Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive human malignancies and it is the fourth leading
cause of cancer-related death in the US (1). Due to its late presentation, early
local invasion and metastatic potential long-term survivors are
rare. Clinical trials performed in PDAC patients using different
chemotherapeutic agents have only slightly improved their survival
over the last few decades, and the overall 5-year relative survival
rate remains at 6.9% (2,3). In addition, more than 80% of patients
have advanced regional disease or distant metastasis at the time of
diagnosis and tumors are often inoperable. Moreover, those patients
who undergo surgical tumor resection exhibit a high incidence of
local recurrence, peripheral organic metastasis and peritoneal
dissemination (4).
At the molecular level, a high percentage of PDACs
overexpress a number of growth factors and their receptors,
including the epidermal growth factor family, transforming growth
factor family and fibroblast growth factor family (5). Fibroblast growth factors (FGFs) are
comprised of ~20 molecules with a wide range of biological actions.
They have the ability to stimulate angiogenesis, and are involved
in cell differentiation, migration, tissue repair and regeneration
(6). The two most extensively
studied FGFs are FGF-1 and FGF-2 (7). FGF-2 is the prototypic heparin-binding
protein with growth, anti-apoptotic and angiogenic activity
(8). It is overexpressed in PDAC
(9), and the FGF-2 receptor has
been reported to correlate with tumor metastasis, stage and
retro-peritoneal invasion (10). In
addition, low expression of FGF-2 has been associated with longer
post-operative survival in PDAC patients (11). In general, FGF-2 can exerts its
biological effects by binding to FGFR-2, which leads to the
activation of a number of signaling cascades; in which the most
dominant is the MAPK/ERK pathway (12).
Cyclooxygenases (COXs) are key enzymes in the
synthesis of prostaglandins. There are two types of COX isoenzymes:
COX-1, which is expressed in many normal tissues and responsible
for many physiological functions; and COX-2, an inducible
prostaglandin synthase that is upregulated in different tumor
tissues and in response to inflammation (13). Celecoxib is a selective COX-2
inhibitor that was first used for adjuvant treatment in patients
with familial adenomatous polyposis (14). Since the introduction of celecoxib
in cancer therapy, a number of studies have investigated molecular
targets and the clinical effects of selective COX-2 inhibitors, as
well as molecular mechanisms of their antitumor activity, either
via selective COX-2 inhibition or COX-2-independent mechanisms of
action. It has been shown in pre-clinical studies of pancreatic
cancer that selective COX-2 inhibitors exert their antitumor effect
by inhibiting cell proliferation and promoting apoptosis (15). Furthermore, although precise
biological mechanisms underlying the antitumor effect of
COX-independent action remain unclear, it is possible that the
effect of COX-2 inhibitors may be mediated, at least in part, by
the suppression of FGF-2 (16). It
has been shown that oral COX-2 inhibitors suppress angiogenesis and
the growth of gastrointestinal tumor explants in nude mice,
possibly via mechanisms associated with the reduced expression of
FGF-2 and VEGF (17). Furthermore,
in esophageal adenocarcinoma, treatment of Seg-1 cells with NS-398
in vitro significantly reduced FGF-2 expression and induced
an antitumor effect (18).
Based on these findings, it is possible that the
antitumor mechanisms of celecoxib may be associated with FGF-2
signal regulation. Therefore, the aim of the present study was to
examine the hypothesis that selective COX-2 inhibitor celecoxib may
synergistically suppress the expression of FGF-2 (possibly through
the activation of ERK1/2 and increase in the secretion of MMPs) and
the expression of its receptor, FGFR-2; thereby, inhibiting
proliferation, invasion and migration, and stimulating the
apoptosis of human pancreatic cancer PANC-1 cells in
vitro.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was
obtained from HyClone (Logan, UT, USA), and penicillin and
streptomycin were acquired from Gibco-BRL (Gibco, NY, USA).
Celecoxib was obtained from Sigma-Aldrich (St. Louis, MO, USA) and
dissolved in anhydrous dimethyl sulfoxide (DMSO) (Sigma, St. Louis,
MO, USA) at a concentration of 100 mmol/l for storage solution.
Human FGF-2 was obtained from Cell Signaling Technology (Boston,
MA, USA), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) was obtained from Sigma Chemicals (Sigma). Other
reagents were purchased from Nanjing Chemical Reagent Co. (Nanjing,
China) unless otherwise described.
Cell culture
PANC-1 cells derived from a human PDAC were acquired
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). Cells were cultured in DMEM with 10% fetal bovine serum
(FBS; Gibco, Grand Island, NY, USA) and 1% ampicillin-streptomycin.
Cells were grown in a humidified incubator with an atmosphere of 5%
CO2 at 37°C.
Cell viability assay
PANC-1 cells were cultured in 96-well plates at a
density of 1×104 cells/well and incubated in medium with
10% FBS. After overnight growth, the cells were treated with 5, 10,
20, 40, 80 and 160 µmol/l of celecoxib in serum-free
conditions. The control group was set in serum-free medium. After
incubation for 24, 48 and 72 h at 37°C, 20 µl of MTT
solution [5 mg/ml in phosphate-buffered saline (PBS)] was added
into each well; and cells were further incubated for 4 h at 37°C.
Next, 100 µl of DMSO was added into each well at 37°C. A
spectrophotometer (Bio-Rad, Hercules, CA, USA) was used to
determine the optical density (OD) value of each well at 490 nm.
Each experiment was performed at least in triplicate, and results
are presented as a relative ratio to the OD at the beginning of the
experiment.
Cell apoptosis assay
In order to quantify apoptosis, the cells were
stained with Annexin V and propidium iodide (PI) using an Annexin
V-FITC/PI apoptosis kit (BD Biosciences, San Jose, CA, USA)
according to the manufacturer's instructions. In brief, PANC-1
cells were cultured into 6-well plates with DMEM containing 10% FBS
for 24 h. Next, the cells were further treated for 24 h with an
indicated concentration of celecoxib or FGF-2. After treatment, the
cells were digested with trypsin, washed twice with cold PBS, and
resuspended in 1X binding buffer at a concentration of
1×105 cells/ml. Next, 100 µl of the solution
(1×105 cells) was mixed with 5 µl of Annexin
V-FITC and 5 µl of PI, and incubated for 15 min in the dark
at room temperature. Finally, 400 µl of 1X binding buffer
was added to each sample; then, the cells were kept on ice and
immediately subjected to flow cytometry on a FACSCalibur flow
cytometer (Becton-Dickinson, San Jose, CA, USA). Cell Quest
software (Becton-Dickinson) was used to analyze the data.
