Introduction
Diffuse large B-cell lymphoma (DLBCL) accounts for
30–40% of adult non-Hodgkin lymphomas. According to its
heterogeneity in clinical course, morphology, immunophenotype and
genetics, DLBCL is subdivided into more than 10 subtypes (1).
Nuclear factor-κB (NF-κB) is an important signal
transduction pathway associated with cell proliferation, apoptosis,
tumor treatment, and immune regulation (2–4).
Abnormal activation of NF-κB signal transduction is considered a
significant feature of DLBCL (5–7), and
was found to be correlated with many clinicopathological features,
prognosis and response to therapy in lymphomas. Therefore, genes
related to NF-κB activation, such as tumor necrosis factor,
α-induced protein 3 (TNFAIP3, also known as A20), mucosa-associated
lymphoid tissue lymphoma translocation gene 1 (MALT1), caspase
recruitment domain-containing membrane-associated guanylate kinase
protein 1 (CARMA1), and B-cell leukemia/lymphoma 10 (BCL10), should
be assessed in association with DLBCL.
MALT1 and A20 regulate NF-κB activation through
multiple processes (8,9). A20 is located on chromosome band
6q23.3, and encodes the A20 protein, which is a negative regulator
of NF-κB. It is associated with pathogenesis, progression and
therapy of several tumors. Abnormalities of A20, such as mutation
and methylation, have been described in many tumors. For instance,
Bavi et al (10) found that
A20 alteration is prevalent in colorectal carcinoma, especially A20
promoter methylation. This results in reduced A20 protein levels,
which are correlated with poor outcome in colorectal carcinoma.
Genetic abnormalities of A20 have also been found in extranodal
marginal zone B cell lymphoma of mucosa-associated lymphoid tissue
(MALT), DLBCL, mantle cell lymphoma, and Hodgkin lymphoma (11,12).
In addition, high MALT1/A20/NF-κB levels and their relationships to
pathogenesis and therapy of several lymphomas have been described
(13–15). However, the role of the A20 protein
in DLBCL remains unclear. MALT1 is an upstream regulatory factor of
A20. Because of its proteolytic activity, MALT1 also promotes NF-κB
activation by cleaving A20 (16).
Once activated, NF-κB regulates its target genes, including
survivin, a member of the inhibitor of apoptosis protein (IAP)
family. Survivin has a double function in regulating cell growth,
modulating apoptosis as well as the cell cycle. Studies have
assessed the effects of survivin on the clinicopathological course,
prognosis, and treatment of lymphomas (17,18).
Therefore, we hypothesized that survivin affects the growth of
aggressive B-cell lymphoma cells in association with abnormal
activation of NF-κB resulting from MALT1 and A20.
Phorbol myristate acetate (PMA), also known as
12-O-tetradecanoylphorbol-13-acetate (TPA), is a potent
tumor promoter. Ionomycin (IONO) is an ionophore secreted by
Streptomyces conglobatus that induces calcium transport into
the cell. PMA is often used in conjunction with ionomycin to
stimulate immune responses (19).
For instance, it was shown that PMA/IONO mimics T-cell antigen
receptor signaling by activating PKC-θ, and induces recruitment of
A20 into a complex of MALT1 and Bcl-10, leading to MALT1-mediated
processing of A20 (20). In
addition, PMA/IONO inhibits growth of tumor cells, inducing
apoptosis (21). However, studies
assessing the effect of PMA/IONO on DLBCL cells are scarce.
Therefore, this study aimed to determine whether PMA/IONO affects
the growth of DLBCL OCI-LY1 cells, exploring the underlying
molecular mechanisms. Notably, we found that PMA/IONO promotes
apoptosis and inhibits the growth of DLBCL cells, and these effects
are associated with A20 upregulation.
Materials and methods
Cell culture and treatment
Diffuse large B-cell lymphoma OCI-LY1 cells were a
kind gift from Dr B. Hilda (Albert Einstein College of Medicine,
New York, NY, USA). They were maintained at 37°C in 5% carbon
dioxide in Iscove's modified Dulbecco's media (IMDM) and
supplemented with 10% FCS, 1% penicillin and streptomycin.
Phorbol-12-myristate-13-acetate (PMA) and ionomycin (IONO) (both
from Sigma-Aldrich, USA) were reconstituted in DMSO. Cells were
treated with mixtures containing different concentrations of PMA
and IONO (PMA/IONO). According to a previous study (20), PMA + IONO combinations were: 200
ng/ml + 0.167 µM, 200 ng/ml + 1 µM, and 200 ng/ml + 2
µM. Various treatment times were assessed, including 0.5, 2,
6, 24, 48 and 72 h.
MTT assay
OCILY1 cells at logarithmic growth phase
(1.25×105/ml), seeded in 96-well plates, were incubated
at 37°C in 5% carbon dioxide in the presence of various test drugs
(PMA + IONO combinations) for 24, 48, and 72 h. Then, MTT solution
(Genview, USA) was added to each well followed by 4-h incubation;
the resulting formazan crystals were dissolved by addition of DMSO.
Absorbance was read at 570 nm on a Rayto RT-6000 microplate reader
(Rayto Life and Analytical Sciences Co., Ltd., Shenzhen,
China).
Cell cycle and apoptosis assays
OCI-LY1 cells (7×105/ml) were seeded in
6-well plates and incubated with 200 ng/ml PMA and 1 µM IONO
in combination for 24, 48, and 72 h, respectively. After
collection, cells were stained with Annexin V-FITC and propidium
iodide (PI) kit (BD Biosciences, USA) in the dark for 5 min.
Finally, cell apoptosis was quantified by flow cytometry on a BD
FACSAria™ III flow cytometer (BD Biosciences).
