Introduction
Epidemiological surveys have shown that breast
cancer is the most common malignant cancer in females. The process
of tumor progression and metastasis is multipart and evolutionary,
involving numerous phases and events at the cellular and molecular
levels. Tumor cells need to detach from each other, migrate, pierce
through vasculature and establish angiogenesis (1). Metastasis is one of the leading causes
of breast cancer-related mortality among females. It is well known
that metastasis and invasion are basic properties of breast cancer
cells and the main cause of cancer-related deaths. Several studies
have reported that the invasion and metastasis of breast cancer
involve multiple biological processes, including cell
proliferation, motility, adhesion, degradation of the basement
membrane, invasion and metastasis (2,3).
Therefore, efforts to develop additional breast cancer strategies
for the treatment and diagnosis of breast cancer are of great
importance.
Extravasation from the vessels is mediated by the
action of extracellular proteases, among which the matrix
metalloproteinases (MMPs) play crucial roles. It is well
established that MMPs, which are vital enzymes that degrade the
extracellular matrix, play central roles in tumor invasion and
metastasis. As a main member of the MMPs, upregulation of the
activity and expression of MMP-9 is frequently observed in breast
cancers with invasive and metastatic capability (3). The induction of cyclooxygenase-2
(COX-2) is a characteristic feature of the tumor environment; it
leads to invasiveness, angiogenesis, and metastatic potential.
COX-2 is overexpressed in breast cancer cell lines and plays an
important role in breast cancer invasion (4). MMP-9 and COX-2 are constitutively
activated in most cancers and offer effective targets for
therapeutic development. MMP-9 and COX-2 are involved in tumor
progression via a wide variety of signaling molecules and
mechanisms. Furthermore, the transcription factors nuclear
transcription factor-κB (NF-κB) and activator protein-1 (AP-1)
which are direct targets of MMP-9 and COX-2, have been correlated
with increased metastatic potential in cancer. Recent studies have
demonstrated that NF-κB and AP-1 promote the migration and invasion
of cancer cells via the regulation of a number of genes (5,6).
Resveratrol is a natural dietary phytochemical that
has a preventive effect against breast cancer. It is an active
ingredient in grapes, wine, mulberries, peanuts and other
vegetation. Resveratrol has antioxidant, anti-inflammatory, and
antisenescence properties, and its potential as an anticancer drug
is currently being evaluated in clinical trials for various types
of cancers (7,8). Its anticancer ability is the key to
its therapeutic potential. It inhibits cell growth, tumor
proliferation, invasion, metastasis and angiogenesis by
downregulating many molecular signaling targets, including MMP-9,
COX-2, NF-κB and AP-1 (7,9). However, the well-known clinical
applications of resveratrol in cancer have been limited owing to
its poor bioavailability. Therefore, it is important to make
resveratrol in more effective forms with respect to efficacy and
stability (10,11).
Nanoparticle research has comprehensively increased
in recent years to include applications in areas such as advanced
nanomedicine. Moreover, nanotechnology has seen remarkable
improvements and has potential for medicinal applications,
including drug development and delivery. Recently, gold
nanoparticles (AuNPs) are used extensively as compounds for
pharmaceutical products targeting several diseases, including
cancer, neurodegenerative disease and hepatitis, owing to their
unfamiliar optoelectronic and physicochemical properties (12,13).
Therefore, the biological synthesis of AuNPs is a focus of current
research. AuNPs are considered more eco-friendly and cost-effective
than other chemical and physical methods owing to the reduced use
of hazardous reagents and solvents, improved material and energy
efficiency from the chemical process, and enhanced design of
non-toxic products (14,15). Phytochemicals have been used
recently to biologically synthesize AuNPs. The nanoformulation of
phytochemicals shows enhanced cellular uptake, bioavailability, and
anticancer activity as compared to phytochemicals (16). Although AuNPs have been shown to
exert antitumor effects in various models, only a few studies have
reported the antitumor properties of AuNPs and the mechanism by
which AuNPs interact with human breast cancer cells. A recent study
found that AuNPs in the 50–300 nm size range caused significant
time- and dose-dependent toxicity in human breast cancer MCF-7
cells through the reactive oxygen species (ROS)-/Jun N-terminal
kinase (JNK)-mediated mitochondrial pathway (17–19).
However, it is still unclear whether AuNPs have a direct beneficial
effect on breast cancer metastasis beside the effects involving
various molecular targets. In the present study, we demonstrated
the biological synthesis of AuNPs using resveratrol, which acts as
a reducing and stabilizing agent, and investigated the
anti-invasive effect of resveratrol-capped gold nanoparticles
(Rev-AuNPs) on 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced breast cancer cells, with a focus on the key
molecular events involved. Rev-AuNP treatment resulted in
inhibitory effects on TPA-induced migration and invasion as well as
the activation of multiple gene products linked to MMP-9, COX-2,
NF-κB, AP-1, phosphoinositide 3-kinase/Akt (PI3K/Akt) and
extracellular signal-regulated kinase (ERK) in human breast cancer
cells. In addition to the key events in breast cancer progresssion,
the crucial role of the induction of heme oxygenase-1 (HO-1) in
response to Rev-AuNP treatment was investigated in TPA-stimulated
breast cancer cells.
Materials and methods
Materials and reagents
Resveratrol and other chemicals were purchased from
Sigma-Aldrich (St. Louis, MO, USA). BD BioCoat™ Matrigel™ invasion
chambers were obtained from BD Biosciences (San Jose, CA, USA).
Antibodies against phosphorylated p38 (p-p38), p-JNK, p-ERK, MMP-2
and MMP-9 were purchased from Cell Signaling Technology (Beverly,
MA, USA). HO-1 small interfering RNA (siRNA), and antibodies
against COX-1, COX-2, ERK, JNK, p38, c-Jun, c-Fos and NF-κB and TBP
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco-BRL Invitrogen (Carlsbad, CA, USA). FuGENE6
and X-tremeGENE siRNA transfection reagents were purchased from
Roche (Indianapolis, IN, USA).
Green synthesis and characterization of
Rev-AuNPs
Rev-AuNPs were prepared by the reduction method
using chloroauric acid according to a previous study (20), with slight modifications. In the
synthesis protocol, resveratrol (500 mM) in dimethyl sulfoxide
(DMSO) was added to a stirred HAuCl4 solution (1 mM),
and kept at room temperature for 10 min. The color change from
yellow to deep ruby red indicated the formation of gold
nanoparticles. The mixture was stirred for another 10 min. The
obtained gold nanoparticles are stable for >1 year. The
UV-visible spectrum of the sampled resveratrol or Rev-AuNP solution
was measured using an Ultrospec 6300 Pro (GE Healthcare Life
Sciences, Buckinghamshire, UK) at wavelengths in the 300–800 nm
range. Average particle sizes and zeta potentials of different
formulations were determined by a dynamic light scattering (DLS)
method using Data Transfer Assistance (DTA) software and a
Zetasizer Nano ZS90 (Malvern Instruments Ltd., Malvern, UK). The
morphology and distribution of the Rev-AuNPs were examined using a
transmission electron microscope (TEM; Hitachi, Tokyo, Japan).
Cell culture
The human breast cell line MCF-7 was obtained from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Cells were grown in RPMI-1640 medium supplemented with 10%
heat-inactivated FBS and 1% penicillin-streptomycin at 37°C in a
humidified incubator with a 5% CO2 atmosphere.
