Introduction
Gastric cancer is the fourth most common cancer and
the second leading cause of cancer-related mortality worldwide
(1). The etiology of gastric cancer
is multifactorial and includes Helicobacter pylori (HP)
infection, smoking tobacco and dietary habits (2). However, vitamin C and some carotenoids
have been inversely associated with risk of gastric cancer
(3). Catechins, for example, are
major components of polyphenol in green tea, one of the most widely
consumed beverages in Asia. Green tea catechins (GTCs) in
particular possess various physiological effects such as
antibacterial, anti-oxidative and anti-carcinogenic activities
(4–6). However, epidemiological evidence of
any anti-carcinogenic effects related to green tea consumption is
still controversial. Although several case-control studies have
reported that green tea consumption decreases the risk of gastric
cancer (7–9), no inverse relationship between green
tea consumption and the risk of gastric cancer has been observed in
cohort studies (10–12).
The most abundant physiologically active constituent
of GTCs is (–)-epigallocatechin-3-gallate (EGCG). The
anti-carcinogenic effects of EGCG have been extensively studied in
many experimental systems, including the skin, lung, colon and
prostate (13–16). This component appears to suppress
cancer cell proliferation by inhibiting nuclear factor-κB,
activator protein-1 and the epidermal growth factor receptor signal
transduction pathway (17–19). Yamane et al (20) reported that EGCG suppresses cellular
kinetics of the gastric mucosa in rat models of
N-methyl-N′-nitro-nitrosoguanidine (MNNG)-induced gastric cancer.
Although the oral administration of EGCG in rodent models has shown
an inhibitory effect on chemically-induced gastric tumorigenesis
(20,21), the molecular mechanism of protection
has not been elucidated. Intestinal type gastric cancer occurs at
later stages and undergoes relatively well-defined histological
steps, including chronic atrophic gastritis, intestinal metaplasia
and dysplasia (22).
Hypergastrinemic insulin-gastrin (INS-GAS) mice mimic these human
gastric carcinogenic sequences (23,24).
Although infection with HP accelerates gastric carcinogenesis in
INS-GAS mice, uninfected male mice develop gastric cancer at around
20 months old regardless of the infection status (23). In the present study, we evaluated
the effect of GTC supplementation on gastric carcinogenesis in
INS-GAS mice in terms of gastric mucosal dysplasia.
Materials and methods
Materials
Polyphenon 60S® was purchased from Mitsui
Norin Co., Ltd., (Shizuoka, Japan) and contained 60.3% catechins,
including EGCG (27.2%), epicatechin gallate (7.7%),
epigallocatechin (15.2%), epicatechin (6.8%), gallocatechin gallate
(2.9%) and catechin gallate (0.5%). The mixture was dissolved in
distilled water at a concentration of 2,000 ppm.
Animals and the study design
Thirty-eight male INS-GAS mice from an FVB/N
background were used in the present study. The INS-GAS mice were
supplied by the Massachusetts Institute of Technology (Boston, MA,
USA) and bred at the Division of Laboratory Animal Resources at the
University of Fukui. Animals were housed on hardwood bedding in a
micro-isolator with solid-bottom polycarbonate cages and maintained
at a constant temperature with a 12-h light/dark cycle. Mice were
fed a regular chow diet (CLEA rodent diet CE-2; CLEA Japan, Inc.,
Tokyo, Japan) and provided distilled water ad libitum. At
the age of 9 weeks, mice were divided into four groups: i) group 1,
mice were supplemented with the GTC solution for 4 weeks (n=8); ii)
group 2, the controls were given distilled water for 4 weeks (n=8);
iii) group 3, mice were supplemented with the GTC solution for 28
weeks (n=13); and iv) group 4, the controls were given distilled
water for 28 weeks (n=9). All mice in groups 1 and 3 were given the
GTC solution (2,000 ppm) instead of distilled water. The GTC
solution and water were replaced weekly. There was no difference in
the food consumption between the GTC-treated group and the control
group in our preliminary experiment. After 4 or 28 weeks of GTC
supplementation, at the age of 13 or 37 weeks, the mice were
sacrificed by CO2 inhalation. Necropsy was performed
under non-fasting conditions, and gastric tissue and serum samples
were collected. All protocols were approved by the University of
Fukui's Committee on Animal Care (permit no. 22047).
Histopathology and
immunohistochemistry
During necropsy, linear strips of the stomach
extending from the squamocolumnar junction through the proximal
duodenum were fixed in 10% neutral buffered formalin overnight,
processed routinely, paraffin-embedded, cut into 2-µm
sections, and stained with hematoxylin and eosin. Histological
evaluation for inflammation and dysplasia were graded on an
ascending scale from 0–4 by a pathologist blinded to sample
identity using previously outlined criteria (25). To evaluate cell proliferation of the
gastric mucosa, 4 samples selected randomly from groups 3 and 4
were stained using Ki-67 immunohistochemistry. The slides were
deparaffinized and hydrated in xylene and graded ethanol to water.
The slides were incubated for 30 min with purified mouse anti-human
Ki-67 monoclonal antibody (material no. 550609, dilution 1:50; BD
Biosciences, San Diego, CA, USA) using the Vector M.O.M kit ImPRESS
Peroxidase Polymer (Vector Laboratories, Inc., Burlingame, CA, USA)
according to the manufacturer's instructions. After washing, the
slides were incubated with diaminobenzidine for 5 min and
counterstained with hematoxylin, dehydrated and mounted. The Ki-67
staining was evaluated by counting the number of positive nuclei
per single gland, and the labeling index was expressed as the mean
number of positively stained nuclei from 10 well-oriented glands as
previously described (26).
