Introduction
Ovarian cancer is the fifth common cause of
cancer-related deaths in women, and the rate of mortality is the
highest in all the gynecologic malignancies. The high rates of
mortality in women with ovarian cancer are due to its late
diagnosis, with approximately three-fourths of patients diagnosed
with advanced disease (1). Although
in recent years, with the advances in chemotherapy, the treatment
for ovarian cancer has shown significant improvement with
consequent reduction in the rate of mortality, the treatment is
susceptible to chemotherapeutic drug resistance, in particular the
emergence of multidrug resistance (MDR). The 5-year rate of
survival of patients with ovarian cancer is only 27% (2).
A very few subgroups of tumor cells have the ability
to self-renew, differentiate, and form secondary/tertiary tumors
after serial xenotransplantation into immune-compromised animal
models and are called cancer stem cells (CSCs) or cancer-initiating
cells (3). With the development of
CSCs, ovarian CSCs have been isolated from ovarian solid tumors,
ovarian cell lines, and ovarian cancer ascites (4). Increasing number of studies have
demonstrated that CSCs are closely associated with drug resistance.
ATP-binding cassette (ABC), subfamily G, member 2 (ABCG2), is
highly expressed in various stem cell populations, and has become
one of the stem cell markers (5,6).
Furthermore, ABCG2 is an important MDR transporter, which can
efflux various chemotherapeutic drugs and may contribute to drug
resistance of cancer cells (7–10).
Current evidence suggests that ABCG2 gene
transcription is regulated by a number of trans-acting elements
including hypoxia-inducible factor 1α (HIF-1α), estrogen receptor,
and peroxisome proliferator-activated receptor. Among these,
HIF-1α, a master transcription factor that regulates
hypoxia-responsive genes, has been recognized to play a critical
role in tumor, metastasis, and chemoradiation resistance (11–15).
Ursolic acid (UA; 3β-hydroxy-urs-12-en-28-oic acid)
is a naturally-derived pentacyclic triterpene acid widely present
in medicinal and other plants (16). UA has a number of biological
properties including antioxidation, anti-inflammation, anticancer,
and hepatoprotection (17–19). However, the exact mechanism through
which the anticancer and reversal of multidrug resistant properties
occurs remains unclear. Therefore, major improvements are required
in the development of safe and effective method for the reversal of
multidrug resistance.
Materials and methods
Cells and cell culture
Ovarian cancer cell line SKOV3 was obtained from the
International Peace Maternity and Child Health Hospital (Shanghai,
China). This cell line had been used in our previous study which
was cooperating with International Peace Maternity and Child Health
Hospital and approved by the Ethics Committee of the International
Peace Maternity and Child Health Hospital (20). The HEY and A2780 cells were
purchased from the Shanghai Cell Collection (Shanghai, China)
(http://www.cellbank.org.cn). All cells
were maintained in RPMI-1640 (Hyclone, Logan, UT, USA),
supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand
Island, NY, USA) at 37°C.
Ovarian cancer sphere culture: Single cancer cells
were-plated in the cell culture dish (100×200 mm style; Corning,
NY, USA), which had been treated with poly-(2-hydroxyethyl
methacrylate (poly-HEMA) (Sigma, St. Louis, MO, USA), for
continuous suspension culture. The cells were maintained in
embryonic stem (ES) medium (serum-free Dulbecco's modified Eagle's
medium-F12; Hyclone), supplemented with 10 ng/ml basic fibroblast
growth factor (bFGF; Gibco), 5 μg/ml insulin (Sigma), 1 mM
L-glutamine (Sigma), 10% knockout serum replacement and
penicillin/streptomycin (1000 U/ml and 100 mg/ml; Invitrogen,
Carlsbad, CA, USA) (21). Stem
cells grown under these conditions formed non-adherent spherical
clusters. To induce the hypoxic environment, the HERA cell
CO2 incubator (Thermo, Germany) was chosen and
maintained with 1% O2, 5% CO2, and 94%
N2.
Quantitative PCR
Total RNAs were extracted using the RNeasy
extraction kit (Qiagen, Zürich, Switzerland). After reverse
transcription of RNA to cDNA, quantitative polymerase chain
reaction (qPCR) using the converted cDNA as template was performed
in triplicate using SYBR Green PCR Master Mix. PCR was performed
using initial denaturation at 95°C for 2 min, followed by 40 cycles
for 10 sec at 95°C and 30 sec at 60°C. The threshold cycle (CT)
values of each sample were used in the post-PCR data analysis.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an
internal control for mRNA-level normalization. The following
primers were used: CD44 F: ACCCCATCCCAGACGAAGACAGTC, R:
GGGATGAAGGTCCTGCTTTCCG; NANOG F: GCA AAAAAGGAAGACAAGGTCC, R:
CCTTCTGCGTCA CACCATTG; OCT-4 F: CGAAGAGAAAGCGAACCAGT ATC, R:
AGAACCACACTGGACCACATC; ABCG2 F: GGT TTCCAAGCGTTCATTCAAA, R:
TAGCCCAAAGTAAAT GGCACCTA; HIF-1α F: CCACAGGACAGTACAGGATG, R:
TCAAGTCGTGCTGAATAATACC; GAPDH: F: GGT GGTCTCCTCTGACTTCAACA, R:
CCAAATTCGTTGT CATACCAGGAAATG.
Cell viability assay
For cell viability assay using Cell Counting Kit-8
(CCK-8), cells were seeded onto 96-well plates at 1×105
cells/well and the medium was treated with the drug (cisplatin-DDP,
UA) for 48 h. After 2 h of incubation with culture medium
containing the CCK-8 reagent (Tongji, Tokyo, Japan), the absorbance
was read at 450 nm using a microplate enzyme-linked immunosorbent
assay reader.
Apoptosis assay using Annexin V
staining
Apoptotic cells were quantified using an Annexin
V-APC/7-aminoactinomycin D (7-AAD) kit (Becton, Dickinson and Co.,
San Jose, CA, USA) and detected using flow cytometry according to
the manufacturer's protocol. After treatment with UA and/or DDP,
respectively, cells were resuspended in 100 μl 1X binding
buffer and incubated with 5 μl Annexin V-APC and 5 μl
7-AAD for 15 min in the dark. After staining, 400 μl of 1x
binding buffer was added to the cells, and samples were analyzed
using flow cytometry. Cells in the early stage of apoptosis stained
only-positive for Annexin V, while those in the late stage stained
positive for both Annexin V and 7-AAD.
Western blots
Whole-cell lysate for sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and cells were washed
twice with phosphate-buffered saline containing protease
inhibitors. The protein content was determined using the
bicinchoninic acid protein assay using a commercial kit (BSA
Protein Assay Reagent; Merck & Co., White House Station, NJ,
USA). The following antibodies were used: rabbit anti-CD44
antibody, rabbit anti-Nanog antibody, mouse anti-OCT-4 antibody,
rabbit anti-p-AKT-308 antibody, rabbit anti-p-AKT-492 antibody,
rabbit-AKT antibody, rabbit-PI3K antibody (all Cell Signaling
Technology, Beverly, MA, USA), mouse anti-HIF-1α antibody (Novus
Biologicals LLC, Littleton, CO, USA), rabbit anti-ABCG2 antibody,
rabbit-anti-P-gp antibody (all Abcam, Cambridge, MA, USA). Equal
loading was confirmed using GAPDH. Densitometric analysis was
performed using the Scion Imaging software (Scion Corp., Tokyo,
Japan), using GAPDH as a control for each sample.
Immunofluorescence staining
Cells were seeded onto glass slides and fixed with
4% paraformaldehyde, permeabilized with Triton X-100, and
subsequently incubated with the monoclonal mouse anti-ABCG2
antibody and mouse anti-HIF-1α (1:50) overnight at 4°C. Samples
were incubated with goat anti-mouse immunoglobulin G antibody
(Biyuntian Biological, Nanjing, China), and cell nuclei were
stained with 4′,6-diamidino-2-phenylindole (DAPI; Biyuntian
Biological). Images were captured using a 80i laser confocal
microscope (Nikon, Tokyo, Japan). Images were further digitally
processed for contrast enhancement using Adobe Photoshop.
Plasmid DNA amplification, extraction,
and purification
The plasmids were transformed into DH5a competent
cells, extracted, and purified according to the plasmid extraction
kit instructions (Tiangen, Beijing, China). The concentration of
the obtained plasmid DNA was measured using spectrophotometry, and
the absorbance of the plasmid was measured at 260 nm. The
OD260 titration was between 1.8 and 2.1, indicating that
there was no contamination in the plasmid DNA.
Plasmid transfection
Cell culture plate was prepared using 40% cell
density and 30 μl serum-free RPMI-1640 was added to a
plastic tube. Plasmid DNA (1 μg) was added to the tube and
mixed by pipetting. HilyMax was added to prepare DNA (μg):
HilyMax (μl) = 1:3 and mixed by pipetting, and the tube was
incubated at room temperature for 15 min. DNA-HilyMax complex was
added to the cell culture well. The plate was incubated at 37°C in
a CO2 incubator for 4 h. Later the medium was changed
and incubation was continued for 48 h.
Statistical analysis
All data are represented as mean ± standard
deviation. Statistical differences between two datasets were
compared using Student's t-test; nonparametric data were compared
using the Mann-Whitney U test. SPSS Statistics 21 was used for the
statistical analysis.
