Introduction
Gastric cancer is a multifactorial and fatal
disease, and its overall mortality ranked third among
cancer-related deaths worldwide in 2012 (1). The incidence and mortality rate of
gastric cancer vary geographically, with the highest in East Asia,
including China and Japan (2,3).
Currently, surgical resection along with chemoradiation are the
standard therapeutic procedures (4,5). More
research efforts to identify molecular biomarkers can facilitate
early diagnosis or new drug development for gastric cancers.
Several critical genes have been discovered to correlate with
different types of gastric cancers (intestinal and diffuse types)
and have been suggested as potential prognostic biomarkers in
previous reviews (6–9).
Ubiquitin-dependent proteolysis is a significance
physiological process involved in differentiation, signal
transduction and the cell cycle (10,11).
Ubiquitin-activating enzyme (E1), ubiquitin-conjugating enzyme (E2)
and ubiquitin ligase (E3) are the three different enzymes involved
in this system. UbcH10 belongs to the E2 gene family coding for a
protein of 19.6 kDa and plays an important role in the destruction
of mitotic cyclins and cell cycle progression (12–14).
Pulmonary tumors are among the neoplasms with
reportedly high levels of UbcH10 and UbcH10 has been shown to play
an important role in tumor progression (15). UbcH10 was found to be a useful
target for diagnosis and therapy in a subsequent study (16). UbcH10 also presents a high
expression level in anaplastic thyroid carcinomas (17), endometrial malignant neoplasms
(18), nasopharyngeal carcinoma
(19), liver cancer (20), breast (21) and colon carcinoma (22,23),
and brain metastasis (24). High
expresssion of UbcH10 is correlated with cell invasion and tumor
node metastasis (TNM) stage in some of the above-mentioned cancer
types. Furthermore, knockdown of UbcH10 expression by RNA
interference significantly reduced the proliferation of cancer
cells (17,25) and enhanced cell apoptosis in
vitro (26).
To date, there is no study concerning UbcH10 and
gastric carcinoma. To investigate this, two gastric cancer cell
lines with a high UbcH10 expression level, KATO III and SGC-7901,
were selected for gene knockdown experiments. UbcH10 was also
transfected into NCI-N87 and HS-746T, two cell lines with low
UbcH10 expression and low multiplication capacity. Cell
proliferation, apoptosis and cell signal transductions were
examined in these cell lines.
To examine the clinical relevance of the findings,
paraffin-embedded gastric carcinoma tissue samples from 59 patients
were analyzed by immunohistochemistry.
Materials and methods
Clinical samples
Tumor samples from 59 cases of gastric carcinoma
were obtained from the Minhang Central Hospital, (Shanghai, China)
from 2012 to October 2014. The procedure was performed with patient
informed consent and in accordance with the Helsinki Declaration
and upon the approval of The Institutional Review Board of Minhang
Hospital.
Cell lines
The human gastric carcinoma cell lines used in the
present study were SGC-7901, AGS, NCI-N87, HS 746T, MKN-45, KATO
III, NCI-SNU-1, SNU-5 and SNU-16 and were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). These
cell lines were cultured in Dulbecco's modified Eagle's medium
(DMEM) containing 10% fetal calf serum, glutamine and
ampicillin/streptomycin (all from Gibco Laboratories, Carlsbad, CA,
USA) in a 5% CO2 atmosphere. GAX023, GAXC031 and GAX066
are primary tumor cell lines established from PDX models (PDX-PDC;
patient-derived xenograft, patient-derived cell line) at Shanghai
ChemPartner as previously described (27–29).
Reagents
The anti-UbcH10 (A-650) antibody was purchased from
Boston Biochem (Boston, MA, USA). Anti-ERK1/2 (#4695),
anti-phospho-ERK1/2 (T202/Y204, #4370), anti-p38 MAPK (D13E1,
#8690), anti-phospho-p38 MAPK (Thr180/Tyr182, #9211), anti-Akt
(#9272), anti-phospho-Akt (Ser473, #9271), anti-actin (13E5, #4970)
and anti-GAPDH (14C10) antibodies were purchased from Cell
Signaling Technology (Danvers, MA, USA). Annexin V-FITC Apoptosis
Detection Kit I (cat. 556547) was obtained from BD Pharmingen (San
Diego, CA, USA). Blocking buffer (PBS), goat anti-mouse (IRDye800CW
926-32210) and goat anti-rabbit (IRDye800CW 926-32211) were
purchased from Odyssey. Hoechst 33258 (#861405) and MTT reagents
(M2128) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Plasmid construction
The CDS sequences (121-660) of UBE2C transcript
variant 1 mRNA (GeneBank NM_007019) were chemically synthesized and
cloned into mammalian cell expression vector
pLVX-UBE2C-IRES-eGFP.
Transfection of small interfering
RNA
Gastric carcinoma cells were transfected using
Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA,
USA) according to the manufacturer's instructions.
siRNA directed against the UbcH10 transcript
with the sequence: 5′-CCUGCAAGAAACCUACUCAdTdT3′ anti-sense, and a
scrambled siRNA were obtained from GenePharma (Shanghai, China).
In vitro transfections were performed using 0.8 μg
(54 pM) siRNA in 24-well plates using Lipofectamine 2000
transfection reagent (1×105 cells/well) following the
manufacturer's protocol. Transfection of siRNA was performed at
least 5 times for each cell line. Each experiment was repeated at
least twice. Non-specific siRNAs (scrambled siRNAs) were used as
negative controls.
Western blot assay
Cells were lysed in T-PER tissue lysis buffer
containing protease inhibitor (both from Thermo Fisher Scientific,
Waltham, MA, USA), and phosphatase inhibitor cocktail (Sigma, St.
Louis, MO, USA). Twenty micrograms of protein/sample was loaded on
4–12% SDS-PAGE gels (Life Technologies, Carlsbad, CA, USA).