Invasion assay
For the cell invasion assay, the cells were
pretreated with the indicated celecoxib or FGF-2 concentration for
2 h, digested with trypsin and placed in the upper compartment of a
Matrigel (BD Biosciences)-coated chamber, as previously described
(19). In brief, equal
concentrations of cells (5×104 cells/well) were
suspended in 300 µl of DMEM containing 0.1% of BSA, and the
cell suspensions were added to the upper compartment of a 24-well
Boyden chamber (Millipore Co., Billerica, MA, USA). Another 600
µl of DMEM containing 15% FBS was added to the lower
compartment in order to stimulate cell invasion. Then, the cells
were incubated for 24 h, and the non-invaded cells were removed
from the top compartment of the membrane with a cotton swab.
Remaining cells on the lower surface of the membrane were fixed
with 4% paraformaldehyde and stained in 0.01% crystal violet
solution. The number of invading cells was quantified from six
random high-power fields (HPFs) visualized at a magnification of
×100.
Scratch migration assay
For the evaluation of PANC-1 cell migration, the
cells were cultured in 6-well plates (1×106 cells/well)
for 24 h, washed with PBS twice and treated with the indicated
concentration of celecoxib or FGF-2 for 2 h. Then, the cells were
scraped with the fine end of a 1-ml pipette tip; and the plates
were washed twice with PBS to remove the detached cells, and were
incubated with DMEM containing 10% FBS. Cell migration was
photographed using 10 HPFs at 0- and 24-h post-induction of the
injury. Remodeling was measured as the relative diminishing area
across the induced injury, and expressed as migration area
percentage.
Western blot analysis
For western blot analysis, PANC-1 cells were washed
twice with cold PBS and lysed in RIPA buffer (PBS containing 1%
Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 ng/ml of
phenylmethylsulfonyl fluoride and 10 µg/ml of aprotinin).
Then, the samples that contained 50 µg of protein/lane were
fractionated on a 10% SDS-polyacrylamide gel by electrophoresis,
and the separated proteins were transferred onto polyvinylidene
difluoride (PVDF) membranes (Millipore) using a semi-dry transfer
cell (Bio-Rad). Next, the blots were incubated in Tris-buffered
saline (TBS; 10 mmol/l of Tris-HCl pH 8.0 and 150 mmol/l of NaCl)
containing 5% (w/v) non-fat dry milk and 0.1% Tween-20 for 2 h at
room temperature. Then, the immunoblots were incubated first with
different specific primary antibodies, followed by incubation with
appropriate secondary antibodies. β-actin was used as a control.
Signals were quantified using the public domain NIH ImageJ 1.49
(http://rsbweb.nih.gov/ij/download.html; US National
Institutes of Health, USA).
The following primary antibodies were used: mouse
anti-human FGF-2 antibody (catalog no. ab130094; dilution 1:500;
Abcam, Cambridge, MA, USA), rabbit anti-human FGF receptor 2
antibody (catalog no. 11835; dilution 1:500), rabbit anti-human
p44/42 MAPK antibody (catalog no. 9102; dilution 1:1,000), rabbit
anti-human phospho-p44/42 MAPK antibody (catalog no. 9101; dilution
1:1,000), rabbit anti-human MMP-2 antibody (catalog no. 4022;
dilution 1:400), rabbit anti-human MMP-9 antibody (catalog no.
3852; dilution 1:400) (all from Cell Signaling Technology) rabbit
anti-human TIMP-1 antibody (catalog no. ab109125; dilution 1:400;
Abcam), mouse anti-human β-actin antibody (catalog no. sc-130301;
dilution 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA,
USA).
RNA extraction and quantitative real-time
PCR (qRT-PCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was
used for the extraction of total RNA from the PANC-1 cells. Next,
cDNA was synthesized using a PrimeScript RT reagent kit and qRT-PCR
was carried out using a SYBR-Green PCR kit (both from Takara,
Dalian, China), according to the manufacturer's instructions. GAPDH
was used as an internal control, and relative expression levels
were assessed using the ΔΔCt method. PCR primers for
qRT-PCR assay were as follows (5′-3′): FGF-2-forward,
AGAGCGACCCTCACATCAAG and FGF-2-reverse, TCGTTTCAGTGCCACAACG;
FGFR-2-forward, TCCTATGACATTAACCGTGTT and FGFR-2-reverse,
TTTAACACTGCCGTTTAT; MMP-2-forward, GTGCTGAAGGACACACTAAAGAAGA and
MMP-2-reverse, TTGCCATCCTTCTCAAAGTTGTAGG; MMP-9-forward,
GCGGAGATTGGGAACCAGCTGTA and MMP-9-reverse, GACGCGCCTGTGTACACCCACA;
TIMP-1-forward, CATCCTGTTGTTGCTGTGGCTGAT and TIMP-1-reverse,
GTCATCTTGATCTCATAACGCTGG; MAPK-1-forward, CAGTTCTTGACCCCTGGTCC and
MAPK-1-reverse, GTACATACTGCCGCAGGTCA; GAPDH-forward,
GGAGCGAGATCCCTCCAAAAT and GAPDH-reverse, GGCTGTTGTCATACTTCTCATGG.
All experiments were performed three times in triplicate.
Statistical analysis
Statistical analyses were performed using the SPSS
software package (version 13.0; SPSS, Inc., Chicago, IL, USA). All
data are presented as mean ± SEM. Data were analyzed by one-way
analysis of variance (ANOVA), and all statistical tests were
two-sided. A P-value of <0.05 was considered to indicate a
statistically significant result.
