Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the most malignant forms of head and neck cancers and is
particularly prevalent in Asia and South China. Currently, surgery
or total laryngectomy, followed by radiotherapy or chemotherapy, is
the primary treatment strategy for LSCC. However, the prognosis of
patients with LSCC is poor due to the advanced stage at which the
disease typically presents. Associated complications, such as
trachyphonia, dysphagia, dyspnea and coughing, can seriously affect
the quality of life of patients (1). Due to the high mortality rate and
morbidity associated with LSCC, effective treatment remains a
considerable clinical challenge (2). Therefore, new and effective preventive
and therapeutic strategies are urgently needed.
Epidemiological and case-control studies have
indicated a strong correlation between the consumption of certain
vegetables and the decreased risk of carcinogenesis (3,4).
Recently, it has been suggested that phenethyl isothiocyanate
(PEITC), an important tumoricidal component found in cruciferous
vegetables such as broccoli and cauliflower may possess anticancer
properties against various malignancies, including breast, colon
and prostate cancers (5–9). Several mechanisms have been proposed
for these actions, including the generation of reactive oxygen
species and initiation of cell cycle arrest (10). However, the role of PEITC in human
laryngocarcinoma cells remains largely unknown.
The primary aim of the present study was to
determine the actions and potential mechanisms of PEITC in
laryngocarcinoma, including LSCC, by studying its effects on
proliferation, apoptosis, cell cycle and metastasis in human
laryngeal cancer Hep-2 and normal bronchial epithelial 16HBE cells
in vitro in order to provide a basis for targeted therapies
and drug screening in patients with LSCC.
Materials and methods
Cell lines, reagents and kits
Human laryngeal cancer cell line Hep-2 and human
normal bronchial epithelial cell line 16HBE were cryopreserved in
our laboratory and stored in liquid nitrogen. PEITC was obtained
from Sigma-Aldrich (St. Louis, MO, USA). Other reagents included
dimethylsulfoxide (DMSO) (Sigma-Aldrich), fetal bovine serum (FBS;
HyClone, Logan, UT, USA), RPMI-1640 medium and 0.25% trypsin
solution (Invitrogen, Carlsbad, CA, USA). Experimental equipment
included Cell Counting Kit-8 (CCK8; Dongji, Japan), Annexin
V-FITC/propidium iodide (PI) apoptosis detection kit, PI cell cycle
analysis kit (both from Lianke, China), TUNEL apoptosis detection
kit (Roche, Indianapolis, IN, USA) and a Transwell insert chamber
coated with Matrigel (BD Biosciences, San Jose, CA, USA). Primary
antibodies against Bcl-2, Bax, Bcl-xl, PI3K class III, PI3K p110α,
PI3K p110β, p-Akt, p-c-Raf, p-NF-κB-p65, p-ERK, cyclin D1, CDK4 and
CDK6 and GAPDH were all purchased from Cell Signaling Technology
(Danvers, MA, USA).
Cell culture and treatments
Hep-2 and 16HBE cells were cultured in RPMI-1640
medium supplemented with 10% FBS and 20 µg/ml antibiotics
(ampicillin and kanamycin) at 37°C in a humidified atmosphere of 5%
CO2. The cells were harvested in their logarithmic
growth phase by trypsinization for use in the experiments. The
cells were seeded in 96-well plates at a density of
1×103 cells/well for normal culture. They were divided
into groups in 6-well plates and treated by adding PEITC to give
the following final concentrations: Hep-2 cells, 0, 2.5, 5, 7.5 and
10 µM; and 16HBE cells, 0, 5, 10, 15 and 20 µM. All
experiments were performed at least three times.
Cell proliferation assay
Hep-2 and 16HBE cells were treated with PEITC as
described above, for 0, 24, 48 and 72 h before being incubated with
10 µl CCK-8 for 1 h at 37°C. DMSO was used as a negative
control. Six repeats were prepared for each treatment group.
Absorbances were detected at 450 nm, and IC50 values
were calculated by sigmoidal dose-response nonlinear regression
analysis using GraphPad Prism software version 5.04 (GraphPad
Software, San Diego, CA, USA).
Flow cytometry with Annexin V-FITC for
the detection of apoptosis
After being treated with PEITC for 24 h as described
above, the cells were harvested by trypsinization, centrifuged and
washed in cold phosphate-buffered saline (PBS). The cells were then
stained with 5 µl Annexin V-FITC solution and 10 µl
of PI solution for 15 min. Stained cells were analyzed using a
FACSCanto™ II spectrometer (BD Biosciences). Data were analyzed
using FlowJo version 7.6.5 software (FlowJo LLC, Ashland, OR,
USA).
Flow cytometry with PI for cell cycle
analysis
After treatment with PEITC for 24 h as described
above, the cells were fixed in 70% ethanol overnight. The cells
were centrifuged and the cell pellets were recovered and
resuspended in 1 mg/ml RNase and 20 µl 0.5% Triton X-100.
The cells were then incubated with 5 µl of 1 mg/ml PI
solution for 30 min at room temperature. Cell cycle distribution
was analyzed using FlowJo software, and the percentages of cells at
each phase of the cell cycle were calculated.
TUNEL assay for detection of
apoptosis
Hep-2 cells were treated with PEITC for 24 h as
described above, and then washed in PBS, air dried and fixed with
freshly prepared 4% paraformaldehyde. Terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL) was performed according
to the manufacturer's protocol (Roche). In brief, the cells were
incubated with TUNEL reaction mixture for 1 h at 37°C. The slides
were washed in PBS and stained with 4′,6-diamidino-2-phenylindole
(DAPI) before being viewed under microscopy. Six fields were
randomly selected from every sample, and 100 cells were randomly
selected from every field. The apoptotic rate was calculated as the
total number of apoptotic cells/100 × 100%.
