Introduction
Thyroid cancer is the most prevalent endocrine
malignancy and the eighth most common cancer in females. Presently,
thyroid cancer incidence has continuously increased worldwide
(1,2). Moreover, the American Cancer Society
has reported that the incidence of thyroid cancer is increasing
most rapidly compared to other cancers (3). Papillary thyroid carcinomas (PTC) and
follicular thyroid carcinomas (FTC) account for 95% of all thyroid
cancer cases. They are clinically classified as well-differentiated
thyroid carcinomas (WDTC) due to their biological behavior
resembling normal follicular cells and good responsiveness to
surgery and radioiodine therapy (4,5).
However, PTC and FTC are usually curable when discovered at early
stages, but survival rates may be reduced from 100% in stages I and
II to 50% at stage IV (6). In
addition, pathological divergences, such as patterns of metastasis,
of PTC and FTC also have significant impact on cancer
aggressiveness and treatment. PTC usually invades neighboring
tissues and occasionally metastasizes to regional lymph nodes,
whereas FTC often metastasizes to bone and lung. Thus the general
treatment for WDTC, thyroidectomy with radioiodine therapy, may
have different consequences for PTC and FTC (5). Prophylactic lymph node and central
neck dissection is an example of different thyroid cancer
management between PTC and FTC. Several studies showing that
prophylactic lymph node and central neck dissection in PTC
potentially decreases cancer recurrence, revealing microscopic
lymph node metastases undetectable by other techniques and
increasing disease-specific survival. In contrast, dissections are
not recommended for most FTC, except in malignant lymph node cases
(7,8). Therefore, FTC is considered to be more
aggressive than PTC. For these reasons, early detection and
classification of thyroid cancer is necessary for optimal
treatment.
Neck ultrasound, laryngeal exam, thyroid function
blood test, chest X-ray, thyroid scan with low-dose radioactive
iodine and fine needle aspiration (FNA) biopsy are available
techniques for thyroid cancer diagnosis. FNA with cytopathological
examination is the most effective technique for thyroid cancer
diagnosis and classification. However, approximately 10–30% of FNA
results are misdiagnosed due to inadequate aspirated materials,
cytodiagnostic errors, missed sampling and nodule compositions
(9,10). In addition, detections of specific
genetic alterations from FNA and thyroid cancer tissue samples have
been studied for decades and some biomarkers have been identified
(e.g. BRAF and RAS mutation). However, determination of disease
status based on genomic analysis and gene expression data alone are
limited due to translational modifications such as mRNA splicing
and post-translational modifications. Thus, proteomics is an option
to identify potential biomarkers for cancer diagnosis that can
fulfill the discordance of genetic biomarkers and improve the
effectiveness of thyroid cancer diagnosis (4,5,11,12).
Several studies, including those from our group, have revealed
potential protein biomarkers from thyroid tissues, e.g., galectin
3, cathepsin B, cytokeratin 19 (CK19) and E-cadherin (13–17).
Unfortunately, these biomarkers do not have enough specificity to
be used clinically. Galectin 3 is one of the most studied thyroid
cancer biomarkers. We have shown galectin 3 to have higher
expression in PTC, compared to FTC and benign tissues (15). However, it is overexpressed in other
cancers such as breast cancer, lung cancer, esophageal cancer, and
laryngeal cancer, making it difficult to discriminate between
thyroid cancer and other cancers (15,18,19).
In addition, galectin 3 is also expressed by macrophages and
activated endothelial cells, which can interfere with diagnosis
(18).
Current interest focuses on using a panel of
multiple biomarkers for diagnosis, which should be more reliable
than using single biomarkers. Several studies on thyroid cancer
have revealed that panel biomarkers have higher specificities than
a single marker alone (17,20,21).
The specificity of using a panel of three biomarkers for detection
of thyroid cancer, namely galectin 3, CK19 and monoclonal antibody
against microvillus surface antigen (HBME), increased by 18, 14 and
4%, respectively, when compared to those of galectin 3, CK19 and
HBME alone (21). However, there is
still a need for classification of thyroid cancer for appropriate
treatment, so it would be beneficial to have a panel of biomarkers
for detection, as well as classification of thyroid carcinomas. In
this study, we aimed to identify novel biomarkers for thyroid
cancer diagnosis and classification using proteomics for improved
thyroid cancer management.
Materials and methods
Cell culture
Human papillary thyroid carcinoma (B-CPAP) cell line
and human follicular thyroid carcinoma (FTC-133) cell line were
kindly provided by Professor Johan Lillehaug, University of Bergen,
Norway. B-CPAP cells were cultured in RPMI medium supplemented with
10% heat-inactivated fetal bovine serum (FBS) and 1%
penicillin-streptomycin-amphotericin-B solution. FTC-133 cells were
cultured in 1:1 mixture of DMEM: Ham's F-12 media supplemented with
10% heat-inactivated FBS, 1% penicillin-streptomycin-amphotericin-B
solution and 1% 200 mM L-glutamine. The cells were incubated at
37°C in 5% CO2.
Human specimens
Five human papillary carcinoma (PTC) tissues and
their adjacent normal tissues, as well as five human follicular
carcinoma (FTC) tissues and their adjacent normal tissues were
obtained from Phramongkutklao Medical Center, Bangkok, Thailand
after approval of the research protocol (S012H) by the
Institutional Review Board of the Royal Thai Army Medical
Department, Thailand. Histopathology confirmed diagnoses of the
tissues. Tissues were stored at −80°C until ready to be
processed.
Protein extraction
Cells were harvested from culture flasks in 0.25 M
sucrose with protease inhibitor cocktail (ratio of 500:1)
(Sigma-Aldrich P8340, St. Louis, MO, USA) using cell scraper and
then centrifuged at 500 × g at 4°C for 10 min. The cell pellets
were lysed using lysis buffer containing 9 M urea, 2% CHAPS, 2%
DTT, 5% ampholine (pH 3-10) and 500:1 protease inhibitor cocktail
for 1 h at room temperature, soni-cated and then centrifuged at
12,000 × g at 4°C for 10 min. Bradford assay was used to determine
protein concentration in supernatants.
Invasion and migration assays
Cell invasion and migration assays were performed
using Transwell chambers (pore size 8.0 μm, Corning). For
invasion assay, the upper chambers were coated with Matrigel (30
μg protein/well). Cells (104) were seeded onto
the upper chamber and 600 μl medium containing 10% FBS was
added to the lower chamber, and incubated at 37°C for 24 h with 5%
CO2. Non-migrating or non-invading cells on the upper
chambers were removed using cotton swabs. The migrating or invading
cells on the upper chamber were fixed in 25% methanol for 15 min
and then stained with 0.5% crystal violet for 15 min. Excess dye
was rinsed with RO water for 30 sec, swabbed and then cells dried
at 70°C overnight. Photographs were taken and then dye was eluted
using HCl-methanol solution (1:9 ratio) for 5 min. Absorbance was
measured at 550 nm.
Two-dimensional SDS-PAGE and image
analysis
Immobiline™ Drystrips (7 cm, pH 3-10 nonlinear) were
rehydrated for 16 h with 150 μg protein samples. The first
dimension was performed at 7,000 Vh by using Ettan IPGphor 3 (GE
Healthcare Co.). For the second dimension, strips were incubated
with equilibration buffer containing 1,4-dithio-erythritol (DTT)
for 10 min followed by incubation with equilibration buffer
containing iodoacetamide (IAA) for 10 min. Proteins were separated
in 12.5% SDS-PAGE gel at 10 mA/gel using PowerPac Basic™ (Bio-Rad)
apparatus. Gels were stained with Coomassie blue R-250 and then
scanned by using Labscan 5.0 software. Protein spots were analyzed
by ImageMaster 2D Platinum 7.0 program and differential protein
expression between the cell lines was measured as percent volume.
Protein spots with expression >1.3-fold were selected for
identification.
In-gel digestion
Selected spots were excised and then destained with
50% acetonitrile (ACN) in 0.1 M NH4HCO3
followed by reduction and alkylation using 10 mM DTT for 45 min at
60°C and 100 mM IAA at room temperature for 30 min in the dark,
respectively. Finally, dried gels were digested with 0.01 μg
of trypsin (Promega Co.) in digestion buffer at 37°C overnight and
then supernatants were collected for protein identification.