Results
Effect of celecoxib and FGF-2 on the
proliferation and apoptosis of PANC-1 cells
In the present study, the influence of celecoxib was
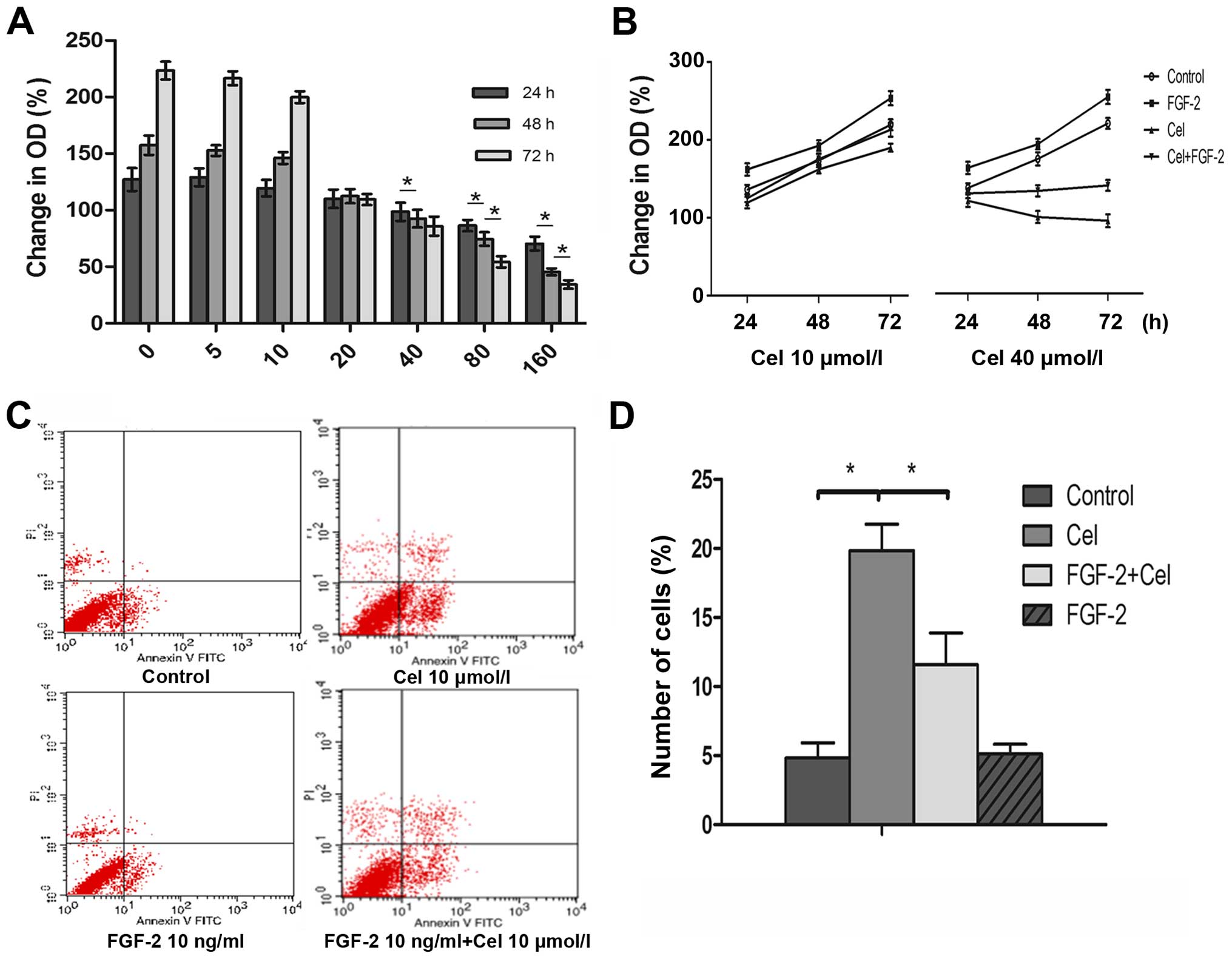
examined on PANC-1 cell growth. Celecoxib inhibited cell
proliferation in a dose- and time-dependent manner (Fig. 1A), and this effect was preventable
through the presence of exogenous FGF-2 (10 ng/ml) in culture
medium with celecoxib (Fig. 1B). No
inhibitory effect on PANC-1 cell growth was observed within the
first 24 h for celecoxib concentrations of up to 40 µmol/l.
Longer periods of incubation reduced MTT signals starting at a
concentration of 20 µmol/l. Hence, for further experiments
in vitro, celecoxib concentrations between 5 and 10
µmol/l were used.
The ability of FGF-2 to reverse the inhibitory
effect of celecoxib on cell proliferation could be attributable to
the inhibition of apoptosis. Celecoxib treatment for 24 h induced a
significant 4-fold increase in the apoptotic index, from 4.8±1.1%
observed in control untreated cells to 19.84±1.9% in cells treated
with celecoxib (10 µmol/l) for 24 h (Fig. 1C and D). FGF-2 alone had no
influence on apoptotic level. However, FGF-2 (10 ng/ml)
significantly reduced celecoxib-induced apoptosis from 19.84±1.9%
in cells treated with celecoxib (10 µmol/l) alone to
11.58±2.3% in cells treated with celecoxib and FGF-2.