Cell invasion assay
A cell invasion assay was conducted using BioCoat™
Matrigel™ invasion chambers according to the manufacturer's
instructions. Briefly, the Matrigel coating was re-hydrated in 0.5
ml of Dulbecco's modified Eagle's medium (DMEM) for 30 min
immediately before the experiments. Cells (5×104)
suspended in 0.5 ml of serum-free medium were added to the upper
chamber of the Matrigel-coated filter inserts. After treatment with
sanguinarine for 1 h, 0.5 ml of serum-free medium containing 50 nM
TPA was added to the bottom well as a chemoattractant. The chambers
were then incubated for 24 h. After incubation, cells on the upper
side of the chamber were removed using cotton swabs, and cells that
had migrated were fixed and stained with 2% ethanol containing 0.2%
crystal violet powder. Invading cells were enumerated under a light
microscope at a magnification of ×10.
In vitro wound healing repair assay
For the in vitro wound healing repair assay
(cell migration assay), the cells were seeded in a 24-well culture
dish until reaching 90% confluency. The cells were then maintained
in serum-free medium for 12 h. The monolayers were carefully
scratched using a 200-µl pipette tip. Cellular debris was
removed by washing with phosphate-buffered saline, after which the
cells were incubated in serum-free medium. The migrated cells were
then fixed with cold 75% methanol for 30 min and washed 3 times
with phosphate-buffered saline. The cultures were photographed at 0
and 24 h to monitor the migration of cells into the wounded area,
after which the closure of the wounded area was calculated.
Gelatin zymography assay
The enzyme activities of MMP-2 and MMP-9 in
conditioned medium were determined using a gelatin zymography
protease assay. Briefly, cells (2×105) were seeded into
6-well plates and allowed to grow to 80% confluency. The cells were
then maintained in serum-free medium for 12 h prior to treatments
with sanguinarine and TPA for 24 h. Conditioned media were
collected, cleared by centrifugation, and mixed with 2X SDS sample
buffer (Invitrogen), followed by electrophoresis in a
polyacrylamide gel containing 0.1% (w/v) gelatin. Following
electrophoresis, the gels were incubated in renaturing buffer (2.5%
Triton X-100) with gentle agitation to remove SDS, followed by
incubation in developing buffer (50 mM Tris-HCl buffer, pH 7.4 and
10 mM CaCl2) overnight at 37°C to allow digestion of the
gelatin. Gels were then stained with SimplyBlue SafeStain
(Invitrogen) until clear bands suggestive of gelatin digestion were
visible.
Western blot analysis
Cells were harvested in ice-cold lysis buffer
consisting of 1% Triton X-100, 1% deoxycholate and 0.1% SDS. The
protein content of the cell lysates was then determined using the
Bradford reagent (Bio-Rad Laboratories, Hercules, CA, USA).
Proteins in each sample (50 µg of total proteins) were
resolved by 12% SDS-PAGE, transferred to a polyvinylidene
difluoride membrane, and exposed to the appropriate antibodies. The
proteins were visualized by enhanced chemiluminescence detection
(Amersham Biosciences, Piscataway, NJ, USA) using horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies. Images were captured using an ImageQuant 350 analyzer
(Amersham Biosciences).
Real-time PCR
Total cellular RNA was isolated using an RNA Spin
Mini RNA Isolation kit (GE Healthcare) according to the
manufacturer's instructions. One microgram of total RNA was
reverse-transcribed using Maxime RT PreMix (Intron Biotechnology,
Seongnam, Korea) and anchored oligo-dT15 primers.
Real-time PCR was performed with SYBR-Green Master Mix (Applied
Biosystems, Foster City, CA, USA) using a Chromo4 instrument
(Bio-Rad). The relative amount of target mRNA was determined using
the Ct method by normalizing target mRNA Ct values to those for
GAPDH (ΔCt). The real-time PCR cycling conditions were 95°C
for 5 min, 40 cycles for 30 sec at 95°C, 20 sec at 55°C and 30 sec
at 72°C, followed by fluorescence measurement. The primer sequences
used were as follows: MMP-9 sense (5′-ttccctggagacctgagaacc-3′) and
MMP-9 antisense (5′-cggcaagtcttccgagtagtttt3′); COX-2 sense
(5′-tacaagcagtggcaaaggc-3′) and COX-2 antisense
(5′-agatcatctctgcctgagtatctt-3′); GAPDH sense (5′-aggtggtctcct
ctgacttc-3′) and GAPDH antisense (5′-taccaggaaatgagcttgac-3′).
Transient transfection and
dual-luciferase assay
To determine the promoter activity, we used a
Dual-Luciferase Reporter Assay system (Promega, Madison, WI, USA).
Cells were transfected with the NF-κB or AP-1 luciferase reporter
plasmids (Stratagene, Grand Island, NY, USA) using the FuGENE6
reagent (Roche Applied Science, Basel, Switzerland) according to
the manufacturer's instructions. Renilla luciferase control
plasmid pRL-CMV (Promega) was co-transfected as an internal control
to determine the transfection efficiency. Twenty-four hours after
transfection, cells were incubated with the indicated reagents for
1 h, and then treated with TPA for 24 h. The luciferase activity
was assayed with a Dual-Luciferase Assay kit according to the
manufacturer's instructions. Luminescence was measured with a
GloMax™ 96 Microplate Luminometer (Promega).
Transient transfection of siRNA
Transfection of MCF-7 cells with siRNA was performed
using the X-tremeGENE siRNA transfection reagent (Roche Applied
Science), according to the manufacturer's instructions.
Commercially available human HO-1-specific siRNAs and
negative control siRNAs were used for transfection. Briefly,
X-tremeGENE siRNA transfection reagent (10 µl) was added to
100 µl of serum-free medium containing 2 µg of each
siRNA oligo, and the mixture was incubated for 20 min at room
temperature.
Statistical analysis
Each experiment was repeated at least 3 times, and
all results are expressed as means ± SE. Statistical analysis was
performed using SPSS (version 18.0) to determine significant
differences among groups. One-way analyses of variance (ANOVA)
followed by Tukey's post hoc tests were used for comparisons
between 3 or more groups. Differences with P<0.05 were
considered statistically significant.
