Introduction
Each year, more than 100,000 women die of ovarian
cancer worldwide (1). Epithelial
ovarian cancer (EOC) accounts for the majority of all ovarian
malignancies and is one of the most lethal among gynecologic
malignancies among women. In many cases, the diagnosis is delayed
due to its asymptomatic nature, and, as a consequence,
approximately two-thirds of patients with EOC have already
developed peritoneal carcinomatosis (2,3). The
prognosis of EOC patients is closely related to the stage at
diagnosis (4,5).
Most ovarian cancer patients are managed with
surgical resection, followed by systemic chemotherapies. Despite
recent advances in therapeutic agents, such as platinum-taxane
combination chemotherapy, the 5-year survival rate is still less
than 40% (6). EOC shows an
unfavorable oncologic outcome, based on its asymptomatic features
at an earlier clinical stage, and numerous intraperitoneal and/or
distant metastases. Despite the relatively high susceptibility of
EOC to paclitaxel plus platinum compounds, which are first-line
chemotherapeutic agents against EOC, the intrinsic or acquired
resistance of tumor cells to these chemotherapies makes the
treatment of EOC difficult. In order to overcome chemo-resistance,
various additional molecular-targeting therapies combined with
conventional anti-neoplastic agents have been developed. High-grade
serous ovarian cancer (HGSOC), which is observed much more
frequently at an advanced stage, comprises approximately 60% of all
histological subtypes of EOC. Recent studies have revealed that
most cases of HGSOC carry TP53 mutations, in contrast to other
types of EOC, which have a much lower incidence of TP53 mutations
(7–9). A recent study using high-throughput
sequencing technology revealed that TP53 mutations occurred in 96%
of 316 HGSOC samples (10). This
suggests that somatic mutation of TP53 is a nearly universal event
in HGSOC.
TP53 is located on chromosome 17p and encodes the
p53 protein. Wild-type p53 functions predominately as a
transcriptional factor, with a potent tumor-suppressive function
via its multiple activities, including induction of cell cycle
arrest, apoptosis, differentiation and senescence (11). Recent studies have shown that
missense TP53 mutations not only eliminate their own
tumor-suppressive function, but also gain oncogenic properties that
promote tumor growth, termed gain-of-function (GOF) (12-14).
Furthermore, TP53 mutations may be associated with poor prognosis
and malignant phenotypes in several types of cancers, including EOC
(15–19). Considering the universality of the
TP53 mutation in EOC, several novel drugs restoring the p53 pathway
have been widely investigated to be utilized in cancer therapy.
PRIMA-1 (p53 reactivation and induction of massive
apoptosis) and its methylated form PRIMA-1MET are small
molecules that can convert mutant p53 to an active conformation,
which restores wild-type functions of p53 in several types of
cancers, such as breast, neck and thyroid cancer and melanoma
(20–23). PRIMA-1MET is one of the
most promising drugs for clinical use to restore wild-type
functions to mutant p53 (24).
Although PRIMA-1MET is the first compound of such drugs
evaluated in clinical trials, the antitumor effects of
PRIMA-1MET on EOC remain unclear.
In our present study, we investigated whether
PRIMA-1MET induces growth suppression and apoptosis in
EOC cells. Using EOC cells with either wild-type or mutant p53, and
with either chemo-sensitivity or chemo-resistance, we demonstrated
that PRIMA-1MET was able to effectively induce cell
death. Therefore, PRIMA-1MET can be a promising
therapeutic strategy to induce cytotoxic effects and reactivate the
p53 pathway in EOC, particularly in HGSOC.
Materials and methods
Cell culture
Human EOC lines, A2780, OVCAR-3, ES-2, SKOV-3,
CaOV-3, TOV21G and OV-90, were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). NOS2 and NOS3 cell
lines derived from serous EOC were previously established in our
institute (25). The NOS2CR and
NOS2TR cells with chronic resistance to cisplatin and paclitaxel
were previously established from the parental NOS2 cells in our
institute (26). Furthermore, we
recently established another two chronic
cisplatin/paclitaxel-resistant cell lines from the parental NOS3
cells: NOS3CR (cisplatin) and NOS3TR (paclitaxel). All EOC cell
lines were maintained at 37°C with 5% CO2 in RPMI-1640
medium (Sigma) supplemented with 10% fetal bovine serum (FBS),
streptomycin (100 µg/ml), and penicillin (100 U/ml).
PRIMA-1MET was purchased from Santa Cruz Biotechnology,
Inc. PRIMA-1MET was diluted in dimethyl sulfoxide (DMSO)
to create a 50-mmol/l stock solution and stored at −20°C.
Antibodies to p53 (610184) were purchased from BD Pharmingen.
Antibodies to cleaved-PARP, PARP and β-actin were purchased from
Cell Signaling Technology.
Direct sequencing of TP53 mutations
Genomic DNA was extracted from the NOS2 and NOS3
cells using the Genomic DNA purification kit (Promega, Madison, WI,
USA). The exons and flanking introns of TP53 were amplified by
polymerase chain reaction (PCR). The primers used are shown in
Table I. The resulting PCR products
were sequenced and the mutation status was confirmed.
 | Table IThe specific primers used for direct
sequencing of the TP53 gene. |
Table I
The specific primers used for direct
sequencing of the TP53 gene.
| Primer | Sequence | Length (bp) |
|---|
| p53 | | |
| Exon 2–4 | F:
GTGTCTCATGCTGGATCCCCACT | 23 |
| R:
GGATACGGCCAGGCATTGAAGT | 22 |
| Exon 5–6 | F:
TGCAGGAGGTGCTTACGCATGT | 22 |
| R:
CCTTAACCCCTCCTCCCAGAGAC | 23 |
| Exon 7–9 | F:
ACAGGTCTCCCCAAGGCGCACT | 22 |
| R:
TTGAGGCATCACTGCCCCCTGAT | 23 |
| Exon 10 | F:
GTCAGCTGTATAGGTACTTGAAGTGCAG | 28 |
| R:
GCTCTGGGCTGGGAGTTGCG | 20 |
| Exon 11 | F:
CCTTAGGCCCTTCAAAGCATTGGTCA | 26 |
| R:
GTGCTTCTGACGCACACCTATTGCAAG | 27 |
RNA extraction
RNA extraction from the cells was undertaken using
the Qiagen RNeasy Mini kit according to the manufacturer's
protocols. The cells were lysed in 250 µl of buffer RLT and
filtered through the filtration spin column. The samples were
applied to the RNeasy Mini spin column. Total RNA bound to the
membrane and contaminants were removed by washing consecutively
with buffers RW1 and RPE. RNA was eluted in RNase-free water.
Extracted RNA was immediately stored at −80°C. The RNA
concentration was determined with the NanoDrop 1000
spectrophotometer.
Reverse transcription
To obtain complementary DNA (cDNA), 1 µg of
RNA and 0.2 µg of random primers (Promega) were used. After
incubation at 72°C for 4 min, the mixture of RNA and random primers
were placed on ice for 4 min. M-MLV RT 1X reaction buffer, M-MLV
Reverse Transcriptase RNase Minus, and 10 mM dNTP (Promega) were
added to the mixture and then incubated at 42°C for 90 min,
followed by 70°C for 15 min. cDNA was stored at −20°C.
Quantitative real-time PCR (qRT-PCR)
Quantitative RT-PCR (qRT-PCR) was performed on the
Takara PCR thermal cycler using the SYBR Green detection system
(Takara, Tokyo, Japan). Cycling conditions consisted of a 3-min hot
start at 95°C, followed by 40 cycles of denaturation at 95°C for 10
sec, annealing at 58–60°C for 10 sec, extension at 72°C for 10 sec,
and then a final inactivation at 95°C for 10 sec. Dissociation
curve analyses were carried out at the end of the cycling to
confirm that one specific product was measured in each reaction.