Celecoxib compromises FGF-2-induced
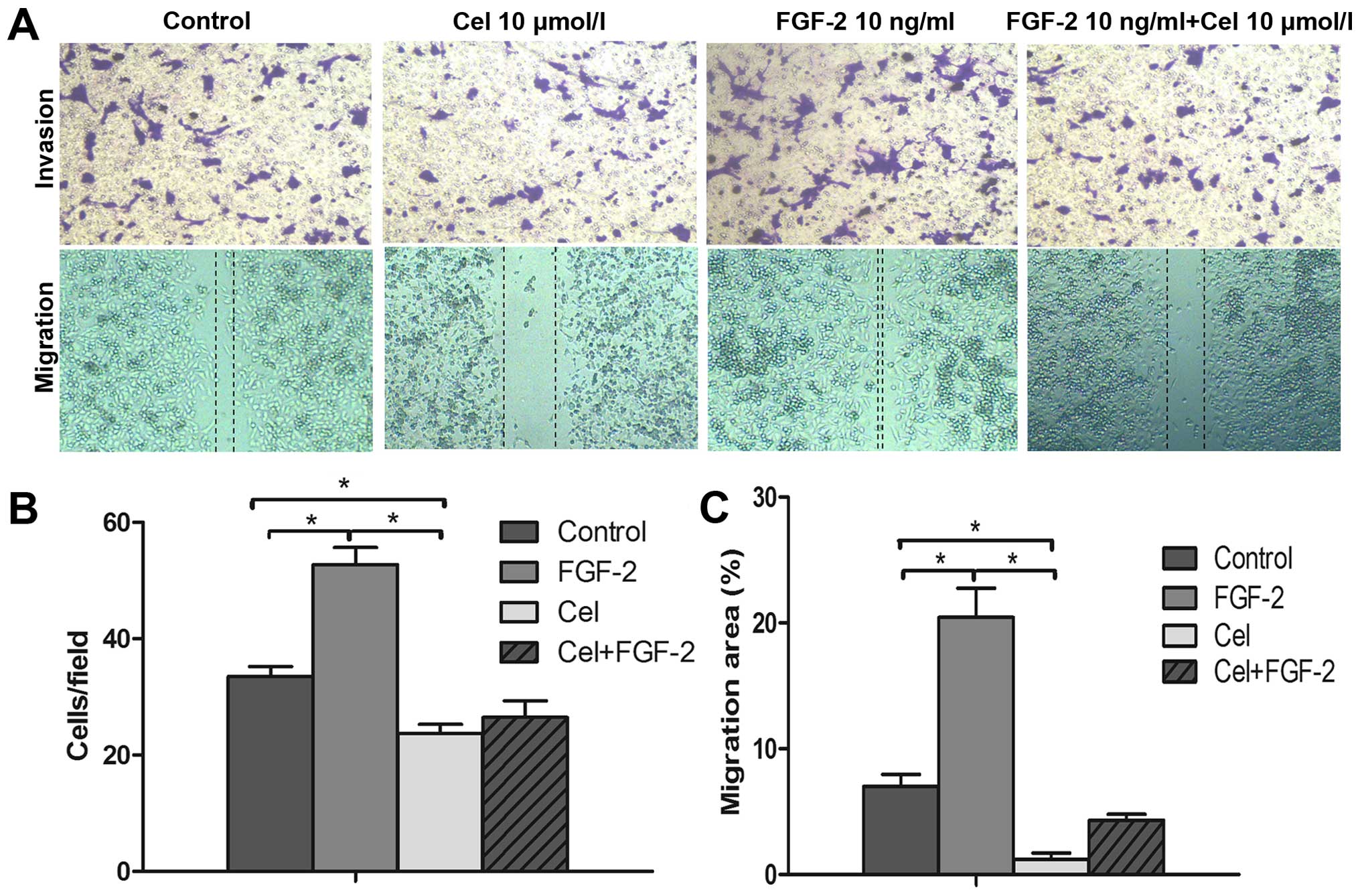
invasion and migration of PANC-1 cells
Next, the effects of celecoxib on the invasion and
migration of PANC-1 cells were investigated. As shown in Fig. 2A, celecoxib (10 µmol/l)
strongly inhibited cell invasion and migration. Compared with the
control group, the invasion and migration abilities of the PANC-1
cells were significantly decreased (P<0.05; Fig. 2B and C). In contrast, treatment with
FGF-2 notably enhanced the invasion and migration abilities of the
PANC-1 cells. However, when PANC-1 cells were treated with FGF-2
(10 ng/ml) and celecoxib (10 µmol/l), the enhanced invasion
and migration abilities induced by FGF-2 were abolished by
celecoxib (Fig. 2).
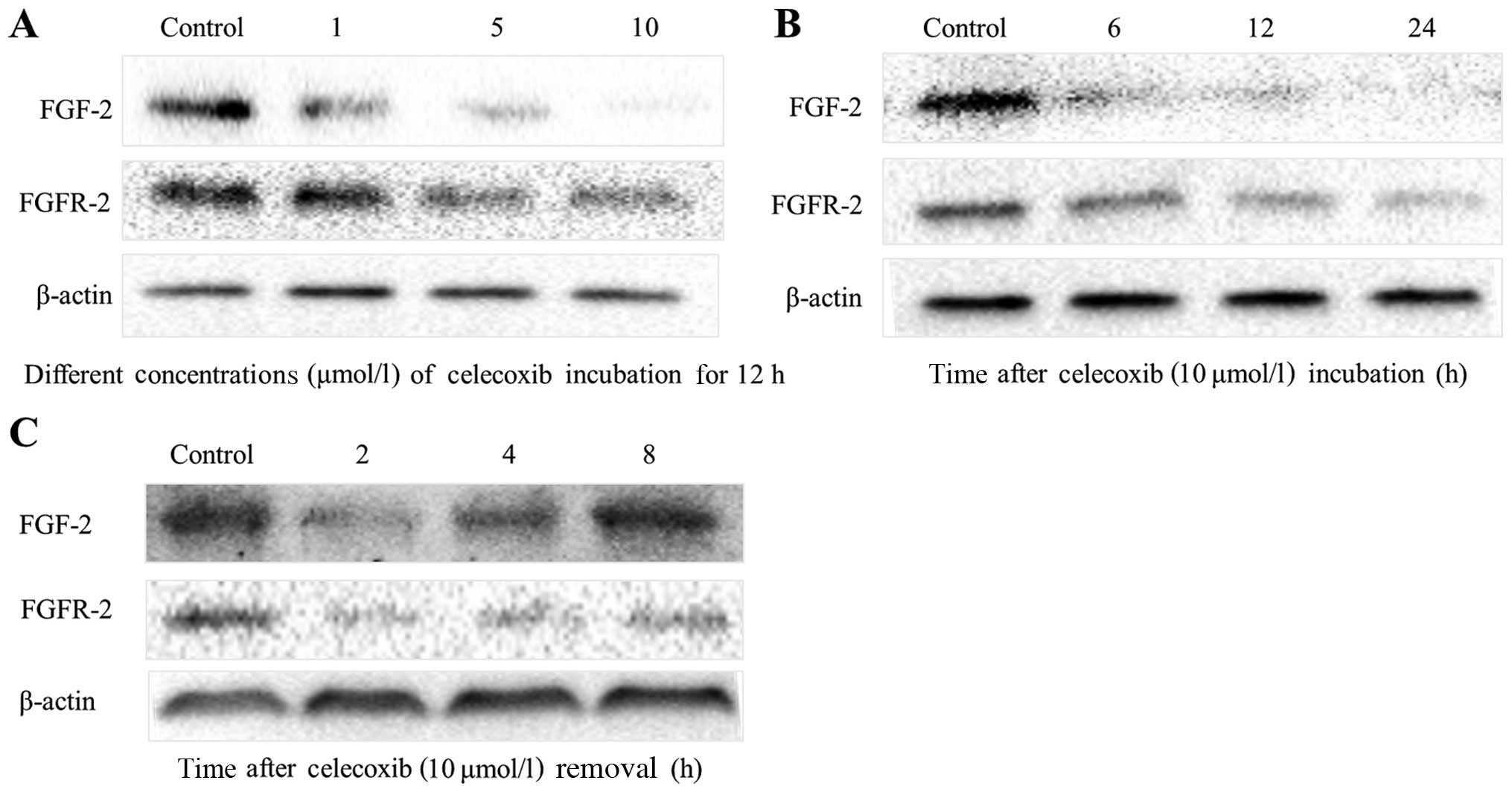
Celecoxib inhibits FGF-2 and FGFR-2
expression in PANC-1 cells
The treatment of PANC-1 cells with 5 and 10
µmol/l of celecoxib resulted in a marked suppression in
cellular FGF-2 content, as indicated by western blot analysis
(Fig. 3). Celecoxib suppressed
FGF-2 expression in a dose-dependent manner; that is, after
incubation with celecoxib for 12 h, cells expressed a marginally
detectable level of FGF-2 (Fig.