Results
Characterization of the Rev-AuNPs
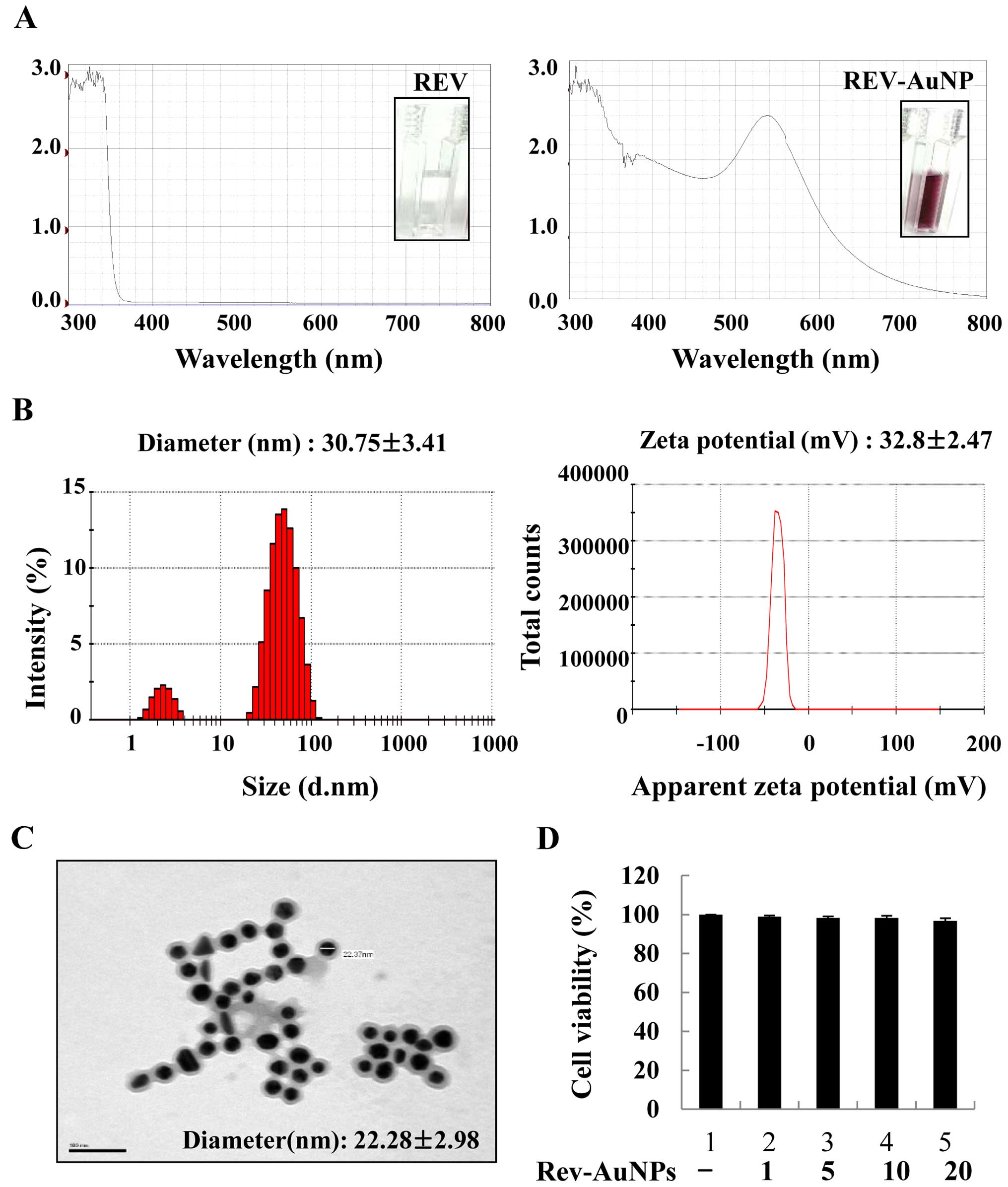
When resveratrol was used as a reductant, the
Rev-AuNPs turned pink in color and exhibited a characteristic
surface plasmon resonance band with maximum absorbance at 540 nm
(Fig. 1A). Rev-AuNPs were
characterized based on their size, distribution and zeta potential
using DLS. Based on the DLS analysis, the Rev-AuNPs had a diameter
of 30.75±3.41 nm and a zeta potential of −32.8±2.47 mV (Fig. 1B). The obtained Rev-AuNPs were
visualized by TEM and were predominantly spherical in shape
(22.28±2.98 nm). The images revealed that the Rev-AuNPs were well
dispersed without any aggregation, suggesting that resveratrol
acted as both a reductant and a stabilizing agent (Fig. 1C). The cytotoxic effect of Rev-AuNPs
on MCF-7 breast cancer cells was determined. Rev-AuNPs at a
concentration of 10 µM were non-toxic to MCF-7 breast cancer
cells (Fig. 1D). Therefore, for all
experiments, we used <10 µM Rev-AuNPs and determined
their anti-invasive properties in TPA-stimulated MCF-7 breast
cancer cells.
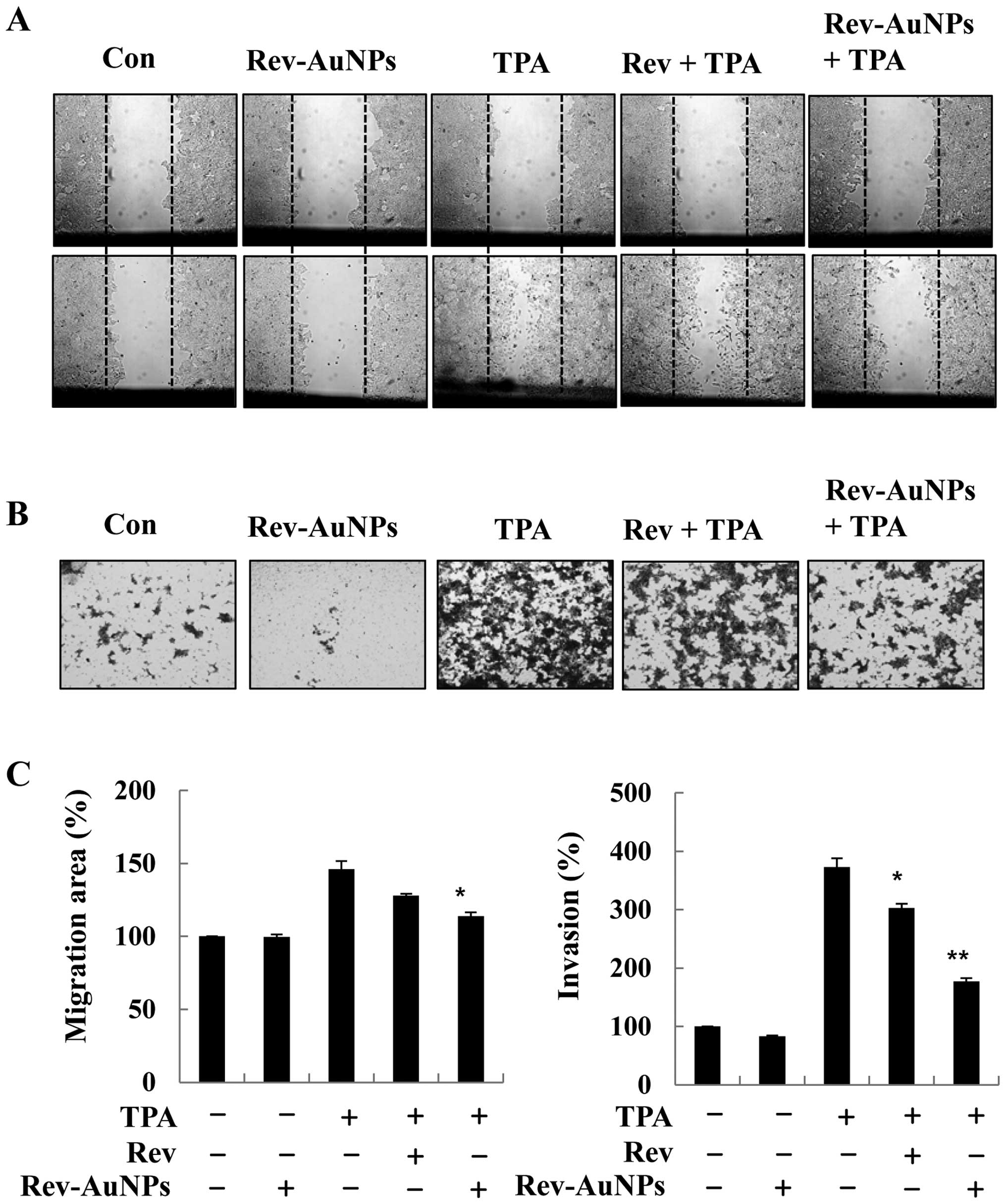
Effect of Rev-AuNP on TPA-induced breast
cancer cell migration and invasion
Breast cancer cell migration and invasion through
the basement membrane are important steps in breast cancer
malignancy. Since proteolytic digestion of the extracellular matrix
and migration of cancer cells across the blood vessel-lining
endothelial monolayers play crucial roles in the metastasis process
(2), we investigated the effects of
Rev-AuNPs on the migration and invasion of MCF-7 breast cancer
cells and compared the observed effects to those of resveratrol.