Relative quantification was performed using the ΔΔCT method
(27). Expression normalization was
conducted by the expression of GAPDH, a housekeeping gene shown to
have stable expression in cancer cell lines (28). The specific primers for each gene
are shown in Table II. All
experiments were performed in triplicate.
 | Table IIThe specific primers used for
quantitative real-time RT-PCR. |
Table II
The specific primers used for
quantitative real-time RT-PCR.
| Primer | Sequence | Length (bp) |
|---|
| PRX3 | F:
GACGCTCAAATGCTTGATGA | 20 |
| R:
GATTTCCCGAGACTACGGTG | 20 |
| GPX1 | F:
AAGAGCATGAAGTTGGGCTC | 20 |
| R:
CAACCAGTTTGGGCATCAG | 19 |
| GAPDH | F:
TTGGTATCGTGGAAGGACTCA | 22 |
| R:
TGTCATCATATTTGGCAGGTT | 21 |
Protein extraction and western blot
analysis
Cultured ovarian cancer cells were washed with PBS
and lysed in RIPA buffer (Millipore). The cells were scrapped into
lysis buffer, centrifuged at 12,000 × g at 4°C for 15 min, and then
diluted in 2X sample buffer [125 mM Tris-HCl (pH 6.8), 4% SDS, 10%
glycerol, 0.01% bromophenol blue, and 10% 2-mercaptoethanol]. Equal
amounts of protein (10 µg) were mixed with the 2X sample
buffer and were boiled at 95°C for 5 min. The samples were loaded
and separated by 7.5–15% SDS-polyacrylamide gel electrophoresis
(PAGE) with running buffer. The separated proteins were transferred
to polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 1% skim milk, incubated with each primary antibody
overnight at 4°C, washed with TBS-T buffer (10 mM Tris-HCl pH 7.4,
150 mM NaCl, 0.05% Tween-20) and incubated with the secondary
antibodies. The proteins were visualized using enhanced
chemiluminescence (GE Healthcare Bio-Sciences, Uppsala,
Sweden).
Cell viability assay
The effect of PRIMA-1MET on the viability
of human EOC cells was evaluated with the CellTiter-Glo Luminescent
Cell Viability Assay (Promega), which quantifies living cells by
ATP signal intensity. The luminescent signal was determined with a
luminometer. Cells were seeded in triplicate in 96-well plates at a
density of 2,000 cells/well. After a 24-h culture, the cells were
treated with various concentrations of PRIMA-1MET, and
then incubated for 24–72 h. Control cells were treated with the
same concentration of DMSO as that of the
PRIMA-1MET-treated cells.
Detection of apoptosis by staining with
Annexin V-FITC and propidium iodide
Cells (2×105) were cultured in 6-well
plates for 24 h before treatment with DMSO (control) or an
appropriate concentration of PRIMA-1MET for 24 h. The
cells were trypsinized, washed once with PBS, and then stained with
Annexin V-FITC and propidium iodide (PI) to determine the
early/late apoptotic cell population (MBL, Japan).
Results
Protein expression of p53 and the TP53
status in EOC cells
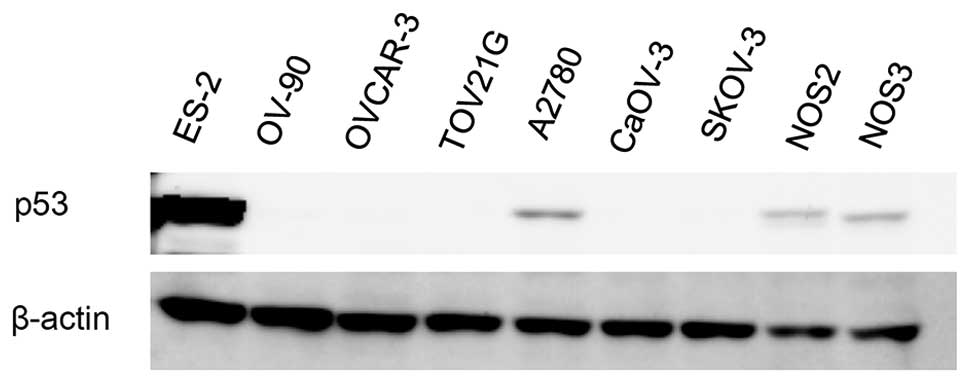
Firstly, we evaluated the levels of p53 protein
expression in the EOC cells by western immunoblot analysis. The
mutation status of TP53 in the EOC cells was acquired from previous
studies. The mutation status of TP53 of NOS2 and NOS3 cells was
evaluated by direct sequencing. The TP53 status of EOC cells is
shown in Table III. The protein
expression of p53 is shown in Fig.
1. EOC cells with wild-type p53, A2780 and NOS2, displayed a
basal expression of p53 to some extent. On the contrary, we could
not detect the protein expression of p53 in the EOC cells with
mutant p53, except for the ES-2 cells. This result demonstrates
that EOC cells bearing mutant p53 do not always express a higher
level of p53 than those bearing wild-type p53.
 | Table IIITP53 status of the EOC cell
lines. |
Table III
TP53 status of the EOC cell
lines.
| Cancer cell
lines | p53 status |
|---|
| ES-2 | S241F |
| OV-90 | S215R |
| OVCAR-3 | R248Q |
| TOV21G | Wild-type |
| A2780 | Wild-type |
| CaOV-3 | Q136Term |
| SKOV-3 | Null |
| NOS2 | Wild-type |
| NOS3 | L257P |
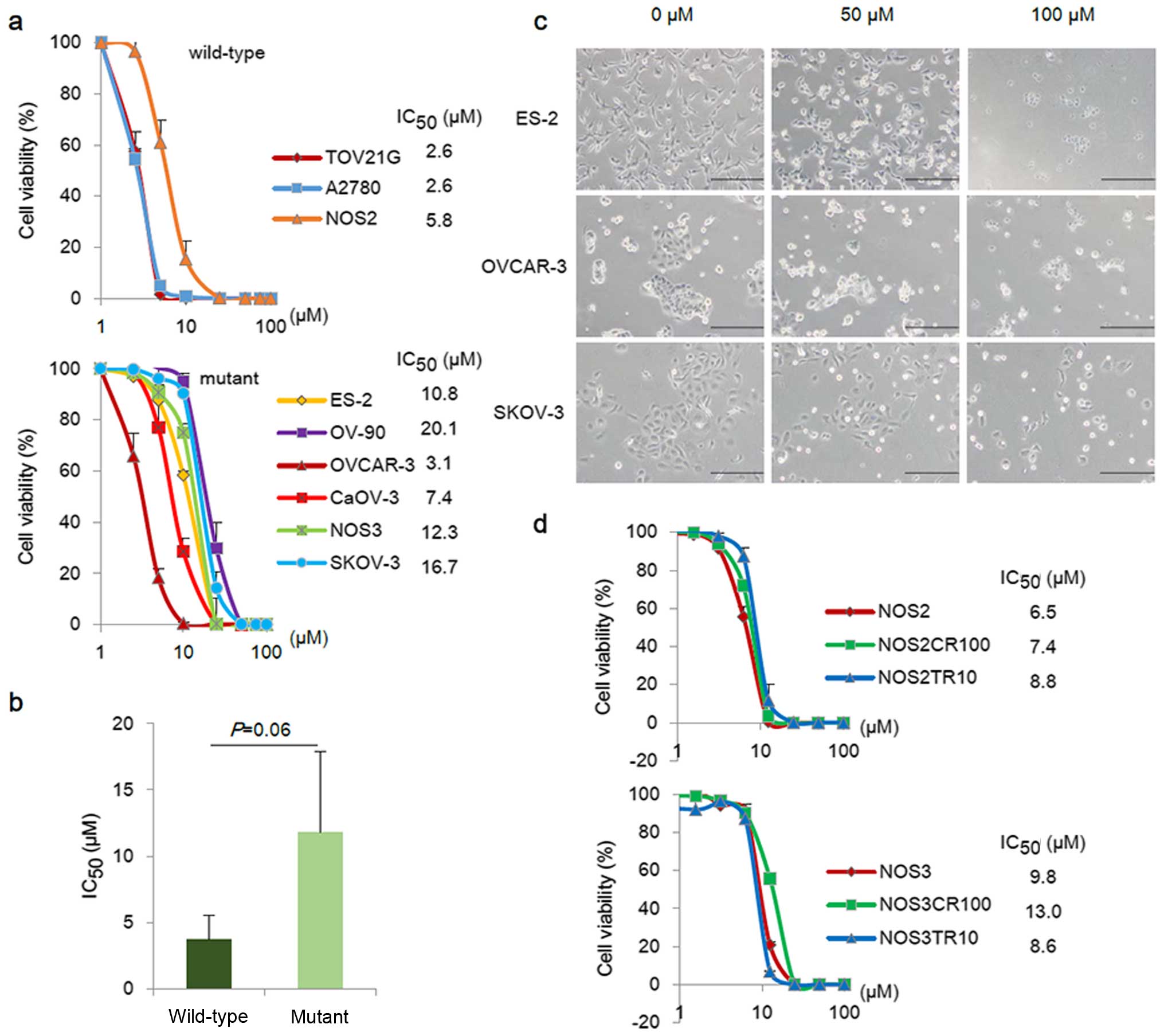
The effect of PRIMA-1MET on
cell death and apoptotic morphological changes in EOC cells
