1. Introduction
During evolution, the skin has been constantly
exposed to complex agents, such as physical (sun rays) and
biological assaults (microbes and or allergens); in response to
these assaults, the skin has developed specific networks of
molecular and cellular sensors to counteract all these aggressors
(1). The skin is the largest organ
with immune function, and represents an intrinsic network of
non-immune and immune cell and molecules that interrelate (2,3).
Throughout ontogeny, apart from being exposed to biological
aggressors, skin is also exposed to potentially harmful
environmental compounds (cigarette smoke, automobile emissions,
industrial soot and groundwater) (4).
Due to these many and complex aggressions, skin
cancer appears in many forms and it is one of the most common
malignancies affecting humans. Owing to various factors, the
incidence of skin cancer has increased over the years (5,6). Skin
cancer is a multifactorial disease in that it has a strong genetic
component. In addition, several environmental factors also play a
role in this increased incidence. Among the environmental factors,
the most significant is the exposure to ultraviolet (UV) radiation
(7), doubled by the exposure to
certain chemicals, medication use or stress. All these connected
factors may modulate many skin physiopathological processes
(8–11), and trigger the initiation and
progression of skin cancer (5).
Skin carcinogenesis is a complex, multistep process
(12,13), and the study of early alterations in
the skin and of the mechanisms involved, as well as the development
of novel therapeutic strategies are of interest for both scientific
research and clinical practice. Moreover, the striking increase in
both the number of new topical chemical entities and their
biological effects creates the need to understand the intimate
molecular mechanisms that underlie their in vivo effects.
Developing in vivo systems for the rapid evaluation of
potential drugs is always the concern of researchers. Mouse models
of skin carcinogenesis remain one of the most commonly available
and cost-effective animal models. In this type of model, agents,
either applied topically or systemically, can be studied at the
earliest possible stage in the development of drugs/new therapies.
In contradiction to the undemanding condition of this model, there
is a complex array of mouse strains with distinctive biological
behavior to this standard protocol, and this review intends to
highlight this distinction and to flag the reported
particularities.
2. Human skin versus animal model skin:
Similarities and peculiarities in carcinogenesis
Immunologically, mice are the first option when
studying immunotoxicology. From this point of view, >15 years
ago, the sequencing of human and mice genomes revealed that
approximatley 300 genes are unique to mice in comparison to humans
(14).
A comprehensive review, published >10 years ago,
demonstrated that although there are differences in the skin of
mice versus human skin, mice are reliable in vivo models
that can be used to furnish relevant toxicological information
(15). Human therapies are becoming
more complex, targeting various cells/proteins/genes; hence, the
information gathered from animal models should be carefully
weighted in terms of extrapolating data from mice to humans. This
is the reason that we have witnessed so many examples of therapies
that have had perfect outcomes in experimental animal models, but
have then failed to be as effective in humans (16–21).
The main immunological differences regarding the
skin is that in mouse skin and mucosa, the predominant T cells are
γ/δ T cell receptors (TCRs), whereas in humans, α/β TCRs prevail
(22).
T cells that are specific for mouse skin have their
TCRs encoded by a single Vγ and Vδ gene. These cells seem
oligoclonal (Vγ5-Vδ1 genes are encoding for this specific TCR), are
found in the epidermis, and they mainly appear as dendritic
epidermal T cells. In human skin, T cells with α/β TCRs predominate
in human skin, mainly in the dermis. Up to now, TCR γ/δ T cells
were not identified in normal human skin (22) and were reported only in lymphoma
cases (23). Even in mice, TCR γ/δ
phenotype T cells can have different densities between different
skin sites and different strains (24). In mice, TCR γ/δ T cells play
important roles in tissue homeostasis and during tissue repair.
These cells secrete growth factors (e.g., for keratinocytes and
insulin-like growth factors), through which the crosstalk between
γ/δ T cells from the skin and keratinocytes takes place, actively
contributing to the physiology of normal skin and further
contributing to wound healing (25). In humans, α/β TCR T cells are
activated by CD1 antigen-presenting molecules. It has been
demonstrated that a large number of T cells with CD1a-autoreactive
phenotype are homed to the skin, producing interleukin (IL)-22 in
response to CD1a expressed on a different skin cell population,
namely Langerhans cells (LCs) (26). Thus, while in mouse skin T cells
collaborate mainly with keratinocytes, in human skin, T cells
associate in functionality with specific dendritic cells, such as
LCs.
3. Inflammation triggering
carcinogenesis
Inflammation involves the secretion of a number of
mediators from immune and non-immune cells and occurs for the
purpose of the restoration of damaged tissue. Inflammation was
known from the beginning of the last century (27), and is a multi-stage process,
beginning with the injury inflicted upon a tissue and ending with
the reconstruction of the damaged tissue.
Environmental factors can inflict this injury,
whether a macro-physical trauma or a micro-trauma (e.g., overuse,
friction and sunburn). At the cellular level, the disruption of the
cellular membrane releases the intracellular contents into
extracellular spaces. Metabolically, hypoxic changes occur, and the
cells become deprived of oxygen (secondary hypoxic injury), the
sodium pump fails, and cellular membrane disruption continues in
adjacent cells, thus enhancing the destruction at a cellular level.
This disruption generates mediators (e.g., histamine and
bradykinin) that represent the first signals triggering an
inflammatory response. The inflammatory response triggers
hemodynamic changes: arteries dilate enhancing blood flow, inactive
capillaries and venules open, the total blood flow increases, the
rate of flow decreases and leukocytes bening to adhere to the
vessel wall. Due to critical permeability alterations, leukocytes
transmigrate to the injured site. Following the increase in
chemoattractant gradient concentration, leukocytes migrate to the
injured site. Neutrophils are the first cells that arrive, being
the temporary first line of defense [short-lived cells
(approximately 7 h)]; neutrophils are followed by macrophages that
build up the second line of defense and can survive for months.
These two type of cells process, through phagocytosis,
debris/microbes, enhancing the clearance process through lymph
vessels (28).
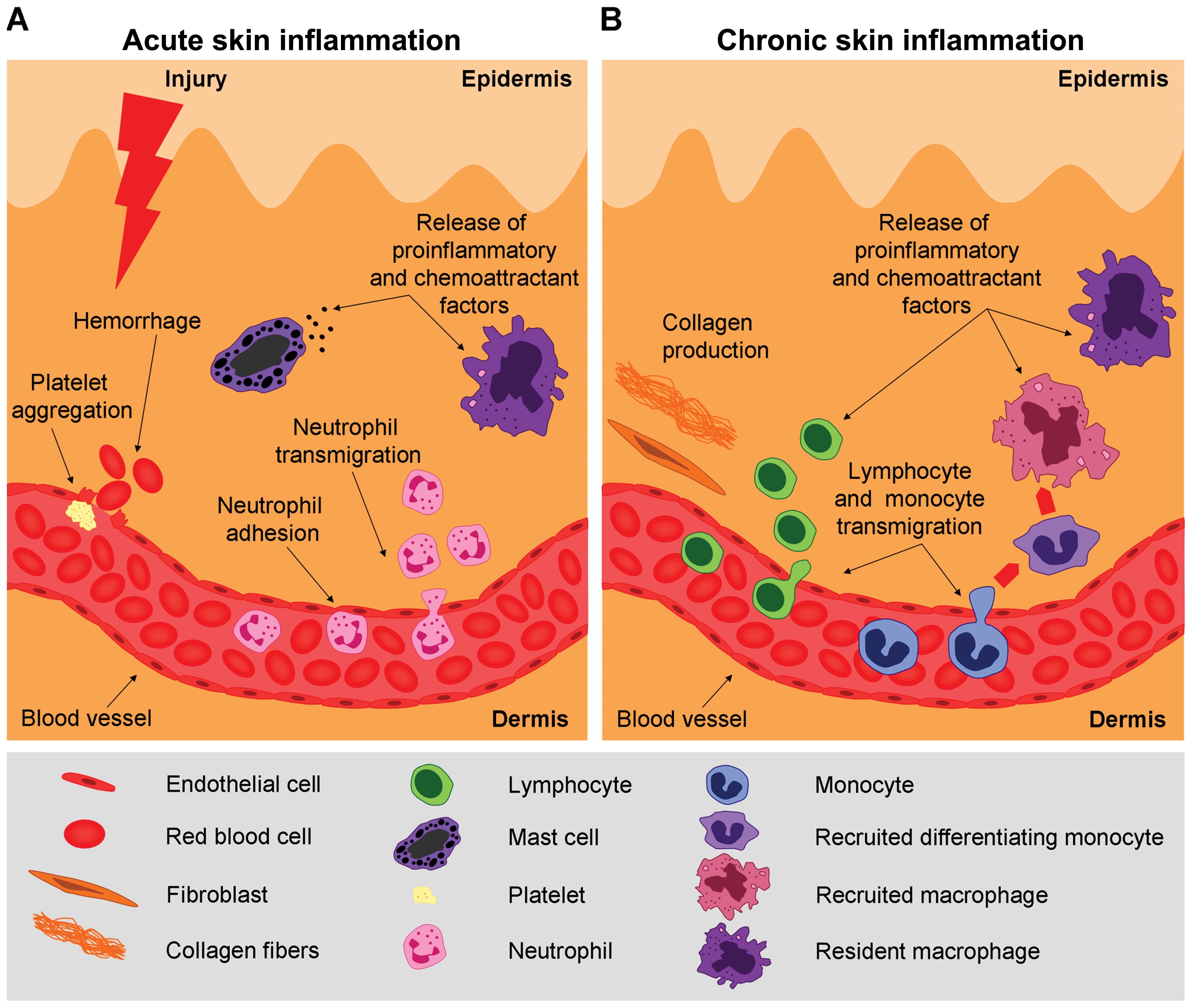
In the framework of this review, we focus on a key
issue in the process of inflammation, the acute versus the chronic
stages of this process (Fig. 1)
that can trigger skin carcinogenesis. There is a scientific
consensus to incriminate chronic inflammation as linked to
carcinogenesis (29) and in mouse
experimental models, this chronic inflammation is experimentally
sustained in order to favor tumorigenesis.
