Introduction
The D-type cyclins, cyclin D1, D2 and D3, are
positive regulators of G1 phase progression. They form a complex
with the cyclin-dependent kinase Cdk4 or Cdk6 to promote cell cycle
entry. Overexpression of the D-type cyclins can shorten the G1
phase and reduce the cell's dependency on mitogens. This function
of D-type cyclins in cell cycle control provides them with
potentially strong oncogenic power. Cyclins D1, D2 and D3 share
both structural and functional similarities but they are expressed
in a tissue-specific manner (1,2).
Deregulation of cyclin D1 expression is a key
pathogenic event in the development of mantle cell lymphoma (MCL).
MCL is a relatively rare disease representing approximately 6–8% of
non-Hodgkin lymphomas (NHLs) and affecting predominantly males. The
median overall survival is approximately 3–5 years. MCL is marked
by chromosomal translocation t(11;14)(q13;32) leading to the
juxtaposition of the CCND1 gene to the IGH gene
resulting in high expression of cyclin D1. In contrast, cyclin D1
is not typically expressed in normal lymphocytes (3,4).
Cyclin D1 overexpression is considered as a diagnostic marker of
MCL. However, several cases of MCL with overexpression of cyclin D2
or D3 instead of cyclin D1 have been reported (5).
Diffuse large B-cell lymphoma (DLBCL) represents 30
to 40% of NHLs and comprises a heterogeneous group of tumors
(6). In contrast to MCL, DLBCL
cases are not associated with any specific genetic aberration.
Instead, there are various genetic aberrations occurring with
different frequencies that accompany this disease. They include
rearrangements and mutations of BCL2, BCL6,
c-MYC, CDKN2A and TP53 genes (7–11).
Heterogeneity of the tumor is reflected in variable patient
outcome. Gene expression profiling provides stratification to
subgroups with different prognosis. In addition, individual
biological markers with prognostic significance have been described
(12–14).
DLBCLs are generally considered as cyclin
D1-negative. However, some DLBCL cases expressing cyclin D1 without
association with t(11;14) have been recently reported. It seems
though that they represent only a minority subgroup of DLBCLs
(15–21). Expression of cyclin D2 has been also
repeatedly studied in DLBCL. The fraction of cyclin D2-positive
cases varies between 13 and 62% and this feature was shown to be an
independent indicator of poor survival (18,22–24).
Cyclin D2 overexpression is closely associated with CD5-positive
cases de novo (24). The
CD5-positive cases de novo represent a DLBCL subtype
predominantly occurring in females and characterized by a higher
age at diagnosis and a significantly poorer survival compared to
CD5-negative DLBCLs (25). The
frequency of cyclin D3 overexpression in DLBCL cases ranges between
20 and 41% (18,24,26)
and seems to be associated with poor response to chemotherapy and
shorter overall survival (26).
We previously performed detailed analysis of cyclin
D1 expression in a collection of 33 tumor samples of MCL cases
(27). In this study, we present a
widely extended study. We analyzed the expression of cyclin D1, D2
and D3 mRNAs in patients with MCL and DLBCL using qRT-PCR and
investigated the impact on disease outcome. We showed that high
expression of cyclin D2 tended to decrease the overall survival
rate among DLBCL patients.
Materials and methods
Tissue samples
We studied a cohort of 30 patients diagnosed with
MCL in the years 2007–2012 and 104 patients diagnosed with DLBCL in
the years 2001–2013 at University Hospital Brno. All patients
underwent surgical biopsy of the tumor tissue and were diagnosed by
a pathologist according to the WHO classification. The fresh-frozen
tissue samples as well as formalin-fixed, paraffin-embedded (FFPE)
tumor tissue blocks were available for all patients. A cohort of
MCL patients (M1–M30) consisted of 23 men and 7 women. Median age
at diagnosis was 66.5 years. Three cytomorphological subtypes were
recognized: common (20 cases), blastoid (6 cases) and a pleomorphic
variant (3 cases). A cohort of DLBCL patients (B1–B104) consisting
of 64 men and 40 women with median age 57.0 years exhibited
centroblastic (45), immunoblastic (8), mediastinal (18), anaplastic (2) and other or unspecified morphological
variants (10). Based on
immunohistochemical staining, the DLBCL cases were subclassified
into germinal center B-cell–like (GCB) [39/104 (37.5%)] and non-GCB
[65/104 (62.5%) groups according to Hans et al (28) and into subgroups 1 [62/104 (59.6%)]
and 2 [42/104 (40.4%)] according to Muris et al (29). Twenty-three patients developed DLBCL
as a secondary tumor. DLBCL patients were treated with either
standard R-CHOP therapy (61/81 de novo cases and 19/23
secondary cases) or intensive therapy (18/81 de novo cases
and 4/23 secondary cases). Two patients with de novo disease
underwent no therapy. All patients were informed consent and they
signed written consent allowing inclusion into this study as
approved by the Ethics Committee of the University of Brno. As a
control, tissues from three healthy donors and the MOLP-8 and
Jurkat cell lines were used.
Cell line
The MOLP-8 cell line expressing a high level of
cyclin D1 (30) was kindly provided
by Dr Eva Bartova, Institute of Biophysics, Academy of Sciences
(Czech Republic). The Jurkat cell line expressing a high level of
cyclin D3 (31) was kindly provided
by Dr Ales Hampl, Faculty of Medicine, Masaryk University (Czech
Republic). MOLP-8 and Jurkat cells were cultured in RPMI-1640
medium (L-glutamine, NaHCO3; Sigma-Aldrich, Prague,
Czech Republic) supplemented with 20% fetal calf serum and 1%
penicillin/streptomycin in 5% CO2 at 37°C.
Immunohistochemistry
Endogenous peroxidase activity was blocked with 3%
hydrogen peroxide in methanol, for 10 min. Antigen retrieval was
performed in citrate buffer, pH 6.0 (Dako, Glostrup, Denmark) at
121°C for 4 min. The CD5-specific mouse monoclonal antibody (clone
4C7; Leica Biosystems, USA) diluted 1:50 was applied at 4°C
overnight. The cyclin D1-specific rabbit monoclonal antibody (clone
SP4; Zytovision, Germany) diluted 1:50 was applied at 4°C
overnight. Reactive sites were identified using biotinylated
secondary antibody, peroxidase ABC (Vector Laboratories, USA), DAB
(Dako), and counterstained with Mayer's haematoxylin.
