Introduction
Epithelial ovarian cancer (EOC) is commonly detected
at a late stage, and is commonly diagnosed with abdominal ascites
and widespread intraperitoneal dissemination. Aggressive
cytoreductive surgery, if possible, is the primary clinical
treatment for advanced ovarian cancer (1,2).
Platinum and paclitaxel combination chemotherapy or i.p. cisplatin
based-chemotherapy is used as a first-line neoadjuvant before
cytoreductive surgery or adjuvant treatment after surgery (3,4). Most
patients develop platinum chemoresistance or multi-drug resistance
during the chemotherapy progress, which is a major obstacle for
chemotherapy in ovarian cancer patients (5). Therefore, the identification of
platinum-resistance mechanisms and the exploration of
chemoresistance reversing therapeutic targets for human EOC are
required.
MACC1 was found to be overexpressed in colon cancer,
gastric carcinoma, lung cancer, hepatocellular carcinoma and
ovarian cancer, and may be used as a biomarker for the poor
prognosis and the high risk of metastasis in these malignant tumors
(6–10). It was found that downregulation of
MACC1 by siRNA sensitized pancreatic cancer cells to gemcitabine
treatment, which may be involved in the inhibition of the Ras/ERK
signaling pathway (11). Another
study showed that knockdown of MACC1 enhanced the apoptosis and
growth inhibitory rates of human glioblastoma cells, and could
increase glioblastoma cell sensitivity to cisplatin chemotherapy
(12). These data indicated that,
in addition to invasion and metastasis, MACC1 may also play other
unknown roles in the pathological processes of cancer cells, such
as chemoresistance.
Herein, we hypothesized that MACC1 may be implicated
in the chemoresistance of cisplatin in ovarian cancer. To verify
this hypothesis, we inhibited the expression of MACC1 in
cisplatin-resistant ovarian cancer A2780/DDP and COC1/DDP cells by
RNA interference. Chemosensitivity and the apoptosis rate in
different ovarian cancer cells were determined following treatment
with different concentrations of cisplatin. Expression levels of
apoptosis-associated proteins and caspase-3 activity were assessed.
The relationship between MACC1 and cisplatin resistance was
investigated.
Materials and methods
Cell transfection
Human ovarian carcinoma A2780/DDP and COC1/DDP cells
were purchased from the China Center for Type Culture Collection
(Wuhan, China), and cultured in complete RPMI-1640 medium (Hyclone,
Logan, UT, USA), at 37°C in 5% CO2. Cells were harvested
in the logarithmic phase of growth for all experiments as described
below. Recombinant MACC1-psuper-EGFP-shRNA eukaryotic plasmids and
the negative control plasmid, as constructed in our previous study
(13), were transfected into the
A2780/DDP and COC1/DDP cells, respectively, which was performed
following the protocol of Lipofectamine 2000 reagent (Invitrogen,
Carlsbad, CA, USA). Stably transfected A2780/DDP and COC1/DDP cells
were isolated by G418 (Sigma, St. Louis, MO, USA). Three cell
groups used for the next research steps were named: blank control
cells (B), negative control cells (NC) and MACC1-knockdown cells
(M).
sqRT-PCR
Cell total RNA was isolated using TRIzol reagent
(Invitrogen), and first strand cDNA was synthesized from 1
µg total RNA according to the protocol of the RevertAid
First Strand cDNA Synthesis kit (Fermentas, EU). Primers used in
the sqRT-PCR were MACC1, 5′-CCTTCGTGGTAATAATGC TTCC-3′ (sense) and
5′-AGGGCTTCCATTGTATTGAGGT-3′ (antisense); and β-actin,
5′-ACGCACCCCAACTACAACTC-3′ (sense) and 5′-TCTCCTTAATGTCACGCACGA-3′
(antisense). PCR cycling parameters (19 cycles) were: denaturation
(94°C for 30 sec), annealing (56°C for 30 sec) and extension (72°C
for 30 sec). Equal amounts of PCR products were electrophoresed on
1.2% agarose gels and visualized by ethidium bromide staining. The
specific bands of the PCR products were analyzed by Image-Pro Plus
6.0 system, and β-actin was used as a control for normalization.
sqRT-PCR was performed three times independently.
Western blot analysis
The antibodies used in the western blotting,
following the manufacturer's protocols, included rabbit anti-human
polyclonal MACC1 (Sigma), rabbit anti-human polyclonal
phospho-ERK1/2 and mouse anti-human monoclonal P-gp (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), rabbit anti-human polyclonal
Bcl-2, rabbit anti-human monoclonal Bcl-XL, mouse
anti-human monoclonal Bax, and rabbit anti-human polyclonal Bad
(Beyotime Biotechnology, Haimen, Jiangsu, China). Total protein was
extracted using RIPA lysis buffer for western blot analysis and IP
(Beyotime Biotechnology), and the protein concentration was
determined using Bradford assay. Equal amounts of protein (30
µg) were separated by 10% SDS-PAGE and transferred onto PVDF
membranes. The detection of hybridized protein was performed by
enhanced chemiluminescence kit (Zhongshan Goldenbridge
Biotechnology, Peking, China), and β-actin was used as a control
for normalization. The relative values of the specific bands were
analyzed by Image-Pro Plus 6.0 system three times
independently.
