Introduction
Breast cancer is the most frequently diagnosed
cancer and the leading cause of cancer-related death among females
worldwide (1). In most cases, it is
the metastasis of malignant tumor cells, rather than the primary
solid tumor, that is the main cause of death (2). Metastasis is a complex process
involving cell adhesion, migration, invasion and extracellular
matrix (ECM) degradation. Hence, it is urgent to develop
therapeutic strategies and agents with the ability to inhibit
growth, migration, invasion and proteolytic degradation of ECM in
breast cancer cells.
Integrin is a family of ubiquitous transmembrane
glycoprotein receptors comprised of 18 α and 8 β subunits with
different combinations and ligand specificity (3). They link the ECM to the intracellular
actin cytoskeleton and stimulate the intracellular signaling
pathway that modulates proliferation, migration and invasion of
cells. Compared with normal cells, metastatic tumor cells often
overexpress certain integrins. For example,
αVβ3, αVβ5 and
α5β1, a subfamily of integrins, have been
demonstrated to be upregulated in lung, melanoma, breast and brain
tumors, which make them ideal pharmacological targets for the
development of antitumorigenic and antiangiogenic compounds
(4,5). Recently, a tripeptide sequence
Arg-Gly-Asp (RGD) which is a conserved motif present in ECM
proteins, has been identified as an important tool in targeting
drugs and imaging agents because of its high affinity to
αVβ3, αVβ5 and
α5β1 integrins. Therefore, RGD has been
investigated as an ideal promising ligand for the development of
antitumor or antimetastatic agents.
Many natural peptides containing the RGD motif have
been examined as effective agents with antitumor and
anti-metastatic activity (6).
Lunasin is such a novel 43-amino acid peptide originally isolated
from soybean seed with the sequence:
SKWQHQQDSCRKQLQGVNLTPCEKHIMEKIQGRGDDDDDDDDD (7). To date, lunasin and its analogues have
been successively identified in barley (8), wheat (9) and other plants, e.g. Amaranth, a plant
cultivated in Mexico (10), and a
traditional Chinese herb, Solanum nigrum L. (11). There is accumulating evidence
showing that the bioactivity of lunasin is highly associated with
its unique peptide sequences consisting of three functional
regions: a C-terminal tail with 8 aspartic acid residues (poly-D),
helping lunasin bind directly to histones affecting the H3 and H4
acelylation/deacelylation process (12); a predicted and structurally
conserved helix region targeting lunasin to the chromatin.
Moreover, as a cell adhesion motif, RGD potentiates the ability of
lunasin to internalize into cells and compete with ECM to interact
with integrins, such as αVβ3,
αVβ5 or α5β1, which is
crucial in angiogenesis, tumor progression and metastasis. It was
found that lunasin inhibited cell proliferation and induced
apoptosis in breast, colon and leukemia cancers owing to its RGD
motif (13). As reported, lunasin
inhibited the metastasis of colon cancer cells by direct binding
with α5β1 integrin through suppression of
focal adhesion kinase (FAK)/ERK/NF-κB signaling in vitro and
in a mouse model (14). Lunasin has
also been reported to exert anti-inflammatory effects on human
macrophages via inhibition of the αVβ3
integrin-mediated Akt/NF-κB pathway (15). Additionally, when combined with
other chemopreventive agents anacardic acid or oxaliplatin, lunasin
potentiated their antitumor effects on MDA-MB-231 (16), and KM12L4 (14) cells, respectively, indicating that
this peptide has a promising role as a co-adjuvant in cancer
therapy.
Although research has proven that lunasin is an
effective bioactive peptide in many cancer therapies, the effect
and mechanism of lunasin in regards to the metastasis of breast
cancer cells are largely unknown. In this study, we assessed the
potential inhibitory effects of lunasin on the growth, migration,
invasion and ECM degradation of breast cancer cells. We also
verified that lunasin exerts its antimetastatic effect by
decreasing the activity and expression of MMP-2/-9 and inactivating
the associated proteins in the FAK/Akt/ERK and NF-κB pathways.
Materials and methods
Cell lines
Human breast cancer cell lines MCF-7 and MDA-MB-231
were purchased from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). MCF-7 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand
Island, NY, USA) supplemented with 10% FBS (Biological Industries,
Kibbutz Beit-Haemek, Israel), 100 mg/ml streptomycin and 100 U/ml
penicillin in a 5% CO2 atmosphere at 37°C. MDA-MB-231
cells were cultured in L15 (Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% FBS, 100 mg/ml streptomycin and 100 U/ml
penicillin (Beyotime Institute of Biotechnology, Shanghai, China)
at 37°C.
Reagents
Lunasin, SKWQHQQDSCRKQLQGVNLTPCEKHIMEIGRDDDDDDDDD
was synthesized by GL Biochem (Shanghai, China). The purity of the
peptide was higher than 95%. Stock solutions of synthetic lunasin
(1 mM) were prepared with sterile distilled water and stored at
−20°C.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) and PMSF were obtained from Sigma-Aldrich. BCA
protein assay kit, RIPA lysis buffer and nuclear/cytoplasmic
protein extraction kit were purchased from Beyotime Institute of
Technology. The primary antibodies to phospho-Akt (Ser473) rabbit
mAb, Akt rabbit mAb, phospho-Src family (Tyr416) rabbit mAb, Src
rabbit mAb, phospho-FAK (Tyr397) rabbit mAb, FAK rabbit mAb,
phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit mAb, p44/42
MAPK (Erk1/2) rabbit mAb, phospho-NF-κB p65 (Ser536) rabbit mAb,
IκBα mouse mAb, phospho-IκBα (Ser32) rabbit mAb, NF-κB p65 rabbit
mAb, β-actin mouse mAb, Lamin B2 (D8P3U) rabbit mAb, anti-rabbit
IgG, HRP-linked antibody, anti-mouse IgG and HRP-linked antibody
were all purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA).
Cell viability assay
Cell viability was assessed using an MTT assay.
MCF-7 and MDA-MB-231 cells were plated at a density of
1×104 cells/well in a 96-well plate overnight and then
treated with various concentrations of lunasin peptide (0–320
µM) for 24 or 48 h. At the end of the treatment, 20
µl of MTT (5 mg/ml) was added and incubated at 37°C for 4 h.
The supernatant was aspirated and the formazan crystals that formed
were dissolved in 150 µl DMSO for 20 min. The absorbance at
490 nm was measured with a microplate reader (Spectra Max 190;
Molecular Devices, LLC, Sunnyvale, CA, USA). The viability was
expressed as the percentage of the lunasin-treated group to the
control group, considered as 100%. All data were analyzed from
three independent experiments with six replicates and the results
are expressed as the mean ± SD.
Wound healing assay
The wound healing assay was performed as previously
reported with some modifications (17). MCF-7 and MDA-MB-231 cells were
seeded into a 6-well plate until growth to 70% confluence with
complete medium. A plastic tip (1 mm) was used to make a scratch on
the cell monolayer as previously described (18). Then the wound area was washed three
times with PBS to remove cell debris and the cells were incubated
with lunasin (0–20 µM) for 24 h. The cells were allowed to
migrate into the wound surface and the average distance of the
migrating cells was observed using inverted microscopy (Leica
Microsystems GmbH, Wetzlar, Germany) at different times. The
migration rate was expressed as the migrated distance of the cells
in the experimental group to that of the control group. All data
were analyzed from three independent experiments performed in
triplicate and the results are expressed as the mean ± SD.