Immunoblotting
Tissue samples were lysed in solution containing 150
mM NaCl, 50 mM NaF, 50 mM Tris (pH 8.0), 5 mM EDTA, 1% NP-40 and 1
mM phenylmethylsulfonyl fluoride in ice for 30 min, and the cell
extract was centrifuged at 17,000 × g for 30 min to remove cell
debris. The protein concentration was determined by the Bradford
assay. Solubilized proteins were resolved by 10% SDS-PAGE and
transferred onto a nitrocellulose membrane. Blots were blocked in
0.1% Tween-20 and 5% low-fat milk in PBS for 1 h and probed with
CD1.1 (Abcam, Cambridge, UK), Ab-4 (Thermo Fisher Scientific,
Fremont, CA, USA), Ab-1 (Neomarkers, Fremont CA, USA) mouse
monoclonal antibodies and actin rabbit monoclonal antibody (BD
Biosciences, USA) at 4°C. Blots were developed with the Dako
peroxidase-conjugated secondary antibody (Dako) using the ECL
chemiluminescence detection kit (GE Healthcare UK Limited, Little
Chalfont, UK).
Real-time quantitative RT-PCR
The cyclin D1, D2 and D3 transcripts were
quantitated by real-time reverse transcription PCR (qRT-PCR). Total
RNA was extracted from the frozen tissue samples using the
Nucleospin RNA kit (Macherey-Nagel, Hoerdt, France). RNA was
reverse transcribed by ProtoScript II RT (New England BioLabs,
Hitchin, UK) and cDNA was amplified and quantified using
TaqMan® Universal PCR Master Mix (Applied Biosystems,
Foster City, CA, USA) and TaqMan Gene Expression Assays for
CCND1 (Hs00765553_m1), CCND2 (Hs00153380_m1),
CCND3 (Hs00236949_m1) and GAPDH in a 7500 Real-Time
PCR system (Applied Biosystems). Quantitative real-time PCR was
performed using these conditions: an initial cycle at 95°C for 10
min, followed by 50 biphasic cycles 95°C/15 sec and 60°C/1 min.
Initial template concentration was calculated from the cycle number
when the amount of PCR product passed a threshold set in the
exponential phase of the PCR reaction. The threshold cycles (Ct)
were recorded for the target gene and reference gene (GAPDH)
in all samples. Relative gene expression was analyzed with the
2−ΔΔCT method using the GAPDH gene as the
endogenous control and negative control as a calibrator. Each PCR
reaction was carried out in duplicates. At least two independent
analyses were performed for each sample.
Statistical analyses
Standard descriptive statistics were applied in the
analysis; absolute and relative frequencies for categorical
variables and median supplemented with minimum-maximum range for
continuous variables. The influence of monitored parameters on
survival and progression-free survival was assessed by hazard ratio
estimates from univariate Cox models. Graphic visualization of
patient survival according to the monitored parameters was
performed using Kaplan-Meier survival curves. Statistical
significance of differences in survival among groups of patients
was tested using the log-rank test. α=0.05 was used as a level of
statistical significance. Analyses were performed in statistical
software IBM SPSS Statistics 22.0.0.1 for Windows (IBM Corporation,
2014).
Results
Quantification of cyclin D1, D2 and D3
transcripts in MCL and DLBCL
We used quantitative real-time PCR to analyze the
level of cyclin D1, D2 and D3 mRNAs in all MCL and DLBCL samples,
healthy specimens (negative controls) and the MOLP-8 cells
(positive control). As an internal standard, GAPDH mRNA was
used. The ratio of CCND1/GAPDH
(CCND2/GAPDH and CCND3/GAPDH,
respectively) in healthy specimens was designated as 1.0 and the
normalized data of patient samples were calculated relative to this
value. The cut-off level for altered cyclin D expression was set up
as the mean value of CCND/GAPDH ratios determined in
3 samples from healthy donors plus 3 standard deviations (SD) and
was determined as 2.8 for cyclin D1, 3.4 for cyclin D2, and 2.9 for
cyclin D3. Values below these values were considered as negative.
The MOLP-8 control cells featuring high expression of cyclin D1 and
low expression of cyclin D2 and D3, reached a relative fold
increase (RFI) of 13.0 for cyclin D1, 0.01 for cyclin D2 and 0.2
for cyclin D3 mRNAs.
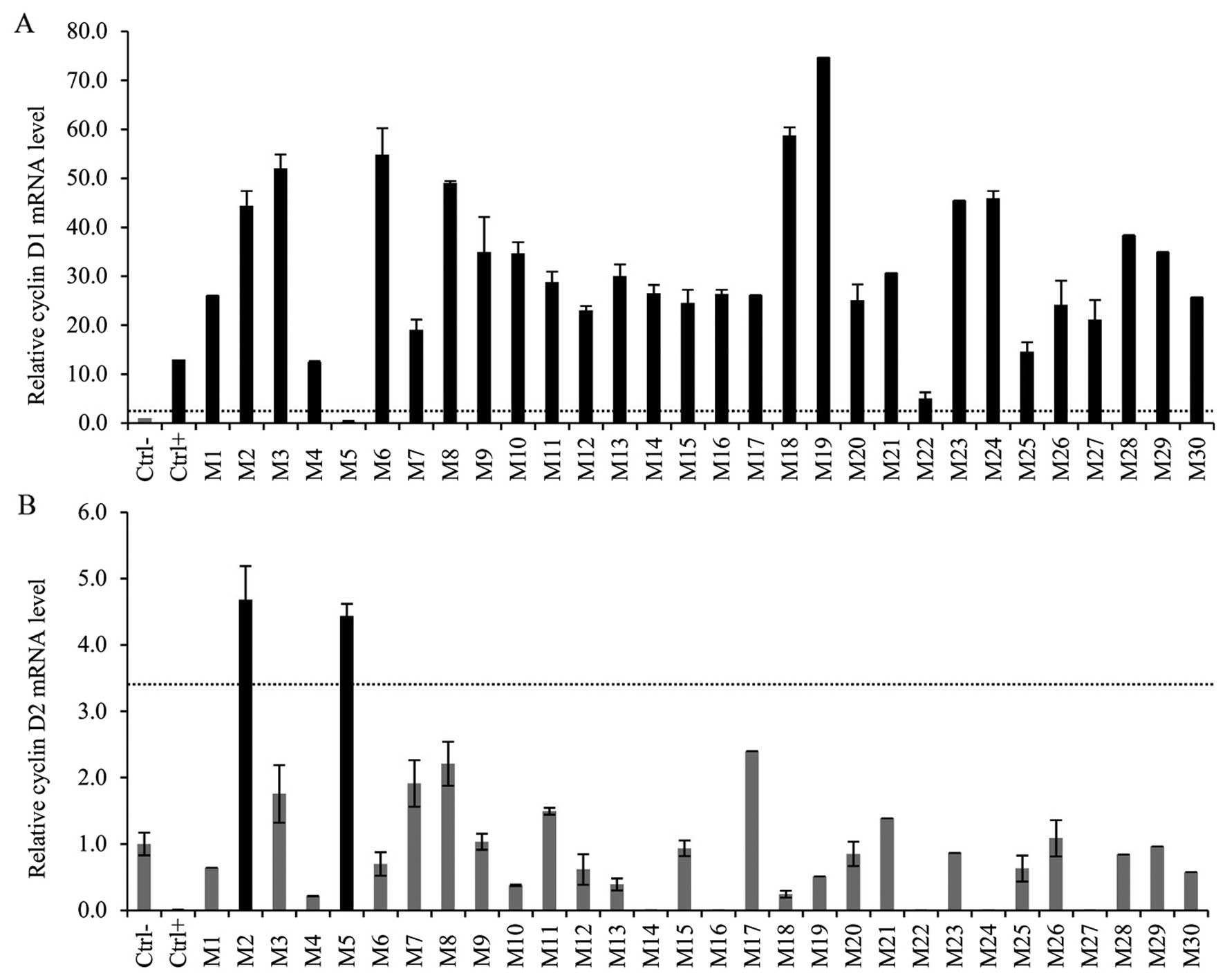
The CCND1 mRNA expression of 7 MCL cases was
analyzed in our previous study (27). Twenty-three new MCL patients were
enrolled. We confirmed overexpression of CCND1 mRNA in 29
out of 30 cases (97%). The median relative fold increase was 27
(range 5.0–74.7). One case, M5, was previously reported as cyclin
D1-negative (27 - case 25B) and analysis of the mRNA level
confirmed this result (Fig. 1A).