3A). However, FGF-2 levels increased rapidly within 8 h of
celecoxib (10 µmol/l) removal, and returned to normal
levels. In contrast, incubation of PANC-1 cells with celecoxib (10
µmol/l) for 12 h resulted in a significant decrease in
FGFR-2 expression; and after removal of celecoxib, FGFR-2
inhibition persisted for some time.
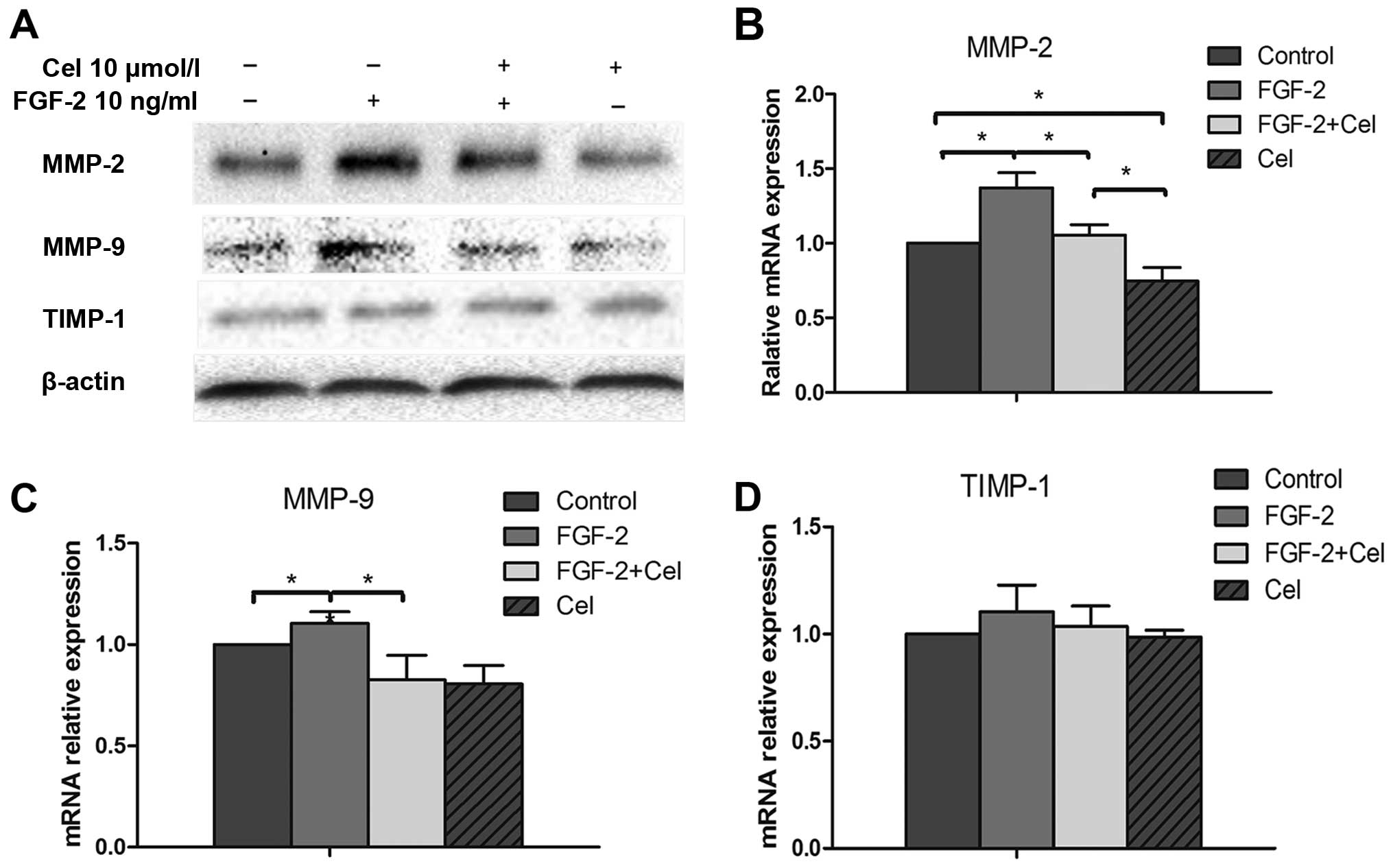
Celecoxib suppresses MMP-9 and MMP-2
expression in PANC-1 cells
Matrix metalloproteinases (MMPs) serve as reliable
markers for tumor cell invasion and migration. In the present
study, PANC-1 cells were treated with or without 10 ng/ml of FGF-2
and with or without 10 µmol/l of celecoxib; and mRNA and
protein levels of MMP-2, MMP-9 and TIMP-1 were examined by qRT-PCR
and western blot analysis, respectively. As shown in Fig. 4, FGF-2 treatment resulted in
increased MMP-2 and MMP-9 protein expression, compared to the
untreated control cells. In contrast, western blot and qRT-PCR
analyses revealed that the expression levels of MMP-2 and MMP-9
were significantly decreased in cells exposed to 10 µmol/l
of celecoxib (P<0.05; Fig.