Protein identification using
LC-MS/MS
Trypsin-digested peptides were identified by
LC-MS/MS. In brief, C18 EASY-Nlc™ column (Thermo
Scientific, Rockford, IL, USA) was used to concentrate and desalt
digested peptides. Peptides were eluted off the column by using
solutions A and B which were composed of 0.1% formic acid in 97%
water with 3% ACN and 0.1% formic in 97% ACN, respectively, and
injected into nano ESI MS/MS (Amazon speed ETD, Bruker Co.) to
generate MS/MS spectra. Parent mass peaks within the range of
50-3000 m/z were selected for MS/MS analysis and the MS/MS spectra
were processed using Bruker Compass 1.4 software. Mascot search
engine (www.matrixscience.com) was used to
identify proteins with the following parameters: NCBI database in
Homo sapiens taxonomy, enzyme used: trypsin, missed cleavage
allowed: one, fixed modification: carbamidomethyl (C) and variable
modification: phospho (ST) and phospho (Y), peptide tolerance: 1.2,
MS/MS mass tolerance: 0.6 kDa, and peptide charges: 1+, 2+ and 3+.
Identified proteins with consistent molecular weight and pI with
their positions in the gels, Mascot score >25 and p≤0.05 were
considered as candidate biomarkers.
Western blot analysis
Proteins were separated on 12.5% SDS-PAGE and
electro-transferred onto PVDF membranes. Membranes were blocked
with 5% bovine serum albumin (BSA) at 4°C, overnight and then
probed with antibody against Annexin A1 (1:1000, Chemicon
International Inc.), annexin A2 (1:1000, Abcam Inc.), heterogeneous
nuclear ribonucleoprotein K or hnRNP K (1:500, Cell signaling
Technology Inc.), 14-3-3σ (1:500, Abcam Inc.), pyruvate kinase
(1:1000, Santa Cruz Biotech Inc.), enolase 1 (1:1000, Abcam Inc.),
glyceraldehyde-3-phosphate dehydrogenase or GAPDH (1:1000, Abcam
Inc.), triose phosphate isomerase or TPI (1:2000, Abcam Inc.),
copine 1 (1:2000, Abcam Inc.), cathepsin D (1:1000, Abcam Inc.),
cofilin 1 (1:500, Abcam Inc.), heat shock 27 kDa protein or HSP27
(1:10000, Abcam Inc.), β-actin (1:10000, Cell signaling Technology
Inc.), proliferating cell nuclear antigen or PCNA (1:5000, Abcam
Inc.) and tubulin as loading control (1:5000, Cell Signaling
Technology Inc.) for 2 h at room temperature. Then, membranes were
washed with TBS/T buffer (TBS, 0.1% Tween-20) and then blocked with
5% skim milk for 30 min. The membranes were incubated with the
corresponding secondary antibodies for 45 min and washed again.
Finally, membranes were incubated with ECL reagent for 5 min and
exposed to film. Labscan 5.0 instrument was used to scan exposed
film and band intensities were measured using ImageQuan TL software
(GE Healthcare Co.).
Pathway analysis
The network most significantly influenced when
comparing PTC and FTC cell lines was predicted by using Ingenuity
Pathway Analysis (IPA®, Qiagen Redwood City, CA, USA;
www.qiagen.com/ingenuity). Accession
numbers and fold-changes of proteins were provided in a dataset.
Criteria input in the software are as follows: Reference set,
Ingenuity Knowledge Base (genes only); Relationship to consider,
Direct and Indirect relationships; Networks, inter action; Data
source, all; Confidence, Experimentally Observed; Species, Human;
Tissues and cell lines, Other cell line; and Mutation, all.
Biomarker validation
Proteins from human tissues were extracted using a
hand homogenizer. In brief, 20 mg of tissue was lysed by 150
μl of RIPA buffer (150 mM NaCl, 1.0% Triton X-100, 0.5%
sodium deoxycholate, 0.1% SDS, 50 mM Tris pH 8.0 and 1 mM EDTA)
containing protease inhibitor and then homogenized by hand
homogenizer and incubated on ice for 1 h. Samples were centrifuged
at 12,000 × g at 4°C for 10 min and the supernatants were
collected. Protein concentrations in samples were determined by
using Bradford assay. Then, protein markers were validated by
western blot analysis as previously described.
Statistical analysis
Experimental data are presented as the mean ± SE.
The statistical significances of data between samples were
determined using the appropriate statistics, namely ANOVA for spot
analysis and unpaired Student's t-test or paired Student's t-test
for others. Data were considered significantly different at
p-values <0.05.
Results
The differential diagnosis of well-differentiated
thyroid carcinomas such as papillary thyroid carcinoma and
follicular thyroid carcinoma requires skilled pathology examination
from fine-needle aspiration of the thyroid nodule. Even though both
types of carcinomas are treatable by thyroidectomy and iodine
ablation, follicular thyroid carcinoma is considered to be more
aggressive and has higher recurrence than papillary thyroid
carcinomas (4,22–24).
Treatment regimens using molecular-targeted chemotherapy also
differ, so it is prudent to distinguish between papillary thyroid
carcinoma and follicular thyroid carcinoma (25). Herein, we provide a panel of
biomarkers that will not only differentiate between these two
well-differentiated thyroid carcinomas but also diagnose normal
from cancerous lesions.
Cell line characterization
In order to understand the differences between
papillary thyroid carcinoma and follicular thyroid carcinoma, cell
lines were characterized. Papillary thyroid carcinoma (B-CPAP) and
follicular thyroid carcinoma (FTC-133) cell lines exhibited
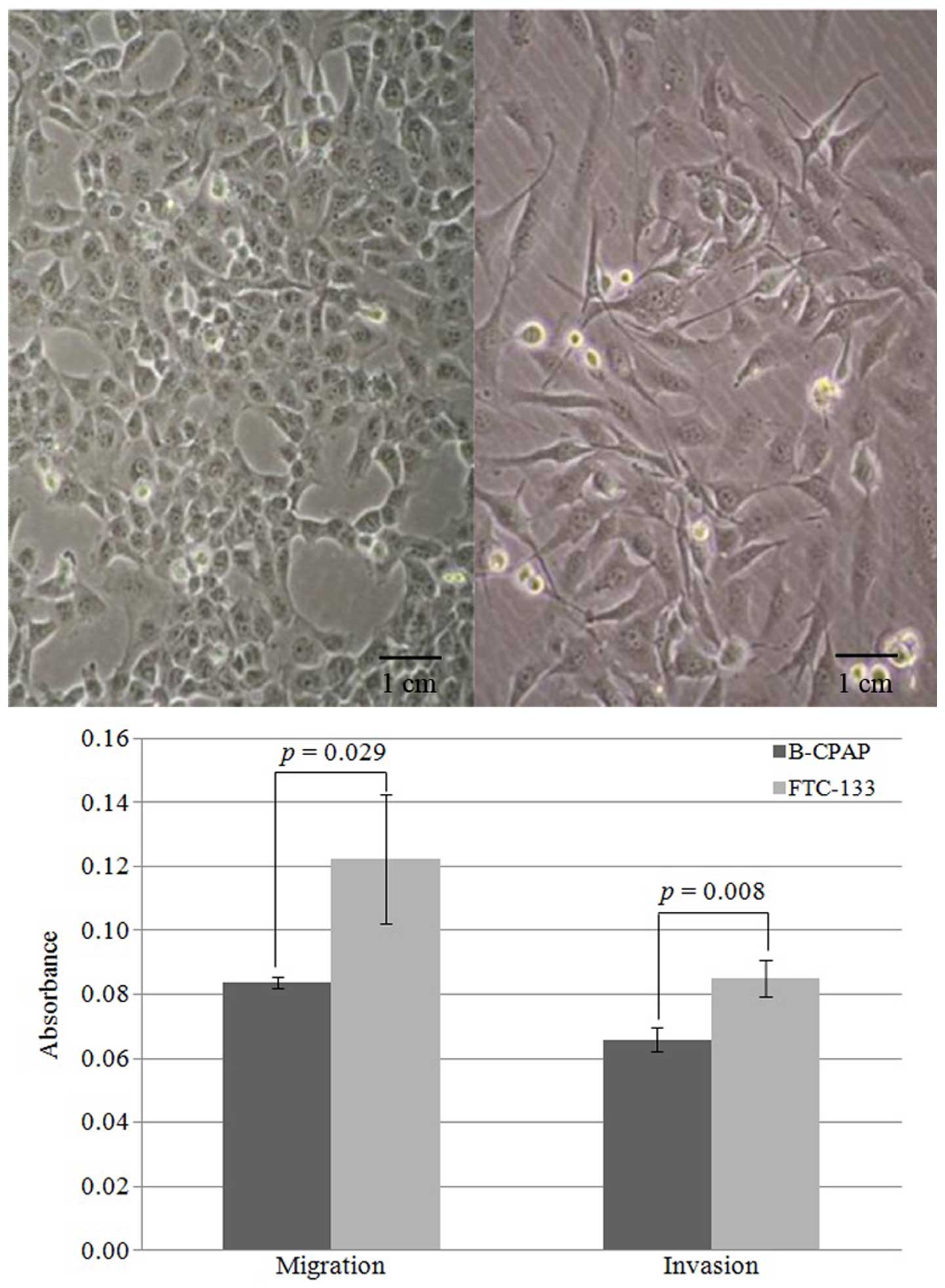
different morphologies and invasiveness (Fig. 1). B-CPAP cells are epithelial-like
whereas FTC-133 cells appear spindle-like (Fig. 1 top panel). The doubling times for
B-CPAP and FTC-133 cells are 22.8±1.5 and 27.9±1.2 h, respectively.