For all newly enrolled samples, the t(11;14) translocation was
assessed by FISH and competitive RT-PCR was performed as previously
described (27), both with
affirmative results: all new cases exhibited translocation t(11;14)
and expressed a high level of CCND1 mRNA (data not shown).
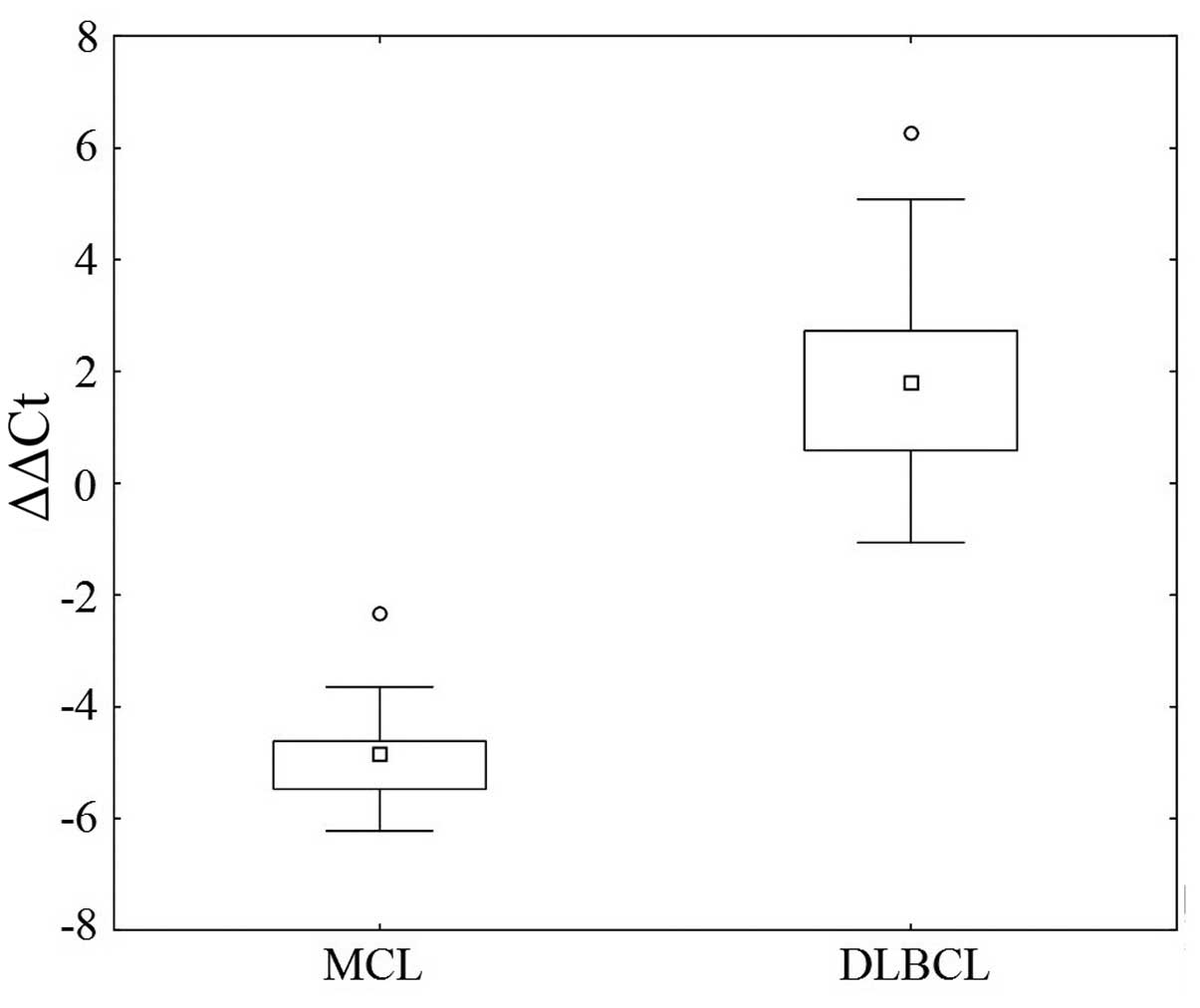
Analysis of the CCND1 mRNA in 104 specimens from the DLBCL
patients did not reveal any positivity. The relative fold increase
of all samples was under the cut-off level reaching a median 0.3
(range 0.01–2.1). Fig. 2
illustrates the quantity of the CCND1 mRNA in the MCL and
DLBCL cases.