Proteins were separated and transferred onto a nitrocellulose
membrane. Membranes were incubated with blocking buffer (LI-COR,
Inc., Lincoln, NE, USA) and incubated with primary antibodies
overnight, followed by the Alexa Fluor 680-conjugated secondary
antibody (Invitrogen). Blots were scanned on the Odyssey system
(LI-COR, Inc.). Anti-p-ERK1/2 (Thr202/Tyr204), anti-ERK1/2,
anti-Akt, anti-p-Akt (Ser473), anti-p38, anti-pp38 and anti-cyclin
D1 antibodies were purchased from Cell Signaling Technology.
Anti-actin (Cell Signaling Technology) or anti-GAPDH antibody
(Epitomics, Burlingame, CA, USA) was used as a loading control.
MTT assay
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay was performed to study the proliferation of the tumor cells.
Cells were plated in 96-well plates at a density of 104
cells/well to attach for 24 h and transfected with 50 nM different
siRNAs. At 0, 24, 48 and 72 h after treatment, 10 μl of MTT
reagent (5 mg/ml; Sigma) was added to each well. After a 4-h
incubation at 37°C, the supernatant was gently aspirated and
formazan crystals were dissolved in 100 μl of dimethyl
sulfoxide (DMSO). The absorbance of each well was measured by an
ELISA plate-reader (Bio-Rad, Hercules, CA, USA) at a wavelength of
570 nm.
Cell cycle and apoptosis analysis
The cell cycle or apoptosis distribution was
analyzed by flow cytometry. Cells were seeded into a 6-well plate,
followed by transfection of siRNA or over-expression plasmids, and
cultivation was maintained for 36 h. For cell cycle analyses, the
harvested cells were washed with PBS and fixed with ice-cold 70%
ethanol at 4°C overnight. The fixed cells were resuspended in PBS
(containing 0.2 mg/ml RNAse and 10 mM PI) and incubated in the dark
for 30 min at 4°C.
For cell apoptosis analyses, the cells were
harvested and washed with PBS and stained with Annexin V and PI
staining (Annexin V-FITC Apoptosis Detection Kit; BD Biosciences,
San Jose, CA, USA). A FACSCalibur flow cytometer (Becton-Dickinson,
San Jose, CA, USA) was used for data acquisition and FlowJo
(TreeStar, Palo Alto, CA, USA) was used for data analysis.
Immunohistochemistry
Protein cellular distribution of UbcH10 was assessed
by immunohistochemical analysis. Formalin-fixed, paraffin-embedded,
6-mm tissue sections were deparaffined. Antigen retrieval was
performed by water heating method at 100°C for 10 min with 0.01 M
citrate buffer (pH 6.0). Endogenous peroxidase activity was
quenched with methanol-hydrogen peroxide (3%) for 15 min. After
blocking with unrelated antiserum, the slides were incubated with
the UbcH10 primary monoclonal antibody (Boston Biochem, Cambridge,
MA, USA) at 1:200 dilution and incubated overnight at 4°C. After
washing with Tris-buffered saline and Tween-20 (TBST), the sections
were incubated with secondary antibodies followed by
peroxidase-labeled streptavidin (Dako, Carpinteria, CA, USA) for 20
min at room temperature, and visualized with 3,3′-diaminobenzidine
and counterstained with hematoxylin. Unrelated IgG was used instead
of the primary antibody as a negative control.
Results
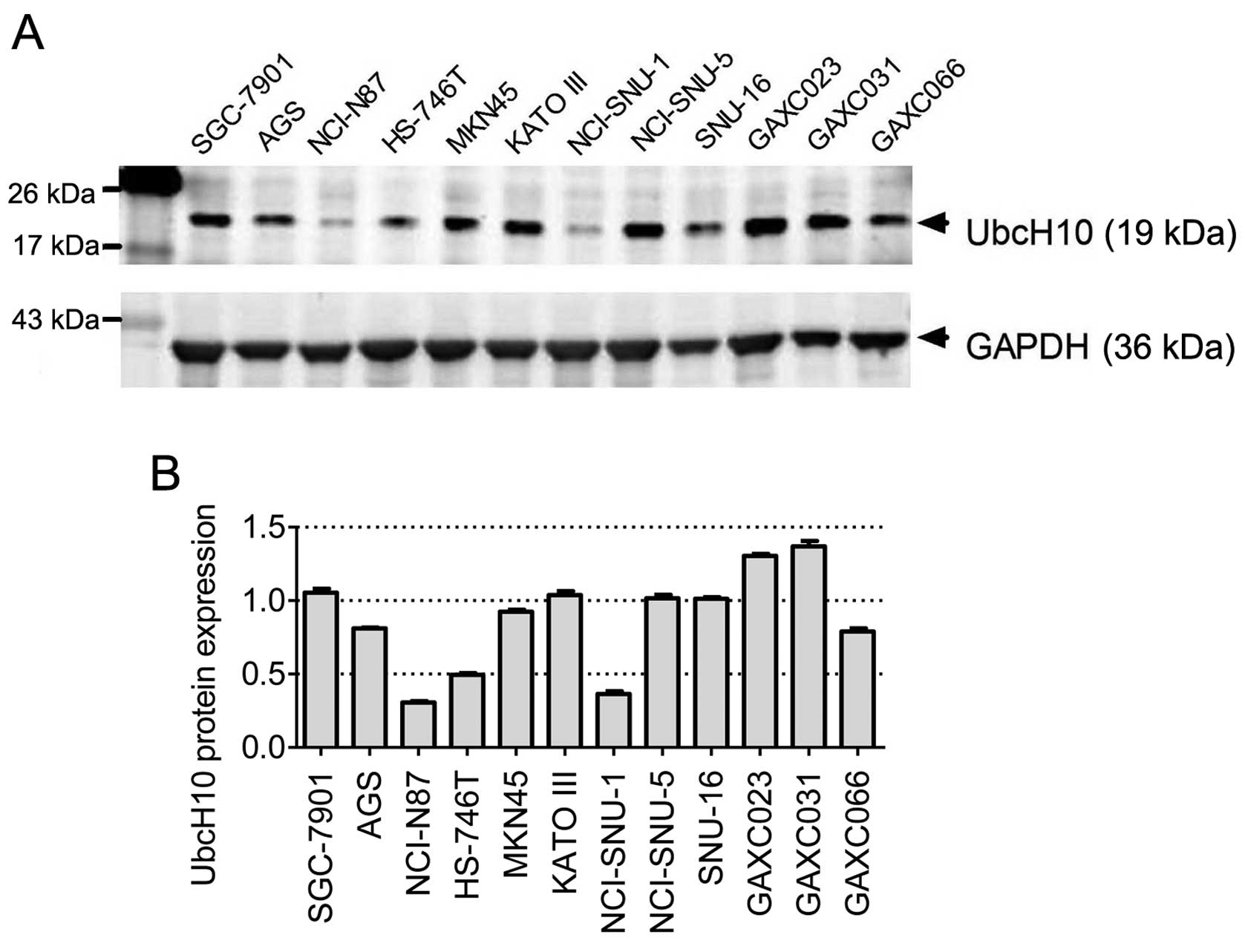
UbcH10 expression in 12 gastric carcinoma
cell lines
To investigate the role of UbcH10 in gastric cancer,
12 gastric cancer cell lines (SGC-7901, AGS, NCI-N87, HS-746T,
MKN-45, KATO III, NCI-SNU-1, SNU-5, SNU-16, GAXC023, GAXC031 and
GAXC066) were examined to determine the UbcH10 expression levels by
western blot analyses. Among these cell lines, GAXC023, GAXC03 and
GAXC066 are primary tumor cell lines established recently in our
laboratory from gastric cancer PDX models (30).