Invasion assay
The invasion assay was performed using a Transwell
chamber (8 µm pore polycarbonate, Corning Costar, Cambridge,
MA, USA) coated with the diluted BD Matrigel™ basement
membrane matrix (BD Biosciences, Bedford, MA, USA). MCF-7 and
MDA-MB-231 cells treated with lunasin (0–20 µM) for 24 h
were trypsinized and suspended at a final concentration of
1×106 cells/ml in serum-free DMEM and L15 medium,
respectively. Cell suspension was added into each Transwell upper
chamber of and the medium with 5% FBS was applied to the bottom of
the chamber as a chemoattractant. The chamber was incubated at 37°C
for 24 h. After incubation, the non-invaded cells in the upper
chamber were removed from the Transwell membrane with a cotton
swab. The invaded cells in the lower chamber were fixed with 100%
methanol and then stained with 1% crystal violet in 2% ethanol. The
invaded cells were counted and photographed under a microscope at
five different fields of the chamber. The data are presented as the
average number of invaded cells in the experimental group to the
control group. All data were analyzed from three independent
experiments performed in triplicate and the results are expressed
as the mean ± SD.
Gelatin zymography
The proteolytic activity of MMP-2 and MMP-9 in the
supernatant was analyzed by gelatin zymography assay as previously
described (19). MCF-7 and
MDA-MB-231 cells were treated with lunasin (0–20 µM) in
serum-free medium for 24 h. After incubation, the supernatant of
the cells was collected and centrifuged at 1,000 × g at 4°C for 10
min. Samples were then loaded on 8% SDS-polyacrylamide gel
containing 0.1% gelatin. The electrophoresis was performed at 100
V. When finished, the gel was washed with elution buffer (2.5%
Triton X-100, 50 mM Tris-HCl, 5 mM CaCl2 and 1 µM
ZnCl2, pH 7.6) and washing buffer (50 mM Tris-HCl, 5 mM
CaCl2 and 1 µM ZnCl2, pH 7.6) twice,
followed by reaction with incubation solution (50 mM Tris-HCl, 5 mM
CaCl2, 1 µM ZnCl2 and 0.02% Brij-35,
pH 7.6) for 48 h at 37°C. Finally, the gel was stained with
staining buffer (0.05% Coomassie Blue R-250, 30% methanol and 10%
acetic acid) and destained with destaining buffer A (30% methanol
and 10% acetic acid), B (20% methanol and 10% acetic acid) and C
(10% methanol and 50% acetic acid) by turn. The results were
photographed and analyzed by Biospectrum Imaging system (UVP, Inc.,
Upland, CA, USA) and Image J software. The data are presented as
the degree of grey in the enzymatic region in the lunasin-treated
group vs. the control group. All data were analyzed from three
independent experiments performed in triplicate and the results are
expressed as the mean ± SD.
Western blot analysis
MCF-7 and MDA-MB-231 cells were cultured in a 6-well
plate at a density of 2×105 cells/well. After
incubation, the cells were pretreated with different concentrations
of lunasin (0–20 µM) for 24 h. Then, the cells were
harvested and lysed with the RIPA cell lysis buffer in the presence
of a protease inhibitor PMSF. The samples were incubated for 30 min
on ice and centrifuged at 8,000 × g for 15 min at 4°C. The cell
extracts were collected and stored at −80°C until use in subsequent
experiments. Total cellular and nuclear proteins were extracted
according to the instructions of the nuclear and cytoplasmic
protein extraction kit. The nuclear extracts were used to determine
NF-κB protein levels and the cytoplasmic extracts were used to
determine IκB levels. The protein concentration was determined
using the BCA protein assay kit. Equal amounts of protein were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to a polyvinylidene
fluoride (PVDF) membrane. Western blot analysis was carried out as
previously described (20). The
protein bands were visualized by enhanced chemiluminescence
detection reagents (Applygen Technologies Inc., Beijing, China)
using a Biospectrum Imaging system.
Statistical analysis
All data are presented as the mean ± standard
deviation (SD) of 3 independent experiments performed in
triplicate. Statistical analysis was performed by Student's t-test
or one-way analysis of variance (ANOVA). In all cases, P<0.05
was considered statistically significant, P<0.01 was considered
extremely significant.
Results
Cytotoxic effect of lunasin on MCF-7 and
MDA-MB-231 breast cancer cells
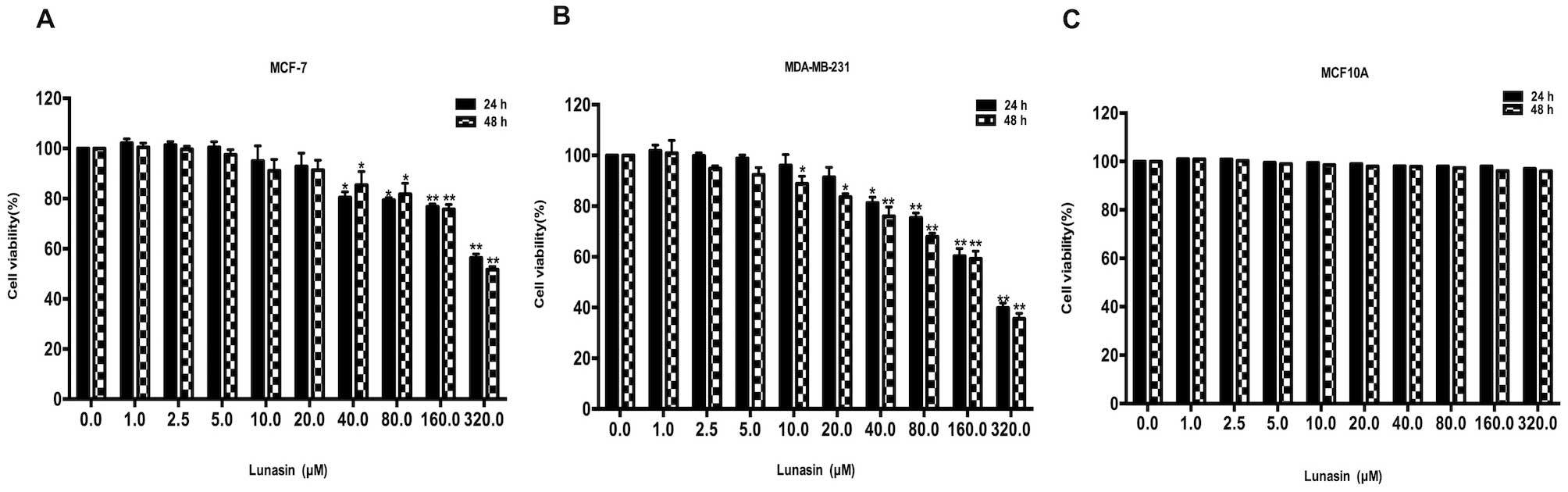
To evaluate the cytotoxic effect of lunasin on human
breast cancer MCF-7 and MDA-MB-231 cells, the viability was
determined by MTT assay. As shown in Fig. 1A and B, lunasin did not exhibit
strong cytotoxic effects on MCF-7 and MDA-MB-231 cells until the
concentration reached 20 µM. With the increase in lunasin
concentration and incubation time, the viability of the cells was
markedly decreased, which demonstrated that lunasin may exert its
inhibitory effect in concentration- and time-dependent manners.
After treatment with lunasin for 24 and 48 h, the IC50
values in the MCF-7 cells were 508.6 µM and 431.9 µM,
respectively. The cell viability of the MDA-MB-231 cells following
treatment with lunasin (Fig. 1B)
was much lower than that of the MCF-7 cells. The IC50
was 224.7 µM at 24 h and 194.9 µM at 48 h. The
cytotoxic effect of lunasin was also investigated in human normal
breast MCF-10A cells (Fig. 1C). As
previosly reported (21), lunasin
selectively kills cancer cells without having an effect on normal
cells.