The level of CCND2 mRNA scored above the
cut-off level in 2 out of 30 MCL cases; in sample M5 which was
earlier classified as cyclin D1-negative/cyclin D2-positive (27 -
case 25B), and sample M2 with high expression of cyclin D1
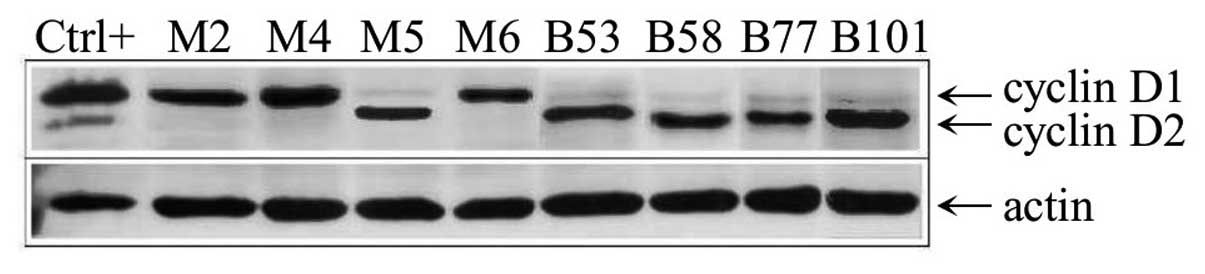
(Fig. 1B). The level of the cyclin
D2 protein in the samples was assessed by immunoblotting using the
Ab-4 antibody and revealed a high level of cyclin D2 protein only
in sample M5 (Fig. 3). In the DLBCL
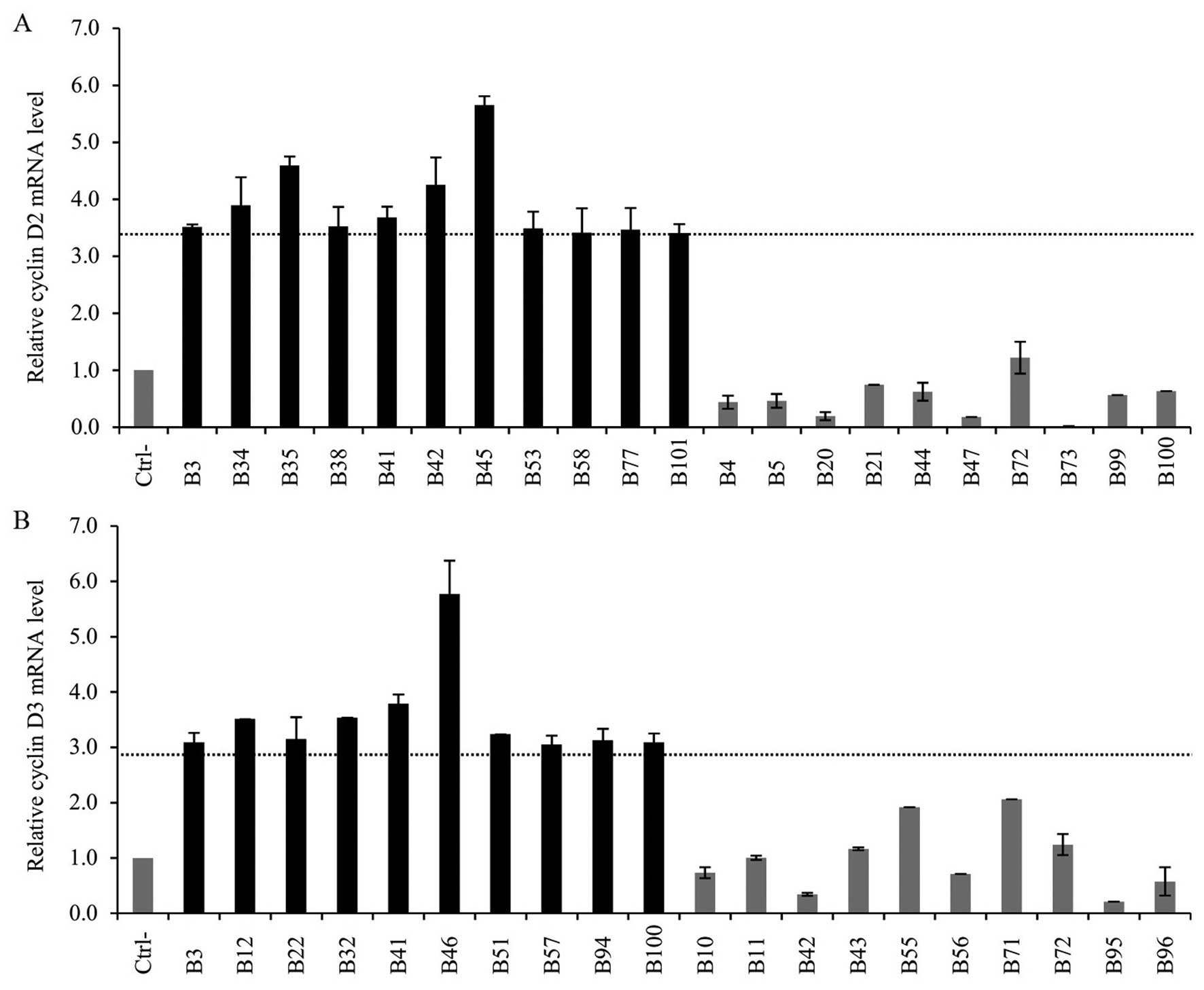
samples, the CCND2 mRNA was increased in 11 cases out of 104
(10.6%) (Fig. 4A). The median
relative fold increase reached 3.6 (range 3.4–5.7). Detection of
the cyclin D2 protein in the DLBCL cases by immunoblotting
confirmed the results (Fig. 3).
Cyclin D2 positivity was not associated with any specific
morphologic subtype. The majority of cyclin D2-positive cases were
ranked in the centroblastic variant (4/45) reaching a frequency of
8.9%. Among the immunoblastic cases, only one was cyclin
D2-positive [1/8 (12.5%)]. Similarly, expression of cyclin D2 was
not associated with any immunohistochemically derived subgroup,
neither GCB/non-GCB nor 1/2. On the other hand, the cyclin D2
positivity was distinctively more frequent among secondary cases
[6/23 (26.1%)] in comparison to cases de novo [5/81
(6.2%)].
Finally, we quantified the CCND3 transcripts
(Fig. 4B). Overexpression of
CCND3 mRNA was found in 6 of the MCL cases (19.3%) reaching
a median of 3.3 (range 3.0–4.3). The M2 specimen exhibited high
expression of all three cyclin D mRNAs. Concurrent overexpression
of cyclin D2 and D3 mRNAs occurred in specimen M5. In the cohort of
DLBCL cases, cyclin D3 mRNA overexpression occurred in 10 cases
(9.6%) reaching a median relative fold increase of 3.19 (range
3.1–5.8). Two specimens, B3 and B41, expressed cyclin D2
concurrently with cyclin D3 mRNA. The level of the cyclin D3
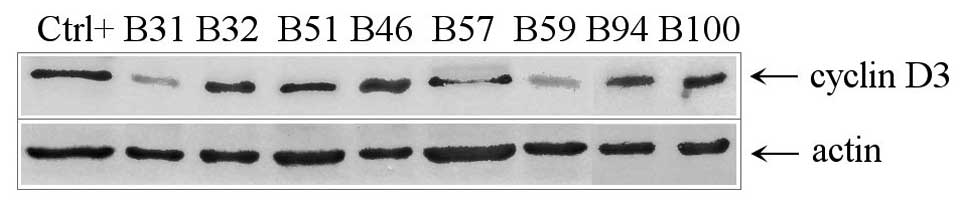
protein was assessed by immunoblotting using the Ab-1 antibody in
the DLBCL samples confirming the results of qRT-PCR (Fig. 5).