Diverse UbcH10 expression levels were observed in
these 12 cell lines, with relative high expression levels observed
in KATO III, SGC-7901, GAXC-023 and GAXC-031 cells, and much lower
expression levels noted in NCI-N87, HS-746T and NCI-SNU-1 cells
(Fig. 1A and B).
Knockdown of UbcH10 expression in gastric
tumor cells inhibits proliferation
SGC-7901 and KATO III, both of which express UbcH10
at a high level, were selected for the RNA interference study.
siRNA transfection led to 90 and 98% inhibition of UbcH10
expression in the SGC-7901 and KATO III cells, respectively
(Fig. 2A).
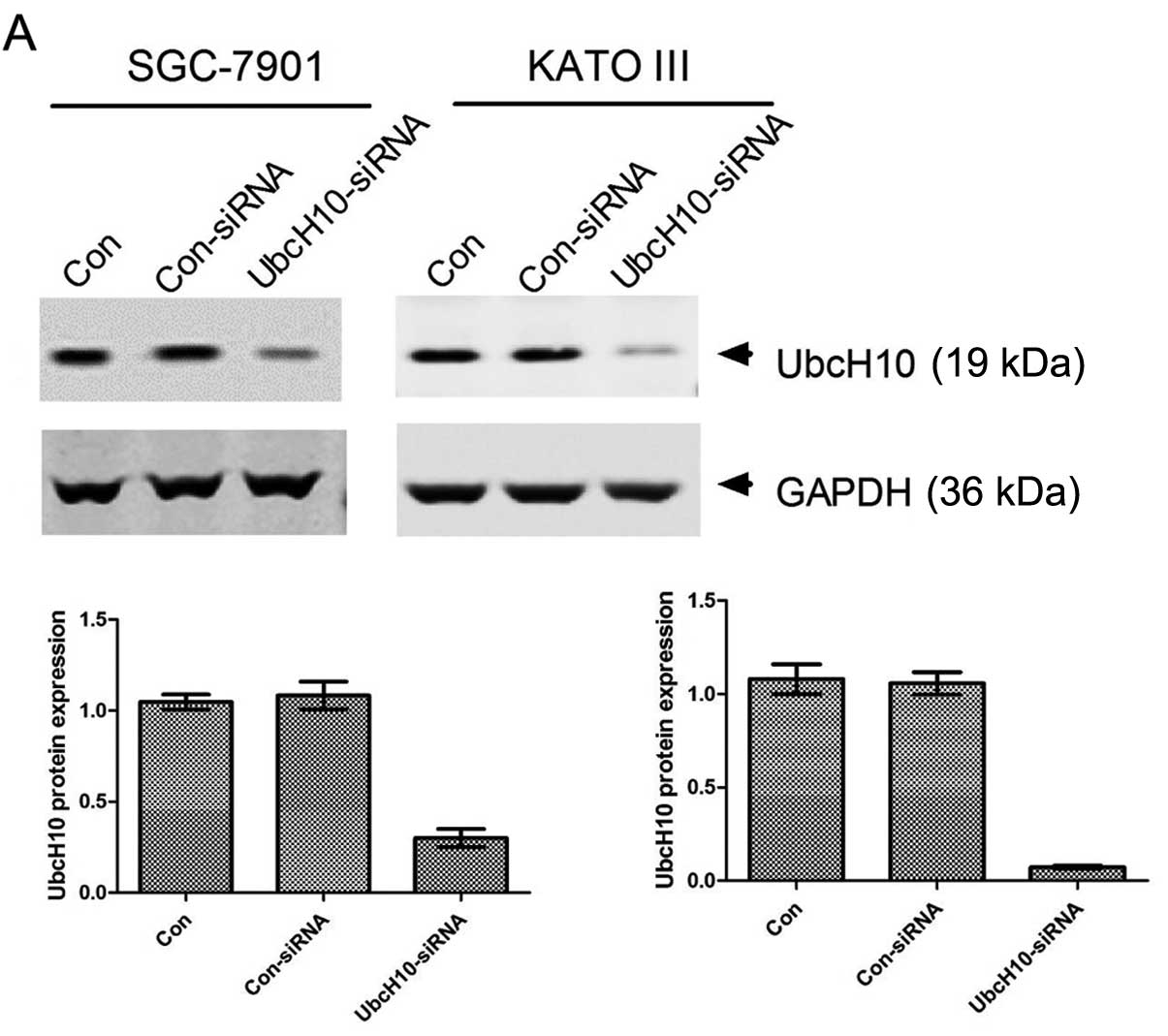 | Figure 2Knockdown of UbcH10 expression by
siRNA inhibits cell proliferation and cell cycle progression. (A)
Inhibition of UbcH10 expression in SGC-7901 and KATO III cells.