Transwell invasion assay
Cell invasion assays were performed using Transwell
migration chambers with Matrigel-coated inserts (BD Biosciences)
according to the manufacturer's protocol. In brief,
1×105 Hep-2 cells were suspended in 200 ml serum-free
RPMI-1640 medium and treated with PEITC for 48 h as described
above. The cells were seeded in Matrigel-coated inserts in the
upper chamber; the lower chamber contained RPMI-1640 medium with
10% FBS as the chemoattractant. After incubation for 48 h at 37°C
in a humidified atmosphere of 5% CO2, any cells that had
not penetrated the membrane were removed using cotton swabs; the
cells that had successfully migrated to the bottom surfaces of the
membranes were fixed with 4% polyoxymethylene and stained with 0.1%
crystal violet for 20 min. They were counted under a microscope at
a magnification of ×100.
Western blotting
Hep-2 cells were treated with PEITC for 24 h as
described above. Total cell lysates were extracted from the
harvested cells using complete protease inhibitor 'cocktail'
(Roche) and 2 mM dithiothreitol (DTT). The proteins were resolved
by 12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF)
membranes before being incubated with the following primary
antibodies: anti-Bcl-2, anti-Bax, anti-Bcl-xl, anti-PI3K class III,
anti-PI3K p110α, anti-PI3K p110β, anti-p-Akt, anti-p-PDK1,
anti-GSK3-β, anti-p-c-Raf, anti-p-NF-κB-p65, anti-p-ERK,
anti-cyclin B1, anti-CDK4 and anti-CDK6. GAPDH was used as an
internal control. After being stained with their respective
secondary antibodies, the proteins were detected by Odyssey
infrared imaging (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Statistical analyses were carried out by one-way
ANOVA using SPSS statistical software version 16.0 (SPSS, Inc.,
Chicago, IL, USA). All data are expressed as mean ± SD. P-values
<0.05 were considered to indicate a statistically significant
result.
Results
Effects of PEITC treatment on the
viability of the Hep-2 and 16HBE cells
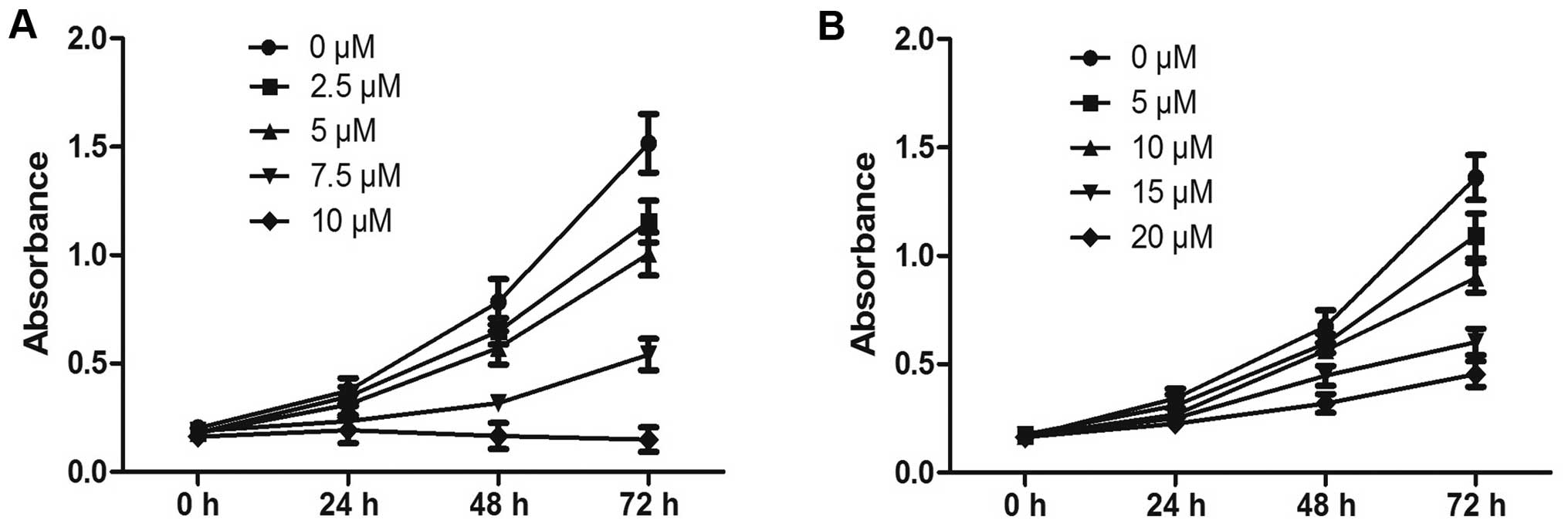
The influence of PEITC treatment on proliferation in
human Hep-2 laryngeal tumor cells and normal 16HBE bronchial
epithelial cells was determined by CCK-8 assays. The results showed
that PEITC exerted profound dose- and time-dependent
antiproliferative effects on the growth of Hep-2 cells when
administered between 0 and 10 µM for treatment times from
0–72 h (Fig. 1A). The inhibitory
efficiency at 10 µM PEITC in Hep-2 cells 24 h post-treatment
exceeded 52%. In contrast, the same treatment conditions had little
effect on the proliferation of the 16HBE cells (Fig. 1B). These results confirmed that
Hep-2 tumor cells exhibited greater sensitivity to PEITC than
normal 16HBE bronchial epithelial cells.