4A–C), compared with the control cells. No change in TIMP-1
expression was observed when PANC-1 cells were treated with FGF-2
or celecoxib alone or in combination (Fig. 4D). Celecoxib was also able to
suppress the FGF-2-induced enhanced expression of MMP-2 and MMP-9,
which is suggestive of its anti-FGF-2-like capacity (Fig. 4A–C).
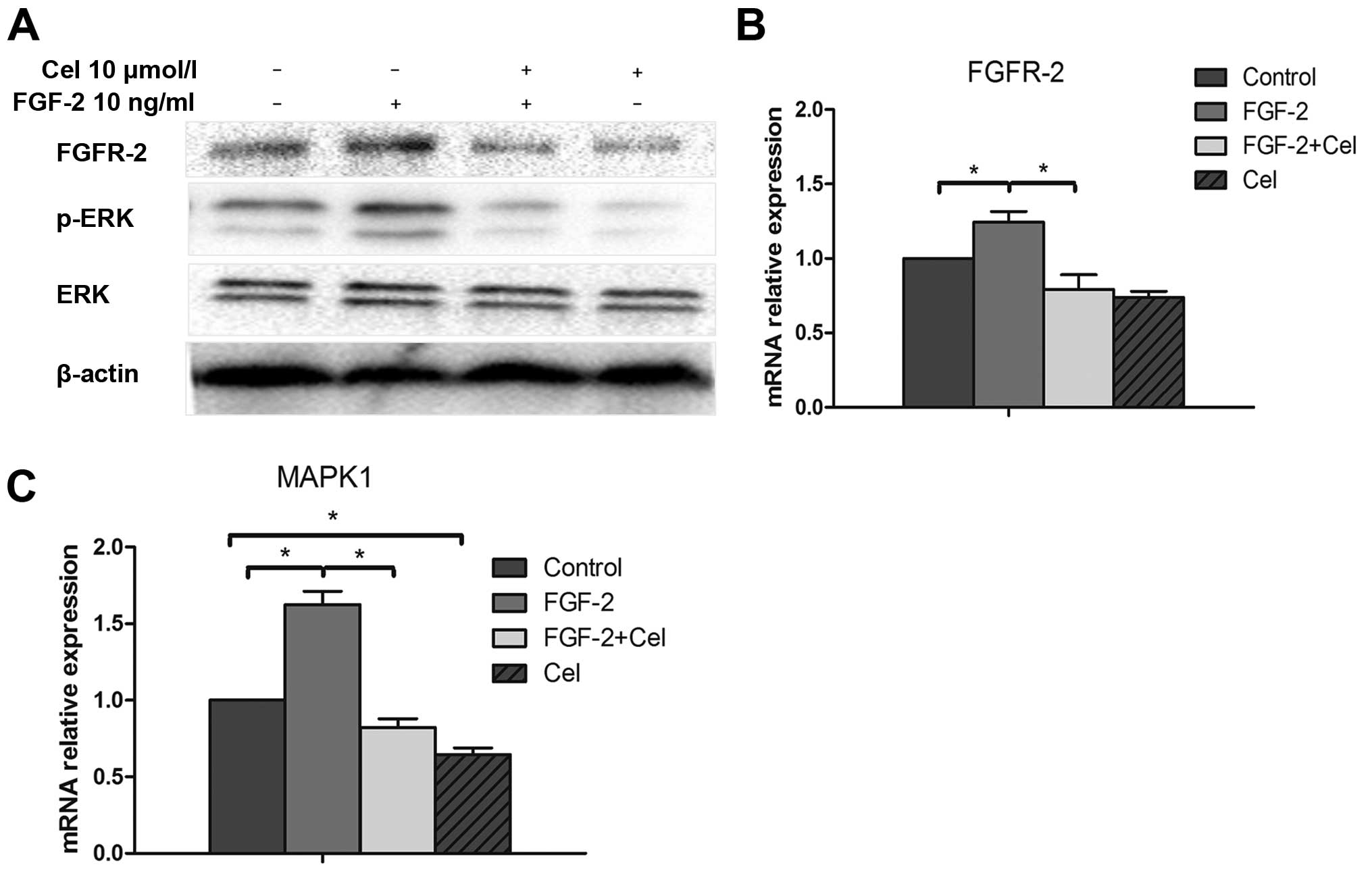
Celecoxib inhibits FGFR-2 expression and
ERK1/2 phosphorylation activated by FGF-2
Mitogenic signaling through FGFRs often involves the
activation of the MAPK pathway. Therefore, we sought to determine
whether the FGF-2-mediated activation of MAPK was altered as a
consequence of changes in FGFR-2 levels. Western blot analysis
revealed that FGF-2 treatment caused the increase in its receptor
FGFR-2 expression, as well as increased levels of phosphorylated
ERK, compared to the control untreated PANC-1 cells (Fig. 5A). In contrast, treatment with
celecoxib inhibited FGFR-2 expression and suppressed phosphorylated
ERK1/2 altered by FGF-2. In addition, qRT-PCR analyses also
revealed that the transcriptional activity of FGFR-2 and MAPK1
decreased in the cells treated with celecoxib, compared to the
cells in the control group (P<0.05; Fig. 5B and C).
Discussion
Pancreatic ductal adenocarcinoma (PDAC) is a very
aggressive and lethal type of tumor, in which its metastatic
invasion is associated with inflammation (20). The COX-2 enzyme is an important
mediator of prostaglandin synthesis and is significantly
overexpressed in many different types of cancers (21). COX-2 inhibitors can exert their
effects by decreasing the expression of COX-2. Nevertheless, some
selective COX-2 inhibitors can also provoke responses in
COX-2-negative cancer cells, which could not be explained by COX-2
inhibition, However, the mechanisms of this biological effects are
poorly understood (22). Celecoxib,
a selective COX-2 inhibitor, has been previously shown to inhibit
the growth of human pancreatic cancer cell lines (23). In addition, in pancreatic xenograft
experiments, treatment with celecoxib inhibited tumor growth,
metastasis and angiogenesis (24).