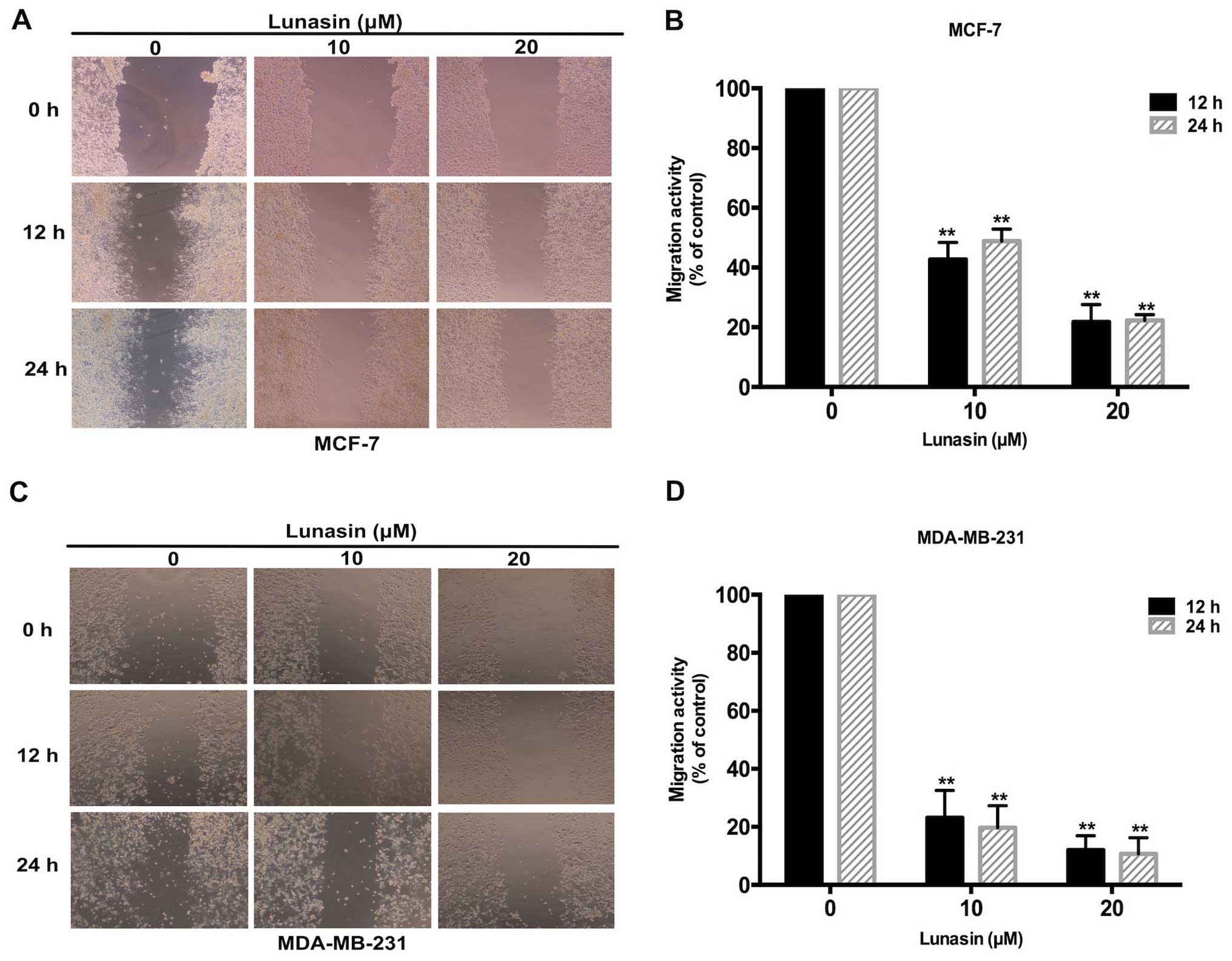
Lunasin inhibits the migration and
motility of breast cancer cells in vitro
A scratch wound assay was carried out to evaluate
the migration and motility of the breast cancer cells following
treatment with lunasin. According to the MTT assay results, 0–20
µM lunasin was selected for its non-cytotoxicity to cells.
As shown in Fig. 2A and B, lunasin
effectively inhibited the migration of MCF-7 cells after a 24-h
treatment. Compared with the control group, the migration rate in
the lunasin group decreased gradually with an increase in lunasin
concentration. The same phenomenon was also observed in the
MDA-MB-231 cells following treatment with lunasin (Fig. 2C and D). All the results indicated
that lunasin significantly suppressed the migration of MCF-7 and
MDA-MB-231 breast cancer cells in a dose-dependent manner.
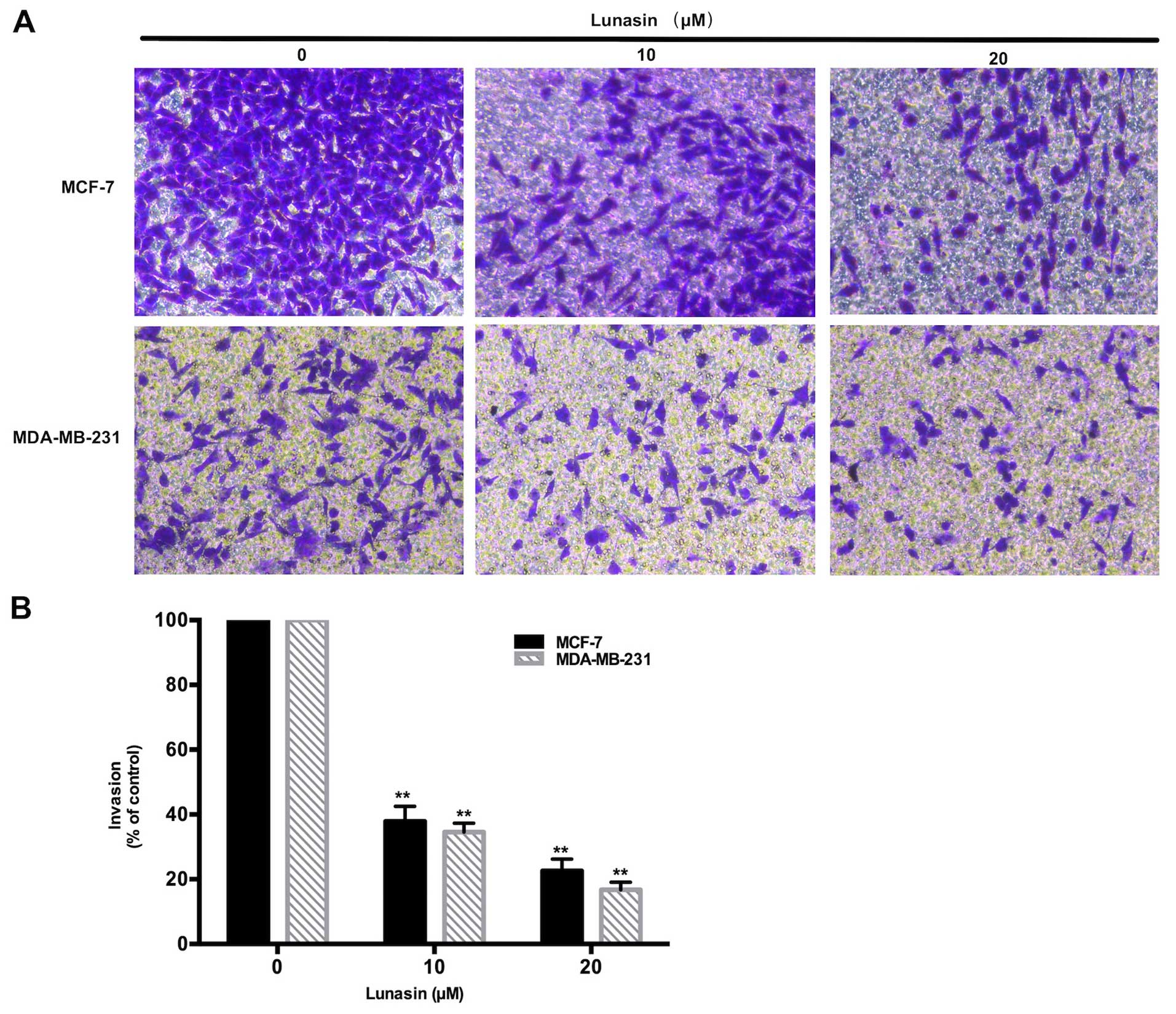
Lunasin inhibits the invasion of breast
cancer cells
The inhibitory effects of lunasin on the invasion of
breast cancer cells were investigated by Transwell invasion assay.