Effects of PEITC on apoptosis in the
Hep-2 and 16HBE cells
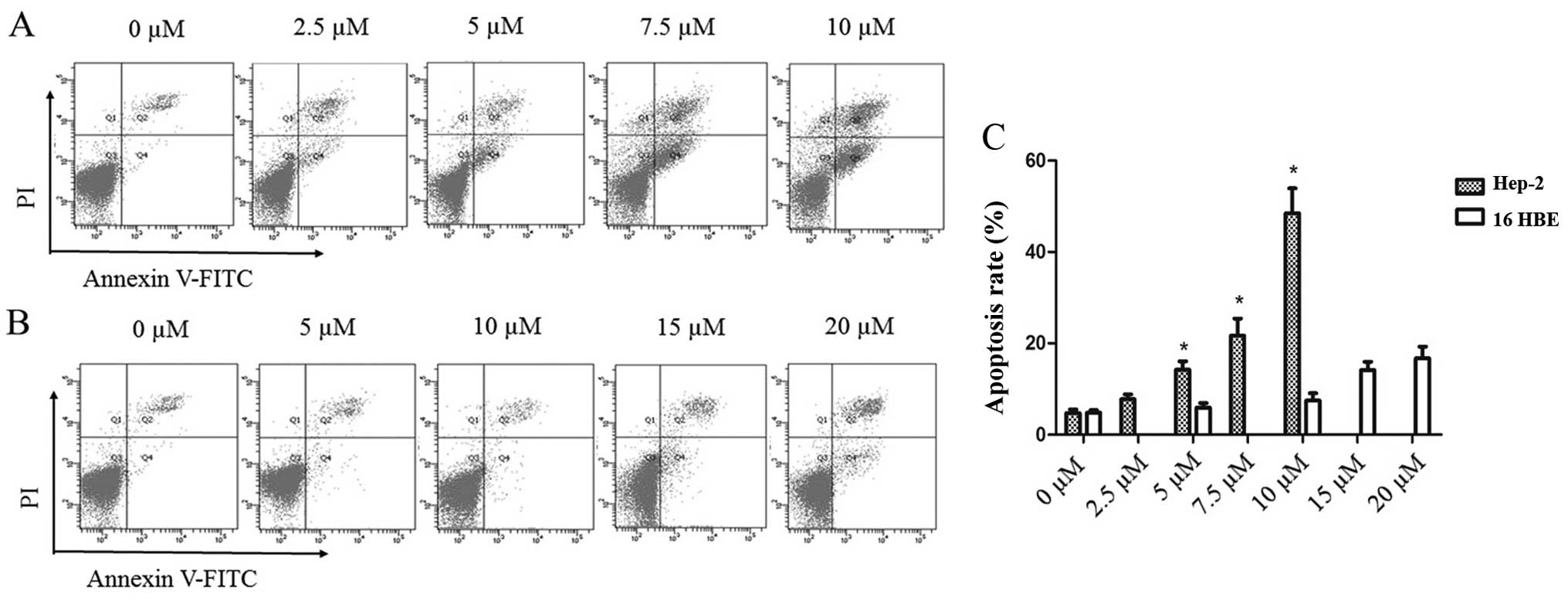
To further explore the effects of PEITC treatment on
laryngeal tumor cells, the levels of apoptosis in the Hep-2 and
16HBE cells were determined by Annexin V-FITC/PI double-staining
flow cytometry after treatment with various concentrations of PEITC
for 24 h. The results demonstrated that the percentages of both
early (FITC+/PI−) and late apoptotic Hep-2
cells (FITC+/PI+) gradually increased with
increasing concentrations of PEITC in a dose-dependent manner
(Fig. 2A). At 10 µM PEITC,
the percentages of apoptotic cells after 24 h treatment were 48.5
and 7.5% in the Hep-2 and 16HBE cells, respectively (Fig. 2C). In addition, the levels of
apoptosis in the 16HBE cells remained comparatively low at higher
concentrations of PEITC, with only 14.1 and 16.7% apoptotic cells
at 15 and 20 µM PEITC, respectively (Fig. 2B). These results demonstrated that
normal 16HBE bronchial cells exhibited higher tolerance to PEITC
treatment than the Hep-2 tumor cells. In summary, PEITC appeared
effective in inducing apoptosis in laryngeal cancer cells between 0
and 10 µM PEITC in vitro while having little or no
toxicological impact on normal non-tumorous bronchial cells.
Effects of PEITC on cell cycle arrest in
the Hep-2 cells
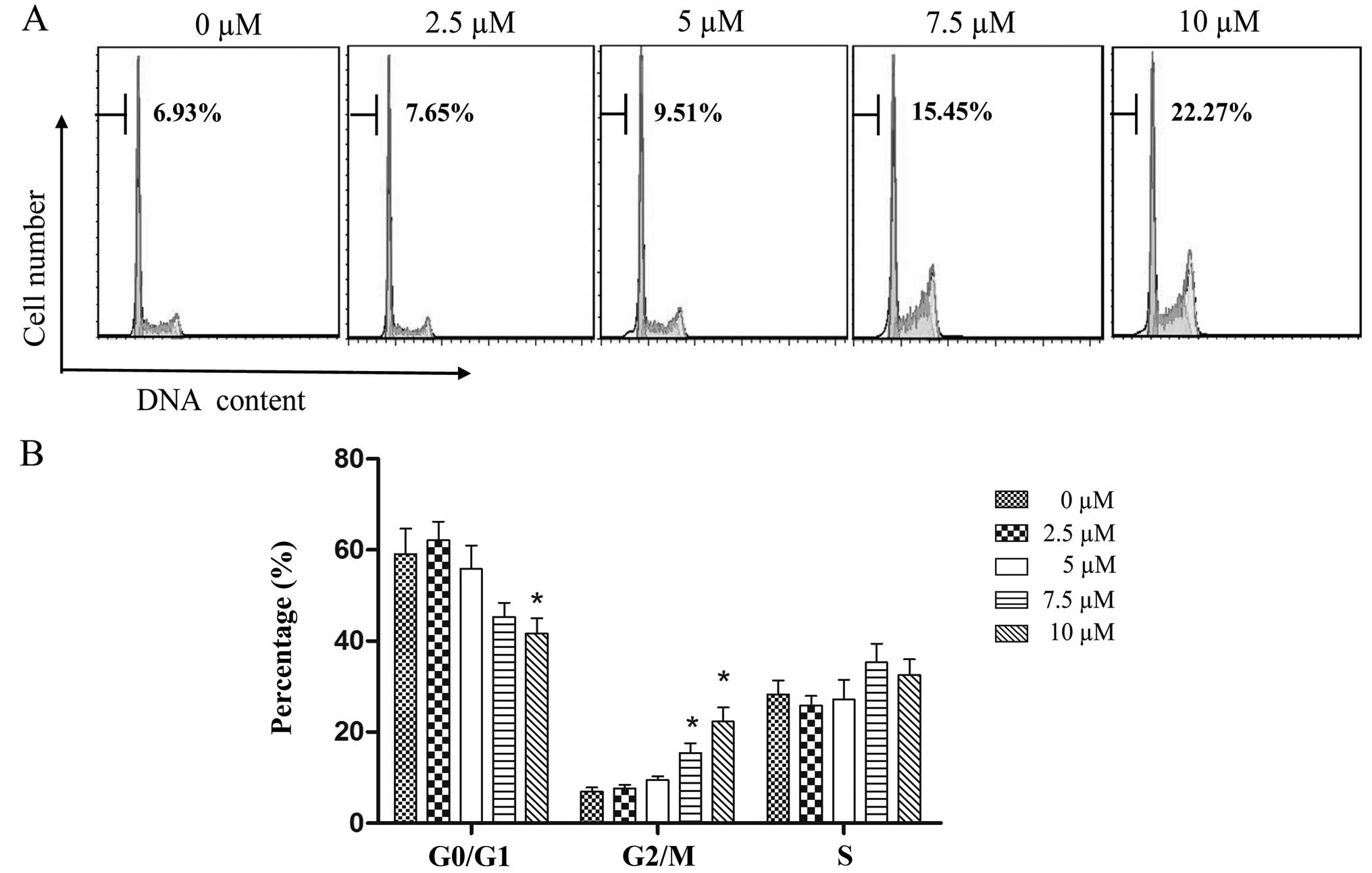
