Introduction
Breast cancer is the most frequently diagnosed
cancer and the second leading cause of cancer-related deaths among
women worldwide (1). The high
breast cancer mortality rates are mainly caused by the metastasis
of tumor cells (2), which can
induce radiation therapy and chemotherapy resistance in advanced
breast cancer. Therefore, the therapeutic strategies including the
development of effective antimetastatic agents have potential
benefits for breast cancer treatment.
Growing evidence indicates that the epithelial
mesenchymal transition (EMT), a developmental process which
involves loss of cell-cell junctions and re-organization of the
actin cytoskeleton, resulting in loss of apical-basal polarity and
acquisition of a spindle-like mesenchymal morphology, plays an
important role in breast cancer metastasis (3,4).
E-cadherin, a key factor included in EMT, has been associated with
the metastasis process in breast cancer. The loss function of
E-cadherin elicits active signals that induce tumor cell migration,
invasion and metastatic dissemination (5,6).
Moreover, levels of E-cadherin have also been found to correlate
with enhanced metastasis and associate with poor prognosis and
relapse in breast cancer patients (7). Transcription of E-cadherin is
repressed by zinc finger proteins of the Slug/Snail family and
Smad-interacting protein (8,9). To
date, numerous clinicopathological studies have shown positive
correlations between the expressions of the transcription factors
Snail and Slug, which are the key inducible factors of EMT, and
poor clinical outcomes in breast, ovary, colorectal and lung cancer
(10).
Reactive oxygen species (ROS), continuously
generated from mitochondrial respiratory chain during intracellular
metabolism, are a family of molecules in response to environmental
stimuli, including superoxide radical (O2−), hydrogen
peroxide (H2O2), hydroxyl radical (·OH) and
singlet oxygen. Accumulating evidence suggests that ROS are
emerging as critical signaling stimuli that mediate a variety
cellular functions including cell cycle progression, apoptosis and
motility (11–13). ROS signaling pathway has been
reported to be intimately involved with EMT in tumor progression
(14,15). Various factors such as epidermal
growth factor (EGF), hepatocyte growth factor (HGF), insulin-like
growth factor (IGF)-I and interleukin-1β (IL-1β) induce EMT in the
progression of tumor cells through ROS generation (16–19).
It is also suggested that ROS play an essential role in TPA-induced
sustained PKC-ERK activation which is responsible for EMT and
migration of HepG2 (20). Another
study showed that long-term oxidative stress may induce invasive
potential of mammary epithelial cells (21).
Hispolon, a poly-phenol compound, which was first
isolated from Inonotus hispidus and has since been isolated
from many tropical mushrooms (22,23).
Previous studies have shown that hispolon inhibits tumor growth and
metastasis through various signal pathways (22,24–26).
Also we have shown that hispolon-induced apoptosis in human gastric
cancer cells through a ROS-mediated mitochondrial pathway (22,24).
However, whether hispolon could exert anti-migration activity in
breast cancer cells as well as the underlying mechanisms have not
been elucidated. In the present study, we found that hispolon
inhibited TPA-induced migration of MCF-7 cells by recovering
E-cadherin activity. In addition, this effect was dependent on the
ROS/p-ERK/slug/E-cadherin related signaling pathway.
Materials and methods
Materials and cell culture
Transwell chambers were purchased from Corning
Costar (USA). Antibodies specific for ERK, phospho-ERK, JNK,
phospho-JNK, p38, phospho-p38 MAPK, AKT, phosphor-AKT, Snail, Slug
and E-cadherin were all purchased from Cell Signaling Technology
(Beverly, MA, USA). Human breast cancer MCF-7 cells were maintained
in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100
U/ml penicillin G and streptomycin 100 mg/ml, and were incubated at
37°C in a humidified atmosphere of 95% air and 5%
CO2.
Chemicals and inhibitors
Hispolon was synthesized as previously described and
its purity was established on the basis of the spectral
(1H, 13C NMR and mass) data. Hispolon stocks
(20 mM) were prepared in dimethylsulphoxide (DMSO) and stored at
−20°C. TPA and NAC were purchased from Sigma (St. Louis, MO, USA).
SB203580 (p38 inhibitor), SP600125 (JNK inhibitor) and LY294002
(AKT inhibitor) were obtained from Calbiochem (San Diego, CA, USA).
U0126 (ERK inhibitor) was from Cell Signaling Technology.
Cell viability assay
The effect of hispolon on the viability of MCF-7
cells was evaluated using MTT method. Briefly, cells were grown in
96-well microtiter plates for drug treatment. Following incubation
for the indicated times, cells were incubated with MTT (0.5 mg/ml)
for 4 h. The formazan precipitate was dissolved in 150 µl
DMSO, and the absorbance was detected at 490 nm with a Model ELX800
microplate reader (Bio-Tek Instruments). Each test was performed in
triplicate experiments.
Cell migration assay
The cell migration assay was conducted using
Transwell chambers according to the manufacturer's instructions.
Briefly, 5×104 MCF-7 cells suspended in 500 µl of
serum-free medium and seeded into the upper chamber of the inserts.
After treatment with different concentrations of hispolon, 750
µl of serum-free medium containing 100 ng/ml of TPA was
added to the bottom wells as a chemoattractant. The chambers were
incubated at 37°C for 24 h. After incubation, the filter inserts
were removed from the wells and the cells on the upper side of the
filter were removed using cotton swabs. Cells that had invaded to
the underside of the filter were first fixed with methanol (15
min), and then stained with 2% ethanol containing 0.2% crystal
violet powder (15 min). After being dried, the stained cells were
enumerated under light microscope at ×10 objective.
Intracellular ROS detection
DCFH-DA is a cell-permeable fluorescent dye specific
for ROS and is used to detect intracellular ROS levels. Cells were
seeded in 12-well plates and were cultured in serum-free medium for
24 h, and then treated with 100 ng/ml TPA for different periods of
time. At the designated time points, cells were washed twice with
phosphate-buffered saline (PBS) and fixed with 10% formaldehyde for
10 min. Intracellular ROS levels were assessed by staining with
DCFH-DA (5 µM) for 30 min at 37°C. Fluorescent images were
captured using an inverted fluorescence microscope (Nikon Eclipse
TE300; Nikon, Melville, NY, USA).
Western blotting
Total cell extracts were prepared using an M-PER
mammalian protein extraction reagent kit (Pierce) according to the
manufacturer's instructions. The protein concentration of each
extract was determined by the Bradford assay. Cell extracts were
separated by electrophoresis on 6–15% sodium dodecyl
sulfate-polyacrylamide gel (SDS-PAGE) and then transferred to
nitrocellulose membranes. The membranes were probed with a primary
antibody followed by a secondary antibody conjugated to horseradish
peroxidase. Protein bands on the membranes were detected by
enhanced chemiluminescence [Western Lightning (Perkin-Elmer Boston,
MA, USA) or SuperSignal West Femto (Pierce, Rockford, IL,
USA)].
Statistical analysis
Numerical data are presented as means ± SD of
different determinations. Statistical significance between
treatment and control groups was analyzed using Student's t-test.
Values of p<0.05 were considered to indicate a statistically
significant result.
