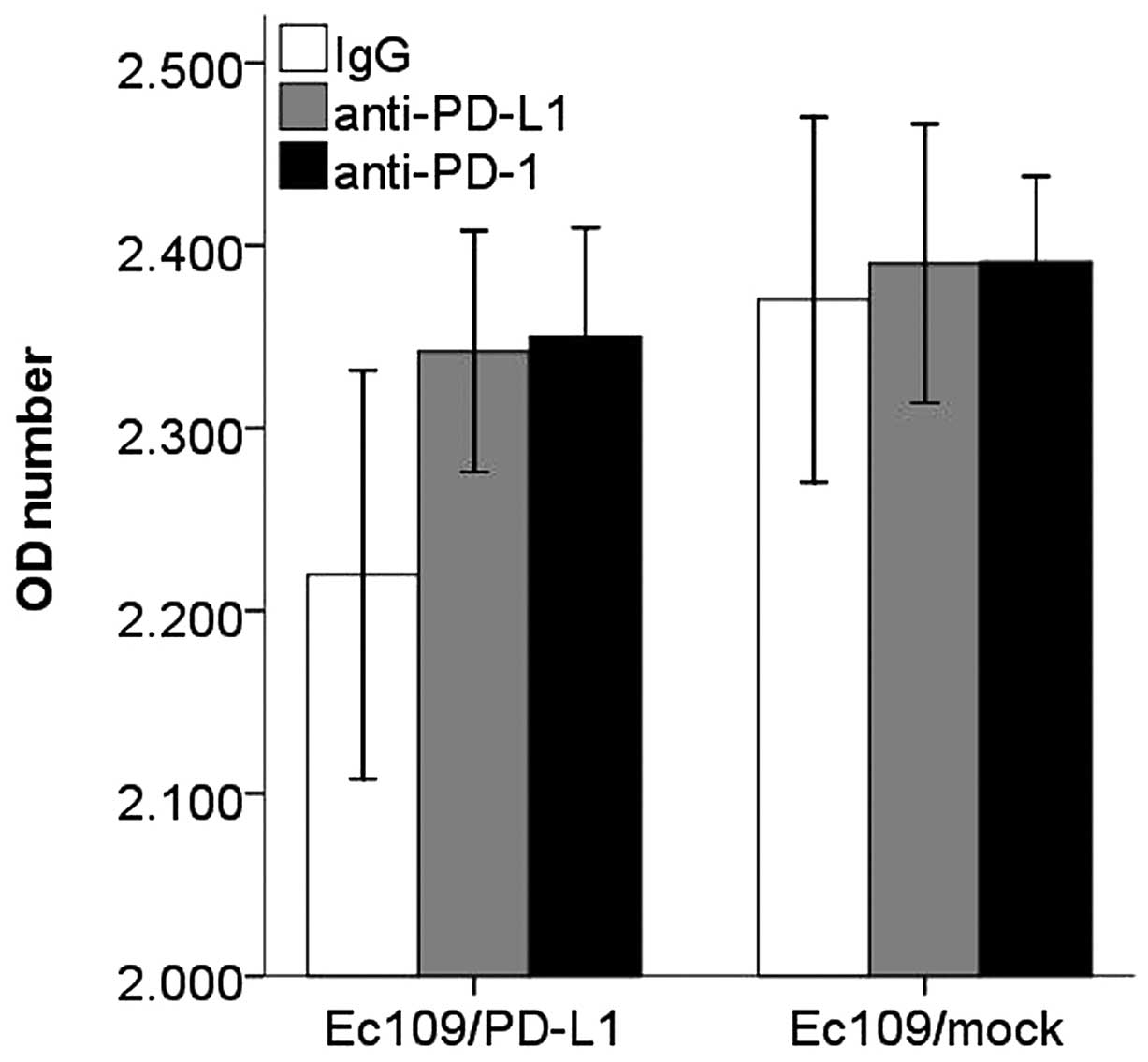
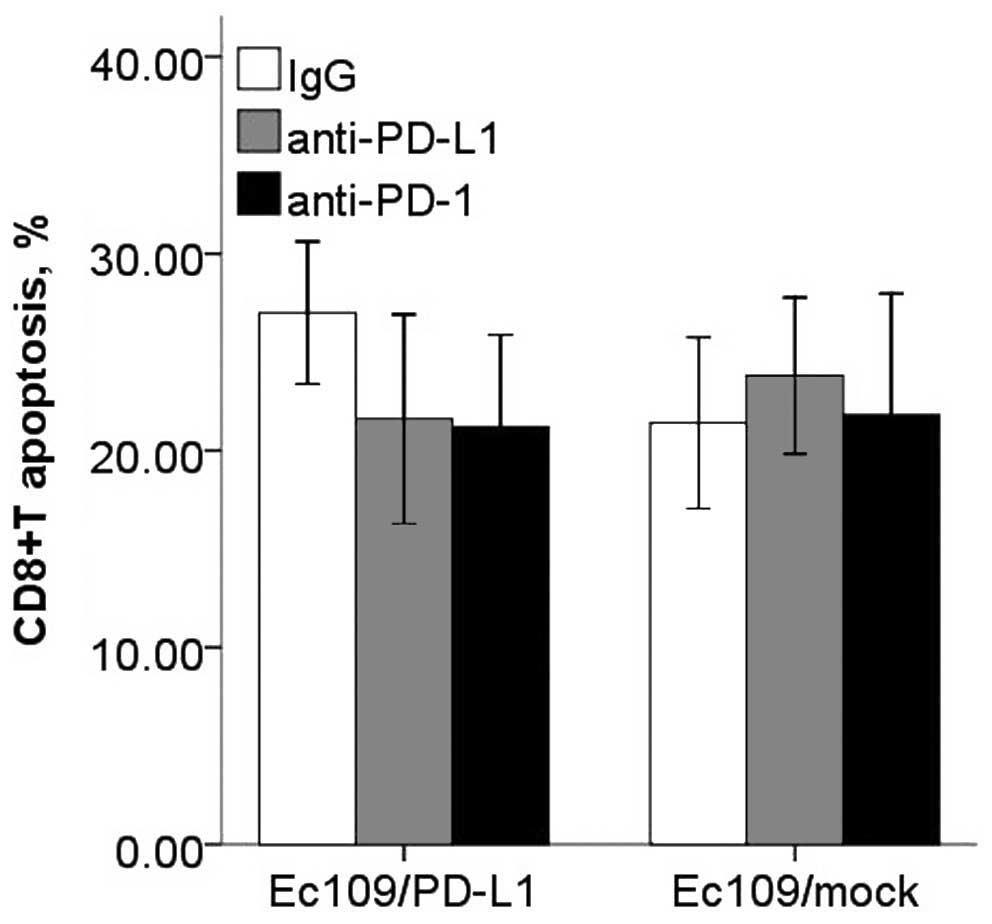
After co-cultured, the Ec109/PD-Ll cells caused a
decrease in CD8+ T cell proliferation, IFN-γ production