The differences between these two stages of
inflammation reside on different immunological mechanisms. During
the acute inflammatory stage, innate cells secrete mediators that
attract Th1-type T lymphocytes. Th1 cells secrete cytokines with
antitumor activity [e.g., IL-2 and interferon (IFN)-γ]. Apart from
T cells, factors secreted by B cells (e.g., antitumor
immunoglobulins) activate cytotoxic T lymphocytes (CTLs). Hence, a
cellular armamentarium is built up to protect against tumor
development.
During the chronic stages of inflammation, when
there is a constant activation of the immune response without
actually resolving the damage inflicted to tissue, there is an
accumulation of regulatory T cells, Th2 cells and activated B
cells. In chronic inflammation, immune cells secrete
pro-tumorigenic factors [e.g., IL-4, -6, -10 and -13, and
transforming growth factor β (TGF-β)] that inactivate CTL
cytotoxicity, thus favoring tumor development (30).
The soluble mediators and cellular effectors of
inflammation are common to the tumor microenvironment and can
reside in the tumor site. Inflammatory conditions can preclude a
malignant transformation and/or an oncogenic alteration sustains
the inflammatory microenvironment favorable for tumor development
(31), a phenomenon that is
exploited in chemically induced skin carcinogenesis.
Chronic inflammation and
tumorigenesis
In skin there is an established link between tissue
damage, inflammation and cancer development. Tumorigenesis is based
on constitutive pathway activation, while inflammation is a
self-limiting process in normal conditions (32). In the framework of this review,
chronic inflammation of the skin can be one of the traits for tumor
initiation and progression. In animal models, chronic inflammation
may be used to trigger tumorigenesis. The long-term production and
the consequent accumulation of inflammatory factors
(cytokines/chemokines) can induce locally an immunosuppressive
milieu associated with tumor development and progression (29).
Cytokines, long-time players in inflammation, are
produced in the skin by an enhanced group of resident cells
[keratinocytes, LCs, melanocytes, mast cells (MCs) and
macrophages], as well as by recruited inflammatory cells (e.g.,
neutrophils, eosinophils and lymphocytes) (2). Generally, cytokines are synthesized
following cell activation and act locally within the tissue, having
a paracrine function in neighboring cells that express specific
receptors or have an autocrine action in the cells producing them
in an auto-regulatory loop. When the inflammatory stimulus is
prolonged, cytokine production becomes excessive, and finally, this
has a deleterious effect both on site and on distal sites from the
original inflammation site, similar to the effects of hormones.
Cytokine receptors have a mainly homologous structure; thus,
various cytokines can have pleiotropic effects, acting on several
targets. Beyond that, cytokines can have a synergistic effect on
the same cells, while acting antagonistically on other cell types
(33).
As it has already been acknowledged, in each tissue,
as with the skin, a complex cytokine network is developed and this
network is precisely regulated (33,34);
any deregulation of this cascade can trigger a tumorigenic process
(Fig. 2).
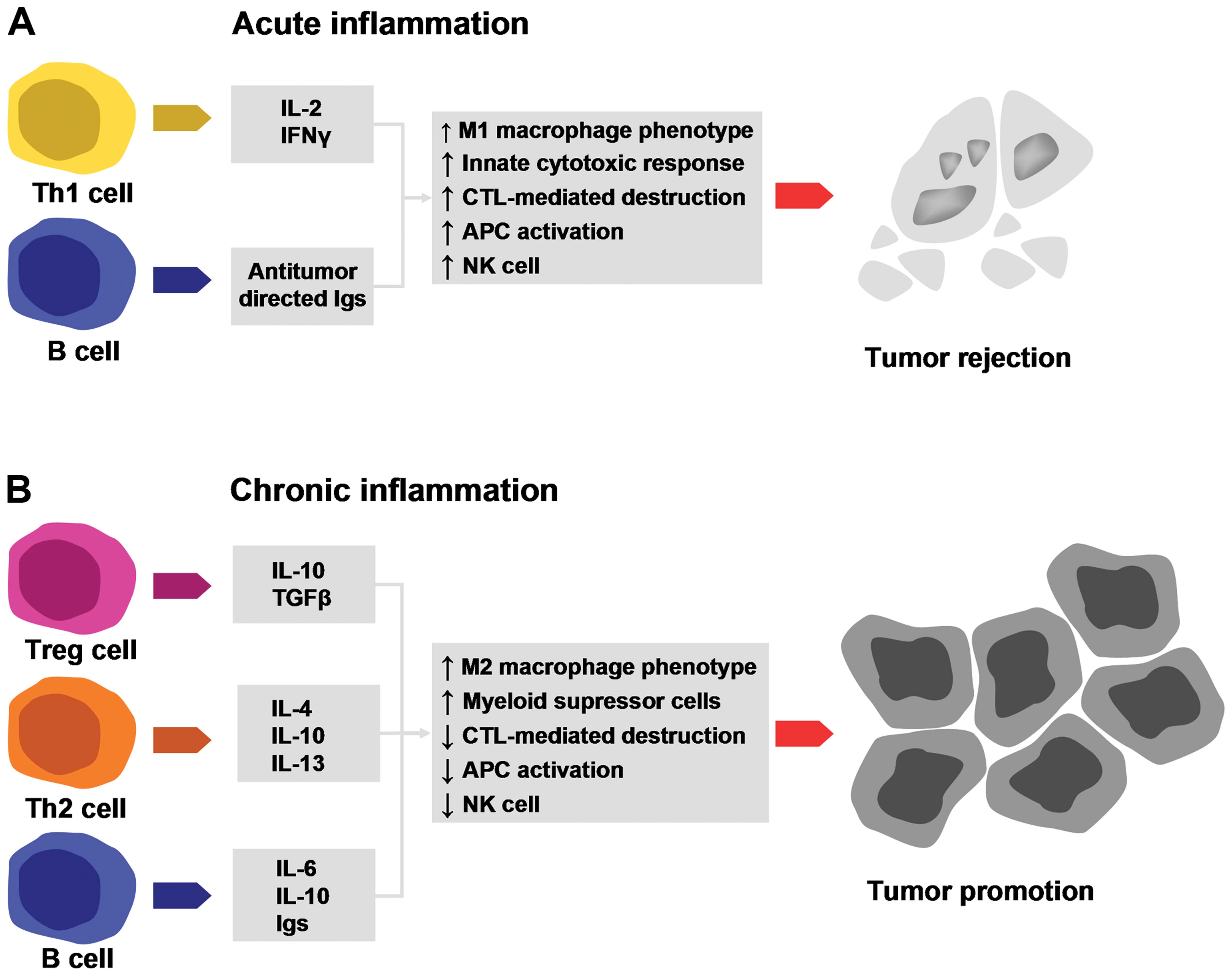 | Figure 2Elements of acute and chronic
inflammation that are linked to tumorigenesis. (A) T and B
lymphocytes secrete factors that induce the M1 macrophage
phenotype, promote the innate immune response, promote cytotoxic T
lymphocyte (CTL)-mediated destruction, enhance the
antigen-presenting capacity and increase natural killer (NK) cell
activity. All these processes have a potent antitumorigenic effect;
(B) T and B lymphocytes secrete factors that induce the M2
macrophage phenotype, enhance myeloid suppressor activity, reduce
CTL activity, decrease the antigen-presenting capacity and increase
NK cell activity. All these processes have a potent pro-tumorigenic
effect (copyright from ref. 29).
IL, interleukin; IFN-γ, interferon-γ; TGF-β, ransforming growth
factor β; Ig, immunoglobulin. |
Cell migration is critical for several normal
processes, such as embryogenesis, the immune response,
inflammation; however, it is also one of the key events in cancer
cell metastasis (35). All the
molecular perturbations underlying chronic inflammation as triggers
for skin tumorigenesis are again the center of cell migration
research. Ehm2 (a novel cancer promoter) belongs to the FERM family
of proteins (4.1 protein, ezrin, radixin, moesin), that are
involved in membrane-cytoskeletal interactions, and are linked to
the metastatic event in several types of cancer, including skin
cancer. In a study published in 2013, the effect of Ehm2 knockdown
on cell migration, adhesion, growth, cell cycle progression and
apoptosis were reported. The authors demonstrated that Ehm2 was
expressed at 3-fold higher levels in tissues with acute
inflammation, compared to the chronic state. Following the
knockdown of Ehm2, the expressoin of another protein was found to
be decreased, namely that of neural Wiskott-Aldrich syndrome
protein (Nwasp) (36). Nwasp is
significant in the invasion processes as the binding of cortactin
to Arp2/3 and Nwasp are key elements for invadopodia formation in
melanoma cells (37). These
reported results propose that Ehm2 proteins are interesting
connection molecules between inflammation and the skin cancer
metastatic process. In melanoma, following the knockdown of Ehm2
protein, the expression of Nwasp was downregulated, directly
affecting cell migration (36).
There are intracellular pathways that are
deregulated in tumorigenesis and in a non-healing wound. Thus, both
processes involve common molecular and signaling pathways, such as
Ras, Hedgehog and WNT (38).
Epithelial-mesenchymal transition (EMT) is a process
through which epithelial cells lose some of their characteristics
(cell polarity and cell-cell adhesion) and gain migratory and
invasive properties, becoming mesenchymal stem cells (39). When a wound is healing, the EMT
process is activated (40).
Epidermal keratinocytes acquire migratory phenotypes (41) and then return to normal upon wound
closure during the rebuilding of the basement membrane. In tumors,
epithelial cells can harbor oncogenic mutations, undergoing EMT, a
process associated with the acquisition of cancer stem cell
properties (42). Another recently
discovered cellular process, transdifferentiation, is a mechanism
that governs the transformation of a mature somatic cell in another
mature somatic cell lacking the intermediate pluripotent state or
progenitor cell type phases (43).
In adult tissues, transdifferentiation is not as accelerated as in
more immature ones; however, in wounding, mesenchymal stem cells
undergo transdifferentiation into epidermal cells, endothelial
cells and pericytes (44,45).
Another mechanism that can link inflammation with
tumorigenesis is the fact that during wound healing, fibroblasts
deposit excess collagen (during fibrosis), which leads to scar
formation. This connective tissue is a microenvironment that is
tumor permissive (46). As stated
above, in this microenvironment, the presence/production of growth
factors, cytokines and chemokines is similar in chronic wounds as
in tumors, with a slight difference being in the expression levels
in terms of kinetics (31) (see
also Fig. 2).
