Introduction
Melanoma is an aggressive and highly metastatic
tumor with poor prognosis and an increasing incidence rate in
recent decades (1,2). The process of angiogenesis is a key
step essential for tumor development and metastasis, including
melanoma (3). Vascular endothelial
growth factor (VEGF), a major angiogenic factor for vascular
endothelial cells, is overexpressed in most human cancers (4,5).
Targeting VEGF signaling pathway is a rapidly growing chemotherapy
class for tumor anti-angiogenic therapy (6,7). Thus,
a better understanding of the regulation of VEGF by other factors
would provide more effective strategies for the development of new
anti-angiogenic therapy.
Tumor hypoxia is a common feature of the
microenvironment of many solid tumors (8,9). VEGF
expression is well known to be upregulated by hypoxia (10,11).
Hypoxia-inducible factor 1 (HIF-1) is responsible for the
transcriptional activation of the VEGF gene in cells exposed to
hypoxia by binding to a hypoxia response element of VEGF gene
promoter (12,13). Signal transducer and activator of
transcription-3 (STAT3) was originally described as latent
cytoplasmic transcription factor in response to extracellular
signaling proteins (14,15). STAT3 is activated by phosphorylation
in response to various cytokines and growth factors and is
constitutively activated with high frequency in diverse human
cancer cell lines and tissues (16). STAT3 has also been shown to be a
transcriptional activator of the VEGF gene by directly binding to
the VEGF promoter to upregulate its transcription (17). Enhancer of zeste homolog 2 (EZH2), a
catalytic subunit of the Polycomb repressive complex 2, catalyzes
the trimethylation of lysine 27 in histone 3 (H3K27me3) and
facilitates heterochromatin formation thereby silences gene
transcription (18,19). Interestingly, EZH2 can also
methylate non-histone proteins such as the transcription factor
nuclear receptor RORA, Talin-1 and STAT4 (20–22).
Moreover, EZH2 can directly bind to STAT3 protein and then mediate
its lysine methylation to enhance STAT3 activity (23).
Nuclear transcription factor-Y alpha (NF-YA) is a
transcription factor that specifically binds to CCAAT motifs of the
promoter regions in a variety of genes (24). By the binding to CCAAT motifs in the
promoter of genes, NF-YA can serve as either an activator or a
repressor in the transcriptional regulation of its target genes
(25). NF-YA target genes were
found to be involved in cell proliferation (26), DNA repair and cell death (27,28).
However, the role of NF-YA in invasion and angiogenesis of tumor
cells has not been reported.
Here we demonstrate that NF-YA is upregulated in
human melanoma cell lines with highly invasive potential.
Overexpression of NF-YA promotes cell invasion and angiogenesis of
human melanoma cells. Moreover, the expression and secretion of
VEGF can be upregulated by the overexpression of NF-YA gene in
melanoma cells. The activity of STAT3 is responsible for VEGF
upregulation (17). We also show
that NF-YA can induce the activity of STAT3 and NF-YA-induced
upregulation of VEGF can be inhibited by STAT3 inhibitor.
Furthermore, knock-down of NF-YA target gene EZH2 also attenuates
the angiogenesis induced by the overexpression of NF-YA. Our
results suggest that NF-YA contributes to tumor angiogenesis
through EZH2-STAT3 signaling in human melanoma cells, and that
NF-YA is therefore a potential therapeutic target for treatment of
human melanoma.
Materials and methods
Reagents
STAT3 inhibitor WP1066 (working concentration: 5
µM) was purchased from Selleck Chemicals (cat no. S2796;
Houston, TX, USA). The mouse anti-human monoclonal antibodies to
NF-YA (cat no. sc-17753; 1:1,000 dilution), STAT3 (cat no.
sc-293151; 1:1,000 dilution), EZH2 (cat no. sc-166609; 1:1,000
dilution) and β-actin (cat no. sc-8432; 1:2,000 dilution) were from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Mouse anti-human
monoclonal antibody to tyrosine phosphorylated STAT3 (pY-STAT3, at
the 705 residue; cat no. #9138; 1:1,000 dilution) was from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Mouse anti-human
monoclonal antibody to VEGF (cat no. ab155932; 1:1,000 dilution)
was from Abcam (Cambridge, MA, USA). Rabbit anti-methylated lysine
polyclonal antibody (Methy-K, cat no. ADI-KAP-TF121-E; 1:1,000
dilution) was from Enzo Life Sciences (Farmingdale, NY, USA).
Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG antibody
(cat no. ab6789) and goat anti-rabbit IgG antibody (cat no. ab6721)
were from Abcam. Protein A/G PLUS-Agarose was from Santa Cruz
Biotechnology, Inc. (cat no. sc-2003). Alexa-568-labeled goat
anti-mouse secondary antibodies (cat no. A-11031; Thermo Fisher
Scientific Inc. Kalamazoo, MI, USA). 4,6-diamidino-2-phenylindole
(DAPI) was from Sigma (cat no. D9542; St. Louis, MO, USA).
Cell culture
A375P, Malme-3M, A375-M2 and SK-MEL-28 human
melanoma cell lines were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). Human umbilical vein
endothelial cells (HUVECs) and HEK293T cell lines were from the
Institute of Cell and Biochemistry Research of the Chinese Academy
of Science (Shanghai, China). HEK293T, A375P, Malme-3M, A375-M2 and
SK-MEL-28 cells were cultured with Dulbecco's modified Eagle's
medium (DMEM; Hyclone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (Gibco, Invitrogen, Grand Island, NY, USA) in a
humidified atmosphere containing 5% CO2 at 37°C. HUVECs
were cultured in Kaighn's modified Ham's F-12K medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco, Invitrogen), 0.1 mg/ml heparin (American
Pharmaceutical Partners, Schaumburg, IL, USA), and 0.03–0.05 mg/ml
ECGS (BD Biosciences, Franklin Lakes, NJ, USA), at 37°C and 5%
humidified atmospheric CO2.
Plasmids
The human NF-YA (NM_002505) gene stably
overexpressing vector using lentiviral vector pCDH-CMV-MCS-EF1-Puro
(System Biosciences, Mountain View, CA, USA) was constructed by
Genesent Biotech (Shanghai, China). The shRNA lentiviral vector
pLKO.1 was from Open Biosystems (Huntsville, AL, USA). Lentivirus
vectors pLKO. 1 containing short hairpins against NF-YA or EZH2 or
scrambled shRNA controls were purchased from Sigma. The target
sequences of the two genes were used as follows: NF-YA,
AGTCCAAGGGCAGCCATTAAT (sh-1-N) and CCATCATGCAAGTACCTGTTT (sh-2-N);
EZH2, CCCAACATAGATGGACCAAAT (sh-1-E) and CCCAACATAGATGGACCAAAT
(sh-2-E).