and increased apoptosis compared with the control group. However,
blockade of PD-L1 or PD-1 with antibodies resulted in enhanced
CD8+ T cells proliferation, IFN-γ production and
decreased apoptosis (Figs.
5Figure 6Figure 7–8).
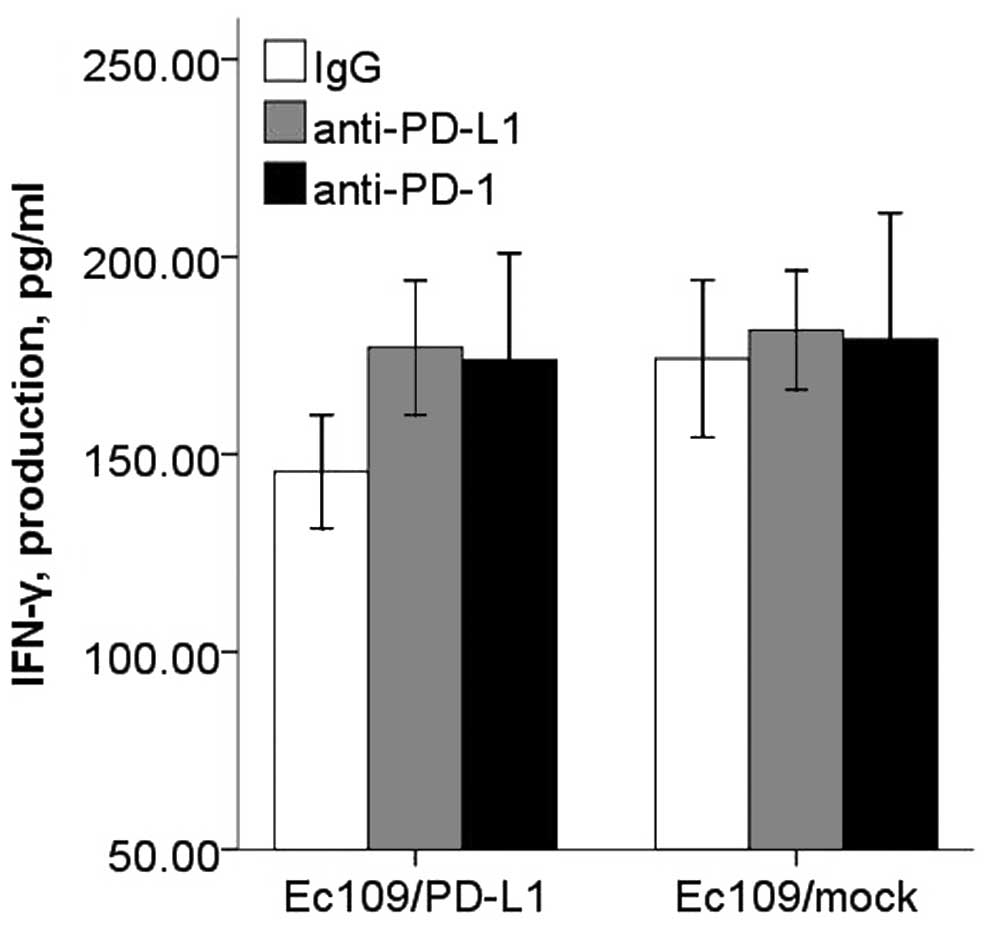
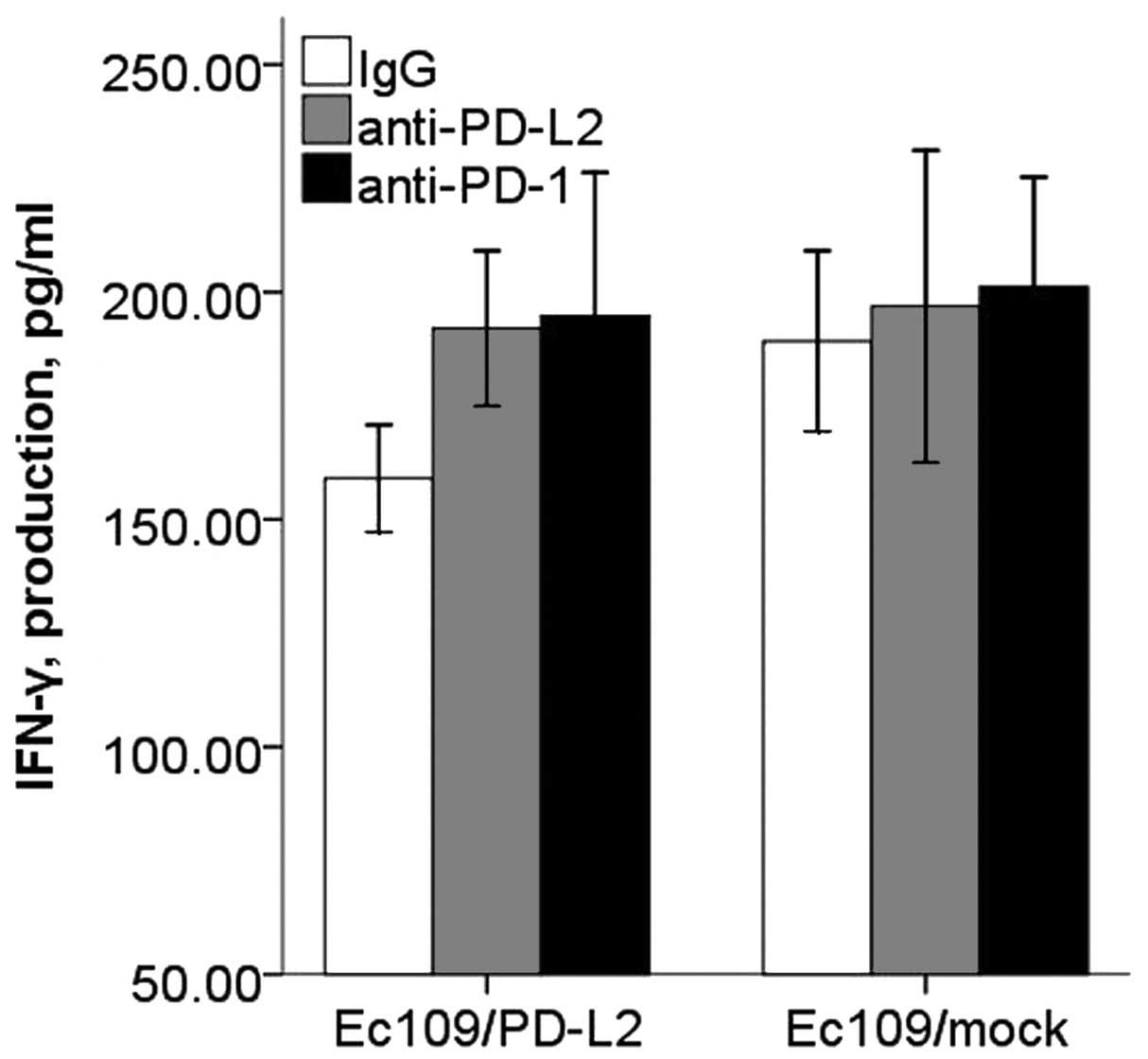
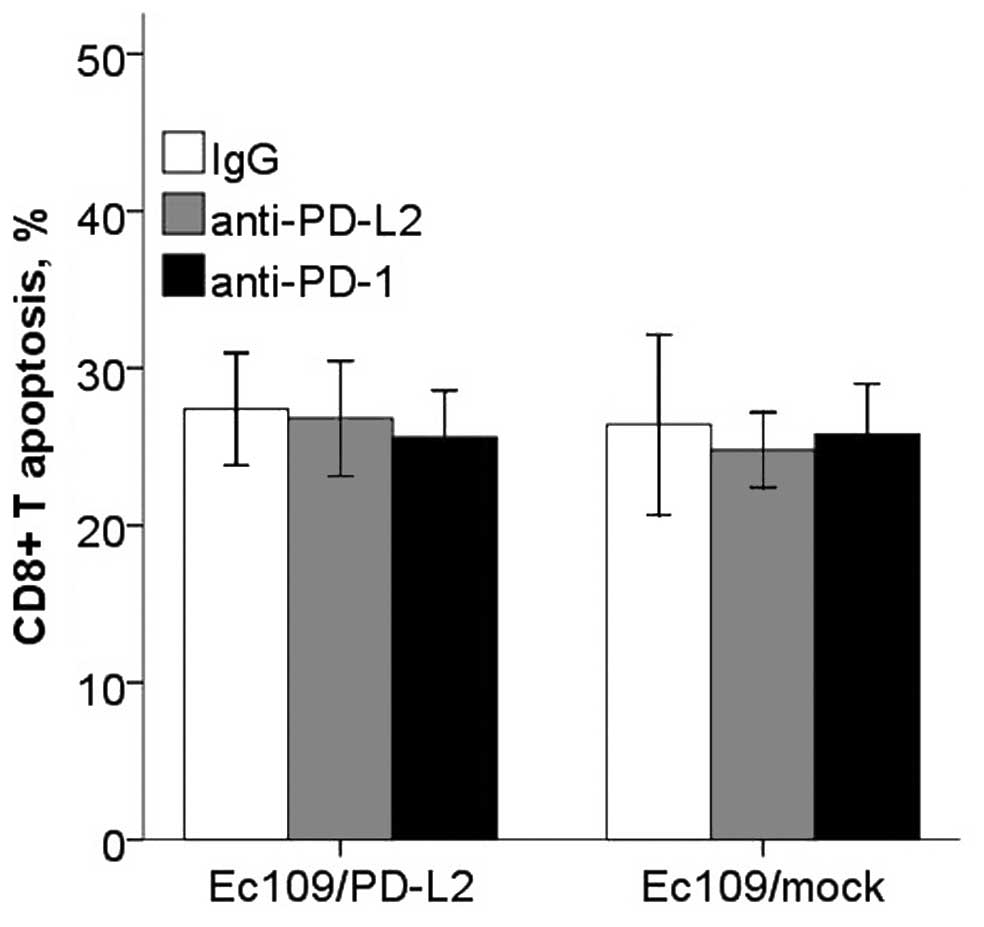
There was significant inhibitory effect of PD-L2 on
the CD8+ T cell IFN-γ secretion, and this inhibitory
effect could be restored with PD-L2 or PD-1 blocking antibody
(Fig. 9). However, CD8+
T cells proliferation and apoptosis in PD-L2 signal test were not
altered significantly (Figs. 10
and 11).