For cell cycle distribution, OCI-LY1 cells
(7×105/ml) were treated as aforementioned for apoptosis
assessment. After collection, cells were fixed in 70% ethanol for
24 h at 4°C, washed three times with PBS, and stained with RNase (1
mg/ml; Sigma) and PI solution (100 µl/ml) for 30 min before
analysis by flow cytometry.
Quantitative real-time PCR
Total RNA was extracted from OCI-LY1 cells using
TRIzol reagent (Beyotime, China) according to the manufacturer's
instructions. cDNA synthesis from 2 µg RNA was carried out
with M-MLV Reverse Transcription kit (Invitrogen, USA).
Quantitative real-time PCR was performed using 500 ng of template,
SYBR Green Master Mix (10 µl), 100 µM of each primer
(forward and reverse) and Nuclease-Free Water to 20 µl final
volume. Primers were designed by Premier 5.0, and are shown in
Table I. Quantitative real-time PCR
was performed on an ABI Real-Time PCR system 7500 Fast thermal
cycler (Applied Biosystems, Inc., USA). Cycle conditions were: 50°C
for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 sec and 60°C
for 1 min. Data were analyzed using the Sequence Detection Software
version 1.6.3 supplied by Applied Biosystems (ABI); the comparative
cycle threshold (ΔΔCt) method was adopted for quantitation.
 | Table IPrimer sequences used in RT-qPCR. |
Table I
Primer sequences used in RT-qPCR.
| Gene name | Sequence |
|---|
| Survivin | F:
5′-GCCAGATTTGAATCGCGGGA-3′ |
| R:
5′-GCAGTGGATGAAGCCAGCCT-3 |
| β-actin | F:
5′-TGGCACCCAGCACAATGAA-3′ |
| R:
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
Western blotting
OCI-LY1 cells (7.5×105 cells/ml) were
treated in 6-well plates with PMA/IONO (200 ng/ml, 200 ng/ml +
0.167 µM, and 200 ng/ml + 2 µM) for 2 h, or PMA/IONO
(200 ng/ml + 1 µM) for 30 min, 2 h and 6 h, respectively.
Treated cells were collected and lysed with RIPA buffer on ice for
15 min for total protein extraction. Protein concentration was
determined by the colorimetric BCA assay. Twenty micrograms of
total protein from each sample was resolved by 10% SDS-PAGE and
transferred onto PVDF membranes. After blocking with 5%
skimmed-milk in T-TBS, mouse anti-MALT1 polyclonal antibody
(1:500), rabbit anti-survivin monoclonal antibody (1:500) (both
from Santa Cruz Biotechnology, USA), mouse anti-TNFAIP3 monoclonal
antibody (1:250; Abcam) and mouse anti-β-actin monoclonal antibody
(1:1,000; Santa Cruz Biotechnology) were added overnight at 4°C.
Then, horseradish peroxidase-conjugated anti-rabbit or anti-mouse
IgG (1:3,000; Beijing Dingguo Changsheng Biotechnology Co., Ltd.,
Beijing, China) was added for 1 h at room temperature. Western
blotting detection was carried out by enhanced chemiluminescence
(ECL). Quantitative analysis was performed with analysis software
Image-Pro Plus (Media Cybernetics).
siRNA transfection
siRNAs for A20 were designed and synthesized by
Shanghai GenePharma Co., Ltd. (China). A total of 3 specific siRNAs
were transfected into OCI-LY1 cells using Lipofectamine RNAiMAX
(Invitrogen) according to the manufacturer's instructions at a
final concentration of 20 nM. Efficacy of knockdown was evaluated
by RT-qPCR and western blot analysis. The most effective siRNA was
used in subsequent experiments. The sequences of the siRNAs are
shown in Table II.
 | Table IIsiRNA sequences for A20. |
Table II
siRNA sequences for A20.
| Gene name | Sequence |
|---|
| siRNA-1 |
5′-CCCUCAUCGACAGAACAUTT-3′ |
| siRNA-2 |
5′-AUGUUUCUGUCGAUGAGGGTT-3′ |
Statistical analysis
Data were analyzed with SPSS 19.0 (SPSS, USA). All
experiments were repeated three times. Values are reported as the
mean ± standard deviation (SD). Tests for homogeneity of variance
were carried out before analysis of variance. One-way or univariate
analysis of variance was used according to data characteristics.
Based on normality test results, correlation analysis was performed
either by the Pearson's or Spearman's method. P<0.05 was
considered to indicate a statistically significant result.