Fig. 3 shows that after incubation
for 24 h, lunasin (10–20 µM) markedly decreased the number
of invasive cells in both the MCF-7 and MDA-MB-231 cells. The
invasiveness of the MCF-7 and MDA-MB-231 cells became less
aggressive with an increase in lunasin concentration. These
findings were consistent with the wound scratch assay, which
indicated that lunasin suppresses the metastasis of breast cancer
cells.
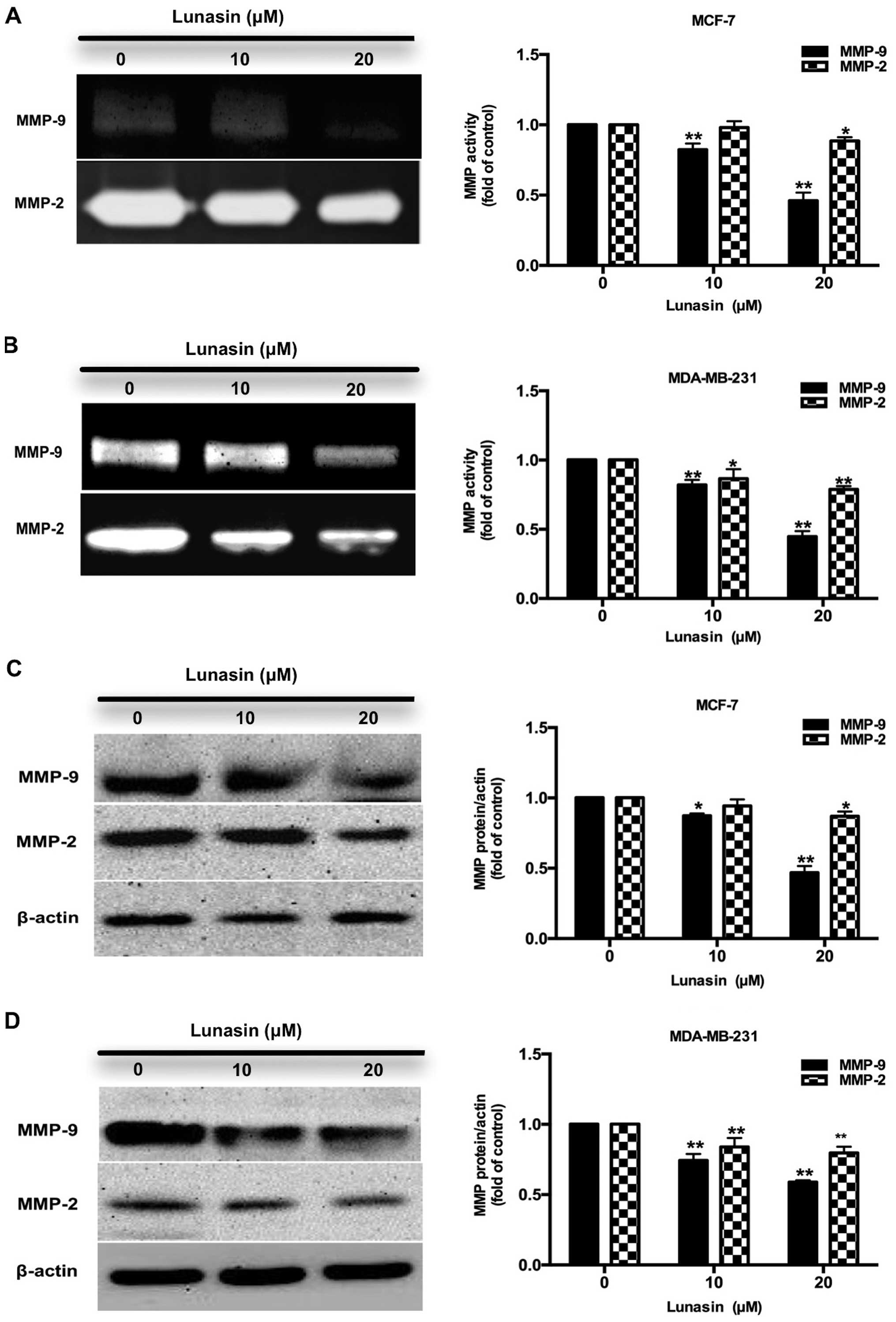
Lunasin reduces the activity and
expression of MMP-2/-9 in breast cancer cells
Matrix metalloproteinases (MMPs) are a family of
proteins that can degrade the ECM leading to tumor metastasis,
apoptosis and carcinogensis (22).
Among the MMP proteins, MMP-2 and MMP-9 are highly expressed and
correlated with the metastasis of breast tumors (23). Hence, in this study the activity and
expression of MMP-2 and MMP-9 were investigated by gelatin
zymography and western blot analysis to examine the possible
anti-invasive ability of lunasin. As shown in Fig. 4A and B, lunasin (10–20 µM)
reduced the activity and expression of MMP-9 in the MCF-7 cells.
However, there were no significant changes in the activity and
expression of MMP-2. In the MDA-MB-231 cells (Fig. 4C and D), lunasin sharply reduced the
activity and expression of MMP-9 and decreased that of MMP-2
gradually. The differences in MMP-2 expression may be attributed to
the different characteristics between the two breast cancer cell
lines.