Real-time reverse transcription
polymerase chain reaction assay of cytokines
Total RNA was extracted from the corpus of the
stomach using TRI reagent (Sigma-Aldrich, St. Louis, MO, USA),
according to the manufacturer's instructions. Total RNA (2
µg) was converted into complement (c)-DNA using a
High-Capacity cDNA Archive kit (Applied Biosystems, Foster City,
CA, USA), according to the manufacturer's protocol. Real-time
quantitative polymerase chain reaction (PCR) was performed using
StepOne Plus (Life Technologies, Carlsbad, CA, USA) under the
following conditions: 50°C for 2 min, 95°C for 10 min followed by
45 cycles at 95°C for 15 sec and 60°C for 1 min. The cDNA was
amplified using the Real-Time PCR Master Mix (Toyobo, Osaka,
Japan). The commercially available primer and probe mixes used in
these experiments included interferon (IFN)-γ, tumor necrosis
factor (TNF)-α, interleukin (IL)-1β, and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) (Applied Biosystems). The PCR reaction was
performed in duplicate, and the data were analyzed using a
comparative cycle threshold method (27). The relative expression of the target
gene was normalized to GAPDH and expressed as the fold change
compared to an average value of the controls that were given
distilled water for 4 weeks (group 2).
Serum gastrin levels
The blood samples at necropsy were centrifuged and
stored at −20°C. Serum gastrin levels in groups 3 and 4 were
measured using a radioimmunoassay (BML Corp., Tokyo, Japan).
Statistical analysis
Non-parametric data, including two histological
parameters and gene expression assays, were analyzed using
Mann-Whitney U test. Parametric data, including body weight, the
serum gastrin level, and Ki-67 labeling were analyzed using
Student's t-test. Statistical computing was performed using Prism 6
software for Windows (Graphpad Software, Inc., San Diego, CA, USA).
Data are shown as mean ± standard error of mean. Statistical
significance was defined as a P-value <0.05.
Results
Administration of GTCs decreased body
weights and serum gastrin levels
We compared body weights between the
GTC-administered group and the control group to confirm that GTCs