Results
PMA/IONO decreases OCI-LY1 cell
proliferation
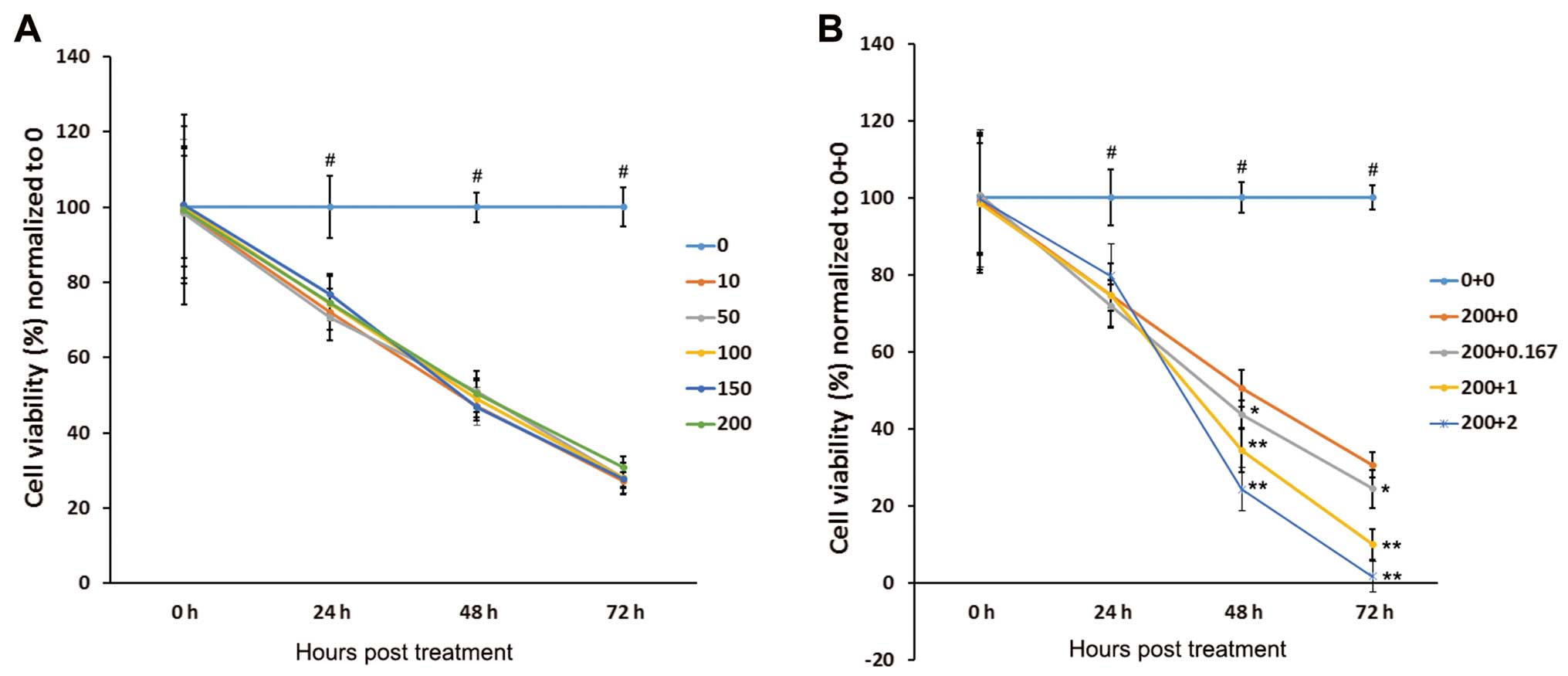
OCI-LY1 cell proliferation was analyzed by MTT
assay. As shown in Fig. 1A, OCI-LY1
cell proliferation was inhibited by PMA, but not in a
concentration-dependent manner. Notably, treatment with PMA/IONO
resulted in markedly inhibited proliferation of the OCI-LY1 cells.
For the initial 24 h, OCI-LY1 cell proliferation was similar after
PMA monotherapy and treatment with the PMA/IONO combinations
(P<0.05). However, at later time-points (48 and 72 h), cell
viability showed statistical differences between the PMA/IONO and
PMA groups (all P<0.05, Fig.
1B).
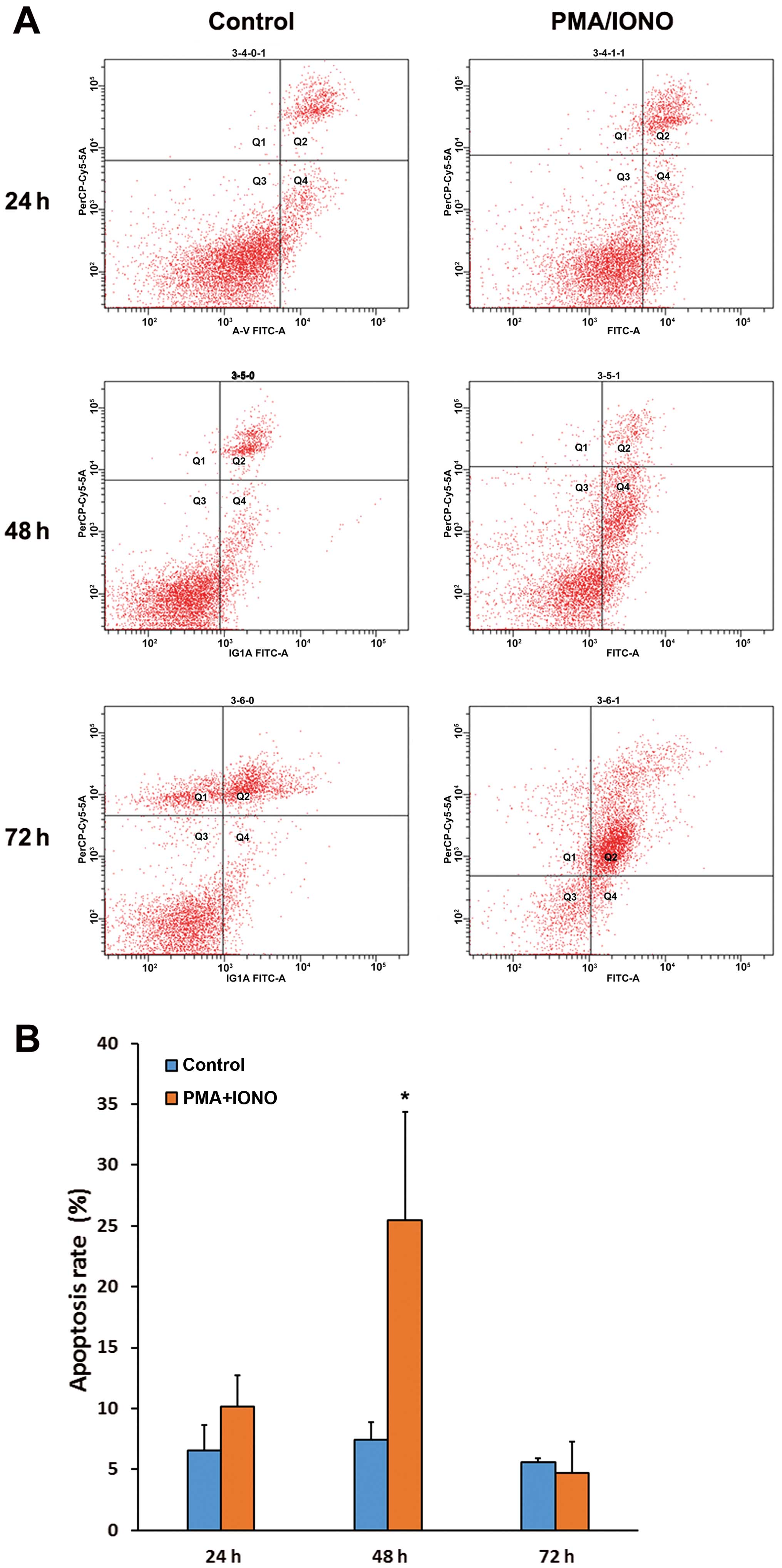
PMA/IONO induces apoptosis in OCI-LY1
cells
Compared with the control cells, apoptosis rates
showed no significant differences after treatment with PMA/IONO for
24 and 72 h (P>0.05); however, the apoptosis rate of OCI-LY1
cells was higher after treatment with PMA/IONO at 48 h compared
with the controls, with 25.5±8.84 and 7.43±1.42%, respectively
(P=0.015), as shown in Fig. 2.