The effect of Rev-AuNPs on MCF-7 breast cancer cell migration and
invasion was determined by a wound healing assay with or without
TPA stimulation for 24 h. We found that TPA-induced MCF-7 breast
cancer cell migration was significantly inhibited by the Rev-AuNPs
(Fig. 2A). The invasive ability of
the MCF-7 breast cancer cells was determined by a Matrigel
Transwell invasion assay. TPA treatment significantly promoted the
invasiveness of the MCF-7 breast cancer cells compared to that
observed in the untreated cells. However, Rev-AuNP pretreatment
significantly inhibited TPA-induced invasion of the MCF-7 breast
cancer cells (Fig. 2B). Notably,
the inhibitory effect of Rev-AuNPs on migration and invasion was
stronger that of resveratrol at the same concentration (Fig. 2C).
Effect of Rev-AuNP on TPA-induced MMP-9
and COX-2 activation in breast cancer cells
MMP-9 and gelatinase are considered to be primarily
responsible for the degradation of major extracellular matrix
components and subsequent metastasis through the basement membrane
(21). Therefore, gelatin
zymography, western blotting and real-time PCR were performed to
detect changes in MMP-9 activity, protein and mRNA expression
levels in the TPA-stimulated MCF-7 breast cancer cells. MMP-9
activity as well as protein and mRNA expression were predominantly
upregulated upon TPA stimulation. Nevertheless, pretreatment with
Rev-AuNPs suppressed TPA-induced over-activation of MMP-9 activity,
protein and mRNA expression (Fig. 3A
and B). COX-2 is observed in many types of cancer and has been
shown to stimulate metastasis. We explored the inhibitory activity
of Rev-AuNPs on TPA-induced COX-2 expression at the protein and
mRNA levels using western blotting and real-time PCR. The
TPA-induced COX-2 mRNA and protein expression levels were also
significantly attenuated by the Rev-AuNPs (Fig. 3A and C). It was interesting to
observe that the inhibitory effect of Rev-AuNPs on the activation
of MMP-9 and COX-2 was stronger than that of resveratrol at the
same concentration. Taken together, these results indicated that
the inhibition of MMP-9 and COX-2 activation by Rev-AuNPs was
responsible for the suppression of migration and invasion in the
TPA-stimulated MCF-7 breast cancer cells.
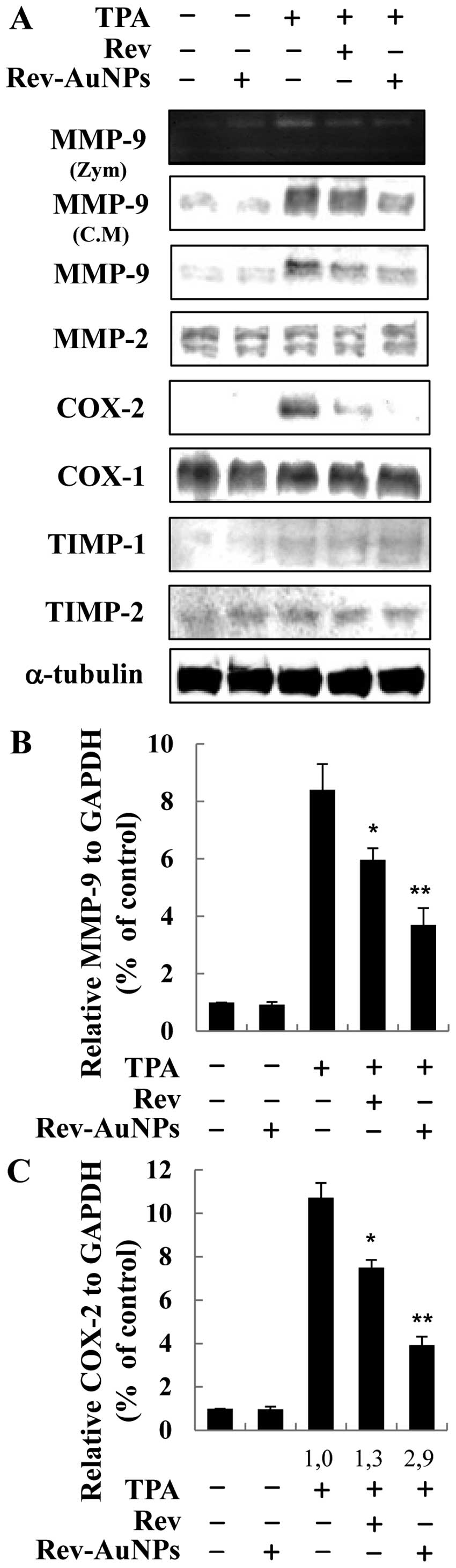 | Figure 3Effects of gold-conjugated resveratrol
nanoparticles (Rev-AuNPs) on MMP-9, MMP-2, COX-1, COX-2, TIMP-1 and
TIMP-2 expression in TPA-induced human breast cancer cells. (A)
MCF-7 cells were treated with resveratrol (Rev) (10 µM) or
Rev-AuNPs (10 µM) or 1 h, followed by TPA treatment (50
ng/ml) for 24 h. MMP-9 enzymatic activity was analyzed by gelatin
zymography (Zym), secretion by western blotting and intracellular
protein expression by western blotting. The protein levels of
MMP-9, MMP-2, COX-1, COX-2, TIMP-1 and TIMP-2 were evaluated by
western blotting. CM, conditioned medium. The relative MMP-9
(B) and COX-2 (C) mRNA expression levels (2−ΔCt)
were quantified by real-time PCR and calculated by subtracting the
Ct value for GAPDH from the Ct value for
MMP-9 and COX-2, which were determined by real-time
RT-PCR: ΔCt = Ct (MMP-9 or COX-2) -
Ct (GAPDH). Quantitative analyses of MMP-9 and
COX-2 mRNA expression are expressed as the means ± SE of 3
independent experiments in each group. *P<0.05,
**P<0.01 significant compared to cells treated with
TPA alone. |
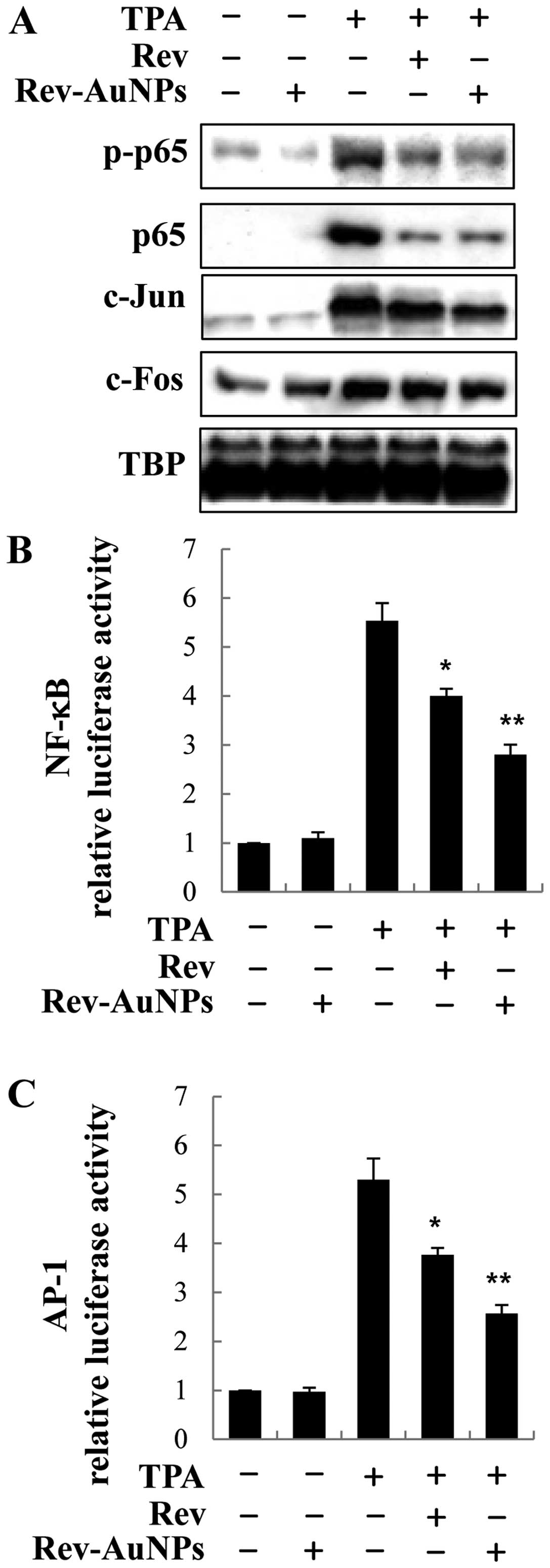
Effect of Rev-AuNP on TPA-induced NF-κB
and AP-1 activation in breast cancer cells
NF-κB and AP-1 are essential regulators of MMP-9 and
COX-2, which are critically involved in cancer metastasis. Hence,
we observed the effects of Rev-AuNPs on NF-κB and AP-1 signaling in
activated breast cancer cells (6).