were reliably administered at a sufficient dosage. In groups 1 and
2, for which the duration of administration was 4 weeks, the body
weight was significantly lower in the group receiving GTCs
(29.8±1.1 g) than in the control group (34.7±0.7 g). Similarly, in
groups 3 and 4, for which the duration of administration was 28
weeks, the body weight was significantly lower in the group
receiving GTCs (33.7±0.8 g) than in the control group (40.3±0.7 g)
(Table I). Furthermore, when the
serum gastrin levels were compared between the 28-week
GTC-administered group and control groups, the level of gastrin
(863±65.6 pg/ml) was significantly lower in the GTC-administered
group than in the control group (1,912±507.4 pg/ml) (P≤0.05). The
low gastrin level in the GTC-administered group during non-fasting
states indicated that the gastrin level was continuously suppressed
by the administration of GTCs.
 | Table IBody weights (g) after
supplementation of GTCs for 4 and 28 weeks. |
Table I
Body weights (g) after
supplementation of GTCs for 4 and 28 weeks.
| Group | Duration of GTCs
administration
|
|---|
| 4 weeks | 28 weeks |
|---|
| Control | 34.7±0.7 | 40.3±0.7 |
| GTCs | 29.8±1.1a | 33.7±0.8a |
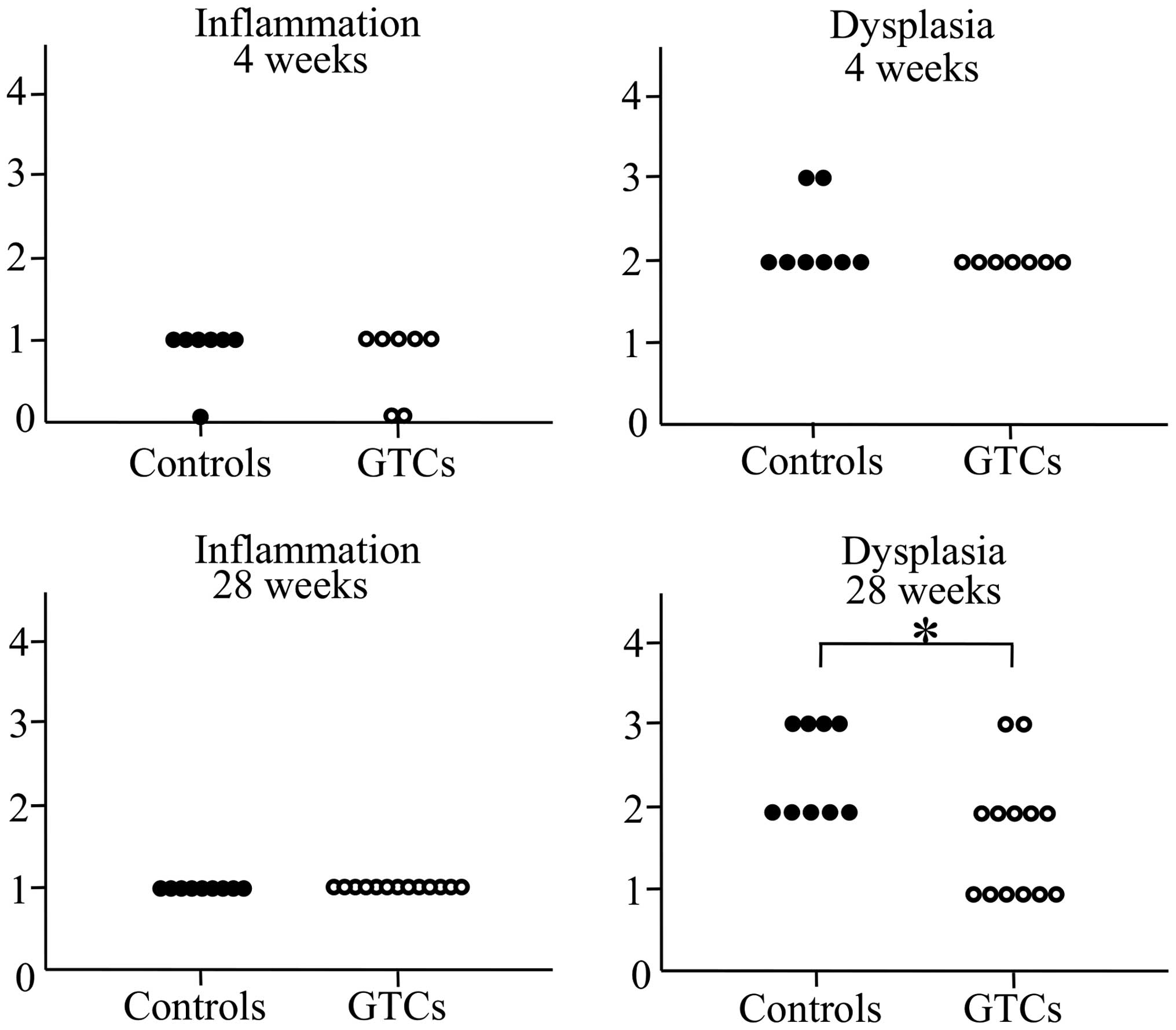
Administration of GTCs attenuated gastric
dysplasia
To evaluate the effects of GTC administration on
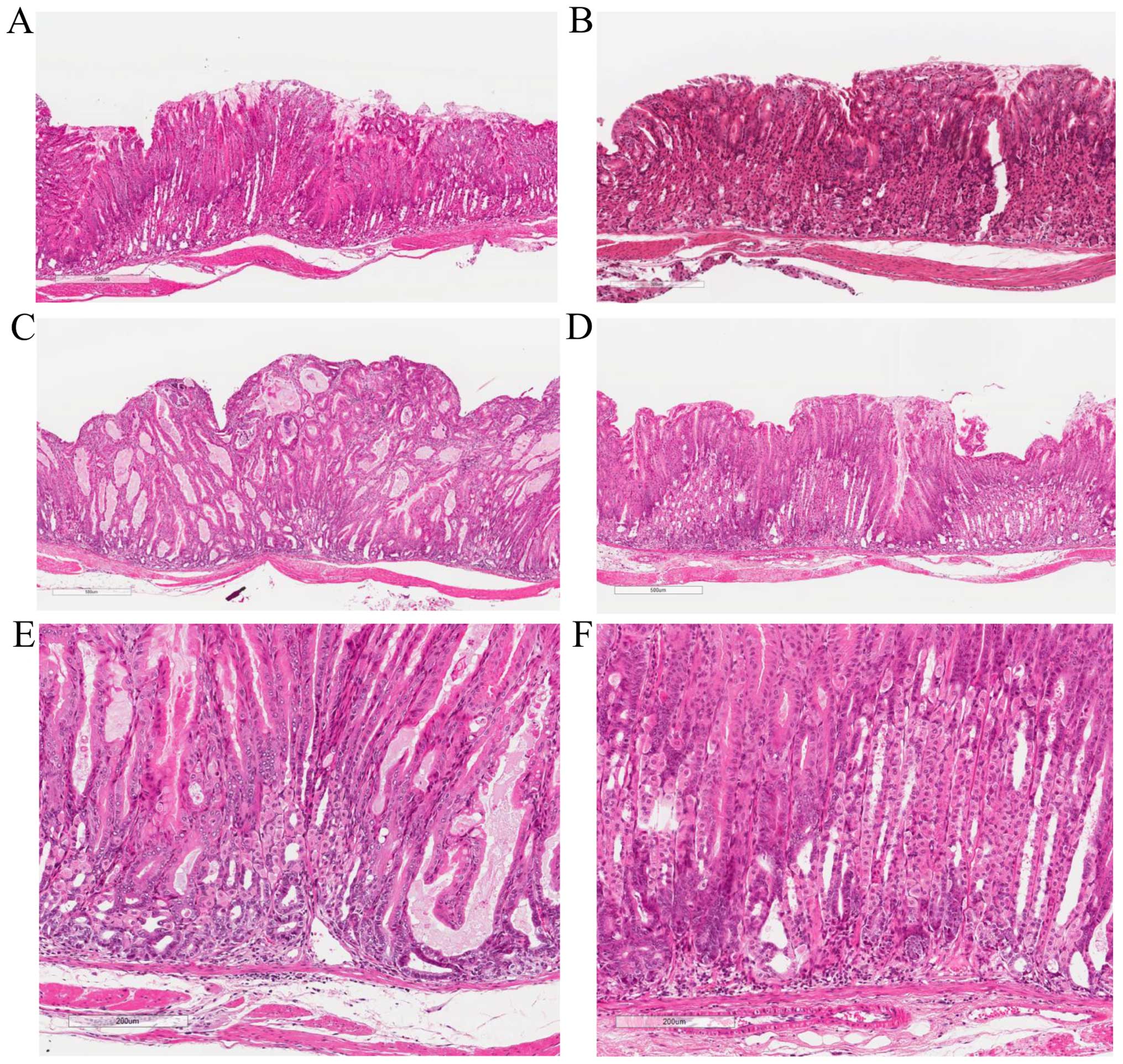
gastritis, we performed histopathological examinations of the
gastric mucosa. The mice developed minimal to mild corpus
gastritis. Similar to the results of previous studies using INS-GAS
mice (23,24,26),
atrophy of the gastric fundic gland, hyperplasia of the glandular
epithelium, and intestinal epithelium metaplasia and dysplasia were
observed (Fig. 1). No differences
in inflammation scores were observed between the GTC-administered
group and the control group in either the 4-week or the 28-week
administration clusters. In the 4-week groups, no differences in
the dysplasia scores were observed between the GTC-administered
group and the control group. However, the dysplasia score was
significantly lower in the 28-week GTC-administered group than in
the 28-week control group (Fig. 2).
