Introduction
Lung cancer, the most common type of cancer,
represents a major public health problem worldwide (1,2). Lung
cancers have been ranked as the main cause of cancer-related
morbidity and mortality (3–5). Unfortunately, lung cancer therapy has
not been explored extensively in the field of pharmaceutics
(6). Because of the poor outcomes
associated with lung cancer, drugs with high efficacy are needed to
treat this malignancy (7–9).
Small molecular agents have previously exhibited
efficacy in anticancer therapies (10,11).
YL4073 has thus far exhibited a profound effect on liver cancer
(12). It is possible that YL4073
may have novel pharmacological application as a medicinal product
with inhibitory activity in other cancers, such as Lewis lung
cancer.
Autophagy could induce cell death and is activated
in response to stress and nutrient deprivation (13). Autophagy is a complex catabolic
mechanism of lysosomal degradation of proteins and other
sub-cellular constituents (14–16).
Several signaling pathways have been identified involved in cancer
associated with the response to autophagy (17,18).
Although autophagy has different roles in the modulation of cancers
(19,20), it is known as a mechanism of cell
death and tumor suppression (21–23).
In the present study, we identified YL4073 as a
potent anticancer agent capable of inducing tumor cell autophagy.
We focused our research mainly on lung carcinoma, owing to its poor
prognosis and lack of effective therapies for this tumor type in
clinical settings, in an attempt to provide preclinical study
profiles for clinical treatment. We have shown that YL4073, a small
molecular agent, was a potent targeted autophagy associated
protein, which induced autophagy in LL/2 cells. Cell autophagy was
followed by apoptosis indicating the involvement of
Akt/m-TOR/p70S6K and TSC/MAPK/AMPK pathways. In addition, YL4073
significantly inhibited the growth of LL/2 tumors in vivo.
Our results indicated that YL4073 has significant anticancer
activity and autophagy was suggested to be involved in this
activity. To the best of our knowledge, this study was the first to
demonstrate that YL4073 was able to induce autophagy in cancer
cells.
Materials and methods
Materials
Dimethyl sulfoxide (DMSO), propidium iodide (PI),
acridine orange, Z-VAD-FMK, and 3-methyladenine (3-MA) were
purchased from Sigma Chemical Co. (St. Louis, MO, USA). Cell
Counting Kit-8 (CCK-8) was purchased from Dojindo Laboratory
(Dojin, Japan); all chemicals employed in this study were of pure
analytic and culture grade. The primary antibodies for LC-3/LC3-II,
Beclin 1, Atg5/Atg12, p-mTOR, p-Akt, p-P70S6K, p-TSC, p-AMPK,
p-p44/42 MAPK, P53, PTEN, and p-Histone H3 were purchased from Cell
Signaling Technology (Beverly, MA, USA); horseradish peroxidase
(HRP)-conjugated anti-rabbit/mouse secondary antibodies were
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
β-actin and GAPDH were obtained from Boster Biotechnology (Wuhan,
China). Protein assay kit was purchased from Bio-Rad (Hercules, CA,
USA). For all in vitro assays, YL4073 and 3-MA were
dissolved in DMSO to prepare a stock solution of 40 mM and 5 M,
respectively, and stored at 4°C. The stock solutions were diluted
in the relevant media to the final DMSO concentration of 0.05% v/v
when used. For all in vivo studies, YL4073 was suspended in
ultra-pure water and Cremophor EL/ethanol (50:50, Cremophor EL, 95%
ethyl alcohol; Sigma Chemical Co.) and administered at 10 ml/kg/day
of body weight by intraperitoneal injection.
Cell culture
Murine Lewis lung carcinoma LL/2, mammary carcinoma
cell 4T1, fibroblast NIH-3T3, and human proximal tubular cell human
kidney-2 (HK-2) cells were obtained from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in RPMI-1640 or
Dulbecco's modified Eagle's medium (DMEM; Life Technologies,
Bedford, MA, USA) containing 10% heat-inactivated FBS (Gibco-BRL,
Grand Island, NY, USA), 100 U/ml penicillin, and streptomycin in a
humid chamber at 37°C and 5% CO2.
Cell viability by CCK-8 assay
Cells were plated in 96-well plates for 24 h and
cultured with YL4073 for 72 h; 10 µl CCK-8 solution was
added to each well. The IC50 of YL4073 was calculated
and optical density (OD) was measured at 450 nm with a Multiskan
Spectrum instrument (Thermo Lab Systems, USA) after cells were
incubated with CCK-8 for 2-4 h at 37°C.
EdU-DNA incorporation assay
5-Ethynyl-2′-deoxyuridine (EdU) is a nucleoside
analogue of thymidine, which can be incorporated into DNA during
DNA synthesis. An EdU-DNA incorporation assay kit (RiboBio, China)
was used to detect the percentage of uptake in proliferating LL/2
cells. Briefly, 3×103 LL/2 cells were seeded in 96-well
plates for 24 h and treated with YL4073 for 48 h. Then, the cells
were incubated with EdU for 2–3 h at 37°C, and the plates observed
with an inverted fluorescent microscope (Carl Zeiss, Germany).
EdU-positive cells were counted in five fields per well; the
average was calculated according to the following formula: EdU (%)
= (EdU-positive cells)/(Hoechst-positive cells) × 100. The
experiment was done in triplicate.
Detection of acidic vesicular
organelles
For detection of acidic vesicular organelles (AVO)
in vitro (24),
1×105 LL/2 cells were plated in 6-well plates and
treated with 20 µM YL4073 or 2 mM 3-MA for 48 h. The cells
were then stained with 1 µg/ml acridine orange for 15 min,
after which images were obtained under a fluorescent microscope
equipped with a digital camera (25,26).
Autophagy and apoptosis analysis by flow
cytometry (FCM)
To further confirm autophagy of the cells, we first
analyzed LL/2 cells by FCM after AVO staining. Cell culture and
drug treatment were carried out as described above. After being
treated with 1 µg/ml AVO for 10 min, cells were collected
and immediately analyzed by FCM (Beckman Coulter, Miami, FL, USA).