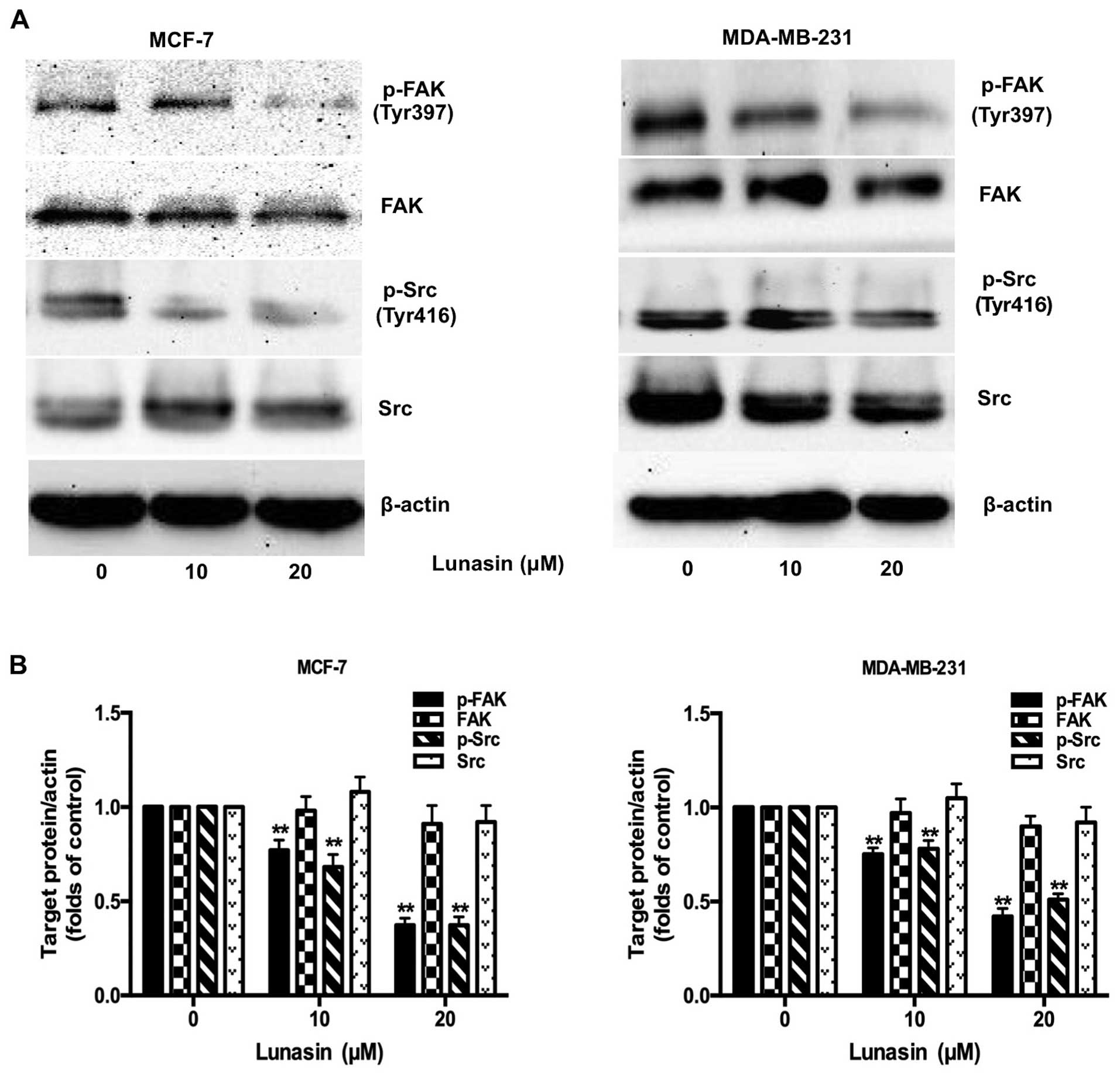
Lunasin suppresses the phosphorylation of
FAK and Src
FAK, a cytoplasmic protein tyrosine kinase, is
crucial for proliferation, migration, adhesion and invasion of
cancer cells (24). It has been
reported that FAK is activated by integrin-clustering and
autophosphorylated at Tyr397 (25).
Once phosphorylated, FAK supplies a binding site for Src to form
the FAK-Src complex, which recruits more signaling molecules
participating in integrin-mediated signaling transduction (26). Therefore, we assessed the expression
of phosphorylated FAK and Src in breast cancer cells after
treatment with lunasin for 24 h. As shown in Fig. 5A and B, lunasin strongly inhibited
the phosphorylation of FAK and Src in both MCF-7 and MDA-MB-231
cell lines. It did not have significant effects on total FAK and
Src, which demonstrated that lunasin exerted its antimetastatic
effect by preventing the phosphorylation of FAK and Src from
forming the FAK-Src complex.
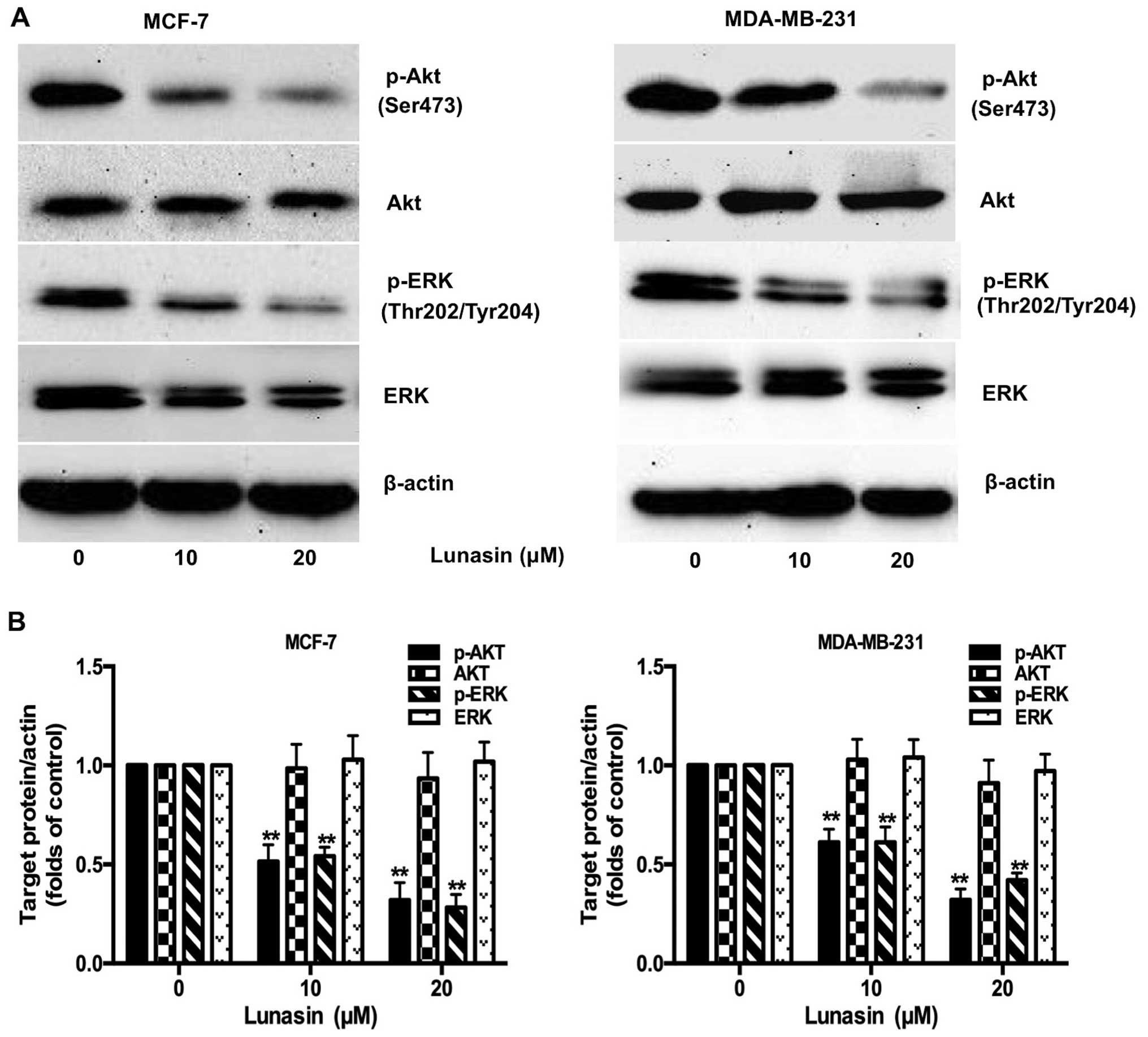
Lunasin suppresses the phosphorylation of
ERK and Akt
Studies have found that the Ras/MEK/ERK and the
PI3K/Akt pathways, the downstream signaling molecules activated by
the FAK-Src complex, are correlated to the survival and metastasis
of cancer cells (27). Hence, the
phosphorylation of key molecules in these pathways, Akt and ERK,
were examined further by western blot analysis. As shown in
Fig. 6, the phosphorylation of Akt
and ERK was attenuated by lunasin (10–20 µM) in a
concentration-dependent manner. There were no significant
differences in the total amount of Akt and ERK. The results
illustrated that lunasin may inhibit the metastasis of breast
cancer cells by blocking the PI3K/Akt/ERK pathway.