To investigate the effects of PEITC treatments on
laryngocarcinoma in greater detail, flow cytometric analysis with
PI single-staining was used to examine cell cycle distribution in
the Hep-2 cells following a 24-h treatment with increasing
concentrations of PEITC. As shown in Fig. 3, the proportion of cells in the
G0/G1 phase decreased from 59.13 to 41.65% as PEITC (P<0.05)
concentrations increased from 0–10 µM; whereas the
corresponding proportion of G2/M phase cells significantly
increased from 6.93 to 22.27% (P<0.05); and the proportion of S
phase cells increased marginally from 28.25 to 32.49%. These
results demonstrated that PEITC has the greatest influence in
promoting cell cycle arrest in Hep-2 cells at the G2/M phase.
TUNEL detection of cell apoptosis in the
Hep-2 cells
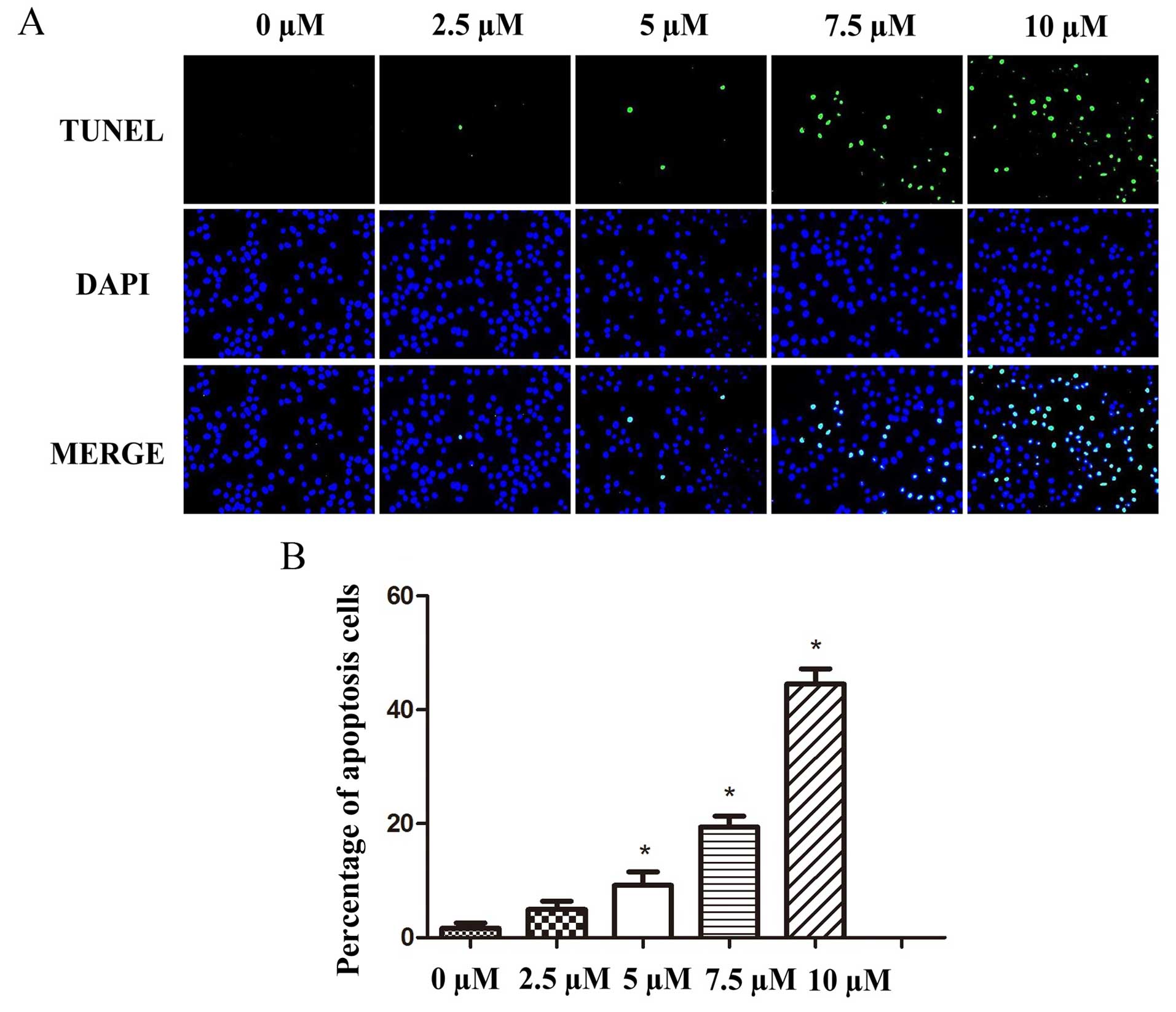
TUNEL assays were performed to verify the flow
cytometric results and to further explore the pro-apoptotic effects
of PEITC treatment on the laryngeal tumor cells. The micrographs
were analyzed by fluorescence microscopy 24 h post-treatment and
showed that the mean percentages of apoptotic increased from
1.32±2.57 at a concentration of 0 PEITC to 4.80±1.77, 8.98±4.41,
19.50±1.58 and 44.23±2.31% at concentrations of 2.5, 5, 7.5 and 10
µM PEITC, respectively. These results confirmed that PEITC
induced apoptosis in the Hep-2 cells in a dose-dependent manner
(Fig. 4).