Moreover, FTC-133 cells demonstrated increased migration and
invasion compared to B-CPAP cells (Fig.
1 bottom panel), consistent with the clinical observation that
follicular thyroid carcinomas are more aggressive than papillary
carcinomas.
Biomarker identification using
proteomics
Classical proteomics using two-dimensional
polyacrylamide gel electrophoresis, followed by trypsin digestion
and LC/MS/MS, were performed to identify the global protein
expression differences between B-CPAP and FTC-133 cells. The
proteomic patterns showed one hundred and one protein spots
differing in expression by >1.3-fold, as analyzed using ANOVA
and these spots were identified by mass spectrometry (data not
shown). Fourteen spots appeared in only B-CPAP cells and 39 spots
appeared in only FTC-133 cells. In addition, 45 spots had higher
expression in B-CPAP cells and 39 spots had higher expression in
FTC-133 cells when compared to one another. For B-CPAP cells,
functional classification revealed the predominant role of proteins
involved in cell growth and proliferation (24%), structure (14%),
glycolysis (14%), anti-apoptosis and drug resistance (12%),
invasion and metastasis (10%), stress response (10%) and other
functions (16%). On the other hand, proteins involved in cell
growth and proliferation (30%), invasion and metastasis (12%),
anti-apoptosis and drug resistance (10%), stress response (10%),
glycolysis (10%) and other functions (20%) were mainly found in
FTC-133 cells.
Fourteen spots were selected for further study,
representing proteins covering the major functions in the two cell
lines, which showed the most significant and/or high fold-changes
in expression. For cell growth and proliferation, proliferating
cell nuclear antigen (PCNA), heterogeneous nuclear
ribonucleoprotein K (hnRNP K) and annexin A1 were selected. For
glycolysis, triose phosphate isomerase (TPI),
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), enolase and
pyruvate kinase were selected. For invasion and metastasis, we
selected cathepsin D, annexin A2 and cofilin 1. For anti-apoptosis
and drug resistance, stress response, structure, and other
functions, 14-3-3σ, heat shock 27 kDa protein (HSP27), β-actin, and
copine 1 were selected, respectively.
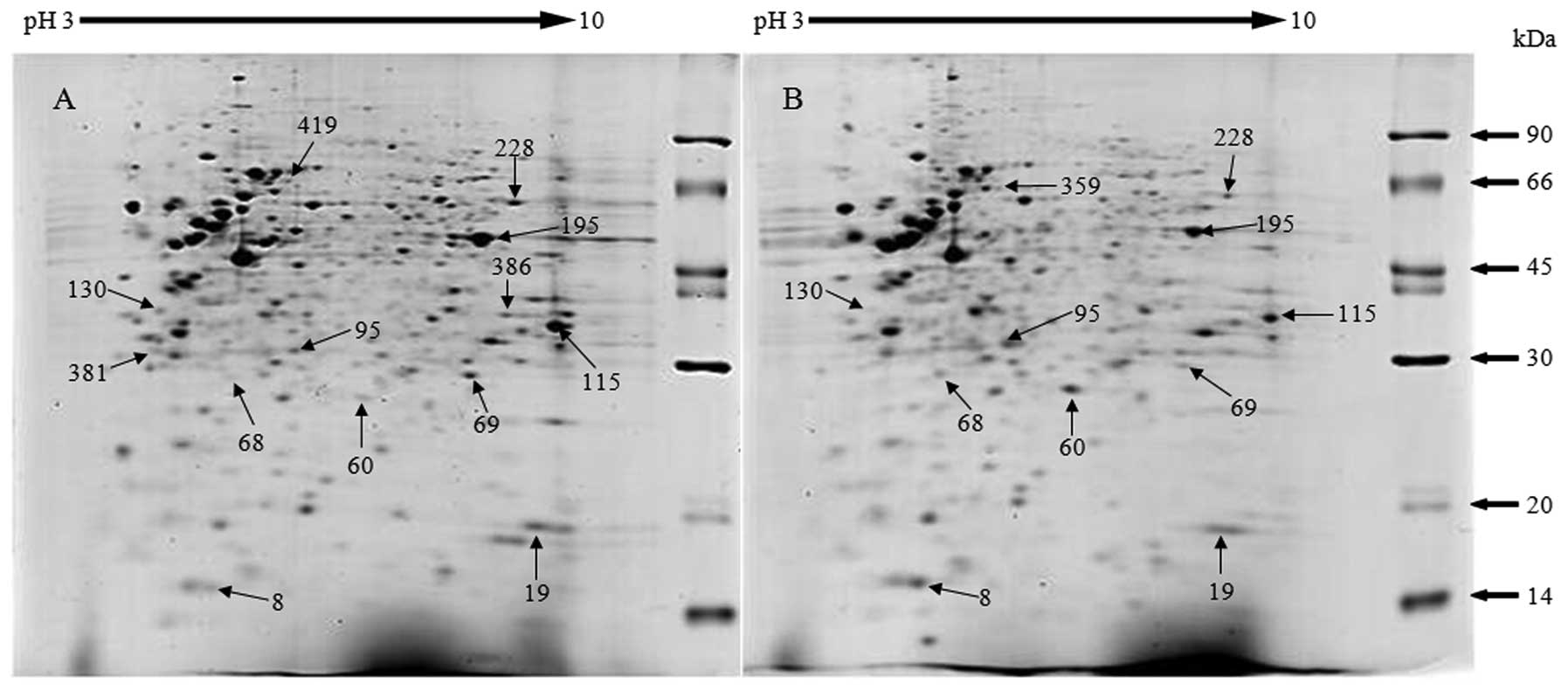
Representative gels of lysates of B-CPAP and FTC-133
cells are shown in Fig. 2, with 14
selected spots differing between the two cell lines labeled. The
identities of three spots present in only B-CPAP cells, one spot in
only FTC-133 cells, six spots that increased in B-CPAP and four
spots that increased in FTC-133 cells are listed in Table I with their functions. Validations
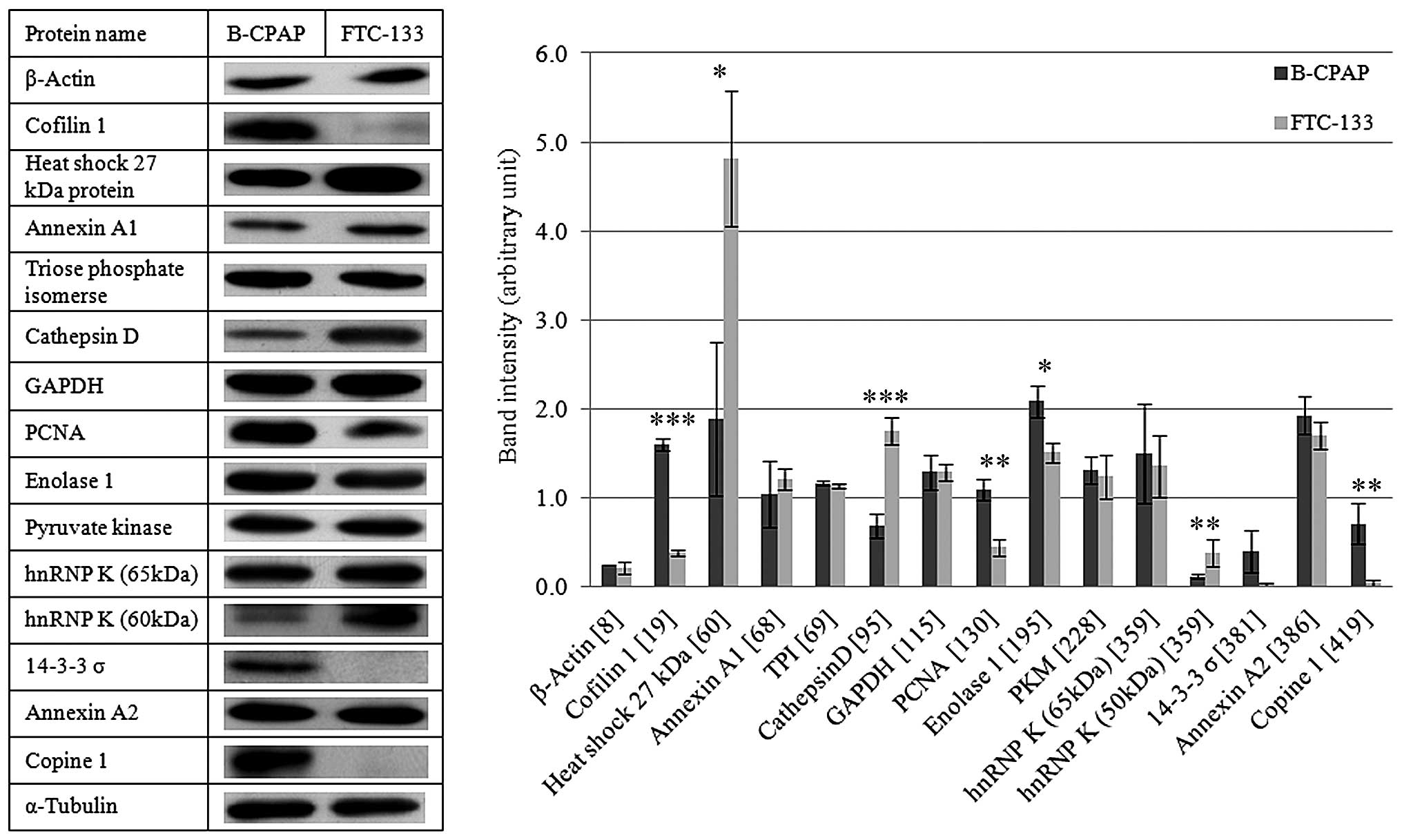
of these identified proteins were performed using immunoblots, as
shown in Fig. 3. Cofilin 1, PCNA,
enolase 1, and copine 1 showed higher expression in B-CPAP cells,
whereas HSP27, cathepsin D and hnRNP K had higher expression in
FTC-133 cells with statistical significance. Although the fourteen
selected proteins showed differential expression in two-dimensional
SDS-PAGE, only seven proteins showed statistically significant
differences in expression between the two cell lines by
immunoblotting. However, immunoblots were performed using
one-dimensional SDS-PAGE and can detect the total expression of the
protein of interest based on the epitope recognized by the capture
antibody. However, in two-dimensional PAGE, spots differing in
isoelectric point and molecular weight are detected. Thus, there
could be other isoforms or cleavage forms of the protein with
differing expression that would affect the total expression
level.