In conclusion, we can ascertain that a close
association exists between chronic tissue damage, inflammation and
cancer (32); in this context
tumors may develop, although uncommonly, also at the site of
chronic skin wounds (47).
4. History of experimental skin
carcinogenesis
Where the story begins
One of the first recorded studies regarding two-step
chemically induced carcinogenesis is that of Frei and Stephens
published >45 years ago (48).
It was actually the first study to demonstrate that tumors induced
in mouse ears correlate with the induction of hyperplasia as an
inflammatory response. Leucocytes migrating to the site were found
to correlate with the rate of induction of hyperplasia; however, at
the same time, no clear association between inflammation,
hyperplasia and tumorigenesis was observed.
From these decisive results, chemically induced
mouse skin carcinogenesis was the main animal model of cutaneous
tumorigenesis (49–51). This model was used for evaluating
anti-tumor drugs, but also for understanding the nature of
epithelial cancers as a multi-stage process (52).
Several protocols have been developed for
'two-stage' carcinogenesis in which tumor incidence, tumoral
latency, multi-staging and the progression of the skin cancer are
studied. In the model of two-stage skin carcinogenesis, the tumor
is initiated after a single sub-carcinogenic dose of
7,12-dimethylbenz[a]-anthracene (DMBA). This irreversible event
leads to visible tumors only after the repeated application of a
promoter agent, such as the phorbol ester, 12-O-tetra
decanoylphorbol-13-acetate (TPA). Therefore, unlike one-step
carcinogenesis, in two-stage carcinogenesis, the initiation and
promotion phases can be noticeably separated, both functionally and
temporally (52). This distinction
of phases offers a tremendous advantage when studying the effects
of environmental factors and/or drugs in the different stages of
tumorigenesis.
Another advantage offered by this two-stage model is
that tumor development can be visually monitored during the
lifespan of a mouse. With this dynamic monitoring, non-invasive
evaluation methods can be used (see below for images of in
vivo confocal microscopy). Moreover, at the end of the
experimental period, the transformed tissue can be harvested and
thoroughly examined. This animal model yields good reproducible
results; thus, when assessing cytostatics, chemopreventive and/or
dietary agents for skin tumorigenesis, reliable results are
obtained (53).
Over the past years, genes and cell signaling
pathways, the molecular mechanisms that underlie tumorigenesis,
were studied using this animal model (54,55).
Considering the broad utilization of this animal
model, its flexibility to extensive experimental approaches, as
well as its unproblematic development for any animal husbandry,
two-stage skin carcinogenesis developed in mice can be used for the
model of human carcinogenesis when cancers of epithelial origin are
studied (56).
Strain differences
One of the first studies acknowledging different
strain susceptibility was published >30 years ago (57). In this early study, authors used
two-stage skin carcinogenesis with a polycyclic aromatic
hydrocarbon (DMBA) as the initiating carcinogen, and subsequently,
as the promoter of carcinogenesis, croton oil. The study used LACA
and BALB/c mice, namely one susceptible and one resistant strain.
In this early study, the authors reported that DMBA was more
effective in the LACA strain compared to the BALB/c one. Moreover,
this was one of the first studies demonstrating the metabolic
activation of DMBA and its transformation in the active carcinogen,
a process common to both mouse strains, whether resistant or
sensitive. This early study flagged strain differences in terms of
the DMBA response, although DMBA was the actual promoter of
carcinogenesis in both studied strains. At that time, mechanisms
such as DNA repair were still under investigation and thus, strain
differences as regards the induction of carcinogenesis could be
only suggested to involve these mechanisms. Dissimilar to
carcinogens, such as DMBA, phorbol ester promoters (component of
the utilized croton oil) appear to shunt any metabolic activation,
while being degraded and inactivated by epidermal cells. These
early experiments with different promoters did not provide any
evidence that the distinct biological behavior of different mouse
strains is sustained by different promoter degradation (57).
After another 10 years, the era of transgenic mice
began and the subject of strain specificity in terms of developing
chemically induced carcinogenesis gained another dimension. The
role of specific genes as determinants for particular behavior was
acknowledged. Strain differences in terms of susceptibility to
specific toxic agents are in fact an in vivo tool to be
exploited and, in this light, transgenic animals that comprise
specific genes can be manipulated. At that time, several
experimental pathways were highlighted, pathways that now are at
the post-graduate research level. It was then stated that
transgenic animals can be used in several ways: to introduce a
human gene encoding a drug metabolizing enzyme, or to delete and/or
modulate the expression patterns of specific within target cells
(58).
In these early experiments, it was shown that the
promoter of a stress-regulated gene linked to a reporter gene (lacZ
or green fluorescent protein) can be inserted into the mouse
genome; then upon experimental tumorigenesis, sensitive cells can
be identified as the cells are carrying the reporter gene.
Transgenic mice can be used for the high-throughput screening of
compounds, an invaluable experimental model that can offer results
regarding the tissue and cellular specificity of certain drugs
(58). As early as the 1990s,
authors highlighted that transgenic animal studies are complex, and
that altering one gene does not mean one straightforward protein
alteration. The introduction of a specific human cytochrome P450
gene can have no metabolic effect due to the overall background
activity in the rodent. Drug uptake, metabolism, detoxification and
repair, as parts of the complex process of toxicity, differ in
primates and rodents; for example, a toxic response in one species
can be totally irrelevant in another (58); however, transgenic animals in the
toxicological domain have gained momentum and are speeding up the
drug discovery processes.
Almost at the same time period, another team
investigated the association between single gene mutations and
carcinogenesis, the histological type triggered by this gene
mutation and whether a novel papillomavirus (at that time) had a
co-carcinogenic function (59). A
two-step protocol (DMBA followed by TPA) was used to induce
papillomas in several inbred, hybrid, and various genetically
altered mice. Studying the histological types of tumors, the
authors reported an increased panel of histology beginning with
early follicular papillomas, along with mixed papillomas, or
exophytic papillomas, hyperplastic papillomas, fibropapillomas,
squamous cell carcinomas (SCCs), and MC tumors. Moreover, in these
mouse models, high-, intermediate-, and non-responding groups can
be obtained. The authors demonstrated that tumor induction was
conditioned by skin-specific mutant genes. Papillomavirus antigens
or viral genomic DNA was not present in the induced tumors. Genetic
differences were first flagged in the late 1990s as underlying the
strain differences (59).
Laboratory animals subjected to specific genetic
manipulations can be used to not only study compounds used to
induce carcinogenesis, but also to study the effects of topical
chemical toxins. This was an important conclusion of the late
1990s, paving the way for the future genomic era of skin toxicology
and dermato-oncology.
5. Refining the model of carcinogenesis
As discussed in the previous section, several
decades of studies have investigated the induction of
carcinogenesis in animal models. The topical administration of
compounds which induce skin carcinogenesis in mice provides an easy
model for studying local, systemic and environmental factors
influencing tumor susceptibility, growth and progression. This
model of chemically induced carcinogenesis almost as old as modern
dermato-oncology and skin toxicology continues to provide a
cost-effective model needed for the identification of biological,
immunological and molecular pathways implicated in skin
tumorigenesis. Besides these somewhat fundamental findings, this
model is frequently used for topical antitumoral drug testing. For
instance, the two-stage mouse carcinogenesis model was typically
used for testing drugs effective for skin cancer prevention
(60,61). Studies regarding chemoprevention
testing were reported in the SENCAR mouse, a model evaluating
phenylretinamides as tumor suppressors via retinoid
receptor-independent mechanism(s) (62).
In essence, this model uses the two-stage
application of chemicals to the skin for the initiation and
promotion of cutaneous tumors. When using a two-stage model of
induction, after a single application of the initiator mutagen,
DMBA, repeated applications of a pro-inflammatory phorbol ester
(TPA), or phorbol 12-myristate 13-acetate (PMA) are carried out.
The literature indicates that tumors that appear can be benign
papillomas that regress or progress to SCC (63). Moreover, the direct appearance of
SCC was reported without the presence of any pre-cancerous lesions.
Thus, two-stage chemically induced carcinogenesis provides an
opportunity to monitor early and late events in cancer development
and progression (63).
Our experience has shown that, when we used this
protocol (64), in three different
mice strains, widely covering the susceptibility area in terms of
chemically induced carcinogenesis (high susceptibility, nude mouse
CD1-Foxn 1nu strain; medium susceptibility, CD-1 strain; and low
susceptibility, C57BL/6 strain) we obtained various results. Thus,
from the C57BL/6 mice, only a small percentage (5%) developed skin
lesions. Tumor formation began approximately after 25 weeks from
the first DMBA application. Macroscopically, they displayed only
one formation, round shaped, rough at palpation with a wide base.
We did not observe metastases at necropsy and the histopathological
identification (Fig. 3) revealed a
poorly differentiated carcinoma. In order to maintain the
homogeneity of the mouse model, we established a clone of tumor
cells with which we established an in vivo mouse model of
skin carcinoma using the C57BL/6 mouse strain.
In the CD1-Foxn 1nu mouse group, approximately 20%
of the mice survived during the experiment. In the survivors, tumor
formations appeared around 8 weeks from the first DMBA application
and the mice were sacrificed 10 weeks after the appearance of the
lesions. Macroscopically, the mice developed multi-formations, 1–3
mm in diameter, with an irregular appearance and a narrow base. No
metastases were observed at necropsy and the histological
examination depicted cutaneous papillomas (Fig. 4). All the presented original animal
experiments were carried out in accordance with EU guidelines
(65).
The mice in the CD1 group all developed skin
formations. In this case, the tumor was clinically detectable as
early as 5–6 weeks after the initial application. Macroscopically,
there were multi formations, elongated with an irregular shape,
soft at palpation, with a narrow base and a rapid growth rate. No
metastases were observed at necropsy. A technology that we and
others are developing in animal models is in vivo
reflectance confocal microscopy (RCM). This modern imaging
technique allows for the non-invasive 'quasi-histological',
real-time evaluation of both human and murine skin structure
(66–68,71).
Using in vivo confocal microscopy for investigating skin
cancer in animal models enables the observation of abnormal tissue
architecture, along with the identification of atypical structures
and all these observations are made during the real-time assessment
of blood flow through dermal vessels. Publications regarding the
use of this technology in mouse models are not abundant, but cover
an area of continuous development. This technique is a new approach
for monitoring tumor progression and the therapeutic effects of
anticancer agents in skin cancer (68,69,72).