Cells were transiently transfected with the scrambled or
UbcH10-siRNA using Lipofectamine 2000. Con, untreated cells;
Con-siRNA, scrambled siRNA; UbcH10-siRNA, UbcH10 targeted siRNA.
Cell lysates were prepared and analyzed for the protein expression
of UbcH10 by western blotting 36 h post-transfection. The UbcH10
expression level is presented in the lower panels. The ratio of
UbcH10 to actin was quantified using software ImageJ. Columns, mean
(n=3); bars, mean ± SD. (B) SGC-7901 and KATO III cells were
transfected with scramble or UbcH10 siRNA, respectively, and cell
proliferation was analyzed by MTT assay in the subsequent 4 days.
Values of optical density (OD) were obtained by the absorbance at
the dual wavelengths 570/700 nm (*P<0.05,
***P<0.001; two-way ANOVA with Bonferronni
post-tests). (C) SGC-7901 and KATO III cells were harvest in 70%
ethanol 36 h after transfection with scramble or UbcH10-siRNA for
cell cycle analyses by flow cytometry. Results were analyzed using
FlowJo software. Bars, mean ± SD (**P<0.01,
***P<0.001; two-way ANOVA with Bonferronni
post-tests). |
Then, MTT cell proliferation assay was used to test
the effect of UbcH10 knockdown. Cell growth was significantly
slower in the UbcH10-knockdown SGC-7901 and KATO III cells when
compared with those transfected with scramble RNAs (Fig. 2B).
Flow cytometric analysis with PI staining was then
used to study cell cycle distribution. UbcH10-knockdown SGC-7901
cells displayed markedly reduced percentages of S and G2/M phase
populations (Fig. 2C).
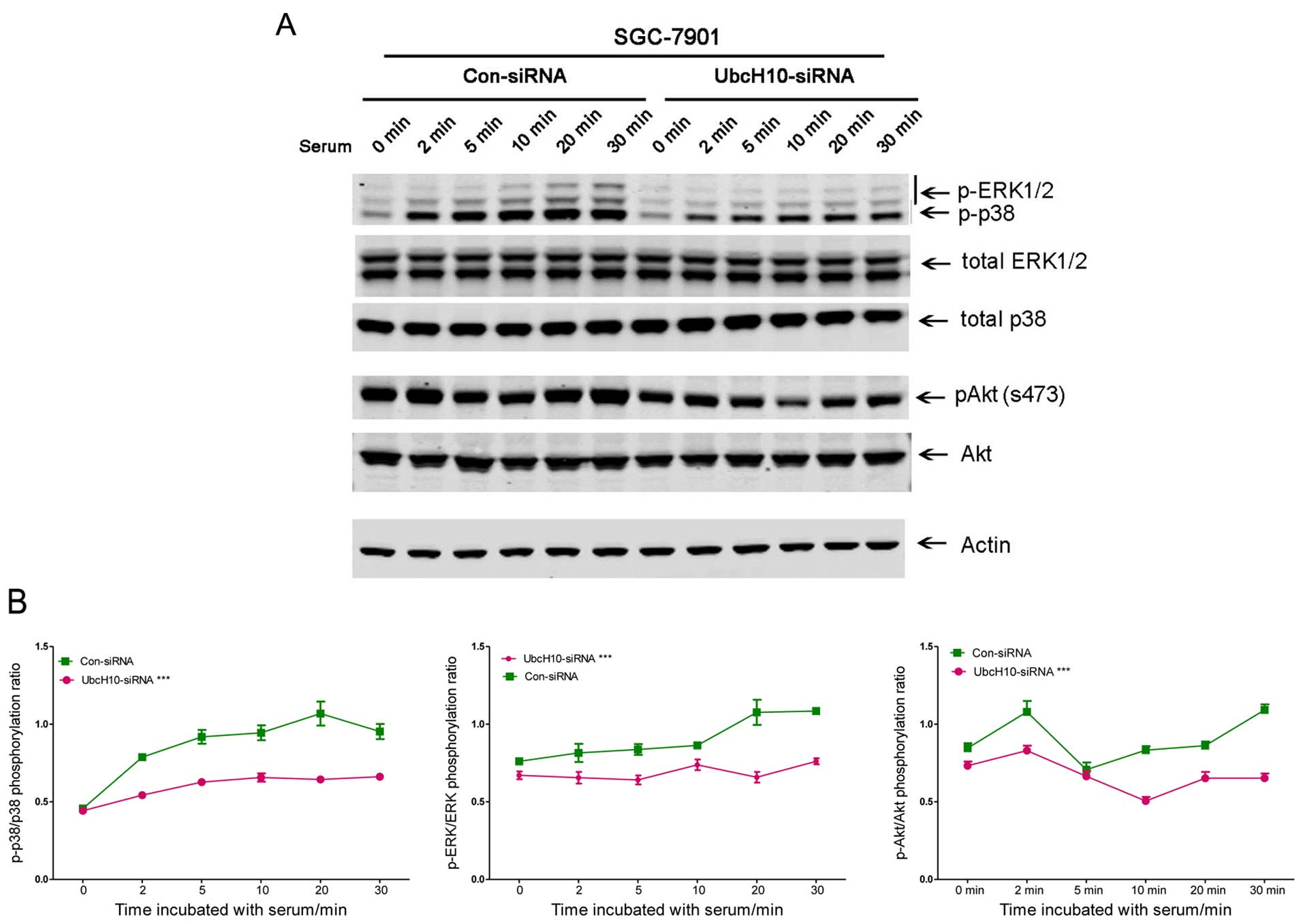
Inhibition of UbcH10 expression reduces
cell fitness
Cell proliferation can be induced by changes in
signaling pathways. Cells with or without UbcH10 knockdown were
analyzed using western blot analyses. The cells with siRNA
transfection were seeded in DMEM without serum overnight. Serum
(1%) was then used to stimulate cell signaling.
Phospho-ERK, phospho-Akt and phospho-p38 were
attenuated with UbcH10 knockdown in the SGC-7901 cells (Fig. 3A and B). Similar changes were also
observed in the same experiment using KATO III cells (data not
shown).