To assess the effect of PRIMA-1MET on EOC
cells, the anti-proliferative effects of various concentrations of
PRIMA-1MET (approximately 0–100 µM) were
determined in a total of 9 EOC cell lines: TOV21G, A2780, ES-2,
OV-90, OVCAR-3, CaOV-3, SKOV-3, NOS2 and NOS3. Fig. 2a shows the cell viability of
wild-type p53 cell lines (TOV21G, A2780, and NOS2) and mutant p53
cell lines (ES-2, OV-90, OVCAR-3, CaOV-3, NOS3 and SKOV-3) treated
with PRIMA-1MET for 48 h. PRIMA-1MET reduced
cell viability after 48 h in all EOC cell lines in a dose-dependent
manner. The IC50 values of PRIMA-1MET ranged
from 2.6 to 20.1 µM, which were independent of the mutation
status of TP53 (Fig. 2b).
Furthermore, PRIMA-1MET treatment induced a
morphological change which was consistent with the apoptotic change
within 6–24 h (Fig. 2c). We next
investigated whether PRIMA-1MET had sufficient effects
on cisplatin- and paclitaxel-resistant cell lines, which were
previously developed from the parental NOS2 and NOS3 cells (NOS2CR,
NOS2TR, NOS3CR, and NO3TR). Dose-responsive cell viability assays
with PRIMA-1MET were performed to evaluate the
sensitivities of the chemo-resistant cells. As shown in Fig. 2d, PRIMA-1MET displayed
anti-proliferative effects on both the parental and chemo-resistant
cells. The IC50 values of the NOS2, NOS2CR, and NOS2TR
cells were 6.5, 7.4, and 8.8 µM, respectively. The
IC50 value of the NOS3CR cells was slightly higher than
the values of the NOS3 and NOS3TR cells (not significant).
PRIMA-1MET had sufficient growth-suppressing activity
regardless of the mutation status of the TP53 and the
chemo-sensitivity in the EOC cells.
PRIMA-1MET induces apoptosis
in a dose-dependent manner in the EOC cells
We next performed an Annexin V-FITC/PI staining
assay to investigate whether PRIMA-1MET actually induced
apoptosis in the EOC cells. Treatment with PRIMA-1MET
for 16 h against EOC cells, TOV21G and A2780, increased the
fraction of early and late apoptotic cells (Fig. 3a). In the TOV21G cells, the
fractions of early and late apoptotic cells were significantly
increased from 1.1 and 4.3% following control vehicle treatment to
3.3 and 54.5% following 20 µM of PRIMA-1MET
treatment, respectively (Fig. 3b).
In the A2780 cells, the proportion of late apoptotic cells was
significantly elevated from 5.3% following control vehicle
treatment to 17.6% following 20 µM treatment (Fig. 3b). To determine whether
PRIMA-1MET also induced apoptosis in chemo-resistant EOC
cells, we evaluated cell apoptosis in another manner using
fluorescence microscopy. The cells after a 24-h treatment with
PRIMA-1MET were fixed with 4% paraformaldehyde, stained
with Hoechst 33342, and then we identified apoptosis with
fluorescence microscopy. The cells which had fragmented or
condensed nuclei were defined as undergoing apoptosis and counted
manually with fluorescence microscopy (29). The representative images of
condensed nuclei are shown in Fig.
3c. PRIMA-1MET treatment significantly increased the
fractions of apoptotic cells with fragmented or condensed nuclei in
both parental cells and their chemo-resistant cells in a
dose-dependent manner (Fig. 3d).
These results indicate that PRIMA-1MET induces apoptotic
cell death in both chemo-sensitive and chemo-resistant EOC
cells.
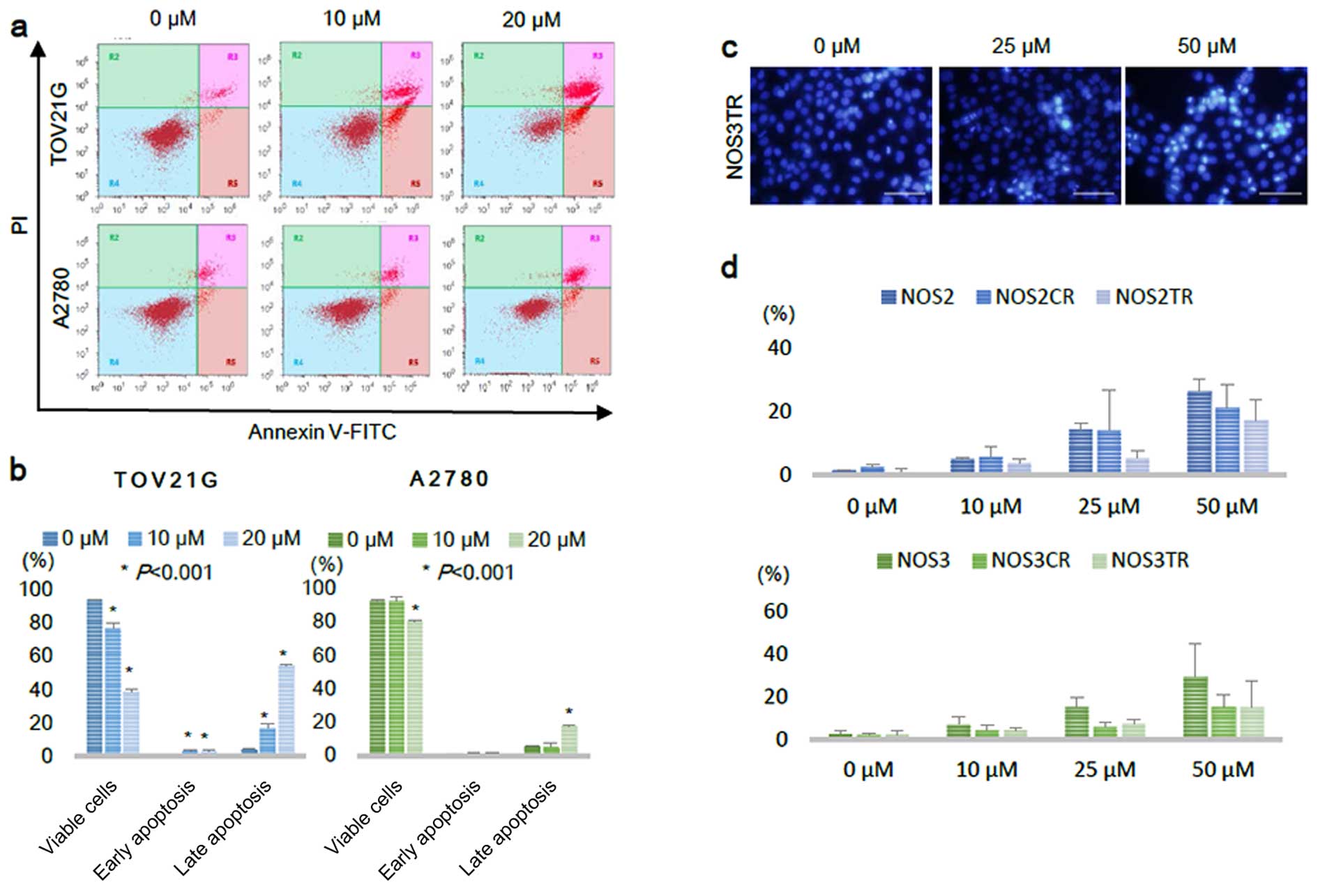 | Figure 3PRIMA-1MET induces
apoptosis in a dose-dependent manner in EOC cell lines. (a) TOV21G
and A2780 cells were treated with 0, 10, 20 µM of
PRIMA-1MET for 16 h, and then stained with Annexin
V-FITC and PI. The Annexin V-positive and PI-negative cells were
defined as early apoptotic, and the Annexin V-positive and
PI-positive cells were defined as late apoptotic (dead cells). (b)
Viable, early apoptotic, and late apoptotic cells were quantified
from triplicate samples. Asterisk indicates statistical
significance (P<0.001). Error bars represent standard
deviations. (c) Representative images of Hoechst 33342 staining of
NOS3TR cells after 24 h of PRIMA-1MET treatment.