 | Table IIdentification of selected proteins
by LC/MS/MS and Mascot database search. |
Table I
Identification of selected proteins
by LC/MS/MS and Mascot database search.
| Spot ID | Accession no. | Protein name | MW/pI | Coverage | Peptide match | Mascot score | Fold change | P-value | Function |
|---|
| 8 | gi|28336 | β-Actin | 42128/5.22 | 16 | 5 | 260 | 3.1521 | 0.006 | Structure
protein |
| 19 | gi|5031635 | Cofilin 1 | 18719/8.22 | 30 | 5 | 216 | 2.255 | 0.002 | Invasion and
metastasis |
| 60 | gi|4504517 | Heat shock 27 kDa
protein | 22826/5.98 | 36 | 8 | 358 | 3.9332 | 0.000 | Stress
response |
| 68 | gi|4502101 | Annexin A1 | 38918/6.57 | 4 | 2 | 76 | 1.6415 | 0.022 | Cell growth and
proliferation |
| 69 | gi|4507645 | Triosephosphate
isomerase (TPI) | 26938/6.45 | 55 | 12 | 548 | 1.3202 | 0.049 | Glycolysis |
| 95 | gi|4503143 | Cathepsin D | 45037/6.10 | 6 | 3 | 119 | 2.4007 | 0.000 | Invasion and
metastasis |
| 115 | gi|31645 |
Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) | 36202/8.26 | 30 | 9 | 311 | 2.2176 | 0.001 | Glycolysis |
| 130 | gi|49456555 | Proliferating cell
nuclear antigen (PCNA) | 29029/4.57 | 32 | 9 | 395 | 1.7506 | 0.005 | Cell growth and
proliferation |
| 195 | gi|4503571 | Enolase 1 | 47481/7.01 | 41 | 19 | 657 | 1.6857 | 0.029 | Glycolysis |
| 228 | gi|35505 | Pyruvate kinase
(PK) | 58411/7.58 | 30 | 17 | 714 | 1.7031 | 0.017 | Glycolysis |
| 359 | gi|460789 | Heterogeneous
nuclear ribonucleoprotein K (hnRNPK) | 51325/5.13 | 5 | 2 | 71 | Present in
FTC133 | 0.000 | Cell growth and
proliferationx |
| 381 | gi|350610434 | 14-3-3σ | 26584/4.90 | 14 | 4 | 150 | Present in
BCPAP | 0.000 | Anti-apoptosis and
drug resistance |
| 386 | gi|114794644 | Annexin A2 | 35448/8.21 | 33 | 10 | 470 | Present in
BCPAP | 0.000 | Invasion and
metastasis |
| 419 | gi|23397696 | Copine 1 | 59649/5.52 | 11 | 7 | 236 | Present in
BCPAP | 0.000 | Others |
To elucidate the significance of the differentially
expressed proteins in cell lines and to predict the relevant
biological network involved in the carcinogenesis of papillary
thyroid carcinoma and follicular thyroid carcinoma, the complete
dataset containing 101 proteins with their fold-changes were
entered into Ingenuity Pathway Analysis (IPA). IPA revealed the top
network, with a score of 23, containing 16 focus molecules involved
in cell death and survival, tumor morphology and cancer (Fig. 4). The network revolves around
interactions with tumor protein p53 (p53), extracellular
signal-regulated kinase 1/2 (ERK1/2) and interleukin-6 (IL-6),
which are proteins regulating cell growth and proliferation. The
expression of p53 is significantly higher in FTC-133 by at least
3-fold than in B-CPAP, but there were no statistically significant
differences in IL-6 and ERK1/2 expression, as determined by Western
blots (data not shown). Overexpression of p53 in lymph node
metastasis of papillary carcinomas has been reported using
immunohistochemistry, suggesting that p53 is associated with cancer
aggressiveness (26). This is
consistent with our observations that higher overexpression of p53
is found in the more invasive FTC-133 cells rather than in B-CPAP
cells, from both pathway analysis validation and invasion assay.
Thus, IPA revealed p53 to be a potential marker with clinical
relevance to determine aggressiveness of thyroid cancers.