The decrease in cell signaling and proliferation may
be a sign of reduced cell fitness. To investigate this, the role of
UbcH10 was then studied in cisplatin (10 μM)-induced gastric
tumor cell apoptosis with flow cytometry using FITC-Annexin V and
PI staining. Knockdown of UbcH10 in these 2 gastric cancer cell
lines resulted in increases in apoptosis and eventual death
following exposure to cisplatin treatment (Fig. 3C).
The data suggest that knockdown of UbcH10 in gastric
cancer cells reduced tumor cell proliferation, serum-induced signal
transduction and increased susceptibility to cisplatin-induced cell
death.
Overexpression of UbcH10 in gastric tumor
cells promotes cell proliferation and inhibits apoptosis
To confirm these findings, we constructed the UbcH10
expression plasmid for the overexpression studies in the NCI-N87
and HS-746T cells, Two of the gastric cancer cell lines showed low
UbcH10 expression (Fig. 1).
The western blot analysis showed the enhanced
expression level of UbcH10 in the two cell lines after the
transfection (Fig. 4A).
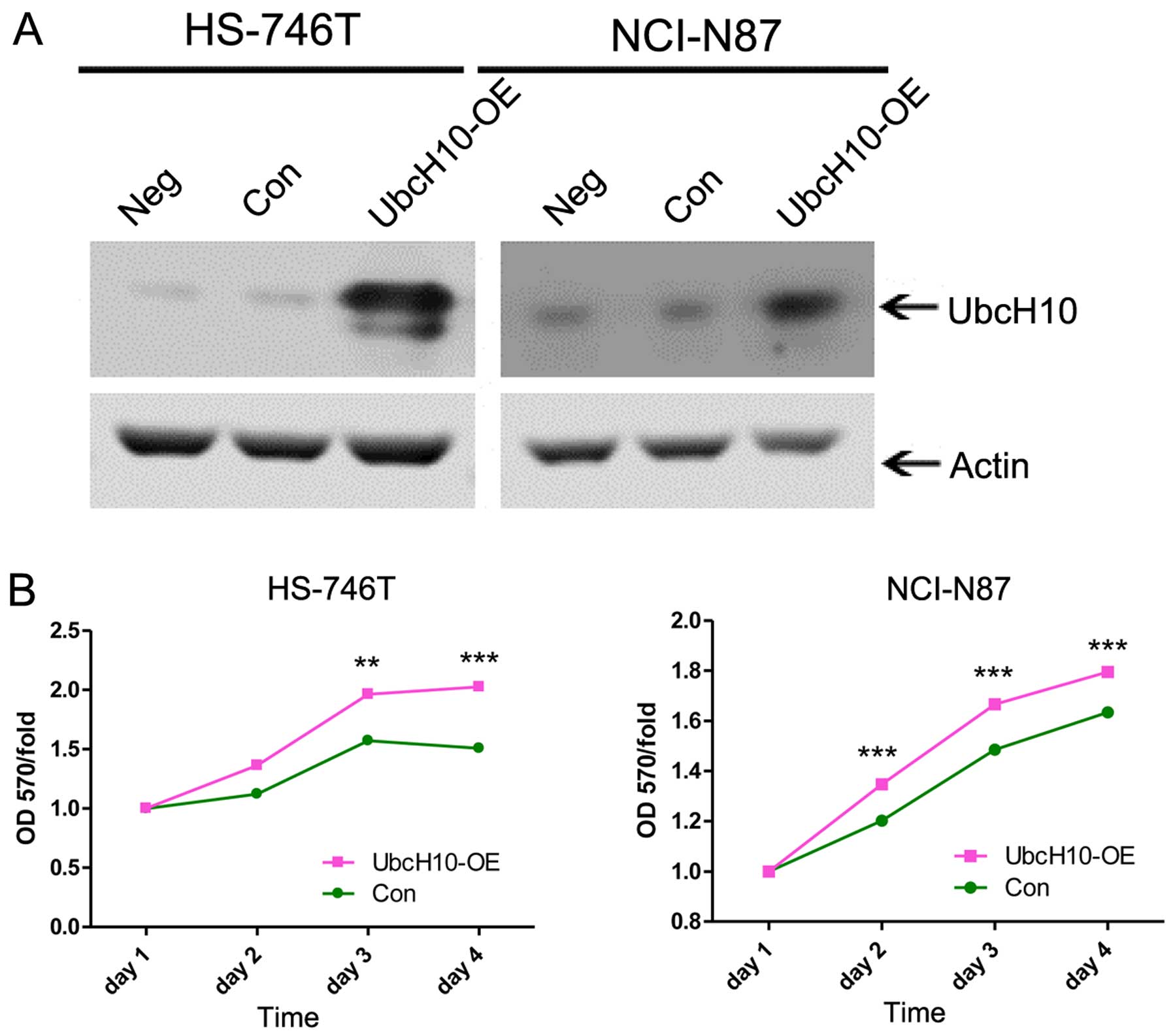 | Figure 4Overexpression of UbcH10 induced by
plasmid transient transfection promotes cell proliferation, cell
cycle and inhibits apoptosis. (A) FLVX-GFP-IRES-UbcH10 was
transiently transfected into HS-746T and NCI-N87 cells,
respectively, as UbcH10-OE; cells treated with FLVX-GFP-IRES empty
plasmid as well as untreated cells were used as negative controls.
(B) HS-746T and NCI-N87 cells were transfected with different
plasmids, and cell proliferation was analyzed by MTT assay in the
subsequent 4 days. Values of optical density (OD) were obtained by
the absorbance at the dual wavelengths 570/700 nm. Bars, mean ± SD
(**P<0.01, ***P<0.001; two-way ANOVA
with Bonferroni post-tests). (C) HS-746T and NCI-N87 cells
transfected with FLVX-GFP-IRES-UbcH10 or control plasmids were
treated with 10 μM cisplatin for 24 h. Cells were fixed with
4% paraformaldehyde followed by Hoechst 33258 staining. The images
were captured by fluorescence microscope, and quantification is
presented in the right panels. Results are representative of 3
independent experiments. Columns, mean (n=3); bars, mean ± SD
(*P<0.05, **P<0.01; t-test). |
MTT assay was used to test the cell proliferation 24
h after transient transfection of UbcH10. In accordance with the
previous gene knockdown data, the overexpression of UbcH10 promoted
tumor cell growth (Fig. 4B).