These results suggested that although INS-GAS mice inevitably
slowly develop gastric cancer from gastritis regardless of the
intervention, the progression of gastric mucosal dysplasia was
inhibited by long-term GTC administration.
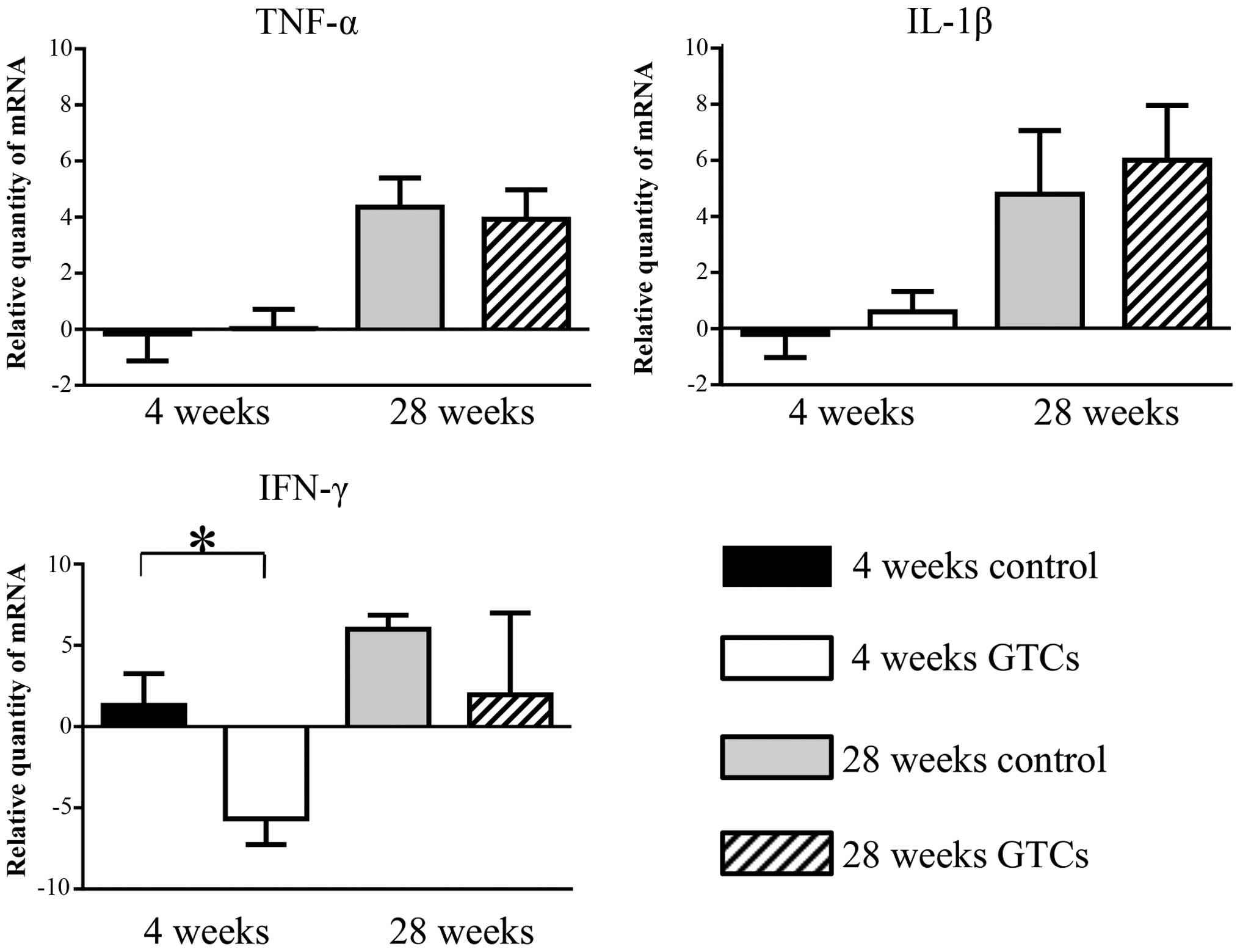
GTCs modulated IFN-γ expression in the
gastric mucosa
To molecularly evaluate the mechanism of changes in
dysplasia, we analyzed messenger (m)-RNA expression of inflammatory
cytokines such as IFN-γ, TNF-α and IL-1β in the gastric tissue
(Fig. 3). In the 4-week
administration cluster, mRNA levels of IFN-γ were significantly
lower in the GTC-administered group than in the control group
(P≤0.05). In addition, although not significantly different, the
28-week mRNA levels of IFN-γ were also lower in the
GTC-administered group than in the control group (P=0.056). We also
analyzed the mRNA expression of TNF-α and IL-1β, but no significant
differences were observed between the GTC-administered group and
the control group in either the 4-week or 28-week administration
clusters. These results suggested that the expression of IFN-γ in
the gastric mucosa was inhibited by GTC administration, but GTC
administration had little or no effect on the expression of other
inflammatory cytokines such as TNF-α and IL-1β.