Furthermore, LL/2 cells were treated with YL4073 for 48 h. Cells
were collected and incubated with 1 ml hypotonic fluorochrome
solution containing 50 ng/ml PI in 0.1% sodium citrate plus 0.1%
Triton X-100, and were immediately analyzed by FCM. Finally, the
cell-permeable caspase inhibitor Z-VAD-FMK and autophagy inhibitor
3-MA were used to study whether caspase family protein kinases
and/or autophagy was involved in YL4073-induced apoptosis and
autophagy. For this assay, LL/2 cells were treated with 20
µM YL4073 combined with 10 mM Z-VAD-FMK or 2 mM 3-MA,
respectively. PI staining was performed and analyzed by FCM 24 and
48 h later.
Western blot analysis
Standard western blot analysis was performed to
identify the possible mechanism of YL4073. Briefly, LL/2 cells were
treated by YL4073 for 48 h and cell proteins were extracted.
Western blotting was further examined via electrophoretic transfer
of sodium dodecyl sulphate-polyacrylamide gel electrophoresis
(SDS-PAGE), separation of proteins on polyvinylidene fluoride
(PVDF) membranes (Millipore, Billerica, MA, USA), and incubation
with primary and secondary antibodies. Protein bands were
visualized using an enhanced chemiluminescence kit (Amersham
Biosciences Corp., Piscataway, NJ, USA).
Pharmacokinetics analysis of YL4073 in SD
rats
SD male rats (weight, 180–200 g) were obtained from
the Beijing Animal Center (Beijing, China) received an
intraperitoneal injection with a single dose of 30 mg/kg YL4073 and
the pharmacokinetic profiles were determined by high-pressure
liquid chromatography (HPLC; Waters, USA). Briefly, the blood of SD
rats (n=3) was collected in heparinized tubes at designated
time-points after drug administration and centrifuged immediately
to separate plasma from blood cells. Samples (90 µl) were
mixed with 100 µl of acetonitrile containing 10 µl
internal standards (5 µg/ml) and centrifuged at 6,000 × g
for 10 min. Then, 100 µl supernatant was evaporated and
re-dissolved in a solution of 50 µl acetonitrile: water
50:50 v/v containing 10% formic acid and subjected to HPLC.
Pharmacokinetic parameters were analyzed using a Pharmacokinetic
software of Drug and Statistics (DAS, edited by Mathematical
Pharmacology Professional Committee of China, version 2.1.1). All
animal experiment protocols were conducted in full compliance with
our universities for the Care and Use of Laboratory Animals and
Experimental Animal Ethics Committee.
Pharmacodynamic analysis of YL4073 in
vivo
Female C57BL/6 mice (6–8 week-old) were obtained
from the Beijing Animal Center (Beijing, China) and used in the
present study. Each mouse received a single injection of harvested
LL/2 cells on the flank in the axillary region for the subcutaneous
mouse tumor model (27). The tumors
were allowed to grow for ten days, and the animals were
subsequently sorted into groups of ten for treatment. Tumor volume
was measured in two dimensions with vernier calipers and calculated
using the following formula: Tumor volume = (length ×
width2) × 0.5 (28).
TUNEL assay in vivo
TUNEL [terminal deoxyribonucleotidyl transferase
(TDT)-mediated dUTP-digoxigenin nick end labeling] assay was
performed to examine the apoptosis induction effect of YL4073 on
LL/2 cells in vivo. LL/2 tumor sections from vehicle and
YL4073-treated mice were subjected to TUNEL assay according to the
manufacturer's instruction. TUNEL-positive cells were counted under
a microscope in three equal-sized fields per slide for quantitative
analysis of apoptotic cells. The percentage of apoptotic cells was
evaluated as follows: Tumor apoptotic index (%) = apoptotic
cells/total cells × 100 (12).
Immunohistochemical analysis in vivo
Immunohistochemical analysis was used to detect the
autophagy induction effect of YL4073 on LL/2 cells in vivo.
LL/2 tumor sections from vehicle and YL4073-treated mice were
incubated with primary LC3-II antibody and the corresponding second
antibody according to the manufacturer's instruction.
Representative images were then observed under an inverted
microscope with a camera system, in three equal-sized fields per
slide (Carl Zeiss) (12).
Statistical analysis
The data were analyzed by SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA) and presented as mean ± SD/SEM.
Kaplan-Meier curves were used to analyze survival and tested with a
log-rank test.
Results
YL4073 inhibited proliferation of murine
cancer cells in vitro
CCK-8 assay was used to measure the effect of YL4073
on cell viability. Our results showed that YL4073 decreased the
viability of 4T1 and LL/2 cell lines with IC50 values of
9.33 and 5.29 µM after treatment for 72 h, respectively
(Table I). LL/2 cells were used to
further study the mechanism of YL4073 in Lewis lung carcinoma. The
IC50 for NIH-3T3 and HK-2 cell lines was >40
µM, higher than that for the cancer cell line.
 | Table IThe effects of YL4073 on tumor cell
viability. |
Table I
The effects of YL4073 on tumor cell
viability.