Results
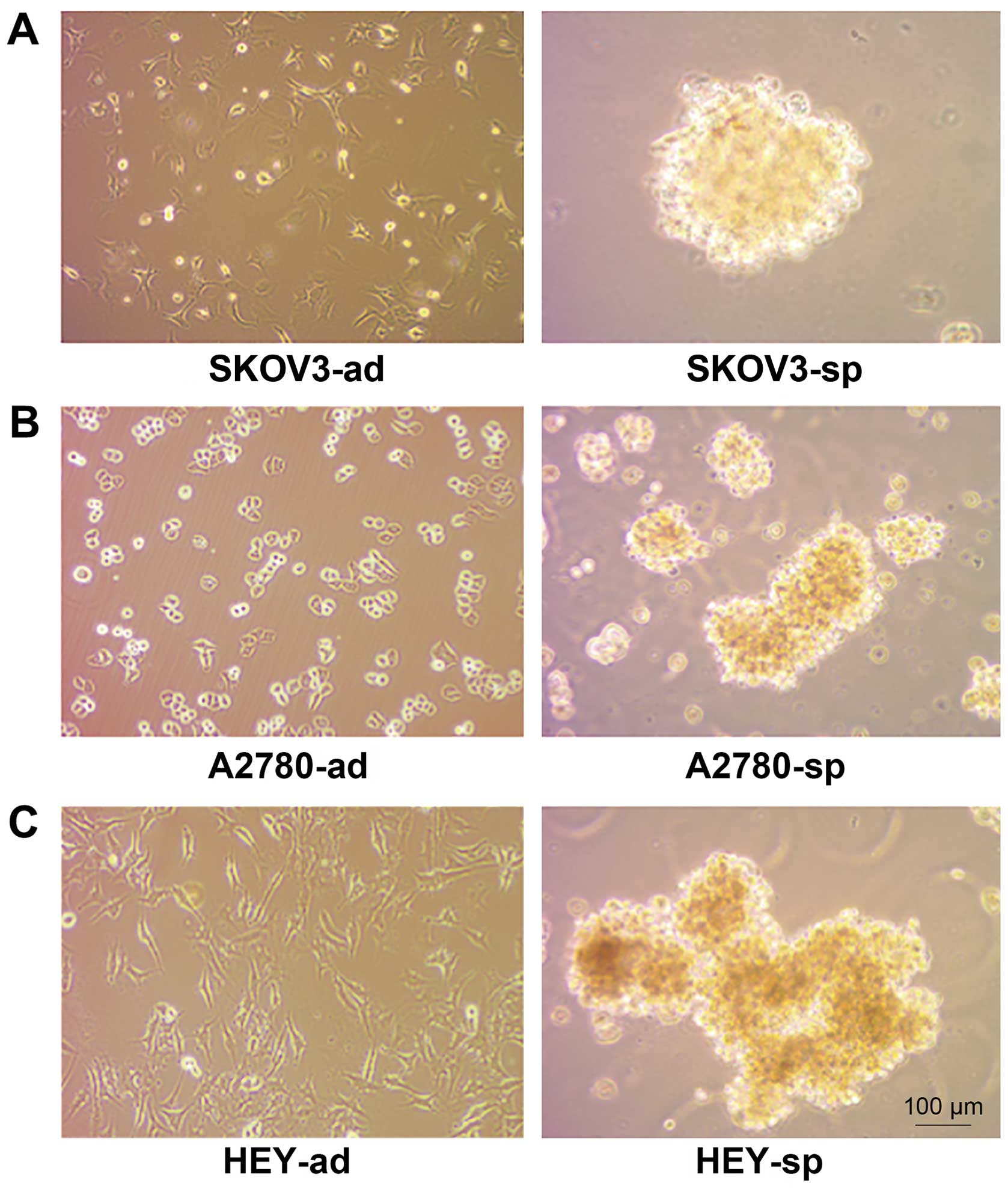
Ovarian CSC sphere culture
Ovarian CSC sphere culture and single cancer cells
(including SKOV3, A2780, HEY), which have been found to have
stemness characteristics when serum-free suspension culture
(21–23) when plated onto the cell culture
dish, were maintained in ES medium. Stem cells grown under these
conditions formed nonadherent spherical clusters. The formation of
sphere cells could be observed on the third day and the sphere
cells matured on the seventh day (Fig.
1).
Ovarian CSCs express stemness-related
genes and drug resistance significantly higher than normal adherent
cells
Ovarian cancer cells were maintained in RPMI-1640
with 10% FBS and ES medium, and the expression of stemness-related
genes (CD44, CD133, Nanog, Oct-4, and ABCG2) was detected (24–30).
The qPCR showed that the expression of CD44, CD133, Nanog, Oct-4,
and ABCG2 in stem cell phenotype of SKOV3 sphere cells was much
higher than those in the SKOV cells (Fig. 2A). The expression levels of these
stemness genes in HEY and A2780 sphere cells were much higher than
in the normal adherent cells (Fig.
2A–C). To investigate whether ovarian CSCs have significantly
higher drug resistance than normal adherent cells, different
concentrations of cisplatin (Sigma) were used to treat cells, and
after 48 h half maximal inhibitory concentration (IC50)
of DDP was analyzed using CCK-8. As shown in Fig. 2D, the median IC50 values
of SKOV3-sp, A2780-sp, and HEY-sp were 28.223, 35.414, and 30.031
μg/ml, respectively. The IC50 values of SKOV3-ad,
A2780-ad, and HEY-ad were 4.910, 7.073, and 6.576 μg/ml
(Fig. 2E), respectively.
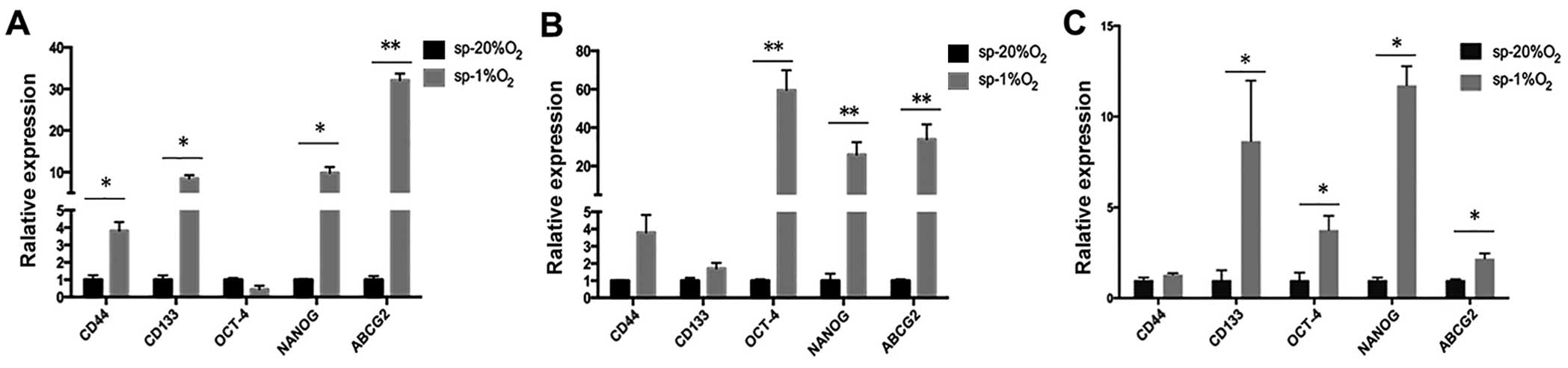
 | Figure 2Ovarian cells have characteristics of
stem cells. (A–C) Quantitative PCR shows that under stem
cell-selective conditions, SKOV3-sp, HEY-sp, and A2780-sp cells
overexpressed stemness genes CD44, CD133, Nanog, Oct-4, and ABCG2
compared with SKOV3-ad (*P<0.05,
**P<0.01). (D) The sphere cells showed stronger drug
resistance as compared with the adherence cells after treatment
with DDP (0, 4, 8, 16, and 32 μg/ml). (E) The cell growth
inhibitory rate of SKOV3-ad, A2780-ad and HEY-ad with DDP (0, 4, 8,
16, and 32 μg/ml). |
It was observed that the IC50 of sphere
cells is much higher than the normal adherent cells, and sphere
cells exhibited higher resistance to chemotherapeutic drugs, with
higher rates of survival.
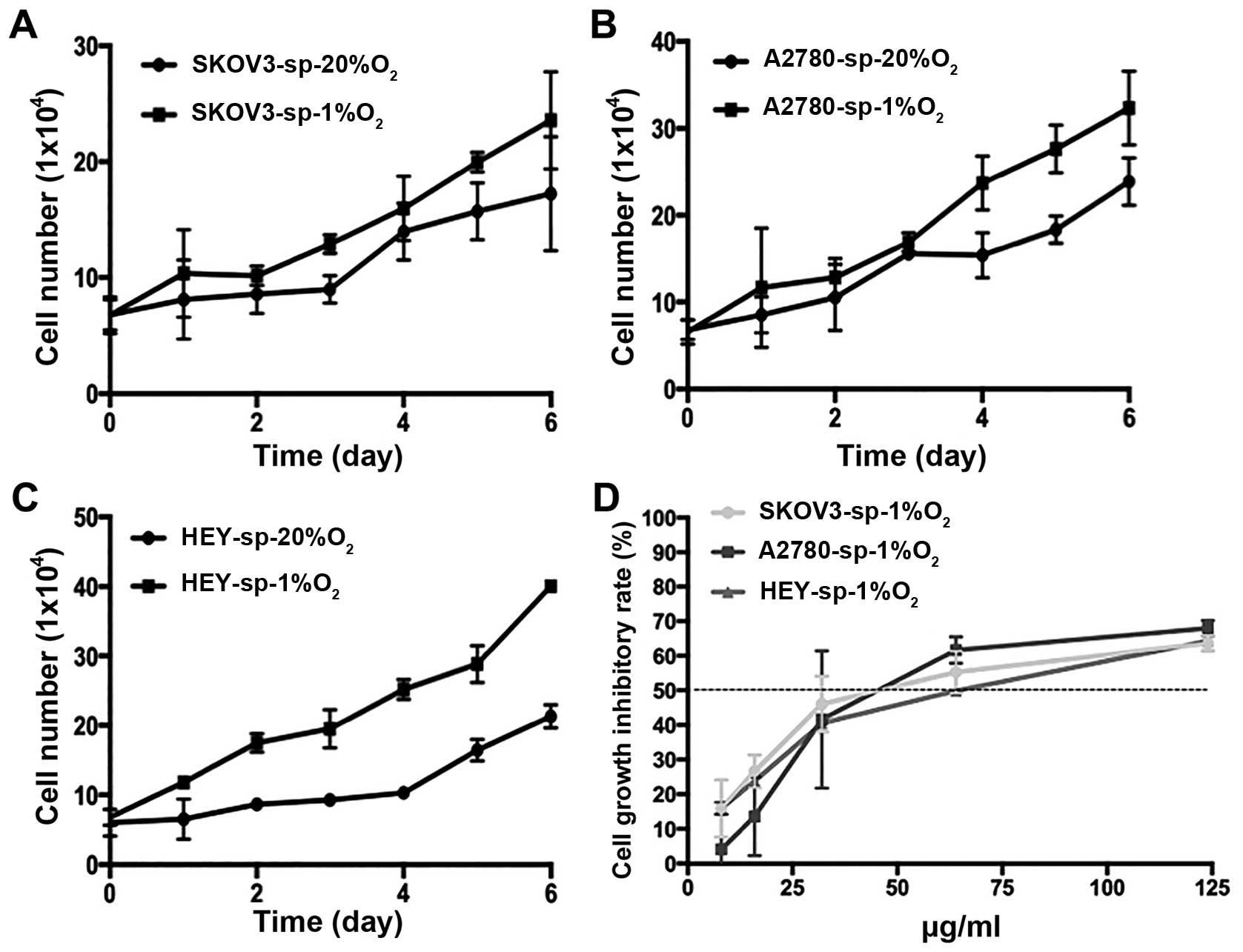
Under hypoxic conditions, ovarian CSCs
grow faster and more drug-resistant than under normoxia
The SKOV3-sp, A2780-sp, and HEY-sp cells grown under
20% or 1% oxygen at different time points and proliferation were
measured by counting the cells (Fig.