Recently, another study demonstrated that the stages
of human and mouse carcinogenesis are not super-imposed. The
authors reported that tumor necrosis factor-α (TNF-α) promotes
endogenously carcinogenesis and that TNF-α-inducing protein (Tipα)
favors EMT and, subsequently, the progression of cancer (73). In this light, the overall
inflammatory process should be reconsidered in this chemically
induced skin carcinogenesis, linking the promoter chemicals to
inflammatory proteins. Bridging the early studies of
'inflammation', as carcinogenesis points toward the role of TNF-α
in tumor-promoting inflammation, this updated study involves
another factor in chemically induced carcinogenesis, namely
immunological players (73). Our
experience suggests that several inflammatory cytokines tested in
serum match the evolution of skin tumors in mouse models (74).
Thoroughly recently reviewed (75), this sequential administrated tandem,
DMBA-TPA, leads to the appearance of a large number of benign
papillomas, more or less developing into SCC. At the molecular
level, tumorigenesis is initiated with the mutational activation of
the Ha-Ras oncoprotein. In human SCC, HA-RAS mutations are rarely
reported, while frequent HA-RAS-mutated tumors are reported in
melanoma following treatment with B-raf inhibitors. This recent
finding indicates the probable existence of Ha-Ras-mutated cancer
stem cells in the actual tumors. In a similar manner, UV-induced
human SCC displays mutations in TP53, a tumor suppressor gene. This
published review emphasizes the differences in skin tumorigenesis
which is chemically induced, but concomitantly shows that these
characteristics are common to humans as well. In making a
comparison of the differences in the skin tumor microenvironment
between the mouse model and humans, common molecular mechanisms
were depicted and the authors recommend the use of mouse models in
skin toxicological testing (75).
In C3H/HeN mice, the exposure of the skin to UV radiation induced
the activation of β-catenin in a time- and dose-dependent manner.
Mice deficient in cyclo-oxygenase-2 (COX-2) subjected to UV
radiation exhibited a lesser activation of β-catenin. In SKH-1
hairless mice exposed to UV radiation, the activatoin of β-catenin
signaling was also observed as well as the development of UV
radiation-induced skin tumors. All the accumulated data from the
study of photocarcinogenesis led to the conclusion that the
activation of the β-catenin pathway can induce skin carcinogenesis
(76).
6. Advancing knowledge using models of skin
carcinogenesis
Genomics
The majority of publications in the genomics
investigating field have focused on studying the environmental
factors, such as UV radiation and carcinogens as potent inductors
of epigenetic alterations.
Exposure to environmental mutagens or simply
spontaneous errors can induce somatic mutations, leading to the
development and/or progression of cancer. The epigenome can also
undergo chemically induced or spontaneous alterations, leading
towards carcinogenesis. Next-generation sequencing can identify
genetic variants, somatic mutations, gene expression profiles, and
epigenetic alterations with single-base resolution. All this new
technology was put in use to provide insight into tumorigenesis
driven by environmental factors (77).
Moreover, nutritional and dietary resources can
modify individual susceptibility through changes in the epigenome.
Epigenetics hence aims to identify the health risks of
environmental toxicants combined with dietary and nutritional ones.
The epigenetic end-points reported by authors highlight global
hypomethylation and specific hypermethylation at diverse tumor
suppressor genes upon environmental exposure. Furthermore, a series
of conditions were flagged that could influence the epigenetic
effects of environmental factors: namely the dynamics of exposure,
dose, gender, organ-target and age were examined for alterations in
the epigenome. The effects of environmental factors can be balanced
by nutritional/dietary agents, reversing their epimutagenic effects
(77).
In a study published in 2014, the genome-wide DNA
methylation profile was investigated in a model of UVB- and
DMBA/TPA-induced skin cancer (78).
In that study, the SKH-1 strain was subjected to UV radiation and
in CD-1 mice, carcinogenesis was induced by DMBA/TPA. The authors
reported >6,000 genes in the UVB group and >5,400 genes in
the DMBA/TPA group that proved an enhanced CpG methylation. Using
ingenuity pathway analysis, the first pathways in which these
deregulated methylation appeared were the ones related to
tumorigenesis. Hence, of the deregulated pathways, cAMP-mediated
signaling, G protein-coupled receptor signaling and PTEN signaling
were associated with UV radiation, while protein kinase A (PKA)
signaling and xenobiotic metabolism signaling were associated with
DMBA/TPA carcinogenesis. As stated in the
inflammation-carcinogenesis section, the inflammatory processes can
sustain tumorigenesis (78).
Thus, in that study, IL-6-related inflammatory
pathways had alterations in the methylation profiles when skin was
subjected to UVB irradiation. Altered genes were classified in the
UVB and DMBA/TPA models, while establishing in silico their
molecular interaction networks. The authors demonstrated that
methylation profiles of skin subjected to environmental factors
(UVB irradiation or chemical carcinogenesis DMBA/TPA) can shed
light on epigenetic gene regulation in skin carcinogenesis
(78).
Actually the first study on the aberrant methylation
of Nr4a3 exon 3 CpG island was reported in a multistep mouse model
of skin carcinogenesis (79).
Studies published in 2014 demonstrated that many
cancer susceptibility loci are located throughout the genome
(80,81). Mapping these loci in a mouse model
of skin cancer, the authors demonstrated strong genetic loci that
render resistance to chemically induced skin papillomas. These loci
are located on chromosomes 4 and 7. Combining congenic mapping
(congenic mouse strain, FVB.MSM-Stmm3) and allele-specific
alteration analysis of these loci located on chromosome 4, it was
demonstrated that Stmm3 (skin tumor modifier of MSM 3) responsible
genes influence the formation of papillomas in two-stage skin
carcinogenesis by regulating papilloma growth rather than
development (80). The same group,
in the same year, demonstrated that Stmm1 may be responsible for
papillomagenesis in two-stage skin carcinogenesis by regulating
epidermal quiescent stem cells (81).
Genetically modified animals have been used as
valuable tools in depicting the carcinogenic process. A number of
studies have used these animals in the animal model of two-stage
skin carcinogenesis in order to depict the roles of certain genes
involved in epithelial carcinogenesis (54,82,83).
In these animals, the functionality of genes can be studied
throughout the evolvement of the carcinogenesis process. Targeting
genetic modifications that can modify cutaneous tissue has allowed
different genetic manipulations (gene overexpression or gene
deletion) in particular skin compartments, recapitulating
spontaneous genetic events in tumorigenesis (84).
In the search for new therapuetic targets from the
panel of signaling pathway molecules, transgenic mouse have been
used. The study by Wilker et al demonstrated that using
LY294002 (a PI3K inhibitor), in a transgenic mouse model that
overexpressed human insulin-like growth factor-1 (IGF-1),
emphasized the role of PI3 kinase and Akt-mediated signaling in
epithelial carcinogenesis (85).
MicroRNAs (miRNAs or miRs) as regulators of gene
expression came into the spotlight in the last 10 years (86,87).
Therefore, it was observed that the exposure to different
carcinogens also alters miRNA expression (88–90).
miRNA expression differs in the different stages of chemically
induced carcinogenesis. Carcinogenic agents are potent miRNA
expression deregulators, while non-carcinogenic chemicals have a
lesser influence on miRNAs. Their expression also differs in
different biological systems. Hence, there are increased changes in
cancer-target tissues in comparison to the non-target tissues
following an acute or a chronic exposure to carcinogens. The
families of deregulated miRNAs in carcinogens regulate genes
involved in xenobiotic metabolism, carcinogen-induced
hypomethylation, DNA repair, apoptosis, cell proliferation, tumor
suppression, cell transformation, oncogenesis, tumor angiogenesis,
tumor progress and malignant transformation (91). Moreover, there are miRNAs with
double functions, such as putative oncogenes or tumor suppressor
genes (92). Carcinogens can
influence the balance between these functions and drive their
functions toward tumorigenesis. As recently demonstrated, miRNAs
specific to carcinogen exposure can be used as biomarkers for
genotoxicity and carcinogenicity (93).
The clear message conveyed from these data is that
new endpoints of environmental toxicants should be found and that
more attention should be paid to finding measure to prevent
environment-related diseases.
Proteomics
As the domain of proteomics is rapidly evolving,
mouse models of chemically induced carcinogenesis have also taken
advantage of this area. The differences in the susceptibility
between strains reside in the genetic diversity that triggers
protein differences. In a recent study, specific protein or
signaling pathway alterations were investigated in association to
this susceptibility (94).
Examining the epidermis proteome in DBA/2 sensitive and
C57BL/6-resistant mice upon the administration of TPA, 19
differentially expressed proteins were found. Five proteins were
calcium-binding proteins: annexin A1, parvalbumin α, S100A8, S100A9
and S100A11. The S100A8 and S100A9 protein levels were found to be
increased when using a different mouse model with the topical
application of tumor promoters, okadaic acid and chrysarobin. When
examining the associatoin between these 19 upregulated proteins, it
was shown that they are active players in several inflammatory
networks involved in skin tumor promotion, such as TNF-α and
nuclear factor (NF)-κB. Nucleic acid mRNAs for various proteins
such as TNF, Nfkb1, IL-22, IL-1b, and chemokines such as Cxcl1,
Cxcl2 and Cxcl5 were found to be highly expressed following the
administratio of TPA in DBA/2 mice in comparison to C57BL/6 mice.
These reported results indicate that inflammatory genes can sustain
the basis of genetic differences in susceptibility in mouse models
of chemically induced carcinogenesis (94). Apart from inflammatory processes
that can account for these different effects, tetraspanins,
cell-surface proteins present on almost all cell and tissue types
have been shown to be involved (95). During two-stage mouse skin chemical
carcinogenesis, CD151 favors tumorigenesis, inducing a more rapid
development, multiplicity, size and progression to SCC in this
model. In human skin developing SCC, CD151 expression is increased.