Lentivirus production and
transduction
For lentiviral production, HEK293T cells
(5×106) were co-transfected with lentiviral plasmids,
packaging vector pCMV-dR8.2 dvpr, and VSV-G (Addgene, Cambridge,
MA, USA) to produce lentiviral particles. The lentiviral particles
were harvested after 48 h by centrifugation and concentrated using
the Lenti™ concentrator kit (Clontech, Lonza, Wokingham, UK). The
lentiviral particles were added to target cells and incubated for
24 h in the presence of 8 µg/ml of polybrene.
Lentivirus-transduced cells were then selected with puromycin for 4
days to generate cells with stable overexpression or knockdown of
the target genes. The expression of the target gene was detected by
western blot analysis.
RNA purification and RT-qPCR
Total RNA was isolated from cultured cells with
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) for RNA extraction.
The reverse transcription was carried out with a PrimeScript
Reverse Transcriptase reagent kit (Takara, Japan). Real-time
quantitative PCR analysis was performed using SYBR Green Master Mix
(Takara) according to the manufacturer's instructions. The mRNA of
β-actin was treated as normalization control. Primers were used as
follows: NF-YA, 5′-GGCAGACCATCGTCTATCAACC-3′ sense and
5′-ATCTGTGCTCCTGCCAAACTGG-3′, antisense; VEGF,
5′-TTGCCTTGCTGCTCTACCTCCA-3′ sense and
5′-GATGGCAGTAGCTGCGCTGATA-3′, antisense; EZH2,
5′-GACCTCTGTCTTACTTGTGGAGC-3′ sense and
5′-CGTCAGATGGTGCCAGCAATAG-3′, antisense; and β-actin,
5′-CACCATTGGCAATGAGCGGTTC-3′ sense and
5′-AGGTCTTTGCGGATGTCCACGT-3′, antisense. All real-time
amplifications were performed in the ABI 7900 Fast Real-Time PCR
system (Applied Biosystems, Foster City, CA, USA). Results of the
real-time PCR data were represented as Ct values of target genes
normalized to its averaged Ct values of β-actin gene to give a ΔCt
value.
Western blot analysis and
immunoprecipitation
For western blot analysis, cells were washed twice
with PBS and then lysed using 200 µl of RIPA lysis buffer
for 30 min on ice. The quantity of protein was measured using a BCA
protein assay (Bio-Rad, Hercules, CA, USA). Samples were then
separated on SDS-PAGE and transferred to nitrocellulose membranes.
The membranes were blocked with 5% BSA in TBST for 1 h at room
temperature. The membranes were then incubated with specific
primary antibodies overnight at 4°C. The antibodies to NF-YA,
STAT3, pY-STAT3, VEGF, Methy-K, and EZH2 were used at a 1:1,000
dilution. The antibody to β-actin were used at a 1:2,000 dilution.
Horseradish peroxidase (HRP)-labeled goat anti-mouse IgG antibody
and goat anti-rabbit IgG antibody were used at a 1:5,000 dilution.
The specifically bound antibodies were detected by
chemiluminescence (ECL) reagent (ECL-Plus kit; Amersham,
Piscataway, NJ, USA) and visualized with Fujifilm LAS-3000 (Multi
Gauge; Fujifilm, Tokyo, Japan). For immunoprecipitation to purify
STAT3 protein, cells were lysed in lysis buffer (50 mM Tris-HCl at
pH 8.0, 150 mM NaCl, 2 mM EDTA and 0.1% NP-40) supplemented with
protease and phosphatase inhibitors (cat no. 1873580 and
4906837001; Roche Diagnostics, Basel, Switzerland) at 4°C for 30
min and cleared by centrifugation at 14,000 rpm at 4°C. The cleared
lysate was incubated for 6 h at 4°C with agarose-conjugated STAT3
antibody.
ELISA analysis
The supernatants of the cells were assayed for VEGF
using appropriate sandwich ELISA kit (cat no. SVE00; R&D
Systems, Minneapolis, MN, USA). The cells were incubated in 24-well
plates (Corning Costar, Corning, NY, USA) for 12 h. The medium was
replaced with serum-free medium for 24 h. VEGF concentrations were
determined by measuring absorbance at 420 nm. VEGF values were
normalized to the total protein extracted from wells.
Matrigel invasion assay
Cells (5×105) were cultured in serum-free
DMEM for 12 h and then seeded into the upper chambers (24-well,
pore size 8-µm; Millipore, Billerica, MA, USA) coated with a
layer of Matrigel (BD Biosciences, San Jose, CA, USA). Following
24-h incubation at 37°C, the cells that did not invade through the
pores were removed from the top chamber by scraping with cotton
swab. The invading cells were fixed with methanol and stained with
hematoxylineosin (H&E) and counted. The number of invasive
cells were counted under a microscope (magnification, ×100).
Immunofluorescence staining
The vector control or NF-YA over-expressed A375P
melanoma cells were seeded onto glass slides and incubated at 37°C
with 5% CO2. Attached for 24 h, cells were then fixed
with 37% formaldehyde in PBS, permeabilized for 10 min in PBS
containing 0.5% Triton X-100 and 1% bovine serum albumin (BSA).
Then, fixed cells were incubated for 1 h in blocking buffer (5% BSA
with 0.1% Triton X-100 in PBS). The slides were then incubated with
anti-pY-STAT3 (1:500) at 4°C overnight followed by incubation with
Alexa-568-labeled secondary antibodies. Nuclear Staining was used
with DAPI. Images were obtained using fluorescence microscopy.
Tube formation assay
To perform the tube formation assay,
5×104 HUVECs were plated in the upper chamber coated
with Matrigel (BD Biosciences) followed by incubation at 37°C
overnight. The human melanoma cells were grown in the lower
chamber. At confluence for melanoma cells, the medium was changed
to serum-free medium. Cultured for 3 days, the tube-like structures
that formed in the gel were photographed (magnification, ×100).
Statistical analysis
The data were analyzed using GraphPad Prism 5.0
(GraphPad Software, La Jolla, CA, USA). Statistical analysis was
performed using a two-tailed Student's t-test. Results of P<0.05
were considered to be significant.