After the cells were treated with the Rev-AuNPs and TPA for 30 min,
we used western blotting to examine the phosphorylation and nuclear
trans-location of NF-κB subunit p65 in the nuclear extract. The
phosphorylation and nuclear translocation of NF-κB subunit p65 were
significantly enhanced after cells were challenged with TPA.
Treatment with Rev-AuNPs apparently inhibited the over-activation
of NF-κB subunit p65. Similarly, the nuclear translocation levels
of AP-1 subunit c-Jun and c-fos were significantly attenuated by
Rev-AuNPs in comparison with the TPA-induced cells. However,
Rev-AuNPs had no obvious effect on the activation of the NF-κB
subunit p65 or AP-1 (Fig. 4A). To
confirm the effect of Rev-AuNPs on the transcriptional activity of
NF-κB and AP-1, we used a lucif-erase reporter assay containing the
NF-κB and AP-1 binding regions. Treatment with TPA-stimulated MCF-7
breast cancer cells increased the promoter activity of NF-κB and
AP-1; treatment with the Rev-AuNPs decreased the TPA-stimulated
promoter activity of NF-κB and AP-1 (Fig. 4B and C).
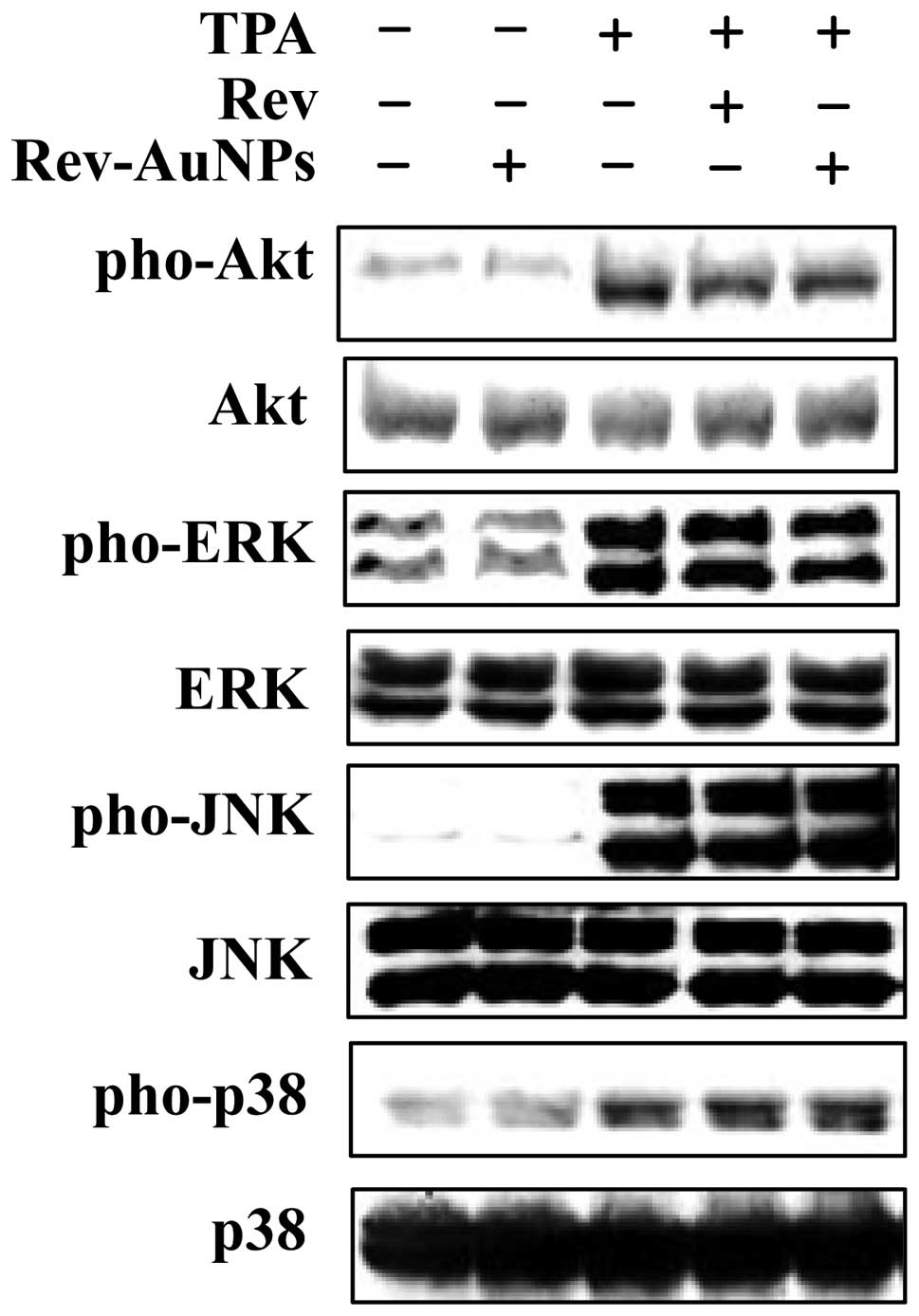
Effect of Rev-AuNP on TPA-induced
activation of Akt and ERK in breast cancer cells
PI3K/Akt and mitogen-activated protein kinases
(MAPKs) are important for the expression of both MMP-9 and COX-2.
They act as specific targets in cancer metastasis (4). We examined whether PI3K/Akt and MAPK
activity was inhibited via treatment with the Rev-AuNPs. We
assessed the phosphorylation levels of PI3K/Akt and MAPKs,
including Akt, JNK, p38 and ERK. When cells were stimulated with
TPA, the levels of phosphorylated Akt, JNK, p38 and ERK were
significantly increased. As shown in Fig. 5, the phosphorylation of Akt and ERK
was markedly inhibited by the Rev-AuNPs. By contrast, Rev-AuNP
pretreatment did not significantly effect TPA-induced JNK and p38
phosphorylation. These results demonstrated that Rev-AuNPs were
able to reduce MMP-9 and COX-2 activation by inhibiting the
activation of the Akt and ERK signaling pathway.