| Cell line | Cell type | IC50
(µM) |
|---|
| LL/2 | Murine Lewis lung
carcinoma cell line | 5.29 |
| 4T1 | Mouse mammary
carcinoma cell line | 9.33 |
| NIH-3T3 | Mouse fibroblasts
cell line | >40 |
| HK-2 | Human proximal
tubular cell line | >40 |
Anti-proliferation effects of YL4073 in
LL/2 cells in vitro
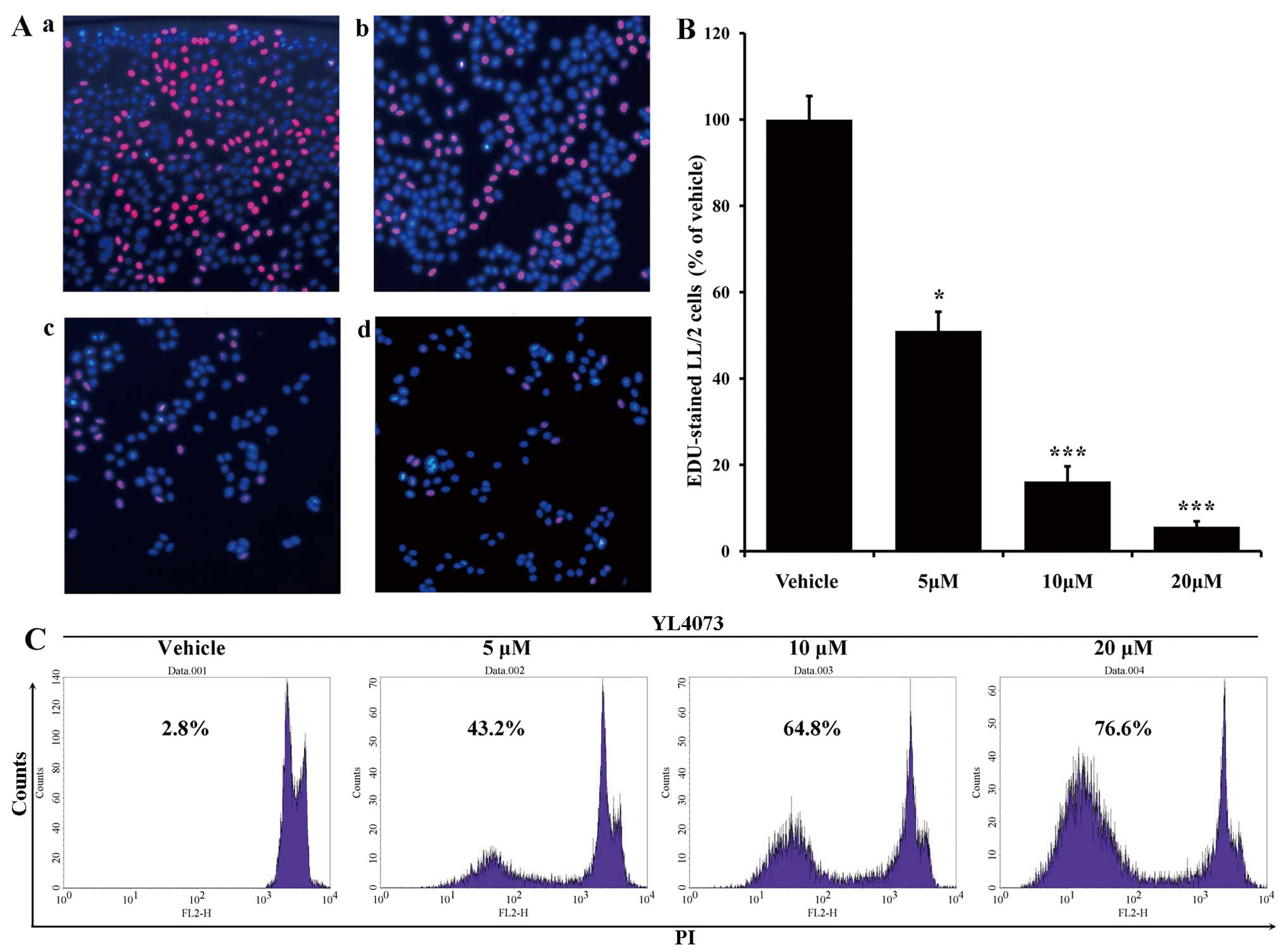
The EdU-DNA incorporation assay validated the
anti-proliferation ability of YL4073. As shown in Fig. 1B and C, the percentage of
EdU-positive cells was 51.05% after 5 µM YL4073 compared
with that after vehicle treatment, whereas the percentage of
EdU-positive cells decreased to 16.18% and 5.67% when cells were
treated with 10 and 20 µM YL4073, respectively.
Effects of YL4073 on LL/2 cell apoptosis
in vitro
Morphological changes of LL/2 cells (stained with
PI) were assessed after treatment with YL4073. As shown in Fig. 1, the percentage of sub-G1 cells in
the YL4073-treated group increased in a concentration-dependent
manner. After cells were treated with 5 µM YL4073, the
apoptosis rate was 43.20%; the rate of apoptosis increased to
64.80% and 76.60% after cells were treated with 10 and 20 µM
YL4073 for 48 h, respectively.