CD151 is associated with the activation of the transcription
factor, signal transducer and activator of transcription 3 (STAT3),
and PKCα-α6β4 integrin. CD151-PKCα is associated with a more
invasive SCC. In mouse models at least, CD151 was proven to be a
future antitumor therapy target (96). In a recent study, in a model of
DMBA-induced skin carcinogenesis using PKCα knockout mice, it was
shown that PKCα suppresses tumor formation, but not tumor growth
and progression to skin carcinogenesis (97). Using IL-6 and tnF-α-deficient mice
in chemically induced skin carcinogenesis, it was also demonstrated
that IL-6 is not the main pro-inflammatory cytokine that promotes
tumorigenesis of the skin. The authors concluded using this model
that individual cytokines have distinct and discrete roles in tumor
promotion (98).
Proteomics still has an important input in the
nearby future in this domain as its rapid evolving methodologies
find their place in quantifying the proteome behind the subtle
process of tumorigenesis (99,100).
7. Testing avenues using animal models of
skin carcinogenesis
Determining how close we are to the human
scenario
The presented animal model runs through the
characteristics of human multi-stage carcinogenesis. As reported
several years ago, it seems that mutations within stem cell niches
can be the initial step in the flow of events leading to
tumorigenesis (101,102). At the genetic level, mutations and
genes that are activated resemble those identified in humans.
Therefore, when mutations in ras family members appear,
receptors of the epidermal growth factor (EGFR) are activated,
signaling pathways of Stat3 and Akt-mediated are activated and the
mutations of TGF-β1 and Tp53 occur, and these are all
genetic processes that seem common in both species (49).
As humans are subjected to multiple carcinogenic
doses, mainly accumulating low doses, this two-stage skin
carcinogenesis protocol uses both carcinogens and promoting agents,
as in an environmental scenario. Furthermore, in human cancers,
there is a long latency that is also mimicked by the promotional
component for tumor development (103). As a result, there is a commonly
agreed opinion that this mouse model can be utilized to study the
mechanistic basis of human epithelial cancers.
Drug testing using the model of skin
carcinogenesis
The toxicological drug testing area is a domain that
frequently uses this mouse model.
The effect of nano-sized titanium dioxide particles
(TiO2), as a growing component used in cosmetics,
sunscreens and food additives, was tested in the model of two-stage
skin chemical carcinogenesis using DMBA and afterwards the tested
compound (104). The topical
application of non-coated rutile-type TiO2 did not
trigger any UV-induced skin carcinogenesis in rats, probably due to
the lack of penetration of TiO2 into the epidermis. The
same authors switched to chemically induced skin carcinogenesis to
test the effect of silicone-coated TiO2. They used mice,
human c-Ha-ras proto-oncogene transgenic mice (rasH2) and resistant
CD-1 ones. The compound did not influence the DMBA carcinogenesis
in sensitive mice or resistant mice, possibly due to the lack of
penetration through the epidermis (104).
In another study, the efficacy of topically applied
liposome-encapsulated tamoxifen (TAM) in a model of
DMBA-TPA-induced skin tumorigenesis in order to potentially
decrease its systemic toxicity was tested. TAM was encapsulated in
special liposomes and the incidence of papillomas was examined
(105). This recent study
demonstrated the inhibition of skin carcinogenesis when liposome
conditioned drug was used, this finding brining good news for the
future use of liposomal systems/drugs in skin cancer (105).
Agaro-oligosaccharides (AGOs) studied in in
vitro models have been proven to suppress nitric oxide (NO)
levels, as well as the production of prostaglandin e (Pge) and
pro-inflammatory cytokines (106).
Continuing this endeavour, in the two-stage mouse model of skin
carcinogenesis, the administration of AGOs delayed the appearance
of tumors and decreased the number of tumors. PGE2 was shown to be
suppressed by AGO intake in a model of TPA-induced ear edema, while
COX-2 and microsomal PGE synthase-1 were found to be downregulated.
As stated above, this was a mouse model that reported results of
the anti-tumor effects of agos, as well as of the
anti-inflammatory, PGE-mediated effects (106).
As demonstrated in another study, the topical
administration of the βG inhibitor, D-glucaro-1,4-lactone (1,4-GL),
or D-glucuronic acid-γ-lactone (GUL) its precursor, to SENCAR mice
with skin carcinogenesis, epidermal hyperplasia was significantly
reduced along with a decrease in inflammation (107). Moreover, Ha-ras mutations were
reduced upon experimental therapy. That study demonstrated that
whether administered topically or through diet, therapy with GUL or
1,4-GL was effective in hindering experimental skin tumorigenesis
(107).
Natural products testing
As there is a continuous effort to establish dietary
routes of harmful compounds (108), over the past years, several papers
were published showing results of natural compound testing in
animal models of skin carcinogenesis and these models are
particularly suited for the evaluation of the effects of dietary
factors/dietary manipulations on tumor development.
Potential natural compounds can be evaluated for
their effects on cutaneous tumor initiation, promotion and/or
progression; several years ago, a study was published on the
anti-inflammatory effects of resveratrol in this model (109). Kleiner et al also showed
that the delivery of isopimpinellin from citrus, prior to exposure
to DMBA, significantly inhibited tumor initiation (110). Singh et al showed that
silymarin was effective at blocking tumor formation and at
promoting even the regression of established tumors, this effect
being dependent on the sequence of delivery (111). The topical application of
rapamycin was reported as a chemopreventive agent, leading to the
regression of papillomas in a model of chemically induced
carcinogenesis (55,112).
For testing the hydroalcoholic extract of Brazilian
red propolis (HERP), an animal model of on dermal carcinogenesis
was used. A single DMBA agent was used and animals treated with
HERP exhibited significantly decreased tumor multiplicity
throughout the 5 weeks of tumor promotion. Administered orally in a
murine model of chemically induced SCC, this natural product
exerted a significant modulatory effect on the formation,
differentiation and progression of tumors (113).
In a previous study (114), geraniol, an acyclic monoterpene
alcohol, was tested in a mouse model of DMBA-induced skin
carcinogenesis without any inflammatory compound, and skin tumors
occurred in all mice. The animals were orally administered geraniol
and monitored for lipid peroxidation and antioxidant status. The
yielded revealed positive results; geraniol at certain doses
prevented tumor formation and regulated the antioxidant status. As
the authors explained, the model yielded clear-cut results;
geraniol inhibited tumor cell proliferation, modulated
detoxification agents and enhanced free radical scavenging
(114).
In another study, in Swiss albino mice exposed to
DMBA, the chemopreventive efficacy of rosmarinic acid was reported
(115). As in the above-mentioned
study, phase I and II detoxification agents, lipid peroxidation
by-products, antioxidants and apoptotic biomarkers were assessed.
The authors reported that they succeeded in inducing SCC in all the
mice within 15 weeks of topically applying only DMBA. The
investigated parameters were found modified in the SCC
tumor-bearing animals, while extremely positive clinical results
were obtained in the animals treated orally with rosmarinic acid.
The treated animals did not develop tumors, while animals already
bearing SCC tumors exhibited a normalization of all the tested
detoxification agents, lipid peroxidation by-products, antioxidants
and apoptotic markers (p53, Bcl-2, caspase-3 and caspase-9)
(115).
SHR mice subjected to DMBA protocol were also tested
for the efficacy of melatonin and metformin. The published results
indicated that melatonin and metformin, significantly attenuated
tumorigenesis and decreased the overall lipid peroxidation levels
(116).
Green tea is also a natural product that exerts
benefitial effects on skin carcinogenesis in animal models. Green
tea polyphenols (GTPs), mainly epigallocatechin-3-gallate (EGCG)
were tested, among other types of cancer, in skin cancer models as
well. It was demonstrated that GTPs/EGCG can promote
anti-neoplastic processes, apoptosis, cell cycle arrest and can
suppress metastasis in tumor cells, but not in normal cells. The
reported difference possibly reside in the molecular mechanisms
triggered by GTPs/EGCG in signaling pathways in transformed cells
versus normal ones (117).
This model is also suitable for investigating the
effects of dietary intake on carcinogenesis as caloric restriction
is known to inhibit/delay the phase in which the tumor is promoted
(118). The altered energy balance
that can induce tissue-specific alterations, along with systemic
effects can be evaluated in this type of animal model (119,120).
Studies on the chemoprevention of skin cancer have
taken advantage of the two-step mouse model of carcinogenesis. The
plant flavonoid, silymarin, was proven to be effective against
chemical/photo carcinogenesis. In several models of skin
carcinogenesis, the topical administration of silymarin inhibited
the effects of DMBA, as well as those of tumor promoters, such as
TPA, mezerein, benzoyal peroxide and okadaic acid (121). The same chemopreventive effects
were obtained for UVB-induced skin carcinogenesis (122). It seems that the actual effects of
this flavonoid are its antioxidant, anti-inflammatory and
immunomodulatory characteristics that hinder inflammation and
evolution to tumorigenesis. In clinical studies, this flavonoid was
reported to reduce the toxicity associated with chemotherapy
(123).
Taking into account the data from recent reports, it
can be emphasized that in the last couple of years, the study of
the topical and systemic antitumor effects of 'green' drugs has
benefited from this mouse model. As in any study on chemoprevention
or dietary intervention, the efficacy of the test compound/drug
studied should be carefully examined and coherent conclusions
should be drawn for proper translation into human pathology.
8. Conclusion and perspectives
We can outline several aspects in the
dermato-oncology and skin toxicology testing area that uses
chemically induced skin carcinogenesis. Thus, there are both pros
and cons for the utilization of this model.
There are important strain differences in
histological type, development and clinical evolution of the skin
tumor. These differences are enhanced by the fact that human skin
tumorigenesis can comprise other pathways and we should be aware of
the existing limitations. In this methodology, mice develop
primarily papillomas, without any direct human equivalent. The
subsequent SCC tumors that develop through tumorigenesis
histologically resemble human SCC tumors. For the chemical
initiation, in mice, Hras is the primary target, while in
humans, Tp53 appears to bear the gene mutation in human
non-melanoma skin cancer (124).
The Hras gene from mouse skin closely resembles the gene
found mutated in other human epithelial cancers (lung, colon and
pancreatic cancers) (125). The
last draw-back is that the two-stage model of skin carcinogenesis
model is limited when studying metastasis, as the rate of
metastatic cutaneous tumors is quite low (126).