Results
TPA downregulates E-cadherin expression
through the production of ROS in MCF-7 cells
12-O-Tetradecanoyl-phorbol-13-acetate (TPA)
is one of the most utilized agents for studying the mechanisms of
carcinogenesis. To characterize the effect of TPA on cell
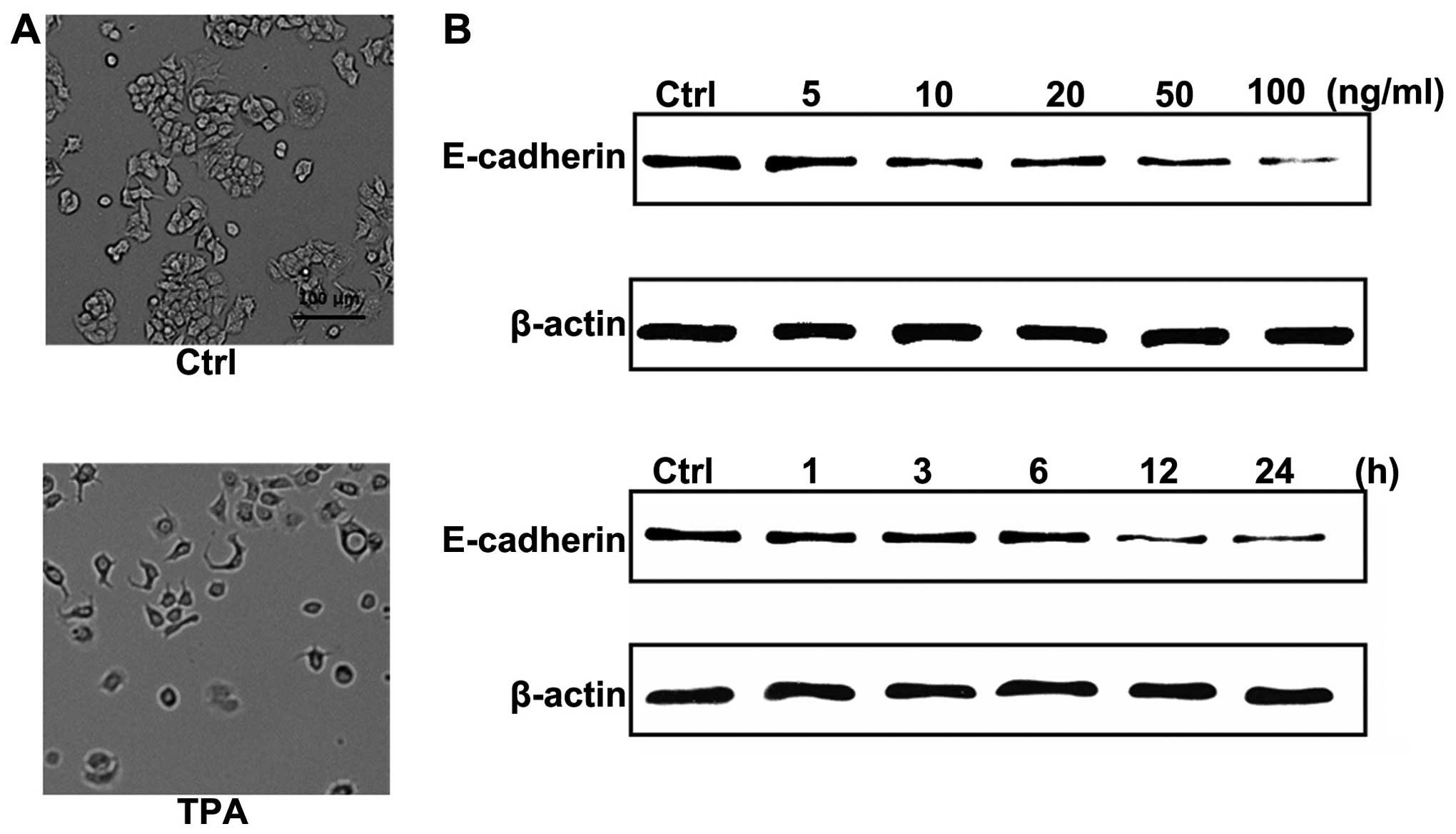
morphology in human breast cancer MCF-7 cells, we treated cells
with TPA (100 ng/ml) for 24 h. As shown in Fig. 1A, treatment with TPA-induced an
obvious morphological change, from a cobblestone-like morphology to
fibroblastic-spindle shape, which is a typical morphology change in
EMT. To further confirm the presence of EMT and investigate the
molecular mechanism by which TPA-induced the morphological change,
we investigated the expression of E-cadherin which plays a key role
during EMT. Western blot analysis showed that TPA downregulated
total E-cadherin protein levels in a dose- and time-dependent
manner in MCF-7 cells (Fig.
1B).
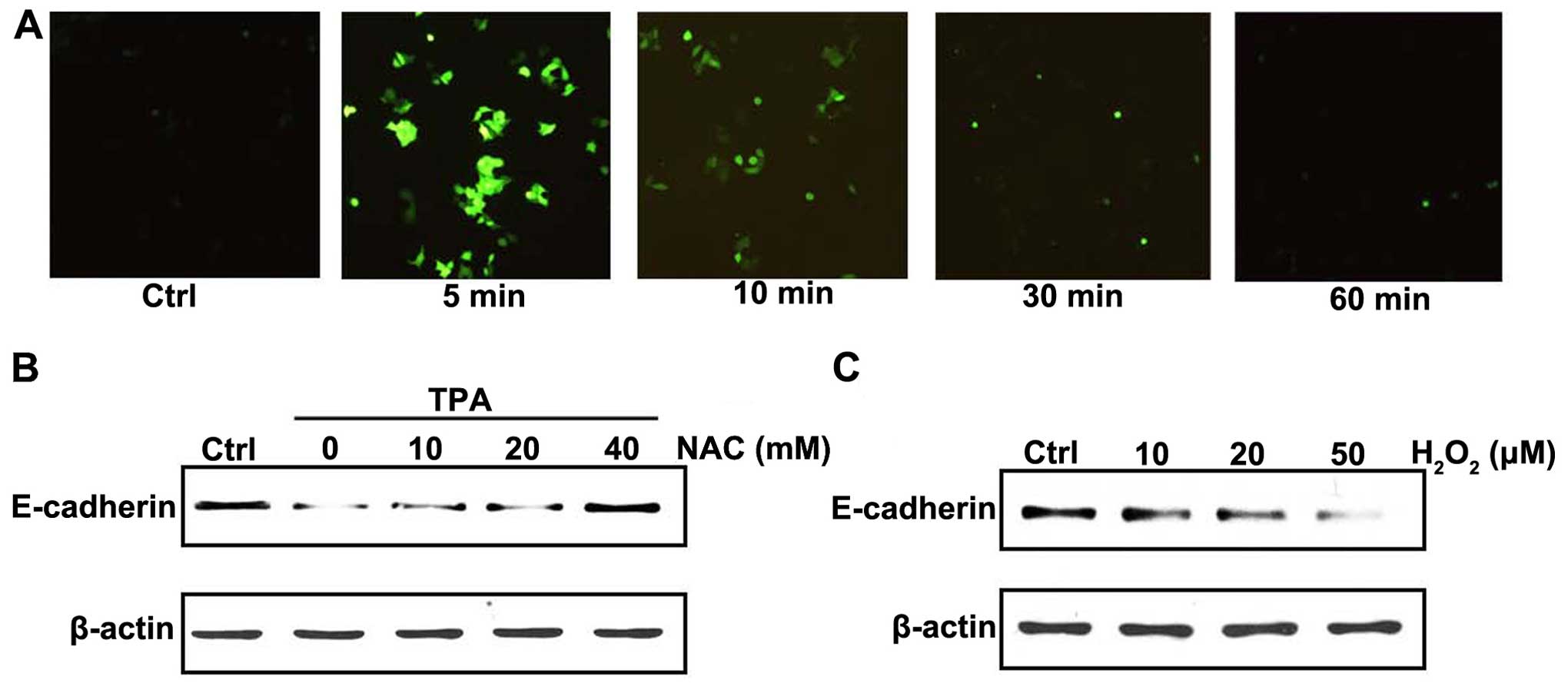
DCFH-DA was used to examine the effect of TPA
treatment on intracellular ROS production. TPA treatment induced
ROS production in a time-dependent manner in MCF-7 cells (Fig. 2A). To confirm the relation of ROS
production and E-cadherin expression, cells were pretreated with
NAC, an antioxidant reagent. As shown in Fig. 2B, downregulation of E-cadherin
induced by TPA was inhibited by pretreating NAC. To further confirm
the effect of ROS on E-cadherin expression, MCF-7 cells were
treated with exogenous H2O2. Notably,
H2O2 treatment downregulated E-cadherin
protein levels in a dose-dependent manner (Fig. 2C). These results suggested that the
effect of TPA on E-cadherin in MCF-7 cells was closely related with
the production of intracellular ROS.