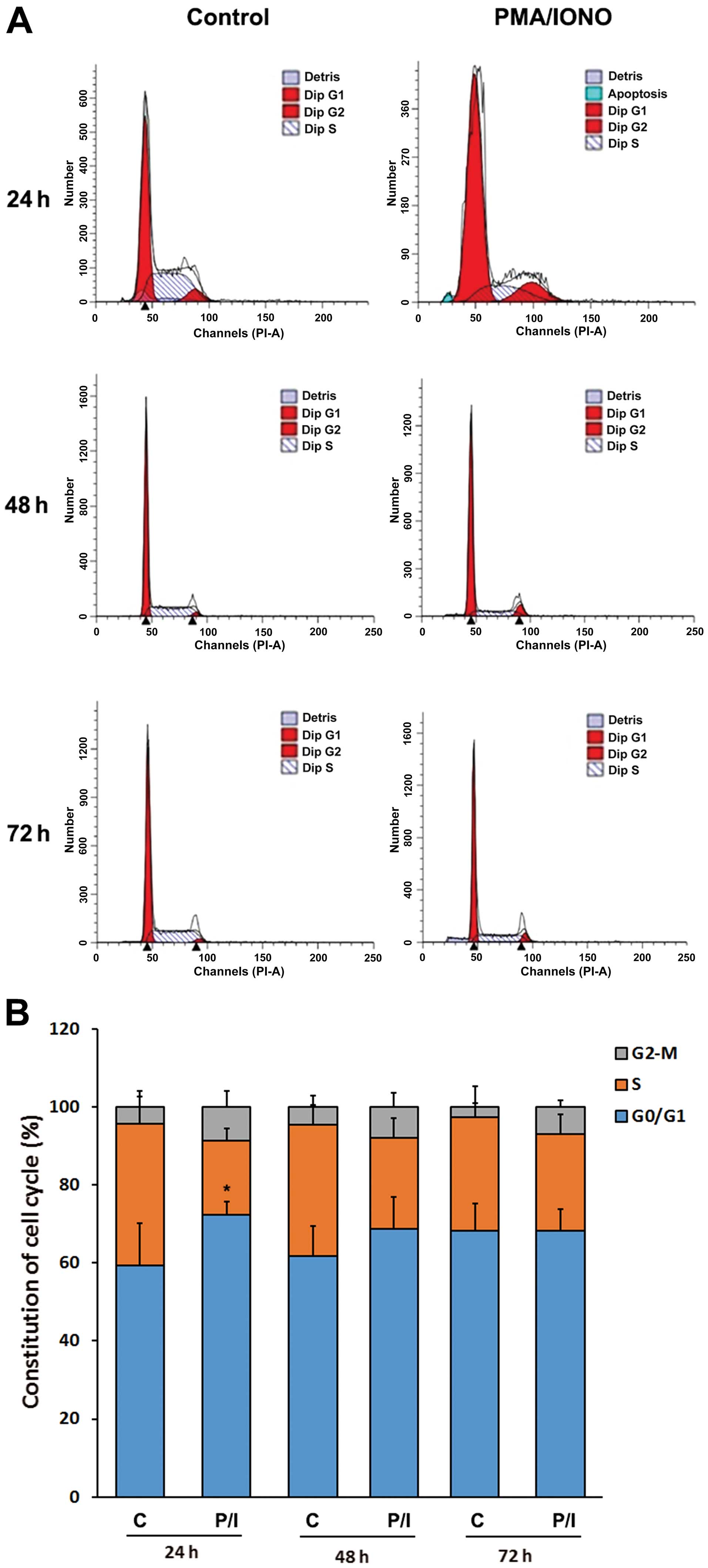
PMA/IONO treatment results in OCI-LY1
cell cycle arrest at the G0/G1 stage
Cell cycle distribution of OCI-LY1 cells was
examined by flow cytometry. Distinct cell cycle distribution
patterns appeared between the control and the PMA/IONO treatment
groups at 24 h (P<0.05); clearly, cells at the G0/G1 stage were
markedly increased after treatment with PMA/IONO (Fig. 3). The differences were less apparent
at later time-points of 48 and 72 h (Fig. 3). These findings indicated that
PMA/IONO caused cell cycle arrest at the G0/G1 stage.