Apoptotic cells were defined by their condensed and fragmented
nuclei with fluorescence microscopy (scale bar, 200 µm). (d)
Apoptosis levels of NOS2 and NOS3 cells, and their chemo-resistant
cells after 0, 10, 25, 50 µM 20 h PRIMA-1MET
treatment. Bars represent the percentages of apoptotic nuclei
counted in each treatment group and are expressed as the mean ±
SD. |
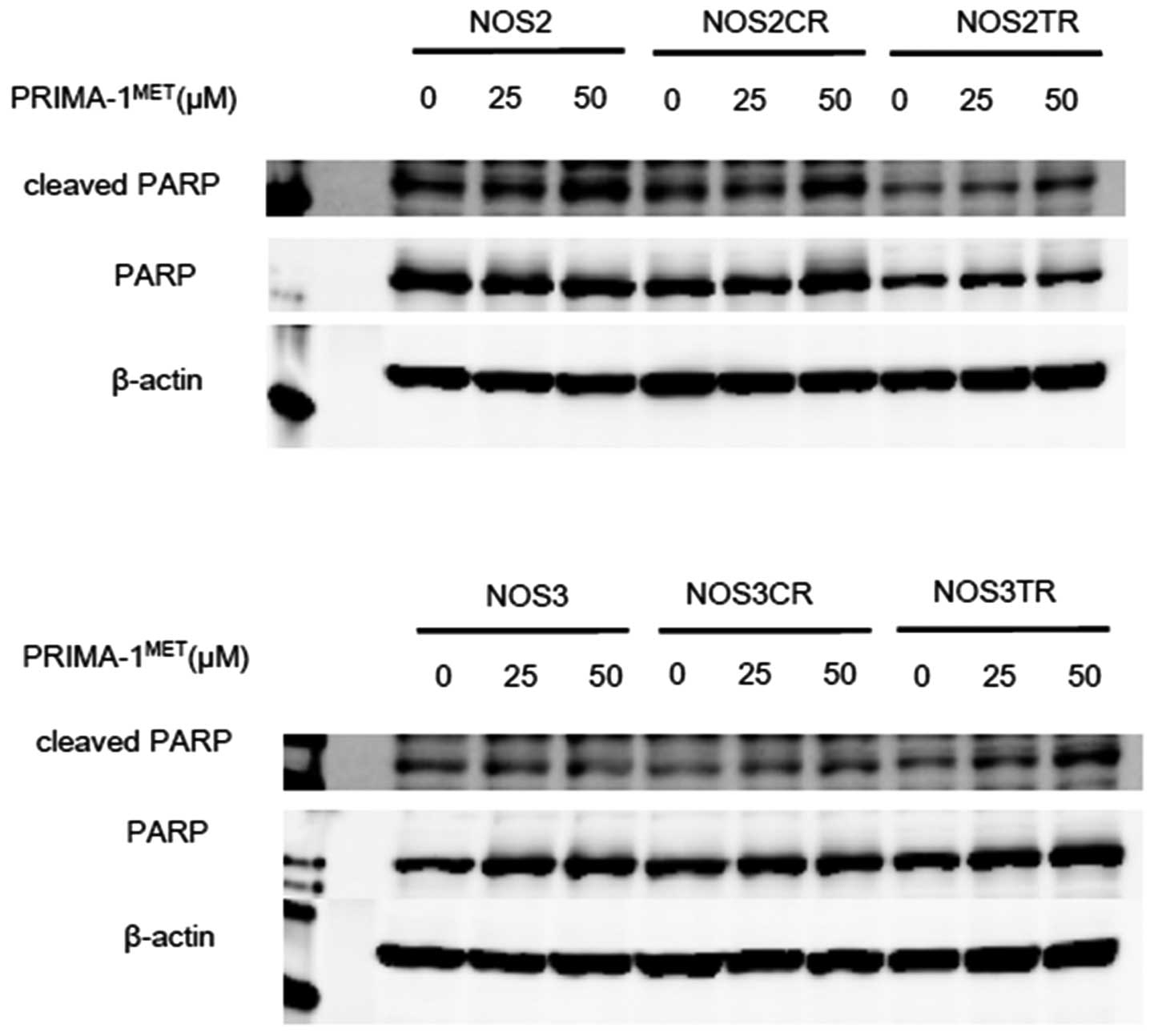
PRIMA-1MET activates PARP
cleavage
In order to confirm whether
PRIMA-1MET-induced cell death is apoptotic, we evaluated
the apoptosis-related protein levels after treatment with
PRIMA-1MET in EOC cells by western blot analysis.
Immunoblot analysis elucidated that PRIMA-1MET induced
dose-dependent PARP cleavage in the NOS2 and NOS3 cells and in
their chemo-resistant cells (Fig.
4). This result showed that PRIMA-1MET induces
apoptosis in EOC cells through PARP cleavage.