The advantages of this model reside in the fact that
upon genetic manipulation, the model can mimic the exact genetic
and phenotypic changes of certain skin disease conditions. By doing
so, drug target validation, preclinical testing and biomarker
discovery can take advantage of rapid and oriented results. The
roles of potential cancer risk modifier genes, such as
proto-oncogenes, and/or tumor suppressor genes in complex processes
such as initiation, promotion and progression can be additionally
studied in these murine models.
Despite the stated differences, authors recommend
the using of mouse models for skin toxicology testing because at
the molecular level, common mechanisms are depicted. As in human
cancer development, genetic alterations caused by carcinogens and
pro-inflammatory cytokines, simultaneous inflammation sustained by
pro-inflammatory cytokines and chemokines favor tumor
progression.
One of the main issues in testing environmental
factors and/or drugs is the similarities between the effects
observed in vitro versus the ones registered in vivo.
Moreover, another outstanding issue is to what extent we can rely
on the experimental animal models when extrapolating the results to
humans.
The newly released ToxCast phase II results,
demonstrate that high-throughput assays for characterizing rodent
toxicants, can integrate both bioactivity and chemical structure.
ToxCast phase I has shown that research on drugs combined with the
intensive use of omics technologies, are mandatory for combining
biological and chemical information in exploring the in
vitro to in vivo connection (127).
This domain still needs to explore in depth the
interrelation between cells of the immune system and tumor cells,
in the continuous search to unravel the intimate molecular
mechanisms that trigger skin tumorigenesis (128,129).
Acknowledgments
The authors would like to thank Dr Bogdan Marinescu
and Dr gheroghita Isvoranu from the 'Victor babes' national
Institute of Pathology Animal Husbandry for providing the tissue
samples from the models of chemically induced skin-carcinogenesis.
The author Constantin Caruntu was granted a young Researchers grant
no. 33891/2014 financed by 'Carol Davila' University of Medicine
and Pharmacy, Bucharest. Project received funding support through
UEFISCDI research project no. ID-PCE-2011-3-0918.
References
|
1
|
Bos JD: The skin as an organ of immunity.
Clin Exp Immunol. 107(suppl 1): 3–5. 1997.PubMed/NCBI
|
|
2
|
Bos JD: Skin immune system: Cutaneous
immunology and clinical immunodermatology. 3rd edition. CRC Press;
Boca Raton, FL: pp. 3–13. 2005
|
|
3
|
Neagu M: The immune system-a hidden
treasure for biomarker discovery in cutaneous melanoma. Advances in
Clinical chemistry. 58. Makowski GS: Academic Press; Burlington,
ON; pp. 89–140. 2012, View Article : Google Scholar
|
|
4
|
Elentner A, Ortner D, Clausen B, Gonzalez
FJ, Fernández-Salguero PM, Schmuth M and Dubrac S: Skin response to
a carcinogen involves the xenobiotic receptor pregnane X receptor.
Exp Dermatol. 24:835–840. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Vries E, Trakatelli M, Kalabalikis D,
Ferrandiz L, Ruiz-de-Casas A, Moreno-Ramirez D, Sotiriadis D,
Ioannides D, Aquilina S, Apap C, et al: EPIDERM Group: Known and
potential new risk factors for skin cancer in European populations:
A multicentre case-control study. Br J Dermatol. 167(Suppl 2):
1–13. 2012. View Article : Google Scholar
|
|
6
|
Diepgen TL and Mahler V: the epidemiology
of skin cancer. Br J Dermatol. 146(Suppl 61): 1–6. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ene CD, Anghel AE, Neagu M and Nicolae I:
25-OH Vitamin D and interleukin-8: Emerging biomarkers in cutaneous
melanoma development and progression. Mediators Inflamm.
2015:9048762015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Căruntu C, Grigore C, Căruntu A,
Diaconeasa A and Boda D: The role of stress in skin diseases.
Intern Med. 8:73–84. 2011.
|
|
9
|
Căruntu C, Ghiţă Ma, Căruntu A and Boda D:
the role of stress in the multifactorial etiopathogenesis of acne.
Ro Med J. 58:98–101. 2011.
|
|
10
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar :
|
|
11
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinologica (Buc).
10:545–558. 2014. View Article : Google Scholar
|
|
12
|
Marks F and Fürstenberger G: Experimental
evidence that skin carcinogenesis is a multistep phenomenon. Br J
Dermatol. 115(suppl 31): 1–8. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bibby Mc: the specificity of early changes
in the skin during carcinogenesis. Br J Dermatol. 104:485–488.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Waterston RH, Lindblad-Toh K, Birney E,
Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R,
Alexandersson M, An P, et al: Mouse Genome Sequencing Consortium:
Initial sequencing and comparative analysis of the mouse genome.
Nature. 420:520–562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mestas J and Hughes CC: Of mice and not
men: differences between mouse and human immunology. J Immunol.
172:2731–2738. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shepherd FA and Sridhar SS: Angiogenesis
inhibitors under study for the treatment of lung cancer. Lung
Cancer. 41(Suppl 1): S63–S72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oehler MK and Bicknell R: The promise of
anti-angiogenic cancer therapy. Br J Ccancer. 82:749–752. 2000.
View Article : Google Scholar
|
|
18
|
Panitch HS, Hirsch RL, Haley AS and
Johnson KP: Exacerbations of multiple sclerosis in patients treated
with gamma interferon. Lancet. 1:893–895. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sykes M: Mixed chimerism and transplant
tolerance. Immunity. 14:417–424. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wood KJ: Passenger leukocytes and
microchimerism: What role in tolerance induction? Transplantation.
75(suppl 9): 17s–20s. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monaco AP: chimerism in organ
transplantation: conflicting experiments and clinical observations.
Transplantation. 75(suppl 9): 13s–16s. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elbe A, Foster CA and Stingl G: T-cell
receptor alpha beta and gamma delta T cells in rat and human skin -
are they equivalent? Semin Immunol. 8:341–349. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gardner RV, Velez MC, Ode DL, Lee JW and
Correa H: Gamma/delta T-cell lymphoma as a recurrent complication
after transplantation. Leuk Lymphoma. 45:2355–2359. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bergstresser PR, Tigelaar RE, Dees JH and
Streilein JW: Thy-1 antigen-bearing dendritic cells populate murine
epidermis. J Invest Dermatol. 81:286–288. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jameson J and Havran WL: Skin gammadelta
T-cell functions in homeostasis and wound healing. Immunol Rev.
215:114–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Jong A, Peña-Cruz V, Cheng TY, Clark
RA, Van Rhijn I and Moody DB: CD1a-autoreactive T cells are a
normal component of the human αβ T cell repertoire. Nat Immunol.
11:1102–1109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lindlahr H: Nature Cure: Philosophy and
practice based on the unity of disease and cure. 20th edition.
Nature Cure Publishing company; Chicago, IL: 1922
|
|
28
|
Ward PA: Acute and chronic inflammation.
Fundamentals of Inflammation. Serhan CN, Ward PA and Gilroy DW:
Cambridge University Press; Cambridge: pp. 1–16. 2010, View Article : Google Scholar
|
|
29
|
Neagu M, Constantin C, Dumitrascu GR, Lupu
AR, Caruntu C, Boda D and Zurac S: Inflammation markers in
cutaneous melanoma - edgy biomarkers for prognosis. Discoveries.
3:e382015. View Article : Google Scholar
|
|
30
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: Crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9(212)2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gonda TA, Tu S and Wang TC: chronic
inflammation, the tumor microenvironment and carcinogenesis. Cell
Cycle. 8:2005–2013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nedoszytko B, Sokołowska-Wojdyło M,
Ruckemann-Dziurdzińska K, Roszkiewicz J and Nowicki RJ: Chemokines
and cytokines network in the pathogenesis of the inflammatory skin
diseases: Atopic dermatitis, psoriasis and skin mastocytosis.
Postepy Dermatol Alergol. 31:84–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Neagu M, Constantin C and Longo C:
Chemokines in the melanoma metastasis biomarkers portrait. J
Immunoassay Immunochem. 36:559–566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assays. J Vis
exp. View Article : Google Scholar : 2014.PubMed/NCBI
|
|
36
|
Bosanquet DC, Ye L, Harding KG and Jiang
WG: Expressed in high metastatic cells (Ehm2) is a positive
regulator of keratinocyte adhesion and motility: The implication
for wound healing. J Dermatol Sci. 71:115–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oser M, Yamaguchi H, Mader CC,
Bravo-Cordero JJ, Arias M, Chen X, Desmarais V, van Rheenen J,
koleske AJ and Condeelis J: Cortactin regulates cofilin and N-WASp
activities to control the stages of invadopodium assembly and
maturation. J Cell Biol. 186:571–587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schäfer M and Werner S: Cancer as an
overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell
Biol. 9:628–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kong D, Li Y, Wang Z and Sarkar FH: Cancer
stem cells and epithelial-to-mesenchymal transition
(EMT)-phenotypic cells: are they cousins or twins? Cancers (Basel).
3:716–729. 2011. View Article : Google Scholar
|
|
40
|
Plikus MV, Guerrero-Juarez CF, Treffeisen
E and Gay DL: Epigenetic control of skin and hair regeneration
after wounding. Exp Dermatol. 24:167–170. 2015. View Article : Google Scholar :
|
|
41
|
Yan C, Grimm WA, Garner WL, Qin L, Travis
T, Tan N and Han YP: Epithelial to mesenchymal transition in human
skin wound healing is induced by tumor necrosis factor-alpha
through bone morphogenic protein-2. Am J Pathol. 176:2247–2258.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leopold PL, Vincent J and Wang H: A
comparison of epithelial-to-mesenchymal transition and
re-epithelialization. Semin Cancer Biol. 22:471–483. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Graf T and Enver T: Forcing cells to
change lineages. Nature. 462:587–594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brittan M, Braun KM, Reynolds LE, Conti
FJ, Reynolds AR, Poulsom R, Alison MR, Wright NA and Hodivala-Dilke
KM: Bone marrow cells engraft within the epidermis and proliferate
in vivo with no evidence of cell fusion. J Pathol. 205:1–13. 2005.
View Article : Google Scholar
|
|
45
|
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma
D and Shimizu H: Mesenchymal stem cells are recruited into wounded
skin and contribute to wound repair by transdifferentiation into
multiple skin cell type. J Immunol. 180:2581–2587. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Egeblad M, Nakasone ES and Werb Z: Tumors
as organs: Complex tissues that interface with the entire organism.