The overexpression of UbcH10 also led to reduced
cisplatin-induced cell apoptosis as detected by Hoechst staining
and apoptotic nuclei quantification (Fig. 4C).
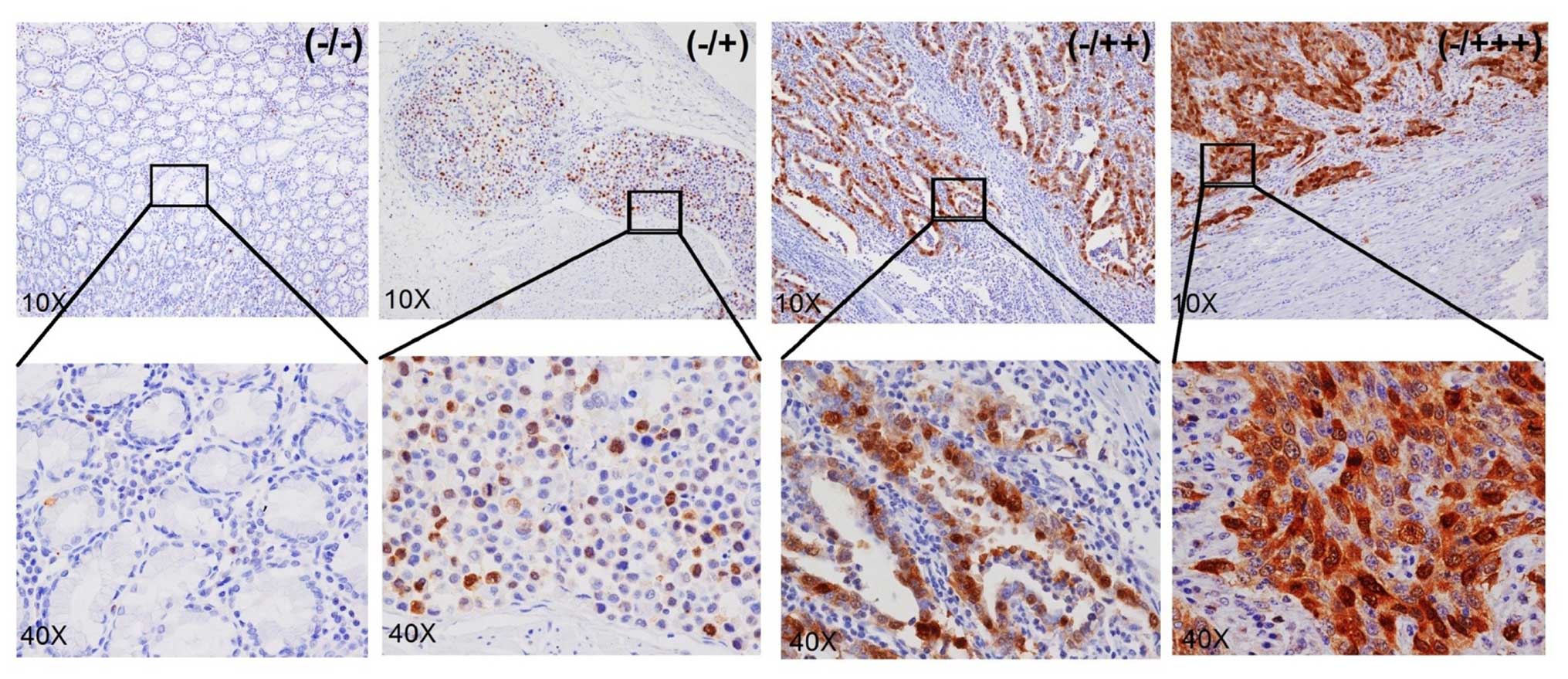
Immunohistochemical (IHC) analysis of
UBcH10 protein expression in gastric carcinoma patient samples
The role of UbcH10 in gastric cancer was supported
both by the UbcH10 overexpression and the knockdown studies. Next,
clinical gastric cancer patient samples were examined to evaluate
the clinical relevance of the findings.
The expression of UbcH10 was examined in 59 cases of
confirmed cases of gastric cancers by IHC analysis. Most of the
cancer tissues showed different levels of UbcH10 expression at the
tumor area; 19 samples displayed clear positive staining (score of
+), 23 samples displayed strong staining (score of ++) and another
7 samples exhibited very strong staining (score of +++) (Fig. 5). No signals were detected in the
neighboring mesenchymal or IgG-negative control. Overall, 49 out of
59 cases (83%) showed significant UbcH10 expression in the
cancerous tissues when compared with that noted in the neigh-boring
mesenchyma (Table I).
 | Table IUbcH10 expression in gastric cancer
patient tumors and mesenchyme. |
Table I
UbcH10 expression in gastric cancer
patient tumors and mesenchyme.
| Staining of
tumors | Staining of
neighboring mesenchyme | No. of samples | Percentage of the
59 samples (%) | Accumulative % |
|---|
| +++ | − | 7 | 11.86 | 11.86 |
| ++ | − | 19 | 32.20 | 44.06 |
| + | − | 23 | 38.98 | 83.04 |
| − | − | 10 | 16.95 | 100.00 |
Discussion
As a critical regulator of cell cycle progression,
UbcH10 is considered as a tumor-promoter gene in the conventional
view among diverse cancers. As a member of the anaphase promoting
complex or cyclosome (APC/C), UbcH10 not only regulates and
controls the cell cycle (31), but
also takes part in initiation, progression and transformation
(15) and significantly shows
increased expression in HCC and other tumor tissues. There are no
reports concerning the relevance of UbcH10 in gastric carcinoma.
Thus, we utilized several gastric carcinoma cell lines for UbcH10
knockdown and overexpression studies.