Relationship between the expression of
cyclin D in DLBCL and disease outcome
Next, we investigated the relationship between the
expression of D-type cyclins and overall survival (OS) and
progression-free survival (PFS) of the DLBCL cases. The median
survival time of the cohort of DLBCL patients was 39.1 months;
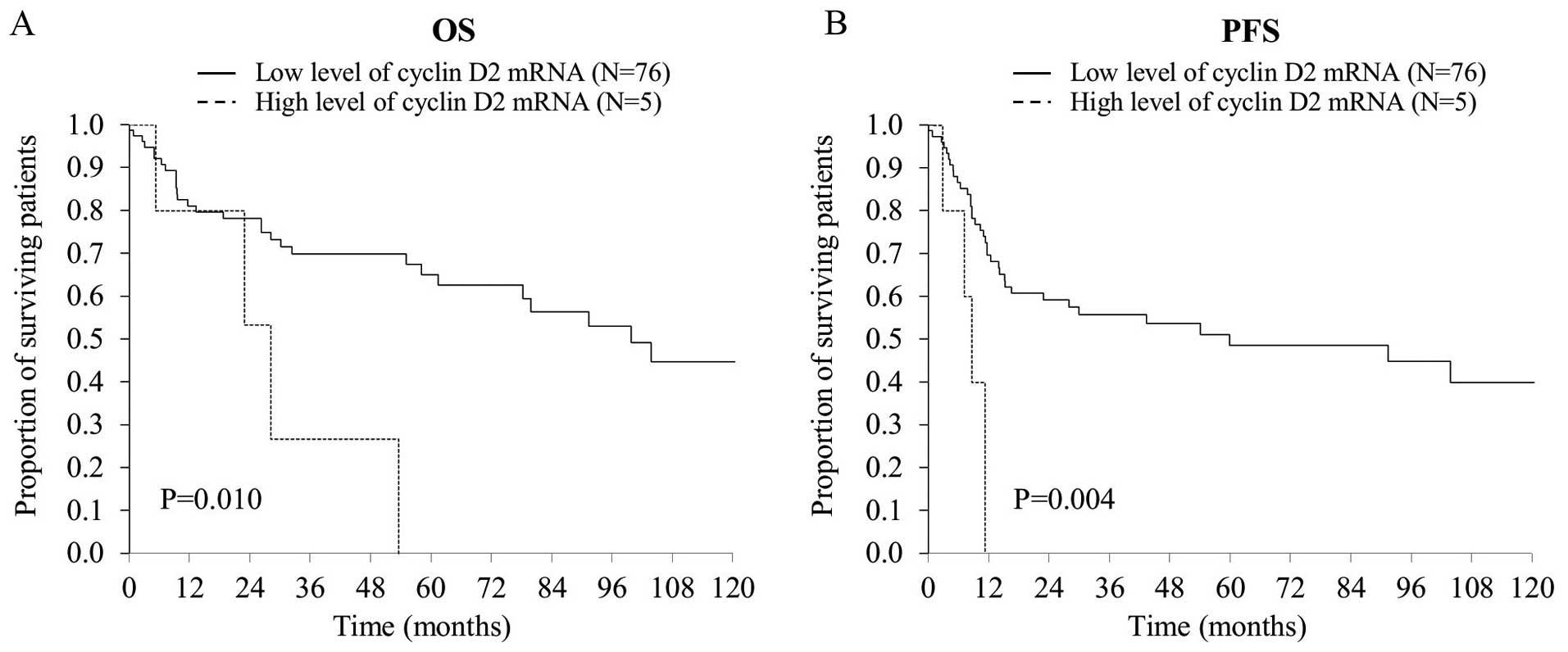
40.4% of patients survived 5 years. Cases exhibiting cyclin D2
expression demonstrated a trend toward worse OS and PFS, but this
effect did not reach statistical significance (P=0.062 and 0.134,
respectively). However, it reached clear statistical significance
(P=0.016 for OS, and P=0.009 for PFS) for DLBCL cases de
novo (Fig. 6). Expression of
cyclin D3 was not associated with OS (P=0.958) and PFS (P=0.822)
(data not shown).
Association of cyclin D2 and D3 with de
novo CD5+ DLBCLs
The CD5 antigen expression was examined by
immunohistochemistry. CD5 positivity was detected in 13 DLBCL cases
(12.5%) including 8 de novo cases. The CD5-positive cases
demonstrated a trend toward worse OS, but this effect did not reach
statistical significance (P=0.124). Cyclin D2 was overexpressed in
25% of de novo CD5+ DLBCLs (2/8) and in 4.1% of
de novo CD5− DLBCLs (3/73). A statistical
analysis of this phenomenon was not performed because of the low
number of cases in the CD5+ arm of the study, although
the higher expression of cyclin D2 appeared to be connected to the
CD5+ phenotype. The concurrent cyclin D3- and
CD5-positivity was found in only one secondary case of DLBCL (D3)
and it was not detected among the DLBCL cases de novo.
Discussion
In the present study, we performed expression
analysis of cyclin D1, D2 and D3 in tumor samples from MCL and
DLBCL cases using quantitative RT-PCR. This method is not performed
as part of routine diagnostic procedures for MCL/DLBCL.
Cyclin D1 overexpression is a diagnostic marker of
MCL and thus it is assessed routinely. Immunohistochemical analysis
and/or fluorescence in situ hybridization (FISH) are almost
exclusively employed techniques. It has been previously shown that
assessment of cyclin D1 mRNA expression by qRT-PCR is helpful and
is a specific tool for the diagnosis of MCL providing an
alternative to FISH and immunohistochemistry (32–35).