Dev Cell. 18:884–901. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dunham LJ: Cancer in man at site of prior
benign lesion of skin or mucous membrane: A review. Cancer Res.
32:1359–1374. 1972.PubMed/NCBI
|
|
48
|
Frei JV and Stephens P: the correlation of
promotion of tumour growth and of induction of hyperplasia in
epidermal two-stage carcinogenesis. Br J Cancer. 22:83–92. 1968.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kemp CJ: Multistep skin cancer in mice as
a model to study the evolution of cancer cells. Semin Cancer Biol.
15:460–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Verma AK, Wheeler DL, Aziz MH and
Manoharan H: Protein kinase Cepsilon and development of squamous
cell carcinoma, the nonmelanoma human skin cancer. Mol Carcinog.
45:381–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rundhaug JE and Fischer SM: Tumor
promoters and models of promotion. comprehensive toxicology. 12.
Sipes IG, McQueen CA and Gandolfi AJ: Elsevier Sciences Ltd; New
York, NY: pp. 325–348. 1997
|
|
52
|
Abel EL, Angel JM, Kiguchi K and
DiGiovanni J: Multi-stage chemical carcinogenesis in mouse skin:
Fundamentals and applications. Nat Protoc. 4:1350–1362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Digiovanni J: Modification of multistage
skin carcinogenesis in mice. Modification of tumor development in
rodents. 33. Ito N and Sugano H: Karger, Basel; pp. 192–229.
1991
|
|
54
|
Segrelles C, Lu J, Hammann B, Santos M,
Moral M, Cascallana JL, Lara MF, Rho O, Carbajal S, Traag J, et al:
Deregulated activity of Akt in epithelial basal cells induces
spontaneous tumors and heightened sensitivity to skin
carcinogenesis. Cancer Res. 67:10879–10888. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Amornphimoltham P, Leelahavanichkul K,
Molinolo A, Patel V and Gutkind JS: Inhibition of Mammalian target
of rapamycin by rapamycin causes the regression of
carcinogen-induced skin tumor lesions. Clin Cancer Res.
14:8094–8101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bassi DE and Klein-Szanto AJP: Current
protocols in pharmacology. Carcinogen-induced animal models of head
and neck squamous cell carcinoma. John Wiley & Sons, Inc;
Hoboken, NJ: pp. 14.12.11–14.12.19. 2007
|
|
57
|
Ashman LK, Murray AW, Cook MG and
Kotlarski I: Two-stage skin carcinogenesis in sensitive and
resistant mouse strains. Carcinogenesis. 3:99–102. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wolf CR and Henderson CJ: Use of
transgenic animals in understanding molecular mechanisms of
toxicity. J Pharm Pharmacol. 50:567–574. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sundberg JP, Sundberg BA and Beamer WG:
Comparison of chemical carcinogen skin tumor induction efficacy in
inbred, mutant, and hybrid strains of mice: Morphologic variations
of induced tumors and absence of a papillomavirus cocarcinogen. Mol
carcinog. 20:19–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
McCormick DL and Moon RC: Antipromotional
activity of dietary N-(4-hydroxyphenyl)retinamide in two-stage skin
tumorigenesis in CD-1 and SENCAR mice. Cancer Lett. 31:133–138.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Warren BS and Slaga TJ: Mechanisms of
inhibition of tumor progression. Basic Life Sci. 61:279–289.
1993.PubMed/NCBI
|
|
62
|
Xu H, Cheepala S, McCauley E, Coombes K,
Xiao L, Fischer SM and Clifford JL: Chemoprevention of skin
carcinogenesis by phenylretinamides: retinoid receptor-independent
tumor suppression. Clin Cancer Res. 12(3 Pt 1): 969–979. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Miller SJ, Wei ZG, Wilson C, Dzubow L, Sun
TT and Lavker RM: Mouse skin is particularly susceptible to tumor
initiation during early anagen of the hair cycle: Possible
involvement of hair follicle stem cells. J Invest Dermatol.
101:591–594. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Marinescu B, Isvoranu G, Constantin C,
Coman C, Zurac S, Căruntu C, Boda D, Neagu M and Călin M:
Experimental model of chemically induced skin carcinogenesis in
mice. Rev Rom Med Vet. 20:97–104. 2010.
|
|
65
|
Home Office: Animals (Scientific
Procedures) Act 1986: Code of Practice for the Housing and Care of
Animals Used in Scientific Procedures. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/228831/0107.pdf.
accessed January 20, 2016.
|
|
66
|
Diaconeasa A, Boda D, Neagu M, Constantin
C, Căruntu C, Vlădău L and Guţu D: The role of confocal microscopy
in the dermato-oncology practice. J Med Life. 4:63–74.
2011.PubMed/NCBI
|
|
67
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17(085003)2012. View Article : Google Scholar
|
|
68
|
Li Y, Gonzalez S, Terwey TH, Wolchok J, Li
Y, Aranda I, Toledo-Crow R and Halpern AC: Dual mode reflectance
and fluorescence confocal laser scanning microscopy for in vivo
imaging melanoma progression in murine skin. J Invest Dermatol.
125:798–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li Z, Huang P, Zhang X, Lin J, Yang S, Liu
B, Gao F, Xi P, Ren Q and Cui D: RGD-conjugated dendrimer-modified
gold nanorods for in vivo tumor targeting and photothermal therapy.
Mol Pharm. 7:94–104. 2010. View Article : Google Scholar
|
|
70
|
Căruntu C, Boda D, Guţu De and Căruntu A:
In vivo reflectance confocal microscopy of basal cell carcinoma
with cystic degeneration. Rom J Morphol Embryol. 55:1437–1441.
2014.
|
|
71
|
Căruntu C, Boda D, Căruntu A, Rotaru M,
Baderca F and Zurac S: In vivo imaging techniques for psoriatic
lesions. Rom J Morphol Embryol. 55(Suppl 3): 1191–1196. 2014.
|
|
72
|
Croix CS, Zipfel WR and Watkins SC:
Potential solutions for confocal imaging of living animals.
Biotechniques. 43(Suppl 1): 14–19. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fujiki H, Sueoka E and Suganuma M: Tumor
promoters: From chemicals to inflammatory proteins. J Cancer Res
Clin Oncol. 139:1603–1614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Neagu M, Constantin C, Martin D, Albulescu
L, Iacob N and Ighigeanu D: Whole body microwave irradiation for
improved dacarbazine therapeutical action in cutaneous melanoma
mouse model. Radiol Res Pract. 2013(414816)2013.
|
|
75
|
Schwarz M, Münzel PA and Braeuning A:
Non-melanoma skin cancer in mouse and man. Arch Toxicol.
87:783–798. 2013. View Article : Google Scholar
|
|
76
|
Prasad R and Katiyar SK: Ultraviolet
radiation-induced inflammation activates β-catenin signaling in
mouse skin and skin tumors. Int J Oncol. 44:1199–1206.
2014.PubMed/NCBI
|
|
77
|
Huang YF, Yeh HY and Soo VW: Inferring
drug-disease associations from integration of chemical, genomic and
phenotype data using network propagation. BMC Med Genomics. 6(Suppl
3): S42013. View Article : Google Scholar
|
|
78
|
Yang AY, Lee JH, Shu L, Zhang C, Su ZY, Lu
Y, Huang MT, Ramirez C, Pung D, Huang Y, et al: Genome-wide
analysis of DNA methylation in UVB- and DMBA/TPA-induced mouse skin
cancer models. Life Sci. 113:45–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Uekusa S, Kawashima H, Sugito K, Yoshizawa
S, Shinojima Y, Igarashi J, Ghosh S, Wang X, Fujiwara K, Ikeda T,
et al: Nr4a3, a possibile oncogenic factor for neuroblastoma
associated with CpGi methylation within the third exon. Int J
Oncol. 44:1669–1677. 2014.PubMed/NCBI
|
|
80
|
Saito M, Okumura K, Miura I, Wakana S,
Kominami R and Wakabayashi Y: Identification of Stmm3 locus
conferring resistance to late-stage chemically induced skin
papillomas on mouse chromosome 4 by congenic mapping and
allele-specific alteration analysis. Exp Anim. 63:339–348. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Okumura K, Saito M, Isogai E, Miura I,
Wakana S, Kominami R and Wakabayashi Y: Congenic mapping and
allele-specific alteration analysis of Stmm1 locus conferring
resistance to early-stage chemically induced skin papillomas. PLos
One. 9:e972012014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Han G, Lu SL, Li AG, He W, Corless CL,
Kulesz-Martin M and Wang XJ: Distinct mechanisms of
TGF-beta1-mediated epithelial-to-mesenchymal transition and
metastasis during skin carcinogenesis. J Clin Invest.
115:1714–1723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matsumoto T, Jiang J, Kiguchi K, Ruffino
L, Carbajal S, Beltrán L, Bol DK, Rosenberg MP and DiGiovanni J:
Targeted expression of c-Src in epidermal basal cells leads to
enhanced skin tumor promotion, malignant progression, and
metastasis. Cancer Res. 63:4819–4828. 2003.PubMed/NCBI
|
|
84
|
Chen J and Roop DR: Genetically engineered
mouse models for skin research: Taking the next step. J Dermatol
Sci. 52:1–12. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wilker E, Lu J, Rho O, Carbajal S, Beltrán
L and DiGiovanni J: Role of PI3K/Akt signaling in insulin-like
growth factor-1 (IGF-1) skin tumor promotion. Mol Carcinog.
44:137–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer - a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar
|
|
88
|
Syed DN, Khan MI, Shabbir M and Mukhtar H:
MicroRNAs in skin response to UV radiation. Curr Drug Targets.