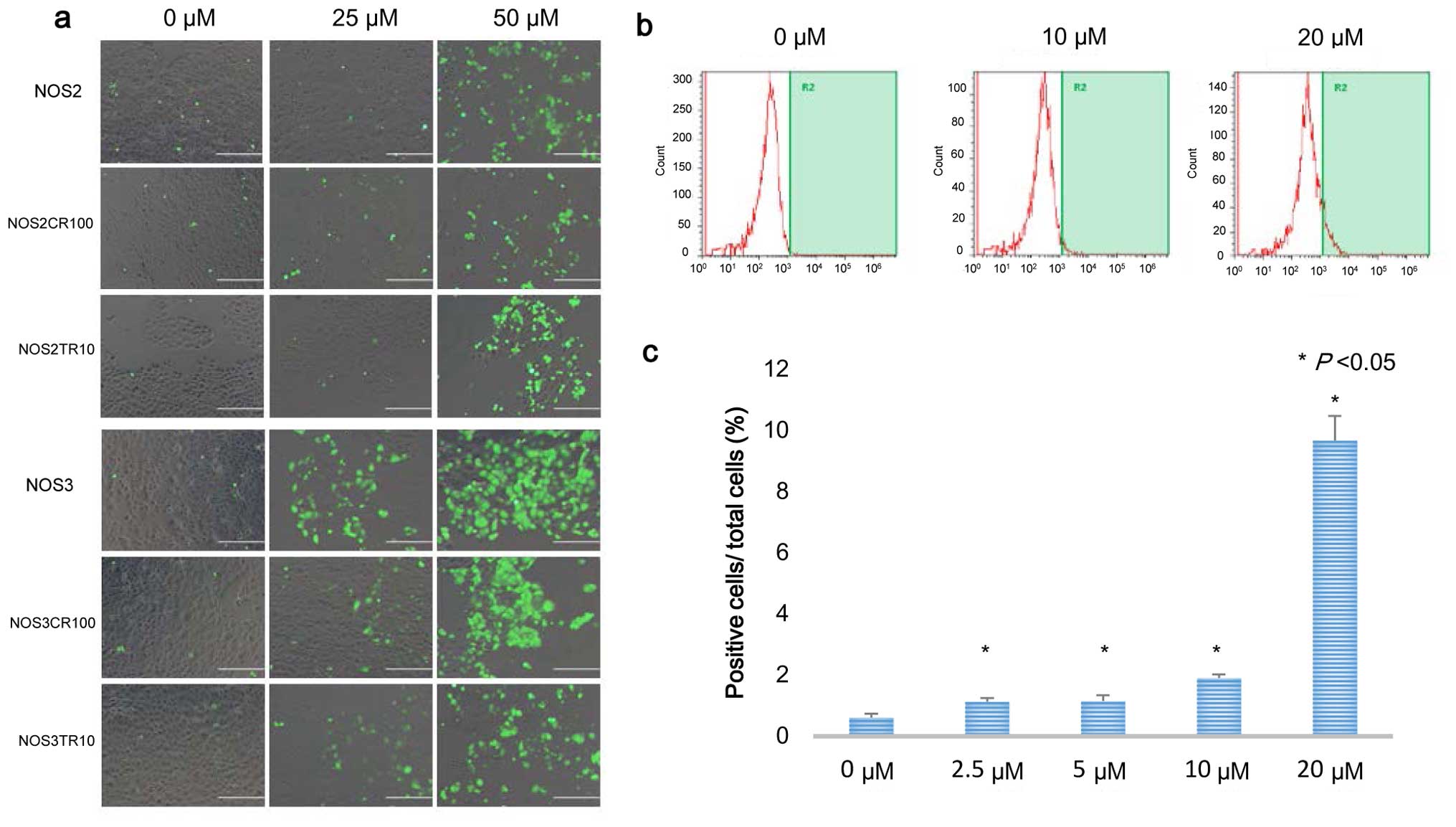
PRIMA-1MET increases
intracellular ROS in EOC cells
As it was reported that PRIMA-1MET
induces intracellular ROS accumulation, we investigated
intracellular ROS accumulation using
5–6-chloromethyl-2′7′-dichlorodihydroflorescein diacetate, acetyl
ester (CM-H2DCFDA; Molecular Probes Invitrogen,
Carlsbad, CA, USA) (20,30). PRIMA-1MET treatment for
24 h promoted intracellular ROS accumulation in the NOS2 and NOS3
cells, and their chemo-resistant cells (Fig. 5a). To quantify the proportion of
fluorescence-positive cells in the TOV21G cells after a 24-h
treatment with PRIMA-1MET, fluorescence activated cell
sorting (FACS) was performed. The proportion of
fluorescence-positive cells was increased in the cells treated with
PRIMA-1MET in a dose-dependent manner, and the increase
was significant (Fig. 5b and c).
These results demonstrate that PRIMA-1MET promotes
intracellular ROS accumulation in EOC cells.
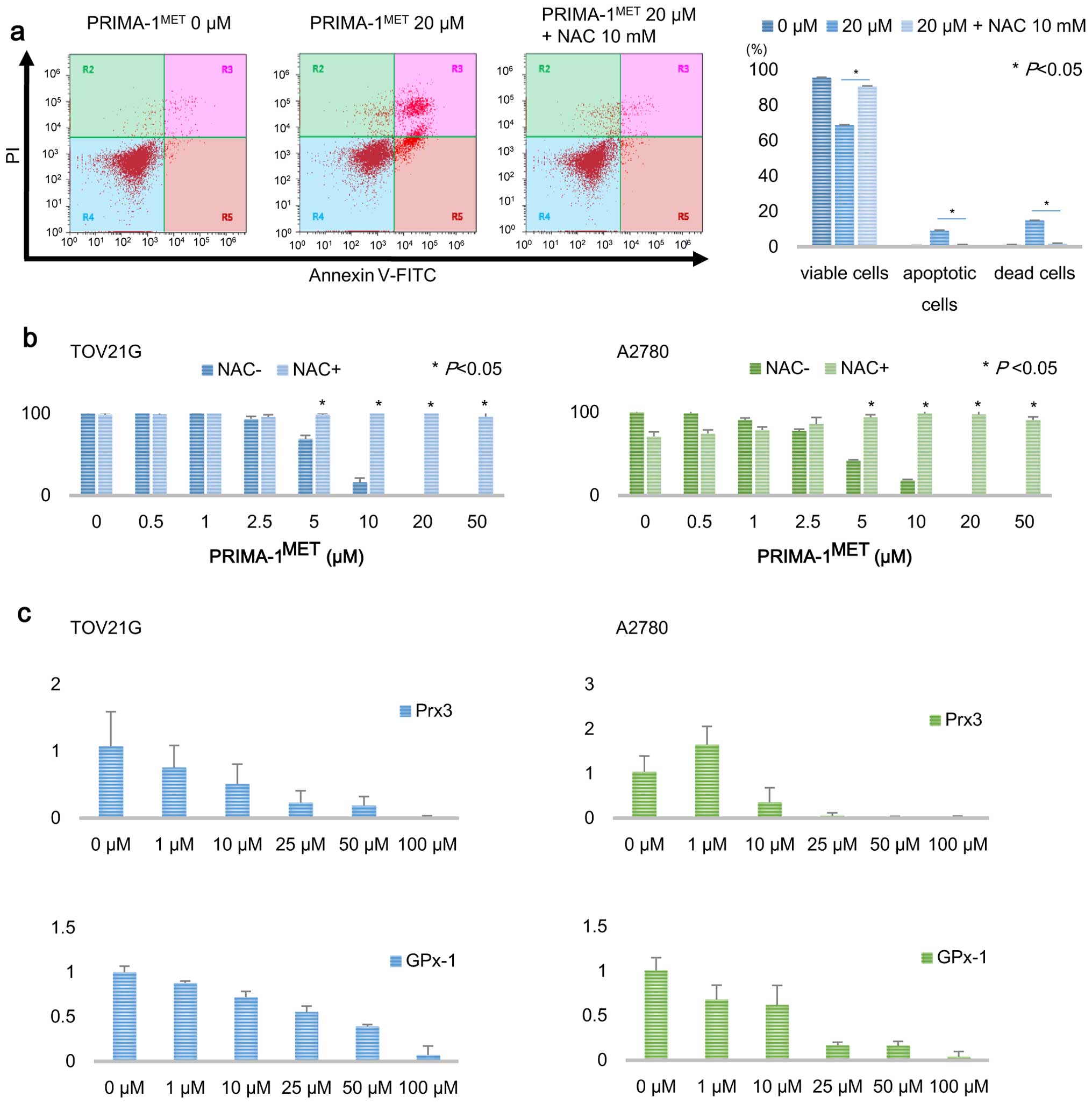
ROS scavenger rescues apoptosis induced
by PRIMA-1MET
To determine whether intracellular ROS accumulation
by treatment with PRIMA-1MET induces apoptosis, we used
a ROS scavenger N-acetyl cysteine (NAC). The compound NAC was added
to cultured cells with 20 µM PRIMA-1MET medium at
a final concentration of 10 mM. Sixteen hours after co-treatment
with PRIMA-1MET and NAC, apoptotic cells were assessed
by Annexin V-FITC and PI staining. The addition of NAC inhibited
apoptosis and the growth-suppressing effect induced by
PRIMA-1MET treatment (Fig.