Results
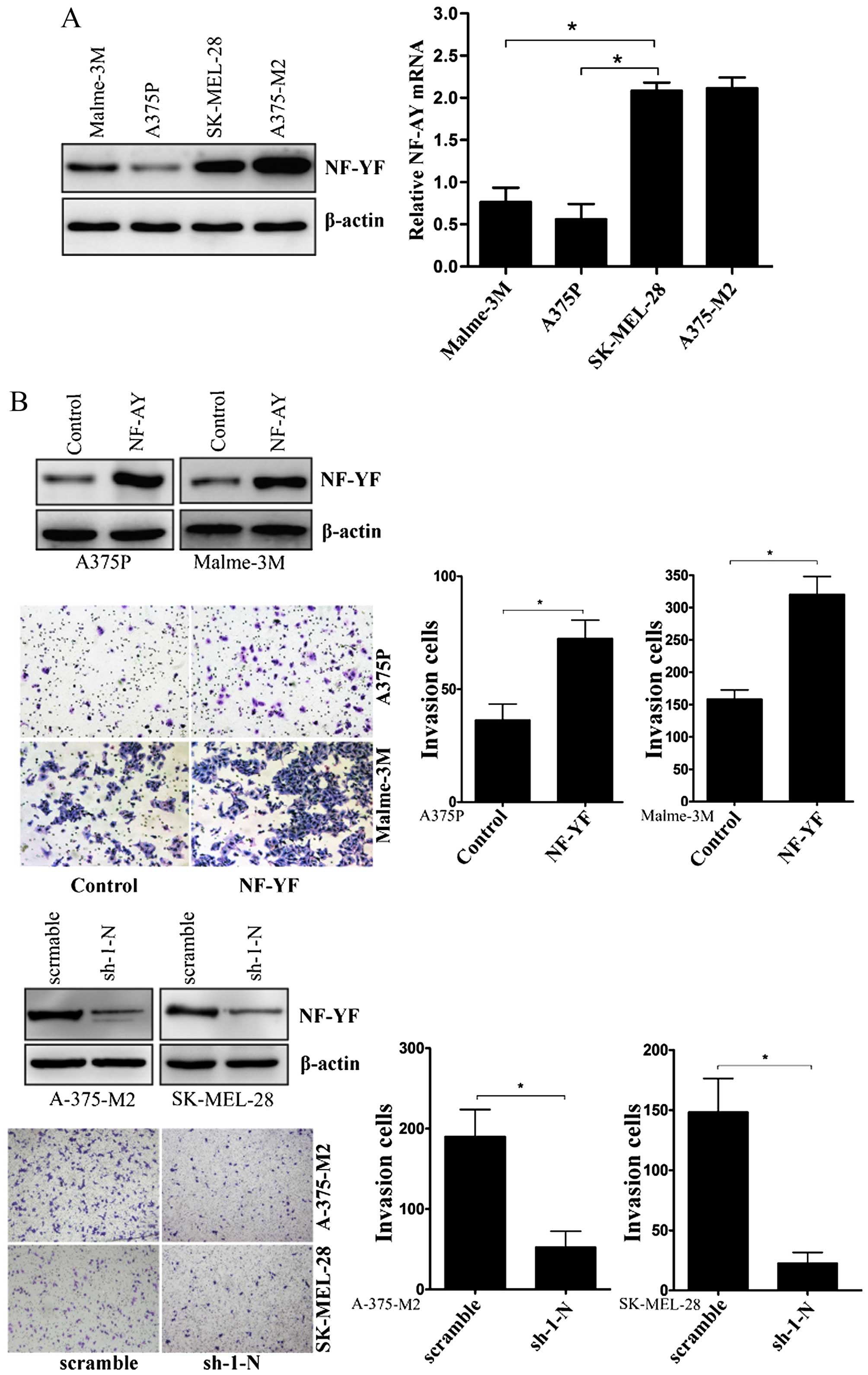
NF-YA is upregulated in highly invasive
human melanoma cells and promotes cell invasion
To examine the role of NF-YA in human melanoma
cells, we first screened four human melanoma cell lines with
different invasive capacities. Western blot analysis indicated that
the expression of NF-YA was detectable in all of these cell lines
(Fig. 1A). By performing real-time
PCR, we also found that the NF-YA mRNA expression patterns were
consistent with the protein expression pattern (Fig. 1A). Malme-3M, A375P, SK-MEL-28 and
A375-M2 human melanoma cell lines differ in their invasive
properties. A375P cell lines are poorly metastatic, Malme-3M cell
lines are moderately metastatic, while A375-M2 and SK-MEL-28 are
highly metastatic (29–31). The data show that NF-YA expression
patterns are consistent with the invasive potentials of these human
melanoma cell lines. Next, we stably over-expressed NF-YA gene in
A375P and Malme-3M cells (Fig. 1B).
As shown in Fig. 1B, melanoma cells
with NF-YA overexpression exhibited significant increase of cell
invasion, whereas, knock-down of NF-YA significant inhibited the
invasion of A-375-M2 and SK-MEL-28 cells (Fig. 1B).
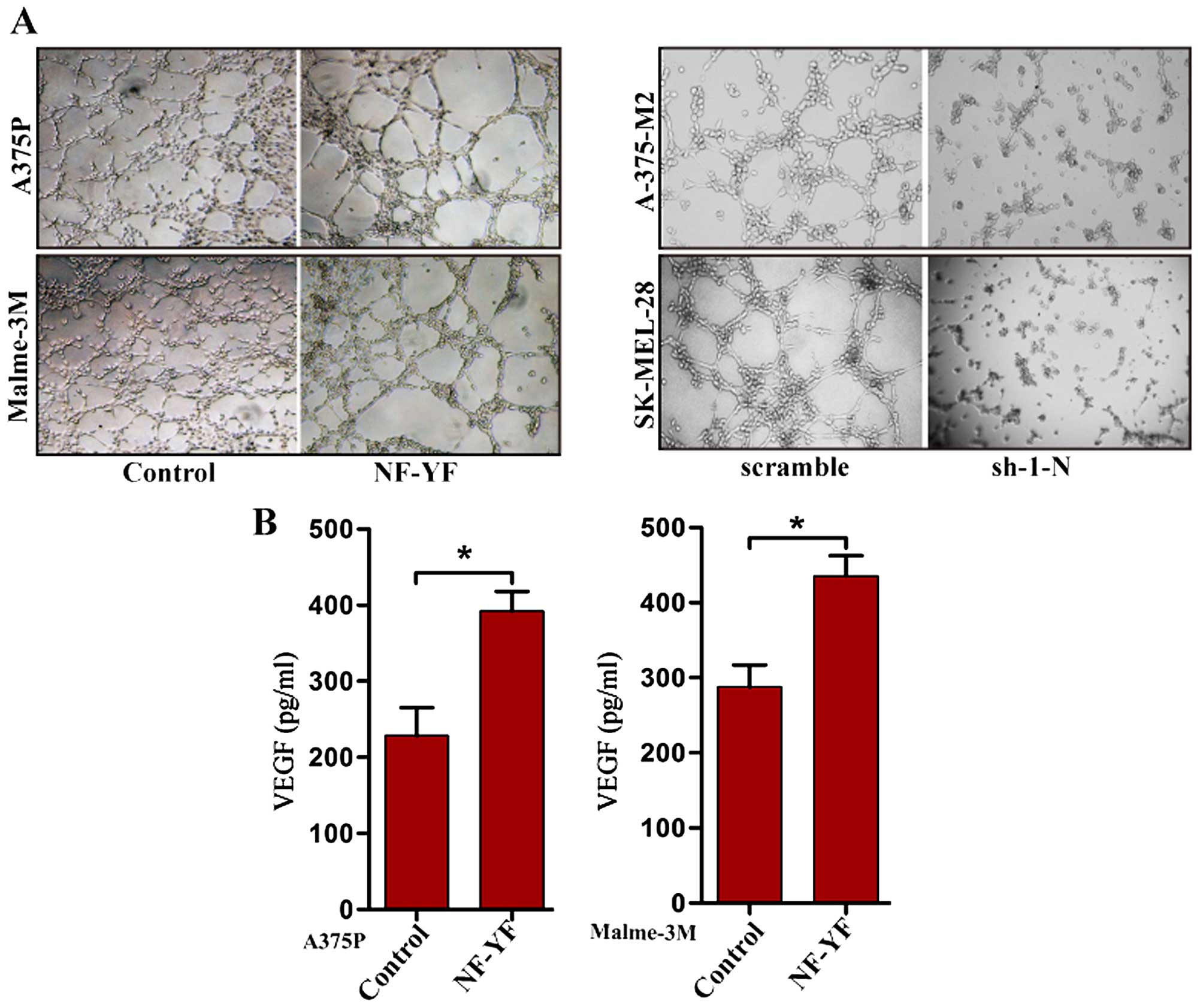
NF-YA promotes angiogenesis induced by
melanoma cells
Invasive tumor cells usually increase vascular
endothelial cell proliferation and angiogenic activity. As a
transcriptional factor, NF-YA may be involved in the regulation of
angiogenesis to vascular endothelial cells by NF-YA target genes.
Thus, we performed a tube formation assay in human umbilical vein
endothelial cells (HUVECs) co-cultured with melanoma cells. HUVECs
were co-cultured with vector control or NF-YA stably over-expressed
A375P or Malme-3M melanoma cells through the collagen matrix. As
shown in Fig. 2A, HUVECs