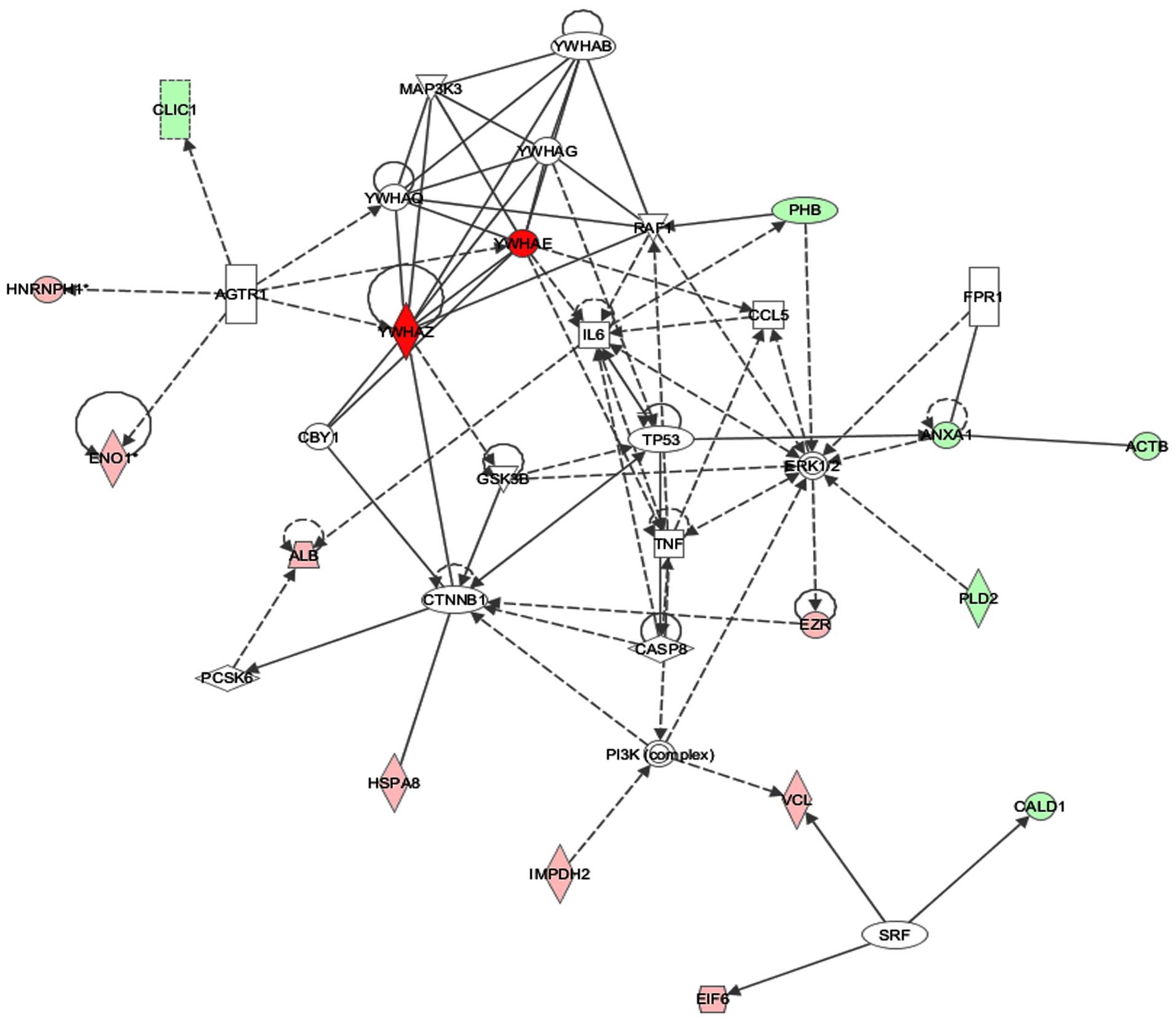 | Figure 4Network analysis using Ingenuity
Pathway Analysis (IPA). IPA was used to analyze the biological
network of one hundred and one identified proteins, which differed
significantly in expression by more than 1.3-fold between B-CPAP
and FTC-133 cell lines. IPA revealed the top network containing 16
focus molecules with a score of 23; this network is associated with
cell death and survival. Proteins shown in green are focus
molecules that had decreased expression in B-CPAP cell line,
whereas proteins in red are focus molecules with higher expression
than in B-CPAP cell line. The intensities of the color correspond
to the fold-changes in expression. Solid or dashed lines indicate
direct or indirect interactions. No arrows, single arrows or double
arrows indicate binding, unidirectional act on, or bidirectional
act on, respectively. The following proteins were identified in the
network by IPA: ACTB (β-actin), ANXA1 (annexin A1), CALD1
(caldesmon 1), CLIC1 (chloride intracellular channel 1), PHB
(prohibitin), PLD2 (phospholipase D2), ALB (albumin), EIF6
(eukaryotic translation initiation 6), ENO1 (enolase1), EZR
(ezrin), HNRNPH1 (heterogeneous nuclear ribonucleoprotein H1),
HSPA8 (heat shock 70 kDa protein 8), IMPDH2
(inosine-5′-monophosphate dehydrogenase), VCL (vinculin), YWHAE
(14-3-3ε protein), YWHAZ (14-3-3ζ protein), YWHAB (14-3-3β
protein), YWHAG (14-3-3γ protein), YWHAQ (14-3-3τ protein), AGTR1
(angiotensin II receptor), CASP8 (caspase 8), CBY1 (chibby
homolog), CCL5 (chemokine C-C motif ligand 5), CTNNB1 (catenin),
ERK1/2 (extracellular signal-regulated kinase), FPR1 (formyl
peptide receptor 1), GSK3B (glycogen synthase kinase 3β), IL6
(interleukin 6), MAP3K3 (mitogen-activated protein kinase 3), PCSK6
(proprotein convertase), PI3K (phosphoinositide 3-kinase), RAF1
(v-Raf1 murine leukemia viral oncogene homolog 1), SRF (serum
receptor factor), TNF (tumor necrosis factor), and TP53 (tumor
protein p53). |
Biomarker validation
In order to assess the potential use of the fourteen
identified proteins from our proteomic study as biomarkers, the
expression levels of these proteins were evaluated in human thyroid
cancer tissues and their adjacent normal tissues. Table II lists clinical pathology
information of five PTC and five FTC tissues from surgical
dissection. Western blot analysis revealed seven proteins, namely
enolase 1, TPI, cathepsin D, copine 1, annexin A2, PCNA and cofilin
1, to be significantly increased in cancerous tissues, whereas
HSP27 showed decreased expression in cancerous tissues when
compared to their normal adjacent tissues. All eight proteins can
distinguish between PTC and normal tissues. However, all proteins,
except for annexin A2, can distinguish between FTC and normal
tissues. Four proteins have the potential to be used as biomarkers
for classifying thyroid cancer, since annexin A2 and cofilin 1 were
significantly upregulated, and HSP27 and PCNA were significantly
downregulated in PTC tissues when compared to FTC tissues (Fig. 5).
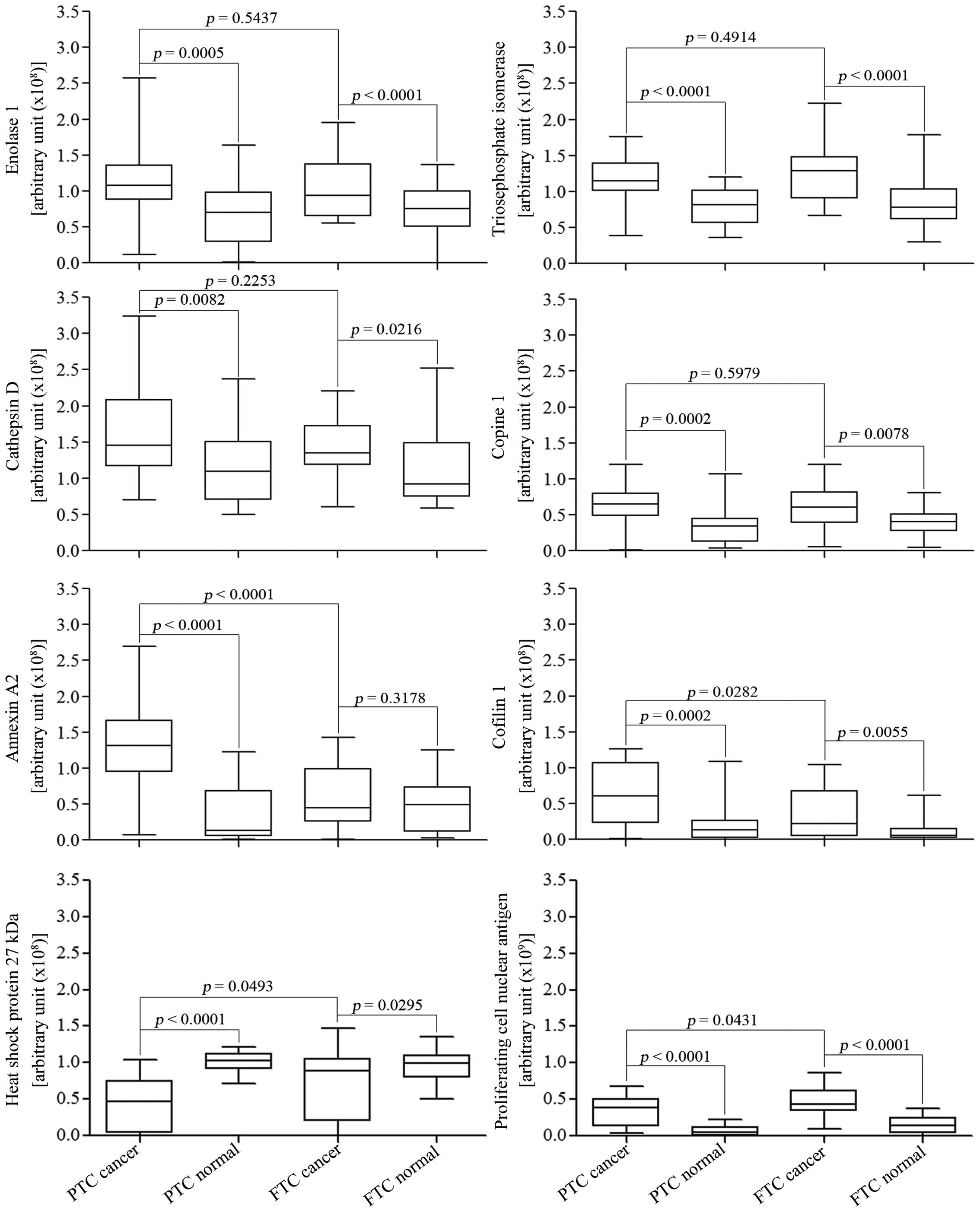 | Figure 5Validation of potential biomarkers
for differentiating and classifying PTC, FTC and normal thyroid
tissues using western blots. Immunoblots of 5 PTC and 5 FTC tissues
with their normal adjacent tissues were performed in triplicate,
and band intensities were measured using ImageQuanTL software. Out
of the fourteen proteins selected from cell lines, eight proteins
showed significant differences in expression when comparing between
cancer types, and between cancer and normal tissues, using
Student's t-test and paired t-test, respectively. The eight
proteins are enolase 1, triose phosphate isomerase (TPI), cathepsin
D, cofilin 1, PCNA, copine 1, annexin A2, and heat shock 27 kDa
protein (HSP27). Among them, annexin A2 and cofilin 1 are
significantly upregu-lated and HSP27 and PCNA are significantly
downregulated in PTC when compared to FTC. All eight can
differentiate between PTC and normal tissues, whereas all but
cathepsin D 34 kDa and annexin A2 can be used to differentiate
between FTC and normal tissues. |
 | Table IIClinical pathology of human thyroid
cancer tissues. |
Table II
Clinical pathology of human thyroid
cancer tissues.