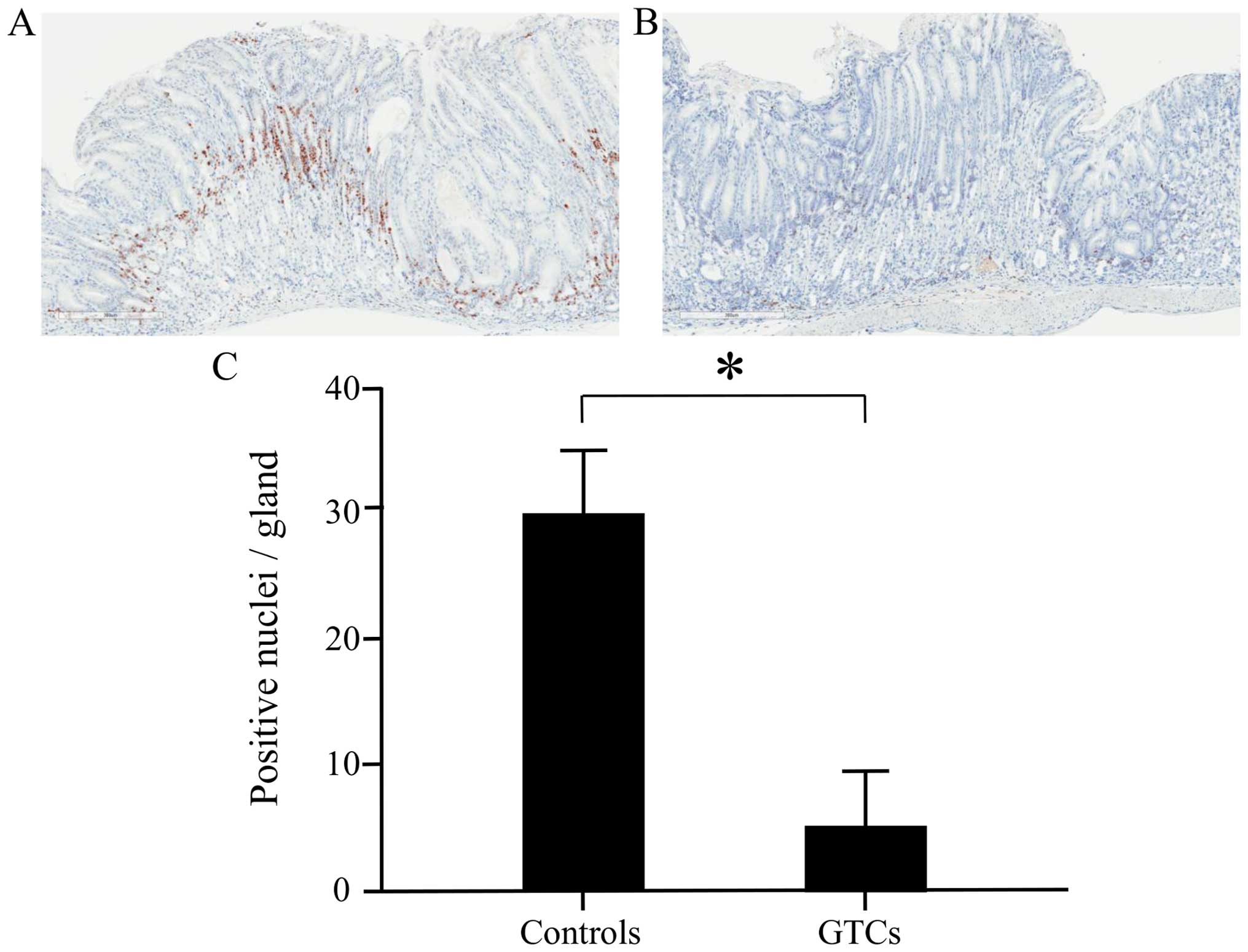
Administration of GTCs suppressed
epithelial cell proliferation in the gastric mucosa
To evaluate the effect of GTC administration on cell
proliferation in the gastric mucosal epithelium, analysis was
performed in the 28-week administration cluster using the Ki-67
immunohistochemical staining labeling index. We observed Ki-67
positive nuclei from the glandular cervical region to the crypt
epithelium (Fig. 4A and B). The
labeling index of Ki-67 was significantly lower in the
GTC-administered group than in the control group (Fig. 4C). This result suggested that cell
growth in the gastric mucosal epithelium was inhibited by GTC
administration.
Discussion
In the present study, we analyzed the preventive
effects of GTC administration on gastric cancer using a mouse
model. INS-GAS mice exhibit high gastrin levels and gastric fundic
gland atrophy, and develop gastric cancer after approximately 20
months in their natural life cycle (23). In the present study, we observed a
significant reduction in body weight due to GTC administration at 4
and 28 weeks, and suppressed gastric mucosa dysplasia progression
after 28 weeks, suggesting a tumor-inhibitory effect from GTCs.
Furthermore, GTC administration also appeared to suppress mRNA
expression of IFN-γ in the gastric mucosa and proliferation of
gastric mucosal epithelial cells.