3A–C). As shown in Fig. 3A and
B, the initial concentrations of SKOV3-sp, A2780-sp, and HEY-sp
were 6.7×104, 6.5×104 and 6.0×104,
respectively, after 7 days of culture under different conditions.
The sphere cells grew faster than under normoxia. In this
experiment, it can be observed that hypoxia can promote the
proliferation of sphere cells. At the same time, under hypoxic
condition, the sphere cells developed more drug resistance than
under normoxia. The IC50 values of SKOV3-sp, A2780-sp,
and HEY-sp were 51.653, 53.889, and 60.774 μg/mL,
respectively (Fig. 3D). Thus, under
hypoxic condition, the IC50 increased significantly.
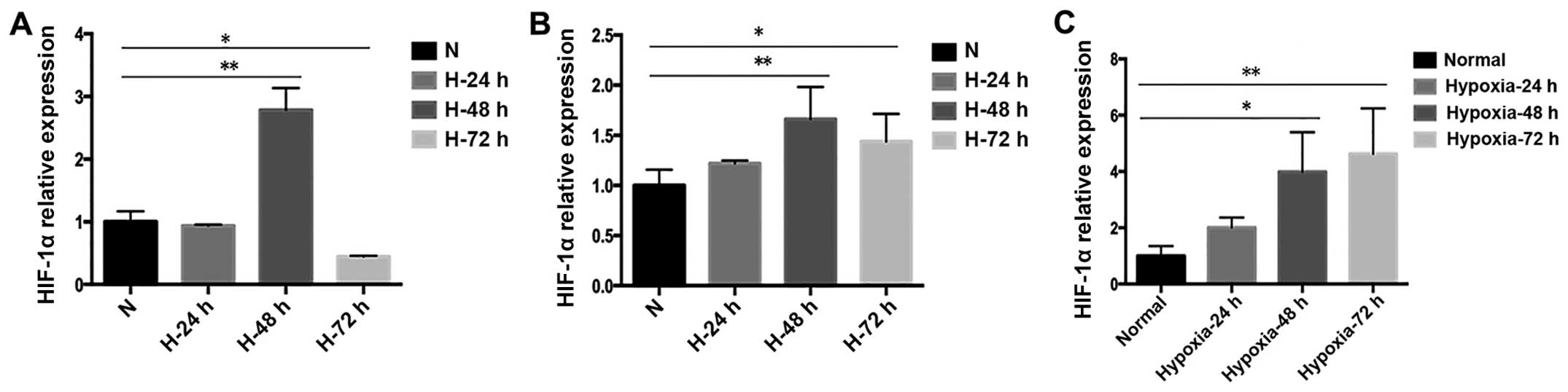
Under hypoxic conditions, the ovarian
CSCs exhibit higher stemness than under normal conditions
Recent advances in cancer research have demonstrated
that the enhanced expression and activation of HIFs are frequent in
cancer cells during the progression of cancer and is associated
with the acquisition of a more malignant behavior, drug resistance,
and poor rate of survival in patients with cancer (31–34).
Hence the expression of HIF-1α at different time points was tested
(Fig. 4). It was observed that when
SKOV3-sp, HEY-sp, and A2780-sp cells were cultured under hypoxic
condition (1% O2) for 24, 48, and 72 h, the expression
of HIF-1 in SKOV3 was higher at 48 h than at 24 or 72 h (Fig. 4A), and the expression of HIF-1α in
HEY-sp was the same as SKOV3-sp (Fig.
4B). However, in A2780-sp, it was found that after 72 h
hypoxia, the expression of HIF-1α was higher than at 24 or 48 h
(Fig. 4C). In subsequent
experiments, 48 h was selected as the time point for culture since
the expression of HIF was significantly increased in all the three
cell strains. When cultured under normal condition, the expression
of HIF-1α did not show difference between adherent and sphere
cells. The expression of HIF-1α in the HEY-sp, and A2780-sp cells
after 48 h under hypoxia was significantly increased and was
statistically significant (Fig.
5).
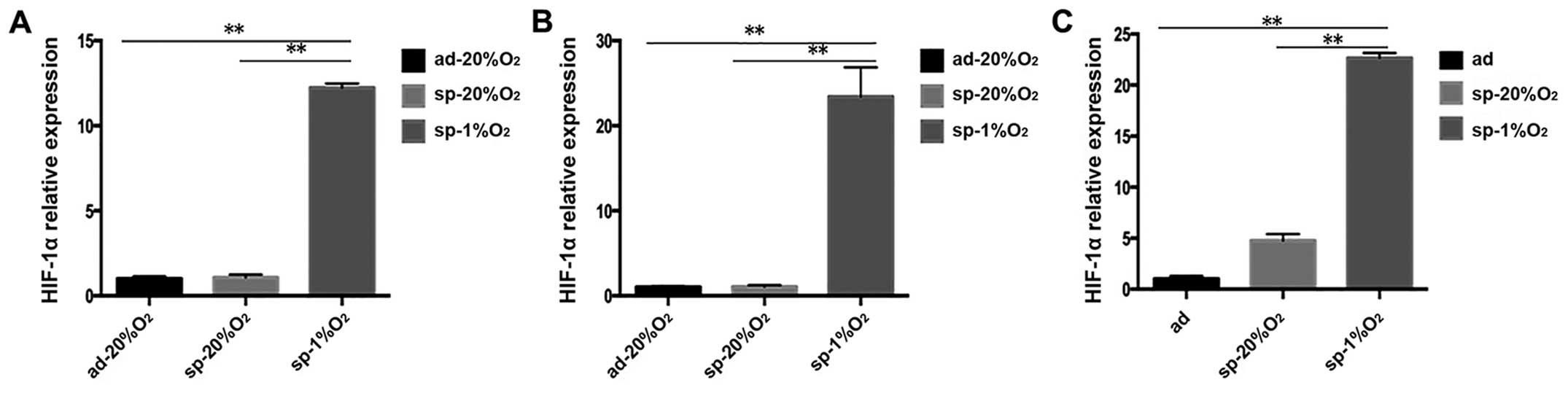
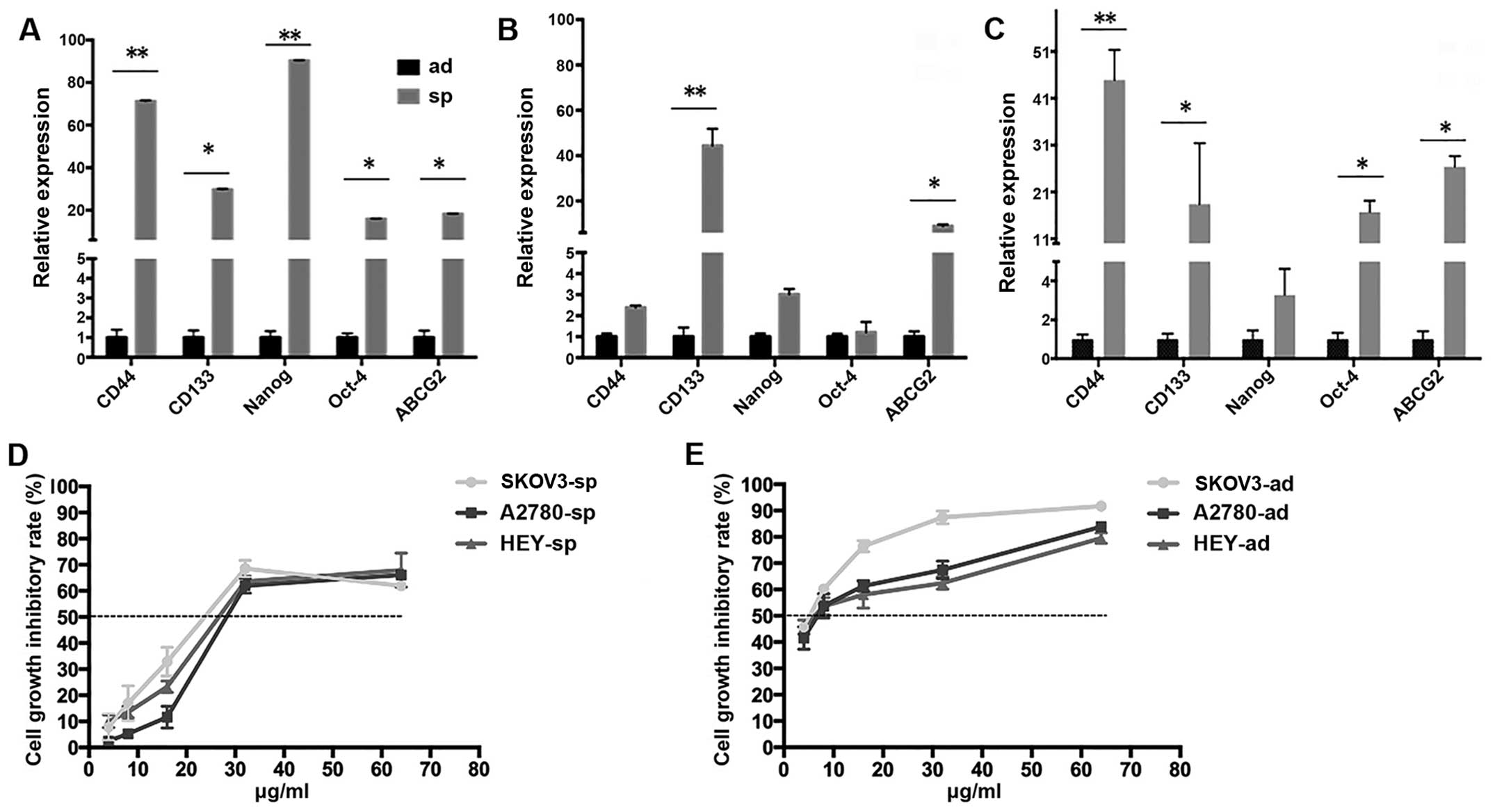
In addition, when the sphere cells were cultured
under hypoxic condition for 48 h, in SKOV3-sp cells, the expression
of stemness genes CD44, CD133, and Nanog was significantly
increased (P<0.05) and the drug resistance gene was apparently
more elevated (P<0.01) (Fig.
6A). In HEY-sp cells after 48 h hypoxia culture, the expression
of stemness genes Nanog and Oct-4 and drug resistance gene ABCG2
was significantly increased (P<0.01) (Fig. 6B). In the A2780-sp, the expression
of stemness genes CD133, Nanog, and Oct-4 and drug resistance gene
ABCG2 was higher than in normal culture (P<0.05) (Fig. 6C). It was found that after hypoxia
culture, with the increase in HIF-1α, the expression of stemness
genes and drug gene ABCG2 was elevated.