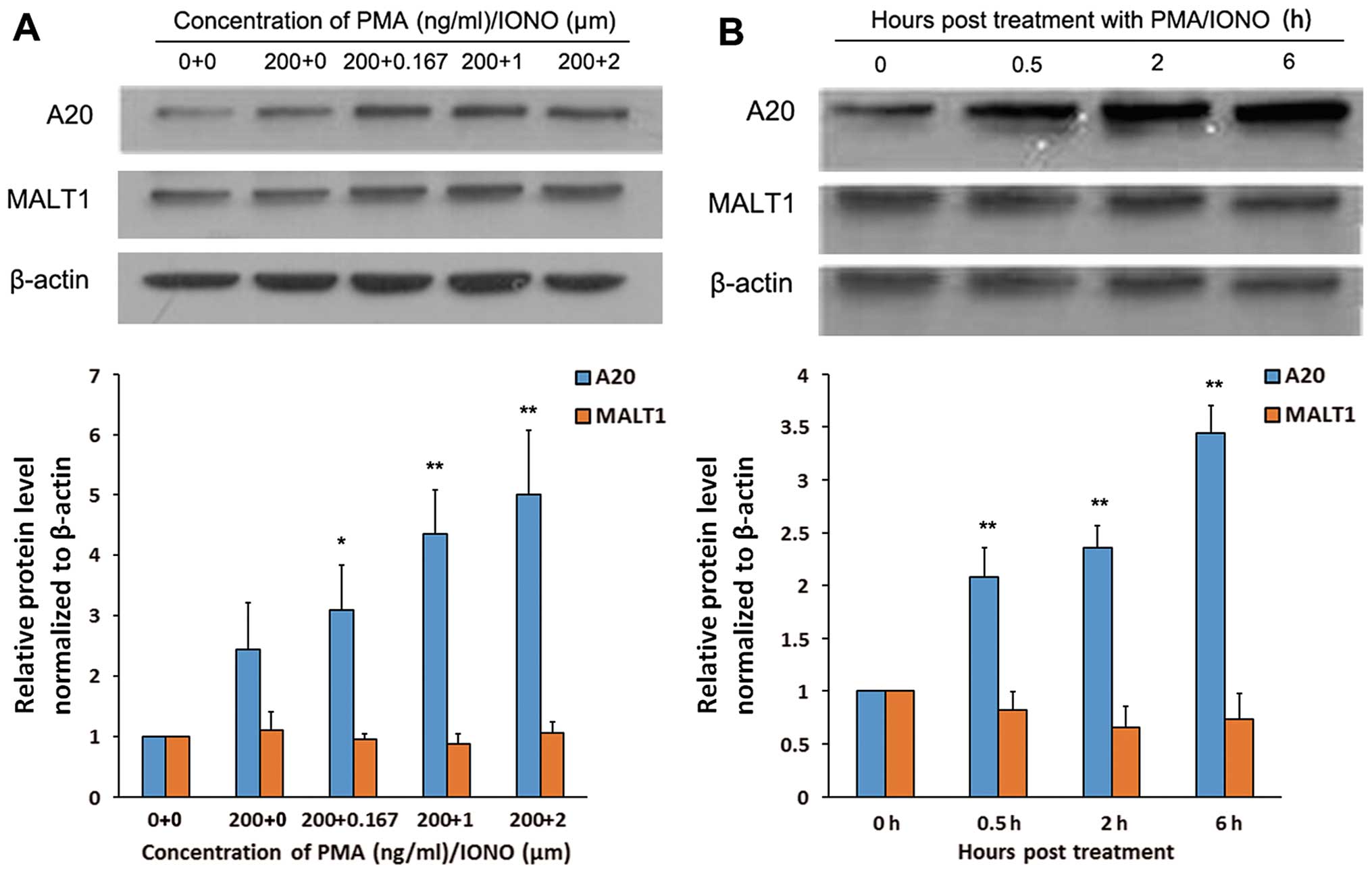
PMA/IONO induces protein expression of
A20 but not MALT1 in OCI-LY1 cells
As shown in Fig. 4,
treatment of OCI-LY1 cells with 200 ng/ml PMA resulted in increased
A20 protein levels. However, combining PMA and IONO further induced
A20 protein expression, in an IONO concentration-dependent manner
(Fig. 4A). In addition, when
PMA/IONO at 200 ng/ml + 1 µM were assessed at different
times, western blot analysis indicated that A20 protein expression
was increased in a time-dependent fashion (Fig. 4B). Meanwhile, no differences in
MALT1 protein levels were found among OCI-LY1 cells treated with
PMA monotherapy and PMA/IONO combinations, at any concentrations or
times (Fig. 4).