Discussion
Despite the presence of large numbers of TILs in
cancer tissues, the immune system often fails to prevent tumor
development and progression (24–27).
Recent studies have suggested a novel mechanism that tumor may
evade host immune response through the expression of PD-L1 and
PD-L2. PD-L1 and PD-L2 have been thought to be involved in the
negative regulation of cellular and humoral immune responses by
engaging PD-1 receptor on activated T and B cells (28,29).
However, there is no previous study on the
expression of PD-L1, PD-L2 and PD-1 simultaneously in ESCC tissues.
Our results show PD-L1, PD-L2 and PD-1 were aberrantly over
expressed in ESCC tissues. PD-L1 and PD-L2 proteins both located on
cytoplasm and cell membrane of tumor cells. We found that 46.2% of
ESCC tissues evaluated in this study were positive for PD-L1 and
42.5% ESCC tissues were positive for PD-L2. Furthermore,
PD-L1-positive patients had significantly poorer prognosis than the
negative patients. Though PD-L2 expression was correlated with an
impaired survival, this difference was not statistically
significant. However, there was no significant relationship between
either PD-L1 or PD-L2 expression with the age, gender, lesion
location, differentiated degree and pathologic stage.
PD-L1 expression has been detected in most human
cancers, such as gastric, pancreatic, kidney, breast, ovarian and
bladder urothelial cancers. In renal cell carcinoma, tumor PD-L1
expression has been shown to correlate with rapid cancer
progression, cancer death and overall mortality (22,30).
In urothelial cell carcinoma, tumor-associated PD-L1 expression was
found to be significantly associated with a high frequency of
postoperative recurrence, poorer survival rate, and advanced tumor
stage (31). In pancreatic cancer,
patients with cancer-cell associated PD-L1-positive expression had
a significantly poorer prognosis than patients with PD-L1-negative
tumors (32,33). In gastric carcinoma, patients with
PD-L1-positive tumors also had a significantly decreased
probability of survival compared with patients with PD-L1-negative
tumors (34).
The research on PD-L2 in malignant tumors is still
relatively rare. In a study of pancreatic cancer, no correlation
was found between PD-L2 expression and survival (32). In ovarian cancer, although PD-L2
expression was correlated with an impaired survival, this did not
reach statistical significance (35). Similarly, in hepatocellular
carcinoma a minority had high PD-L2 expression, and again, although
PD-L2 expression was correlated with an impaired disease-free
survival, this difference was not statistically significant
(36). Only one report suggested
that the expression of PD-L2 was significantly correlated with
poorer prognosis in ESCC (23).
Thus, the majority of studies have found a significant correlation
between impaired survival and PD-L1 expression, but much less so
for PD-L2. Currently, the expression of PD-L2 research conclusion
remains controversial in tumor tissues.
TILs are considered as a manifestation of the host
immune response (37). Several
clinical studies have suggested that TILs play a critical role and
have prognostic significance in certain human tumors including
esophageal cancer (38–40). PD-1 mainly expressed on TILs. In our
research, we showed for the first time that the count of
PD-1+ TILs was negatively correlated with both PD-L1 and
PD-L2 expression in ESCC. The results of the current study
indicated that the expression of PD-L1 and PD-L2 on ESCC inhibits
PD-1+ TILs activity or promote PD-1+ TILs
apoptosis, ultimately promoting immune evasion via the PD-1/PD-Ls
pathway.
In order to further study the effects of PD-L1 and
PD-L2 on immune cells, we measured the PD-L1, PD-L2 and PD-1
expression of Ec109 cells by FCM. The percentage of PD-L1 positive
cells on Ec109 was 24.5±4.2%. The Ec109 cells did not express PD-L2
and PD-1. Then stable transfected Ec109 cell line was established
and PD-L1/PD-L2 gene was expressed successfully. Transcription of
PD-L1 gene and PD-L2 gene were identified by qRT-PCR. This has not
been reported in ESCC, and can be used as a model applied to
further studies on PD-L1 and PD-L2.