In the first part of our study, we confirmed this. The MCL cases
with translocation t(11;14) and a high level of the cyclin D1
protein also showed a high level of cyclin D1 mRNA. Second, the
cyclin D1-positive MCL cases exhibited significant overexpression
of cyclin D1 mRNA ranging from a 5- to 75-fold increase over the
values found in the non-neoplastic specimens, thus reliably
distinguishing cyclin D1 positivity from negativity. This was
clearly documented also by analysis of the cyclin D1-negative MCL
case M5 (27 -case 25B) as reported previously, which scored
substantially under the cut-off level (Fig. 1A).
qRT-PCR is rapid, sensitive, specific, reproducible
and thus a convenient technique for the routine analysis of gene
expression. In comparison to other methods, it allows quantitative
assessment of CCND1 mRNA. However, for diagnostic purposes,
knowledge of the exact level of CCND1 mRNA (or protein) is
often dispensable. Simple qualitative 'positive-negative'
resolution may be sufficient. In addition, according to our
experience, RNA isolated from formalin-fixed paraffin-embedded
tumor tissue blocks is often degraded to such an extent that
prevents reliable qRT-PCR analysis. From a practical point of view,
the necessity to use fresh or deeply frozen tumor tissues may be
limiting in some clinical institutions. Having the option to use
formalin-fixed paraffin-embedded tumor tissue blocks clearly shifts
the preference to FISH and IHC.
The CCND1 mRNA below the cut-off limit was
detected in MCL case M5 that was previously described as cyclin
D1-negative/cyclin D2-positive. In the previous report, cyclin D2
positivity was assessed by immunoblotting using the cyclin
D2-specific antibody Ab-4 (27 - case 25B). In the present study,
the level of cyclin D2 mRNA was analyzed by qRT-PCR. In this
particular case, the analyses detected a high level of cyclin D2
mRNA indeed, thus confirming the earlier conclusion. Interestingly,
one of the cyclin D1-positive MCL cases, M2, scored also positive
for CCND2 mRNA by qRT-PCR analysis. Although we failed to
detect the cyclin D2 protein in this case, the
CCND1/CCND2 mRNA values were rather balanced as
determined by competitive RT-PCR analysis (27 - case 27). On the
other hand, a few more cases scored comparably balanced in
CCND1/CCND2 mRNAs by competitive RT-PCR (27 - cases
30 and 32); none of them scored positive by the qRT-PCR analysis of
CCND2 mRNA (cases M6 and M7).
Expression of CCND3 above the cut-off limit
was observed in 6 MCL cases, including case M2 which scored
positive for all three types of cyclin D, and M5 with concurrent
high expression of cyclin D2. The competitive RT-PCR analysis
showed that the level of cyclin D3 was generally very low compared
to cyclin D1 and/or D2 (27). This
suggests that although the 6 MCL cases exhibited a significantly
elevated level of cyclin D3 mRNA relative to the non-neoplastic
specimen, the real level may be very low.
Nevertheless, although the low incidence of cyclin
D1-negative and cyclin D2- or D3-positive MCL cases has been
confirmed (5), the presence of
cyclin D2 or D3 is not specific for MCL and cannot be used as a
criterion for reliable diagnosis of this disease. For example,
Quintanilla-Martinez et al (36) found cyclin D2 in most B-cell
non-Hodgkin lymphomas using immunohistochemistry. Cyclin D3 was
also found in non-Hodgkin and classical Hodgkin lymphomas (18,37,38).
In the present study, we confirmed that expression of cyclin D2 and
D3 is not MCL-specific as we found several positive cases among the
DLBCL patients.
In contrast to several studies (15–18,20,21) we
did not detect any cyclin D1-positive case in the cohort of 104
DLBCL patients. Apart from case reports, the incidence of cyclin D1
positivity among DLBCL cases described by others ranges from 1.5 to
4.3% with the exception of Vela-Chávez et al study (20) indicating 15%. The referred studies
usually employed immunohistochemical detection of the cyclin D1
protein, while we performed quantitative assessment of CCND1
mRNA. This may be an explanation of the difference. In addition,
the possibility of misdiagnosed positive DLBCL cases is not
excluded as Ok et al (39)
recently identified 6 tumors originally classified as cyclin
D1-positive DLBCL and reclassified them later as likely pleomorphic
MCL. Other authors also considered such misdiagnosed cases
(15,21). Recently, a report of two blastoid
B-cell lymphoma, most probably DLBCL cases, displaying the
CCND1 gene rearrangement and high cyclin D1 expression
suggests the existence of a diagnostic 'gray zone' between DLBCL
and MCL (40). On the other hand,
with respect to the size of our cohort (104 patients) and suggested
the low prevalence of cyclin D1 positivity (1.5–4.3%) we cannot
reliably exclude the rare occurrence of the cyclin D1-positive
DLBCL case. Nevertheless, our results rather support the former
idea of DLBCL cases as cyclin D1-negative or at least the very rare
occurrence of cyclin D1 positivity among DLBCL cases (26,41).
The frequency of cyclin D2 positivity in DLBCL was
found to vary between 13 and 62% in previous studies and it has
been suggested as an adverse marker of DLBCL (12,18,22,23,28,42).
The analyses were performed either immunohistochemically or by
qRT-PCR. In our study, overexpressed CCND2 mRNA was found in
11 DLBCL cases (10.6%). Cyclin D2 expression was negatively related
with overall survival but this effect was not statistically
significant (P=0.062). However, when analyzing de novo and
secondary DLBCL cases separately, we found that the cyclin D2
positivity, although much less frequent among the de novo
cases [5/81 (6.2%)], represents a clear adverse marker. This result
is in agreement with previous studies (13,22,28).
Overexpression of cyclin D2 was also found to be
associated with CD5 positivity in DLBCL, especially in de
novo cases (24). In our
cohort, the low number of cyclin D2-positive cases also did not
allow statistical analysis. Nevertheless, the tendency was rather
clear. Cyclin D2 was overexpressed in 2 of 8 CD5-positive cases,
while only in 3 of 73 CD5-negative cases.
The expression of cyclin D3 in DLBCL has been also
studied by various groups and again with rather inconsistent
results. The positivity assessed by immunohistochemistry scored
between 20 and 62% (18,22,26).
While Filipits et al (26)
demonstrated that high expression of cyclin D3 in tumors (detected
in 38% cases) is associated with a significantly lower complete
remission rate and shorter 3-year overall survival, Hans et
al (22), detecting cyclin D3
overexpression in 62% of cases, found no effect on survival. In our
cohort, cyclin D3 mRNA positivity occurred in 9.6% DLBCL cases and
we did not find any impact on overall survival (P=0.958). Part of
the explanation, similarly to MCL cases, may be the actual level of
cyclin D3 mRNA which seems to be substantially lower in comparison
to cyclin D1 and D2 as indicated by competitive RT-PCR (27).