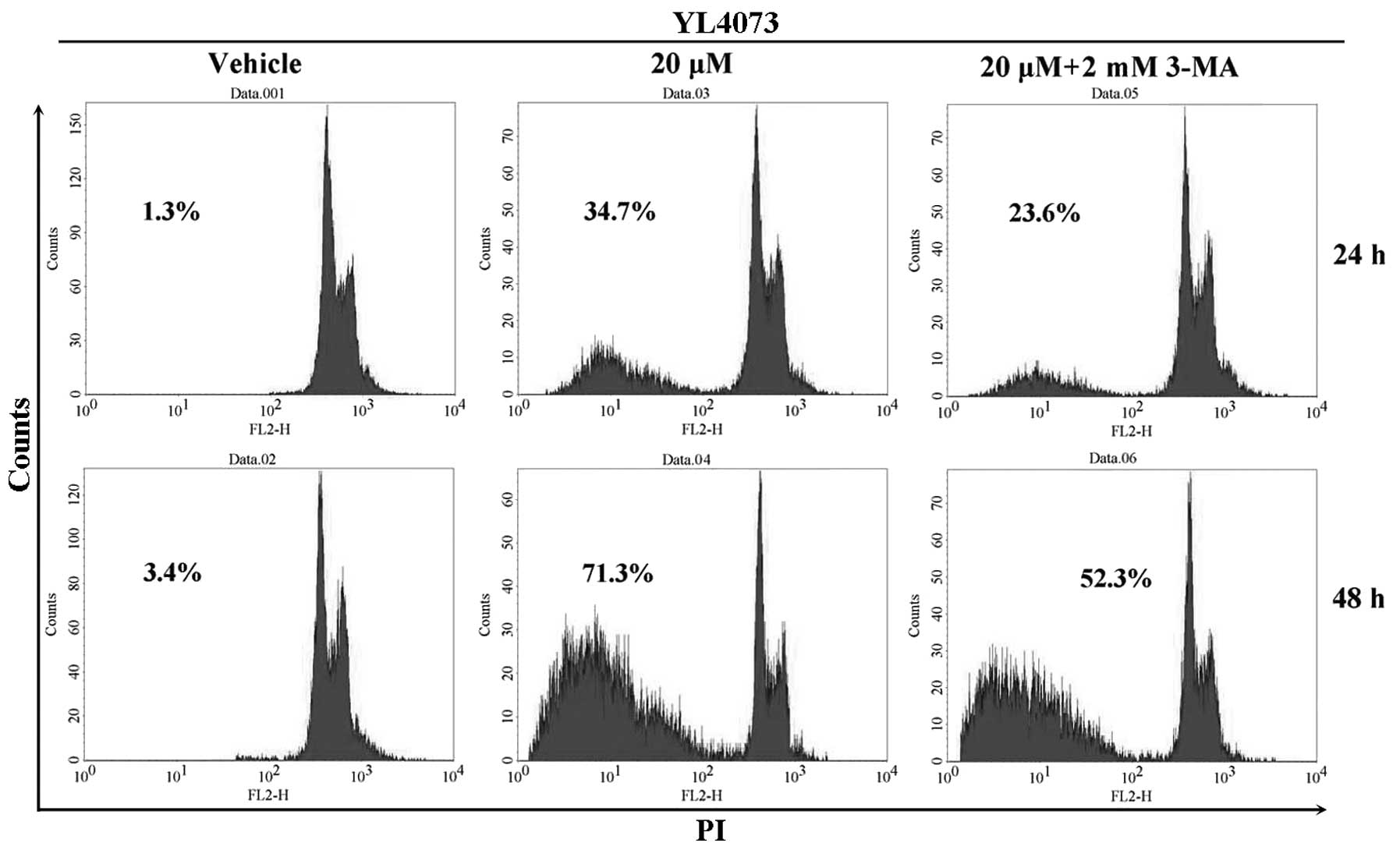
Effect of caspase on YL4073-induced
apoptosis
The effect of autophagy inhibitor 3-MA on
YL4073-induced apoptosis was assayed by FCM. The rate of apoptosis
after treatment with 20 µM YL4073 plus 2 mM 3-MA decreased
from 34.7 to 23.6% and from 71.3% to 52.3% after 24 and 48 h
treatment, respectively, compared with that after YL4073 treatment
alone (Fig. 2). Furthermore,
similar results were observed in caspase inhibitor
Z-VAD-FMK-treated LL/2 cells (data not shown). These results
suggest that YL4073-induced apoptosis in LL/2 cells is associated
with autophagy, which may be one of mechanisms of the anticancer
effect of YL4073.
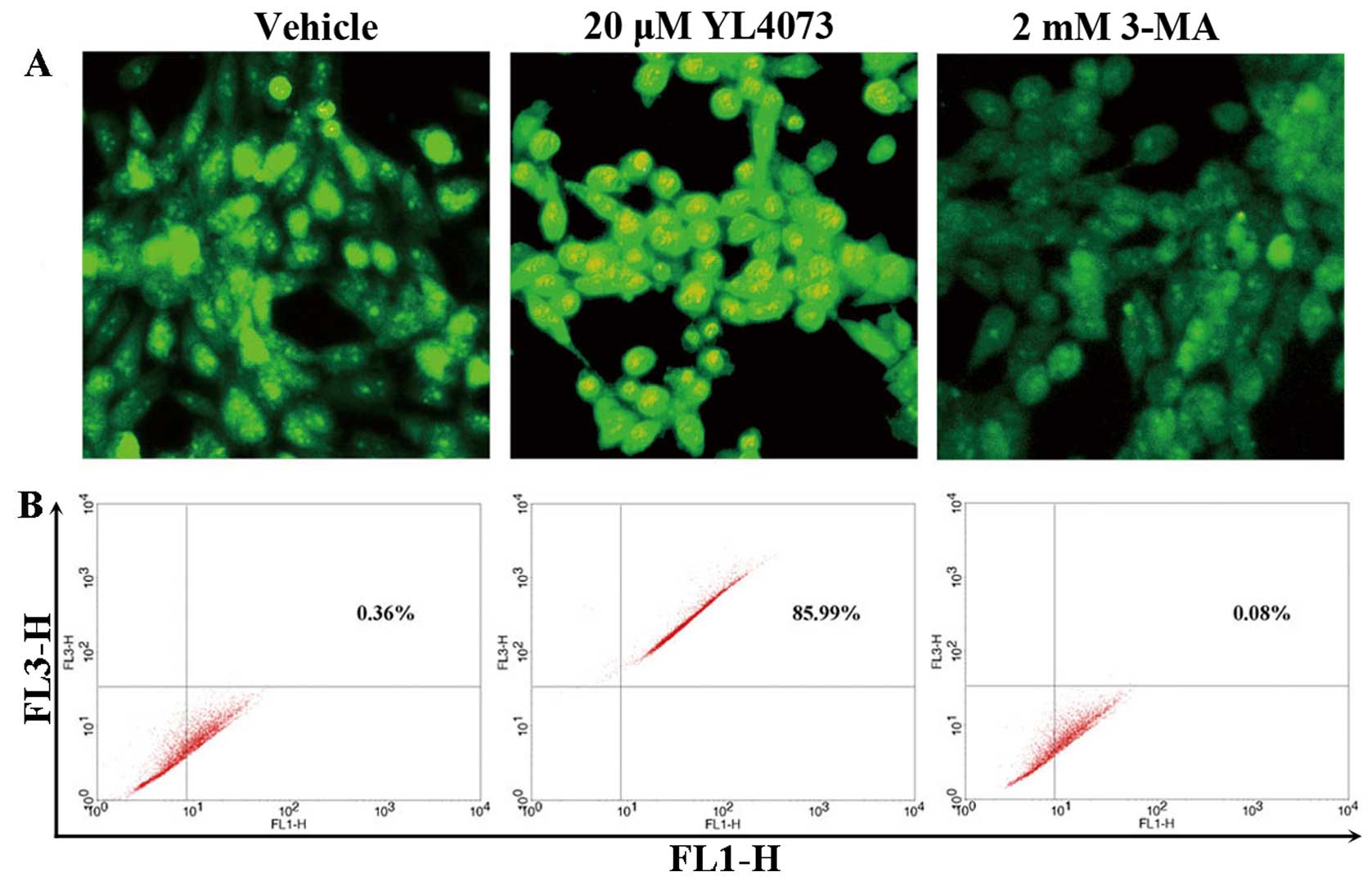
Induction of autophagy in LL/2 cells
examined with AVO assay
Cell death by autophagy has been proposed recently
(29,30). Autophagy is characterized by AVO
formation, which was detected and measured by staining with
acridine orange (31,32). As shown in Fig. 3A, staining LL/2 cells with acridine
orange showed the accumulation of AVO in the cell cytoplasm after
exposure to 20 µM YL4073. This was inhibited by addition of
2 mM 3-MA, which inhibited autophagosome sequestration (33,34).