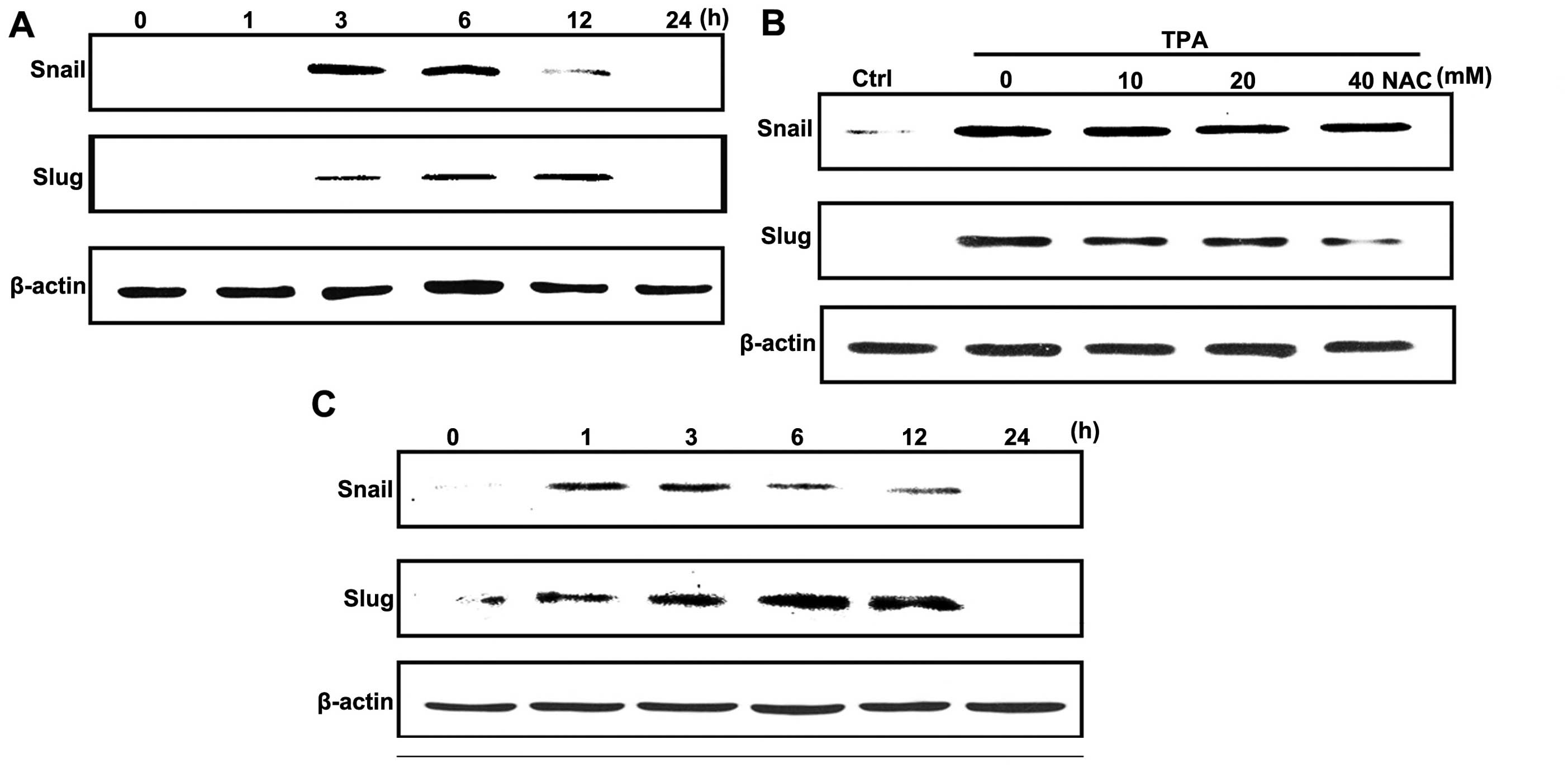
Slug expression is upregulated by
TPA-induced ROS production
To investigate whether TPA downregulated E-cadherin
expression by modulating the transcriptional regulation of
E-cadherin, we examined the protein levels of E-cadherin
transcriptional repressors, Snail and Slug. Treatment with TPA
significantly increased Snail and Slug expression in a
time-dependent manner (Fig. 3A). To
determine whether ROS production was involved in TPA-induced
increasing levels of Snail and Slug proteins, cells were pretreated
with NAC in the presence of TPA. As shown in Fig. 3B, NAC pretreatment diminished
TPA-stimulated Slug levels, whereas the increase in Snail levels
were not affected. Notably, exogenous H2O2
increased both Snail and Slug levels in a different manner compared
to TPA (Fig. 3C).