In conclusion, we used qRT-PCR to study the
expression of cyclin D1, D2 and D3 in MCL and DLBCL cases. We
showed that the cyclin D1 expression was limited to MCL and did not
occur in DLBCL cases. Overexpression of cyclin D2, rare in MCL,
occured in a distinct portion of the DLBCL cases and may serve as a
negative prognostic marker. Expression of cyclin D3 was found in
comparable frequency in both types of studied lymphomas but did not
display any statistically significant effect on disease
outcome.
Acknowledgments
We thank Eva Bartova and Ales Hampl/Dasa Dolezalova
for providing us with the MOLP-8 and Jurkat cells. This study was
supported by grant NT/13784-4/2012 of the Internal Grant Agency of
the Ministry of Health of the Czech Republic, and by MH CZ - DRO
(FNBr, 65269705).
References
|
1
|
Sherr CJ: D-type cyclins. Trends Biochem
Sci. 20:187–190. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Musgrove EA, Caldon CE, Barraclough J,
Stone A and Sutherland RL: Cyclin D as a therapeutic target in
cancer. Nat Rev Cancer. 11:558–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banks PM, Chan J, Cleary ML, Delsol G, De
Wolf-Peeters C, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe
ES, et al: Mantle cell lymphoma. A proposal for unification of
morphologic, immunologic, and molecular data. Am J Surg Pathol.
16:637–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartkova J, Lukas J, Strauss M and Bartek
J: Cell cycle-related variation and tissue-restricted expression of
human cyclin D1 protein. J Pathol. 172:237–245. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salaverria I, Royo C, Carvajal-Cuenca A,
Clot G, Navarro A, Valera A, Song JY, Woroniecka R, Rymkiewicz G,
Klapper W, et al: CCND2 rearrangements are the most frequent
genetic events in cyclin D1(−) mantle cell lymphoma. Blood.
121:1394–1402. 2013. View Article : Google Scholar :
|
|
6
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Hematopoietic and Lymphoid Tissues. 4th edition. IARC;
Lyon, France: 2008
|
|
7
|
Ye BH, Rao PH, Chaganti RS and
Dalla-Favera R: Cloning of bcl-6, the locus involved in chromosome
translocations affecting band 3q27 in B-cell lymphoma. Cancer Res.
53:2732–2735. 1993.PubMed/NCBI
|
|
8
|
Gascoyne RD, Adomat SA, Krajewski S,
Krajewska M, Horsman DE, Tolcher AW, O'Reilly SE, Hoskins P,
Coldman AJ, Reed JC, et al: Prognostic significance of Bcl-2
protein expression and Bcl-2 gene rearrangement in diffuse
aggressive non-Hodgkin's lymphoma. Blood. 90:244–251.
1997.PubMed/NCBI
|
|
9
|
Savage KJ, Johnson NA, Ben-Neriah S,
Connors JM, Sehn LH, Farinha P, Horsman DE and Gascoyne RD: MyC
gene rearrangements are associated with a poor prognosis in diffuse
large B-cell lymphoma patients treated with R-CHOP chemotherapy.
Blood. 114:3533–3537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barrans S, Crouch S, Smith A, Turner K,
Owen R, Patmore R, Roman E and Jack A: Rearrangement of MYC is
associated with poor prognosis in patients with diffuse large
B-cell lymphoma treated in the era of rituximab. J Clin Oncol.
28:3360–3365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu-Monette ZY, Wu L, Visco C, Tai YC,
Tzankov A, Liu WM, Montes-Moreno S, Dybkaer K, Chiu A, Orazi A, et
al: Mutational profile and prognostic significance of TP53 in
diffuse large B-cell lymphoma patients treated with R-CHOP: Report
from an International DLBCL Rituximab-CHOP Consortium Program
Study. Blood. 120:3986–3996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alizadeh AA, Eisen MB, Davis RE, Ma C,
Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lossos IS, Czerwinski DK, Alizadeh AA,
Wechser MA, Tibshirani R, Botstein D and Levy R: Prediction of
survival in diffuse large-B-cell lymphoma based on the expression
of six genes. N Engl J Med. 350:1828–1837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horn H, Ziepert M, Becher C, Barth TF,
Bernd HW, Feller AC, Klapper W, Hummel M, Stein H, Hansmann ML, et
al German High-Grade Non-Hodgkin Lymphoma Study Group: MyC status
in concert with BCL2 and BCL6 expression predicts outcome in
diffuse large B-cell lymphoma. Blood. 121:2253–2263. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ehinger M, Linderoth J, Christensson B,
Sander B and Cavallin-Ståhl E: A subset of CD5- diffuse large
B-cell lymphomas expresses nuclear cyclin D1 with aberrations at
the CCND1 locus. Am J Clin Pathol. 129:630–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez-Justo M, Huang Y, Ye H, Liu H,
Chuang SS, Munson P, Prada-Puentes C, Kim I, Du MQ and Bacon CM:
Cyclin D1-positive diffuse large B-cell lymphoma. Histopathology.
52:900–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teruya-Feldstein J, Gopalan A and
Moskowitz CH: CD5 negative, cyclin D1-positive diffuse large B-cell
lymphoma (DLBCL) presenting as ruptured spleen. Appl
Immunohistochem Mol Morphol. 17:255–258. 2009. View Article : Google Scholar
|
|
18
|
Metcalf RA, Zhao S, Anderson MW, Lu ZS,
Galperin I, Marinelli RJ, Cherry AM, Lossos IS and Natkunam Y:
Characterization of D-cyclin proteins in hematolymphoid neoplasms:
Lack of specificity of cyclin-D2 and D3 expression in lymphoma
subtypes. Mod Pathol. 23:420–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lucioni M, Novara F, Riboni R, Fiandrino
G, Nicola M, Kindl S, Boveri E, Jemos V, Arcaini L, Zuffardi O, et
al: CD5(−) diffuse large B-cell lymphoma with peculiar cyclin
D1+ phenotype. Pathologic and molecular characterization
of a single case. Hum Pathol. 42:1204–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vela-Chávez T, Adam P, Kremer M, Bink K,
Bacon CM, Menon G, Ferry JA, Fend F, Jaffe ES and
Quintanilla-Martínez L: Cyclin D1 positive diffuse large B-cell
lymphoma is a post-germinal center-type lymphoma without
alterations in the CCND1 gene locus. Leuk Lymphoma. 52:458–466.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hsiao SC, Cortada IR, Colomo L, Ye H, Liu
H, Kuo SY, Lin SH, Chang ST, Kuo TU, Campo E, et al: SOX11 is
useful in differentiating cyclin D1-positive diffuse large B-cell
lymphoma from mantle cell lymphoma. Histopathology. 61:685–693.