GTCs have been reported to show various
physiological activities such as antibacterial, antitumor and
anti-inflammatory actions (4–6). In
the present study, the GTC-administered groups had a significant
reduction in body weight compared to the control groups, which is
in agreement with previous reports on abdominal cavity fat and
overall weight reduction following green tea powder administration
in mice (28). The suggested
mechanisms involve GTC-suppressed fatty acid synthase activity in
the liver, increased activity of the enzymes involved in lipolysis
(29), and increased expression of
lipolytic enzymes in adipocytes (30). In addition, the catechin intake and
exercise have been reported to have inhibitory effects on the
increase in mouse body weight (31). In the present study, GTCs
administered via drinking water showed sufficient physiological
activity to result in body weight reduction.
In the 28-week administration cluster, the serum
gastrin level was significantly lower in the GTC group than in the
control group. Gastric fundic gland atrophy and the reduction of
the gastric acid secretion capacity are seen in INS-GAS mice as
early as 6 months after birth in their natural life cycle, and
further increases in the gastrin levels are inevitably observed as
time progresses (23). Sato et
al (32) reported that GTC
administration reduces the serum gastrin level and protects against
stress-initiated acute gastric mucosal damage in water-immersion
mouse models. Furthermore, gastrin and histamine receptor
antagonists reportedly suppress the progression of gastric mucosal
atrophy due to chronic gastritis in Helicobacter
felis-infected INS-GAS mice, ultimately resulting in the
suppression of gastric carcinogenesis (27). The mechanism by which GTCs reduce
gastrin levels in INS-GAS mice is not fully understood, but it is
thought that the reduction inhibits the progression of dysplasia in
the gastric mucosa. The present study supported this hypothesis, as
dysplasia of the gastric mucosa was indeed significantly suppressed
in the 28-week GTC-administered group compared to the control
group.
The GTC-administered group also had reduced gastric
mucosal IFN-γ expression, suggesting that IFN-γ is involved in the
suppression of dysplasia. An HP infection is a class I gastric
carcinogen; the infection causes chronic gastritis, which
progresses into atrophic gastritis, intestinal metaplasia, and
dysplasia and ultimately leads to carcinogenesis (22). Increased expressions of inflammatory
cytokines such as IFN-γ and IL-1β are seen in HP-infected gastric
mucosa, rein forcing their important role in inflammatory
carcinogenesis (33,34). Furthermore, IFN-γ knockout mice fail
to develop HP-infected gastritis or gastric mucosa atrophy
(35). In this study, gastric
mucosal IFN-γ expression was suppressed by GTC administration at 4
weeks, and although the difference did not remain significant at 28
weeks, a reduction was still noticeable. To date, GTC
administration has been reported to inhibit the local production of
inflammatory cytokines such as IFN-γ and TNF-α in mouse models with
dextran sulfate sodium-induced colitis, autoimmune hepatitis and
arthritis (36–38). In addition, it is reported that GTC
decrease IL-1β expression in melanoma cells (39). The inhibitory effect of GTC on mRNA
expression of IL-1β and TNF-α was not observed in this study. Host
factor may be associated with the differences in susceptibility to
GTC.
In the present study, the inhibition of cell
proliferation of the gastric mucosal epithelium was observed in the
GTC-administered groups, similar to the GTC-induced growth
inhibitory effects of gastric mucosal epithelial cells in an
MNNG-induced gastric carcinogenesis model. Many studies have thus
far reported cytostatic effects of GTC in various cancer cell lines
such as lung, breast and colorectal cancer (40–42),
which are thought to involve TNF-α release inhibition and an
inhibitory effect on DNA methylation. Some mechanisms have been
proposed for the effect of GTCs on cell proliferation. GTC induces
apoptosis, cell cycle arrest and modulation intracellular cell
signaling. EGCG trigger cell growth arrest pathway at G1 stage
cycle through regulation of cyclin D1, cdk4, cdk6, p21/WAF1/CIP1
and p27/KIP1 and induced apoptosis through caspase-3 and caspase-9
activation (43). Furthermore, cell
growth inhibition and apoptosis induction by EGCG have been
identified in gastric carcinoma cell lines as well. These
mechanisms involve Id1 and EGCG-regulated surviving expression, but
the specifics of these mechanisms have not yet been elucidated
(44,45).
We reported the potential preventive effects of GTCs
on gastric carcinogenesis in INS-GAS mice via the suppression of
IFN-γ expression and gastric mucosal epithelial cell growth
inhibition. In addition, GTCs possess anti-oxidant and
anti-vascularity effects, which should be equally closely examined
in future studies. Understanding the protective mechanisms of GTCs
against gastric carcinogenesis at a molecular level may lead to new
therapeutic and preventative interventions for treating gastric
cancer in humans.
Acknowledgments
The INS-GAS mice were supplied by Professor James G.
Fox (Massachusetts Institute of Technology). We would like to thank
Editage (www.editage.jp) for English language
editing. The present study was supported by the JSPS Grant-in-Aid
for Scientific Research (C) (grant no. 23510346).
References
|
1
|
Ferro A, Peleteiro B, Malvezzi M, Bosetti
C, Bertuccio P, Levi F, Negri E, La Vecchia C and Lunet N:
Worldwide trends in gastric cancer mortality (1980–2011), with
predictions to 2015, and incidence by subtype. Eur J Cancer.
50:1330–1344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gonzalez CA and Riboli E: Diet and cancer
prevention: Contributions from the European Prospective
Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer.