| Case | Gender | Age | Diagnosis |
|---|
| PTC1 | Male | 73 | Papillary thyroid
carcinoma, both lobes |
| PTC2 | Female | 80 | Papillary thyroid
carcinoma, follicular variant |
| PTC3 | Male | 70 | Papillary thyroid
carcinoma at right robe |
| PTC4 | Male | 23 | Papillary thyroid
carcinoma at left lobe |
| PTC5 | Male | 33 | Papillary thyroid
carcinoma |
| FTC1 | Male | 51 | Follicular thyroid
carcinoma at left lobe |
| FTC2 | Female | 48 | Follicular thyroid
carcinoma at left lobe |
| FTC3 | Female | 36 | Follicular thyroid
carcinoma at right lobe |
| FTC4 | Female | 51 | Follicular thyroid
carcinoma at right lobe |
| FTC5 | Male | 26 | Follicular thyroid
carcinoma |
Discussion
In this study, we identified novel potential
biomarkers for thyroid cancer diagnosis and classification. Novel
options for thyroid cancer diagnosis are necessary because of the
30% inconclusive diagnosis from fine needle aspirated biopsies and
the increasing incidence of thyroid incidentalomas (10). Moreover, there is increasing
evidence on the impact of prophylactic lymph node and central neck
dissection on PTC, revealing potential benefits of classifying
thyroid cancer in the disease management (7,8). It is
widely accepted that biomarkers are useful tools for detecting
cancer, monitoring disease progression and possibly identifying
novel therapeutic targets. Although thyroid cancer biomarkers have
been studied for several decades, only few biomarkers for thyroid
cancer classification are available, such as BRAF mutation
and RET/PTC rearrangement in PTC, and RAS mutation
and PPARγ rearrangement in FTC (13,27).
However, it is difficult to determine point mutations in genes or
gene rearrangements, and these genomic biomarkers may not be
correlated with actual clinical pathology (13). Thus, using protein biomarkers is a
better option for thyroid cancer diagnosis and classification.
In this study, proteomic analysis was performed on
thyroid cancer cell lines, B-CPAP and FTC-133, to represent
papillary and follicular carcinomas, respectively. Out of one
hundred and one proteins with differential expression, 14 potential
biomarkers were identified from the cell line study. Validation of
these proteins on thyroid cancer tissues from Thai patients
revealed that expression of enolase 1, TPI, cathepsin D, copine 1,
annexin A2, cofilin 1, PCNA and HSP27 are significantly different
between normal and cancer tissues. Moreover, four proteins namely
annexin A2, cofilin 1, PCNA and HSP27 showed significantly
different expression between PTC and FTC tissues.
Among the eight newly discovered biomarkers from our
study, two are glycolytic enzymes, namely enolase 1 and triose
phosphate isomerase, which are overexpressed in thyroid cancer. The
overexpression of glycolytic enzymes supports anaerobic
proliferation (Warburg effect) and this phenomenon has been widely
accepted as a major source of ATP production in cancer cells
(28,29). TPI is a glycolytic enzyme that
converts dihydroxyacetone phosphate to glyceraldehyde-3-phosphate
(28). Some studies have reported
that TPI has potential as a biomarker for lung squamous cell
carcinoma and pancreatic cancer (30,31).
Enolase 1 commonly exists in the cytoplasm of cells and acts as a
glycolytic enzyme that converts phospho-D-glycerate to
phosphoenolpyruvate (28). The
expression of enolase 1 is upregulated in various types of cancers,
e.g., breast and cervical cancers (29). In contrast, the expression of
enolase 1 is downregulated in non-small cell lung cancer, and is
associated with poor survival rates. Enolase 1 may be alternatively
translated to maltose binding protein 1 (MBP1) and then acts as a
MYC inhibitor, inducing cell death and suppressing cell growth
(32). Enolase 1 is also associated
with cancer invasion and metastasis by acting as a
plasminogen-binding receptor. When enolase 1 integrates into the
cell membrane, it promotes plasmin activity leading to cancer cell
invasion and metastasis by degrading the extracellular matrix
(33).
Cathepsin D is a member of the cathepsin protease
family found in lysosomes and phagosomes that is activated at
acidic pH (34). Members of the
family, namely cathepsins B, D and L, have been reported to cleave
thyroglobulin in the thyroid resulting in the release of thyroid
hormones, triiodothyronine and thyroxine (16,35).
Cathepsins B and L are cysteine proteases that are more effective
in proteolytic cleavage of thyroglobulin in thyroid lysosomes but
are less stable than cathepsin D, an aspartic protease (35). Cathepsin D is overexpressed in
breast cancer and proposed to be involved in cancer metastasis,
cell proliferation and tumor angiogenesis, and fibroblast
proliferation, thus being a marker for aggressiveness of breast
cancer (34). Kraimps et al
reported the overexpression of cathepsin D in thyroid neoplastic
tissues and proposed cathepsin D to be a prognostic marker for poor
survival of advanced stage thyroid cancer, consistent with our
results that cathepsin D expression was upregulated in thyroid
carcinomas compared to normal tissues (36). Previously, our group demonstrated
cathepsin B overexpression in malignant thyroid tissues when
compared to follicular adenomas (15,16).
However, when comparing the two cell lines, cathepsin B did not
show differential expression by at least 1.3-fold and was thus not
selected for the tissue validation in this study. Using
immunoblotting, we did detect the presence of the active form and
heavy chain of cathepsin B, including their isoforms, at different
expression levels between the two cell lines, without affecting the
overall protein expression (data not shown). This suggests the
involvement of post-translational modifications in cathepsin B and
we plan to investigate the role of these modifications in thyroid
carcinogenesis.
Annexin A2 is a calcium-dependent, anionic
phospholipid binding protein that exists in monomeric and
heterotetrameric forms. The monomeric annexin A2 is located in the
cytoplasm, whereas the heterotetramer is located in the plasma
membrane. However, annexin A2 is more strongly expressed in the
cell membrane rather than the cytoplasm (37,38).
The overexpression of annexin A2 has been found in several cancers
including gastric carcinoma, breast cancer, colorectal cancer,
pancreatic cancer, prostate cancer, high-grade gliomas and kidney
cancer (37,38). Moreover, the overexpression of
annexin A2 has been shown to affect biological processes such as
proliferation, apoptosis, invasion and metastasis. Membrane annexin
A2 acts as a receptor or a binding protein for several types of
proteases, e.g., cathepsin B, plasminogen and tissue plasminogen
activator, and extracellular matrix proteins. Therefore, the
expression of annexin A2 on the cell membrane is associated with
cancer cell invasion, lymph node metastasis and poor prognosis
(37–39).
Cofilin 1 is a small ubiquitous protein (~19 kDa)
that regulates actin polymerization and depolymerization through
different phosphorylation patterns. Actin dynamics is related to
cell motility, which is an essential mechanism for cancer invasion
and metastasis (40,41). The overexpression of cofilin 1 has
been reported in various cancers, e.g., ovarian cancer and oral
squamous cellular carcinoma (OSCC) (40–42).
In papillary thyroid carcinomas, the overexpression of cofilin 1
has been reported in fine needle biopsy samples (14). Moreover, high phosphorylation levels
of cofilin 1 have been reported in melanoma, and breast and
prostate cancer. The upregulation of both expression and
phosphorylation of cofilin 1 is associated with cancer invasion,
metastasis and poor prognosis (40–42).