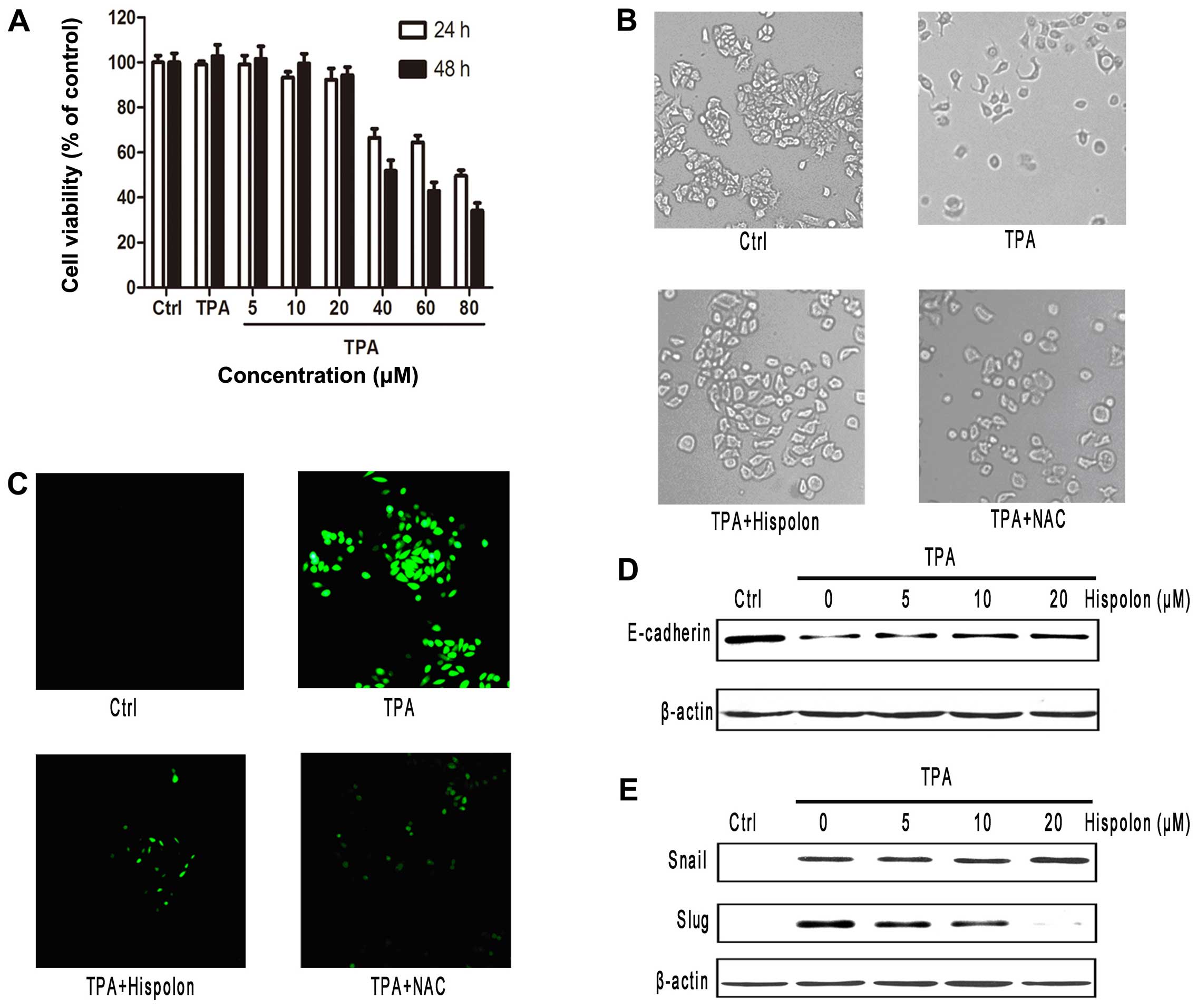
Hispolon inhibits TPA-induced EMT
Hispolon is a natural polyphenol compound isolated
from medical fungus. We first evaluated the effect of hispolon on
the viability of breast cancer MCF-7 cells. At the concentrations
tested, between 5 and 20 µM, for duration of 24 h, hispolon
demonstrated negligible antiproliferative effects on the cells
(Fig. 4A). To ascertain that any
possible anti-migration effects of hispolon observed was not due to
their antiproliferative activities, nonlethal concentrations (≤20
µM) were used for the following experiments.
From the morphological aspect, hispolon markedly
inhibited the TPA-induced morphologic change. Following treatment
with TPA, MCF-7 cells acquired spindle-like cell morphology.
Notably, pretreatment with 20 µM hispolon significantly
inhibited TPA-induced changes in cell morphology (Fig. 4B). To show whether hispolon
inhibited the change of cell morphology by expression of
E-cadherin, we examined the protein level of E-cadherin by western
blotting in dose-response analysis. As shown in Fig. 4D, downregulation of E-cadherin was
clearly abolished by hispolon in a dose-dependent manner. Since
Snail and Slug are considered to be master regulators of
E-cadherin, the protein expression levels of transcription factors
were evaluated by western blotting. The Slug expression level was
upregulated by TPA stimulation in MCF-7 cells, which was abolished
by hispolon in a dose-dependent manner. However, the expression of
Snail was not affected by hispolon (Fig. 4E).
To determine whether ROS production was involved in
hispolon against TPA-induced EMT, we examined hispolon-mediated
cellular ROS level changes. The cells were pretreated with the
antioxidant reagent NAC followed by treatment with TPA. Strikingly,
the cellular ROS levels were reduced after pretreatment with
hispolon (Fig. 4C). As shown in
Fig. 4B and C, NAC decreased the
intracellular ROS levels and inhibited the morphological change
which was induced by treatment of TPA in MCF-7 cells. These results
demonstrated that hispolon inhibited TPA-induced EMT through
repressing the ROS production.
Hispolon inhibits TPA-induced EMT by
activation of ERK
Numerous studies have demonstrated that tumor
metastasis originates from abnormal regulation of the activation of
intracellular signal molecules such as mitogen-activated protein
kinase and tyrosine kinase (MAPK), including ERK, p38, c-Jun
N-terminal kinase (JNK) and AKT kinase which are important
downstream signaling cascades involved in tumor cell migration.
Herein, we examined the expressions of phospho-AKT, AKT,