co-cultured with NF-YA stably overexpressed melanoma cells
exhibited significantly increased tubular networks formation
compared with the vector control. Additionally, HUVECs co-cultured
with NF-YA stably knock-down melanoma cells exhibited significantly
decreased tubular networks formation compared with the scramble
control (Fig. 2A). Vascular
endothelial growth factor (VEGF) is a major modulator to
angiogenesis (6). Here, we also
found that the secretion of VEGF was augmented by NF-YA stably
overexpressed melanoma cells (Fig.
2B).
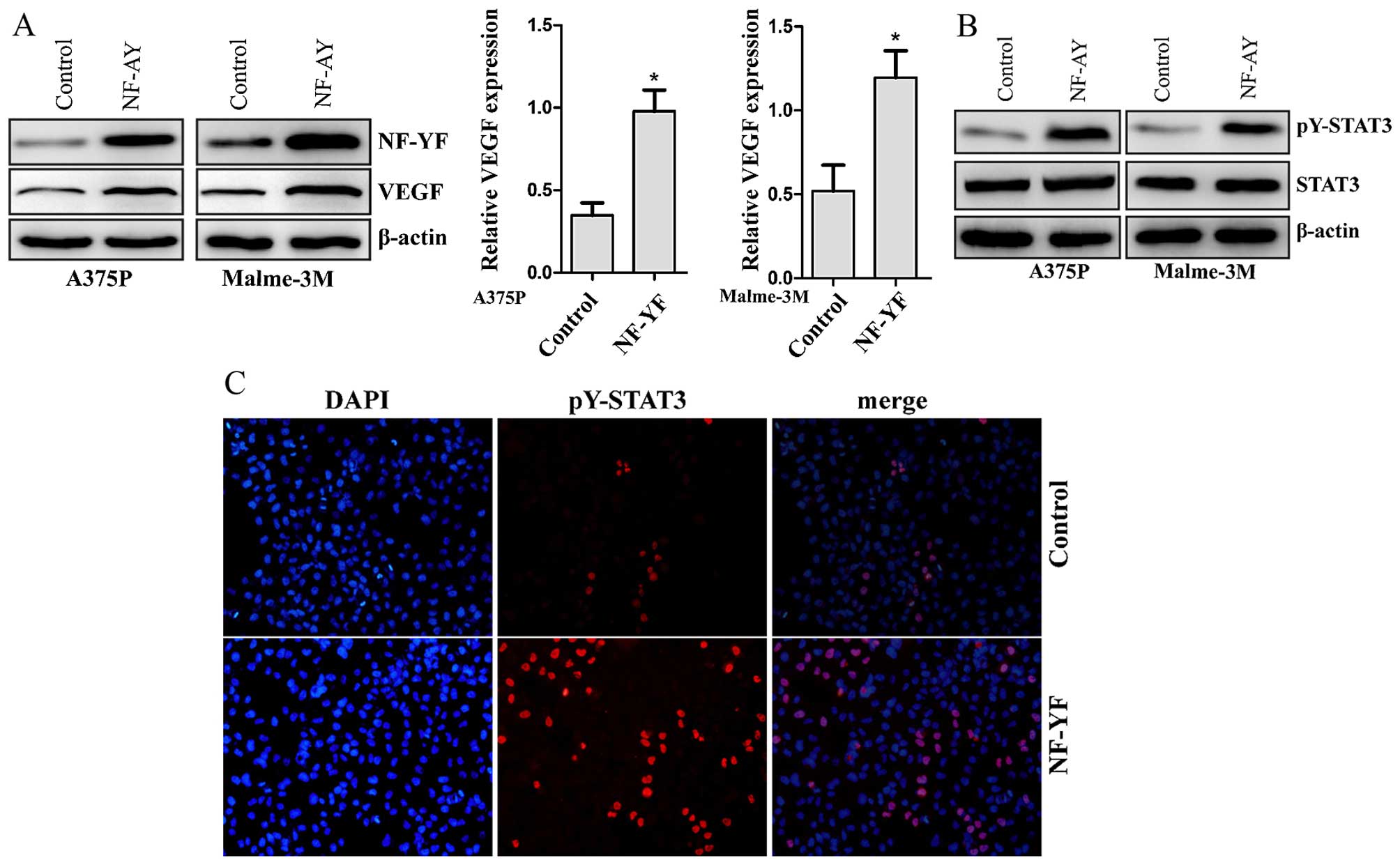
NF-YA upregulates the expression of VEGF
and increases the activity of STAT3
Elevated VEGF secretion was found in NF-YA stably
overexpressed melanoma cells together with an increased tubular
networks formation. We then performed western blot analysis and
real-time PCR to examine whether NF-YA affect the expression of
VEGF in protein and transcription levels. Overexpression of NF-YA
significantly increased the expression of VEGF in A375P or Malme-3M
melanoma cells at both the transcription and protein levels
(Fig. 3A). To further investigate
the signaling pathway that mediated VEGF overexpression, we
detected the activity of STAT3 in NF-YA overexpressed cells. STAT3
is a latent transcription factor, which is phosphorylated in active
forms. Active STAT3 directly regulates the promoter of the VEGF
gene and up-regulates its expression (17). Tyrosine phosphorylated STAT3 at the
705 residue (pY-STAT3) is a constitutively active form of STAT3
(32). We assessed the expression
of total and phosphorylated STAT3 (pY-STAT3) in vector control and
NF-YA overexpressed melanoma cells with western blot. We found that
the STAT3 total expression levels remained unchanged after
overexpression of NF-YA in melanoma cells, whereas STAT3
phosphorylation was enhanced (Fig.