MTT assay
Cells were planted (1×104 cells/well)
into 96-well plates, and 100 µl complete RPMI-1640 medium
containing 10% FBS was added into each well. Three duplicate wells
were set up for each group. The cells were cultured for 24 h, and
then medium containing cisplatin at 0, 10, 20, 30, 40, 50, and 60
µmol/l, respectively was added. The cells were incubated for
48 h, and the previous medium was replaced by 100 µl
complete RPMI-1640 medium in each well. After 24 h, 20 µl
MTT reagent (5 mg/ml; Sigma) was added into each well. Cells were
then incubated for another 4 h and then the former medium was
aspirated and 150 µl DMSO was added. The absorbance of the
sample was measured by a microplate spectrophotometer (Thermo
Spectronic, Madison, WI, USA) at 492 nm. All experiments were
conducted in triplicate. Cell growth inhibition rate and the half
inhibitory concentration (IC50) value of cisplatin
(LOGIT assay) were calculated. Cell growth inhibition rate = (1 −
sample OD/control OD) × 100%.
Flow cytometric analysis
Every cell group was captured for 48 h in medium
containing cisplatin at 0, 10, 20, 30, 40, 50 and 60 µmol/l,
respectively. The cells were incubated for another 24 h with
complete RPMI-1640 medium. Approximately 1×106 cells
were treated into a single-cell suspension with PBS solution, and
were prepared following the manufacturer's protocol in the Annexin
V-FITC Apoptosis Detection kit (Beyotime Biotechnology). Then,
rates of apoptosis were analyzed with FACScan system (BD
Biosciences, San Jose, CA, USA).
Caspase-3 activity assay
Every cell group was cultured for 48 h in medium
containing cisplatin at 0, 10, 20, 30, 40, 50 and 60 µmol/l,
respectively, and then incubated for another 24 h with completed
RPMI-1640 medium. Approximately 1×106 cells were
collected, total protein was extracted using RIPA lysis buffer for
western blot analysis and IP (Beyotime Biotechnology), and the
protein concentration was controlled using Bradford assay (between
1 to 3 mg/ml as requested). Equal volumes of protein (50 µl)
were prepared following the manufacturer's protocol in the
Caspase-3 Activity Detection kit (Beyotime Biotechnology). The
absorbance values were measured by a microplate spectrophotometer
(Thermo Spectronic) at 405 nm. The pyrolysis liquid without the
cell samples was used as a blank control. The value of ΔA405
(absorbance value of sample at 405 nm minus absorbance value of
blank control at 405 nm) represents the activity of caspase-3. All
experiments were performed in triplicate.
Statistical analysis
Average values are expressed as mean ± standard
deviation (SD). Measurement data were analyzed by one-way ANOVA,
non-parametric test and Bonferroni test using SPSS 17.0 software
package. A difference was considered significant at P<0.05.
Results
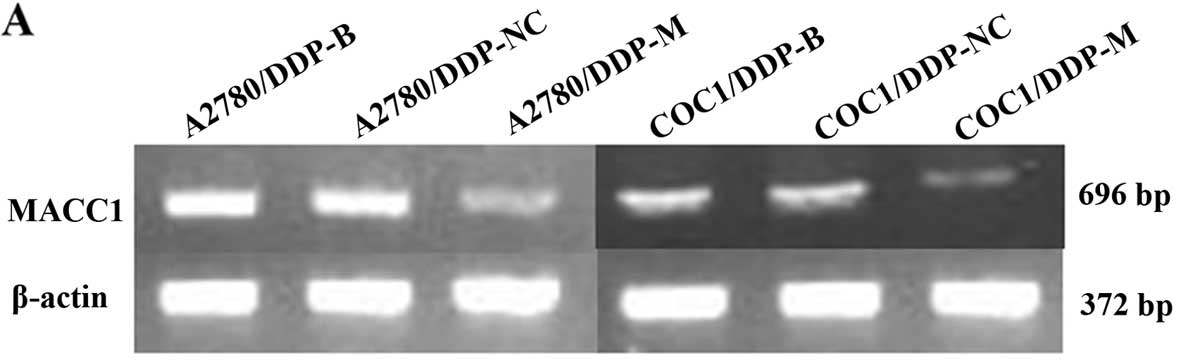
MACC1 mRNA and protein expression is
altered after RNA interference
As a result of MACC1 knockdown, significant
downregulation of MACC1 was observed in the A2780/DDP-M and
COC1/DDP-M cells (P<0.05). No differences were noted between the
A2780/DDP-B and A2780/DDP-NC cells, and between the COC1/DDP-B and
COC1/DDP-NC cells (Fig. 1).