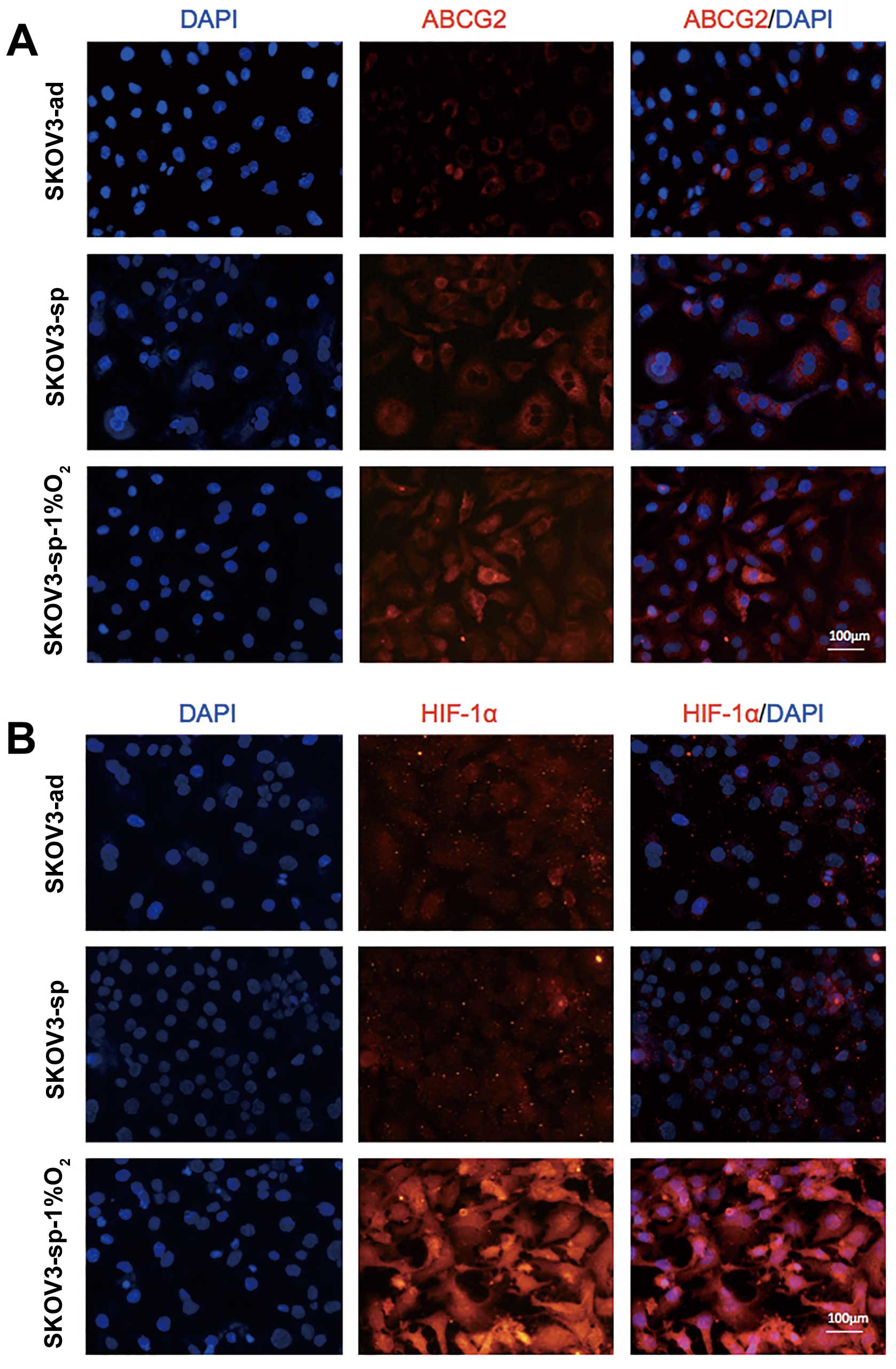
To further understand the expression of HIF-1α in
ABCG2, the increase in the expression of ABCG2 in SKOV3 cells under
hypoxic condition was chosen (Fig.
6), and immunofluorescence staining was performed on SKOV3-ad
and SKOV3-sp under different culture conditions. The ABCG2 proteins
are mainly found on the plasma membrane (Fig. 7A). It was observed that SKOV3-sp is
expressed at higher level than the SKOV3-ad. When SKOV3-sp was
cultured under different conditions for 48 h, the sphere cells
under hypoxic condition expressed ABCG2 at much higher level than
under normal condition (Fig. 7A).
At the same time, the expression of HIF-1α between SKOV3-ad and
SKOV3-sp did not show any difference, and when the SKOV3-sp was
cultured under hypoxic condition, the expression of HIF-1α
increased significantly (Fig.
7B).
UA inhibits the proliferation and
enhances the sensitivity of cisplatin in ovarian CSCs
In this experiment, it was examined whether UA could
inhibit the proliferation of ovarian cells and when UA combined
with cisplatin can enhance the sensitivity of cisplatin. As shown
in Fig. 8B, UA inhibits the
adherent and sphere cells. The IC50 values at 48 h for
SKOV3-ad, A2780-ad, and HEY-ad were 19.370, 25.257, and 19.349
μg/ml, respectively. The IC50 values of SKOV3-sp,
A2780-sp, and HEY-sp were 31.669, 36.745, and 39.239 μg/ml,
respectively. Among these, the IC10 values of SKOV3-sp,
HEY-sp, and A2780-sp were 2.934, 5.359, and 2.557 μg/ml
(Fig. 8C). Less than
IC10, cell survival was not found to be significantly
different from untreated cells. Hence, for cell proliferation
experiments, the cells were treated with UA in the concentration
range of IC10.
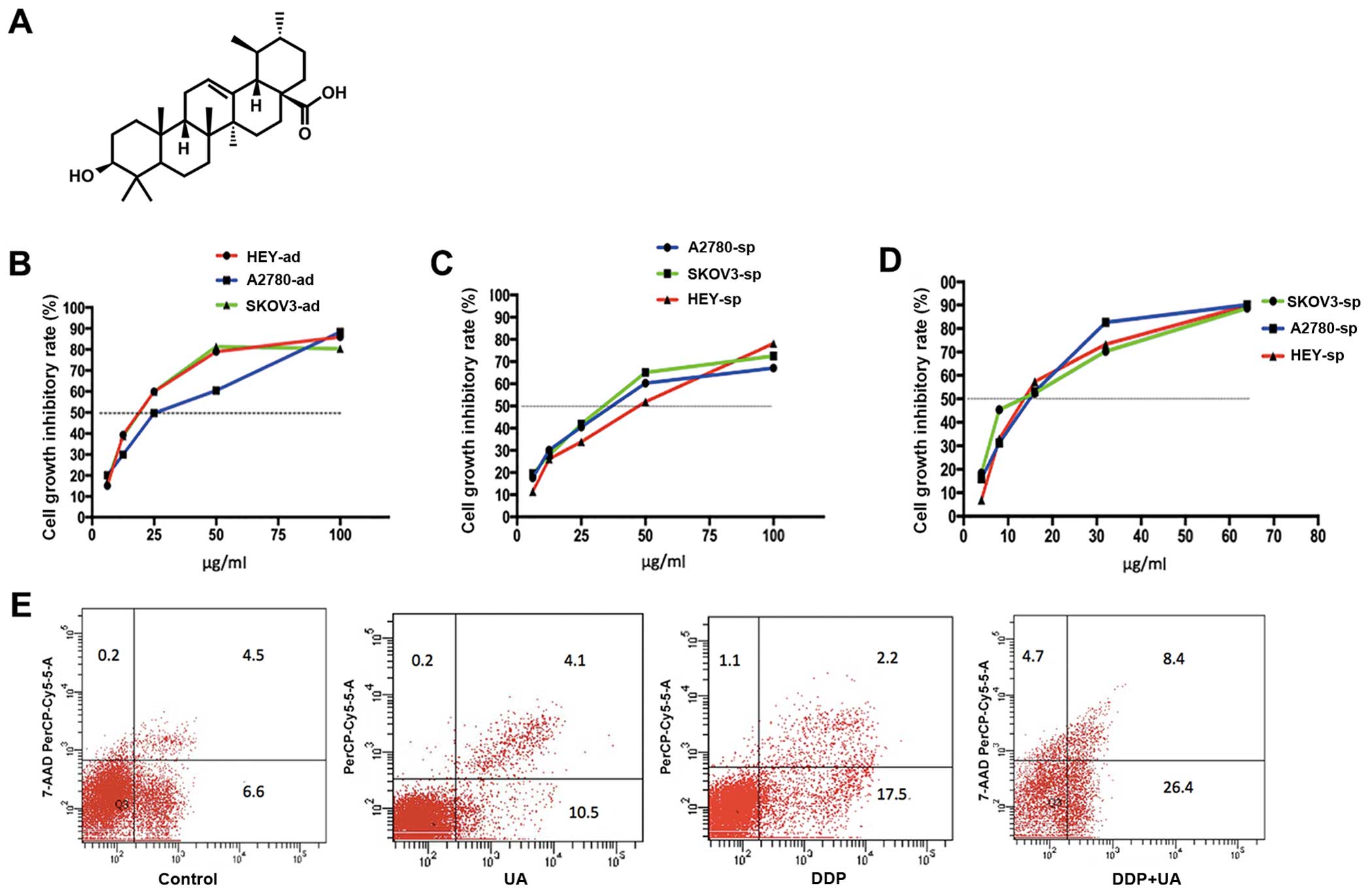 | Figure 8Effects of ursolic acid on
proliferation and effect on the sensitivity of cisplatin under
hypoxic condition. (A) The chemical structure of ursolic acid. (B)
Ursolic acid inhibited the proliferation of SKOV3-ad, HEY-ad, and
A2780-ad (6.25, 12.5, 25, 50, and 100 μg/mL). (C) Ursolic
acid inhibited the proliferation of SKOV3-sp, HEY-sp, and A2780-sp
(6.25, 12.5, 25, 50, and 100 μg/ml). (D) The cell growth
inhibitory rate when ovarian cells were treated with low-dose
ursolic acid (IC10) combined with cisplatin (4, 8, 16,
32, and 64 μg/mL). (E) Apoptosis in SKOV3-sp after treatment
with ursolic acid and cisplatin for 48 h under hypoxic
condition. |
To investigate the effect of UA on the sensitivity
of cisplatin under hypoxic conditions, the IC10 of UA
combined with cisplatin for 48 h was chosen, and then analyzed
using CCK-8. It can be observed that the IC50 values of
SKOV3-sp, HEY-sp, and A2780-sp were 12.681, 14.759, and 13.302
μg/ml. The IC50 values were significantly lower
than when cisplatin was used alone (Fig. 8D). It can lead to the increase in
the sensitivity of cisplatin due to UA under hypoxic condition.