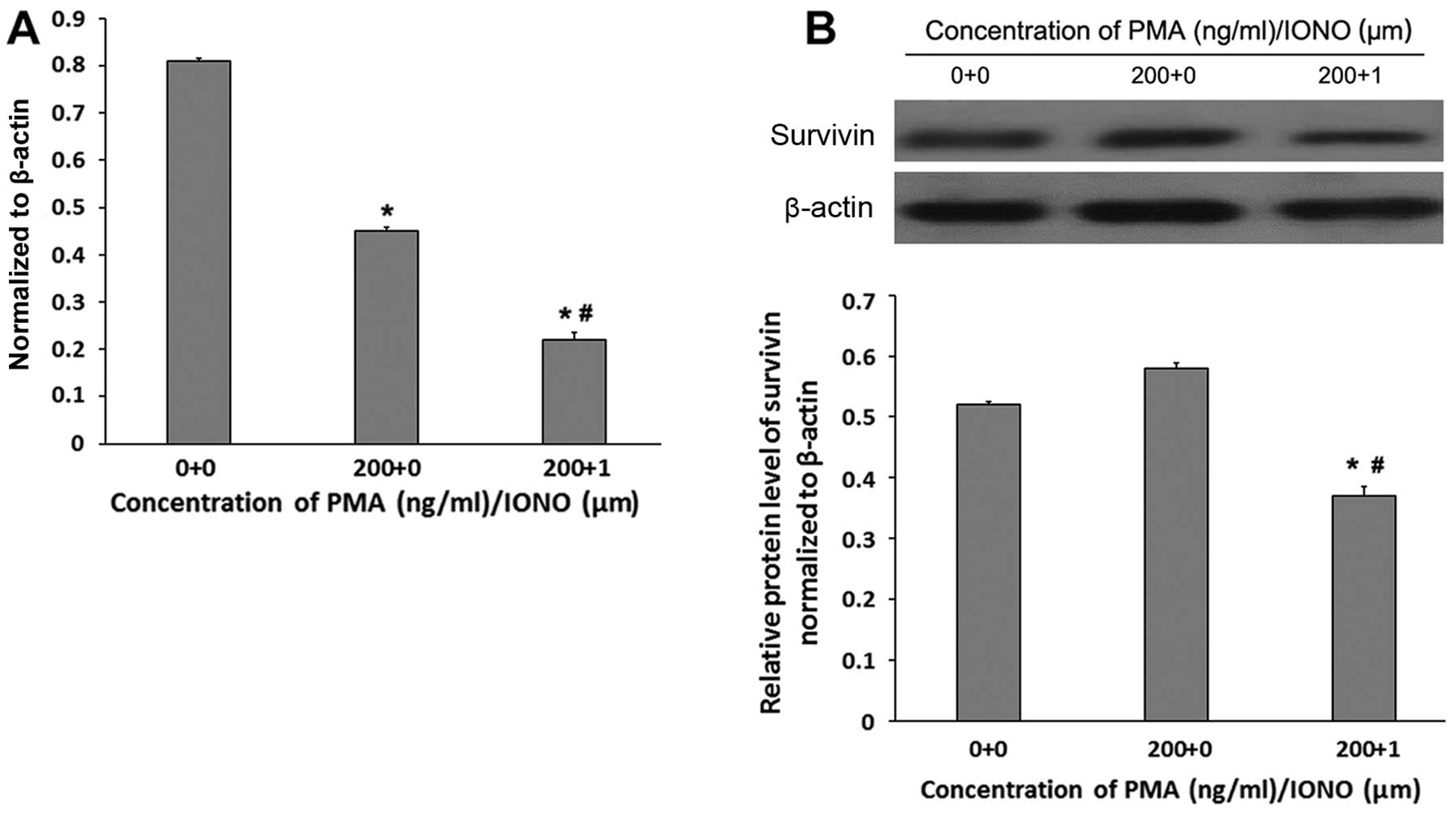
PMA/IONO decreases survivin expression at
the gene and protein levels
Survivin mRNA levels were significantly lower in
OCI-LY1 cells treated for 48 h with PMA + IONO (0.22±0.06) compared
with the PMA (0.45±0.08) and the control (0.81±0.14) groups (all
P<0.05, Fig. 5A). In
concordance, survivin protein amounts were lower in the OCI-LY1
cells treated with PMA + IONO (0.37±0.02) compared with values
obtained after treatment of PMA (0.58±0.06) and no treatment
(0.52±0.07) (all P<0.01, Fig.
5B). Although a significant difference was obtained in survivin
mRNA levels between the PMA and control groups (P<0.05, Fig. 5A), similar survivin protein amounts
were obtained between the latter 2 groups (P>0.05, Fig. 5B).
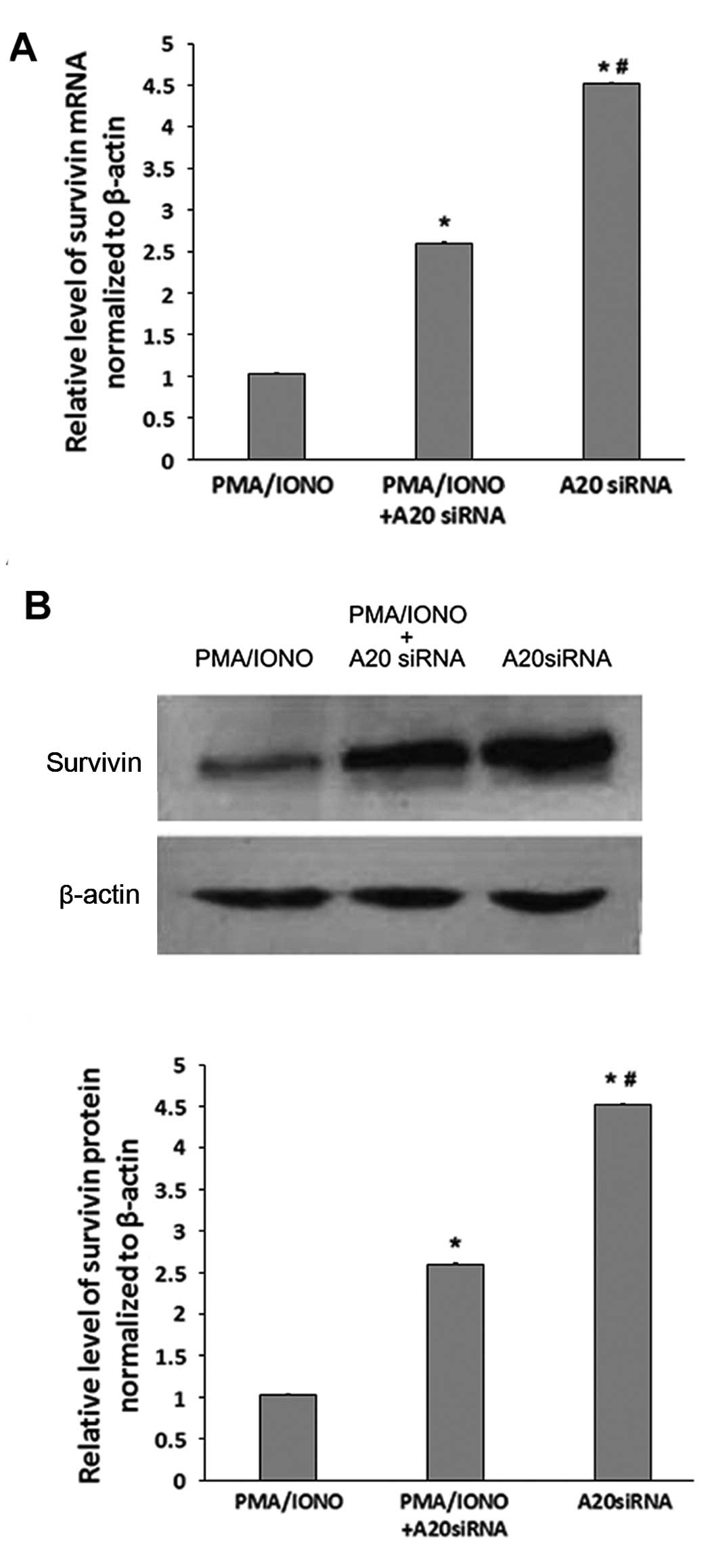
A20 silencing increases survivin
expression at the gene and protein levels
As shown in Fig. 6A,
survivin mRNA levels were increased significantly in the
A20-knockdown OCI-LY1 cells; however, survivin mRNA amounts were
decreased in the OCI-LY1 cells after A20 silencing and treatment
with PMA/IONO. Similar findings were obtained at the protein level
for survivin expression (Fig.