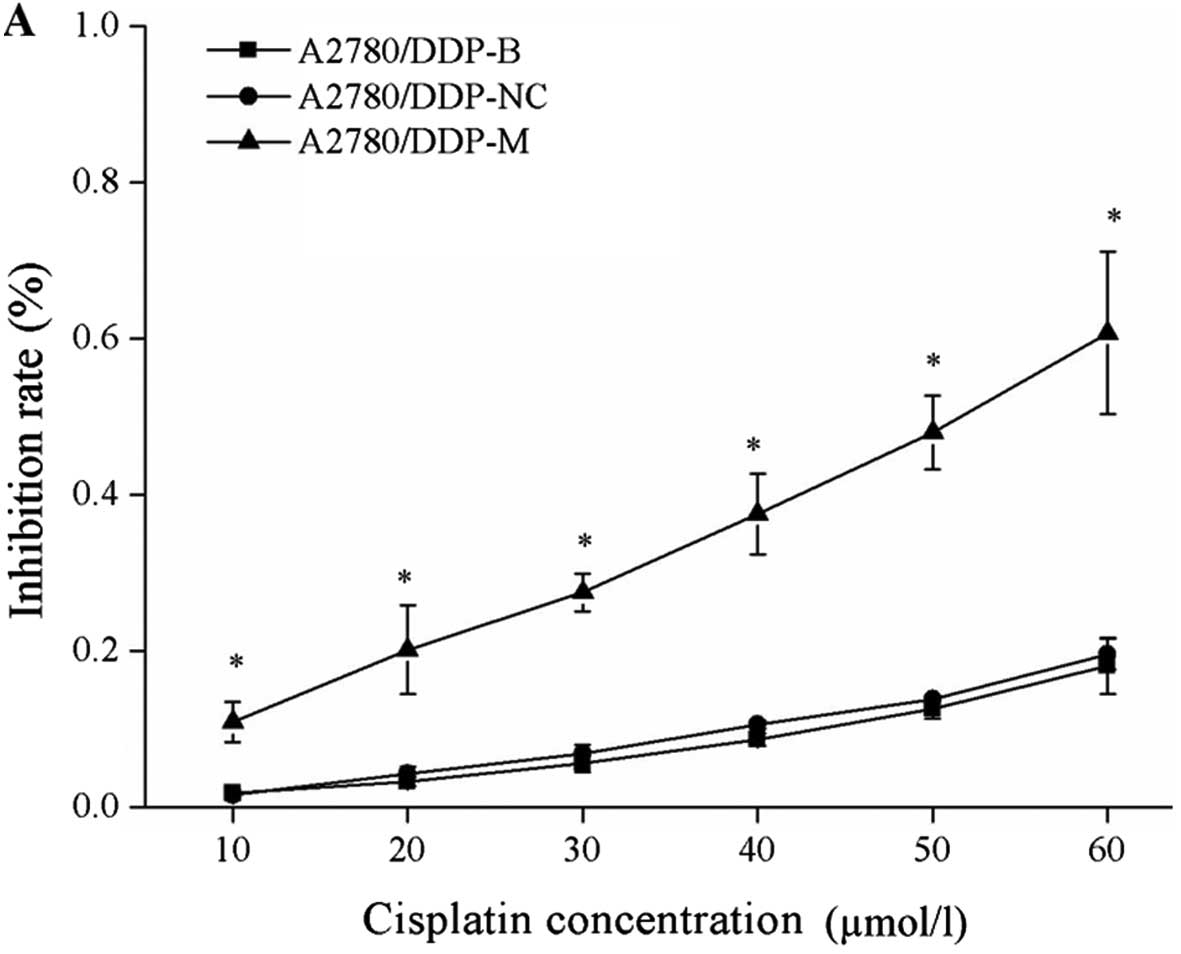
Suppression of cell proliferation after
MACC1 knockdown
After incubation with different concentrations of
cisplatin, cell growth inhibition rates were obviously increased in
the A2780/DDP-M and COC1/DDP-M cells (χ2=9.029, P=0.011;
χ2=6.226, P=0.044). With higher concentrations of
cisplatin, increased rates of cell growth inhibition were noted
(P<0.05). No differences were found between the A2780/DDP-B and
A2780/DDP-NC cells, between the COC1/DDP-B and COC1/DDP-NC cells
(Fig. 2).
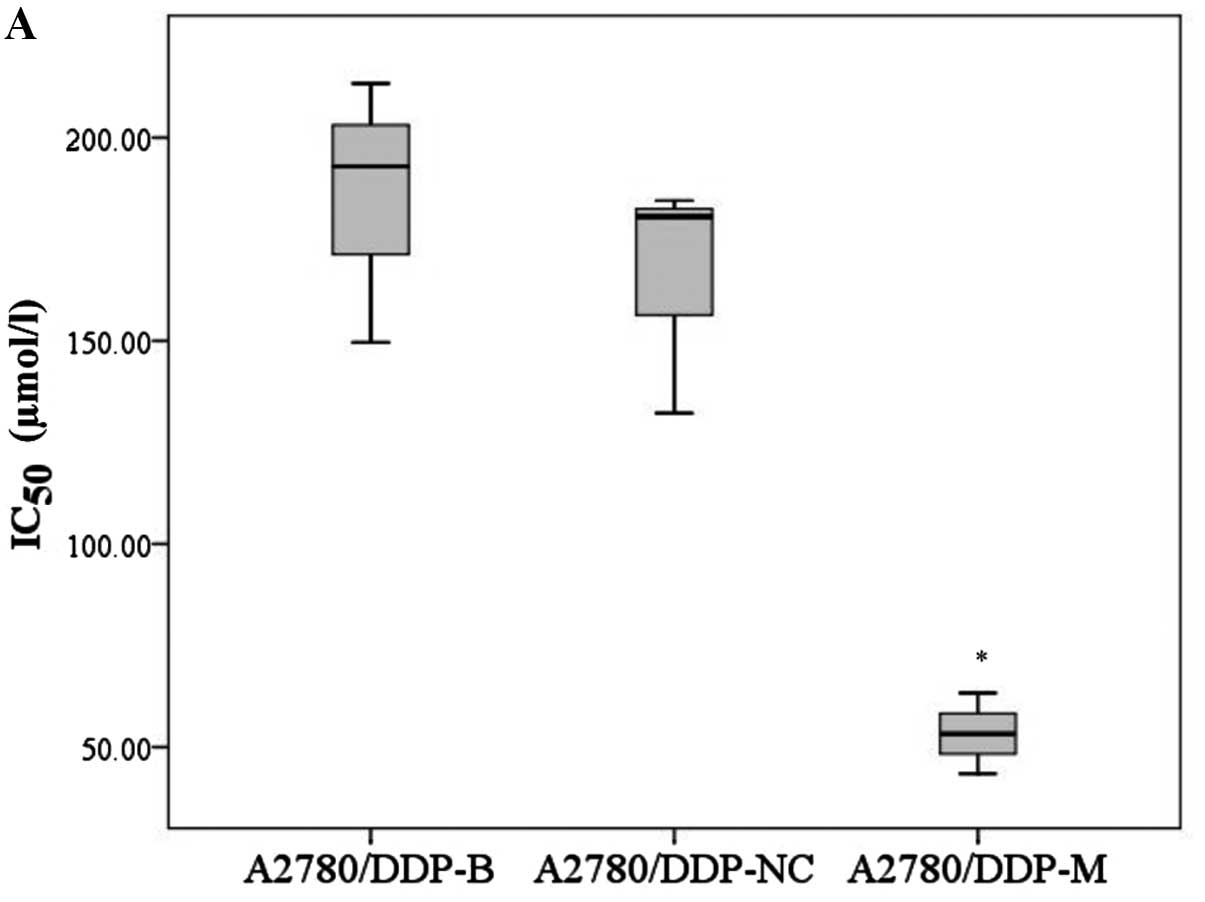
In addition, the IC50 values of
A2780/DDP-M and COC1/DDP-M cells for cisplatin were obviously
decreased compared with the control cells (F=22.760, P=0.002;