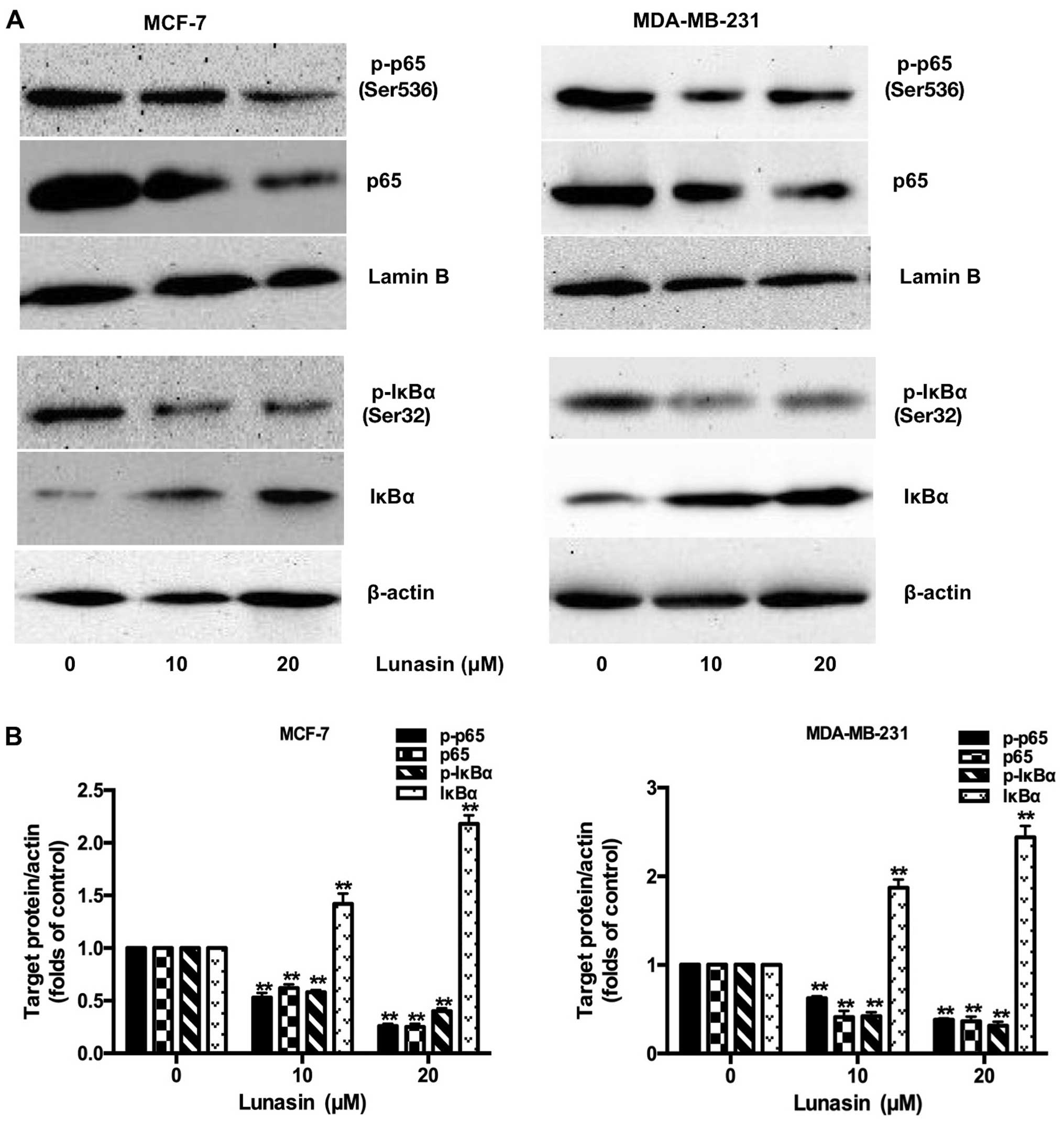
Lunasin inactivates the translocation
process of NF-κB into the nucleus
NF-κB, an important transcriptional factor,
regulates the expression of many genes involved in mammary
carcinogenesis, survival and metastasis of breast cancer cells
(28). The activation of NF-κB is
controlled by the targeted phosphorylation and subsequent
degradation of IκB, which prevents NF-κB from translocation into
the nucleus. To further explore the underlying mechanisms of
lunasin, the expression of the NF-κB inhibitor, IκBα and p-IκBα in
the cytoplasm, the translocation of NF-κB, p65 and p-p65 in the
nucleus, were all examined in our study using western blot
analysis. The results showed that the phosphorylation and
degradation of IκBα were markedly inhibited by lunasin (10–20
µM) with enhanced IκB expression (Fig. 7). Accordingly, the translocation of
activated NF-κB, p-p65 and p65 in the nucleus, was decreased
following treatment with lunasin.
Discussion
Metastasis is a series of complex processes
consisting of cancer cell proliferation, cell motility, cell
adhesion to ECM and ECM proteolysis. It has been reported that 90%
of breast cancer deaths are attributed to the metastasis of cancer
cells. Therefore, it is urgent to develop new therapeutic agents to
inhibit the metastasis of breast cancer. Lunasin, a 43-amino acid
peptide derived from soybeans, is a bioactive peptide which has
been demonstrated as an effective chemopreventive and antitumor
agent in cancer therapy. However, knowledge of the inhibitory
effects of lunasin on the metastasis of breast cancer cells and its
underlying mechanism is limited. In this study, we demonstrated
that lunasin suppressed the proliferation, migration and invasion
of breast cancer cells. Meanwhile, the activity and expression of
MMP-2/-9, phosphorylation of FAK, Src, Akt, ERK, p65, IκBα and
translocation of p65 were decreased, which indicated that lunasin
inhibited the metastasis of breast cancer cells via suppression of
the FAK/Akt/ERK and NF-κB pathways.
In this present study, we found that lunasin
suppressed the proliferation of MCF-7 and MDA-MB-231 breast cancer
cells with IC50 values of 508.6 µM and 224.7
µM, respectively. These values were slightly higher than
those of previous research (29),
in which the IC50 value of MDA-MB-231 was 181 µM
following treatment by synthetic lunasin. In order to examine the
antimetastatic potential of the peptide, 10–20 µM lunasin
was selected because of its non-cytotoxicity to MCF-7 and
MDA-MB-231 breast cancer cells. Migration and invasion are two
critical processes during the metastasis of cancer cells. The
migratory and invasive abilities of cancer cells enable them to
migrate to adjacent tissues finally leading to metastasis. In the
present study, the inhibitory effects of lunasin on migration and
invasion were observed by scratch wound and Transwell assays. As
shown in Figs. 2 and 3, lunasin was able to effectively block
the migration and invasion of MCF-7 and MDA-MB-231 cells in a
concentration-dependent manner. Furthermore, as we examined,
lunasin also inhibited the proteolytic degradation of the ECM.