In addition, the low-dose UA (IC10) was
chosen for combining with median dose of cisplatin to detect cell
apoptosis under hypoxic condition. As shown in Fig. 8D, when low-dose UA was used alone,
the rate of apoptosis was 14.6% and the cell apoptosis did not
change. However, compared to treatment with cisplatin alone,
combining cisplatin with UA increased the rate of apoptosis from
19.7 to 34.8% (Fig. 8E).
UA inhibits proliferation and reverses
drug resistance of ovarian CSC by suppressing ABCG2 and HIF-1α
under different culture conditions
In this experiment, it was observed that the ovarian
CSCs were more drug resistant under hypoxic condition, and the
expressions of ABCG2 and HIF-1α were significantly increased
simultaneously. The expression of ABCG2 has been proved to be
closely associated with drug resistant cancer stem cells (35–38).
Here, 3, 10, and 30 ml (IC10, IC25, and
IC50) were chosen to treat SKOV3-sp cells for 48 h to
explore whether UA inhibit the expression of ABCG and HIF-1α. It
was observed that under normal condition, different concentrations
of UA could inhibit the stemness gene CD44, CD133, Nanog, and OCT-4
in a dose-dependent manner (Fig.
9A). Later, the mRNA of ABCG2 and HIF-1α in SKOV3-sp treated
with UA under hypoxic condition was tested. As shown in Fig. 9B, under hypoxic condition UA could
suppress the expression of stemness genes CD44, CD133, Nanog, and
Oct-4. High doses of UA could significantly inhibit ABCG2 and
HIF-1α. Immunofluorescence staining on SKOV3-sp under hypoxic
condition was performed, and SKOV3-sp was treated with UA (3, 10,
and 30 μg/ml for 48 h). In Fig.
9C and D, it shows an increase in the concentration of UA, the
expression of HIF-1α gradually reduced under hypoxic condition. The
following conclusions were reached: UA could suppress the
expression of ABCG2 and HIF-1α under hypoxic conditions and in a
dose-dependent manner.
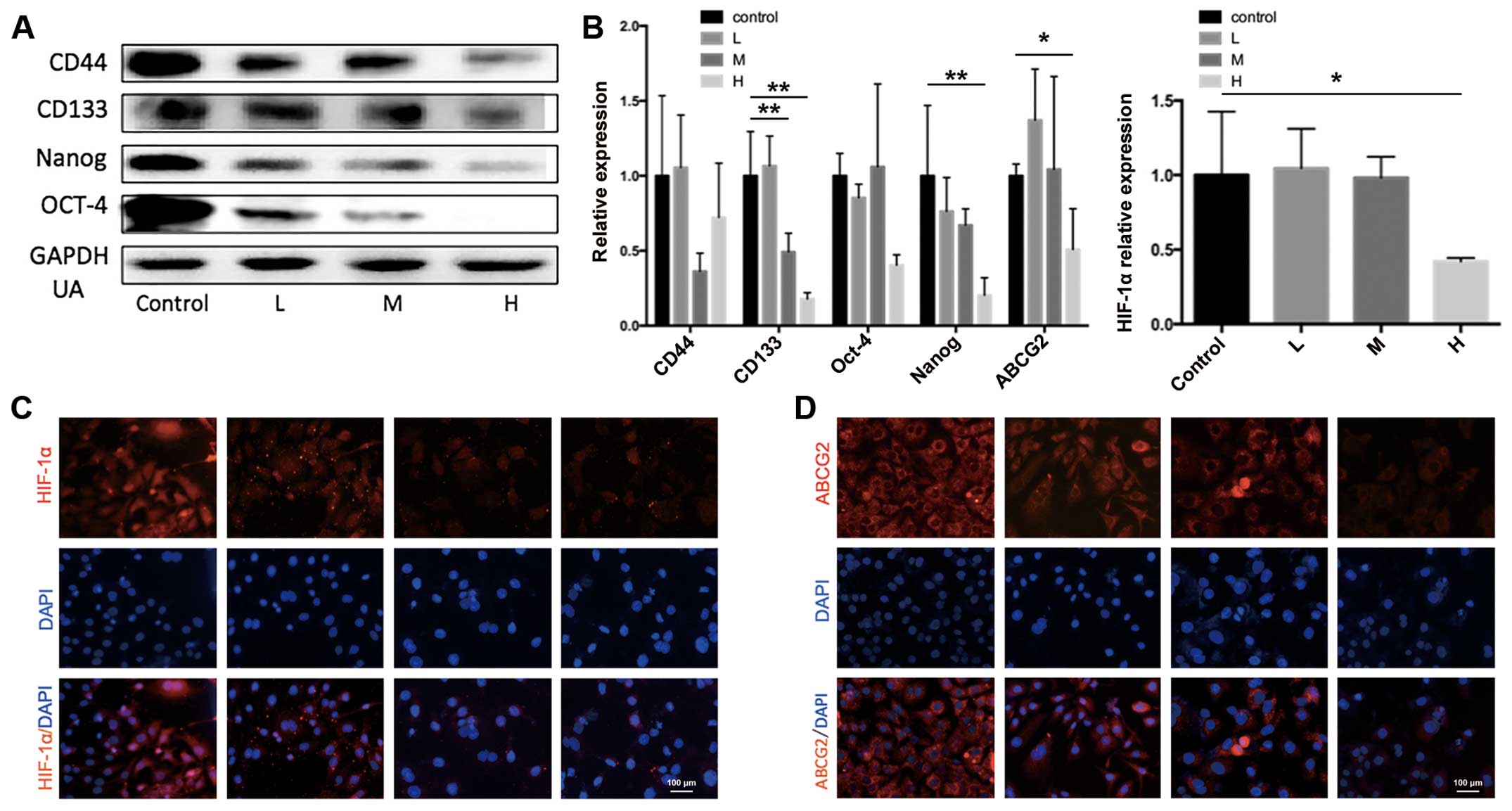 | Figure 9Ursolic acid inhibits ABCG2 and
HIF-1α in SKOV3-sp under hypoxic condition. (A) SKOV3-sp cells were
treated with different concentrations of ursolic acid (3, 10, and
30 μg/mL) for 48 h and CD44, CD133, Nanog, and OCT-4 were
detected using western blot analysis. (B) Under hypoxic condition,
SKOV3-sp cells were treated with ursolic acid (3, 10, and 30
μg/mL), mRNAs of ABCG2 and HIF-1α were detected using
quantitative PCR. *P<0.05, **P-values
represent that ursolic acid group is different compared with
control group under hypoxia condition. (C) Immunofluorescence stain
detected the expression of HIF-1α in SKOV3-sp after treatment with
ursolic acid for 48 h under hypoxic condition. (D)
Immunofluorescence stain detected the expression of ABCG2 in
SKOV3-sp after treatment with ursolic acid for 48 h under hypoxic
condition. DAPI, 4′,6-diamidino-2-phenylindole; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; HIF, hypoxia-inducible
factor; PCR, polymerase chain reaction. |
HIF-1α inhibitor YC-1 combined with UA
suppressed the stemness gene and ABCG2 under hypoxic condition
YC-1, which is
3-(5′-hydroxymethyl-2′-furyl)-1-benzyl-indazole, is a treatment for
circulatory disorders, the inhibition of platelet aggregation, and
vasoconstriction by inhibiting the soluble guanylate cyclase drugs.
In 2001, Chun et al found that YC-1 inhibited the activity
of HIF-1 (39) in breast cancer
(40) and liver cancer (41), and was found to have the anti-tumor
effect. To investigate whether inhibition of expression of HIF-1α
by YC-1 under hypoxic condition in SKOV3-sp cells is correlated
with apoptosis, stemness, and drug-resistant gene ABCG2, the
concentrations of YC-1 selected were 6, 14, and 32 μM
(IC10, IC25, and IC50 for 48 h)
was selected (Fig. 10A). It was
found that with an increase in the concentration of YC-1, the
expression of HIF-1α was gradually reduced after treatment with
YC-1 for 48 h under hypoxic condition (Fig. 10B). Later low-dose YC-1 (6
μM) was used to treat SKOV3-sp for 48 h. As shown in
Fig. 10C, when low-dose YC-1 was
used, the rate of apoptosis was 25.1%, which was much higher than
7.9% under hypoxic condition.
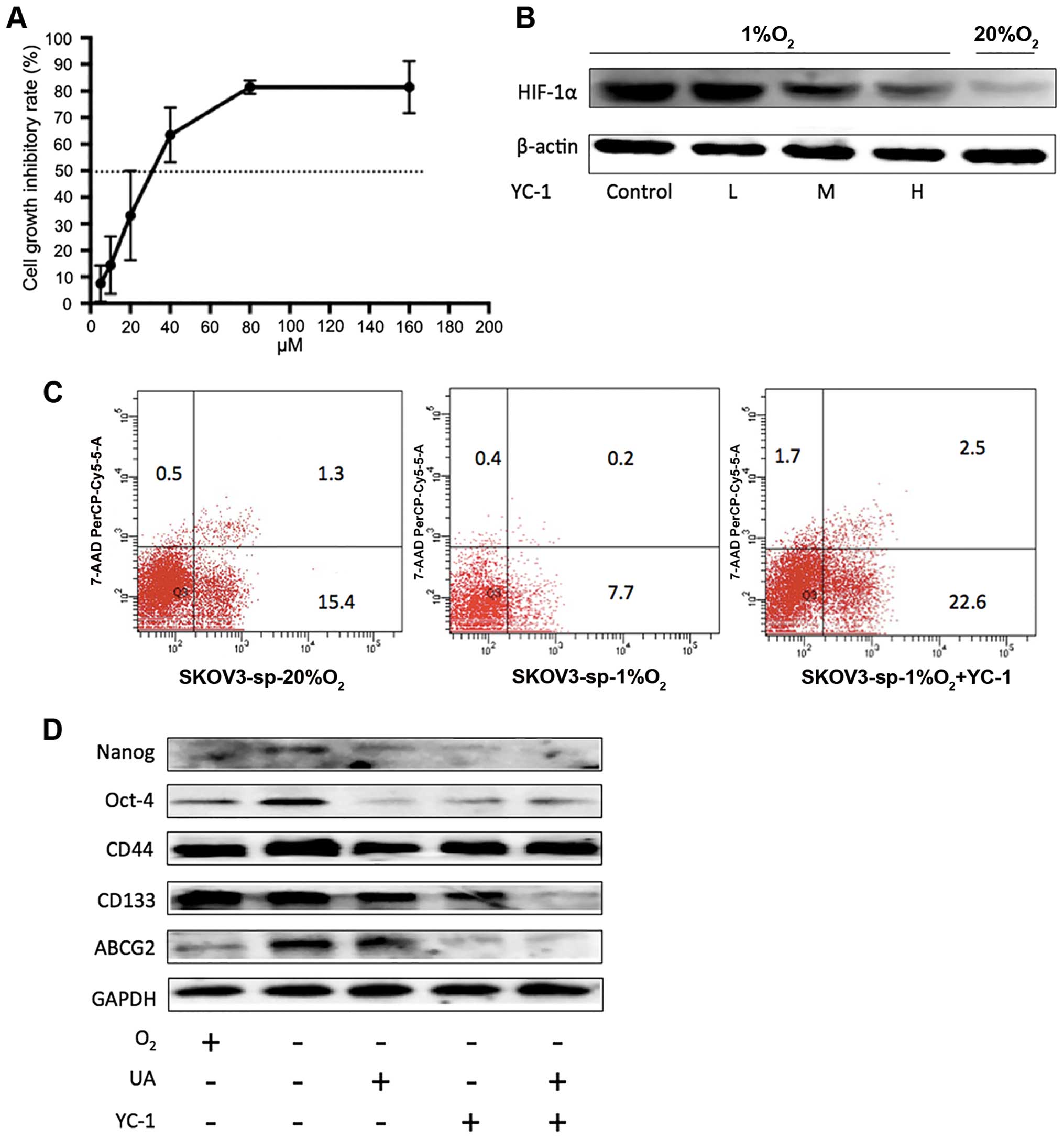 | Figure 10Inhibition of YC-1 on SKOV3-sp under
hypoxic condition. (A) SKOV3-sp cells were treated with YC-1 (5,
10, 20, 40, 80, and 160 μM) for 48 h under hypoxic
condition. (B) Different concentrations of YC-1 (6, 14, and 32
μM) were used to treat SKOV3-sp for 48 h to determine the
inhibition on HIF-1α under hypoxic condition. (C) The apoptosis of
SKOV3-sp cells after treatment with low-dose YC-1 (6 μM)
under hypoxic condition. (D) The inhibition of YC-1 combined with
ursolic acid on the stemness genes and ABCG2 under hypoxic
condition. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HIF,
hypoxia-inducible factor; UA, ursolic acid. |
When YC-1 was combined with UA under hypoxic
condition, compared with YC-1 alone or UA, the stemness genes
Nanog, OCT-4, CD44, and CD133 were significantly inhibited. In
addition, the expression of ABCG2 was significantly decreased
(Fig. 10D). These results suggest
that after the inhibition of HIF-1α, the expression of ABCG2 was
degraded.