6a and b). Furthermore, to examine the effect of
PRIMA-1MET on the expression of antioxidant enzymes
including Prx3 and GPx-1, which scavenge intracellular ROS to
sustain homeostasis, we treated TOV21G and A2780 cells with
PRIMA-1MET for 20 h and, thereafter, evaluated the mRNA
levels of antioxidant enzymes by real-time RT-PCR. The mRNA levels
of Prx3 and GPx-1 were significantly decreased after 20 h of
treatment with PRIMA-1MET in a dose-dependent manner
(Fig. 6c). Our results suggest that
the antitumor effects of PRIMA-1MET may be mediated by
intracellular ROS accumulation, and that the intracellular ROS
accumulation and the cytotoxic effect induced by
PRIMA-1MET may be due to downregulation of Prx3 and
GPx-1.
Discussion
Most EOC patients experience recurrent disease,
despite a high rate of complete clinical remission. Although
recurrent EOC patients frequently receive chemotherapy, they are
basically incurable due to the acquisition of chemo-resistance.
Resistance to cytotoxic agents is a major obstacle to complete
cure, and a number of attempts to overcome chemo-resistance have
been made in EOC (31,32). While much effort has been made to
restore chemo-sensitivity to resistant cells, no promising
molecules have been identified. Thus, there is a need to develop
novel therapeutics for EOC. Despite the fact that
PRIMA-1MET has been confirmed to exhibit
tumor-suppressing effects on various types of cancer cells, there
have been few reports on the effect of PRIMA-1MET on
chemo-resistant cells in EOC (33–35).
In our present study, we attempted to verify whether
PRIMA-1MET has antitumor effects on EOC cells.
PRIMA-1MET is a prodrug converted to MQ
with potential to bind to cysteine residues and change the
conformation of the core domain of mutant p53 (30). PRIMA-1/PRIMA-1MET has
been reported to synergize with cytotoxic agents to induce
apoptotic cell death (33,36,37).
Recently, Mohell et al reported that combined treatment with
APR-246 and platinum or other drugs could give rise to an improved
strategy for recurrent high-grade serous ovarian cancer (37). In this study, chemo-resistant cells
incubated with PRIMA-1MET exhibited apoptosis, which was
characterized by morphological features, such as chromosomal DNA
condensation and fragmentation. Furthermore, the effect of
PRIMA-1MET on cell viability of chemo-resistant cells
was similar to that of the parental cells with either wild-type p53
(NOS2) or mutant p53 (NOS3). These findings suggest that
PRIMA-1MET may have the possibility to be used for
patients with chemo-resistant EOC bearing not only mutant p53 but
also wild-type p53.
EOC cells easily spread to the peritoneal cavity and
form disseminated metastases with a large amount of ascites. During
this metastatic process, tumors may gradually acquire stem-like
properties and become chemo-resistant. To our knowledge, there has
been no report examining the efficacy of PRIMA-1MET in
cancer stem cells (or cancer stem-like cells). On the other hand, a
recent study suggested the possibility that PRIMA-1MET
may overcome the chemo-resistance of EOC cells (37). Indeed, PRIMA-1MET may be
able to target cancer stem cells with chemo-resistant properties
although additional studies are warranted.
In the present study, we investigated the efficacy
of PRIMA-1MET in the growth suppression and apoptosis
induction in ovarian cancer cell lines (n=9) in vitro. We
demonstrated that PRIMA-1MET suppressed cell viability
and induced massive apoptosis, regardless of the TP53 mutational
status. Furthermore, ovarian cancer cell lines carrying wild-type
p53 were slightly more sensitive than those carrying mutant p53
(not significant). To date, previous reports have shown that
PRIMA-1/PRIMA-1MET were more effective on pancreatic and
small cell lung cancer cells expressing mutant p53 than on those
expressing wild-type p53 or null (38,39).
Interestingly, despite the fact that there is evidence that
PRIMA-1MET restores the wild-type p53 function to mutant
p53, several recent studies have shown that PRIMA-1MET
displayed cytotoxic effects on Ewing sarcoma cells, acute myeloid
leukemia cells, and human myeloma cells irrespective of the TP53
mutational status (40–42). This controversy is because
PRIMA-1MET not only restores wild-type p53 function to
mutant p53, but also induces apoptosis in a p53-independent manner
through intracellular ROS accumulation and endoplasmic reticulum
(ER) stress (40,41). Indeed, in this study, we
demonstrated that incubation with PRIMA-1MET resulted in
an antitumor effect with intracellular ROS accumulation in ovarian
cancer cells, and co-treatment with PRIMA-1MET and an
ROS scavenger, NAC, blocked the cytotoxic effects, suggesting that
the effects of PRIMA-1MET are due to an intracellular
ROS increase in EOC cells. Our results were partly consistent with
previous reports, and support the antitumor effects of
PRIMA-1MET being universal irrespective of the TP53
mutational status in EOC cells. Unknown diverse mechanisms of
PRIMA-1MET may provide a convincing strategy for
overcoming chemo-resistance in not only EOC but also other
cancers.
ROS can generate oxidative stress in cells inducing
DNA damage, protein degradation, peroxidation of lipids, and
finally cell death at a high concentration. It is well known that
cancer cells are normally more tolerant to high levels of oxidative
stress than normal cells (43). One
of the underlying mechanisms of cancer cells to survive under high
oxidative condition is overexpression of antioxidant enzymes to
scavenge ROS (44). An inhibitor of
glutathione synthesis, buthionine sulfoximine (BSO) was used in a
clinical situation (45). In the
present study, we demonstrated that PRIMA-1MET induced
intracellular accumulation and suppressed the expression of
antioxidant enzymes, Prx3 and GPx-1, in EOC cells. Prx3 is one of
the 2-Cys peroxiredoxin family (PRX 1–4), and operates as a
reductase to metabolize ROS (46).
Cunniff et al reported that knockdown of Prx3 increased
oxidative stress and mitochondrial dysfunction in malignant
mesothelioma cells, suggesting that Prx3 plays a critical role in
cell cycle progression and sustaining the mitochondrial structure
(47). Furthermore, a recent report
by Song et al showed that Prx3 was highly upregulated in
colon cancer stem cells, and that knockdown of Prx3 led to
decreased cellular viability (48).
In addition, several studies have already shown that GPx-1 protects
cancer cells upon exposure to severe oxidative stress (49,50).
According to our findings, PRIMA-1MET suppressed the
expression of both Prx3 and GPx-1, suggesting that it may induce an
intracellular ROS increase mediated by downregulation of Prx3 and
GPx-1.