χ2=6.489, P=0.039). No differences were noted between
the A2780/DDP-B and A2780/DDP-NC cells, and between the COC1/DDP-B
and COC1/DDP-NC cells (Fig. 3).
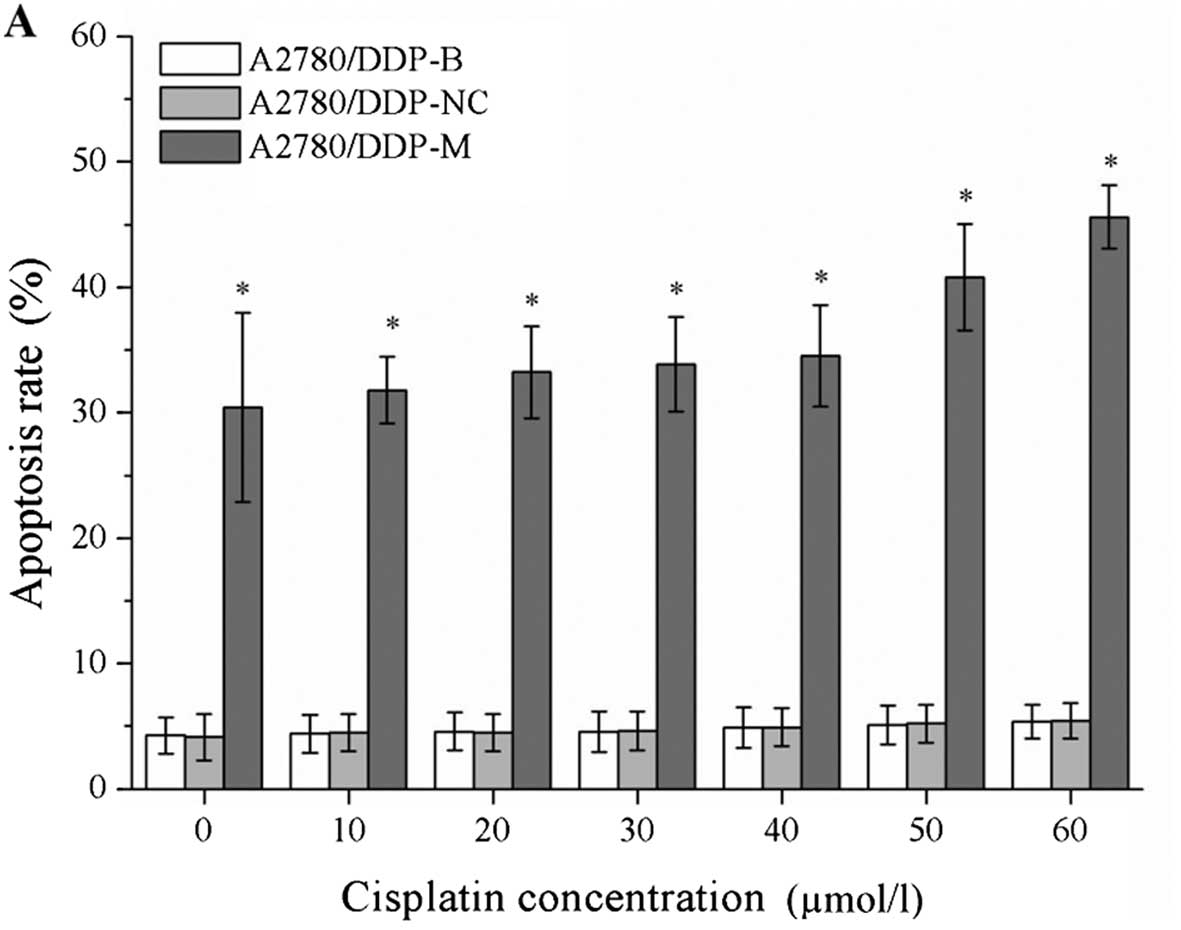
Cell apoptosis is induced by RNA
interference
After incubation with different concentrations of
cisplatin, the cell apoptosis rates were markedly increased in the
A2780/DDP-M and COC1/DDP-M cells (χ2=41.375, P=0.000;
χ2=41.362, P=0.000). For a higher concentration of
cisplatin, greater rates of cell apoptosis were observed
(P<0.05). No differences were noted between the A2780/DDP-B and
A2780/DDP-NC cells, and between the COC1/DDP-B and COC1/DDP-NC
cells (Fig. 4).