Furthermore, we have analyzed the PD-1/PD-Ls signal
pathway on the function of CD8+ T cells for the frst
time by co-culturing Ec109 and CD8+ T cells. We chose
the CD8+ T lymphocytes in the function experiment,
because CD8+ T cells are generally thought to play a
central role in antitumor immune response and the presence of
CD8+ T was reported as a prognostic factor in esophageal
cancer (38,39). CD8+ T cells can also
produce IFN-γ, which is an important activator of macrophages and
inducer of class II major histocompatibility complex (MHC) molecule
expression, and IFN-γ has antiviral, immunoregulatory and antitumor
properties (41).
The Ec109 cells and purifed activated
CD8+ T cells were co-cultured for 48 h. PD-L1
significantly inhibited the CD8+ T cells proliferation,
IFN-γ secretion and enhanced the apoptosis, which could be restored
with the presence of PD-L1 and PD-1 blocking antibody. PD-L2
significantly inhibited the IFN-γ secretion of CD8+ T
cells, and this could be restored with the presence of PD-L2 and
PD-1 blocking antibody. But no significant result was obtained in
the proliferation and apoptosis experiments. PD-1/PD-Ls
interactions lead to phosphorylation of two tyrosines at the
intracellular tail of PD-1. These tyrosines are part of an
immunoreceptor tyrosine-based inhibitory motif (ITIM) and an
immunoreceptor tyrosine-based switch motif (ITSM). ITSM then
recruits either of two structurally-related protein tyrosine
phosphatases (42), which suppress
activation of PI3K/Akt (43).
Consequently, the survival factor Bcl-xL is downregulated and
expression of transcription factors associated with effector cell
function including GATA-3, T-bet and Eomes are lost (44). The net result of these PD-1-induced
cascades is an impairment of proliferation, cytokine production,
cytolytic function, and survival of the CD8+ T cells
(45).
However, the results of PD-L1 and PD-L2 are not
identical. These data indicate that PD-L1 and PD-L2 may have
different roles in tolerance induction, as Rozali et al
(46) concluded that PD-L2 could
play a role in the modulation of T-cell function, but the exact
molecular pathway was yet to be elucidated. Of note, PD-1 may not
be the only receptor for PD-L2. This can be inferred from helminth
infection and allergic animal models, showing enhanced disease
severity when PD-L2 blocking antibodies were used, but not when
PD-1 blocking antibodies were used (47,48).
Furthermore, PD-L2 mutants with abolished PD-1 binding capacity
could still exert functional effects on T cells from normal and
PD-1-defcient mice (49). Thus, the
role of PD-L2 still is not clear.
Activation of the immune system is recognized as an
important treatment strategy against cancer (50). In fact, therapeutic antibodies for
blocking PD-1 and PD-L1 have been developed and are undergoing
human clinical testing (51,52).
Although PD-1 and PD-L1 directed therapy is currently undergoing
investigation in several types of malignancies, including both
solid tumors and hematologic malignancies, PD-1 and PD-L1 therapy
has been most studied in patients with metastatic melanoma.
Antibodies targeting PD-1 in clinical development include
nivolumab, pembrolizumab and pidilizumab. The first antibody to
target PD-L1 in clinical trials was MDX-1105. Antibodies currently
in clinical development that target PD-L1 include MPDL3280A,
MEDI4736 and MSB0010718C. These clinical trials result in durable
responses and relative safety in patients with a wide range of
cancers (20,21). These therapeutic antibodies for
blocking PD-1 and PD-L1 have broad application prospects.
However, immunotherapy in ESCC is still immature.
Our finding revealed that PD-L1, PD-L2 and PD-1 were aberrantly
expressed in ESCC and they might thwart effective antitumor
immunity by interaction with tumor-T-cell, which provides an
important clue to reveal the cellular immune deficiency mechanism
in ESCC. Thus, more research in animal models, and in human are
necessary to fully delineate the immune regulation functions of
PD-Ls as well as molecule mediation mechanism involved in ESCC. How
to selectively block these inhibitory molecules will be an
attractive approach for ESCC immunotherapy.
Acknowledgments
The present study was supported by the assistance of
the Central Laboratory in the Affiliated Cancer Hospital of
Zhengzhou University, the Biological Treatment Center and Institute
of Digestive of the Second Affiliated Hospital of Zhengzhou
University.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen DJ and Ajani J: An expert opinion on
esophageal cancer therapy. Expert Opin Pharmacother. 12:225–239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lagergren J and Lagergren P: Oesophageal
cancer. BMJ. 341:c62802010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: MAGIC Trial Participants: Perioperative
chemotherapy versus surgery alone for resectable gastroesophageal
cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siewert JR, Lordick F, Ott K, Stein HJ,
Weber WA, Becker K, Peschel C, Fink U and Schwaiger M: Induction
chemotherapy in Barrett cancer: Influence on surgical risk and
outcome. Ann Surg. 246:624–628; discussion 628–631. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J, Simes J, Australasian Gastro-Intestinal and Trials
Group: Survival benefts from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong H and Chen L: B7-H1 pathway and its
role in the evasion of tumor immunity. J Mol Med (Berl).