6B).
Discussion
In this study, we demonstrated that PMA/IONO
promotes apoptosis and inhibits the growth of DLBCL cells, and
these effects are likely mediated by A20 upregulation.
At present, A-CHOP is the classical treatment for
DLBCL. Because of its heterogeneity, many cases with DLBCL cannot
achieve a good response to this therapy. Indeed, Zelenetz pointed
out that treatment for DLBCL has exceeded A-CHOP (22). Therefore, it is important to
identify new therapeutic targets for this lymphoma; some genes
regulating NF-κB activation may be valuable in the treatment of
DLBCL (23).
As aforementioned, PMA/IONO decreased OCI-LY1
proliferation, in time- and IONO concentration-dependent manners.
These findings corroborate previous studies showing that PMA and
IONO in combination affect the growth of T and B cells and
macrophages in vitro (24,25).
Decreased cell proliferation might be explained by the cell cycle
arrest of OCI-LY1 cells treated with PMA/IONO at the G0/G1 phase as
stated above. In addition, we found that OCI-LY1 cell apoptosis was
increased after treatment with PMA/IONO for 48 h; of note this
effect was not observed at 72 h, possibly due to the overall cell
death rate at this time-point for both groups. The role of A20 in
DLBCL remains unclear. In this study, the relationship between
PMA/IONO and the expression of various proteins, such as A20, MALT1
and survivin, in OCI-LY1 cells were also assessed.
Notably, we recently found that A20 abnormalities
are involved in DLBCL cell proliferation and drug resistance, as
well as poor prognosis (unpublished data). A20 expression levels in
the OCI-LY1 cells were increased after treatment with PMA/IONO.
Markedly, A20 protein levels increased after treatment with
PMA/IONO, which caused no change in MALT1 protein expression. This
finding indicates that the change in A20 protein expression is not
linked to proteolytic activation by MALT1 protein in the OCI-LY1
cells treated with PMA/IONO. All in all, these results suggest that
PMA/IONO induced apoptosis and decreased growth in OCI-LY1 cells
and this is associated with its effects on A20 protein expression
in OCI-LY1 cells. Ca2+ overload is one of the mechanisms
of apoptosis of OCI-LY1 cells exposed to PMA/IONO (26). Another mechanism of apoptosis and
proliferation inhibition may be associated with A20 upregulation.
In this case, it is possible that A20 expression inhibits NF-κB
activation. We also found that survivin expression was decreased in
the OCI-LY1 cells after PMA/IONO treatment. The reduced survivin
levels may result from inhibition of NF-κB signaling caused by A20
upregulation. These data were confirmed by A20 silencing in OCI-LY1
cells, which resulted in increased survivin amounts, both at the
gene and protein levels.
In conclusion, this study demonstrated that PMA/IONO
affects the growth of OCI-LY1 cells, an effect associated with A20
induction. As an important negative regulator, A20 also impacts
progression and treatment of DLBCL possibly by inhibiting NF-κB,
indirectly inhibiting target genes such as survivin. Therefore, A20
may be considered a therapeutic target for DLBCL.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81160299).
References
|
1
|
Menon MP, Pittaluga S and Jaffe ES: The
histological and biological spectrum of diffuse large B-cell
lymphoma in the World Health Organization classification. Cancer J.
18:411–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pulvino M, Liang Y, Oleksyn D, DeRan M,
Van Pelt E, Shapiro J, Sanz I, Chen L and Zhao J: Inhibition of
proliferation and survival of diffuse large B-cell lymphoma cells
by a small-molecule inhibitor of the ubiquitin-conjugating enzyme
Ubc13-Uev1A. Blood. 120:1668–1677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramachandiran S, Adon A, Guo X, Wang Y,
Wang H, Chen Z, Kowalski J, Sunay UR, Young AN, Brown T, et al:
Chromosome instability in diffuse large B cell lymphomas is
suppressed by activation of the noncanonical NF-κB pathway. Int J
Cancer. 136:2341–2351. 2015. View Article : Google Scholar
|
|
4
|
Niu M, Shen Y, Xu X, Yao Y, Fu C, Yan Z,
Wu Q, Cao J, Sang W, Zeng L, et al: Piperlongumine selectively
suppresses ABC-DLBCL through inhibition of NF-κB p65 subunit
nuclear import. Biochem Biophys Res Commun. 462:326–331. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Staudt LM: Oncogenic activation of
NF-kappaB. Cold Spring Harb Perspect Biol. 2:a0001092010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Grubor V, Love CL, Banerjee A,
Richards KL, Mieczkowski PA, Dunphy C, Choi W, Au WY, Srivastava G,
et al: Genetic heterogeneity of diffuse large B-cell lymphoma. Proc
Natl Acad Sci USA. 110:1398–1403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Q, Fu W, Jiang H, Du J, Zhang C, Xi
H, Zhou F, Li R and Hou J: Clinicopathological implications of
nuclear factor κB signal pathway activation in diffuse large B-cell
lymphoma. Hum Pathol. 46:524–531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Afonina IS, Elton L, Carpentier I and
Beyaert R: MALT1 - a universal soldier: Multiple strategies to
ensure NF-κB activation and target gene expression. FEBS J.