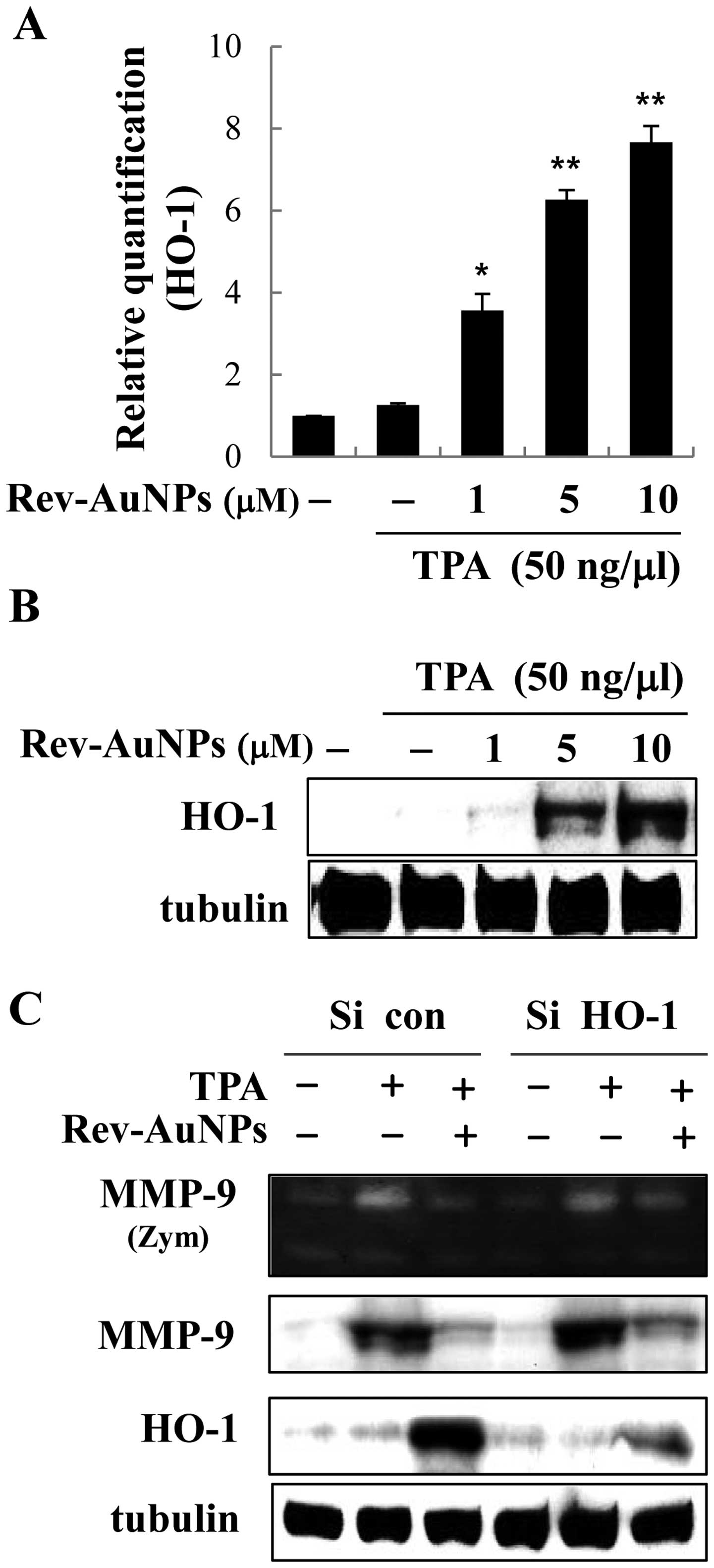
Inhibitory effect of Rev-AuNPs on
TPA-induced MMP-9 activity via HO-1
We hypothesized that the upregulation of HO-1
by Rev-AuNPs may be a key factor in the attenuation of the
TPA-induced invasion response. Accordingly, we determined the
effect of Rev-AuNPs on the expression of HO-1 in MCF-7 breast
cancer cells. We found quite interesting results; Rev-AuNP
treatment gradually upregulated the expression of HO-1 at the mRNA
and protein levels in a dose-dependent manner (Fig. 6A and B). Numerous anticancer drug
candidates prevent tumor progression via the induction of HO-1.
Therefore, we characterized the Rev-AuNP-mediated anti-invasion
effects in response to TPA stimulation with respect to the
overexpression of HO-1. We established an HO-1
knockdown model by transfection of HO-1 siRNA to cells,
which definitely inhibit HO-1 expression. The effective knockdown
of HO-1 was confirmed using a western blot assay. Next, we observed
that TPA-induced increases in MMP-9 activity and protein expression
were suppressed by Rev-AuNPs, but this effect was limited in the
HO-1-knockdown cells (Fig.
6C). Our findings strongly support the notion that the Rev-AuNP
anti-invasive effect was associated with the activation of HO-1 in
the MCF-7 breast cancer cells.
Discussion
Resveratrol has long been part of the daily diet
worldwide and has not been shown to cause any toxicity.
Comprehensive research over the past 20 years has indicated that
resveratrol has beneficial effects against a wide range of
diseases, such as cardiovascular, neurological, metabolic and liver
autoimmune diseases. Various lines of evidence indicate that
resveratrol possesses antioxidant, anti-inflammatory, antibacterial
and antisenescence activities. It is a key factor in multiple
intracellular signaling pathways and targets various signaling
molecules involved in tumor progression. It also inhibits signaling
molecules involved in cancer invasion, such as MMP-9, COX-2, NF-κB
and AP-1 (9,10,22).
Importantly, the capacity of resveratrol to suppress tumor
proliferation and metastasis in a number of cancer cell lines has
provoked increased attention for its use as an anticancer agent.
For this reason, resveratrol has received a great deal of attention
and the use of preparations containing resveratrol is increasing.
Alternative pharmacological techniques are necessary to maximize
the efficiency of resveratrol by improving its targeting and
bioavailability (22,23).
Recently, considerable research has focused on the
use of nanoscale materials as new effective anticancer agents in
humans. Specifically, AuNPs have been developed as an important
tool for medical applications. The biological synthesis of AuNPs is
eco-friendly and an ideal method to develop environmentally
sustainable nanoparticles. The bioconjugation of AuNPs is widely
used to develop biocompatible AuNPs for medical applications
(16,20,24).
In the present study, resveratrol was used as a reductant for the
green synthesis of Rev-AuNPs and its invasive efficacy on MCF-7
breast cancer cells was examined. Spectroscopic, light scattering,
and microscopic techniques were used to characterize the Rev-AuNPs,
including a UV-visible spectrum analysis, DLS and TEM. Using DLS,
we observed that the particle size and surface charge (zeta
potential) of Rev-AuNPs were 30.75±3.41 nm and -32.8±2.47 mV. Using
TEM, we determined the particle size distribution as well as the
morphology of the Rev-AuNPs; their size was 22.28±2.98 nm.
The invasion and metastasis of breast cancer cells
are essential biological characteristics of tumor progression and
most cancer deaths are due to metastasis development. Thus, the
regulation or even regression of the process of metastasis would be
a significant advance in the treatment of breast cancer. TPA as a
protein kinase activator is frequently used as a biomedical
research tool in models of carcinogenesis (2). In the present study, we systematically
investigated the effect of Rev-AuNPs on TPA-stimulated breast
cancer cells by applying several capable experimental methods.