Expression of p-ERK1/2, P-gp, Bcl-2,
Bcl-XL, Bax and Bad in the different cell groups
After MACC1 knockdown, obvious downregulation of
p-ERK1/2, P-gp, Bcl-2 and Bcl-XL was detected in the
A2780/DDP-M and COC1/DDP-M cells (P<0.05). In contrast,
upregulation of Bax and Bad was found in the A2780/DDP-M and
COC1/DDP-M cells (P<0.05). No differences in these proteins were
noted between the A2780/DDP-B and A2780/DDP-NC cells, and between
the COC1/DDP-B and COC1/DDP-NC cells (Fig. 5).
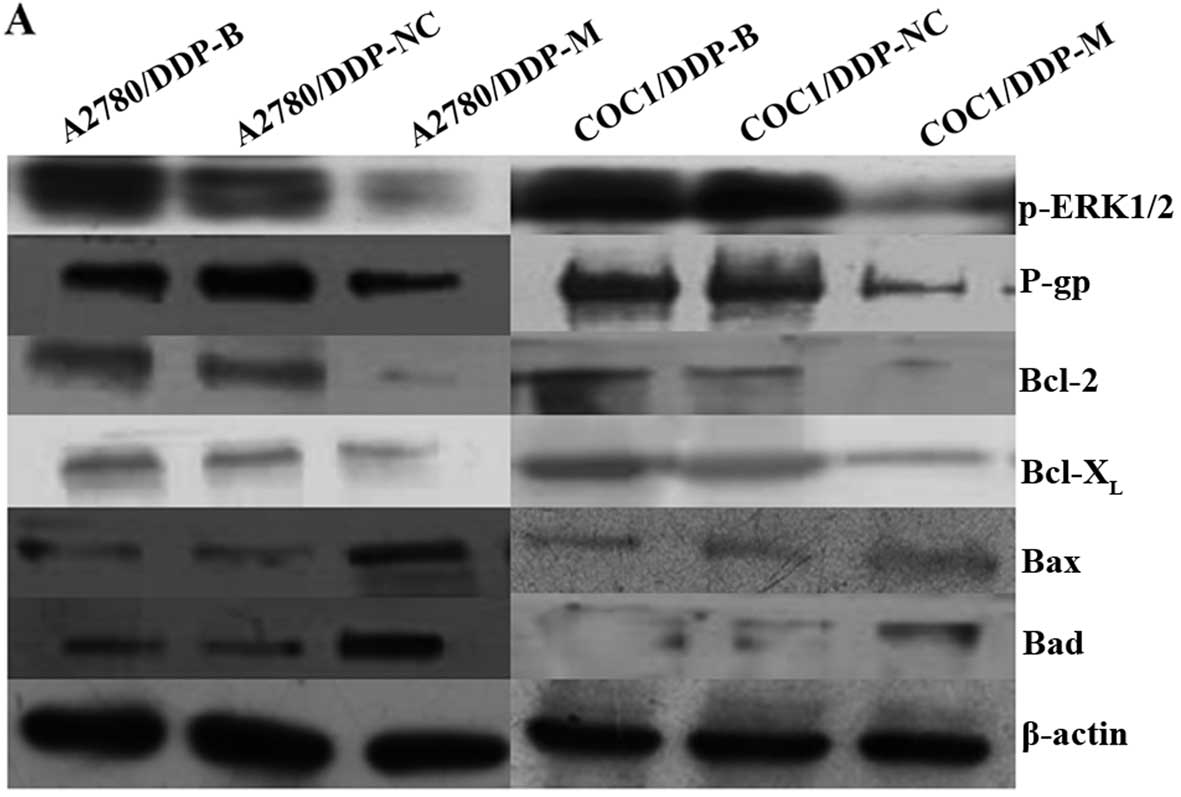 | Figure 5Expression levels of p-ERK1/2, P-gp,
Bcl-2, Bcl-XL, Bax and Bad proteins following MACC1
knockdown as detected by western blotting. (A) Expression levels of
p-ERK1/2, P-gp, Bcl-2, Bcl-XL, Bax and Bad proteins as
detected by western blotting in the different cell groups. (B)
Histogram shows the relative values of p-ERK1/2, P-gp, Bcl-2,
Bcl-XL, Bax and Bad proteins determined by western blotting. Each
bar represents the mean ± SD. *P<0.05 compared with
the blank and negative control groups. |
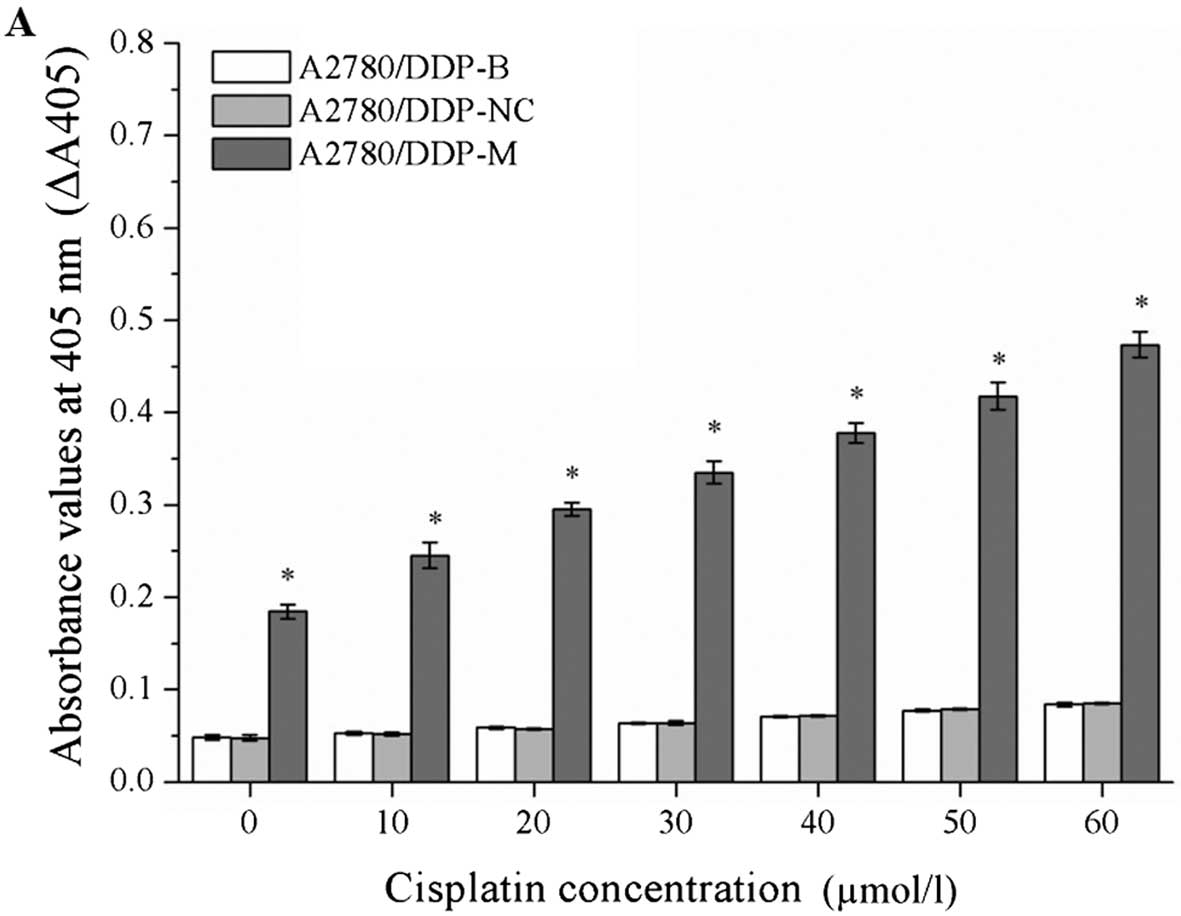
Caspase-3 activity is increased by RNA
interference
After incubation with different concentrations of
cisplatin, the values of ΔA405 were markedly increased in the
A2780/DDP-M and COC1/DDP-M cells (χ2=41.345, P=0.000;
χ2=41.349, P=0.000). At a higher concentration of
cisplatin, a greater value for ΔA405 was observed (P<0.05).
These data indicate that the activity of caspase-3 was increased in
the A2780/DDP-M and COC1/DDP-M cells. No differences were noted
between the A2780/DDP-B and A2780/DDP-NC cells, and between the
COC1/DDP-B and COC1/DDP-NC cells (Fig.
6).
Discussion