To our knowledge, no previous studies have confirmed
intracellular ROS accumulation in benign tumor cells or normal
epithelial cells. In the present study, we did not evaluate whether
PRIMA-1MET induces intracellular ROS accumulation in
such non-malignant or normal cells. In the living body, the
majority of ROS generated by various stimulation can be degenerated
by a higher antioxidant capacity derived from intrinsic ROS
scavengers, resulting in weakened efficacy. We believe that the
actual effects may depend on the local balance between ROS and such
intrinsic scavengers. Certainly, PRIMA-1MET may induce
intracellular ROS accumulation in benign or normal cells as well as
tumor cells. We speculate that the carcinogenetic effect of
PRIMA-1MET in such cells may be minimal. However, we
cannot deny that administration of PRIMA-1MET may have
some risks. Therefore, further investigation concerning the effect
of PRIMA-1MET against benign or normal cells is
necessary when used clinically.
In conclusion, we demonstrated that
PRIMA-1MET exhibited antitumor effects on
chemo-resistant cells through intracellular ROS accumulation and
repressed antioxidant enzymes. To utilize PRIMA-1MET for
EOC patients including chemo-resistant cases, we need to
investigate further how PRIMA-1MET suppresses Prx3 and
GPx-1. PRIMA-1MET is a promising compound for further
development as a potential cytotoxic agent against EOC.
Acknowledgments
The authors wish to thank Dr Kathleen Pishas for the
study and technical support.
References
|
1
|
Sankaranarayanan R and Ferlay J: Worldwide
burden of gynaecological cancer: The size of the problem. Best
Pract Res Clin Obstet Gynaecol. 20:207–225. 2006. View Article : Google Scholar
|
|
2
|
Masoumi Moghaddam S, Amini A, Morris DL
and Pourgholami MH: Significance of vascular endothelial growth
factor in growth and peritoneal dissemination of ovarian cancer.
Cancer Metastasis Rev. 31:143–162. 2012. View Article : Google Scholar :
|
|
3
|
Muñoz-Casares FC, Rufián S, Arjona-Sánchez
Á, Rubio MJ, Díaz R, Casado Á, Naranjo Á, Díaz-Iglesias CJ, Ortega
R, Muñoz-Villanueva MC, et al: Neoadjuvant intraperitoneal
chemotherapy with paclitaxel for the radical surgical treatment of
peritoneal carcinomatosis in ovarian cancer: A prospective pilot
study. Cancer Chemother Pharmacol. 68:267–274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chan JK, Tian C, Monk BJ, Herzog T, Kapp
DS, Bell J and Young RC; Gynecologic Oncology Group: Prognostic
factors for high-risk early-stage epithelial ovarian cancer: A
Gynecologic Oncology Group study. Cancer. 112:2202–2210. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chan JK, Teoh D, Hu JM, Shin JY, Osann K
and Kapp DS: Do clear cell ovarian carcinomas have poorer prognosis
compared to other epithelial cell types? A study of 1411 clear cell
ovarian cancers. Gynecol Oncol. 109:370–376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bell D, Berchuck A, Birrer M, Chien J,
Cramer DW, Dao F, Dhir R, DiSaia P, Gabra H, Glenn P, et al Cancer
Genome Atlas Research Network: Integrated genomic analyses of
ovarian carcinoma. Nature. 474:609–615. 2011. View Article : Google Scholar
|
|
8
|
Havrilesky L, Darcy M, Hamdan H, Priore
RL, Leon J, Bell J and Berchuck A; Gynecologic Oncology Group
Study: Prognostic significance of p53 mutation and p53
overexpression in advanced epithelial ovarian cancer: A Gynecologic
Oncology Group Study. J Clin Oncol. 21:3814–3825. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Risch HA, McLaughlin JR, Cole DE, Rosen B,
Bradley L, Fan I, Tang J, Li S, Zhang S, Shaw PA, et al: Population
BRCA1 and BRCA2 mutation frequencies and cancer penetrances: A
kin-cohort study in Ontario, Canada. J Natl Cancer Inst.
98:1694–1706. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang HJ, Chun SM, Kim KR, Sohn I and Sung
CO: Clinical relevance of gain-of-function mutations of p53 in
high-grade serous ovarian carcinoma. PLoS One. 8:e726092013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giaccia AJ and Kastan MB: The complexity
of p53 modulation: Emerging patterns from divergent signals. Genes
Dev. 12:2973–2983. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Di Agostino S, Strano S, Emiliozzi V,
Zerbini V, Mottolese M, Sacchi A, Blandino G and Piaggio G: Gain of
function of mutant p53: The mutant p53/NF-Y protein complex reveals
an aberrant transcriptional mechanism of cell cycle regulation.
Cancer Cell. 10:191–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blandino G, Levine AJ and Oren M: Mutant
p53 gain of function: Differential effects of different p53 mutants
on resistance of cultured cells to chemotherapy. Oncogene.
18:477–485. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong P, Karaayvaz M, Jia N, Kaneuchi M,
Hamada J, Watari H, Sudo S, Ju J and Sakuragi N: Mutant p53
gain-of-function induces epithelial-mesenchymal transition through
modulation of the miR-130b-ZEB1 axis. Oncogene. 32:3286–3295. 2013.
View Article : Google Scholar :
|
|
15
|
Høgdall EV, Kjaer SK, Blaakaer J,
Christensen L, Glud E, Vuust J and Høgdall CK: P53 mutations in
tissue from Danish ovarian cancer patients: From the Danish
'MALOVA' ovarian cancer study. Gynecol Oncol. 100:76–82. 2006.
View Article : Google Scholar
|
|
16
|
Concin N, Hofstetter G, Berger A,
Gehmacher A, Reimer D, Watrowski R, Tong D, Schuster E, Hefler L,
Heim K, et al: Clinical relevance of dominant-negative p73 isoforms
for responsiveness to chemotherapy and survival in ovarian cancer:
Evidence for a crucial p53-p73 cross-talk in vivo. Clin Cancer Res.
11:8372–8383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Helland A, Holm R, Skomedal H,
Abeler VM, Danielsen HE, Tropé CG, Børresen-Dale AL and Kristensen
GB: TP53 mutations in early-stage ovarian carcinoma, relation to
long-term survival. Br J Cancer. 90:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ueno Y, Enomoto T, Otsuki Y, Sugita N,
Nakashima R, Yoshino K, Kuragaki C, Ueda Y, Aki T, Ikegami H, et
al: Prognostic significance of p53 mutation in suboptimally
resected advanced ovarian carcinoma treated with the combination
chemotherapy of paclitaxel and carboplatin. Cancer Lett.
241:289–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartel F, Jung J, Böhnke A, Gradhand E,
Zeng K, Thomssen C and Hauptmann S: Both germ line and somatic
genetics of the p53 pathway affect ovarian cancer incidence and
survival. Clin Cancer Res. 14:89–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng X, Zhang MQ, Conserva F, Hosny G,
Selivanova G, Bykov VJ, Arnér ES and Wiman KG:
APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and
converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis.
4:e8812013. View Article : Google Scholar
|
|
21
|
Issaeva N, Bozko P, Enge M, Protopopova M,
Verhoef LG, Masucci M, Pramanik A and Selivanova G: Small molecule
RITA binds to p53, blocks p53-HDM-2 interaction and activates p53
function in tumors. Nat Med. 10:1321–1328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russo D, Ottaggio L, Penna I, Foggetti G,
Fronza G, Inga A and Menichini P: PRIMA-1 cytotoxicity correlates
with nucleolar localization and degradation of mutant p53 in breast
cancer cells. Biochem Biophys Res Commun. 402:345–350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roh JL, Kang SK, Minn I, Califano JA,
Sidransky D and Koch WM: p53-Reactivating small molecules induce
apoptosis and enhance chemotherapeutic cytotoxicity in head and
neck squamous cell carcinoma. Oral Oncol. 47:8–15. 2011. View Article : Google Scholar :
|
|
24
|
Lehmann S, Bykov VJ, Ali D, Andrén O,
Cherif H, Tidefelt U, Uggla B, Yachnin J, Juliusson G, Moshfegh A,
et al: Targeting p53 in vivo: A first-in-human study with
p53-targeting compound APR-246 in refractory hematologic
malignancies and prostate cancer. J Clin Oncol. 30:3633–3639. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Misawa T, Kikkawa F, Maeda O, Obata NH,
Higashide K, Suganuma N and Tomoda Y: Establishment and
characterization of acquired resistance to platinum anticancer
drugs in human ovarian carcinoma cells. Jpn J Cancer Res. 86:88–94.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kajiyama H, Shibata K, Terauchi M,
Yamashita M, Ino K, Nawa A and Kikkawa F: Chemoresistance to
paclitaxel induces epithelial-mesenchymal transition and enhances
metastatic potential for epithelial ovarian carcinoma cells. Int J
Oncol. 31:277–283. 2007.PubMed/NCBI
|
|
27
|
Kapitanović S, Cacev T, Antica M, Kralj M,
Cavrić G, Pavelić K and Spaventi R: Effect of indomethacin on
E-cadherin and beta-catenin expression in HT-29 colon cancer cells.