First, we discovered that TPA induced migration and invasion
activity in the breast cancer cells, and in the presence of
Rev-AuNPs, the TPA-induced migration and invasion activity
decreased. The extracellular matrix regulates multiple cellular
functions necessary for tumor invasion and extracellular matrix
components (i.e., MMPs) are key mediators of this progression.
Activation of MMP-9 is correlated with increased cell invasion,
angiogenesis, as well as aggressive breast cancer. A specific
inhibitor of MMP-9 is TIMP-1 and MMP-2 is regulated by TIMP-2;
accordingly, the elucidation of TIMP-1 and TIMP-2 regulation will
provide insight into the process of metastasis (2,3). We
analyzed the inhibitory effect of Rev-AuNPs on MMP-9 activation in
breast cancer cells. The Rev-AuNPs inhibited MMP-9 not by
inhibiting MMP-9 enzymatic activity, but by the suppression of
MMP-9 mRNA and protein expression, whereas the activation of MMP-2,
TIMP-1 and TIMP-2 was not affected. Furthermore, we found that
Rev-AuNPs inhibited TPA-induced expression of COX-2 at the mRNA and
protein levels in the MCF-7 breast cancer cells.
To further investigate the anti-invasive mechanism
of Rev-AuNPs, we examined the effects of Rev-AuNPs on NF-κB and
AP-1 signaling. The NF-κB and AP-1 signaling pathways are essential
for cell migration, survival and proliferation and are associated
with tumor progression (6). Indeed,
we found that Rev-AuNPs significantly inhibited the activation of
NF-kB and AP-1 in TPA-stimulated breast cancer cells. PI3K/Akt and
MAPKs are closely connected with tumor progression (4). Several key molecules involved in
invasion and metastasis, such as MMPs and COX-2, are also regulated
by PI3K/Akt and MAPK signaling. We were further interested in the
inhibitory mechanism of Rev-AuNPs on PI3K/Akt and MAPK, which
regulate a range of biological processes implicated in tumor
progression. PI3K/Akt and ERK play roles in the regulation of
various cancers. We compared the anti-invasive effect between
resveratrol and Rev-AuNPs in MCF-7 breast cancer cells. Notably,
Rev-AuNPs exhibited a better anti-invasive activity than
resveratrol in most parts, and did not exhibit cytotoxicity. The
inhibitory effect of Rev-AuNPs on MMP-9, COX-2, NF-κB, AP-1,
PI3K/Ak and ERK activation was stronger than that of resveratrol
for the same concentrations.
Several beneficial effects of HO-1, including
antioxidant, anti-inflammation and antisenescence activity, have
been described. Overexpression of HO-1 may decrease tumor
progression via decreasing the range of intracellular oxidants.
However, conflicting reports have also indicated that the induction
of HO-1 is associated with tumor progression and is also correlated
with anticancer drug resistance. Nevertheless, many anticancer drug
candidates have been reported to prevent tumor progression via the
induction of HO-1 (21,25,26).
We investigated whether Rev-AuNPs inhibit TPA-induced MMP-9
activation in MCF-7 breast cancer cells via HO-1 expression.
Rev-AuNP-mediated inhibition of MMP-9 activity was also suppressed
by the knockdown of endogenous HO-1 in the MCF-7 breast cancer
cells.
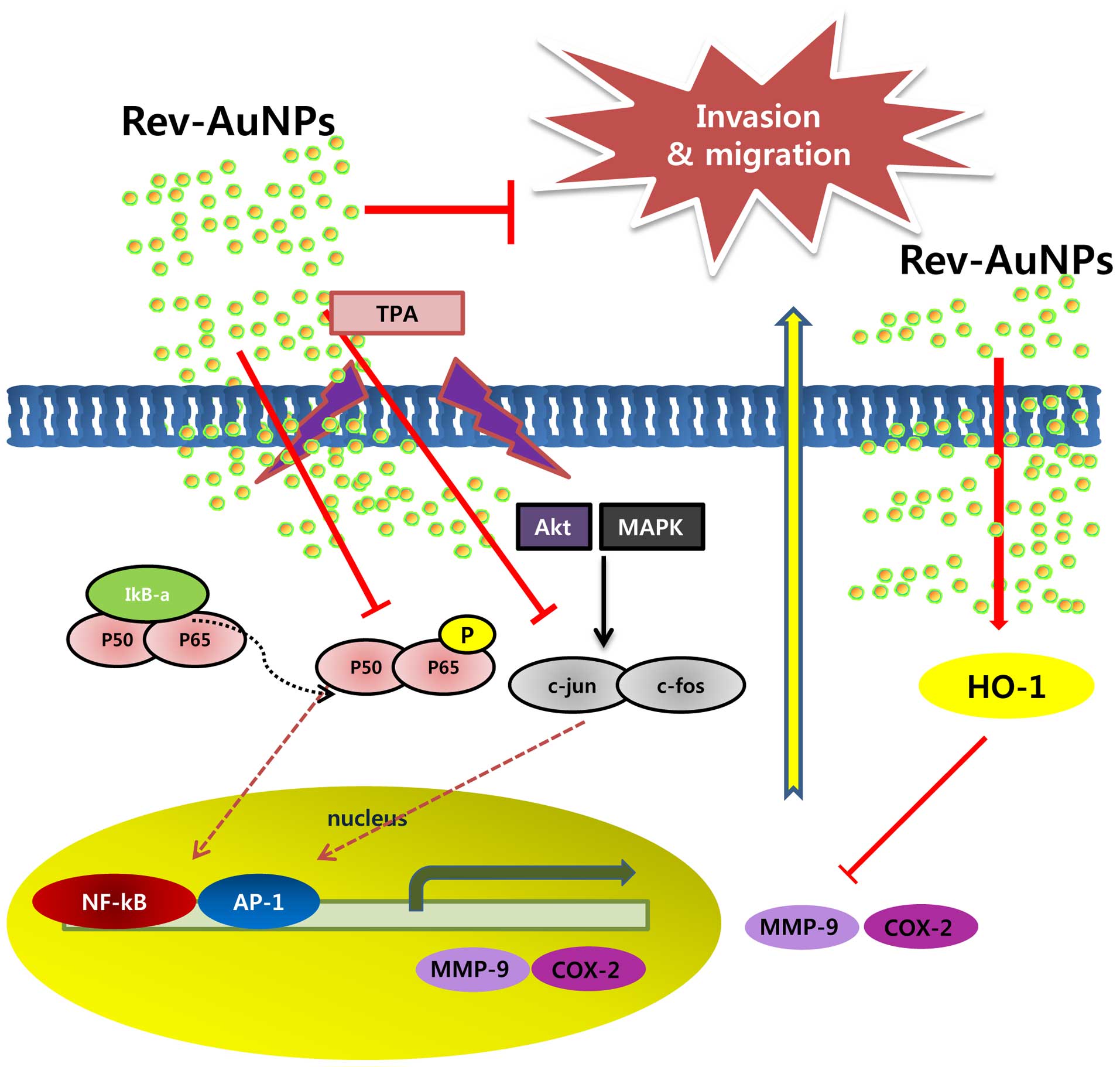
In conclusion, resveratrol was used as a reductant
for the green synthesis of Rev-AuNPs. The synthesized Rev-AuNPs
were completely characterized with respect to particle size, shape,
and surface charge by spectroscopic, light scattering and
microscopic techniques. We found that Rev-AuNPs effectively
inhibited breast cancer cell progression. This inhibition involved
many molecules, including MMP-9, COX-2, NF-κB, AP-1, PI3K/Akt and
ERK (Fig. 7). Although further
investigation is needed to clarify the precise mechanisms by which
Rev-AuNPs inhibit breast cancer progression and to identify the
Rev-AuNPs responsible for the observed effects, Rev-AuNPs may be
considered a potential therapeutic agent for the treatment of
breast cancer. As well, our results that focus on nanoscale
materials as new effective anticancer agents and clinical
utilization are demonstrated, concluding with considerations to
develop AuNPs for clinical applications.