Platinum compounds are the mainstay of chemotherapy
for ovarian cancer, particularly for advanced patients. Cisplatin,
a conventional chemotherapy drug, has long-term clinical
application in ovarian cancer (14). Unfortunately, intrinsic or acquired
platinum resistance remains a critical obstacle for chemotherapy
treatment of epithelial ovarian cancer. After tumor cytoreductive
surgery, only 70–80% of ovarian cancer cases are responsive to
platinum combined chemotherapy (15).
MACC1 has been found to be associated with invasion
and metastasis in numerous types of malignant tumors. Our previous
studies showed that MACC1 mRNA and protein were overexpressed in
ovarian cancer tissues and cells. Inhibition of MACC1 by RNA
interference suppressed the invasion and metastatic potential of
ovarian carcinoma cells in vitro and in vivo, and the
antitumor effects of MACC1 knockdown may involve the inhibition of
the MEK/ERK1/2 pathways (10,13).
Recently, it has been reported that small
interfering RNA targeting MACC1 attenuates cisplatin resistance in
tongue squamous cell carcinoma cells (16). However, another research team
claimed that downregulation of MACC1 expression by RNA interference
technology had no effect on cisplatin resistance in salivary
adenoid cystic carcinoma cells (17). The present results showed that MACC1
was obviously downregulated in the A2780/DDP and COC1/DDP cells by
RNA interference. After MACC1 knockdown, cell growth was repressed,
IC50 values of cisplatin were decreased, and the cell
apoptosis rate was increased in the A2780/DDP and COC1/DDP cells
following treatment with different concentrations of cisplatin.