PI3K/Akt signaling pathway activation
plays an important functional role in UA-induced downregulation of
HIF-1α and reduction of ABCG2
Emerging evidence suggests that PI3K/Akt signaling
mediates regulation and activation of HIF-1α in various human
cancers (42,43). To investigate the relationship
between HIF-1α and ABCG2 and whether UA inhibited ABCG2 though
downregulation of HIF-1α. The PI3K/Akt signaling pathway, small
interfering RNA was used to knockdown the expression of HIF-1α. In
this experiment, four sequences were chosen for design, synthesis,
and confirmation by sequencing and cloned into pGPU6 vector. The
transfection efficiency was detected using western blot and qPCR in
SKOV3-ad under hypoxic condition. As shown in Fig. 11A, it can be observed that the
positive control shRNA of GAPDH was suppressed obviously and the
HIF-1α-Homo-764 site could inhibit the expression of HIF-1α the
level of RNA or protein levels. Next the HIF-1α-Homo-764 was chosen
to transfect SKOV3-ad and after 48 h the ES medium was changed and
the culture dish was treated with poly-HEMA to enrich sphere cells.
The transfection efficiency was observed through the fluorescent
microscope (Fig. 11B). Under
hypoxic condition, no difference was observed between the control
and shRNA of NC groups in the expression of HIF-1α, but in the
shRNA of HIF-1α of the expression of HIF-1α showed a significant
suppression (Fig. 11C). At the
same time, the expression of ABCG2 also appeared to be inhibited
(Fig. 11D).
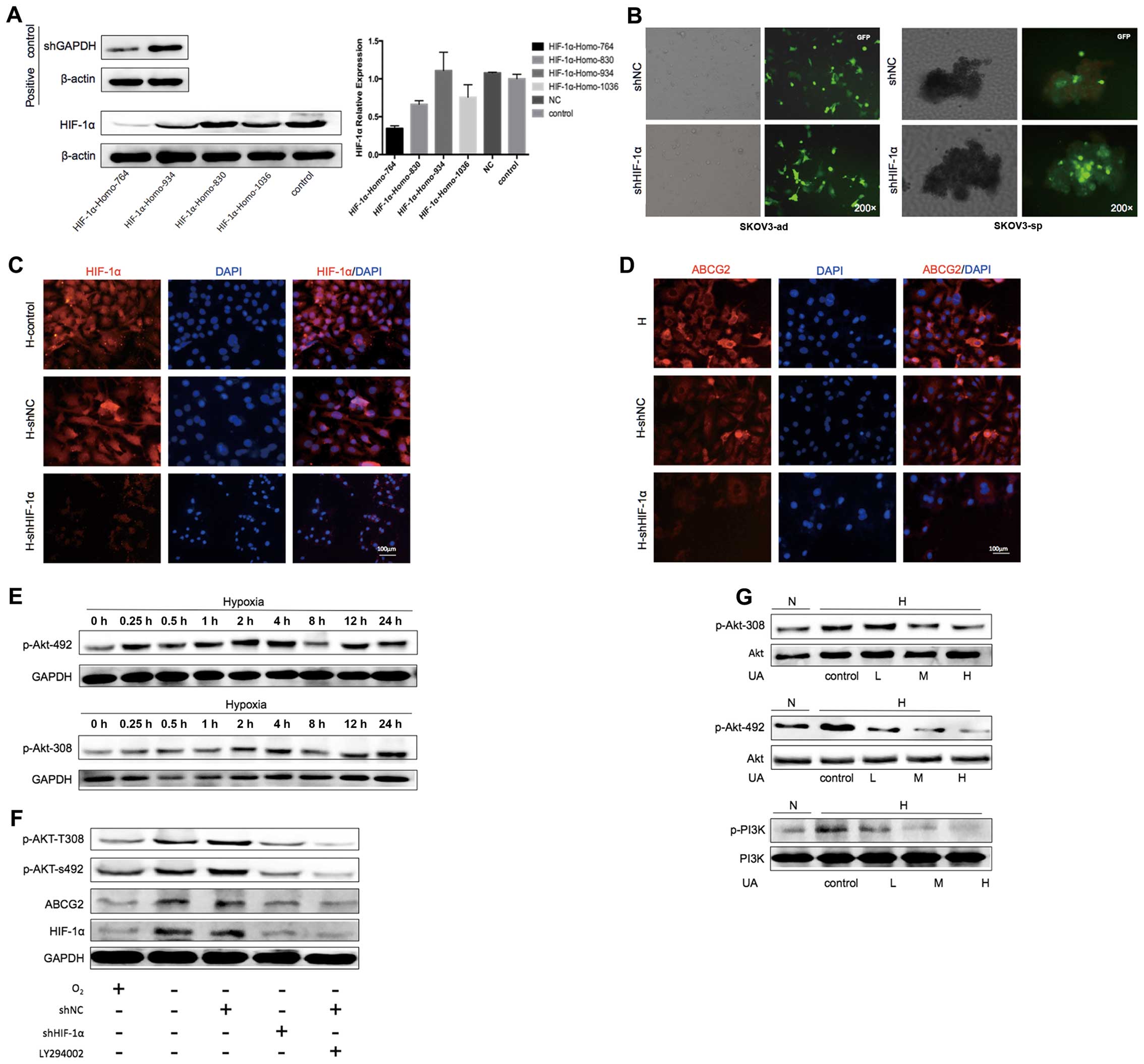 | Figure 11Ursolic acid inhibits ABCG2 though
downregulation of HIF-1α. (A) The transfection efficiency was
detected using western blot and quantitative PCR in the SKOV3-ad
cells under hypoxic condition for 48 h. (B) The transfection
efficiency of HIF-1α-Homo-764 site in SKOV3-ad and SKOV3-sp under
hypoxic condition for 48 h was detected using a fluorescent
microscope. (C) After the knockdown of HIF-1α, the expression of
HIF-1α SKOV3-adin SKOV3-sp under hypoxic condition for 48 h was
detected using fluorescent microscope. (D) After the knockdown of
HIF-1, the expression of ABCG2 in SKOV3-sp under hypoxic condition
for 48 h was detected using fluorescent microscope. (E) Western
blot detected the activation time of phosphorylated Akt under
different durations of hypoxia (0, 0.25, 0.5, 1, 2, 4, 8, 12, and
24 h). (F) Western blot detected the expression of p-Akt-492,
p-Akt-308, HIF-1α, and ABCG2 in SKOV3-sp, which were treated with
LY294002 or knockdown of HIF-1α under hypoxic condition. (G)
Western blot of the expression of p-Akt-492, p-Akt-308, and PI3K
after SKOV3-sp was treated with UA (3, 10, and 30 μg/mL).
DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde
3-phosphate dehydrogenase; GFP, green fluorescent protein; HIF,
hypoxia-inducible factor; PCr, polymerase chain reaction; UA,
ursolic acid. |
To investigate whether the PI3K/Akt signaling was
activated under hypoxic condition, the phosphorylation sites
(p-Akt-492 and p-Akt-308) of the key protein Akt on the activation
time of PI3K/Akt signaling pathway under hypoxic condition were
detected. SKOV3-sp was used under hypoxic condition for 0, 0.25,
0.5, 1, 2, 4, 8, and 24 h and then the expression of p-Akt was
detected. It was observed that with the increase in hypoxia, the
expression of p-Akt-492 and p-Akt-308 increased, reached a peak
under hypoxic condition at 4 h and then declined (Fig. 11E). It was assessed that under
hypoxic condition for 4 h, AKT was activated. To elucidate whether
the PI3K/Akt signaling pathway regulates the expression of ABCG2,
HIF-1α affects ABCG2, the PI3K inhibitor LY29004 (44,45).
Knockdown of HIF-1α by shRNA were used to investigate HIF-1α
causing high expression of ABCG2 under hypoxic condition by the
activation of the PI3K/Akt pathway. It was found that under hypoxic
condition p-Akt-492, p-Akt-308, HIF-1α, and ABCG2 were
significantly increased. The knockdown of HIF-1α and ABCG2 was
inhibited, while p-Akt-492 and p-Akt-308 appeared reduced.
Treatment with LY29004 resulted in a corresponding reduction in the
expression of p-Akt-492 and p-Akt-308. In addition, the expression
of phosphorylated Akt was reduced by the treatment with Ly29004,
leading to further reduction in HIF-1α and ABCG2 (Fig. 11F).