3B). Moreover, immunofluorescence analysis on A375P melanoma
cells indicated increased number of pY-STAT3 positive cells in
NF-YA overexpressed cells compared to the vector control (Fig. 3C).
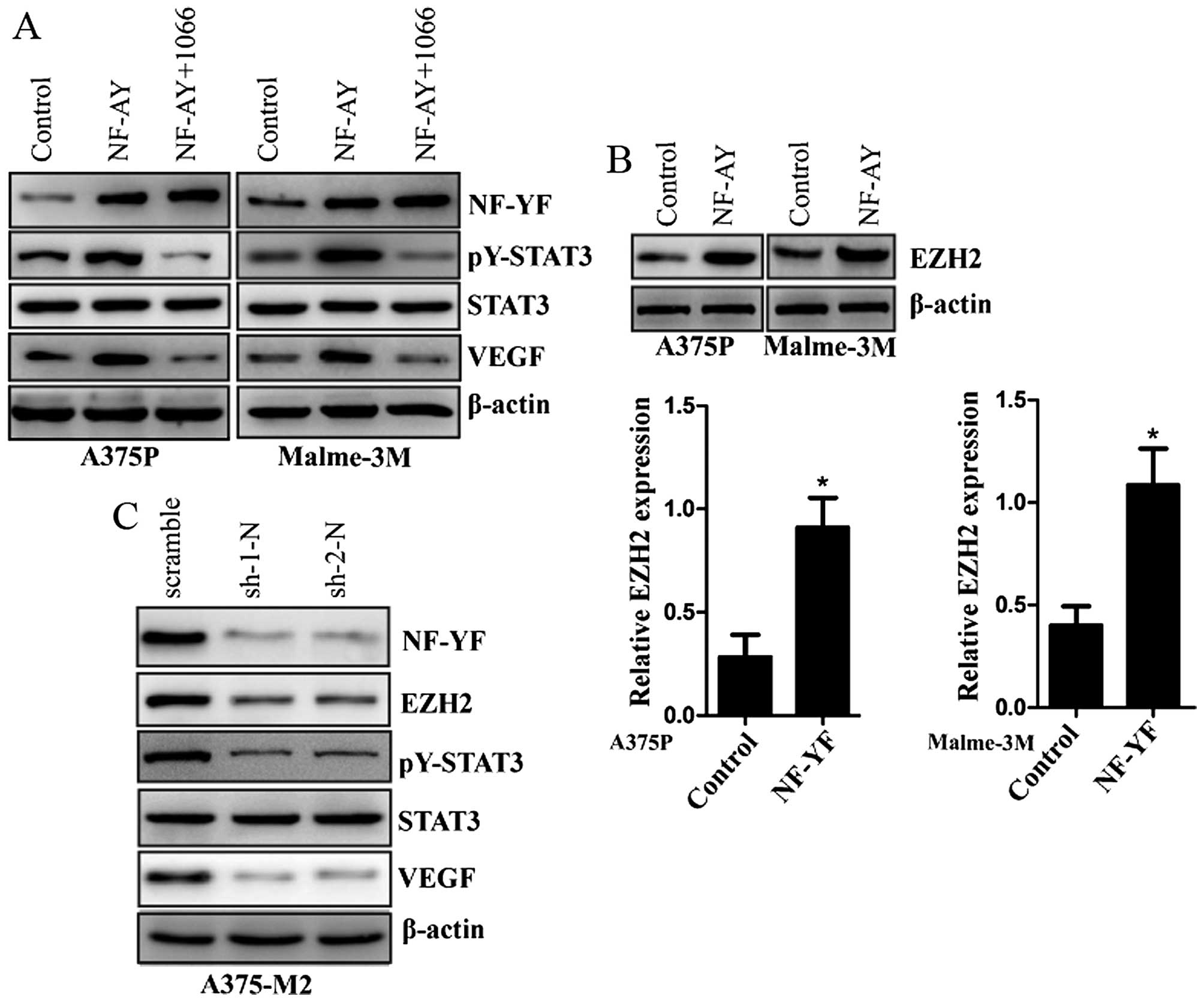
NF-YA induced upregulation of VEGF can be
attenuated by STAT3 inhibition
Because our data indicate that overexpression of
NF-YA upregulates the expression of VEGF and increases the activity
of STAT3, we investigated a possible downstream factor of NF-YA to
mediate this effect. WP1066 is a STAT3 inhibitor by blocking
constitutively activated STAT3 phosphorylation (Tyr 705 residue)
(32,33). We further examined the role of
WP1066 in NF-YA stably overexpressed melanoma cells. With the
treatment of WP1066 in NF-YA stably overexpressed A375P or Malme-3M
melanoma cells, NF-YA induced upregulation of VEGF and increased
activity of STAT3 was inhibited (Fig.
4A). EZH2 was reported to directly bind to STAT3 protein and
then mediate its lysine methylation to enhance STAT3 activity
(23). Actually, NF-YA is a key
regulator of EZH2 expression (34).
In NF-YA stably over-expressed A375P and Malme-3M melanoma cells,
we confirm that the expression of EZH2 is upregulated by the
overexpression of NF-YA at both transcription and protein levels
(Fig. 4B). In addition, we sought
to determine the effects of NF-YA knockdown in melanoma cells.