MMPs, a family of zinc-containing endopeptidases that can degrade
the ECM, play a vital role in this process (30). Among the MMPs, MMP-2 and MMP-9 have
been reported as being highly expressed in aggressive breast tumors
and associated with poor prognosis (31,32).
In our study, it was found that lunasin strongly inhibited the
activity and expression of MMP-9 in both the MCF-7 and MDA-MB-231
cell lines. Nevertheless, the decrease in MMP-2 was much more
slight compared with MMP-9 in the MCF-7 and MDA-MB-231 cell lines.
The results demonstrated that lunasin plays a more important role
in the suppression of MMP-9 rather than MMP-2. In conclusion, the
results substantiated the inhibitory ability of lunasin on the
proliferation, migration, invasion and ECM degradation, which
indicates that lunasin inhibits the metastasis of breast cancer
cells.
Integrins, a family of heterodimer transmembrane
glycoproteins, consist of 18 α and 8 β subunits to form 24
different subtypes (33). In breast
cancer cells, αVβ3,
αVβ5 and α5β1 integrins
are often highly expressed in comparison to other subtypes, which
can bind to ECM proteins such as fibronectin, vitronectin and
collagen through a tripeptide motif Arg-Gly-Asp (RGD) to facilitate
cell adhesion, migration, invasion and intracellular signaling
(34). Hence, peptides containing
the RGD motif are considered to be ideal agents to compete with ECM
to interact specifically with integrins (35). Previous studies suggest that lunasin
possesses an affinity for αVβ3 and
α5β1 in macrophages (15) and cancer cells (36–38)
which enable it to prevent the activation of the integrin-mediated
signaling pathway by direct blockade. In this present study, we
selected two typical breast cancer cell lines, ER-positive MCF-7
cells and ER-negative MDA-MB-231 cells with expression of different
integrin subtypes. Research has confirmed that in MDA-MB-231 cancer
cells, αVβ3 is often highly expressed
(39–41), while MCF-7 cells are
αVβ3 negative but with
αVβ5 and α5β1
expression (42). Thus, we examined
the inhibitory effect on these two breast cancer cells and aimed to
ascertain the underlying mechanism involved. The differences in the
inhibitory effects of lunasin on these two cell lines may be
ascribed to the different integrins and ER expression on their
surface.
FAK, a receptor protein-tyrosine kinase, plays a
central role in the intracellular signaling of integrin. Once
integrin binds to the ECM, FAK is recruited to the clustering
integrins and activated by autophosphorylation. Then Src is further
phosphorylated to form the FAK-Src complex, which initiates the
signal transduction of cell survival, proliferation, migration and
invasion. As examined in our study (Fig. 5), lunasin strongly inhibited the
phosphorylation of FAK and Src in both MCF-7 and MDA-MB-231 cell
lines with no changes in total FAK and Src proteins. The results
were consistent with the study conducted by Dia et al
(14) in which it was reported,
that lunasin suppresses the metastasis of colon cancer cells
through inhibition of the FAK/Src pathway.
Previous studies have shown that decreased levels of
MMP-9 and MMP-2, in breast cancer cells, are highly related to the
inhibition of PI3K/Akt and ERK, both of which are downstream
targets of FAK (43,44). PI3K, a lipid kinase, participates in
multiple cell signaling pathways through the activation of Akt.
Additionally, activated Akt can lead to the invasion and metastasis
of cancer cells by stimulating the secretion of MMPs (45,46).
It is accepted that the MAP family kinases possibly take part in
signaling processes that modulate MMPs, including MMP-9 (47). In order to gain a better
understanding of the mechanism by which lunasin inhibits tumor
metastasis, the expression of Akt and ERK was examined by western
blotting (Fig. 6). The results
confirmed that lunasin markedly blocked the phosphorylation of Akt
and ERK which may stop the cellular signaling to transcription
factors of MMPs.
Moreover, NF-κB is considered to regulate the
survival, proliferation, chemoresistance, angiogenesis, cellular
invasion and migration of cancer cells. Previous studies have
reported that NF-κB may be the transcriptional regulatory factor of
MMPs and other genes, while IκB is an inhibitor which prevents
NF-κB translocation into the nucleus (48). It was reported that PI3K activates
IκB kinases (IKKs) to phosphorylate IκB, leading to its
ubiquitination and proteasomal degradation to release the NF-κB/Rel
complex. Lunasin has been shown to suppress the FAK/ERK/NF-κB
signaling pathway in colon cancer cells (14) and Akt/NF-κB pathway in macrophages
during activation of lipopolysaccharide-induced inflammation
(49). In our study, we also found
that lunasin inhibited the metastasis of breast cancer cells
through preventing IκB phosphorylated and enhancing IκB protein
expression, which decreased the phosphorylation and translocation
of NF-κB.
In summary, lunasin inhibited the cell
proliferation, cell migration, cell invasion and the activity and
expression of MMP-2 and MMP-9 in breast cancer cells. We proposed
that lunasin possibly exerted its inhibitory effect via the
suppression of the integrin-mediated FAK/Akt/ERK and NF-κB
signaling pathways. These results provide a foundation for future
investigation of lunasin as an effective antitumor and
antimetastatic agent for breast cancer therapy.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 8147929 and 81573539), the
Natural Science Foundation of Heilongjiang Province (no. H2015042),
the Excellent Creative Talents Support Project of Heilongjiang
University of Chinese Medicine (no. 2012RCQ12), the Research
Foundation of Heilongjiang University of Chinese Medicine (no.
201004), and the Natural Science Foundation of Heilongjiang
Province (no. H2015042).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Weigelt B, Peterse JL and van't Veer LJ:
Breast cancer metastasis: Markers and models. Nat Rev Cancer.
5:591–602. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Danen EH: Integrins: regulators of tissue
function and cancer progression. Curr Pharm Des. 11:881–891. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eliceiri BP and Cheresh DA: Adhesion
events in angiogenesis. Curr Opin Cell Biol. 13:563–568. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruoslahti E: Specialization of tumour
vasculature. Nat Rev Cancer. 2:83–90. 2002. View Article : Google Scholar
|
|
6
|
Temming K, Schiffelers RM, Molema G and
Kok RJ: RGD-based strategies for selective delivery of therapeutics
and imaging agents to the tumour vasculature. Drug Resist Updat.
8:381–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galvez AF and de Lumen BO: A soybean cDNA
encoding a chromatin-binding peptide inhibits mitosis of mammalian
cells. Nat Biotechnol. 17:495–500. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong HJ, Lam Y and de Lumen BO: Barley
lunasin suppresses ras-induced colony formation and inhibits core
histone acetylation in mammalian cells. J Agric Food Chem.
50:5903–5908. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong HJ, Jeong JB, Kim DS, Park JH, Lee
JB, Kweon DH, Chung GY, Seo EW and de Lumen BO: The cancer
preventive peptide lunasin from wheat inhibits core histone
acetylation. Cancer Lett. 255:42–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Silva-Sánchez C, de la Rosa AP,
León-Galván MF, de Lumen BO, de León-Rodríguez A and de Mejía EG:
Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J
Agric Food Chem. 56:1233–1240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong JB, Jeong HJ, Park JH, Lee SH, Lee
JR, Lee HK, Chung GY, Choi JD and de Lumen BO: Cancer-preventive
peptide lunasin from Solanum nigrum L. inhibits acetylation of core
histones H3 and H4 and phosphorylation of retinoblastoma protein
(Rb). J Agric Food Chem. 55:10707–10713. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hernández-Ledesma B, Hsieh CC and de Lumen
BO: Relationship between lunasin's sequence and its inhibitory
activity of histones H3 and H4 acetylation. Mol Nutr Food Res.