FCM analysis was used to quantify the YL4073-induced increase in
the fractional volume and acidity of AVO. As shown in Fig. 3B, 20 µM YL4073 increased the
intensity of fluorescence in LL/2 cells to 85.99% compared with
vehicle, indicating that 2 mM 3-MA suppressed the development of
AVO in LL/2 cells. YL4073 significantly affected development of AVO
in LL/2 cells compared with vehicle (P<0.01), and the inhibitory
effect of 3-MA on development of AVO was also significant
(P<0.05).
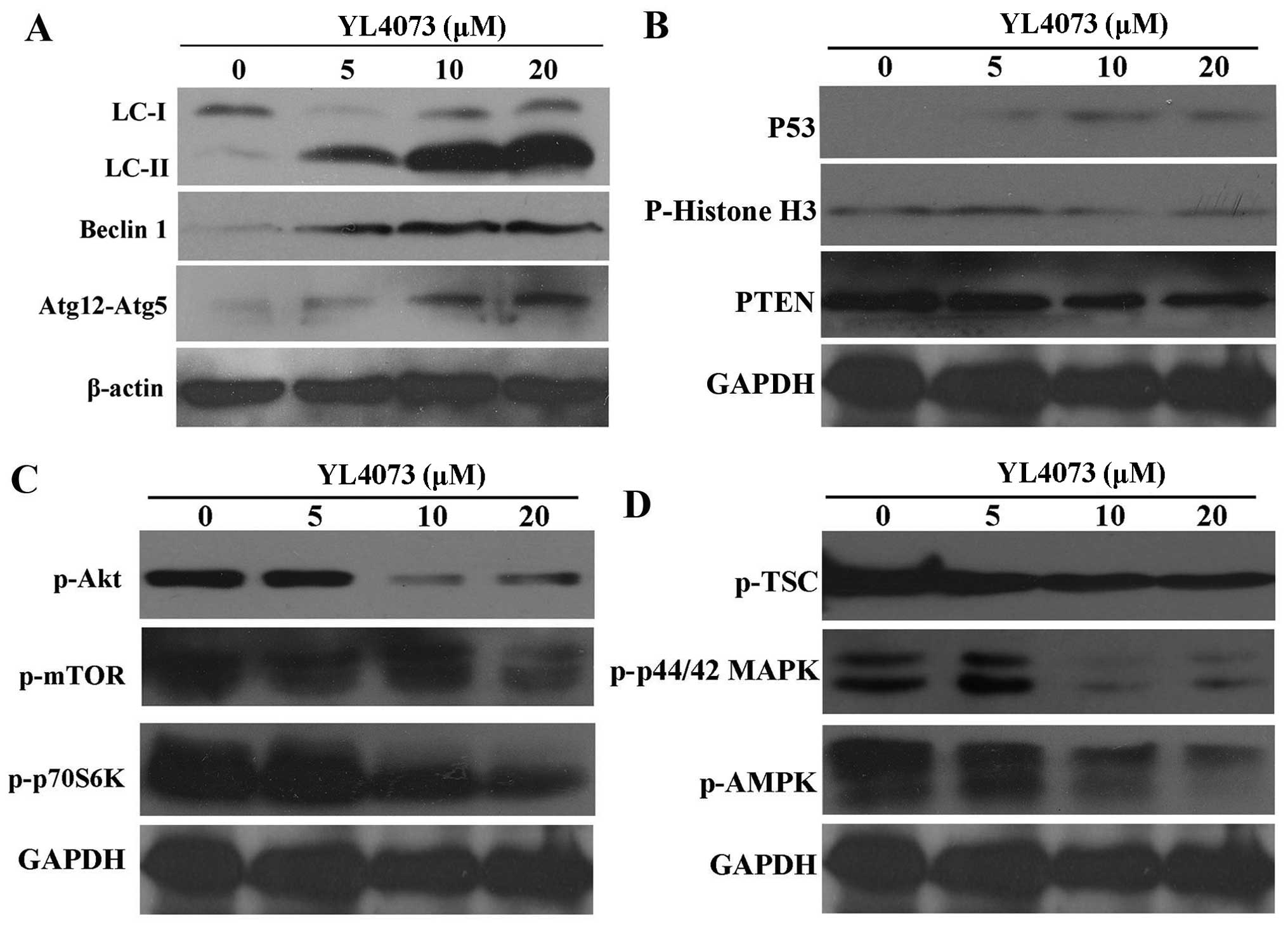
YL4073 induces autophagosome formation in
LL/2 cells
Western blotting was performed to monitor the
alteration of YL4073-mediated autophagy (34). As shown in Fig. 4A, LC3-II protein was detectable in
LL/2 cells following YL4073 treatment for 48 h. Activation of a
series of autophagy proteins was a critical step for autophagosome
formation. To determine whether the key autophagy-related proteins
were synergistically activated in response to YL4073-induced
autophagy, the expression of Beclin 1 and the key regulator of
autophagy, Atg12/Atg5 conjugate, were assessed in LL/2 cells. As
shown in Fig. 4A, YL4073 induced a
marked accumulation of Beclin 1 and intracellular Atg12-Atg5
complex after 48 h compared with vehicle; moreover, p-histone H3
was significantly inhibited by YL4073, while the expression of P53
was slightly activated (Fig. 4B).