Furthermore, in a clinical assessment study of celecoxib
pharmacodynamics in pancreatic cancer patients, celecoxib
significantly decreased the level of COX and PGE2; but this effect
did not induce a significant decrease in tumor growth, suggesting
that COX and PGE2 downregulation may be irrelevant for pancreatic
cancer growth (25). Moreover,
there is evidence suggesting that the mechanism of the antitumor
effect of celecoxib may be mediated by targets other than COX-2
such as the down-regulation of the expression of survivin (26) or AKT (27). It has been previously reported that
PANC-1 cells express a marginally detectable level of COX-2
(28); however, COX-2 inhibitors
continue to exert an antitumor effect in these cells. In the
present study, we investigated the possible regulation of FGF-2 and
its receptor by selective COX-2 inhibitor celecoxib, as well as the
role of FGF-2 in the survival, proliferation, invasion and
migration of PANC-1 cells. We demonstrated that celecoxib
significantly inhibited cell proliferation and stimulated apoptosis
in PANC-1 cells. This was accompanied by a profound but reversible
suppression of FGF-2 expression in PANC-1 cells. Furthermore, the
addition of exogenous FGF-2 to culture medium significantly
ameliorated the antiproliferative and pro-apoptotic effects of
celecoxib in PANC-1 cells. These data suggest that in PANC-1 cells,
COX-2 inhibitor celecoxib suppresses cell proliferation and induces
apoptosis, at least in part, via an FGF-2-dependent pathway. FGF-2
is an important growth and differentiation factor involved in many
physiological and pathological processes. Indeed, it has been shown
in several studies that the tissue-specific expression of FGFs and
FGFRs is critical in the regulation of tissue homeostasis (7–10). The
ectopic expression of FGF ligands or aberrant splicing of FGFRs can
result in the activation of autocrine signaling pathways and cause
uncontrolled cell proliferation (29). Moreover, FGFR-2 overexpression was
correlated with increased pancreatic cancer cell proliferation,
invasion and early liver recurrence in patients following surgical
resection of a tumor (30).
Invasion and metastasis are two hallmarks of cancer,
the main causes of cancer-related death, and the most difficult
clinical treatment issues, particularly in PDAC (3,4).
Numerous studies that explored these processes in PDAC have been
conducted over several decades, but tumor metastasis continues to
remain the main cause of poor outcome in PDAC patients (31–33).
In recent years, studies focusing on the tumor microenvironment
suggest that the remodeling of the tumor extracellular matrix (ECM)
increases the invasion and metastatic capabilities of tumor cells
(32). Among numerous factors
involved in ECM remodeling, MMPs secreted by cancer cells have been
postulated as one of the major facilitators of tumor invasion
(33). Compared to benign tumors,
malignant tumors exhibit increased MMP expression levels, which
lead to the increased degradation of the ECM and promotion of tumor
cell migration and invasion (34).
Indeed, the upregulation of the expression of MMPs and TIMPs in
cancer tissues results in increased tumor invasion and metastasis
(35). The elevated expression of
MMP-2 or MMP-9 is associated with the hydrolysis of the ECM and
induction of cancer cell invasion through the basement membrane
(36). The results of the present
study revealed that celecoxib can inhibit FGF-2 signaling
pathway-mediated MMP regulation in PANC-1 cells during tumor cell
invasion in vitro. Our data revealed that the expression
levels of MMP-2 and MMP-9 were decreased in the PANC-1 cells when
treated with celecoxib. In contrast, the expression levels of MMP-2
and MMP-9 were significantly induced by FGF-2 treatment. It has
been reported that many patients with pancreatic cancer overexpress
FGF-2, which leads to the activation of the ERK1/2 pathway,
increased expression of MMPs and higher invasive potential
(9–11). In addition, in the present study,
TIMP-1 levels did not change after celecoxib treatment, suggesting
that a different pathway is involved in the regulation of TIMP-1
expression in PANC-1 cells.
The MAPK/ERK signaling pathway is one of the most
commonly activated pathways in human cancers (37). It has been shown that this pathway,
particularly ERK1/2, regulates the expression of MMPs (38). Indeed, a decrease in ERK1/2
phosphorylation may be involved in the downregulation of MMPs and
upregulation of the expression of TIMPs (39). In the present study, we showed that
ERK1/2 was activated in PANC-1 cells following treatment with
FGF-2. In addition, FGF-2 increased PANC-1 cell growth and invasion
via binding to its receptor FGFR-2 and phosphorylation of the
ERK1/2 protein, which further resulted in increased secretion of
MMP-2 and MMP-9. In contrast, treatment with celecoxib suppressed
the expression of FGFR-2, inhibited ERK1/2 phosphorylation and
acted against the effect of FGF-2; suggesting that the molecular
mechanisms of celecoxib inhibition in PANC-1 cell invasion and
migration are related to the ERK1/2 pathway and mediated by FGFR-2.
Another possible mechanism is the involvement of the Ras-MAPK
signaling pathway. Pancreatic adenocarcinoma has the highest
incidence of K-ras point mutations (70–90% of all cases)
(40), and PANC-1 cells have an
activating K-ras gene and it has been shown that
K-ras is involved in increasing or activating
extracellularly sequestered FGF-2 (41). In addition, oncogenic Ras-induced
proliferation can be abolished by addition of an anti-FGF-2
blocking antibody (41). Based on
these findings we hypothesized that the antitumor effects of
celecoxib may also be associated with K-ras regulation in
PANC-1 cells.
In conclusion, the present study is the first to
report on the relationship between FGF-2 expression and COX-2
inhibitor celecoxib in PANC-1 cells. The antitumor effect of
celecoxib was exerted through the inhibition of the expression of
FGFR-2 and interruption of the activity of MMPs. These results
provide a rational basis for the further evaluation of the efficacy
of celecoxib in the treatment of PDAC and perhaps other
malignancies, in which FGF2/FGFR2 signaling has an important
role.
Abbreviations:
|
COXs
|
cyclooxygenases
|
|
FGFs
|
fibroblast growth factors
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
MMPs
|
matrix metalloproteinases
|
|
TIMPs
|
tissue inhibitor of
metalloproteinases
|
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun H, Ma H, Hong G, Sun H and Wang J:
Survival improvement in patients with pancreatic cancer by decade:
A period analysis of the SEER database, 1981–2010. Sci Rep.
4:67472014. View Article : Google Scholar
|
|
4
|
Sohn TA, Yeo CJ, Cameron JL, Koniaris L,
Kaushal S, Abrams RA, Sauter PK, Coleman J, Hruban RH and Lillemoe
KD: Resected adenocarcinoma of the pancreas-616 patients: Results,
outcomes, and prognostic indicators. J Gastrointest Surg.