phospho-MAPK and MAPK expression in MCF-7 cells. The
phosphorylation levels of AKT and MAPK were increased by TPA
time-dependently. A time-course analysis showed that the
phosphorylation levels of AKT and MAPK peaked at 5–30 min after TPA
stimulation and gradually recovered to the baseline values 3 h
later (Fig. 5A). These results
indicated that AKT and MAPK signaling pathways were actually
capable of being activated in response to TPA in MCF-7 cells.
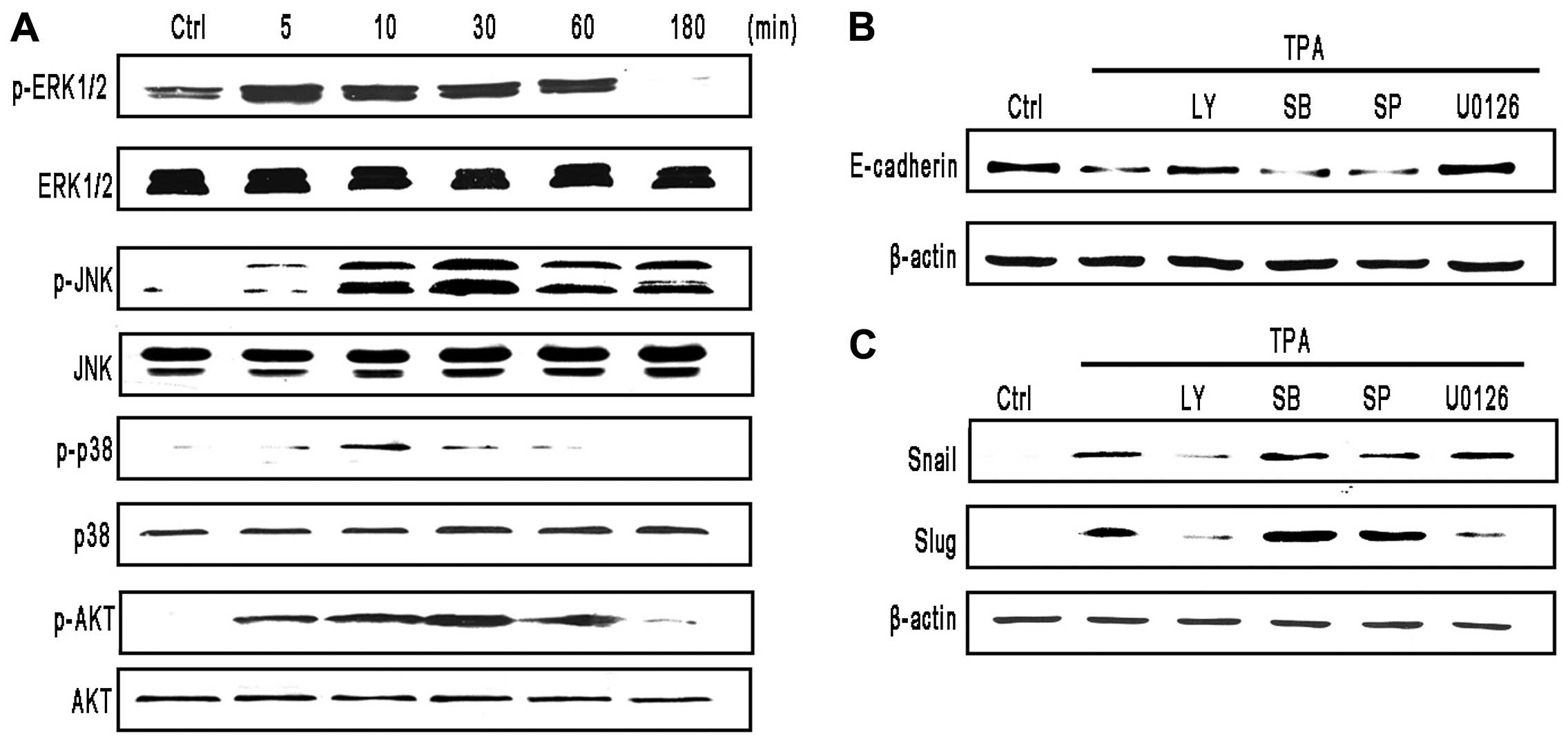 | Figure 5TPA downregulates E-cadherin
expression by ERK and AKT signaling pathway. (A) TPA activates the
MAPK signaling pathway. Cells were treated with 100 ng/ml TPA for
the indicated durations. Phosphorylation of ERK, p38, JNK and AKT
were determined by western blotting using antibodies specific for
phosphorylated, activated forms of ERK (p-ERK), p38 (p-p38), JNK
(p-JNK) and AKT (p-AKT). Membranes were stripped and reprobed with
antibodies to total ERK, p38, JNK and AKT. (B and C) MCF-7 cells
were pretreated with selective MAPK pathway inhibitors, LY294002
(LY) (20 µM), SB203580 (SB) (20 µM), SP600125 (SP)
(20 µM), U0126 (20 µM) for 3 h, followed by treatment
with 100 ng/ml TPA, (B) for 24 h (E-cadherin) (C) for 3 h (Snail)
or 12 h (Slug) and protein levels were analyzed by western
blotting. |
To assess whether MAPK and AKT activation were
involved in TPA-induced EMT, cells were pretreated with special
kinase inhibitors. Notably, we found that the AKT inhibitor
LY294002 and the ERK inhibitor U0126 diminished the inhibitory
effects of TPA on E-cadherin protein levels, but the p38 MAPK and
JNK inhibitor did not (Fig. 5B).
Furthermore, as shown in Fig. 5C,
treatment with the ERK inhibitor U0126 diminished the upregulation
of TPA-induced Slug. Treatment with the AKT inhibitor LY294002
diminished the upregulation of TPA-induced Snail and Slug. To some
extent, ERK and AKT kinases were involved in TPA-induced EMT.
To investigate whether ROS production, ERK and AKT
activation, and the subsequent upregulation of transcription
factors were involved in hispolon inhibiting TPA-induced EMT, cells
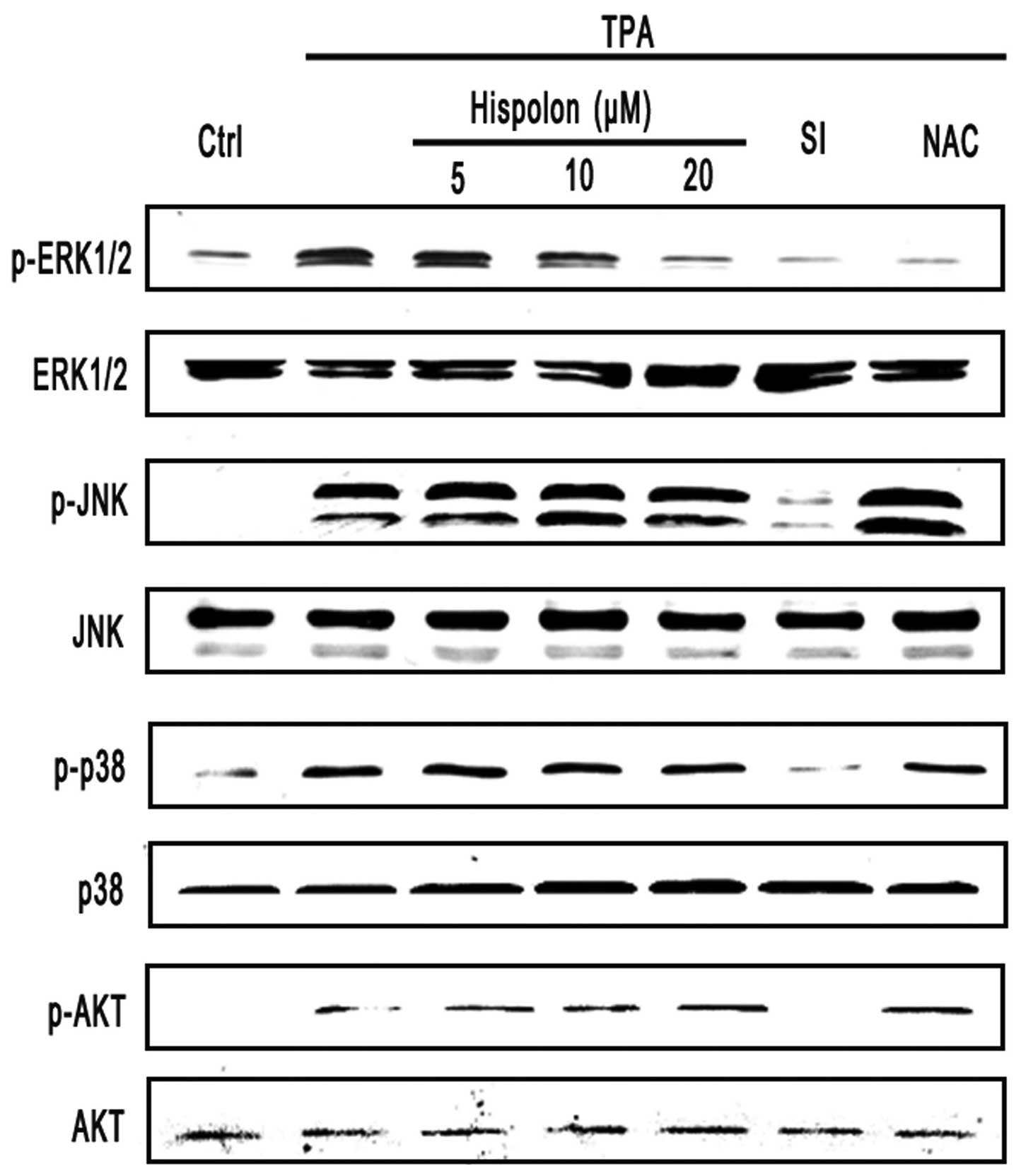
were pretreated with hispolon. Notably, we found that hispolon
markedly diminished TPA-induced ERK phosphorylation but did not
affect TPA-induced phosphorylation of p38 MAPK, JNK and AKT
(Fig. 6). Notably, treatment with
antioxidant NAC showed similar phenomenon (Fig. 6). Taken together, these results
suggested that hispolon down-regulated Slug expression via ERK
signaling by inhibiting the production of intracellular ROS.