Collectively, these observations suggest that YL4073 induces
autophagy and apoptosis in LL/2 cells.
YL4073 inhibits Akt/mTOR/p70S6K and
activates TSC/MAPK/AMPK pathway in LL/2 cells
Western blot analysis was also used to evaluate the
effect of YL4073 on the Akt/mTOR/p70S6K pathway, because it is the
main pathway that down-regulates autophagy (35). Treatment with YL4073 decreased
phosphorylated Akt (p-Akt) effectively for 48 h in LL/2 cells;
p-mTOR activity was also affected (Fig.
4C). These results suggest that the upstream pathway of Akt was
influenced by YL4073 treatment. Furthermore, YL4073 decreased
p-p70S6K and p-TSC gradually in LL/2 cells after 48 h.
Because the AMPK/MAPK pathway upregulated autophagy
in starved cancer cells (36), we
examined the effects of YL4073 treatment on this pathway. As shown
in Fig. 4D, YL4073 decreased p-AMPK
and p-p44/42MAPK for 48 h in LL/2 cells. Collectively, these
results indicated that YL4073 could inhibit both the
Akt/mTOR/p70S6K pathway and the AMPK/MAPK pathway, and both changes
potentially mediated YL4073-induced autophagy.
Pharmacokinetic and pharmacodynamic
profile of YL4073
YL4073 was used as an anticancer therapeutic agent
model in a pharmacokinetics study. We determined certain
pharmacokinetic parameters of YL4073 in blood serum of SD rats by
HPLC. A concentration versus time curve was measured in serum at
each time-point. Our results shown that the plasma concentration of
YL4073 at 5 min was 5.53 µg/ml after treatment with a 30
mg/kg dose. Pharmacokinetic modelling suggested that the plasma
concentration-time profile can be described using a two-compartment
model with an estimated t1/2 of 4.4 h and urinary
clearance representing 5.1% of systemic clearance, with substantial
variation in the plasma concentration. The AUC was 11.9 mg/l per
hour. We used AUC to estimate the efficacy of YL4073; it was also
used to predict toxicity. The results indicated that YL4073 had
high efficacy and low toxicity.
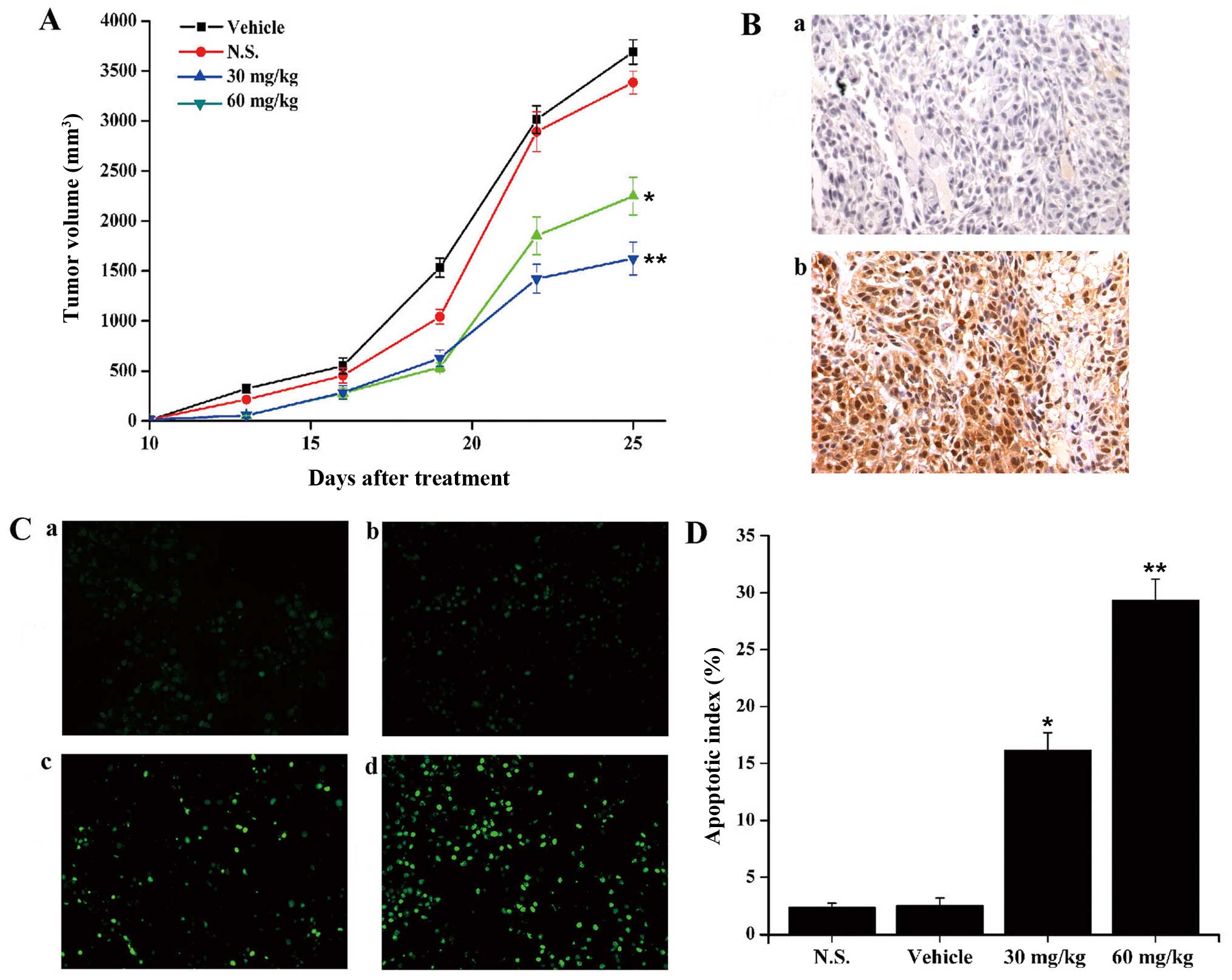
We evaluated whether YL4073 inhibited the growth of
LL/2 subcutaneous tumors by inducing apoptosis and autophagy in
vivo. C57BL/6 mice were inoculated subcutaneously with LL/2
cells, and after ten days, tumor volume reached about 50 to 70
mm3. Intraperitoneal injections of 60 mg/kg/day YL4073,
30 mg/kg/day YL4073, vehicle, and N.S. were administered to
different groups. Tumor growth was observed for 14 days after the
initiation of treatment. On day 14, there was 29.1% and 52.6% tumor
growth inhibition in 60 mg/kg/day and 30 mg/kg/day YL4073 groups
compared with vehicle (P<0.05; Fig.