These data indicate that inhibition of MACC1 improves the cisplatin
sensitivity of ovarian cancer cisplatin-resistant cells, and MACC1
may be involved in the chemoresistance of cisplatin in ovarian
cancer.
The MAPK-ERK1/2 pathway has been implicated in cell
survival, anti-apoptosis, invasion, metastasis, angiogenesis and
chemoresistance of malignancies, including ovarian carcinoma
(18–21). P-glycoprotein (P-gp), also known as
MDR1 or the ATP-binding cassette subfamily B member 1, is involved
in the drug resistance of tumor cells. P-gp is important for
transporting drugs across the cell membrane to pump drugs out of
the cells and to decrease intracellular drug concentrations
(22). Inhibition of MDR1 or P-gp
expression can enhance drug chemosensitivity in malignant tumor
cells, including ovarian cancer (23–26).
Chemotherapy drugs play therapeutic roles in cancer through
different mechanisms, mainly by inhibition of cell growth and
induction of cell apoptosis. The Bcl-2 gene family plays key roles
in cell apoptosis and cancer therapy (27). Bcl-2 and Bcl-XL are
important anti-apoptotic proteins; Bax and Bad are pro-apoptotic
proteins. Overexpression of Bcl-2 leads to drug-resistance to
cisplatin in ovarian tumor cells, and Bcl-2 inhibition by gene
silencing induces cell apoptosis and reverses drug resistance
(28,29). Caspase-3 is the most important
apoptotic protease cascade protein in the caspase family, which can
implement programmed cell death under the regulation of Bcl-2
family proteins (30,31).
Following MACC1 knockdown, expression levels of
p-ERK1/2, P-gp, Bcl-2 and Bcl-XL protein were obviously
decreased, while expression levels of Bax and Bad were
significantly increased in this study. Furthermore, caspase-3
activity was markedly increased in the MACC1-knockdown ovarian
cancer cells. These results elucidate the possible mechanisms of
MACC1 involved in the cisplatin-resistance of ovarian cancer cells.
These mechanisms may include the regulation of P-gp expression
through the ERK1/2 signaling pathway, which affects the expression
of pro-apoptotic and anti-apoptotic proteins, and the activity of
caspase-3 downstream.
In conclusion, the present study demonstrated that
suppression of MACC1 improves the chemosensitivity of cisplatin in
epithelial ovarian cancer cells, which may be through regulation of
the ERK1/2 signaling pathway and P-gp and its downstream apoptotic
proteins.
Acknowledgments
We thank the Clinical Medicine Key Disciplines
Laboratory of Henan Province for assistance with the experiments.
Our work was supported by the Medical Key Scientific Research
Project of Henan Province (122102310545) and the Hospital Youth
Fund Project of the First Affiliated Hospital of Zhengzhou
University.
References
|
1
|
Ledermann JA and Kristeleit RS: Optimal
treatment for relapsing ovarian cancer. Ann Oncol. 21(Suppl 7):
vii218–vii222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schorge JO, Modesitt SC, Coleman RL, Cohn
DE, Kauff ND, Duska LR and Herzog TJ: SGO White Paper on ovarian
cancer: Etiology, screening and surveillance. Gynecol Oncol.
119:7–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parmar MK, Ledermann JA, Colombo N, du
Bois A, Delaloye JF, Kristensen GB, Wheeler S, Swart AM, Qian W,
Torri V, et al ICON AGO Collaborators: Paclitaxel plus
platinum-based chemotherapy versus conventional platinum-based
chemotherapy in women with relapsed ovarian cancer: The
ICON4/AGO-OVAR-2.2 trial. Lancet. 361:2099–2106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bookman MA: The addition of new drugs to
standard therapy in the first-line treatment of ovarian cancer. Ann
Oncol. 21(Suppl 7): vii211–vii217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirahata A, Shinmura K, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC1 as a marker for advanced colorectal
carcinoma. Anticancer Res. 30:2689–2692. 2010.PubMed/NCBI
|
|
7
|
Shirahata A, Sakata M, Kitamura Y,
Sakuraba K, Yokomizo K, Goto T, Mizukami H, Saito M, Ishibashi K,
Kigawa G, et al: MACC 1 as a marker for peritoneal-disseminated
gastric carcinoma. Anticancer Res. 30:3441–3444. 2010.PubMed/NCBI
|
|
8
|
Shimokawa H, Uramoto H, Onitsuka T,
Chundong G, Hanagiri T, Oyama T and Yasumoto K: Overexpression of
MACC1 mRNA in lung adenocarcinoma is associated with postoperative
recurrence. J Thorac Cardiovasc Surg. 141:895–898. 2011. View Article : Google Scholar
|
|
9
|
Shirahata A, Fan W, Sakuraba K, Yokomizo
K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, et
al: MACC 1 as a marker for vascular invasive hepatocellular
carcinoma. Anticancer Res. 31:777–780. 2011.PubMed/NCBI
|
|
10
|
Zhang RT, Shi HR, Huang HL, Chen ZM, Liu
HN and Yuan ZF: Expressions of MACC1, HGF, and C-met protein in
epithelial ovarian cancer and their significance. Nan Fang Yi Ke Da
Xue Xue Bao. 31:1551–1555. 2011.In Chinese. PubMed/NCBI
|
|
11
|
Wang G, Kang MX, Lu WJ, Chen Y, Zhang B
and Wu YL: MACC1: A potential molecule associated with pancreatic
cancer metastasis and chemoresistance. Oncol Lett. 4:783–791.