81:281–287. 2003.
|
|
9
|
Flies DB and Chen L: The new B7s: Playing
a pivotal role in tumor immunity. J Immunother. 30:251–260. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharpe AH and Freeman GJ: The B7-CD28
superfamily. Nat Rev Immunol. 2:116–126. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene super-family, upon programmed cell death. EMBO J.
11:3887–3895. 1992.PubMed/NCBI
|
|
12
|
Nishimura H and Honjo T: PD-1: An
inhibitory immunoreceptor involved in peripheral tolerance. Trends
Immunol. 22:265–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tseng SY, Otsuji M, Gorski K, Huang X,
Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM and Tsuchiya H:
B7-DC, a new dendritic cell molecule with potent costimulatory
properties for T cells. J Exp Med. 193:839–846. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mahoney KM, Freeman GJ and McDermott DF:
The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in
melanoma. Clin Ther. 37:764–782. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massari F, Santoni M, Ciccarese C, Santini
D, Alfieri S, Martignoni G, Brunelli M, Piva F, Berardi R,
Montironi R, et al: PD-1 blockade therapy in renal cell carcinoma:
Current studies and future promises. Cancer Treat Rev. 41:114–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki H, Owada Y, Watanabe Y, Inoue T,
Fukuharav M, Yamaura T, Mutoh S, Okabe N, Yaginuma H, Hasegawa T,
et al: Recent advances in immunotherapy for non-small-cell lung
cancer. Hum Vaccin Immunother. 10:352–357. 2014. View Article : Google Scholar
|
|
20
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thompson RH, Kuntz SM, Leibovich BC, Dong
H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H,
et al: Tumor B7-H1 is associated with poor prognosis in renal cell
carcinoma patients with long-term follow-up. Cancer Res.
66:3381–3385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cordon-Cardo C, Fuks Z, Drobnjak M, Moreno
C, Eisenbach L and Feldman M: Expression of HLA-A,B,C antigens on
primary and metastatic tumor cell populations of human carcinomas.
Cancer Res. 51:6372–6380. 1991.PubMed/NCBI
|
|
25
|
Restifo NP, Esquivel F, Kawakami Y,
Yewdell JW, Mulé JJ, Rosenberg SA and Bennink JR: Identification of
human cancers deficient in antigen processing. J Exp Med.
177:265–272. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ebrahimi B, Tucker SL, Li D, Abbruzzese JL
and Kurzrock R: Cytokines in pancreatic carcinoma: Correlation with
phenotypic characteristics and prognosis. Cancer. 101:2727–2736.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pardoll D: Does the immune system see
tumors as foreign or self? Annu Rev Immunol. 21:807–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T,
Gajewski TF, Fu YX, Zheng P and Liu Y: B7DC/PDL2 promotes tumor
immunity by a PD-1-independent mechanism. J Exp Med. 197:1721–1730.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okazaki T, Maeda A, Nishimura H, Kurosaki
T and Honjo T: PD-1 immunoreceptor inhibits B cell
receptor-mediated signaling by recruiting src homology
2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc
Natl Acad Sci USA. 98:13866–13871. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Thompson RH, Gillett MD, Cheville JC,
Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen
L, et al: Costimulatory B7-H1 in renal cell carcinoma patients:
Indicator of tumor aggressiveness and potential therapeutic target.
Proc Natl Acad Sci USA. 101:17174–17179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakanishi J, Wada Y, Matsumoto K, Azuma M,
Kikuchi K and Ueda S: Overexpression of B7-H1 (PD-L1) significantly
associates with tumor grade and postoperative prognosis in human
urothelial cancers. Cancer Immunol Immunother. 56:1173–1182. 2007.