5A). In addition, immunohistochemical analysis and TUNEL assay
was used to examine whether YL4073 induced autophagy and apoptosis
in vivo. As shown in Fig.
5B–D, the expression levels of LC3-II increased, and the number
of apoptotic cells in YL4073-treated groups was more than that in
the vehicle group, after treatment with YL4073 in vivo. The
results were consistent with the results of WB in vitro.
These results suggest that YL4073 inhibited LL/2 tumor growth in
vivo by inducing autophagy and apoptosis.
Discussion
Although notable progress has been made in the
management of advanced lung cancer, many challenges remain
(37). Chemotherapy has been the
primary treatment for patients with advanced lung cancers (38). However, recent studies suggest no
significant improvement in survival rate in these patients
(39). There is an urgent,
unanswered medical necessity for the development of rational and
effective therapies to treat advanced malignant lung cancers
(40). In this study, we
demonstrated that small molecular compound YL4073 induced autophagy
and apoptosis in murine Lewis lung cancer LL/2 cells both in
vitro and in vivo.
YL4073 has significant anti-proliferation activities
against a panel of murine cancer cell lines, including 4T1 cells
and LL/2 cells; LL/2 cells were most sensitive to YL4073 treatment.
Furthermore, we focused on the effect of YL4073 on LL/2 cells to
study the relationship between the autophagy and apoptosis pathways
involved. The anti-proliferation effect of YL4073 has yet to be
studied in a wider variety of cancer cells. Susceptibility to
apoptosis of tumor cells was an important determinant of effective
therapy.
Based on our results, we consider that YL4073
inhibits the growth of tumors by inducing autophagy. Our results
indicated YL4073 induced LL/2 cell autophagy in a
concentration-dependent manner. We demonstrated that YL4073
promoted an autophagic process in LL/2 cells. Our data show that
YL4073 treatment resulted in the appearance of a series of
autophagic markers, including development of double-membrane
autophagic vacuoles and AVO augmented conversion of LC3-I to LC3-II
(41). Furthermore, YL4073
treatment caused the activation of a group of autophagy-related
proteins, including Beclin 1 and Atg12-Atg5 complex, both of which
are essential for autophagosome formation (42).
Inhibition of the Akt/mTOR/p70S6K and the
TSC/MAPK/AMPK signalling pathways may contribute to the autophagy
of tumors (43). The Akt-mTOR
signalling pathway is an important downregulator of autophagy
(42). The study also employed
EdU-DNA incorporation assays to show that YL4073 could inhibit the
proliferation of tumor cells; p-Akt and p-mTOR were also inhibited
in the presence of YL4073, suggesting that inhibition of the
Akt/mTOR/p70S6K signalling pathway contributed to YL4073-induced
autophagy in LL/2 cells. In addition, the phosphorylation of TSC,
MAPK and AMPK were inhibited by YL4073 treatment. These findings
suggest that inhibition of these two signalling pathways is a
potential therapeutic approach for the treatment of cancer. YL4073
effectively inhibited tumor growth in an established LL/2 lung
cancer model, and induced autophagy in vivo. In addition,
there were no obviously pathological changes in major organs,
including the heart, liver, spleen, lung, kidney and brain,
according to H&E stain (data not shown).
We also attempted to identify the primary target of
YL4073, since it is critical to further study. We have carried out
some limited research to this end; a bioinformatics-based 'reverse
docking' method with computer-aided drug designed has been applied,
which predicted that the possible primary targets might be CXCR3,
glutathione S-transferase, chymotrypsin, and penicillopepsin.
Furthermore, the results of the kinase inhibitory activity
experiment showed that YL4073 did not block kinase activity of MEK,
or Raf (12). Therefore, the
primary molecular target of YL4073 is not yet identified. Our aim
for further study is to use new approaches to identify the primary
target, such as quantitative chemical proteomics, which has been
used as an effective method to detect the primary molecular target
of some agents (44).
In conclusion, we demonstrated that YL4073 has
anticancer activity and that it induced autophagy and apoptosis
both in vitro and in vivo. The anticancer mechanisms
involved LC3, Beclin 1, and Atg5/Atg12 complexes, inhibition of the
Akt/mTOR/p70S6K pathway, and activation of the AMPK/MAPK pathway in
LL/2 cells. These results indicate that YL4073 warrants further
in-depth study and may be a promising agent for future lung cancer
therapy.
Acknowledgments
This study was supported by the National Natural
Sciences Foundation of China (no. 81272459 and 81402947), Natural
Sciences Foundation of Anhui Province (1508085QH162), China
Postdoctoral Science Foundation Funded Project (2015M581974), and
Grants for Scientific Research of BSKY from Anhui Medical
University (XJ201315).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tyczynski JE, Bray F and Parkin DM: Lung
cancer in Europe in 2000: Epidemiology, prevention, and early
detection. Lancet Oncol. 4:45–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh RP, Deep G, Chittezhath M, Kaur M,
Dwyer-Nield LD, Malkinson AM and Agarwal R: Effect of silibinin on
the growth and progression of primary lung tumors in mice. J Natl
Cancer Inst. 98:846–855. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Proctor RN: Tobacco and the global lung
cancer epidemic. Nat Rev Cancer. 1:82–86. 2001. View Article : Google Scholar
|
|
6
|
Li ZG, Zhao YL, Wu X, Ye HY, Peng A, Cao
ZX, Mao YQ, Zheng YZ, Jiang PD, Zhao X, et al: Barbigerone, a
natural isoflavone, induces apoptosis in murine lung-cancer cells
via the mitochondrial apoptotic pathway. Cell Physiol Biochem.