To investigate whether UA could inhibit the PI3K/Akt
pathway, SKOV3-sp was treated with different concentrations of UA
for 4 h. A slight decrease in the expression of p-Akt-492 and
p-Akt-308 was noted, and the effect was more evident with
increasing concentrations, while the expression of PI3K gradually
decreased (Fig. 11G). It can be
concluded that UA inhibits the activation under hypoxic conditions
in the PI3K/Akt signaling pathway.
Discussion
Ovarian cancer is the most lethal among the
gynecologic malignancies as it is diagnosed at an advanced stage in
most patients. In spite of success in initial treatment with a
combination of surgical debulking and chemotherapy, unacceptably
large number of patients (70%) develop terminal, recurrent,
chemotherapeutic resistance (46).
With the development of CSC theory and assays using markers for the
enrichment of CSCs, functional assays have been used to demonstrate
CSCs in ovarian cancer. Studies have shown that the CSCs can be
identified in tumors by their ability to grow in spheres, which are
known as tumor spheres. CSCs from epithelial organs can be expanded
as sphere-like cellular aggregates in a serum-free medium
containing epidermal growth factor and bFGF (47–50).
In these spheres, 4–20% of the cells are stem cells; the others are
progenitor cells in various phases of differentiation, which enrich
the CSC population by sphere formation and it is applicable to
ovarian CSCs (51–53). In this study, the serum-free
suspension culture was chosen to enrich the ovarian CSCs. A small
population of tumorigenic cells from the ovarian cancer cell lines
SKOV3, A2780, and HEY was chosen. Among these sphere cells, it can
be observed that the stem cell markers CD44, CD133, Nanog, and
OCT-4 were expressed more than the normal cells, and ABCG2, which
is widely expressed in various stem cell populations, is highly
expressed in sphere cells and is responsible for the maintenance of
sphere phenotype (5). As a key MDR
transporter, ABCG2 has the capability to efflux various
chemotherapeutic drugs and may contribute to drug resistance of
cancer cells (54). At the same
time, ABCG2, as one of the important stem cell markers, has close
association with CSCs. The expression of ABCG2 in stem cells from
tumor tissues and tumor cells indicates its important role in stem
cell biology. In the present study, the expression of ABCG2 in the
SKOV3-sp, A2780-sp, and HEY-sp was found to increase significantly.
The ovarian cancer sphere cells increased the cisplatin resistance
as compared with the adherent cells.
Hypoxia-induced drug resistance has been observed
in vitro in breast carcinoma neuroblastoma, and colon cancer
(55–57). HIF-1α mediates the cellular response
to hypoxia and the master regulators of stem properties (58,59).
In the present study, the increase in HIF-1α, and the change in the
stemness of ovarian CSCs and the ABCG2 were tested under hypoxic
condition. It was found that under hypoxic condition for 48 h in
the spheres of SKOV3, A2780, and HEY, in addition to increased HIF,
stem genes CD44, CD133, Nanog, Oct-4, and ABCG2 have experienced
different degrees of increase. The sphere cells elevated the
resistance to cisplatin. Here the following inference was obtained:
under hypoxic condition, hypoxia induces drug resistance due to
increased HIF-1α and elevated ABCG2.
UA is one of the active compounds in Chinese
anticancer herbal medicine. In the present study, it was found that
low doses of UA (which was found to be no different from untreated
cells and recognized as non-cytotoxic dose) in combination with
cisplatin could induce apoptosis significantly as compared to
cisplatin alone. Moreover, UA in combination with cisplatin
significantly enhanced the cytotoxicity of cisplatin to suppress
ovarian CSCs. It could be considered that UA increases the
sensitivity of cisplatin under hypoxic condition. Therefore, in the
succeeding experiments, the reversal of resistance mechanisms of UA
under hypoxic conditions was explored.
In this study, it was found that under hypoxic
condition, UA inhibited the expression of HIF-1α and can
simultaneously inhibit the expression of resistance gene ABCG2;
with the increase in the concentration of the inhibition increasing
more obviously. Therefore, it is suspected that UA decreases the
expression of HIF-1α to inhibit ABCG2 to reverse the resistance of
ovarian CSCs. In this experiment, to elucidate whether the
expression of ABCG2 is correlated with the expression of HIF-1α,
the HIF-1α inhibitor YC-1 was chosen to treat the SKOV3 sphere
cells. It was found that with an increase in the concentration of
YC-1, the expression of HIF-1I gradually decreased. Later, the
low-dose YC-1 was chosen to treat SKOV3 sphere cells under hypoxic
conditions; the rate of apoptosis increased significantly. After
treatment with low concentrations of YC-1. At the same time, it was
found that after treatment with YC-1, the increasing ABCG2 under
hypoxia condition appeared suppressed. When UA was combined with
YC-1, the expression of ABCG2 showed a significant decrease.
Under hypoxic condition, enhanced expression of
HIF-1α in high tumorigenic cancer stem/progenitor cells sustained
the activation PI3K/Akt (60,61). A
recent study suggests that the PI3K/Akt signaling mediates the
regulation and activation of HIF-1α (43,45).
Moreover, excessive activation of PI3K/Akt plays an important role
in the chemotherapeutic resistance (62,63).
However, whether the PI3K/Akt signaling pathway, which is activated
by hypoxia, effects resistance through the regulation of the
resistant gene ABCG2 is not clear. Whether the mechanism is
impacted by HIF-1α is worth exploring. Hence in the present study,
the activation time of p-Akt in SKOV3-sp was tested under hypoxic
condition. As a result, it was found that with an increase in
hypoxia, the expression of p-Akt-492 and p-Akt-308 was increased,
and reached a peak under hypoxia for 4 h and then declined. Later
HIF-1α was knocked down by shRNA and the PI3K inhibitor LY294002
was chosen. It was found that inhibiting the PI3K/Akt activity by
the inhibitor LY294002 decreased the expression of HIF-1α in
A2780-sp cells. Knockdown of HIF-1α, to some extent, can inhibit
the expression of phosphorylated Akt. In the succeeding experiment,
it was found that when SKOV3-sp is treated with UA under hypoxia it
can significantly inhibit the expression of the key protein
phosphorylated PI3K and phosphorylated Akt on the PI3K/Akt
signaling pathway. The result indicated that under hypoxic
condition UA could inhibit the PI3K/Akt signaling pathway activated
by the hypoxic condition.
In summary, it was demonstrated that under hypoxic
condition UA could inhibit the proliferation and reversal of
drug-resistant ovarian CSCs by suppressing the expression of
downregulation of HIF-1α and ABCG2. PI3K/Akt signaling pathway
activation plays an important functional role in UA-induced
downregulation of HIF-1α and ABCG2 reduction. This study indicates
that UA, a compound in traditional Chinese medicine, is a promising
agent to reverse drug-resistance in ovarian CSCs.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81173291, 81303106, 81573805) and
the Program of Science and Technology Commission of Shanghai
Municipality (no. 13ZR1462200).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kitamura H, Okudela K, Yazawa T, Sato H
and Shimoyamada H: Cancer stem cell: Implications in cancer biology
and therapy with special reference to lung cancer. Lung Cancer.
66:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szotek PP, Pieretti-Vanmarcke R, Masiakos
PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F,
Maclaughlin DT and Donahoe PK: Ovarian cancer side population
defines cells with stem cell-like characteristics and Mullerian
Inhibiting Substance responsiveness. Proc Natl Acad Sci USA.
103:11154–11159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou S, Schuetz JD, Bunting KD, Colapietro
AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M,
Nakauchi H, et al: The ABC transporter Bcrp1/ABCG2 is expressed in
a wide variety of stem cells and is a molecular determinant of the
side-population phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang G, Wang Z, Luo W, Jiao H, Wu J and
Jiang C: Expression of potential cancer stem cell marker ABCG2 is
associated with malignant behaviors of hepatocellular carcinoma.
Gastroenterol Res Pract. 2013:7825812013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Natarajan K, Xie Y, Baer MR and Ross DD:
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer
drug resistance. Biochem Pharmacol. 83:1084–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noguchi K, Katayama K and Sugimoto Y:
Human ABC transporter ABCG2/BCRP expression in chemoresistance:
Basic and clinical perspectives for molecular cancer therapeutics.
Pharm Genomics Pers Med. 7:53–64. 2014.
|
|
9
|
Wu CP, Sim HM, Huang YH, Liu YC, Hsiao SH,
Cheng HW, Li YQ, Ambudkar SV and Hsu SC: Overexpression of
ATP-binding cassette transporter ABCG2 as a potential mechanism of
acquired resistance to vemurafenib in BRAF(V600E) mutant cancer
cells. Biochem Pharmacol. 85:325–334. 2013. View Article : Google Scholar :
|
|
10
|
Nakanishi T and Ross DD: Breast cancer
resistance protein (BCRP/ABCG2): Its role in multidrug resistance
and regulation of its gene expression. Chin J Cancer. 31:73–99.
2012. View Article : Google Scholar
|
|
11
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar :
|
|
12
|
Oda K, Nishimura T, Higuchi K, Ishido N,
Ochi K, Iizasa H, Sai Y, Tomi M and Nakashima E: Estrogen receptor
α induction by mitoxantrone increases Abcg2 expression in placental
trophoblast cells. J Pharm Sci. 102:3364–3372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Zhang M, Xing L, Wang Y, Xiao Y
and Wu Y: HIF-1α contributes to proliferation and invasiveness of
neuroblastoma cells via SHH signaling. PLoS One. 10:e01211152015.
View Article : Google Scholar
|
|
14
|
Wan J, Chai H, Yu Z, Ge W, Kang N, Xia W
and Che Y: HIF-1α effects on angiogenic potential in human small
cell lung carcinoma. J Exp Clin Cancer Res. 30:772011. View Article : Google Scholar
|
|
15
|
Thomas R and Kim MH: HIF-1 alpha: A key
survival factor for serum-deprived prostate cancer cells. Prostate.
68:1405–1415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang X, Yan L, Xu L and Gao J: Comparative
study on the hepatoprotective and anti-oxidation effects of
oleanolic acid, ursolic acid and asiatic acid. Lishizhen Med
Materia Med Res. 21:pp. 2824–2827. 2012, http://en.cnki.com.cn/Article_en/CJFDTOTAL-SZGY201011046.htm.
|
|
18
|
Ikeda Y, Murakami A and Ohigashi H:
Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol Nutr
Food Res. 52:26–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martin-Aragón S, de las Heras B,
Sanchez-Reus MI and Benedi J: Pharmacological modification of
endogenous antioxidant enzymes by ursolic acid on
tetrachloride-induced liver damage in rats and primary cultures of
rat hepatocytes. Exp Toxicol Pathol. 53:199–206. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Wang W, Qian L, Zhang Q, Lai D
and Qi C: Ursolic acid inhibits the proliferation of human ovarian
cancer stem-like cells through epithelial-mesenchymal transition.