View Article : Google Scholar
|
|
32
|
Nomi T, Sho M, Akahori T, Hamada K, Kubo
A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, et al:
Clinical significance and therapeutic potential of the programmed
death-1 ligand/programmed death-1 pathway in human pancreatic
cancer. Clin Cancer Res. 13:2151–2157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Loos M, Giese NA, Kleeff J, Giese T, Gaida
MM, Bergmann F, Laschinger M, W Büchler M and Friess H: Clinical
significance and regulation of the costimulatory molecule B7-H1 in
pancreatic cancer. Cancer Lett. 268:98–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG and
Xu N: Immunohistochemical localization of programmed death-1
ligand-1 (PD-L1) in gastric carcinoma and its clinical
significance. Acta Histochem. 108:19–24. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating
CD8+ T lymphocytes are prognostic factors of human
ovarian cancer. Proc Natl Acad Sci USA. 104:3360–3365. 2007.
View Article : Google Scholar
|
|
36
|
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M,
Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al: Overexpression
of PD-L1 significantly associates with tumor aggressiveness and
postoperative recurrence in human hepatocellular carcinoma. Clin
Cancer Res. 15:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rosenberg SA: The immunotherapy of solid
cancers based on cloning the genes encoding tumor-rejection
antigens. Annu Rev Med. 47:481–491. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho Y, Miyamoto M, Kato K, Fukunaga A,
Shichinohe T, Kawarada Y, Hida Y, Oshikiri T, Kurokawa T, Suzuoki
M, et al: CD4+ and CD8+ T cells cooperate to
improve prognosis of patients with esophageal squamous cell
carcinoma. Cancer Res. 63:1555–1559. 2003.PubMed/NCBI
|
|
39
|
Schumacher K, Haensch W, Röefzaad C and
Schlag PM: Prognostic significance of activated CD8(+) T cell
infiltrations within esophageal carcinomas. Cancer Res.
61:3932–3936. 2001.PubMed/NCBI
|
|
40
|
Ropponen KM, Eskelinen MJ, Lipponen PK,
Alhava E and Kosma VM: Prognostic value of tumour-infiltrating
lymphocytes (TILs) in colorectal cancer. J Pathol. 182:318–324.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schroder K, Hertzog PJ, Ravasi T and Hume
DA: Interferon-gamma: An overview of signals, mechanisms and
functions. J Leukoc Biol. 75:163–189. 2004. View Article : Google Scholar
|
|
42
|
Chemnitz JM, Parry RV, Nichols KE, June CH
and Riley JL: SHP-1 and SHP-2 associate with immunoreceptor
tyrosine-based switch motif of programmed death 1 upon primary
human T cell stimulation, but only receptor ligation prevents T
cell activation. J Immunol. 173:945–954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Parry RV, Chemnitz JM, Frauwirth KA,
Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB
and Riley JL: CTLA-4 and PD-1 receptors inhibit T-cell activation
by distinct mechanisms. Mol Cell Biol. 25:9543–9553. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nurieva R, Thomas S, Nguyen T,
Martin-Orozco N, Wang Y, Kaja MK, Yu XZ and Dong C: T-cell
tolerance or function is determined by combinatorial costimulatory
signals. EMBO J. 25:2623–2633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Riley JL: PD-1 signaling in primary T
cells. Immunol Rev. 229:114–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rozali EN, Hato SV, Robinson BW, Lake RA
and Lesterhuis J: Programmed death ligand 2 in cancer-induced
immune suppression. Clin Dev Immunol. 2012(656340)2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsumoto K, Inoue H, Nakano T, Tsuda M,
Yoshiura Y, Fukuyama S, Tsushima F, Hoshino T, Aizawa H, Akiba H,
et al: B7-DC regulates asthmatic response by an IFN-gamma-dependent
mechanism. J Immunol. 172:2530–2541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ishiwata K, Watanabe N, Guo M, Tomihara K,
Brumlik MJ, Yagita H, Pardoll D, Chen L and Shin T: Costimulator
B7-DC attenuates strong Th2 responses induced by Nippostrongylus
brasiliensis. J Immunol. 184:2086–2094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang S, Bajorath J, Flies DB, Dong H,
Honjo T and Chen L: Molecular modeling and functional mapping of
B7-H1 and B7-DC uncouple costimulatory function from PD-1
interaction. J Exp Med. 197:1083–1091. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang W, Chen PW, Li H, Alizadeh H and
Niederkorn JY: PD-L1: PD-1 interaction contributes to the
functional suppression of T-cell responses to human uveal melanoma
cells in vitro. Invest Ophthalmol Vis Sci. 49:2518–2525. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|