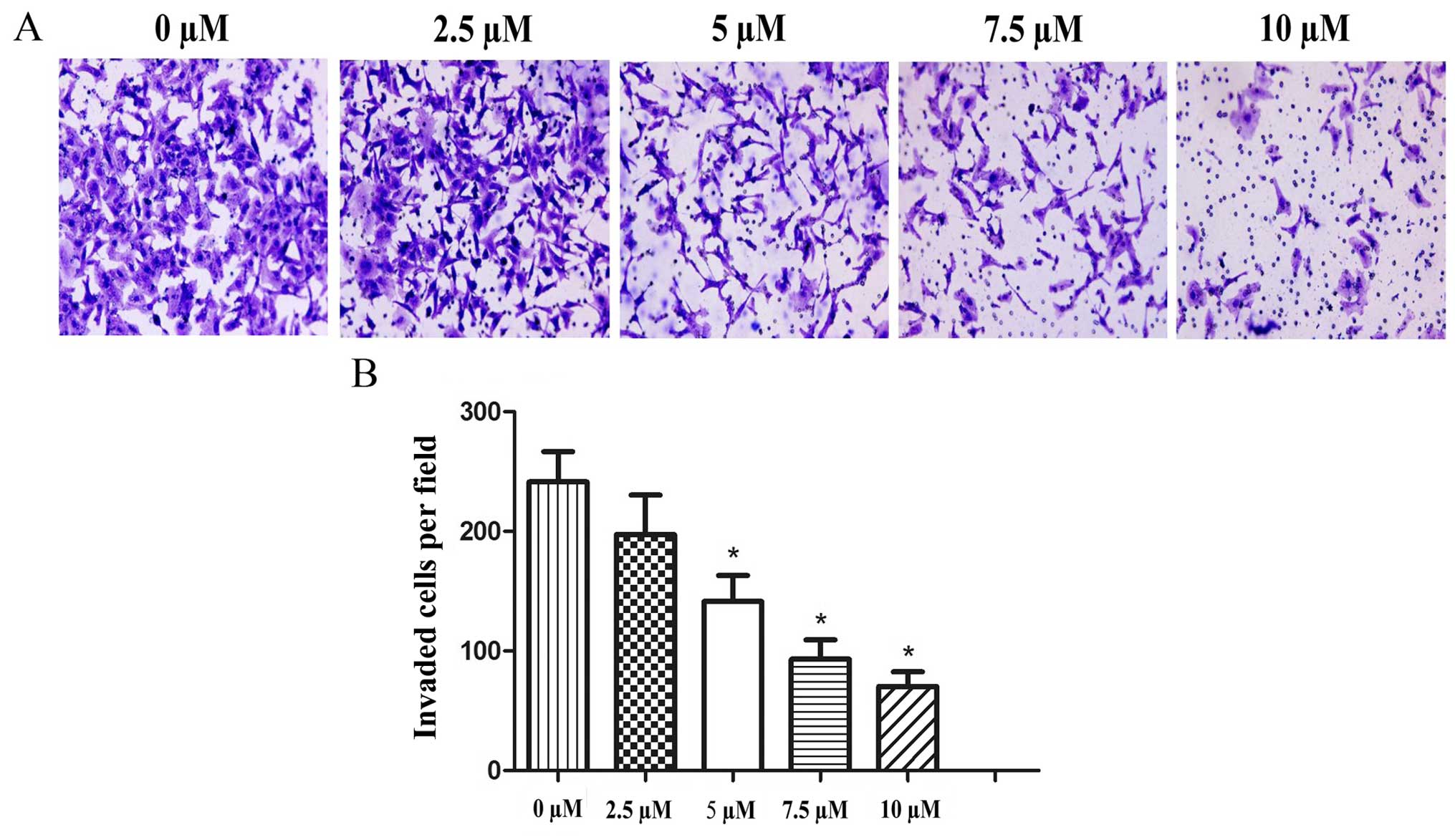
Effects of PEITC treatment on cell
invasion in the Hep-2 cells
A Transwell assay was performed to determine whether
PEITC influences the invasiveness of Hep-2 laryngeal cancer cells.
Following treatment with 0–10 µM PEITC for 48 h, the data
clearly demonstrated that PEITC suppressed the invasive ability of
the Hep-2 cells in a dose-dependent manner (Fig. 5). The effects were most significant
at concentrations ≥5 µM PEITC (P<0.05).