Stable knockdown of NF-YA in A375-M2 melanoma cells resulted in
downregulated expression of EZH2 and VEGF (Fig. 4C), and the activity of STAT3 was
also inhibited in A375-M2 melanoma cells with NF-YA stable
knockdown (Fig. 4C).
NF-YA upregulates VEGF expression through
activating EZH2-STAT3 signaling
Since NF-YA knockdown suppresses the expression of
EZH2 and the activity STAT3, we examined whether knockdown of NF-YA
inhibits the lysine methylation of STAT3 in A375-M2 cells. STAT3
protein from control or NF-YA knockdown A375-M2 cells were
immunoprecipitated with anti-STAT3 antibody and the blots were
analyzed for methylated STAT3 using pan-methyl lysine antibody. The
methylation of STAT3 protein from NF-YA knockdown A375-M2 cells was
significantly reduced compared with the scramble control A375-M2
cells (Fig. 5A). The enhanced STAT3
activity was mediated by its lysine methylation. Consistent with
the methylation of STAT3, STAT3 phosphorylation (Tyr 705 residue)
was also inhibited in NF-YA knockdown A375-M2 cells (Fig. 5A). Next, we determined whether EZH2
knockdown could affect the expression of NF-YA. The result showed
that EZH2 knockdown did not affect the expression of NF-YA in A375P
cells (Fig. 5B). Differently,
knockdown of EZH2 inhibited the expression of VEGF, the methylation
and activity of STAT3 (Fig. 5B).
Together, these data suggest that NF-YA regulates STAT3 activity
and VEGF expression via upregulating EZH2 expression. To further
confirm this, we overexpressed NF-YA in EZH2 knockdown A375P cells.
Although NF-YA was over-expressed in EZH2 knockdown A375P cells,
the expression of VEGF and the activity of STAT3 did not change
(Fig. 5B). Likely, NF-YA did not
affect the cell invasion of A375P cells with EZH2 knockdown
(Fig. 5C). HUVECs co-cultured with
EZH2 knockdown A375P cells did not exhibit significant tubular
networks formation by the overexpression of NF-YA in these EZH2
knockdown cells (Fig. 5D).
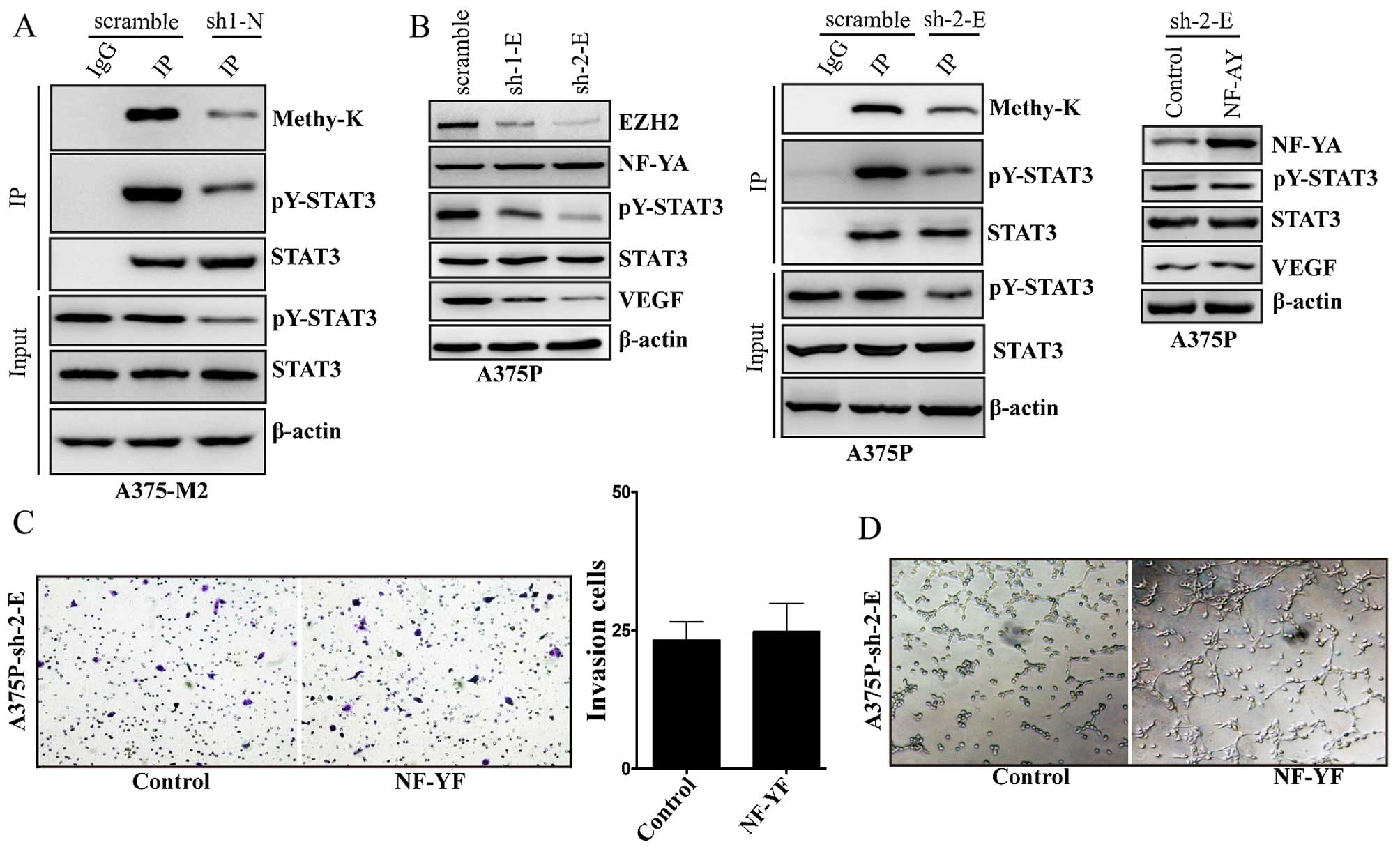 | Figure 5The role of EZH2 in NF-YA induced
VEGF expression and STAT3 activation. (A) Immunoprecipitation of
STAT3 protein using antibodies for STAT3 (IP), or a non-specific
immunoglobulin G (IgG) from scramble control or NF-YA stably
knockdown (sh-1-N) A375-M2 cells, followed by immunoblots with the
indicated antibodies. Methy-K, means rabbit anti-methylated lysine
antibody; input indicate 5% of pre-immunoprecipitated samples. (B)
NF-YA, EZH2, STAT3, pY-STAT3, VEGF and β-actin protein levels were
detected by western blot analysis in scramble control or EZH2
stably knockdown (sh-1-E, sh-2-E) A375P cells (left panel);
immunoprecipitation of STAT3 protein using antibodies for STAT3
(IP), or a non-specific immunoglobulin G (IgG) from scramble
control or EZH2 stably knockdown (sh-2-E) A375P cells, followed by
immunoblots with the indicated antibodies. Methy-K, means rabbit
anti-methylated lysine antibody; input indicate 5% of
pre-immunoprecipitated samples (middle panel); NF-YA, STAT3,
pY-STAT3, VEGF and β-actin protein levels were detected by western
blot analysis in vector control and NF-YA stably overexpressed
A375P cells with EZH2 stably knockdown (sh-2-E) (right panel). (C)
The vector control or NF-YA stably overexpressed A375P-sh-2-E cells
were seeded in a Matrigel-coated upper chamber. Cells invaded
through the Matrigel after incubation for 24 h was detected by
H&E staining and quantified (lower panel). Data expression was
analyzed by quantification of six randomly selected fields.