Hispolon inhibits TPA-induced cell
migration
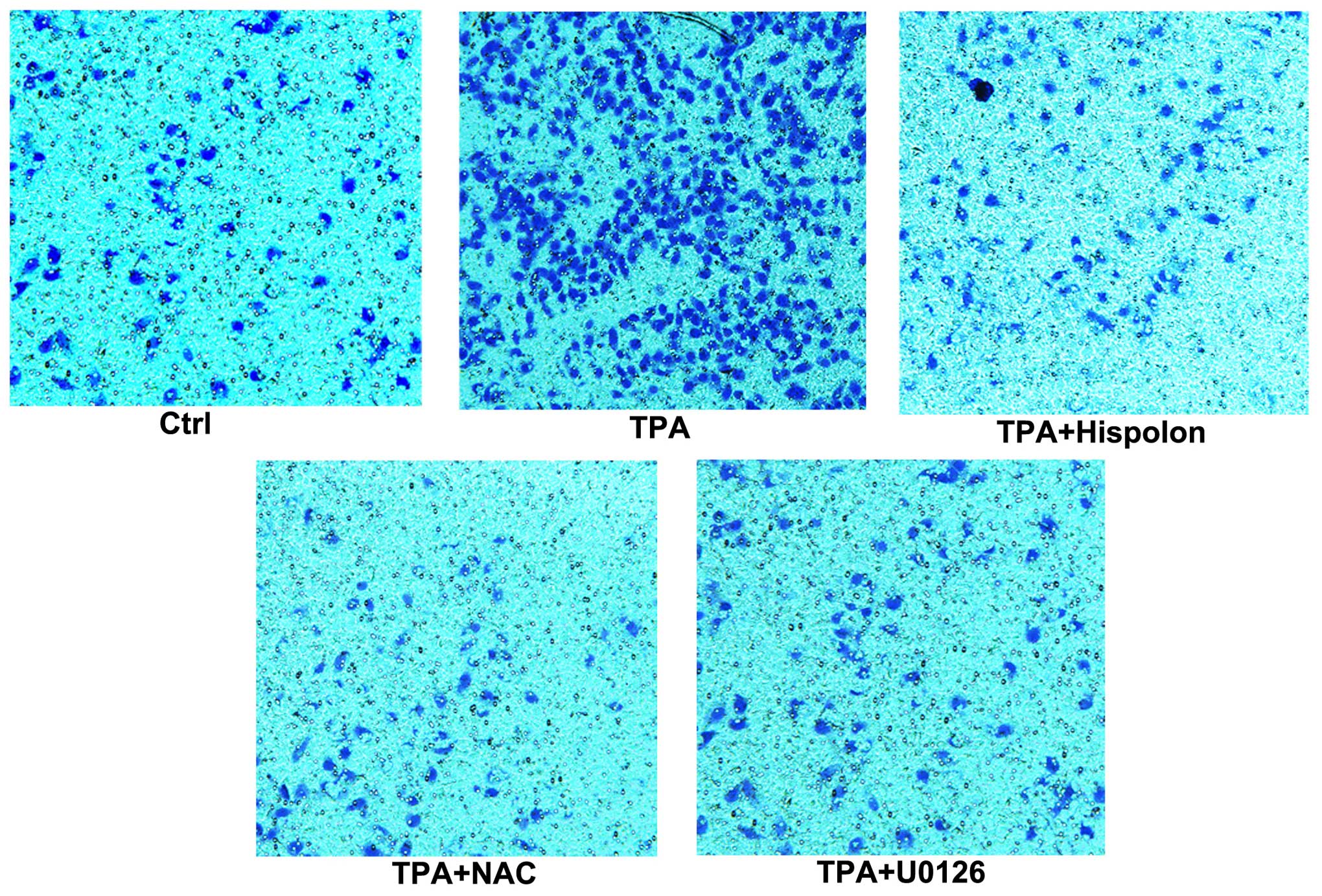
Since hispolon inhibited the TPA-induced EMT in
MCF-7 cells, we next examined whether the inhibitory effect of
hispolon on EMT led to an inhibition of cellular migration in MCF-7
cells. As shown in Fig. 7,
migration assay revealed that hispolon reduced TPA-mediated
cellular migration. U0126 and NAC inhibited TPA-induced cell
migration in MCF-7 cells and to the same extent by hispolon. These
results suggested that hispolon actually inhibits the cellular
migration phenotype of the EMT in MCF-7 cells. It is assumed that
the inhibitory activity of hispolon on the cellular migration
phenotype is due to its inhibitory effect of ROS-ERK signaling
pathway.
Taken together, these results indicated that
hispolon inhibits the TPA-induced EMT, and is characterized by
E-cadherin downregulation, morphologic changes and an increase in
the cellular migrating phenotype by inhibiting
ROS/ERK/Slug/E-cadherin pathway in MCF-7 cells.
Discussion
Metastasis is a major obstacle for cancer therapy
and is a primary cause of mortality in many cancer types, including
breast cancer. TPA is a well-known tumor promoter and exhibits many
biological effects by altering gene expression. Previous studies
revealed that TPA was a potential inducer of tumor
invasion/migration in breast cancer cells and EMT in human prostate
cancer ARCaPE cells (27,28).
Additionally, TPA has been shown to downregulate E-cadherin
expression in human cancer cells, resulting in tumor metastasis
(29,30). Moreover, studies showed that ROS are
essential mediators of TPA-induced cell migration and invasion
(20,31). However, the specific role of ROS in
the downregulation of E-cadherin expression caused by TPA signaling
remains to be elucidated. In the present study, we reported that
ROS-mediated TPA-induced E-cadherin downregulation and cell
migration. ROS exerted their effects via activating ERK and
increasing Slug expression. In addition, our studies suggested that
hispolon, a natural polyphenol compound, could inhibit TPA-induced
cell migration through ROS/ERK/Slug/E-cadherin signaling
pathway.
ROS generation is induced by various growth factors,
cytokines and tumor promoters (17,18,32).
The contribution of an elevation in ROS to carcinogenesis was
detected in several different types of cancer cells, and the
ROS-mediated signaling cascade in tumor metastasis was highlighted.
However, the importance of ROS generation in TPA-induced migration
of breast cancer cells is still undefined. In the present study,
increasing ROS generation followed by cell migration, MAPK
activation and loss of E-cadherin expression were observed in
TPA-treated MCF-7 breast cells, and they were blocked by addition
of the antioxidant NAC through reduction in ROS. These results
suggest that the production of ROS may be a critical mediator in
TPA-induced EMT, and TPA-induced ROS generation was an initial
event in the progression of breast cancer metastasis. Notably, the
time course of exogenous H2O2-induced Slug
expression was significantly different from that of TPA. Moreover,
NAC abolished TPA-induced expression of Slug in MCF-7 cells, but
treatment with exogenous H2O2 increased both
expression of Snail and Slug. These results indicated that the
functional consequences of TPA-induced endogenous ROS signaling
differed from those of exogenous H2O2
treatment. Previous research showed that NF-κB rather than ROS play
a more important role in the TNF-α-induced EMT of MCF-7 cells
(33). However, our present study
suggested EMT transition was mainly due to the production of
cellular ROS stimulated by TPA.
Loss of E-cadherin gene expression, which is the
hallmark of epithelial-mesenchymal transition (EMT), is mainly due
to upregulation of E-cadherin repressors such as Snail and Slug.
Indeed, ectopic expression of Snail or Slug in SKOV3 cells results
in EMT associated enhanced motility, invasiveness and
tumorigenicity (34). In the
present study, we found that TPA could induce Snail and Slug
expression by producing intracellular ROS. However, treatment with
the antioxidant agent NAC totally blocked ROS production but only
partially abolished the TPA-induced EMT and cell migration and did
not inhibit TPA-induced Snail expression. Thus, we predicted that
other ROS independent pathways may contribute to Snail induction
and mediate TPA-induced E-cadherin downregulation. Additionally, we
also found hispolon and NAC abolished TPA-induced expression of
Slug, but not Snail in MCF-7 cells. Although Slug is thought to
function in a redundant manner with Snail, several recent studies
suggested unique functions of Slug. i) Slug was essential for
Notch1:Jagged 1-mediated EMT, tumor growth and metastasis (35); ii) Slug expression was correlated
with poor breast cancer prognosis (36); iii) Slug, but not Snail or Twist was
expressed in ES cells and was part of the ES cell signature
activated in several cancer cells (37); iv) Slug but not Snail expression,
was linked to ductal development in the breast including tubule
maintenance or growth within invasive ductal carcinoma (38). These observations along with the
results of the present study highlighted critical role of Slug in
breast cancer metastasis.