14:1128–1134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Singh A, Willems E, Singh A, Hafeez BB,
Ong IM, Mehta SL and Verma AK: Ultraviolet radiation-induced tumor
necrosis factor alpha, which is linked to the development of
cutaneous SCC, modulates differential epidermal microRNAs
expression. Oncotarget. Feb 22–2016.Epub ahead of print. PubMed/NCBI
|
|
90
|
Skourti E, Logotheti S, Kontos CK,
Pavlopoulou A, Dimoragka PT, trougakos IP, gorgoulis V, scorilas A,
Michalopoulos I and Zoumpourlis V: Progression of mouse skin
carcinogenesis is associated with the orchestrated deregulation of
miR-200 family members, miR-205 and their common targets. Mol
Carcinog. Aug 27–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Chen T: The role of MicroRNA in chemical
carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol
Rev. 28:89–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Corsini LR, Bronte G, Terrasi M, Amodeo V,
Fanale D, Fiorentino E, Cicero G, Bazan V and Russo A: The role of
microRNAs in cancer: diagnostic and prognostic biomarkers and
targets of therapies. Expert Opin Ther Targets. 16(Suppl 2):
s103–s109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Pogribny IP, Beland FA and Rusyn I: The
role of microRNAs in the development and progression of
chemical-associated cancers. Toxicol Appl Pharmacol. Nov
24–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shen J, Abel EL, Riggs PK, Repass J,
Hensley SC, Schroeder LJ, Temple A, Chau A, McClellan SA, Rho O, et
al: Proteomic and pathway analyses reveal a network of inflammatory
genes associated with differences in skin tumor promotion
susceptibility in DBA/2 and C57BL/6 mice. Carcinogenesis.
33:2208–2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hemler ME: Tetraspanin functions and
associated micro-domains. Nat Rev Mol Cell Biol. 6:801–811. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li Q, Yang XH, Xu F, Sharma C, Wang HX,
Knoblich K, Rabinovitz I, Granter SR and Hemler ME: Tetraspanin
CD151 plays a key role in skin squamous cell carcinoma. Oncogene.
32:1772–1783. 2013. View Article : Google Scholar
|
|
97
|
Hara T, Matsumura S, Hakuno F, Takahashi S
and Chida K: PKCα suppresses 7,12-dimethylbenz[a]anthracene-induced
skin tumor formation. Anticancer Res. 32:3097–3101. 2012.PubMed/NCBI
|
|
98
|
Suganuma M, Okabe S, Kurusu M, Iida N,
Ohshima S, Saeki Y, Kishimoto T and Fujiki H: Discrete roles of
cytokines, TNF-α, IL-1, IL-6 in tumor promotion and cell
transformation. Int J Oncol. 20:131–136. 2002.
|
|
99
|
Matei C, Tampa M, Ion RM, Georgescu SR,
Dumitrascu GR, Constantin C and Neagu M: Protein microarray for
complex apoptosis monitoring of dysplastic oral keratinocytes in
experimental photodynamic therapy. Biol Res. 47(33)2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tanase CP, Albulescu R and Neagu M:
Application of 3D hydrogel microarrays in molecular diagnostics:
Advantages and limitations. Expert Rev Mol Diagn. 11:461–464. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Kangsamaksin T, Park HJ, Trempus CS and
Morris RJ: A perspective on murine keratinocyte stem cells as
targets of chemically induced skin cancer. Mol Carcinog.
46:579–584. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Trempus CS, Morris RJ, Ehinger M, Elmore
A, Bortner CD, Ito M, Cotsarelis G, Nijhof JG, Peckham J, Flagler
N, et al: CD34 expression by hair follicle stem cells is required
for skin tumor development in mice. Cancer Res. 67:4173–4181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Klein EA: Can prostate cancer be
prevented? Nat Clin Pract Urol. 2:24–31. 2005. View Article : Google Scholar
|
|
104
|
Sagawa Y, Futakuchi M, Xu J, Fukamachi K,
Sakai Y, Ikarashi Y, Nishimura T, Suzui M, Tsuda H and Morita A:
Lack of promoting effect of titanium dioxide particles on
chemically-induced skin carcinogenesis in rats and mice. J Toxicol
Sci. 37:317–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Bhatia A, Singh B, Raza K, Shukla A,
Amarji B and Katare OP: Tamoxifen-loaded novel liposomal
formulations: Evaluation of anticancer activity on DMBA-TPA induced
mouse skin carcinogenesis. J Drug Target. 20:544–550. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Enoki T, Tominaga T, Takashima F, Ohnogi
H, Sagawa H and Kato I: Anti-tumor-promoting activities of
agaro-oligosac-charides on two-stage mouse skin carcinogenesis.
Biol Pharm Bull. 35:1145–1149. 2012. View Article : Google Scholar
|
|
107
|
Kowalczyk MC, Spears E, Narog M, Zoltaszek
R, Kowalczyk P, Hanausek M, Yoshimi N, Slaga TJ and Walaszek Z:
Modulation of biomarkers related to tumor initiation and promotion
in mouse skin by a natural β-glucuronidase inhibitor and its
precursors. Oncol Rep. 26:551–556. 2011.PubMed/NCBI
|
|
108
|
Meghea A, Murariu A, Tanase C and Codorean
E: Heavy metals contamination of commercial fish foodstuff -
potential health risks on human consumers. Environ Eng Manag J.
8:233–236. 2009.
|
|
109
|
Kundu JK, Shin YK and Surh YJ: Resveratrol
modulates phorbol ester-induced pro-inflammatory signal
transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as
prime targets. Biochem Pharmacol. 72:1506–1515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Kleiner HE, Vulimiri SV, Starost MF, Reed
MJ and DiGiovanni J: Oral administration of the citrus coumarin,
isopimpinellin, blocks DNA adduct formation and skin tumor
initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice.
Carcinogenesis. 23:1667–1675. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Singh RP, Tyagi AK, Zhao J and Agarwal R:
Silymarin inhibits growth and causes regression of established skin
tumors in SENCAR mice via modulation of mitogen-activated protein
kinases and induction of apoptosis. Carcinogenesis. 23:499–510.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Dao V, Pandeswara S, Liu Y, Hurez V, Dodds
S, Callaway D, Liu A, Hasty P, Sharp ZD and Curiel TJ: Prevention
of carcinogen and inflammation-induced dermal cancer by oral
rapamycin includes reducing genetic damage. Cancer Prev Res
(Phila). 8:400–409. 2015. View Article : Google Scholar
|
|
113
|
Pinheiro KS, Ribeiro DR, Alves AV,
Pereira-Filho RN, Oliveira CR, Lima SO, Reis FP, Cardoso JC and
Albuquerque-Júnior RL: Modulatory activity of Brazilian red
propolis on chemically induced dermal carcinogenesis. Acta Cir
Bras. 29:111–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Manoharan S and Selvan MV: Chemopreventive
potential of geraniol in 7,12-dimethylbenz(a) anthracene (DMBA)
induced skin carcinogenesis in Swiss albino mice. J Environ Biol.
33:255–260. 2012.PubMed/NCBI
|
|
115
|
Sharmila R and Manoharan S: Anti-tumor
activity of rosmarinic acid in 7,12-dimethylbenz(a)anthracene
(DMBA) induced skin carcinogenesis in Swiss albino mice. Indian J
Exp Biol. 50:187–194. 2012.PubMed/NCBI
|
|
116
|
Man'cheva TA, Demidov DV, Plotnikova NA,
Kharitonova TV, Pashkevich IV and Anisimov VN: Melatonin and
metformin inhibit skin carcinogenesis and lipid peroxidation
induced by benz(a)pyrene in female mice. Bull Exp Biol Med.
151:363–365. 2011. View Article : Google Scholar
|
|
117
|
Hu G, Zhang L, Rong Y, Ni X and Sun Y:
Downstream carcinogenesis signaling pathways by green tea
polyphenols: A translational perspective of chemoprevention and
treatment for cancers. Curr Drug Metab. 15:14–22. 2014. View Article : Google Scholar
|
|
118
|
Birt DF, Pinch HJ, Barnett T, Phan A and
Dimitroff K: Inhibition of skin tumor promotion by restriction of
fat and carbohydrate calories in SENCAR mice. Cancer Res. 53:27–31.
1993.PubMed/NCBI
|
|
119
|
Moore T, Beltran L, Carbajal S, Strom S,
Traag J, Hursting SD and DiGiovanni J: Dietary energy balance
modulates signaling through the Akt/mammalian target of rapamycin
pathways in multiple epithelial tissues. Cancer Prev Res (Phila).
1:65–76. 2008. View Article : Google Scholar
|
|
120
|
Stewart JW, Koehler K, Jackson W, Hawley
J, Wang W, Au A, Myers R and Birt DF: Prevention of mouse skin
tumor promotion by dietary energy restriction requires an intact
adrenal gland and glucocorticoid supplementation restores
inhibition. Carcinogenesis. 26:1077–1084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Katiyar SK: Silymarin and skin cancer
prevention: anti-inflammatory, antioxidant and immunomodulatory
effects (Review). Int J oncol. 26:169–176. 2005.
|
|
122
|
Vaid M and Katiyar SK: Molecular
mechanisms of inhibition of photocarcinogenesis by silymarin, a
phytochemical from milk thistle (Silybum marianum L. Gaertn.)
(Review). Int J Oncol. 36:1053–1060. 2010.PubMed/NCBI
|
|
123
|
Deep G and Agarwal R: Chemopreventive
efficacy of silymarin in skin and prostate cancer. Integr Cancer
Ther. 6:130–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Benjamin CL and Ananthaswamy HN: p53 and
the pathogenesis of skin cancer. Toxicol Appl Pharmacol.
224:241–248. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kiaris H and Spandidos DA: Mutations of
ras genes in human tumors (review). Int J Oncol. 7:413–421.
1995.PubMed/NCBI
|
|
126
|
Hennings H, Spangler EF, Shores R,
Mitchell P, Devor D, Shamsuddin AK, Elgjo KM and Yuspa SH:
Malignant conversion and metastasis of mouse skin tumors: A
comparison of SENCAR and CD-1 mice. Environ Health Perspect.
68:69–74. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Liu J, Mansouri K, Judson RS, Martin MT,
Hong H, Chen M, Xu X, Thomas RS and Shah I: Predicting
hepatotoxicity using ToxCast in vitro bioactivity and chemical
structure. Chem Res Toxicol. 28:738–751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Bulman A, Neagu M and Constantin C:
Immunomics in Skin Cancer - Improvement in Diagnosis, Prognosis and
Therapy Monitoring. Curr Proteomics. 10:202–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Spandidos DA: A unified theory for the
development of cancer. Biosci Rep. 6:691–708. 1986. View Article : Google Scholar : PubMed/NCBI
|