46:2555–2562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shiota S, Shimizu M, Mizushima T, Ito H,
Hatano T, Yoshida T and Tsuchiya T: Marked reduction in the minimum
inhibitory concentration (MIC) of beta-lactams in
methicillin-resistant Staphylococcus aureus produced by epicatechin
gallate, an ingredient of green tea (Camellia sinensis). Biol Pharm
Bull. 22:1388–1390. 1999. View Article : Google Scholar
|
|
5
|
El-Beshbishy HA: Hepatoprotective effect
of green tea (Camellia sinensis) extract against tamoxifen-induced
liver injury in rats. J Biochem Mol Biol. 38:563–570. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng CW, Shieh PC, Lin YC, Chen YJ, Lin
YH, Kuo DH, Liu JY, Kao JY, Kao MC and Way TD: Indoleamine
2,3-dioxy-genase, an immunomodulatory protein, is suppressed by
(–)-epigallocatechin-3-gallate via blocking of
gamma-interferon-induced JAK-PKC-delta-STAT1 signaling in human
oral cancer cells. J Agric Food Chem. 58:887–894. 2010. View Article : Google Scholar
|
|
7
|
Kono S, Ikeda M, Tokudome S and Kuratsune
M: A case-control study of gastric cancer and diet in northern
Kyushu, Japan. Jpn J Cancer Res. 79:1067–1074. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji BT, Chow WH, Yang G, McLaughlin JK, Gao
RN, Zheng W, Shu XO, Jin F, Fraumeni JF Jr and Gao YT: The
influence of cigarette smoking, alcohol, and green tea consumption
on the risk of carcinoma of the cardia and distal stomach in
Shanghai, China. Cancer. 77:2449–2457. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inoue M, Tajima K, Hirose K, Hamajima N,
Takezaki T, Kuroishi T and Tominaga S: Tea and coffee consumption
and the risk of digestive tract cancers: Data from a comparative
case-referent study in Japan. Cancer Causes Control. 9:209–216.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsubono Y, Nishino Y, Komatsu S, Hsieh CC,
Kanemura S, Tsuji I, Nakatsuka H, Fukao A, Satoh H and Hisamichi S:
Green tea and the risk of gastric cancer in Japan. N Engl J Med.
344:632–636. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nagano J, Kono S, Preston DL and Mabuchi
K: A prospective study of green tea consumption and cancer
incidence, Hiroshima and Nagasaki (Japan). Cancer Causes Control.
12:501–508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoshiyama Y, Kawaguchi T, Miura Y, Mizoue
T, Tokui N, Yatsuya H, Sakata K, Kondo T, Kikuchi S, Toyoshima H,
et al Japan Collaborative Cohort Study Group: A prospective study
of stomach cancer death in relation to green tea consumption in
Japan. Br J Cancer. 87:309–313. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh T and Katiyar SK: Green tea
polyphenol, (–)-epigallo-catechin-3-gallate, induces toxicity in
human skin cancer cells by targeting β-catenin signaling. Toxicol
Appl Pharmacol. 273:418–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Bian S and Yang CS: Green tea
polyphenol EGCG suppresses lung cancer cell growth through
upregulating miR-210 expression caused by stabilizing HIF-1α.
Carcinogenesis. 32:1881–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baek SJ, Kim JS, Jackson FR, Eling TE,
McEntee MF and Lee SH: Epicatechin gallate-induced expression of
NAG-1 is associated with growth inhibition and apoptosis in colon
cancer cells. Carcinogenesis. 25:2425–2432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harper CE, Patel BB, Wang J, Eltoum IA and
Lamartiniere CA: Epigallocatechin-3-gallate suppresses early stage,
but not late stage prostate cancer in TRAMP mice: Mechanisms of
action. Prostate. 67:1576–1589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ahmad N, Gupta S and Mukhtar H: Green tea
polyphenol epigallocatechin-3-gallate differentially modulates
nuclear factor kappaB in cancer cells versus normal cells. Arch
Biochem Biophys. 376:338–346. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong Z, Ma W, Huang C and Yang CS:
Inhibition of tumor promoter-induced activator protein 1 activation
and cell transformation by tea polyphenols, (–)-epigallocatechin
gallate, and theaflavins. Cancer Res. 57:4414–4419. 1997.PubMed/NCBI
|
|
19
|
Adachi S, Shimizu M, Shirakami Y, Yamauchi
J, Natsume H, Matsushima-Nishiwaki R, To S, Weinstein IB, Moriwaki
H and Kozawa O: (–)-Epigallocatechin gallate downregulates EGF
receptor via phosphorylation at Ser1046/1047 by p38 MAPK in colon
cancer cells. Carcinogenesis. 30:1544–1552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamane T, Takahashi T, Kuwata K, Oya K,
Inagake M, Kitao Y, Suganuma M and Fujiki H: Inhibition of
N-methyl-N′-nitro-N-nitrosoguanidine-induced carcinogenesis by
(–)-epigallocatechin gallate in the rat glandular stomach. Cancer
Res. 55:2081–2084. 1995.PubMed/NCBI
|
|
21
|
Katiyar SK, Agarwal R, Zaim MT and Mukhtar
H: Protection against N-nitrosodiethylamine and
benzo[a]pyrene-induced forestomach and lung tumorigenesis in A/J
mice by green tea. Carcinogenesis. 14:849–855. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
23
|
Wang TC, Dangler CA, Chen D, Goldenring
JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ,
et al: Synergistic interaction between hypergastrinemia and
Helicobacter infection in a mouse model of gastric cancer.
Gastroenterology. 118:36–47. 2000. View Article : Google Scholar
|
|
24
|
Fox JG, Wang TC, Rogers AB, Poutahidis T,
Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, et al:
Host and microbial constituents influence Helicobacter
pylori-induced cancer in a murine model of hypergastrinemia.