MAPK and AKT cascades are major signaling pathways
which can drive the metastasis of tumor cells (39). It was demonstrated that increased
ROS levels may enhance MAPK activities in the malignant progression
of cancer cells (17,19). However, the role of ROS in MAPK
activation induced cell migration is not clear. In the present
study, TPA treatment significantly increased ERK, p38 and JNK
expression in breast cancer cells. However, only TPA-induced
activation of ERK was inhibited by NAC treatment. In addition,
E-cadherin inhibition by U0126 significantly reduced TPA-induced
breast cancer cell migration. Taken together, our results indicated
that ROS-dependent ERK activation was involved in TPA-induced
E-cadherin downregulation and cell invasion in breast cancer cells.
AKT kinase is a convergence point for multiple extracellular and
other upstream signals, functioning as a master switch to generate
a plethora of intracellular signals and responses. Downstream
targets of AKT are thought to be involved in survival, growth,
metastasis and metabolic-related pathways (40,41).
Activation of AKT signaling has been detected in cells undergoing
EMT (42). In the present study,
phosphorylation of AKT was not inhibited by pretreatment with
hispolon. The AKT kinase inhibitor LY294002 prevented TPA-induced
upregulation of Snail or Slug, or downregulation of E-cadherin,
suggesting the AKT pathway was not involved in the antimetastasis
effect of hispolon. These results indicated that a different signal
pathway may be involved in the EMT progression, and ERK pathway was
the major mediator in MCF-7 cells treated by hispolon.
For centuries, people have been harnessing the power
of nature to provide medicinal solutions to various diseases. In
particular, rich sources of natural compounds isolated from plants
have potential anticancer activities. Various well-known naturally
occurring agents, such as resveratrol (43), quercetin (44), curcumin (45) and gingerol (46,47)
have been demonstrated to exert inhibitory effects on cancer cell
invasion and metastasis. Hispolon, isolated from medical fungus, is
a natural polyphenol compound with low toxicity, have antiviral,
antiproliferative and immunomodulatory activities (24,25,48).
Moreover, numerous studies have shown the antiproliferation
activity of hispolon in many types of human cancers. Our previous
research showed hispolon could inhibit TPA-induced migration and
invasion of MDA-MB-231 cells by reducing MMP-9 secretion and
expression, mainly through the NF-κB signaling pathway (49). In the present study, we confirmed
that hispolon can inhibit the migration of MCF-7, breast cancer
cells by a completely different mechanism. Previous literature show
ER signaling may globally regulate the EMT program (50) and loss of ER may change the
expression profile of specific matrix macromolecules (51). Moreover, hispolon inhibited cell
growth through modulation of ER in estrogen-positive breast cancer
cells (52). Regarding different ER
status of the two breast cancer cell types involved in our
research, the different mechanism of anti-migration of hispolon may
depend on the different ER status. Although further studies are
needed to establish the exact signal pathway, our results taken
together strengthen the potential of hispolon as a multitarget drug
in anticancer therapy.
In the present study, we demonstrated that hispolon
inhibited migration of breast cancer MCF-7 cells through
E-cadherin. Hispolon could downregulate Slug by inhibiting ERK
signaling, thereby inhibiting the TPA-mediated EMT in breast cancer
cells. Our results provided new insight into the mechanisms of
hispolon inhibition of cancer cell metastasis and suggested that
hispolon could be a potential agent for treatment of breast
cancers.
Acknowledgments
The present study was supported by the following
grants: the Natural Science Foundation of Zhejiang Province (grant
no. LQ14H160016), and the Zhejiang Province Program for the
Cultivation of High-level Innovative Health Talents (2012).
Abbreviations:
|
MAPK
|
mitogen-activated protein kinase
|
|
TPA
|
phorbol 12-myristate 13-acetate
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
ERK
|
extracellular signal-regulated kinase
AKT, protein kinase B
|
|
NAC
|
N-acetyl-cysteine
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weigelt B, Hu Z, He X, Livasy C, Carey LA,
Ewend MG, Glas AM, Perou CM and Van't Veer LJ: Molecular portraits
and 70-gene prognosis signature are preserved throughout the
metastatic process of breast cancer. Cancer Res. 65:9155–9158.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsuji T, Ibaragi S and Hu GF:
Epithelial-mesenchymal transition and cell cooperativity in
metastasis. Cancer Res. 69:7135–7139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyoshi J and Takai Y: Structural and
functional associations of apical junctions with cytoskeleton.
Biochim Biophys Acta. 1778:670–691. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
7
|
Doyle S, Evans AJ, Rakha EA, Green AR and
Ellis IO: Influence of E-cadherin expression on the mammographic
appearance of invasive nonlobular breast carcinoma detected at
screening. Radiology. 253:51–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu WS, Wu JR and Hu CT: Signal cross talks
for sustained MAPK activation and cell migration: The potential
role of reactive oxygen species. Cancer Metastasis Rev. 27:303–314.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boonstra J and Post JA: Molecular events
associated with reactive oxygen species and cell cycle progression
in mammalian cells. Gene. 337:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu CT, Wu JR, Cheng CC, Wang S, Wang HT,
Lee MC, Wang LJ, Pan SM, Chang TY and Wu WS: Reactive oxygen
species-mediated PKC and integrin signaling promotes tumor
progression of human hepatoma HepG2. Clin Exp Metastasis.
28:851–863. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Z, Li Y and Sarkar FH: Signaling
mechanism(s) of reactive oxygen species in epithelial-mesenchymal
transition reminiscent of cancer stem cells in tumor progression.
Curr Stem Cell Res Ther. 5:74–80. 2010. View Article : Google Scholar
|
|
16
|
Hwang YS, Jeong M, Park JS, Kim MH, Lee
DB, Shin BA, Mukaida N, Ellis LM, Kim HR, Ahn BW, et al:
Interleukin-1beta stimulates IL-8 expression through MAP kinase and
ROS signaling in human gastric carcinoma cells. Oncogene.
23:6603–6611. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CW, Yang LY, Shen SC and Chen YC:
IGF-I plus E2 induces proliferation via activation of ROS-dependent
ERKs and JNKs in human breast carcinoma cells. J Cell Physiol.
212:666–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Binker MG, Binker-Cosen AA, Richards D,
Oliver B and Cosen-Binker LI: EGF promotes invasion by PANC-1 cells
through Rac1/ROS-dependent secretion and activation of MMP-2.
Biochem Biophys Res Commun. 379:445–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KH and Kim JR: Reactive oxygen species
regulate the generation of urokinase plasminogen activator in human
hepatoma cells via MAPK pathways after treatment with hepatocyte
growth factor. Exp Mol Med. 41:180–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu WS, Tsai RK, Chang CH, Wang S, Wu JR
and Chang YX: Reactive oxygen species mediated sustained activation
of protein kinase C alpha and extracellular signal-regulated kinase
for migration of human hepatoma cell Hepg2. Mol Cancer Res.