Proliferating cell nuclear antigen (PCNA) or cyclin
is a well-known cell cycle marker that promotes DNA replication by
acting as a cofactor for DNA polymerase δ, a major eukaryotic DNA
polymerase (43). The expression of
PCNA in non-proliferating cells is usually low, but it is elevated
in cells during S-phase or when cells have DNA damage (43). Therefore, PCNA is overexpressed in
many cancers, such as thyroid cancer, breast cancer, pancreatic
cancer and astrocytomas (43,44).
PCNA has been reported to be involved in the molecular
carcinogenesis of papillary thyroid carcinoma and the
overexpression of PCNA was correlated with increased malignancy of
thyroid cancers (44,45). Our results, showing that PCNA
expression is higher in the more invasive FTC than in PTC, are
consistent with these findings.
Copine 1 is a member of a novel family of ubiquitous
calcium dependent, membrane-binding proteins. These proteins are
highly conserved and widely expressed in eukaryotes. The biological
function of copine 1 is poorly understood (46,47).
However, several studies have reported that copine 1 has more than
20 target binding protein partners, such as tyrosine/threo-nine
kinase1/extracellular signal-regulated necrosis factor-α (TNF-α)
and nuclear factor κ-light-chain-enhancer of activated B cells
(NF-κB) (46,48). Therefore, copine 1 may be involved
in various biological processes, e.g., inflammation, apoptosis,
autophagy, growth control, mitosis, gene transcription, exocytosis
and cytoskeleton organization (46–48).
This is the first report to identify the upregulation of copine 1
expression in thyroid cancer, which warrants further studies.
Heat shock protein 27 kDa (HSP27) is a member of the
heat shock protein family. The heat shock protein family is a group
of cellular protective molecules that is activated under stress
conditions such as heat and irradiation. HSP27 is inducible and is
an ATP-independent chaperone activated when in the oligomeric form.
Oligomerization of HSP27 is promoted by several stresses such as
heat and cell-cell contact (49,50).
Clinically, HSP27 is overexpressed in many cancers such as breast
cancer, bladder cancer, ovarian cancer, osteosarcoma, endometrial
cancer and leukemia (50,51). On the contrary, our group has
reported HSP27 downregulation in malignant thyroid tumors as
compared to benign tumors, consistent with our current findings
that HSP27 exhibited decreases in expression in PTC and FTC
compared to their adjacent normal tissues (16). Of note, HSP27 restrains cellular
apoptosis and necrosis, resulting in the promotion of cancer cell
survival and resistance to chemotherapy (50). Anoikis resistance is a hallmark of
cancer metastasis that prevents cancer cell death under detachment
(52). Approximately 20% of FTC
cases usually metastasize to the bone and lung through the blood
circulatory system, i.e., FTC is resistant to anoikis (4,23).
However, the mechanism of anoikis resistance in thyroid cancer,
especially in FTC, remains to be elucidated. We observed the
overexpression of HSP27 in FTC rather than in PTC, suggesting that
HSP27 may be involved in the mechanism of anoikis resistance in
FTC, thus supporting the idea that FTC is more invasive than
PTC.
Our results showed that four out of eight potential
biomarkers (enolase 1, annexin A2, cofilin 1 and cathepsin D) are
associated with invasion and metastasis in various cancers.
Therefore, we hypothesize that the mechanism of thyroid cancer
invasion may be initiated by overexpression of annexin A2 and
enolase 1. These two proteins can intercalate into the plasma
membrane of cancer cells, act as cathepsin D or plasminogen
receptor and then promote the degradation of extracellular matrix
to allow for translocation of cancer cells. Consequently, cofilin 1
induces actin dynamics that initiates cancer cell motility and then
promotes invasion and metastasis. Moreover, annexin A2 and cofilin
1 are significantly upregulated in PTC compared to FTC, thus, these
two proteins may be involved in different modes of metastasis in
PTC and FTC. Future studies on the role of annexin A2 and cofilin 1
in metastasis may improve our knowledge on the mechanisms involved
and reveal specific molecular targets for the treatment of PTC.
In conclusion, we have discovered eight potential
biomarkers for thyroid cancer diagnosis and four possible
biomarkers that differentiate between PTC and FTC. The eight
biomarkers are enolase 1, TPI, cathepsin D, cofilin 1, copine 1,
annexin A2, PCNA and HSP27. All eight can be used to differentiate
between PTC and normal tissues, while enolase 1, TPI, cathepsin D,
cofilin 1, copine 1, PCNA and HSP27 can be used to differentiate
between FTC and normal tissues. For classification of
well-differentiated thyroid carcinomas, annexin A2, cofilin 1, PCNA
and HSP27 can be used. Moreover, p53 may be an additional biomarker
to indicate the aggressiveness of thyroid carcinomas.
We hope that a multi-marker panel using these
potential biomarkers will provide for better diagnosis for early
detection and classification of thyroid cancer. Validation of this
panel using a greater number of samples, samples from less invasive
procedures and/or from various ethnic populations will need to be
conducted in order to confirm the usefulness of this panel for
clinical diagnosis. Further experiments are needed to develop
diagnostic assays based on this panel to improve sensitivity for
detection for use in a clinical setting. Moreover, our results
suggest new molecular insights on thyroid cancer metastasis,
especially for PTC, and thyroid cancer anoikis resistance in FTC,
which can be novel potential targets for thyroid cancer
treatment.
Acknowledgments
This study was partially funded by the New
Researcher Grant from the Thailand Research Fund (TRG 5380025) and
Chulabhorn Research Institute. We would like to express our
gratitude to Professor Johan Lillehaug, University of Bergen,
Norway, for providing us with thyroid cancer cell lines and to the
Laboratory of Pharmacology, Chulabhorn Research Institute, for
monoclonal antibodies for validation of the pathway.
References
|
1
|
International Agency for Research on
Cance: GLOBOCAN 2012: estimated cancer incidence, mortality and
prevalence worldwide in 2012. World Health Organization. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Accessed Sept 2, 2014.
|
|
2
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kondo T, Ezzat S and Asa SL: Pathogenetic
mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer.
6:292–306. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Parameswaran R, Brooks S and Sadler GP:
Molecular pathogenesis of follicular cell derived thyroid cancers.
Int J Surg. 8:186–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barakate DM: Thyroid cancer prognosis.
Thyroid Clinic Sydney. http://www.thyroid.com.au/thyroid-cancer/thyroid-cancer-prognosis/2015.
Accessed Sept 3. 2015.
|
|
7
|
Conzo G, Docimo G, Mauriello C,
Gambardella C, Esposito D, Cavallo F, Tartaglia E, Napolitano S and
Santini L: The current status of lymph node dissection in the
treatment of papillary thyroid cancer. A literature review Clin
Ter. 164:e343–e346. 2013.
|
|
8
|
Cooper DS, Doherty GM, Haugen BR, Kloos
RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F,
Schlumberger M, et al American Thyroid Association (ATA) Guidelines
Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer:
Revised American Thyroid Association management guidelines for
patients with thyroid nodules and differentiated thyroid cancer.
Thyroid. 19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hall TL, Layfield LJ, Philippe A and
Rosenthal DL: Sources of diagnostic error in fine needle aspiration
of the thyroid. Cancer. 63:718–725. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moses W, Weng J, Sansano I, Peng M,
Khanafshar E, Ljung BM, Duh QY, Clark OH and Kebebew E: Molecular
testing for somatic mutations improves the accuracy of thyroid
fine-needle aspiration biopsy. World J Surg. 34:2589–2594. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krause K, Jessnitzer B and Fuhrer D:
Proteomics in thyroid tumor research. J Clin Endocrinol Metab.
94:2717–2724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan Y, Shi L, Liu Q, Dong R, Zhang Q, Yang
S, Fan Y, Yang H, Wu P, Yu J, et al: Discovery and identification
of potential biomarkers of papillary thyroid carcinoma. Mol Cancer.
8:79–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brown LM, Helmke SM, Hunsucker SW,
Netea-Maier RT, Chiang SA, Heinz DE, Shroyer KR, Duncan MW and
Haugen BR: Quantitative and qualitative differences in protein
expression between papillary thyroid carcinoma and normal thyroid
tissue. Mol Carcinog. 45:613–626. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giusti L, Iacconi P, Ciregia F,
Giannaccini G, Donatini GL, Basolo F, Miccoli P, Pinchera A and
Lucacchini A: Fine-needle aspiration of thyroid nodules: Proteomic
analysis to identify cancer biomarkers. J Proteome Res.
7:4079–4088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Subhasitanont P, Srisomsap C, Punyarit P
and Svasti J: Proteomic studies of galectin-3 expression in human
thyroid diseases by immunodetection. Cancer Genomics Proteomics.