2012.PubMed/NCBI
|
|
22
|
Hans CP, Weisenburger DD, Greiner TC, Chan
WC, Aoun P, Cochran GT, Pan Z, Smith LM, Lynch JC, Bociek RG, et
al: Expression of PKC-beta or cyclin D2 predicts for inferior
survival in diffuse large B-cell lymphoma. Mod Pathol.
18:1377–1384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Amen F, Horncastle D, Elderfield K, Banham
AH, Bower M, Macdonald D, Kanfer E and Naresh KN: Absence of
cyclin-D2 and Bcl-2 expression within the germinal centre type of
diffuse large B-cell lymphoma identifies a very good prognostic
subgroup of patients. Histopathology. 51:70–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Igawa T, Sato Y, Takata K, Iwaki N, Tanaka
T, Asano N, Maeda Y, Orita Y, Nakamura N, Nakamura S, et al: De
novo CD5-positive diffuse large B-cell lymphomas show high
specificity for cyclin D2 expression. Diagn Pathol. 8:812013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi M, Seto M, Okamoto M,
Ichinohasama R, Nakamura N, Yoshino T, Suzumiya J, Murase T, Miura
I, Akasaka T, et al: De novo CD5+ diffuse large B-cell
lymphoma: A clinicopathologic study of 109 patients. Blood.
99:815–821. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Filipits M, Jaeger U, Pohl G, Stranzl T,
Simonitsch I, Kaider A, Skrabs C and Pirker R: Cyclin D3 is a
predictive and prognostic factor in diffuse large B-cell lymphoma.
Clin Cancer Res. 8:729–733. 2002.PubMed/NCBI
|
|
27
|
Stefancikova L, Moulis M, Fabian P,
Falkova I, Vasova I, Kren L, Macak J and Smardova J: Complex
analysis of cyclin D1 expression in mantle cell lymphoma: Two
cyclin D1-negative cases detected. J Clin Pathol. 62:948–950. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar
|
|
29
|
Muris JJF, Meijer CJLM, Vos W, van Krieken
JH, Jiwa NM, Ossenkoppele GJ and Oudejans JJ: Immunohistochemical
profiling based on Bcl-2, CD10 and MUM1 expression improves risk
stratification in patients with primary nodal diffuse large B cell
lymphoma. J Pathol. 208:714–723. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuo Y, Drexler HG, Harashima A, Okochi
A, Hasegawa A, Kojima K and Orita K: Induction of CD28 on the new
myeloma cell line MOLP-8 with t(11;14)(q13;q32) expressing
delta/lambda type immunoglobulin. Leuk Res. 28:869–877. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Szepesi A, Gelfand EW and Lucas JJ:
Association of proliferating cell nuclear antigen with
cyclin-dependent kinases and cyclins in normal and transformed
human T lymphocytes. Blood. 84:3413–3421. 1994.PubMed/NCBI
|
|
32
|
Bijwaard KE, Aguilera NS, Monczak Y,
Trudel M, Taubenberger JK and Lichy JH: Quantitative real-time
reverse transcription-PCR assay for cyclin D1 expression: Utility
in the diagnosis of mantle cell lymphoma. Clin Chem. 47:195–201.
2001.PubMed/NCBI
|
|
33
|
Hui P, Howe JG, Crouch J, Nimmakayalu M,
Qumsiyeh MB, Tallini G, Flynn SD and Smith BR: Real-time
quantitative RT-PCR of cyclin D1 mRNA in mantle cell lymphoma:
Comparison with FISH and immunohistochemistry. Leuk Lymphoma.
44:1385–1394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brizova H, Kalinova M, Krskova L, Mrhalova
M and Kodet R: Quantitative measurement of cyclin D1 mRNA, a potent
diagnostic tool to separate mantle cell lymphoma from other B-cell
lymphoproliferative disorders. Diagn Mol Pathol. 17:39–50.
2008.PubMed/NCBI
|
|
35
|
Cao X, Fan L, Fang C, Zhu DX, Dong HJ,
Wang DM, Wang YH, Xu W and Li JY: The expression of SOX11, cyclin
D1, cyclin D2, and cyclin D3 in B-cell lymphocytic proliferative
diseases. Med Oncol. 29:1190–1196. 2012. View Article : Google Scholar
|
|
36
|
Quintanilla-Martinez L, Slotta-Huspenina
J, Koch I, Klier M, Hsi ED, de Leval L, Klapper W, Gesk S, Siebert
R and Fend F: Differential diagnosis of cyclin D2+
mantle cell lymphoma based on fluorescence in situ hybridization
and quantitative real-time-PCR. Haematologica. 94:1595–1598. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Teramoto N, Pokrovskaja K, Szekely L,
Polack A, Yoshino T, Akagi T and Klein G: Expression of cyclin D2
and D3 in lymphoid lesions. Int J Cancer. 81:543–550. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Møller MB, Nielsen O and Pedersen NT:
Cyclin D3 expression in non-Hodgkin lymphoma. Correlation with
other cell cycle regulators and clinical features. Am J Clin
Pathol. 115:404–412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ok CY, Xu-Monette ZY, Tzankov A, O'Malley
DP, Montes-Moreno S, Visco C, Møller MB, Dybkaer K, Orazi A, Zu Y,
et al: Prevalence and clinical implications of cyclin D1 expression
in diffuse large B-cell lymphoma (DLBCL) treated with
immunochemotherapy: A report from the International DLBCL
Rituximab-CHOP Consortium Program. Cancer. 120:1818–1829. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Juskevicius D, Ruiz C, Dirnhofer S and
Tzankov A: Clinical, morphologic, phenotypic, and genetic evidence
of cyclin D1-positive diffuse large B-cell lymphomas with CYCLIN D1
gene rearrangements. Am J Surg Pathol. 38:719–727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bai M, Tsanou E, Agnantis NJ, Chaidos A,
Dimou D, Skyrlas A, Dimou S, Vlychou M, Galani V and Kanavaros P:
Expression of cyclin D3 and cyclin E and identification of distinct
clusters of proliferation and apoptosis in diffuse large B-cell
lymphomas. Histol Histopathol. 18:449–457. 2003.PubMed/NCBI
|
|
42
|
Malumbres R, Chen J, Tibshirani R, Johnson
NA, Sehn LH, Natkunam Y, Briones J, Advani R, Connors JM, Byrne GE,
et al: Paraffin-based 6-gene model predicts outcome in diffuse
large B-cell lymphoma patients treated with R-CHOP. Blood.
111:5509–5514. 2008. View Article : Google Scholar : PubMed/NCBI
|