Exp Mol Pathol. 80:91–96. 2006. View Article : Google Scholar
|
|
28
|
Sugiyama K, Kajiyama H, Shibata K, Yuan H,
Kikkawa F and Senga T: Expression of the miR200 family of microRNAs
in mesothelial cells suppresses the dissemination of ovarian cancer
cells. Mol Cancer Ther. 13:2081–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakahara T, Iwase A, Nakamura T, Kondo M,
Bayasula, Kobayashi H, Takikawa S, Manabe S, Goto M, Kotani T, et
al: Sphingosine-1-phosphate inhibits
H2O2-induced granulosa cell apoptosis via the
PI3K/Akt signaling pathway. Fertil Steril. 98:1001.e1–1008.e1.
2012.
|
|
30
|
Lambert JM, Gorzov P, Veprintsev DB,
Söderqvist M, Segerbäck D, Bergman J, Fersht AR, Hainaut P, Wiman
KG and Bykov VJ: PRIMA-1 reactivates mutant p53 by covalent binding
to the core domain. Cancer Cell. 15:376–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Utsumi F, Kajiyama H, Nakamura K, Tanaka
H, Mizuno M, Ishikawa K, Kondo H, Kano H, Hori M and Kikkawa F:
Effect of indirect nonequilibrium atmospheric pressure plasma on
anti-proliferative activity against chronic chemo-resistant ovarian
cancer cells in vitro and in vivo. PLoS One. 8:e815762013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan Z, Choy E and Hornicek FJ: NSC23925,
identified in a high-throughput cell-based screen, reverses
multidrug resistance. PLoS One. 4:e74152009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bykov VJ, Zache N, Stridh H, Westman J,
Bergman J, Selivanova G and Wiman KG: PRIMA-1(MET) synergizes with
cisplatin to induce tumor cell apoptosis. Oncogene. 24:3484–3491.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Supiot S, Zhao H, Wiman K, Hill RP and
Bristow RG: PRIMA-1(met) radiosensitizes prostate cancer cells
independent of their MTp53-status. Radiother Oncol. 86:407–411.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ali D, Jönsson-Videsäter K, Deneberg S,
Bengtzén S, Nahi H, Paul C and Lehmann S: APR-246 exhibits
anti-leukemic activity and synergism with conventional
chemotherapeutic drugs in acute myeloid leukemia cells. Eur J
Haematol. 86:206–215. 2011. View Article : Google Scholar
|
|
36
|
Nahi H, Lehmann S, Mollgard L, Bengtzen S,
Selivanova G, Wiman KG, Paul C and Merup M: Effects of PRIMA-1 on
chronic lymphocytic leukaemia cells with and without hemizygous p53
deletion. Br J Haematol. 127:285–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohell N, Alfredsson J, Fransson Å,
Uustalu M, Byström S, Gullbo J, Hallberg A, Bykov VJ, Björklund U
and Wiman KG: APR-246 overcomes resistance to cisplatin and
doxorubicin in ovarian cancer cells. Cell Death Dis. 6:e17942015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zandi R, Selivanova G, Christensen CL,
Gerds TA, Willumsen BM and Poulsen HS:
PRIMA-1Met/APR-246 induces apoptosis and tumor growth
delay in small cell lung cancer expressing mutant p53. Clin Cancer
Res. 17:2830–2841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Izetti P, Hautefeuille A, Abujamra AL, de
Farias CB, Giacomazzi J, Alemar B, Lenz G, Roesler R, Schwartsmann
G, Osvaldt AB, et al: PRIMA-1, a mutant p53 reactivator, induces
apoptosis and enhances chemotherapeutic cytotoxicity in pancreatic
cancer cell lines. Invest New Drugs. 32:783–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tessoulin B, Descamps G, Moreau P, Maïga
S, Lodé L, Godon C, Marionneau-Lambot S, Oullier T, Le Gouill S,
Amiot M, et al: PRIMA-1Met induces myeloma cell death
independent of p53 by impairing the GSH/ROS balance. Blood.
124:1626–1636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Russo D, Ottaggio L, Foggetti G, Masini M,
Masiello P, Fronza G and Menichini P: PRIMA-1 induces autophagy in
cancer cells carrying mutant or wild type p53. Biochim Biophys
Acta. 1833:1904–1913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aryee DN, Niedan S, Ban J, Schwentner R,
Muehlbacher K, Kauer M, Kofler R and Kovar H: Variability in
functional p53 reactivation by PRIMA-1(Met)/APR-246 in Ewing
sarcoma. Br J Cancer. 109:2696–2704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tong L, Chuang CC, Wu S and Zuo L:
Reactive oxygen species in redox cancer therapy. Cancer Lett.
367:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Landry WD and Cotter TG: ROS signalling,
NADPH oxidases and cancer. Biochem Soc Trans. 42:934–938. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bailey HH, Mulcahy RT, Tutsch KD,
Arzoomanian RZ, Alberti D, Tombes MB, Wilding G, Pomplun M and
Spriggs DR: Phase I clinical trial of intravenous L-buthionine
sulfoximine and melphalan: An attempt at modulation of glutathione.
J Clin Oncol. 12:194–205. 1994.PubMed/NCBI
|
|
46
|
Cox AG, Peskin AV, Paton LN, Winterbourn
CC and Hampton MB: Redox potential and peroxide reactivity of human
peroxiredoxin 3. Biochemistry. 48:6495–6501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cunniff B, Wozniak AN, Sweeney P, DeCosta
K and Heintz NH: Peroxiredoxin 3 levels regulate a mitochondrial
redox setpoint in malignant mesothelioma cells. Redox Biol.
3:79–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song IS, Jeong YJ, Jeong SH, Heo HJ, Kim
HK, Bae KB, Park YH, Kim SU, Kim JM, Kim N, et al: FOXM1-induced
PRX3 regulates stemness and survival of colon cancer cells via
maintenance of mitochondrial function. Gastroenterology.
149:1006.e9–1016.e9. 2015. View Article : Google Scholar
|
|
49
|
Huang C, Ding G, Gu C, Zhou J, Kuang M, Ji
Y, He Y, Kondo T and Fan J: Decreased selenium-binding protein 1
enhances glutathione peroxidase 1 activity and downregulates HIF-1α
to promote hepatocellular carcinoma invasiveness. Clin Cancer Res.
18:3042–3053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gan X, Chen B, Shen Z, Liu Y, Li H, Xie X,
Xu X, Li H, Huang Z and Chen J: High GPX1 expression promotes
esophageal squamous cell carcinoma invasion, migration,
proliferation and cisplatin-resistance but can be reduced by
vitamin D. Int J Clin Exp Med. 7:2530–2540. 2014.PubMed/NCBI
|