4:747–758. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori K, Shibanuma M and Nose K: Invasive
potential induced under long-term oxidative stress in mammary
epithelial cells. Cancer Res. 64:7464–7472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, He FY and Li YQ: The apoptosis
effect of hispolon from Phellinus linteus (Berkeley & Curtis)
Teng on human epidermoid KB cells. J Ethnopharmacol. 105:280–285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mo S, Wang S, Zhou G, Yang Y, Li Y, Chen X
and Shi J: Phelligridins C-F: Cytotoxic
pyrano[4,3-c][2]benzopyran-1,6-dione and furo[3,2-c]pyran-4-one
derivatives from the fungus Phellinus igniarius. J Nat Prod.
67:823–828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen W, Zhao Z, Li L, Wu B, Chen SF, Zhou
H, Wang Y and Li YQ: Hispolon induces apoptosis in human gastric
cancer cells through a ROS-mediated mitochondrial pathway. Free
Radic Biol Med. 45:60–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang GJ, Deng JS, Huang SS and Hu ML:
Hispolon induces apoptosis and cell cycle arrest of human
hepatocellular carcinoma Hep3B cells by modulating ERK
phosphorylation. J Agric Food Chem. 59:7104–7113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang GJ, Yang CM, Chang YS, Amagaya S,
Wang HC, Hou WC, Huang SS and Hu ML: Hispolon suppresses SK-Hep1
human hepatoma cell metastasis by inhibiting matrix
metallo-proteinase-2/9 and urokinase-plasminogen activator through
the PI3K/Akt and ERK signaling pathways. J Agric Food Chem.
58:9468–9475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SY, Kim YH, Kim Y and Lee SJ:
Frondoside A has an anti-invasive effect by inhibiting TPA-induced
MMP-9 activation via NF-κB and AP-1 signaling in human breast
cancer cells. Int J Oncol. 41:933–940. 2012.PubMed/NCBI
|
|
28
|
Noh EM, Lee YR, Hur H and Kim JS: Radix
clematidis extract inhibits TPA-induced MMP-9 expression by
suppressing NF-κB activation in MCF-7 human breast cancer cells.
Mol Med Rep. 4:879–883. 2011.PubMed/NCBI
|
|
29
|
Wen-Sheng W: ERK signaling pathway is
involved in p15INK4b/p16INK4a expression and
HepG2 growth inhibition triggered by TPA and Saikosaponin a.
Oncogene. 22:955–963. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He H, Davidson AJ, Wu D, Marshall FF,
Chung LW, Zhau HE, He D and Wang R: Phorbol ester
phorbol-12-myristate-13-acetate induces epithelial to mesenchymal
transition in human prostate cancer ARCaP cells. Prostate.
70:1119–1126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Radisky DC, Levy DD, Littlepage LE, Liu H,
Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et
al: Rac1b and reactive oxygen species mediate MMP-3-induced EMT and
genomic instability. Nature. 436:123–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Traore K, Sharma RB, Burek CL and Trush
MA: Role of ROS and MAPK in TPA-induced ICAM-1 expression in the
myeloid ML-1 cell line. J Cell Biochem. 100:1010–1021. 2007.
View Article : Google Scholar
|
|
33
|
Dong R, Wang Q, He XL, Chu YK, Lu JG and
Ma QJ: Role of nuclear factor kappa B and reactive oxygen species
in the tumor necrosis factor-alpha-induced epithelial-mesenchymal
transition of MCF-7 cells. Braz J Med Biol Res. 40:1071–1078. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kurrey NK, K A and Bapat SA: Snail and
Slug are major determinants of ovarian cancer invasiveness at the
transcription level. Gynecol Oncol. 97:155–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leong KG, Niessen K, Kulic I, Raouf A,
Eaves C, Pollet I and Karsan A: Jagged1-mediated Notch activation
induces epithelial-to-mesenchymal transition through Slug-induced
repression of E-cadherin. J Exp Med. 204:2935–2948. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Martin TA, Goyal A, Watkins G and Jiang
WG: Expression of the transcription factors snail, slug, and twist
and their clinical significance in human breast cancer. Ann Surg
Oncol. 12:488–496. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Côme C, Magnino F, Bibeau F, De Santa
Barbara P, Becker KF, Theillet C and Savagner P: Snail and slug
play distinct roles during breast carcinoma progression. Clin
Cancer Res. 12:5395–5402. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hour MJ, Tsai SC, Wu HC, Lin MW, Chung JG,
Wu JB, Chiang JH, Tsuzuki M and Yang JS: Antitumor effects of the
novel quinazolinone MJ-33: Inhibition of metastasis through the
MAPK, AKT, NF-κB and AP-1 signaling pathways in DU145 human
prostate cancer cells. Int J Oncol. 41:1513–1519. 2012.PubMed/NCBI
|
|
40
|
Phung TL, Ziv K, Dabydeen D, Eyiah-Mensah
G, Riveros M, Perruzzi C, Sun J, Monahan-Earley RA, Shiojima I,
Nagy JA, et al: Pathological angiogenesis is induced by sustained
Akt signaling and inhibited by rapamycin. Cancer Cell. 10:159–170.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
42
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Woo JH, Lim JH, Kim YH, Suh SI, Min DS,
Chang JS, Lee YH, Park JW and Kwon TK: Resveratrol inhibits phorbol
myristate acetate-induced matrix metalloproteinase-9 expression by
inhibiting JNK and PKC delta signal transduction. Oncogene.
23:1845–1853. 2004. View Article : Google Scholar
|
|
44
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Woo MS, Jung SH, Kim SY, Hyun JW, Ko KH,
Kim WK and Kim HS: Curcumin suppresses phorbol ester-induced matrix
metalloproteinase-9 expression by inhibiting the PKC to MAPK
signaling pathways in human astroglioma cells. Biochem Biophys Res
Commun. 335:1017–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee HS, Seo EY, Kang NE and Kim WK:
[6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer
cells. J Nutr Biochem. 19:313–319. 2008. View Article : Google Scholar
|
|
47
|
Yagihashi S, Miura Y and Yagasaki K:
Inhibitory effect of gingerol on the proliferation and invasion of
hepatoma cells in culture. Cytotechnology. 57:129–136. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu TL, Huang GJ, Lu TJ, Wu JB, Wu CH, Yang
TC, Iizuka A and Chen YF: Hispolon from Phellinus linteus has
antiproliferative effects via MDM2-recruited ERK1/2 activity in
breast and bladder cancer cells. Food Chem Toxicol. 47:2013–2021.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sun YS, Zhao Z and Zhu HP: Hispolon
inhibits TPA-induced invasion by reducing MMP-9 expression through
the NF-κB signaling pathway in MDA-MB-231 human breast cancer
cells. Oncol Lett. 10:536–542. 2015.PubMed/NCBI
|
|
50
|
Al Saleh S, Al Mulla F and Luqmani YA:
Estrogen receptor silencing induces epithelial to mesenchymal
transition in human breast cancer cells. PLoS One. 6:e206102011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bouris P, Skandalis SS, Piperigkou Z,
Afratis N, Karamanou K, Aletras AJ, Moustakas A, Theocharis AD and
Karamanos NK: Estrogen receptor alpha mediates epithelial to
mesenchymal transition, expression of specific matrix effectors and
functional properties of breast cancer cells. Matrix Biol.
43:42–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jang EH, Jang SY, Cho IH, Hong D, Jung B,
Park MJ and Kim JH: Hispolon inhibits the growth of estrogen
receptor positive human breast cancer cells through modulation of
estrogen receptor alpha. Biochem Biophys Res Commun. 463:917–922.
2015. View Article : Google Scholar : PubMed/NCBI
|