2012.PubMed/NCBI
|
|
12
|
Shang C, Hong Y, Guo Y, Liu YH and Xue YX:
Influence of the MACC1 gene on sensitivity to chemotherapy in human
U251 glioblastoma cells. Asian Pac J Cancer Prev. 16:195–199. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang R, Shi H, Chen Z, Wu Q, Ren F and
Huang H: Effects of metastasis-associated in colon cancer 1
inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells.
J Exp Clin Cancer Res. 30:832011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen S, Jiao JW, Sun KX, Zong ZH and Zhao
Y: MicroRNA-133b targets glutathione S-transferase π expression to
increase ovarian cancer cell sensitivity to chemotherapy drugs.
Drug Des Devel Ther. 9:5225–5235. 2015.
|
|
15
|
Zhang Z, Xie Z, Sun G, Yang P, Li J, Yang
H, Xiao S, Liu Y, Qiu H, Qin L, et al: Reversing drug resistance of
cisplatin by hsp90 inhibitors in human ovarian cancer cells. Int J
Clin Exp Med. 8:6687–6701. 2015.PubMed/NCBI
|
|
16
|
Li HF, Liu YQ, Shen ZJ, Gan XF, Han JJ,
Liu YY, Li HG and Huang ZQ: Downregulation of MACC1 inhibits
invasion, migration and proliferation, attenuates cisplatin
resistance and induces apoptosis in tongue squamous cell carcinoma.
Oncol Rep. 33:651–660. 2015.
|
|
17
|
Li H, Liao X, Liu Y, Shen Z, Gan X, Li H
and Huang Z: The expression of MACC1 and its role in the
proliferation and apoptosis of salivary adenoid cystic carcinoma. J
Oral Pathol Med. 44:810–817. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
19
|
Nicosia SV, Bai W, Cheng JQ, Coppola D and
Kruk PA: Oncogenic pathways implicated in ovarian epithelial
cancer. Hematol Oncol Clin North Am. 17:927–943. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin W, Wu L, Liang K, Liu B, Lu Y and Fan
Z: Roles of the PI-3K and MEK pathways in Ras-mediated
chemoresistance in breast cancer cells. Br J Cancer. 89:185–191.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montagut C and Settleman J: Targeting the
RAF-MEK-ERK pathway in cancer therapy. Cancer Lett. 283:125–134.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Andorfer P and Rotheneder H: Regulation of
the MDR1 promoter by E2F1 and EAPP. FEBS Lett. 587:1504–1509. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Ding Z, Peng Y, Pan F, Li J, Zou
L, Zhang Y and Liang H: HIF-1α inhibition reverses multidrug
resistance in colon cancer cells via downregulation of
MDR1/P-glycoprotein. PLoS One. 9:e988822014. View Article : Google Scholar
|
|
24
|
Wang Q, Wang Z, Chu L, Li X, Kan P, Xin X,
Zhu Y and Yang P: The effects and molecular mechanisms of miR-106a
in multidrug resistance reversal in human glioma U87/DDP and U251/G
cell lines. PLoS One. 10:e01254732015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Januchowski R, Wojtowicz K,
Sujka-Kordowska P, Andrzejewska M and Zabel M: MDR gene expression
analysis of six drug-resistant ovarian cancer cell lines. Biomed
Res Int. 2013:2417632013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang T, Guan M, Jin HY and Lu Y: Reversal
of multidrug resistance by small interfering double-stranded RNAs
in ovarian cancer cells. Gynecol Oncol. 97:501–507. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thomas S, Quinn BA, Das SK, Dash R, Emdad
L, Dasgupta S, Wang XY, Dent P, Reed JC, Pellecchia M, et al:
Targeting the Bcl-2 family for cancer therapy. Expert Opin Ther
Targets. 17:61–75. 2013. View Article : Google Scholar
|
|
28
|
Babu A, Wang Q, Muralidharan R, Shanker M,
Munshi A and Ramesh R: Chitosan coated polylactic acid
nanoparticle-mediated combinatorial delivery of cisplatin and
siRNA/Plasmid DNA chemosensitizes cisplatin-resistant human ovarian
cancer cells. Mol Pharm. 11:2720–2733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang HL, Lin KY, Juan YC, Kumar KJ, Way
TD, Shen PC, Chen SC and Hseu YC: The anti-cancer activity of
Antrodia camphorata against human ovarian carcinoma (SKOV-3) cells
via modulation of HER-2/neu signaling pathway. J Ethnopharmacol.
148:254–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galluzzi L, Kepp O and Kroemer G:
Caspase-3 and prostaglandins signal for tumor regrowth in cancer
therapy. Oncogene. 31:2805–2808. 2012. View Article : Google Scholar
|
|
31
|
Zhang SD, Shan L, Li W, Li HL and Zhang
WD: Isochamaejasmin induces apoptosis in leukemia cells through
inhibiting Bcl-2 family proteins. Chin J Nat Med. 13:660–666.
2015.PubMed/NCBI
|