55:989–998. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Lumen BO: Lunasin: a cancer-preventive
soy peptide. Nutr Rev. 63:16–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dia VP and Gonzalez de Mejia E: Lunasin
potentiates the effect of oxaliplatin preventing outgrowth of colon
cancer metastasis, binds to α5β1 integrin and
suppresses FAK/ERK/NF-κB signaling. Cancer Lett. 313:167–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cam A and de Mejia EG: RGD-peptide lunasin
inhibits Akt-mediated NF-κB activation in human macrophages through
interaction with the αVβ3 integrin. Mol Nutr
Food Res. 56:1569–1581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hsieh CC, Hernandez-Ledesma B and de Lumen
B: Cell proliferation inhibitory and apoptosis-inducing properties
of anacardic acid and lunasin in human breast cancer MDA-MB-231
cells. Food Chem. 125:630–636. 2011. View Article : Google Scholar
|
|
17
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: a convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu L and Deng X: Protein kinase Ciota
promotes nicotine-induced migration and invasion of cancer cells
via phosphorylation of micro- and m-calpains. J Biol Chem.
281:4457–4466. 2006. View Article : Google Scholar
|
|
19
|
Li C, Zhao Y, Yang D, Yu Y, Guo H, Zhao Z,
Zhang B and Yin X: Inhibitory effects of kaempferol on the invasion
of human breast carcinoma cells by downregulating the expression
and activity of matrix metalloproteinase-9. Biochem Cell Biol.
93:16–27. 2015. View Article : Google Scholar
|
|
20
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hernández-Ledesma B, Hsieh CC and de Lumen
BO: Lunasin, a novel seed peptide for cancer prevention. Peptides.
30:426–430. 2009. View Article : Google Scholar
|
|
22
|
McCawley LJ and Matrisian LM: Matrix
metalloproteinases: multifunctional contributors to tumor
progression. Mol Med Today. 6:149–156. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanemaaijer R, Verheijen JH, Maguire TM,
Visser H, Toet K, McDermott E, O'Higgins N and Duffy MJ: Increased
gelatinase-A and gelatinase-B activities in malignant vs. benign
breast tumors. Int J Cancer. 86:204–207. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J and Hochwald SN: The role of FAK
in tumor metabolism and therapy. Pharmacol Ther. 142:154–163. 2014.
View Article : Google Scholar
|
|
25
|
Toutant M, Costa A, Studler JM, Kadaré G,
Carnaud M and Girault JA: Alternative splicing controls the
mechanisms of FAK autophosphorylation. Mol Cell Biol. 22:7731–7743.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schlaepfer DD, Mitra SK and Ilic D:
Control of motile and invasive cell phenotypes by focal adhesion
kinase. Biochim Biophys Acta. 1692:77–102. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Nimwegen MJ and van de Water B: Focal
adhesion kinase: a potential target in cancer therapy. Biochem
Pharmacol. 73:597–609. 2007. View Article : Google Scholar
|
|
28
|
Wu JT and Kral JG: The NF-kappaB/IkappaB
signaling system: a molecular target in breast cancer therapy. J
Surg Res. 123:158–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh CC, Hernández-Ledesma B and de Lumen
BO: Lunasin, a novel seed peptide, sensitizes human breast cancer
MDA-MB-231 cells to aspirin-arrested cell cycle and induced
apoptosis. Chem Biol Interact. 186:127–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pellikainen JM, Ropponen KM, Kataja VV,
Kellokoski JK, Eskelinen MJ and Kosma VM: Expression of matrix
metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special
reference to activator protein-2, HER2, and prognosis. Clin Cancer
Res. 10:7621–7628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–40. 2009.PubMed/NCBI
|
|
33
|
Takada Y, Ye X and Simon S: The integrins.
Genome Biol. 8:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cabodi S, Di Stefano P, Leal MP,
Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D,
Tornillo G, et al: Integrins and signal transduction. Adv Exp Med
Biol. 674:43–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meyer A, Auernheimer J, Modlinger A and
Kessler H: Targeting RGD recognizing integrins: drug development,
biomaterial research, tumor imaging and targeting. Curr Pharm Des.
12:2723–2747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dia VP and Gonzalez de Mejia E: Lunasin
induces apoptosis and modifies the expression of genes associated
with extracellular matrix and cell adhesion in human metastatic
colon cancer cells. Mol Nutr Food Res. 55:623–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
de Mejia EG, Wang W and Dia VP: Lunasin,
with an arginine-glycine-aspartic acid motif, causes apoptosis to
L1210 leukemia cells by activation of caspase-3. Mol Nutr Food Res.
54:406–414. 2010. View Article : Google Scholar
|
|
38
|
Inaba J, McConnell EJ and Davis KR:
Lunasin sensitivity in non-small cell lung cancer cells is linked
to suppression of integrin signaling and changes in histone
acetylation. Int J Mol Sci. 15:23705–23724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meyer T, Marshall JF and Hart IR:
Expression of alpha-V integrins and vitronectin receptor identity
in breast cancer cells. Br J Cancer. 77:530–536. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rashidi LH, Homayoni H, Zou X, Liu L and
Chen W: Investigation of the strategies for targeting of the
afterglow nanoparticles to tumor cells. Photodiagnosis Photodyn
Ther. 13:244–254. 2016. View Article : Google Scholar
|
|
41
|
Shan D, Li J, Cai P, Prasad P, Liu F,
Rauth AM and Wu XY: RGD-conjugated solid lipid nanoparticles
inhibit adhesion and invasion of αVβ3
integrin-overexpressing breast cancer cells. Drug Deliv Transl Res.
5:15–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zeng F, Luo F, Lv S, Zhang H, Cao C, Chen
X, Wang S, Li Z, Wang X, Dou X, et al: A monoclonal antibody
targeting neuropilin-1 inhibits adhesion of MCF7 breast cancer
cells to fibronectin by suppressing the FAK/p130cas signaling
pathway. Anticancer Drugs. 25:663–672. 2014.PubMed/NCBI
|
|
43
|
Pal S, Moulik S, Dutta A and Chatterjee A:
Extracellular matrix protein laminin induces matrix
metalloproteinase-9 in human breast cancer cell line MCF-7. Cancer
Microenviron. 7:71–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao W, Zheng W and Chen T: Ruthenium
polypyridyl complex inhibits growth and metastasis of breast cancer
cells by suppressing FAK signaling with enhancement of
TRAIL-induced apoptosis. Sci Rep. 5:91572015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tian T, Nan KJ, Guo H, Wang WJ, Ruan ZP,
Wang SH, Liang X and Lu CX: PTEN inhibits the migration and
invasion of HepG2 cells by coordinately decreasing MMP expression
via the PI3K/Akt pathway. Oncol Rep. 23:1593–1600. 2010.PubMed/NCBI
|
|
46
|
Jung CH, Kim EM, Park JK, Hwang SG, Moon
SK, Kim WJ and Um HD: Bmal1 suppresses cancer cell invasion by
blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway.
Oncol Rep. 9:2109–2113. 2013.
|
|
47
|
O-charoenrat P, Wongkajornsilp A,
Rhys-Evans PH and Eccles SA: Signaling pathways required for matrix
metalloproteinase-9 induction by betacellulin in head-and-neck
squamous carcinoma cells. Int J Cancer. 111:174–183. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kane LP, Shapiro VS, Stokoe D and Weiss A:
Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 9:601–604.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Mejia EG and Dia VP: Lunasin and
lunasin-like peptides inhibit inflammation through suppression of
NF-kappaB pathway in the macrophage. Peptides. 30:2388–2398. 2009.
View Article : Google Scholar : PubMed/NCBI
|