Gastroenterology. 124:1879–1890. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rogers AB, Taylor NS, Whary MT, Stefanich
ED, Wang TC and Fox JG: Helicobacter pylori but not high salt
induces gastric intraepithelial neoplasia in B6129 mice. Cancer
Res. 65:10709–10715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohtani M, García A, Rogers AB, Ge Z,
Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC, et al:
Protective role of 17 beta -estradiol against the development of
Helicobacter pylori-induced gastric cancer in INS-GAS mice.
Carcinogenesis. 28:2597–2604. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takaishi S, Cui G, Frederick DM, Carlson
JE, Houghton J, Varro A, Dockray GJ, Ge Z, Whary MT, Rogers AB, et
al: Synergistic inhibitory effects of gastrin and histamine
receptor antagonists on Helicobacter-induced gastric cancer.
Gastroenterology. 128:1965–1983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sayama K, Lin S, Zheng G and Oguni I:
Effects of green tea on growth, food utilization and lipid
metabolism in mice. In Vivo. 14:481–484. 2000.PubMed/NCBI
|
|
29
|
Sugiura C, Nishimatsu S, Moriyama T, Ozasa
S, Kawada T and Sayama K: Catechins and caffeine inhibit fat
accumulation in mice through the improvement of hepatic lipid
metabolism. J Obes. 2012:5205102012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MS, Kim CT, Kim IH and Kim Y:
Inhibitory effects of green tea catechin on the lipid accumulation
in 3T3-L1 adipocytes. Phytother Res. 23:1088–1091. 2009. View Article : Google Scholar
|
|
31
|
Murase T, Haramizu S, Shimotoyodome A and
Tokimitsu I: Reduction of diet-induced obesity by a combination of
tea-catechin intake and regular swimming. Int J Obes. 30:561–568.
2006. View Article : Google Scholar
|
|
32
|
Sato H, Matsui T and Arakawa Y: The
protective effect of catechin on gastric mucosal lesions in rats,
and its hormonal mechanisms. J Gastroenterol. 37:106–111. 2002.
View Article : Google Scholar
|
|
33
|
Sayi A, Kohler E, Hitzler I, Arnold I,
Schwendener R, Rehrauer H and Müller A: The CD4+ T
cell-mediated IFN-gamma response to Helicobacter infection is
essential for clearance and determines gastric cancer risk. J
Immunol. 182:7085–7101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Uedo N, Tatsuta M, Iishi H, Baba M, Yano
H, Ishihara R, Higashino K and Ishiguro S: Enhancement by
interleukin-1 beta of gastric carcinogenesis induced by
N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats: A possible
mechanism for Helicobacter pylori-associated gastric
carcinogenesis. Cancer Lett. 198:161–168. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Smythies LE, Waites KB, Lindsey JR, Harris
PR, Ghiara P and Smith PD: Helicobacter pylori-induced mucosal
inflammation is Th1 mediated and exacerbated in IL-4, but not
IFN-gamma, gene-deficient mice. J Immunol. 165:1022–1029. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shirakami Y, Shimizu M, Tsurumi H, Hara Y,
Tanaka T and Moriwaki H: EGCG and polyphenon E attenuate
inflammation-related mouse colon carcinogenesis induced by AOM plus
DDS. Mol Med Rep. 1:355–361. 2008.PubMed/NCBI
|
|
37
|
Haqqi TM, Anthony DD, Gupta S, Ahmad N,
Lee MS, Kumar GK and Mukhtar H: Prevention of collagen-induced
arthritis in mice by a polyphenolic fraction from green tea. Proc
Natl Acad Sci USA. 96:4524–4529. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Mei Y, Feng D and Xu L:
(–)-Epigallocatechin-3-gallate protects mice from concanavalin
A-induced hepatitis through suppressing immune-mediated liver
injury. Clin Exp Immunol. 145:485–492. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ellis LZ, Liu W, Luo Y, Okamoto M, Qu D,
Dunn JH and Fujita M: Green tea polyphenol
epigallocatechin-3-gallate suppresses melanoma growth by inhibiting
inflammasome and IL-1β secretion. Biochem Biophys Res Commun.
414:551–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujiki H, Suganuma M, Okabe S, Sueoka N,
Komori A, Sueoka E, Kozu T, Tada Y, Suga K, Imai K, et al: Cancer
inhibition by green tea. Mutat Res. 402:307–310. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deguchi H, Fujii T, Nakagawa S, Koga T and
Shirouzu K: Analysis of cell growth inhibitory effects of catechin
through MAPK in human breast cancer cell line T47D. Int J Oncol.
21:1301–1305. 2002.PubMed/NCBI
|
|
42
|
Moseley VR, Morris J, Knackstedt RW and
Wargovich MJ: Green tea polyphenol epigallocatechin 3-gallate,
contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human
colon cancer cells. Anticancer Res. 33:5325–5333. 2013.PubMed/NCBI
|
|
43
|
Shankar S, Suthakar G and Srivastava RK:
Epigallocatechin-3-gallate inhibits cell cycle and induces
apoptosis in pancreatic cancer. Front Biosci. 12:5039–5051. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma J, Shi M, Li G, Wang N, Wei J, Wang T,
Ma J and Wang Y: Regulation of Id1 expression by
epigallocatechin-3-gallate and its effect on the proliferation and
apoptosis of poorly differentiated AGS gastric cancer cells. Int J
Oncol. 43:1052–1058. 2013.PubMed/NCBI
|
|
45
|
Onoda C, Kuribayashi K, Nirasawa S, Tsuji
N, Tanaka M, Kobayashi D and Watanabe N:
(–)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer
cell lines by down-regulating survivin expression. Int J Oncol.
38:1403–1408. 2011.PubMed/NCBI
|