A375P-sh-2-E, means A375P cells with EZH2 stably knockdown
(sh-2-E). (D) HUVECs cells were plated in the upper chamber coated
with Matrigel in Transwell filters (0.4 µm). The vector
control or NF-YA stably overexpressed A375P-sh-2-E cells were grown
in the lower chamber. Cultured for 3 days, the tube-like structures
that formed in the gel were photographed (magnification, ×100). |
Discussion
Melanoma is an aggressive and highly metastatic
tumor with a high angiogenic potential (1). Angiogenesis is triggered by molecular
signals from tumor cells when tumor tissues require nutrients and
oxygen (35). A number of growth
factors stimulate angiogenesis by inducing endothelial cell
migration (4), proliferation
(6) and expansion (36). These growth factors include vascular
endothelial growth factor (VEGFs), basic fibroblast growth factor
(bFGF), hepatocyte growth factor (HGF), platelet-derived growth
factor (PDGF), transforming growth factors β (TGFβ), interleukin-6
(IL-6), interleukin-8 (IL-8) and epidermal growth factor (EGF)
(35,37). Among these, VEGF (also known as
VEGF-A) is a protein with vascular permeability activity that was
first identified as a factor secreted by tumor cells (38,39).
VEGF is a well-studied and powerful angiogenic factor.
Anti-angiogenic therapy targeting VEGF signaling is a rapidly
growing chemotherapy class for cancer therapy (6). Thus, a better understanding of the
regulation of VEGF by other factors would provide more effective
strategies for the development of new anti-angiogenic therapy.
The expression of VEGF is well known to be
upregulated by hypoxia (13).
Hypoxia induces the expression of VEGF via hypoxia-inducible
factor-1α (HIF-1α) by binding to a hypoxia response element of VEGF
gene promoter to activate VEGF gene expression in cells exposed to
hypoxia (11). Moreover, STAT3 also
has been shown to be a transcriptional activator of the VEGF gene
by directly binding to the VEGF promoter (12). In the present study, we demonstrated
that NF-YA increases the expression of VEGF and promotes
angiogenesis of human melanoma cells in vitro. The results
suggest that targeting NF-YA has the potential for tumor
anti-angiogenic therapy.
EZH2 was reported to directly bind to STAT3 protein
and mediate its lysine methylation to enhance STAT3 activity
(23). Moreover, NF-YA is a key
regulator of EZH2 expression by up-regulating its transcription
(34). Consistent with this report,
we also showed that overexpression of NF-YA in human melanoma cells
up-regulated the expression of EZH2. Because our data indicate that
NF-YA upregulates the expression of VEGF and increases the activity
of STAT3, STAT3 inhibitor WP1066 was used to study the role of
STAT3 in NF-YA induced up-regulation of VEGF. With the treatment of
WP1066 in NF-YA stably overexpressed A375P or Malme-3M melanoma
cells, NF-YA induced upregulation of VEGF and the increased
activity of STAT3 was inhibited.
EZH2-induced lysine methylation of STAT3 is a
critical modification that leads to STAT3 activation (23). In the present study, we observed
that the lysine methylation of STAT3 protein from NF-YA knockdown
A375-M2 cells was significantly reduced compared with the control
A375-M2 cells. Consistent with the methylation of STAT3, STAT3
phosphorylation (Tyr 705 residue) was also inhibited in NF-YA
knockdown A375-M2 cells. Furthermore, knockdown of EZH2 was shown
to inhibit the expression of VEGF and phosphorylation of STAT3 in
A375P cells. Importantly, the expression of VEGF and
phosphorylation of STAT3 were not affected by the overexpression of
NF-YA in EZH2 stably knockdown A375P cells. Similarly, NF-YA did
not affect the cell invasion and angiogenesis of A375P cells with
stable EZH2 knockdown.
Taken together, the present study demonstrated that
overexpression of NF-YA contributes to cell invasion and
angiogenesis through EZH2-STAT3 signaling in human melanoma cells.
Therefore, our results suggest the possibility that NF-YA might be
a potential therapeutic target in the treatment of human
melanoma.
References
|
1
|
Tas F: Metastatic behavior in melanoma:
Timing, pattern, survival, and influencing factors. J Oncol.
2012:6476842012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geller AC, Clapp RW, Sober AJ, Gonsalves
L, Mueller L, Christiansen CL, Shaikh W and Miller DR: Melanoma
epidemic: An analysis of six decades of data from the Connecticut
Tumor Registry. J Clin Oncol. 31:417–4178. 2013. View Article : Google Scholar
|
|
3
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lamalice L, Le Boeuf F and Huot J:
Endothelial cell migration during angiogenesis. Circ Res.
100:782–794. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schaafhausen MK, Yang WJ, Centanin L,
Wittbrodt J, Bosserhoff A, Fischer A, Schartl M and Meierjohann S:
Tumor angiogenesis is caused by single melanoma cells in a manner
dependent on reactive oxygen species and NF-κB. J Cell Sci.
126:3862–3872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia X, Hong Q, Lei L, Li D, Li J, Mo M,
Wang Y, Shao Z, Shen Z, Cheng J, et al: Basal and therapy-driven
hypoxia-inducible factor-1α confers resistance to endocrine therapy
in estrogen receptor-positive breast cancer. Oncotarget.
6:8648–8662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeh JJ and Kim WY: Targeting tumor hypoxia
with hypoxia-activated prodrugs. J Clin Oncol. 33:1505–1508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao D, Zhou H, Zhao J, Jin L, Yu W, Yan H,
Hu Y and Guo T: PGC-1α integrates glucose metabolism and
angiogenesis in multiple myeloma cells by regulating VEGF and
GLUT-4. Oncol Rep. 31:1205–1210. 2014.PubMed/NCBI
|
|
11
|
Zhang C, Li Y, Cornelia R, Swisher S and
Kim H: Regulation of VEGF expression by HIF-1α in the femoral head
cartilage following ischemia osteonecrosis. Sci Rep. 2:6502012.