In the present study, we found that inhibition of
UbcH10 expression suppressed cell proliferation and altered the
cell to become more sensitive to apoptosis accompanied by cell
cycle change and cell survival signaling attenuation. In agreement
with these data, the overexpression of UbcH10 promoted cell
proliferation and resistance to cisplatin-induced apoptosis. UbcH10
was also overexpressed in 3 PDX-derived primary cell lines
established in our laboratory. These cell lines had only limited
passages in vitro and may be more closely related to current
clinical diseases than traditional cancer cell lines.
More importantly, significant expression of UbcH10
protein was detected in patient tumor tissues, but not in adjacent
mesenchyma among 83% of the gastric carcinoma patients (49 out of
59). In addition, with a limited data set, we also noted that the
highest UbcH10 expression was also correlated with poorly
differentiated cancer, suggesting that UbcH10 expression may be
associated with poor prognosis. This issue may require future
patient follow-up studies or retrospective analyses of a large
volume of clinical data and samples. If confirmed, Ubch10
expression level can serve as a useful biomarker for diagnosis and
prognosis.
Amplifications of certain chromosomal regions have
been identified in many types of cancers. The gene UBE2C
coding UbcH10 was reported to form genomic amplification which led
to the increase in UBE2C expression in colon cancer, thyroid
carcinoma and prostate cancer (32–34). A
UBE2C genomic amplification may contribute to the
overexpression of UbcH10 observed in at least some of the patient
gastric cancer samples and gastric cancer cell lines. A systemic
genomic analysis of the patient samples may offer a more definitive
answer.
Notably, knockdown of UbcH10 expression in 2
overexpressing cell lines resulted in slightly different responses.
In the SGC-7901 cells, such knockdown led to reduced percentages of
S and G2M phase cells, while in the KATO III cells, G2/M phases
were largely unaffected with changes predominantly in the S phases
(data not shown). In addition, the changes in protein
phosphorylation were more prominent in the UbcH10-knockdown KATO
III cells in the absence of serum stimulation. These data suggest
that the exact mechanism of how UbcH10 promotes tumor cell
proliferation and resistance to apoptosis also depends on the
̔wiring̓ of the cells, making detailed mechanistic studies more
difficult.
Manipulating the ubiquitin pathways in cancer
treatment has become one of the current focuses of research,
despite that targeting these pathways has been largely premature in
the clinic. The tumor promoting role of UbcH10 may make it a
valuable therapeutic target.
Overall, our results indicated that overexpression
of UbcH10 leads to increased cancer cell proliferation and reduced
cancer cell apoptosis in gastric cancer cell lines, in agreement
with previous findings in other types of cancers (20,35–38),
further validated the tumor-promoting role of UbcH10. These data,
and more importantly, its overexpression in gastric cancer patient
samples while its absence in adjacent mesenchyma, suggest that
UbCH10 may represent a potential biomarker for the diagnosis and
prognosis or a potential therapeutic target in gastric
carcinoma.
Acknowledgments
The present study was supported by the Shanghai
Municipal Commission of Science and Technology (grant no.
14DZ2252000), and the Minhang Commission of Health Research (grant
no. 2012MW16).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
2
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin Y, Ueda J, Kikuchi S, Totsuka Y, Wei
WQ, Qiao YL and Inoue M: Comparative epidemiology of gastric cancer
between Japan and China. World J Gastroenterol. 17:4421–4428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma MR and Schilsky RL: GI cancers in
2010: New standards and a predictive biomarker for adjuvant
therapy. Nat Rev Clin Oncol. 8:70–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smyth EC and Cunningham D: Gastric cancer
in 2012: Defining treatment standards and novel insights into
disease biology. Nat Rev Clin Oncol. 10:73–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nobili S, Bruno L, Landini I, Napoli C,
Bechi P, Tonelli F, Rubio CA, Mini E and Nesi G: Genomic and
genetic alterations influence the progression of gastric cancer.
World J Gastroenterol. 17:290–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang BG and Kim WH: Molecular pathology of
gastric carcinoma. Pathobiology. 78:302–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yasui W, Sentani K, Sakamoto N, Anami K,
Naito Y and Oue N: Molecular pathology of gastric cancer: Research
and practice. Pathol Res Pract. 207:608–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan IB, Ng I, Tai WM and Tan P:
Understanding the genetic basis of gastric cancer: Recent advances.
Expert Rev Gastroenterol Hepatol. 6:335–341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
A-Hassan E, Heinz WF, Antonik MD, D'Costa
NP, Nageswaran S, Schoenenberger CA and Hoh JH: Relative
microelastic mapping of living cells by atomic force microscopy.
Biophys J. 74:1564–1578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pickart CM and Fushman D: Polyubiquitin
chains: Polymeric protein signals. Curr Opin Chem Biol. 8:610–616.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto Y, Ozaki T, Miyazaki K, Aoyama M,
Miyazaki M and Nakagawara A: UbcH10 is the cancer-related E2
ubiquitin-conjugating enzyme. Cancer Res. 63:4167–4173.
2003.PubMed/NCBI
|
|
13
|
Rape M and Kirschner MW: Autonomous
regulation of the anaphase-promoting complex couples mitosis to
S-phase entry. Nature. 432:588–595. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rape M, Reddy SK and Kirschner MW: The
processivity of multiubiquitination by the APC determines the order
of substrate degradation. Cell. 124:89–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Ree JH, Jeganathan KB, Malureanu L and
van Deursen JM: Overexpression of the E2 ubiquitin-conjugating
enzyme UbcH10 causes chromosome missegregation and tumor formation.
J Cell Biol. 188:83–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Perrotta I, Bruno L, Maltese L, Russo E,
Donato A and Donato G: Immunohistochemical analysis of the
ubiquitin-conjugating enzyme UbcH10 in lung cancer: A useful tool
for diagnosis and therapy. J Histochem Cytochem. 60:359–365. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pallante P, Berlingieri MT, Troncone G,
Kruhoffer M, Orntoft TF, Viglietto G, Caleo A, Migliaccio I,
Decaussin-Petrucci M, Santoro M, et al: UbcH10 overexpression may
represent a marker of anaplastic thyroid carcinomas. Br J Cancer.