24:95–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gill RR, Jaklitsch MT and Jacobson FL:
Controversies in lung cancer screening. J Am Coll Radiol.
10:931–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De la Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: Epidemiology, etiology, and prevention. Clin Chest
Med. 32:605–644. 2011. View Article : Google Scholar
|
|
9
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R,
Grannis FW Jr, et al National comprehensive cancer network:
Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw.
11:645–653; quiz 653. 2013.PubMed/NCBI
|
|
10
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu YZ, Zheng RL, Zhou Y, Peng F, Lin HJ,
Bu Q, Mao YQ, Yu LT, Yang L, Yang SY, et al: Small molecular
anticancer agent SKLB703 induces apoptosis in human hepatocellular
carcinoma cells via the mitochondrial apoptotic pathway in vitro
and inhibits tumor growth in vivo. Cancer Lett. 313:44–53. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuma A, Hatano M, Matsui M, Yamamoto A,
Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T and Mizushima N: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lum JJ, Bauer DE, Kong M, Harris MH, Li C,
Lindsten T and Thompson CB: Growth factor regulation of autophagy
and cell survival in the absence of apoptosis. Cell. 120:237–248.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klionsky DJ and Emr SD: Autophagy as a
regulated pathway of cellular degradation. Science. 290:1717–1721.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohsumi Y: Molecular dissection of
autophagy: Two ubiquitin-like systems. Nat Rev Mol Cell Biol.
2:211–216. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Espert L, Denizot M, Grimaldi M,
Robert-Hebmann V, Gay B, Varbanov M, Codogno P and Biard-Piechaczyk
M: Autophagy is involved in T cell death after binding of HIV-1
envelope proteins to CXCR4. J Clin Invest. 116:2161–2172. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bommareddy A, Hahm ER, Xiao D, Powolny AA,
Fisher AL, Jiang Y and Singh SV: Atg5 regulates phenethyl
isothiocyanate-induced autophagic and apoptotic cell death in human
prostate cancer cells. Cancer Res. 69:3704–3712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanzawa T, Kondo Y, Ito H, Kondo S and
Germano I: Induction of autophagic cell death in malignant glioma
cells by arsenic trioxide. Cancer Res. 63:2103–2108.
2003.PubMed/NCBI
|
|
27
|
Abe J, Kusuhara M, Ulevitch RJ, Berk BC
and Lee JD: Big mitogen-activated protein kinase 1 (BMK1) is a
redox-sensitive kinase. J Biol Chem. 271:16586–16590. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Buck E, Eyzaguirre A, Brown E, Petti F,
McCormack S, Haley JD, Iwata KK, Gibson NW and Griffin G: Rapamycin
synergizes with the epidermal growth factor receptor inhibitor
erlotinib in non-small-cell lung, pancreatic, colon, and breast
tumors. Mol Cancer Ther. 5:2676–2684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bursch W, Ellinger A, Gerner C, Fröhwein U
and Schulte-Hermann R: Programmed cell death (PCD). Apoptosis,
autophagic PCD, or others? Ann NY Acad Sci. 926:1–12. 2000.
View Article : Google Scholar
|
|
30
|
Bursch W, Hochegger K, Torok L, Marian B,
Ellinger A and Hermann RS: Autophagic and apoptotic types of
programmed cell death exhibit different fates of cytoskeletal
filaments. J Cell Sci. 113:1189–1198. 2000.PubMed/NCBI
|
|
31
|
Traganos F and Darzynkiewicz Z: Lysosomal
proton pump activity: Supravital cell staining with acridine orange
differentiates leukocyte subpopulations. Methods Cell Biol.
41:185–194. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
33
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim J and Klionsky DJ: Autophagy,
cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and
mammalian cells. Annu Rev Biochem. 69:303–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shigemitsu K, Tsujishita Y, Hara K,
Nanahoshi M, Avruch J and Yonezawa K: Regulation of translational
effectors by amino acid and mammalian target of rapamycin signaling
pathways. Possible involvement of autophagy in cultured hepatoma
cells. J Biol Chem. 274:1058–1065. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pattingre S, Bauvy C and Codogno P: Amino
acids interfere with the ERK1/2-dependent control of macroautophagy
by controlling the activation of Raf-1 in human colon cancer HT-29
cells. J Biol Chem. 278:16667–16674. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Luistro L, He W, Smith M, Packman K,
Vilenchik M, Carvajal D, Roberts J, Cai J, Berkofsky-Fessler W,
Hilton H, et al: Preclinical profile of a potent γ-secretase
inhibitor targeting notch signaling with in vivo efficacy and
pharmacodynamic properties. Cancer Res. 69:7672–7680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karna P, Zughaier S, Pannu V, Simmons R,
Narayan S and Aneja R: Induction of reactive oxygen
species-mediated autophagy by a novel microtubule-modulating agent.
J Biol Chem. 285:18737–18748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang H, White EJ, Ríos-Vicil CI, Xu J,
Gomez-Manzano C and Fueyo J: Human adenovirus type 5 induces cell
lysis through autophagy and autophagy-triggered caspase activity. J
Virol. 85:4720–4729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shinojima N, Yokoyama T, Kondo Y and Kondo
S: Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in
curcumin-induced autophagy. Autophagy. 3:635–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Codogno P and Meijer AJ: Autophagy and
signaling: Their role in cell survival and cell death. Cell Death
Differ. 12(Suppl 2): 1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li J, Rix U, Fang B, Bai Y, Edwards A,
Colinge J, Bennett KL, Gao J, Song L, Eschrich S, et al: A chemical
and phosphoproteomic characterization of dasatinib action in lung
cancer. Nat Chem Biol. 6:291–299. 2010. View Article : Google Scholar : PubMed/NCBI
|