View Article : Google Scholar
|
|
12
|
Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB,
Lee CN and Hsieh CY: Interleukin-6 promotes cervical tumor growth
by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene.
22:1517–1527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kimura H, Weisz A, Kurashima Y, Hashimoto
K, Ogura T, D'Acquisto F, Addeo R, Makuuchi M and Esumi H: Hypoxia
response element of the human vascular endothelial growth factor
gene mediates transcriptional regulation by nitric oxide: Control
of hypoxia-inducible factor-1 activity by nitric oxide. Blood.
95:189–197. 2000.
|
|
14
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhuang S: Regulation of STAT signaling by
acetylation. Cell Signal. 25:1924–1931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mora LB, Buettner R, Seigne J, Diaz J,
Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, et
al: Constitutive activation of Stat3 in human prostate tumors and
cell lines: Direct inhibition of Stat3 signaling induces apoptosis
of prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|
|
17
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rao RA, Dhele N, Cheemadan S, Ketkar A,
Jayandharan GR, Palakodeti D and Rampalli S: Ezh2 mediated H3K27me3
activity facilitates somatic transition during human pluripotent
reprogramming. Sci Rep. 5:82292015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caganova M, Carrisi C, Varano G, Mainoldi
F, Zanardi F, Germain PL, George L, Alberghini F, Ferrarini L,
Talukder AK, et al: Germinal center dysregulation by histone
methyltransferase EZH2 promotes lymphomagenesis. J Clin Invest.
123:5009–5022. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JM, Lee JS, Kim H, Kim K, Park H, Kim
JY, Lee SH, Kim IS, Kim J, Lee M, et al: EZH2 generates a methyl
degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin
ligase complex. Mol Cell. 48:572–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gunawan M, Venkatesan N, Loh JT, Wong JF,
Berger H, Neo WH, Li LY, La Win MK, Yau YH, Guo T, et al: The
methyltransferase Ezh2 controls cell adhesion and migration through
direct methylation of the extranuclear regulatory protein talin.
Nat Immunol. 16:505–516. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tong Q, He S, Xie F, Mochizuki K, Liu Y,
Mochizuki I, Meng L, Sun H, Zhang Y, Guo Y, et al: Ezh2 regulates
transcriptional and posttranslational expression of T-bet and
promotes Th1 cell responses mediating aplastic anemia in mice. J
Immunol. 192:5012–5022. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim E, Kim M, Woo DH, Shin Y, Shin J,
Chang N, Oh YT, Kim H, Rheey J, Nakano I, et al: Phosphorylation of
EZH2 activates STAT3 signaling via STAT3 methylation and promotes
tumorigenicity of glioblastoma stem-like cells. Cancer Cell.
23:839–852. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Domashenko AD, Wiener S and Emerson SG:
NF-Ya protein delivery as a tool for hematopoietic progenitor cell
expansion. Methods Mol Biol. 916:303–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin YC, Chen YN, Lin KF, Wang FF, Chou TY
and Chen MY: Association of p21 with NF-YA suppresses the
expression of Polo-like kinase 1 and prevents mitotic death in
response to DNA damage. Cell Death Dis. 5:e9872014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Domashenko AD, Danet-Desnoyers G, Aron A,
Carroll MP and Emerson SG: TAT-mediated transduction of NF-Ya
peptide induces the ex vivo proliferation and engraftment potential
of human hematopoietic progenitor cells. Blood. 116:2676–2683.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin S, Fan F, Fan W, Zhao H, Tong T,
Blanck P, Alomo I, Rajasekaran B and Zhan Q: Transcription factors
Oct-1 and NF-YA regulate the p53-independent induction of the
GADD45 following DNA damage. Oncogene. 20:2683–2690. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matuoka K and Chen KY: Possible role of
subunit A of nuclear factor Y (NF-YA) in normal human diploid
fibroblasts during senescence. Biogerontology. 1:261–271. 2000.
View Article : Google Scholar
|
|
29
|
Orgaz JL, Pandya P, Dalmeida R,
Karagiannis P, Sanchez-Laorden B, Viros A, Albrengues J, Nestle FO,
Ridley AJ, Gaggioli C, et al: Diverse matrix metalloproteinase
functions regulate cancer amoeboid migration. Nat Commun.
5:42552014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eustace AJ, Crown J, Clynes M and
O'Donovan N: Preclinical evaluation of dasatinib, a potent Src
kinase inhibitor, in melanoma cell lines. J Transl Med. 6:532008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herraiz C, Calvo F, Pandya P, Cantelli G,
Rodriguez-Hernandez I, Orgaz JL, Kang N, Chu T, Sahai E and
Sanz-Moreno V: Reactivation of p53 by a cytoskeletal sensor to
control the balance between DNA damage and tumor dissemination. J
Natl Cancer Inst. 108:pii: djv289. 2015.PubMed/NCBI
|
|
32
|
Judd LM, Menheniott TR, Ling H, Jackson
CB, Howlett M, Kalantzis A, Priebe W and Giraud AS: Inhibition of
the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and
in vivo. PLoS One. 9:e959932014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou X, Ren Y, Liu A, Han L, Zhang K, Li
S, Li P, Li P, Kang C, Wang X, et al: STAT3 inhibitor WP1066
attenuates miRNA-21 to suppress human oral squamous cell carcinoma
growth in vitro and in vivo. Oncol Rep. 31:2173–2180.
2014.PubMed/NCBI
|
|
34
|
Garipov A, Li H, Bitler BG, Thapa RJ,
Balachandran S and Zhang R: NF-YA underlies EZH2 upregulation and
is essential for proliferation of human epithelial ovarian cancer
cells. Mol Cancer Res. 11:360–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nishida N, Yano H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar
|
|
36
|
Xue Y, Lim S, Yang Y, Wang Z, Jensen LD,
Hedlund EM, Andersson P, Sasahara M, Larsson O, Galter D, et al:
PDGF-BB modulates hematopoiesis and tumor angiogenesis by inducing
erythropoietin production in stromal cells. Nat Med. 18:100–110.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mahabeleshwar GH and Byzova TV:
Angiogenesis in melanoma. Semin Oncol. 34:555–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and proangiogenic therapies. Genes Cancer.
2:1097–1105. 2011. View Article : Google Scholar
|
|
39
|
Detmar M: Tumor angiogenesis. J Investig
Dermatol Symp Proc. 5:20–23. 2000. View Article : Google Scholar
|