93:464–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kefeli M, Yildiz L, Celik H, Tosun M and
Karagoz F: UbcH10 expression in benign, hyperplastic, and malignant
endometrial curetted materials: A tissue microarray study. Int J
Surg Pathol. 20:360–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen Z, Jiang X, Zeng C, Zheng S, Luo B,
Zeng Y, Ding R, Jiang H, He Q, Guo J, et al: High expression of
ubiquitin-conjugating enzyme 2C (UBE2C) correlates with
nasopharyngeal carcinoma progression. BMC Cancer. 13:1922013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ieta K, Ojima E, Tanaka F, Nakamura Y,
Haraguchi N, Mimori K, Inoue H, Kuwano H and Mori M: Identification
of overexpressed genes in hepatocellular carcinoma, with special
reference to ubiquitin-conjugating enzyme E2C gene expression. Int
J Cancer. 121:33–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berlingieri MT, Pallante P, Sboner A,
Barbareschi M, Bianco M, Ferraro A, Mansueto G, Borbone E,
Guerriero E, Troncone G, et al: UbcH10 is overexpressed in
malignant breast carcinomas. Eur J Cancer. 43:2729–2735. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujita T, Ikeda H, Taira N, Hatoh S, Naito
M and Doihara H: Overexpression of UbcH10 alternates the cell cycle
profile and accelerate the tumor proliferation in colon cancer. BMC
Cancer. 9:872009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Chen Y, Hu C, Jing H, Cao Y and
Liu X: Association of clinicopathological features with UbcH10
expression in colorectal cancer. J Cancer Res Clin Oncol.
136:419–426. 2010. View Article : Google Scholar
|
|
24
|
Jiang L, Huang CG, Lu YC, Luo C, Hu GH,
Liu HM, Chen JX and Han HX: Expression of ubiquitin-conjugating
enzyme E2C/UbcH10 in astrocytic tumors. Brain Res. 1201:161–166.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen SM, Jiang CY, Wu JY, Liu B, Chen YJ,
Hu CJ and Liu XX: RNA interference-mediated silencing of UBCH10
gene inhibits colorectal cancer cell growth in vitro and in vivo.
Clin Exp Pharmacol Physiol. 37:525–529. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang L, Bao Y, Luo C, Hu G, Huang C, Ding
X, Sun K and Lu Y: Knockdown of ubiquitin-conjugating enzyme
E2C/UbcH10 expression by RNA interference inhibits glioma cell
proliferation and enhances cell apoptosis in vitro. J Cancer Res
Clin Oncol. 136:211–217. 2010. View Article : Google Scholar
|
|
27
|
Lodhia KA, Hadley AM, Haluska P and Scott
CL: Prioritizing therapeutic targets using patient-derived
xenograft models. Biochim Biophys Acta. 1855:223–234.
2015.PubMed/NCBI
|
|
28
|
Choi SY, Lin D, Gout PW, Collins CC, Xu Y
and Wang Y: Lessons from patient-derived xenografts for better in
vitro modeling of human cancer. Adv Drug Deliv Rev. 79–80:222–237.
2014. View Article : Google Scholar
|
|
29
|
John T, Kohler D, Pintilie M, Yanagawa N,
Pham NA, Li M, Panchal D, Hui F, Meng F, Shepherd FA, et al: The
ability to form primary tumor xenografts is predictive of increased
risk of disease recurrence in early-stage non-small cell lung
cancer. Clin Cancer Res. 17:134–141. 2011. View Article : Google Scholar
|
|
30
|
Sicklick JK, Leonard SY, Babicky ML, Tang
CM, Mose ES, French RP, Jaquish DV, Hoh CK, Peterson M, Schwab R,
et al: Generation of orthotopic patient-derived xenografts from
gastrointestinal stromal tumor. J Transl Med. 12:412014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Y, Hwang WC and Basavappa R:
Structural and functional analysis of the human mitotic-specific
ubiquitin-conjugating enzyme, UbcH10. J Biol Chem. 277:21913–21921.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi Y, Ishii Y, Nishida Y, Ikarashi
M, Nagata T, Nakamura T, Yamamori S and Asai S: Detection of
aberrations of ubiquitin-conjugating enzyme E2C gene (UBE2C) in
advanced colon cancer with liver metastases by DNA microarray and
two-color FISH. Cancer Genet Cytogenet. 168:30–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee JJ, Au AY, Foukakis T, Barbaro M, Kiss
N, Clifton-Bligh R, Staaf J, Borg A, Delbridge L, Robinson BG, et
al: Array-CGH identifies cyclin D1 and UBCH10 amplicons in
anaplastic thyroid carcinoma. Endocr Relat Cancer. 15:801–815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tzelepi V, Zhang J, Lu JF, Kleb B, Wu G,
Wan X, Hoang A, Efstathiou E, Sircar K, Navone NM, et al: Modeling
a lethal prostate cancer variant with small-cell carcinoma
features. Clin Cancer Res. 18:666–677. 2012. View Article : Google Scholar
|
|
35
|
Donato G, Iofrida G, Lavano A, Volpentesta
G, Signorelli F, Pallante PL, Berlingieri MT, Pierantoni MG,
Palmieri D, Conforti F, et al: Analysis of UbcH10 expression
represents a useful tool for the diagnosis and therapy of
astrocytic tumors. Clin Neuropathol. 27:219–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin J, Raoof DA, Wang Z, Lin MY, Thomas
DG, Greenson JK, Giordano TJ, Orringer MB, Chang AC, Beer DG, et
al: Expression and effect of inhibition of the
ubiquitin-conjugating enzyme E2C on esophageal adenocarcinoma.
Neoplasia. 8:1062–1071. 2006. View Article : Google Scholar
|
|
37
|
Troncone G, Guerriero E, Pallante P,
Berlingieri MT, Ferraro A, Del Vecchio L, Gorrese M, Mariotti E,
Iaccarino A, Palmieri EA, et al: UbcH10 expression in human
lymphomas. Histopathology. 54:731–740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vasiljevic A, Champier J,
Figarella-Branger D, Wierinckx A, Jouvet A and Fèvre-Montange M:
Molecular characterization of central neurocytomas: Potential
markers for tumor typing and progression. Neuropathology.
33:149–161. 2013. View Article : Google Scholar
|