3:389–394. 2006.
|
|
16
|
Srisomsap C, Subhasitanont P, Otto A,
Mueller EC, Punyarit P, Wittmann-Liebold B and Svasti J: Detection
of cathepsin B up-regulation in neoplastic thyroid tissues by
proteomic analysis. Proteomics. 2:706–712. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Griffith OL, Chiu CG, Gown AM, Jones SJ
and Wiseman SM: Biomarker panel diagnosis of thyroid cancer: A
critical review. Expert Rev Anticancer Ther. 8:1399–1413. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feilchenfeldt J, Tötsch M, Sheu S-Y,
Robert J, Spiliopoulos A, Frilling A, Schmid KW and Meier CA:
Expression of galectin-3 in normal and malignant thyroid tissue by
quantitative PCR and immunohistochemistry. Mod Pathol.
16:1117–1123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volante M, Bozzalla-Cassione F, Orlandi F
and Papotti M: Diagnostic role of galectin-3 in follicular thyroid
tumors. Virchows Arch. 444:309–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kebebew E, Peng M, Reiff E and McMillan A:
Diagnostic and extent of disease multigene assay for malignant
thyroid neoplasms. Cancer. 106:2592–2597. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Scognamiglio T, Hyjek E, Kao J and Chen
Y-T: Diagnostic usefulness of HBME1, galectin-3, CK19, and CITED1
and evaluation of their expression in encapsulated lesions with
questionable features of papillary thyroid carcinoma. Am J Clin
Pathol. 126:700–708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Viglietto G and De Marco C: Molecular
biology of thyroid cancer. Contemporary Aspects of Endocrinology.
Diamanti-Kandarakis E: InTech; c2011, pp. 189–234. 2011, http://www.intechopen.com/books/contemporary-aspects-of-endocrinology/molecular-biology-of-thyroid-cancer.
|
|
23
|
Schlumberger MJ: Papillary and follicular
thyroid carcinoma. N Engl J Med. 338:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zaydfudim V, Feurer ID, Griffin MR and
Phay JE: The impact of lymph node involvement on survival in
patients with papillary and follicular thyroid carcinoma. Surgery.
144:1070–1077; discussion 1077–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woyach JA and Shah MH: New therapeutic
advances in the management of progressive thyroid cancer. Endocr
Relat Cancer. 16:715–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morita N, Ikeda Y and Takami H: Clinical
significance of p53 protein expression in papillary thyroid
carcinoma. World J Surg. 32:2617–2622. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grogan RH, Mitmaker EJ and Clark OH: The
evolution of biomarkers in thyroid cancer-from mass screening to a
personalized biosignature. Cancers (Basel). 2:885–912. 2010.
View Article : Google Scholar
|
|
28
|
Kim JW and Dang CV: Multifaceted roles of
glycolytic enzymes. Trends Biochem Sci. 30:142–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Capello M, Ferri-Borgogno S, Cappello P
and Novelli F: α-Enolase: A promising therapeutic and diagnostic
tumor target. FEBS J. 278:1064–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mikuriya K, Kuramitsu Y, Ryozawa S,
Fujimoto M, Mori S, Oka M, Hamano K, Okita K, Sakaida I and
Nakamura K: Expression of glycolytic enzymes is increased in
pancreatic cancerous tissues as evidenced by proteomic profiling by
two-dimensional electrophoresis and liquid chromatography-mass
spectrometry/mass spectrometry. Int J Oncol. 30:849–855.
2007.PubMed/NCBI
|
|
31
|
Zhang XZ, Xiao ZF, Li C, Xiao ZQ, Yang F,
Li DJ, Li MY, Li F and Chen ZC: Triosephosphate isomerase and
peroxiredoxin 6, two novel serum markers for human lung squamous
cell carcinoma. Cancer Sci. 100:2396–2401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang YS, Wu W, Walsh G, Hong WK and Mao
L: Enolase-α is frequently down-regulated in non-small cell lung
cancer and predicts aggressive biological behavior. Clin Cancer
Res. 9:3641–3644. 2003.PubMed/NCBI
|
|
33
|
Liu K-J and Shih N-Y: The role of enolase
in tissue invasion and metastasis of pathogens and tumor cells. J
Cancer Mol. 3:pp. 45–48. 2007, http://www.mupnet.com/jocm%203%282%29%2045-48.pdf.
|
|
34
|
Liaudet-Coopman E, Beaujouin M, Derocq D,
Garcia M, Glondu-Lassis M, Laurent-Matha V, Prébois C, Rochefort H
and Vignon F: Cathepsin D: Newly discovered functions of a
longstanding aspartic protease in cancer and apoptosis. Cancer
Lett. 237:167–179. 2006. View Article : Google Scholar
|
|
35
|
Dunn AD, Crutchfield HE and Dunn JT:
Proteolytic processing of thyroglobulin by extracts of thyroid
lysosomes. Endocrinology. 128:3073–3080. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kraimps J-L, Métayé T, Millet C, Margerit
D, Ingrand P, Goujon JM, Levillain P, Babin P, Begon F and Barbier
J: Cathepsin D in normal and neoplastic thyroid tissues. Surgery.
118:1036–1040. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lokman NA, Ween MP, Oehler MK and
Ricciardelli C: The role of annexin A2 in tumorigenesis and cancer
progression. Cancer Microenviron. 4:199–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mussunoor S and Murray GI: The role of
annexins in tumour development and progression. J Pathol.
216:131–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bao H, Jiang M, Zhu M, Sheng F, Ruan J and
Ruan C: Overexpression of Annexin II affects the proliferation,
apoptosis, invasion and production of proangiogenic factors in
multiple myeloma. Int J Hematol. 90:177–185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sidani M, Wessels D, Mouneimne G, Ghosh M,
Goswami S, Sarmiento C, Wang W, Kuhl S, El-Sibai M, Backer JM, et
al: Cofilin determines the migration behavior and turning frequency
of metastatic cancer cells. J Cell Biol. 179:777–791. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang W, Eddy R and Condeelis J: The
cofilin pathway in breast cancer invasion and metastasis. Nat Rev
Cancer. 7:429–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu B, Fukada K, Zhu H and Kyprianou N:
Prohibitin and cofilin are intracellular effectors of transforming
growth factor β signaling in human prostate cancer cells. Cancer
Res. 66:8640–8647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Maiti AK, Ghosh K, Chatterjee U,
Chakrobarti S, Chatterjee S and Basu S: Epidermal growth factor
receptor and proliferating cell nuclear antigen in astrocytomas.
Neurol India. 56:456–462. 2008. View Article : Google Scholar
|
|
44
|
Cvejic D, Selemetjev S, Savin S, Paunovic
I and Tatic S: Changes in the balance between proliferation and
apoptosis during the progression of malignancy in thyroid tumours.
Eur J Histochem. 53:65–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Feng L, Li M, Zhang Q-P, Piao Z-A, Wang
Z-H and Lv S: Utility of BRAF protein overexpression in predicting
the metastasis potential of papillary thyroid carcinoma. Oncol
Lett. 2:59–63. 2011.PubMed/NCBI
|
|
46
|
Tomsig JL, Sohma H and Creutz CE:
Calcium-dependent regulation of tumour necrosis factor-alpha
receptor signalling by copine. Biochem J. 378:1089–1094. 2004.
View Article : Google Scholar
|
|
47
|
Tomsig JL, Snyder SL and Creutz CE:
Identification of targets for calcium signaling through the copine
family of proteins. Characterization of a coiled-coil
copine-binding motif. J Biol Chem. 278:10048–10054. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramsey CS, Yeung F, Stoddard PB, Li D,
Creutz CE and Mayo MW: Copine-I represses NF-kappaB transcription
by endoproteolysis of p65. Oncogene. 27:3516–3526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Parcellier A, Gurbuxani S, Schmitt E,
Solary E and Garrido C: Heat shock proteins, cellular chaperones
that modulate mitochondrial cell death pathways. Biochem Biophys
Res Commun. 304:505–512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Garrido C, Brunet M, Didelot C, Zermati Y,
Schmitt E and Kroemer G: Heat shock proteins 27 and 70:
Anti-apoptotic proteins with tumorigenic properties. Cell Cycle.
5:2592–2601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lebret T, Watson RWG, Molinié V, O'Neill
A, Gabriel C, Fitzpatrick JM and Botto H: Heat shock proteins
HSP27, HSP60, HSP70, and HSP90: Expression in bladder carcinoma.
Cancer. 98:970–977. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Paoli P, Giannoni E and Chiarugi P:
Anoikis molecular pathways and its role in cancer progression.
Biochim Biophys Acta. 1833:3481–3498. 2013. View Article : Google Scholar : PubMed/NCBI
|