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(2015R1D1A1A01059450). The present study was financially supported
by the Ministry of Trade, Industry and Energy (MOTIE) and Korea
Institute for Advancement of Technology (KIAT) through the
Promoting Regional Major Industry (R0004422).
References
|
1
|
Gnerlich JL, Deshpande AD, Jeffe DB,
Seelam S, Kimbuende E and Margenthaler JA: Poorer survival outcomes
for male breast cancer compared with female breast cancer may be
attributable to in-stage migration. Ann Surg Oncol. 18:1837–1844.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bertolini F: Adipose tissue and breast
cancer progression: A link between metabolism and cancer. Breast.
22(Suppl 2): S48–S49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song J, Su H, Zhou YY and Guo LL:
Prognostic value of matrix metalloproteinase 9 expression in breast
cancer patients: A meta-analysis. Asian Pac J Cancer Prev.
14:1615–1621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang S and Han H: Effect of
cycloxygenase-2 silencing on the malignant biological behavior of
MCF-7 breast cancer cells. Oncol Lett. 8:1628–1634. 2014.PubMed/NCBI
|
|
5
|
Jin ML, Park SY, Kim YH, Park G and Lee
SJ: Halofuginone induces the apoptosis of breast cancer cells and
inhibits migration via downregulation of matrix
metalloproteinase-9. Int J Oncol. 44:309–318. 2014.
|
|
6
|
Liao YF, Rao YK and Tzeng YM: Aqueous
extract of Anisomeles indica and its purified compound exerts
anti-metastatic activity through inhibition of NF-κB/AP-1-dependent
MMP-9 activation in human breast cancer MCF-7 cells. Food Chem
Toxicol. 50:2930–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim YS, Sull JW and Sung HJ: Suppressing
effect of resveratrol on the migration and invasion of human
metastatic lung and cervical cancer cells. Mol Biol Rep.
39:8709–8716. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakraborty A, Gupta N, Ghosh K and Roy P:
In vitro evaluation of the cytotoxic, anti-proliferative and
anti-oxidant properties of pterostilbene isolated from Pterocarpus
marsupium. Toxicol In Vitro. 24:1215–1228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harikumar KB, Kunnumakkara AB, Sethi G,
Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S
and Aggarwal BB: Resveratrol, a multitargeted agent, can enhance
antitumor activity of gemcitabine in vitro and in orthotopic mouse
model of human pancreatic cancer. Int J Cancer. 127:257–268.
2010.
|
|
10
|
Amri A, Chaumeil JC, Sfar S and Charrueau
C: Administration of resveratrol: What formulation solutions to
bioavailability limitations? J Control Release. 158:182–193. 2012.
View Article : Google Scholar
|
|
11
|
Pangeni R, Sahni JK, Ali J, Sharma S and
Baboota S: Resveratrol: Review on therapeutic potential and recent
advances in drug delivery. Expert Opin Drug Deliv. 11:1285–1298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nicol JR, Dixon D and Coulter JA: Gold
nanoparticle surface functionalization: A necessary requirement in
the development of novel nanotherapeutics. Nanomedicine.
10:1315–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park H, Tsutsumi H and Mihara H: Cell
penetration and cell-selective drug delivery using α-helix peptides
conjugated with gold nanoparticles. Biomaterials. 34:4872–4879.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Karuppaiya P, Satheeshkumar E, Chao WT,
Kao LY, Chen EC and Tsay HS: Anti-metastatic activity of
biologically synthesized gold nanoparticles on human fibrosarcoma
cell line HT-1080. Colloids Surf B Biointerfaces. 110:163–170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suganya KS, Govindaraju K, Kumar VG, Dhas
TS, Karthick V, Singaravelu G and Elanchezhiyan M: Blue green alga
mediated synthesis of gold nanoparticles and its antibacterial
efficacy against Gram positive organisms. Mater Sci Eng C.
47:351–356. 2015. View Article : Google Scholar
|
|
16
|
Sanna V, Pala N, Dessì G, Manconi P,
Mariani A, Dedola S, Rassu M, Crosio C, Iaccarino C and Sechi M:
Single-step green synthesis and characterization of gold-conjugated
polyphenol nanoparticles with antioxidant and biological
activities. Int J Nanomedicine. 9:4935–4951. 2014.PubMed/NCBI
|
|
17
|
Kondath S, Srinivas Raghavan B,
Anantanarayanan R and Rajaram R: Synthesis and characterisation of
morin reduced gold nanoparticles and its cytotoxicity in MCF-7
cells. Chem Biol Interact. 224C:78–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barathmanikanth S, Kalishwaralal K, Sriram
M, Pandian SR, Youn HS, Eom S and Gurunathan S: Anti-oxidant effect
of gold nanoparticles restrains hyperglycemic conditions in
diabetic mice. J Nanobiotechnology. 8:162010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan HA, Abdelhalim MA, Alhomida AS and Al
Ayed MS: Transient increase in IL-1β, IL-6 and TNF-α gene
expression in rat liver exposed to gold nanoparticles. Genet Mol
Res. 12:5851–5857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kumar CG, Poornachandra Y and Mamidyala
SK: Green synthesis of bacterial gold nanoparticles conjugated to
resveratrol as delivery vehicles. Colloids Surf B Biointerfaces.
123:311–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chao CY, Lii CK, Hsu YT, Lu CY, Liu KL, Li
CC and Chen HW: Induction of heme oxygenase-1 and inhibition of
TPA-induced matrix metalloproteinase-9 expression by
andrographolide in MCF-7 human breast cancer cells. Carcinogenesis.
34:1843–1851. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee CW, Yen FL, Huang HW, Wu TH, Ko HH,
Tzeng WS and Lin CC: Resveratrol nanoparticle system improves
dissolution properties and enhances the hepatoprotective effect of
resveratrol through antioxidant and anti-inflammatory pathways. J
Agric Food Chem. 60:4662–4671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Wang X, Wang C, Chen B, Dai Y, Zhang
R, Song M, Lv G and Fu D: The enhancement effect of gold
nanoparticles in drug delivery and as biomarkers of drug-resistant
cancer cells. ChemMedChem. 2:374–378. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Correard F, Maximova K, Estève MA, Villard
C, Roy M, Al-Kattan A, Sentis M, Gingras M, Kabashin AV and Braguer
D: Gold nanoparticles prepared by laser ablation in aqueous
biocompatible solutions: Assessment of safety and biological
identity for nanomedicine applications. Int J Nanomedicine.
9:5415–5430. 2014.PubMed/NCBI
|
|
25
|
Lee WY, Chen YC, Shih CM, Lin CM, Cheng
CH, Chen KC and Lin CW: The induction of heme oxygenase-1
suppresses heat shock protein 90 and the proliferation of human
breast cancer cells through its byproduct carbon monoxide. Toxicol
Appl Pharmacol. 274:55–62. 2014. View Article : Google Scholar
|
|
26
|
Lin CW, Shen SC, Hou WC, Yang LY and Chen
YC: Heme oxygenase-1 inhibits breast cancer invasion via
suppressing the expression of matrix metalloproteinase-9. Mol
Cancer Ther. 7:1195–1206. 2008. View Article : Google Scholar : PubMed/NCBI
|