Effects of PEITC treatment on protein
expression levels associated with proliferation, apoptosis and cell
cycle signaling pathways
Having demonstrated that PEITC treatment could have
significant effects on proliferation, apoptosis, cell cycle
distribution and invasion in Hep-2 cells, it was necessary to
explore the potential mechanisms. Therefore, western blotting was
performed to identify changes in the expression levels of
regulatory proteins involved in key signaling pathways related to
the occurrence and development of laryngocarcinoma. The pathways of
interest included proliferation: PI3K, Akt, NF-κB and ERK;
apoptosis: Bcl-2, Bcl-xl and Bax; and cell cycle progression:
GSK3-β, cyclin B1, CDK4 and CDK6. Qualitative analyses showed that
as the PEITC concentration increased from 0 to10 µM, the
expression levels of PI3K class III, PI3K p110α, PI3K p110β, p-Akt,
p-PDK1, GSK3-β, p-NF-κB-p65, p-c-Raf, p-ERK, Bcl-2, Bcl-xl, cyclin
B1, CDK4 and CDK6 were downregulated 24 h post-treatment and Bax
was upregulated, whereas t-Akt, t-NF-κB-p65 and t-ERK protein
expression remained unchanged as the PEITC concentration increased
(Fig. 6).
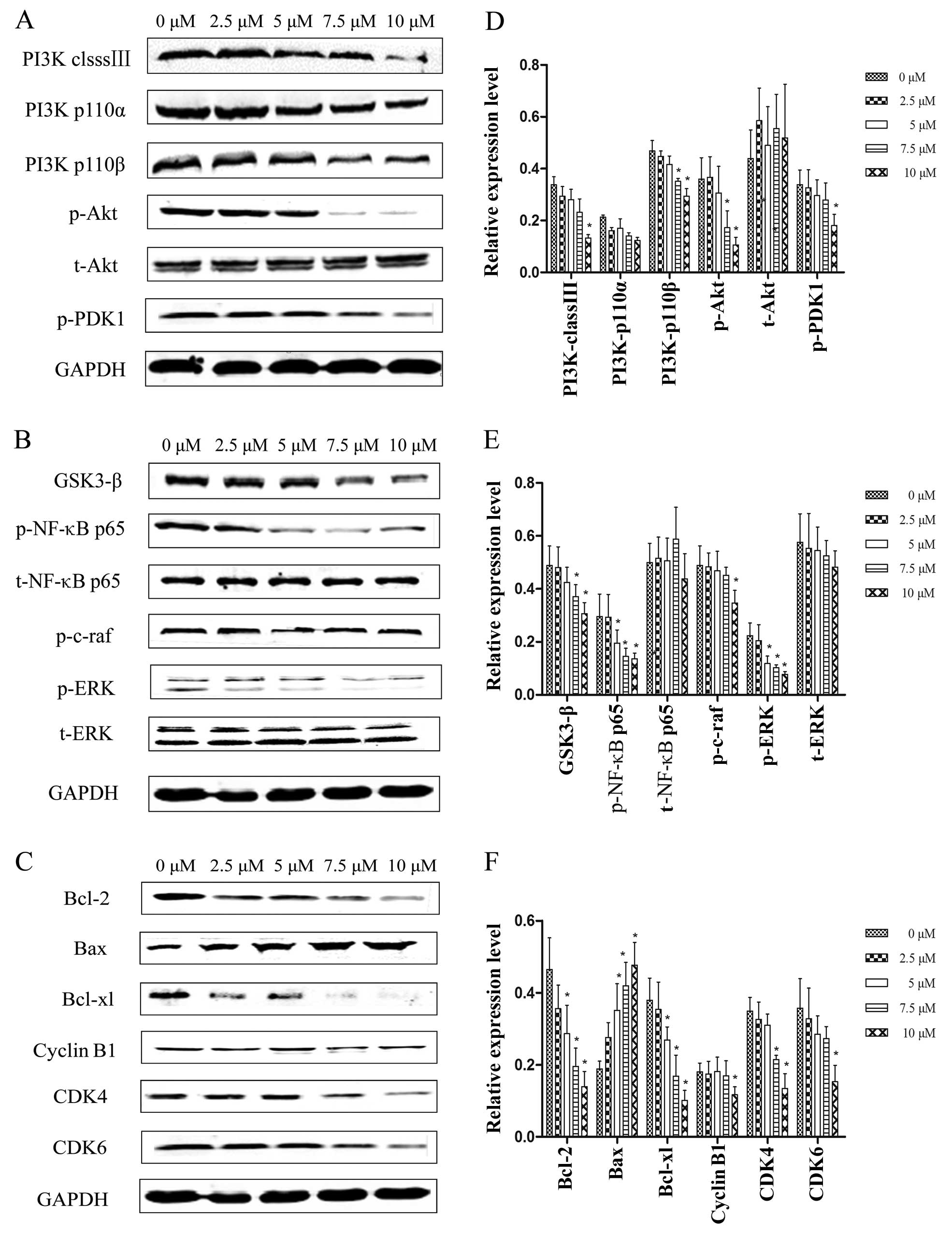 | Figure 6Western blot assays of protein
expression levels in proliferation, apoptosis and cell cycle
progression pathways in the Hep-2 cells treated with PEITC for 24
h. (A) PI3K class III, PI3K p110α, PI3K p110β, p-Akt, t-Akt and
p-PDK1. (B) GSK3-β, p-NF-κB p65, t-NF-κB p65, p-c-Raf p-ERK and
t-ERK. (C) Bcl-2, Bax, Bcl-xl, cyclin B1, CDK4 and CDK6. (D–F) Data
presented are the means ± SD. Results were normalized to GAPDH.
*P<0.05 relative to values at 0 µM PEITC. |
Discussion
Several studies have identified PEITC, a major
active constituent in cruciferous vegetables, as a potential
anticancer agent in various types of malignancies, including
breast, colon and prostate carcinomas (7–9).
However, its actions in laryngocarcinoma, including LSCC, remain
largely unknown. Therefore the purpose of the present study was to
explore its actions and potential mechanisms in human Hep-2
laryngeal tumor cells and normal 16HBE bronchial epithelial
cells.