Oncol Rep. 34:2375–2384. 2015.PubMed/NCBI
|
|
21
|
Lim JJ, Yang K, Taylor-Harding B,
Wiedemeyer WR and Buckanovich RJ: VEGFR3 inhibition chemosensitizes
ovarian cancer stemlike cells through down-regulation of BRCA1 and
BRCA2. Neoplasia. 16:343–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang R, Wang J, Zhong Y, Liu Y, Stokke T,
Trope CG, Nesland JM and Suo Z: Mitochondrial DNA deficiency in
ovarian cancer cells and cancer stem cell-like properties.
Anticancer Res. 35:3743–3753. 2015.PubMed/NCBI
|
|
23
|
Ruiz I, Martín-Arruti M, Lopez-Lopez E and
Garcia-Orad A: Lack of association between deficient mismatch
repair expression and outcome in endometrial carcinomas of the
endometrioid type. Gynecol Oncol. 134:20–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiraga T, Ito S and Nakamura H: Side
population in MDA-MB-231 human breast cancer cells exhibits cancer
stem cell-like properties without higher bone-metastatic potential.
Oncol Rep. 25:289–296. 2011.
|
|
25
|
Kim RJ and Nam JS: OCT4 expression
enhances features of cancer stem cells in a mouse model of breast
cancer. Lab Anim Res. 27:147–152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gazda LS, Martis PC, Laramore MA, Bautista
MA, Dudley A, Vinerean HV and Smith BH: Treatment of
agarose-agarose RENCA macrobeads with docetaxel selects for OCT4(+)
cells with tumor-initiating capability. Cancer Biol Ther.
14:1147–1157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silva IA, Bai S, McLean K, Yang K,
Griffith K, Thomas D, Ginestier C, Johnston C, Kueck A, Reynolds
RK, et al: Aldehyde dehydrogenase in combination with CD133 defines
angiogenic ovarian cancer stem cells that portend poor patient
survival. Cancer Res. 71:3991–4001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nam EJ, Lee M, Yim GW, Kim JH, Kim S, Kim
SW and Kim YT: MicroRNA profiling of a CD133(+) spheroid-forming
subpopulation of the OVCAR3 human ovarian cancer cell line. BMC Med
Genomics. 5:182012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casagrande F, Cocco E, Bellone S, Richter
CE, Bellone M, Todeschini P, Siegel E, Varughese J, Arin-Silasi D,
Azodi M, et al: Eradication of chemotherapy-resistant
CD44+ human ovarian cancer stem cells in mice by
intraperitoneal administration of Clostridium perfringens
enterotoxin. Cancer. 117:5519–5528. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiou SH, Wang ML, Chou YT, Chen CJ, Hong
CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, et al:
Coexpression of Oct4 and Nanog enhances malignancy in lung
adenocarcinoma by inducing cancer stem cell-like properties and
epithelialmesenchymal transdifferentiation. Cancer Res.
70:10433–10444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wenger RH: Cellular adaptation to hypoxia:
O2-sensing protein hydroxylases, hypoxia-inducible
transcription factors, and O2-regulated gene expression.
FASEB J. 16:1151–1162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mathieu J, Zhang Z, Zhou W, Wang AJ,
Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al:
HIF induces human embryonic stem cell markers in cancer cells.
Cancer Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jubb AM, Buffa FM and Harris AL:
Assessment of tumour hypoxia for prediction of response to therapy
and cancer prognosis. J Cell Mol Med. 14:18–29. 2010. View Article : Google Scholar
|
|
34
|
Milosevic M, Warde P, Ménard C, Chung P,
Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M,
et al: Tumor hypoxia predicts biochemical failure following
radiotherapy for clinically localized prostate cancer. Clin Cancer
Res. 18:2108–2114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang Y, Fan Y, Qi Y, Liu D, Wu K, Wen F
and Zhao S: Side population cells separated from A549 lung cancer
cell line possess cancer stem cell-like properties and inhibition
of autophagy potentiates the cytotoxic effect of cisplatin. Oncol
Rep. 34:929–935. 2015.PubMed/NCBI
|
|
36
|
Xie ZY, Lv K, Xiong Y and Guo WH:
ABCG2-meditated multidrug resistance and tumor-initiating capacity
of side population cells from colon cancer. Oncol Res Treat.
37:666–668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Warrier S, Pavanram P, Raina D and Arvind
M: Study of chemo-resistant CD133+ cancer stem cells from human
glioblastoma cell line U138MG using multiple assays. Cell Biol Int.
36:1137–1143. 2012. View Article : Google Scholar
|
|
38
|
Oh PS, Patel VB, Sanders MA, Kanwar SS, Yu
Y, Nautiyal J, Patel BB and Majumdar AP: Schlafen-3 decreases
cancer stem cell marker expression and autocrine/juxtacrine
signaling in FOLFOX-resistant colon cancer cells. Am J Physiol
Gastrointest Liver Physiol. 301:G347–G355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chun YS, Yeo EJ, Choi E, Teng CM, Bae JM,
Kim MS and Park JW: Inhibitory effect of YC-1 on the hypoxic
induction of erythropoietin and vascular endothelial growth factor
in Hep3B cells. Biochem Pharmacol. 61:947–954. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng Y, Li W, Liu Y, Cheng HC, Ma J and
Qiu L: YC-1 exerts inhibitory effects on MDA-MB-468 breast cancer
cells by targeting EGFR in vitro and in vivo under normoxic
condition. Chin J Cancer. 31:248–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kong J, Kong F, Gao J, Zhang Q, Dong S, Gu
F, Ke S, Pan B, Shen Q, Sun H, et al: YC-1 enhances the anti-tumor
activity of sorafenib through inhibition of signal transducer and
activator of transcription 3 (STAT3) in hepatocellular carcinoma.
Mol Cancer. 13:72014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhong H, Chiles K, Feldser D, Laughner E,
Hanrahan C, Georgescu MM, Simons JW and Semenza GL: Modulation of
hypoxia-inducible factor 1alpha expression by the epidermal growth
factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human
prostate cancer cells: Implications for tumor angiogenesis and
therapeutics. Cancer Res. 60:1541–1545. 2000.PubMed/NCBI
|
|
43
|
Mazure NM, Chen EY, Laderoute KR and
Giaccia AJ: Induction of vascular endothelial growth factor by
hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt
signaling pathway in Ha-ras-transformed cells through a hypoxia
inducible factor-1 transcriptional element. Blood. 90:3322–3331.
1997.PubMed/NCBI
|
|
44
|
Bleau AM, Hambardzumyan D, Ozawa T,
Fomchenko EI, Huse JT, Brennan CW and Holland EC: PTEN/PI3K/Akt
pathway regulates the side population phenotype and ABCG2 activity
in glioma tumor stem-like cells. Cell Stem Cell. 4:226–235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kilic-Eren M, Boylu T and Tabor V:
Targeting PI3K/Akt represses Hypoxia inducible factor-1α activation
and sensitizes Rhabdomyosarcoma and Ewing's sarcoma cells for
apoptosis. Cancer Cell Int. 13:362013. View Article : Google Scholar
|
|
46
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Uchida N, Buck DW, He D, Reitsma MJ, Masek
M, Phan TV, Tsukamoto AS, Gage FH and Weissman IL: Direct isolation
of human central nervous system stem cells. Proc Natl Acad Sci USA.
97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim CF, Jackson EL, Woolfenden AE,
Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT and Jacks T:
Identification of bronchioalveolar stem cells in normal lung and
lung cancer. Cell. 121:823–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang L, Mezencev R, Bowen NJ, Matyunina LV
and McDonald JF: Isolation and characterization of stem-like cells
from a human ovarian cancer cell line. Mol Cell Biochem.
363:257–268. 2012. View Article : Google Scholar
|
|
53
|
Soriţău O, Tomuleasa CI, Páll E, Virág P,
Fischer-Fodor E, Foris V, Barbos O, Tatomir C, Kacsó G and Irimie
A: Enhanced chemoresistance and tumor sphere formation as a
laboratory model for peritoneal micrometastasis in epithelial
ovarian cancer. Rom J Morphol Embryol. 51:259–264. 2010.
|
|
54
|
Frelet A and Klein M: Insight in
eukaryotic ABC transporter function by mutation analysis. FEBS
Lett. 580:1064–1084. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Adamski J, Price A, Dive C and Makin G:
Hypoxia-induced cytotoxic drug resistance in osteosarcoma is
independent of HIF-1Alpha. PLoS One. 8:e653042013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiang L, Liu ZH, Huan Q, Su P, Du GJ, Wang
Y, Gao P and Zhou GY: Hypoxia-inducible factor-2a is associated
with ABCG2 expression, histology-grade and Ki67 expression in
breast invasive ductal carcinoma. Diagn Pathol. 7:322012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen J, Ding Z, Peng Y, Pan F, Li J, Zou
L, Zhang Y and Liang H: HIF-1α inhibition reverses multidrug
resistance in colon cancer cells via downregulation of
MDR1/P-glycoprotein. PLoS One. 9:e988822014. View Article : Google Scholar
|
|
58
|
Méndez O, Zavadil J, Esencay M, Lukyanov
Y, Santovasi D, Wang SC, Newcomb EW and Zagzag D: Knock down of
HIF-1alpha in glioma cells reduces migration in vitro and invasion
in vivo and impairs their ability to form tumor spheres. Mol
Cancer. 9:1332010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang L, Lin C, Wang L, Guo H and Wang X:
Hypoxia and hypoxia-inducible factors in glioblastoma multiforme
progression and therapeutic implications. Exp Cell Res.
318:2417–2426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Manohar SM, Padgaonkar AA, Jalota-Badhwar
A, Sonawane V, Rathos MJ, Kumar S and Joshi KS: A novel inhibitor
of hypoxia-inducible factor-1α P3155 also modulates PI3K pathway
and inhibits growth of prostate cancer cells. BMC Cancer.
11:3382011. View Article : Google Scholar
|
|
61
|
Befani CD, Vlachostergios PJ, Hatzidaki E,
Patrikidou A, Bonanou S, Simos G, Papandreou CN and Liakos P:
Bortezomib represses HIF-1α protein expression and nuclear
accumulation by inhibiting both PI3K/Akt/TOR and MAPK pathways in
prostate cancer cells. J Mol Med Berl. 90:45–54. 2012. View Article : Google Scholar
|
|
62
|
Chen Y, Wang BC and Xiao Y: PI3K: A
potential therapeutic target for cancer. J Cell Physiol.
227:2818–2821. 2012. View Article : Google Scholar
|
|
63
|
Fulda S: The PI3K/Akt/mTOR pathway as
therapeutic target in neuroblastoma. Curr Cancer Drug Targets.
9:729–737. 2009. View Article : Google Scholar : PubMed/NCBI
|