282:3286–3297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martinez-Climent JA: The origin and
targeting of mucosa-associated lymphoid tissue lymphomas. Curr Opin
Hematol. 21:309–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bavi P, Abubaker J, Al-Sanea N,
Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Uddin S, Siraj AK
and Al-Kuraya KS: Clinico-pathological significance of TNF
alpha-induced protein3 (TNFAIP3) in Middle Eastern colorectal
carcinoma. Clin Epigenetics. 2:417–418. 2011. View Article : Google Scholar
|
|
11
|
Honma K, Tsuzuki S, Nakagawa M, Tagawa H,
Nakamura S, Morishima Y and Seto M: TNFAIP3/A20 functions as a
novel tumor suppressor gene in several subtypes of non-Hodgkin
lymphomas. Blood. 114:2467–2475. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paik JH, Go H, Nam SJ, Kim TM, Heo DS, Kim
CW and Jeon YK: Clinicopathologic implication of A20/TNFAIP3
deletion in diffuse large B-cell lymphoma: An analysis according to
immunohistochemical subgroups and rituximab treatment. Leuk
Lymphoma. 54:1934–1941. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura S and Matsumoto T: Helicobacter
pylori and gastric mucosa-associated lymphoid tissue lymphoma:
Recent progress in pathogenesis and management. World J
Gastroenterol. 19:8181–8187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nocturne G, Boudaoud S, Miceli-Richard C,
Viengchareun S, Lazure T, Nititham J, Taylor KE, Ma A, Busato F,
Melki J, et al: Germline and somatic genetic variations of TNFAIP3
in lymphoma complicating primary Sjogren's syndrome. Blood.
122:4068–4076. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fontan L and Melnick A: Targeting
lymphomas through MALT1 inhibition. Oncotarget. 3:1493–1494. 2012.
View Article : Google Scholar
|
|
16
|
McAllister-Lucas LM, Baens M and Lucas PC:
MALT1 protease: A new therapeutic target in B lymphoma and beyond?
Clin Cancer Res. 17:6623–6631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He C, Liu Z, Ji J and Zhu H: Prognostic
value of survivin in patients with non-Hodgkin's lymphoma: A
meta-analysis. Int J Clin Exp Med. 8:5847–5854. 2015.PubMed/NCBI
|
|
18
|
Sun L, Zhao Y, Shi H, Ma C and Wei L:
LMP-1 induces survivin expression to inhibit cell apoptosis through
the NF-κB and PI3K/Akt signaling pathways in nasal NK/T-cell
lymphoma. Oncol Rep. 33:2253–2260. 2015.PubMed/NCBI
|
|
19
|
Abate D, Saldan A, Forner G, Tinto D,
Bianchin A and Palù G: Optimization of interferon gamma ELISPOT
assay to detect human cytomegalovirus specific T-cell responses in
solid organ transplants. J Virol Methods. 196:157–162. 2014.
View Article : Google Scholar
|
|
20
|
Coornaert B, Baens M, Heyninck K, Bekaert
T, Haegman M, Staal J, Sun L, Chen ZJ, Marynen P and Beyaert R: T
cell antigen receptor stimulation induces MALT1
paracaspase-mediated cleavage of the NF-kappaB inhibitor A20. Nat
Immunol. 9:263–271. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Debernardis D, Stanzione S, Ottoboni C,
Clerico L, Mancuso T, Parodi S and Russo P: Endogenous tumor
necrosis factor enhances topoisomerase II inhibitors activity in
human ovarian cancer cell lines. J Pharmacol Exp Ther. 279:84–90.
1996.PubMed/NCBI
|
|
22
|
Zelenetz AD: Guidelines for NHL: Updates
to the management of diffuse large B-cell lymphoma and new
guidelines for primary cutaneous CD30+ T-cell
lymphoproliferative disorders and T-cell large granular lymphocytic
leukemia. J Natl Compr Canc Netw. 12(Suppl 5): 797–800.
2014.PubMed/NCBI
|
|
23
|
Carbone A, Gloghini A, Kwong YL and Younes
A: Diffuse large B cell lymphoma: Using pathologic and molecular
biomarkers to define subgroups for novel therapy. Ann Hematol.
93:1263–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Busca A, Saxena M and Kumar A: Critical
role for antiapoptotic Bcl-xL and Mcl-1 in human macrophage
survival and cellular IAP1/2 (cIAP1/2) in resistance to
HIV-Vpr-induced apoptosis. J Biol Chem. 287:15118–15133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fritzenwanger M, Jung C, Franz M, Foerster
M and Figulla HR: Immunomodulatory effects of cardiotrophin-1 on in
vitro cytokine production of monocytes and CD4+
T-lymphocytes. Indian J Med Res. 136:471–476. 2012.PubMed/NCBI
|
|
26
|
Yee J, White RE, Anderton E and Allday MJ:
Latent Epstein-Barr virus can inhibit apoptosis in B cells by
blocking the induction of NOXA expression. PLoS One. 6:e285062011.
View Article : Google Scholar : PubMed/NCBI
|