Our findings demonstrated that treatment with PEITC
significantly suppressed proliferation, induced apoptosis, promoted
G2/M cell cycle arrest and inhibited invasion and thereby
metastasis, in the Hep-2 cancer cells in vitro (P<0.05).
Importantly, the results also demonstrated that treatment within a
safe range of concentrations (from 0–10 µM PEITC) had little
toxicological impact on normal 16HBE cells. These findings were
consistent with published studies on other types of cancer
(11–13); for example, Wang et al showed
that treatment with 5–10 µM PEITC for 8–24 h could inhibit
proliferation and induce apoptosis in cervical cancer cells
(14).
Our results also showed that PEITC significantly
inhibited proliferation in Hep-2 cells in a time- and
dose-dependent manner, and significantly promoted apoptosis and
inhibited cell invasion in a dose-dependent manner (P<0.05).
Similar observations have been reported in previous studies
(15,16). For example, the percentage of Hep-2
cells was reduced to 52% and the percentage of apoptotic cells was
increased to 48% 24 h post-treatment with 10 µM PEITC
relative to the corresponding levels at 0 µM PEITC
(P<0.05). These effects were accompanied by concurrent increases
in the percentages of cells at the G2/M phase, suggesting that
PEITC may inhibit cell growth by inducing cell cycle arrest at the
G2/M checkpoint.
Tumorigenesis is characterized by uncontrolled cell
growth and tumor formation and is associated with alterations in
the expression levels of proteins involved in pro-survival
signaling pathways linked to the occurrence and development of
tumors (17). Furthermore, the
suppression of multiple signaling pathways simultaneously has been
found to have a greater anticancer impact than suppression of a
single signaling pathway alone (18–20).
Proteins such as PI3K, Akt, ERK and NF-κB have been found to play
key roles in the regulation of proliferation, cell cycle
progression and apoptosis in LSCC (21–23).
RNAi is frequently used to inhibit the expression of proteins and
has proven to be important in developing effective therapeutic
strategies against cancers. Using these methods, our results
indicated that PEITC may target several important signaling
pathways associated with apoptosis, cell growth and metastasis
simultaneously, thereby enhancing its antitumor impact on
laryngocarcinoma.
Metastasis and tumor cell invasion are important
factors in the prognosis and recurrence of cancers. Our results
showed that treatment with PEITC inhibited the invasiveness of
Hep-2 cells in vitro and suggested that this effect was due
in part to the suppression of ERK and NF-κB activity. Similar
findings were reported by Gupta et al in a mouse model of
breast cancer in which the administration of PEITC suppressed
development of metastasized tumors (24). However, data on the antimetastatic
effects of PEITC in other forms of cancer remain scarce, and
further investigations is needed to elucidate the molecular
mechanisms.
Currently, surgery followed by radiotherapy or
chemotherapy is the primary treatment for LSCC. However, this
strategy frequently results in damage to normal surrounding tissues
(25,26). Consequently, there is increasing
interest in identifying natural compounds with effective anticancer
properties due their low toxicities (27). The results of the present study
demonstrated that PEITC was effective in suppressing proliferation,
invasion and inducing apoptosis in Hep-2 laryngeal cancer cells
while having little toxicological impact on 16HBE normal bronchial
epithelial cells, particularly within a range of 0–10 µM
PEITC. Although, increased levels of apoptosis were observed in
16HBE cells at higher concentrations of PEITC (up to 20 µM),
these remained comparatively low relative to the levels in the
Hep-2 cells. Similar observations have been reported previously.
PEITC was found to inhibit mammary carcinoma tissues in a mouse
model, but was well-tolerated in normal mammary glands (28); however, another study reported
increased levels of aspartate aminotransferase (AST) at high doses
of PEITC (100–150 mg/kg) but no change was observed at lower doses
(50 mg/kg), suggesting that PEITC may cause dose-dependent toxicity
in normal tissues at high treatment concentrations (29). Variations in treatment times or
analytical time points between different studies may have
contributed to these inconsistencies (30). Therefore, further in vivo
studies are required to define the toxicity profile of PEITC in
laryngocarcinoma in order to ensure that doses can be titrated
accurately in a clinical setting.
In conclusion, the present study demonstrated that
PEITC significantly induced antiproliferative, pro-apoptotic and
antimetastatic effects in Hep-2 human laryngeal cancer cells in a
time- and dose-dependent manner, while presenting little
toxicological damage to normal 16HBE bronchial epithelial cells
in vitro. The findings suggest that its anticancer
activities resulted from its ability to inhibit several critical
pro-survival pathways related to the occurrence and development of
laryngocarcinoma simultaneously via dysregulation of key cell
signaling proteins. In summary, PEITC may offer a valuable
contribution to the development of novel therapeutic strategies for
the treatment of LSCC in the future.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81001214, 81172569
and 81372880).
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morshed K: Association between human
papillomavirus infection and laryngeal squamous cell carcinoma. J
Med Virol. 82:1017–1023. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolonel LN, Hankin JH, Whittemore AS, Wu
AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW,
et al: Vegetables, fruits, legumes and prostate cancer: A
multiethnic case-control study. Cancer Epidemiol Biomarkers Prev.
9:795–804. 2000.PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Annema N, Heyworth JS, McNaughton SA,
Iacopetta B and Fritschi L: Fruit and vegetable consumption and the
risk of proximal colon, distal colon, and rectal cancers in a
case-control study in Western Australia. J Am Diet Assoc.
111:1479–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta P, Kim B, Kim SH and Srivastava SK:
Molecular targets of isothiocyanates in cancer: Recent advances.
Mol Nutr Food Res. 58:1685–1707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et
al: Selective killing of oncogenically transformed cells through a
ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer
Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huong D, Shim JH, Choi KH, Shin JA, Choi
ES, Kim HS, Lee SJ, Kim SJ, Cho NP and Cho SD: Effect of
β-phenylethyl isothiocyanate from cruciferous vegetables on growth
inhibition and apoptosis of cervical cancer cells through the
induction of death receptors 4 and 5. J Agric Food Chem.
59:8124–8131. 2011. View Article : Google Scholar
|
|
9
|
Sakao K, Desineni S, Hahm ER and Singh SV:
Phenethyl isothiocyanate suppresses inhibitor of apoptosis family
protein expression in prostate cancer cells in culture and in vivo.
Prostate. 72:1104–1116. 2012. View Article : Google Scholar :
|
|
10
|
Chan ATC: Nasopharyngeal carcinoma. Ann
Oncol. 21(Suppl 7): vii308–vii312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao D, Choi S, Lee YJ and Singh SV: Role
of mitogen-activated protein kinases in phenethyl
isothiocyanate-induced apoptosis in human prostate cancer cells.
Mol Carcinog. 43:130–140. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang NY, Huang YT, Yu CS, Ko YC, Wu SH, Ji
BC, Yang JS, Yang JL, Hsia TC, Chen YY, et al: Phenethyl
isothiocyanate (PEITC) promotes G2/M phase arrest via p53
expression and induces apoptosis through caspase- and
mitochondria-dependent signaling pathways in human prostate cancer
DU 145 cells. Anticancer Res. 31:1691–1702. 2011.PubMed/NCBI
|
|
13
|
Huong LD, Shin JA, Choi ES, Cho NP, Kim
HM, Leem DH and Cho SD: β-Phenethyl isothiocyanate induces death
receptor 5 to induce apoptosis in human oral cancer cells via p38.
Oral Dis. 18:513–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XF, Wu DM, Li BX, Lu YJ and Yang BF:
Synergistic inhibitory effect of sulforaphane and 5-fluorouracil in
high and low metastasis cell lines of salivary gland adenoid cystic
carcinoma. Phytother Res. 23:303–307. 2009. View Article : Google Scholar
|
|
15
|
Gupta P and Srivastava SK: Antitumor
activity of phenethyl isothiocyanate in HER2-positive breast cancer
models. BMC Med. 10:802012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Chen Z, Hu Y and Huang P:
Inhibition of mitochondrial respiration and rapid depletion of
mitochondrial glutathione by β-phenethyl isothiocyanate: Mechanisms
for anti-leukemia activity. Antioxid Redox Signal. 15:2911–2921.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doerfler W, Hohlweg U, Müller K, Remus R,
Heller H and Hertz J: Foreign DNA integration - perturbations of
the genome - oncogenesis. Ann NY Acad Sci. 945:276–288. 2001.
View Article : Google Scholar
|
|
18
|
Liu SF, Wang H, Lin XC, Xiang H, Deng XY,
Li W, Tang M and Cao Y: NF-kappaB inhibitors induce lytic
cytotoxicity in Epstein-Barr virus-positive nasopharyngeal
carcinoma cells. Cell Biol Int. 32:1006–1013. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang H, Fan D, Zhou G, Li X and Deng H:
Phosphatidylinositol 3-kinase inhibitor (LY294002) induces
apoptosis of human nasopharyngeal carcinoma in vitro and in vivo. J
Exp Clin Cancer Res. 29:342010. View Article : Google Scholar
|
|
20
|
Li SS, Tang QL, Wang SH, Wang S and Yang
XM: Simultaneously targeting bcl-2 and Akt pathways sensitizes
nasopharyngeal carcinoma to tumor necrosis factor-related
apoptosis-inducing ligand. Cancer Biother Radiopharm. 27:88–95.
2012. View Article : Google Scholar
|
|
21
|
Chen C, Chen SM, Xu B, Chen Z, Wang F, Ren
J, Xu Y, Wang Y, Xiao BK and Tao ZZ: In vivo and in vitro study on
the role of 3,3′-diindolylmethane in treatment and prevention of
nasopharyngeal carcinoma. Carcinogenesis. 34:1815–1821. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YQ, Chen C, Chen Z, Xu Y, Wang Y,
Xiao BK, Chen SM and Tao ZZ: Indole-3-carbinol inhibits cell
proliferation and induces apoptosis in Hep-2 laryngeal cancer
cells. Oncol Rep. 30:227–233. 2013.PubMed/NCBI
|
|
23
|
Zhang X: Depression of testes-specific
protease 50 (TSP50) inhibits cell proliferation and induces
apoptosis in laryngocarcinoma. Tumour Biol. 35:10781–10788. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gupta P, Adkins C, Lockman P and
Srivastava SK: Metastasis of breast tumor cells to brain is
suppressed by phenethyl isothiocyanate in a novel in vivo
metastasis model. PLoS One. 8:e672782013. View Article : Google Scholar :
|
|
25
|
Baletic N, Malicevic H, Petrovic Z,
Marinkovic-Eric J and Peric A: Advantages and limitations of the
autofluorescent diagnostics of the laryngeal cancer and
precancerosis. Eur Arch Otorhinolaryngol. 267:925–931. 2010.
View Article : Google Scholar
|
|
26
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the 30 years from 1981 to 2010. J Nat
Prod. 75:311–335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh SV, Kim SH, Sehrawat A, Arlotti JA,
Hahm ER, Sakao K, Beumer JH, Jankowitz RC, Chandra-Kuntal K, Lee J,
et al: Biomarkers of phenethyl isothiocyanate-mediated mammary
cancer chemoprevention in a clinically relevant mouse model. J Natl
Cancer Inst. 104:1228–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manesh C and Kuttan G: Effect of naturally
occurring isothiocyanates on the immune system. Immunopharmacol
Immunotoxicol. 25:451–459. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang LG and Chiao JW: Prostate cancer
chemopreventive activity of phenethyl isothiocyanate through
epigenetic regulation (Review). Int J Oncol. 37:533–539. 2010.
View Article : Google Scholar : PubMed/NCBI
|