4:567–579. 2000. View Article : Google Scholar
|
|
5
|
Cho K, Ishiwata T, Uchida E, Nakazawa N,
Korc M, Naito Z and Tajiri T: Enhanced expression of keratinocyte
growth factor and its receptor correlates with venous invasion in
pancreatic cancer. Am J Pathol. 170:1964–1974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beenken A and Mohammadi M: The FGF family:
Biology, pathophysiology and therapy. Nat Rev Drug Discov.
8:235–253. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
August P, Javerzat S and Bikfalvi A:
Regulation of vascular development by fibroblast growth factors.
Cell Tissue Res. 8:204–210. 2003.
|
|
8
|
Powers CJ, McLeskey SW and Wellstein A:
Fibroblast growth factors, their receptors and signaling. Endocr
Relat Cancer. 7:165–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Turner N and Grose R: Fibroblast growth
factor signalling: From development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuda Y, Yoshimura H, Suzuki T, Uchida
E, Naito Z and Ishiwata T: Inhibition of fibroblast growth factor
receptor 2 attenuates proliferation and invasion of pancreatic
cancer. Cancer Sci. 105:1212–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nandy D and Mukhopadhyay D: Growth factor
mediated signaling in pancreatic pathogenesis. Cancers. 3:841–871.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corson LB, Yamanaka Y, Lai KM and Rossant
J: Spatial and temporal patterns of ERK signaling during mouse
embryogenesis. Development. 130:4527–4537. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morita I: Distinct functions of COX-1 and
COX-2. Prostaglandins Other Lipid Mediat. 68–69:165–175. 2002.
View Article : Google Scholar
|
|
14
|
Steinbach G, Lynch PM, Phillips RK,
Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y,
Fujimura T, et al: The effect of celecoxib, a cyclooxygenase-2
inhibitor, in familial adenomatous polyposis. N Engl J Med.
342:1946–1952. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kirane A, Toombs JE, Ostapoff K, Carbon
JG, Zaknoen S, Braunfeld J, Schwarz RE, Burrows FJ and Brekken RA:
Apricoxib, a novel inhibitor of COX-2, markedly improves standard
therapy response in molecularly defined models of pancreatic
cancer. Clin Cancer Res. 18:5031–5042. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sánchez-Fidalgo S, Martín-Lacave I,
Illanes M and Motilva V: Angiogenesis, cell proliferation and
apoptosis in gastric ulcer healing. Effect of a selective cox-2
inhibitor. Eur J Pharmacol. 505:187–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sawaoka H, Tsuji S, Tsujii M, Gunawan ES,
Sasaki Y, Kawano S and Hori M: Cyclooxygenase inhibitors suppress
angiogenesis and reduce tumor growth in vivo. Lab Invest.
79:1469–1477. 1999.
|
|
18
|
Baguma-Nibasheka M, Barclay C, Li AW,
Geldenhuys L, Porter GA, Blay J, Casson AG and Murphy PR: Selective
cyclooxygenase-2 inhibition suppresses basic fibroblast growth
factor expression in human esophageal adenocarcinoma. Mol Carcinog.
46:971–980. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan
W, Sun Q, Xu J, Wu Z, et al: Hedgehog signaling regulates hypoxia
induced epithelial to mesenchymal transition and invasion in
pancreatic cancer cells via a ligand-independent manner. Mol
Cancer. 12:662013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jamieson NB, Mohamed M, Oien KA, Foulis
AK, Dickson EJ, Imrie CW, Carter CR, McKay CJ and McMillan DC: The
relationship between tumor inflammatory cell infiltrate and outcome
in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol.
19:3581–3590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mohammed A, Janakiram NB, Madka V, Brewer
M, Ritchie RL, Lightfoot S, Kumar G, Sadeghi M, Patlolla JM, Yamada
HY, et al: Targeting pancreatitis blocks tumor-initiating stem
cells and pancreatic cancer progression. Oncotarget. 6:15524–15539.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barlow M, Edelman M, Glick RD, Steinberg
BM and Soffer SZ: Celecoxib inhibits invasion and metastasis via a
cyclooxygenase 2-independent mechanism in an in vitro model of
Ewing sarcoma. J Pediatr Surg. 47:1223–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
El-Rayes BF, Ali S, Sarkar FH and Philip
PA: Cyclooxygenase 2-dependent and -independent effects of
celecoxib in pancreatic cancer cell lines. Mol Cancer Ther.
3:1421–1426. 2004.PubMed/NCBI
|
|
24
|
Wei D, Wang L, He Y, Xiong HQ, Abbruzzese
JL and Xie K: Celecoxib inhibits vascular endothelial growth factor
expression in and reduces angiogenesis and metastasis of human
pancreatic cancer via suppression of Sp1 transcription factor
activity. Cancer Res. 64:2030–2038. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jimeno A, Amador ML, Kulesza P, Wang X,
Rubio-Viqueira B, Zhang X, Chan A, Wheelhouse J, Kuramochi H,
Tanaka K, et al: Assessment of celecoxib pharmacodynamics in
pancreatic cancer. Mol Cancer Ther. 5:3240–3247. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pyrko P, Soriano N, Kardosh A, Liu YT,
Uddin J, Petasis NA, Hofman FM, Chen CS, Chen TC and Schönthal AH:
Downregulation of survivin expression and concomitant induction of
apoptosis by celecoxib and its non-cyclooxygenase-2-inhibitory
analog, dimethylcelecoxib (DMC), in tumor cells in vitro and in
vivo. Mol Cancer. 5:192006. View Article : Google Scholar
|
|
27
|
Pal I and Mandal M: GSK690693 enhances
Celecoxib mediated apoptosis by an Akt mediated pathway in colon
cancer. Cancer Res. 73(Suppl 8): S10442013. View Article : Google Scholar
|
|
28
|
Molina MA, Sitja-Arnau M, Lemoine MG,
Frazier ML and Sinicrope FA: Increased cyclooxygenase-2 expression
in human pancreatic carcinomas and cell lines: Growth inhibition by
nonsteroidal anti-inflammatory drugs. Cancer Res. 59:4356–4362.
1999.PubMed/NCBI
|
|
29
|
Marek L, Ware KE, Fritzsche A, Hercule P,
Helton WR, Smith JE, McDermott LA, Coldren CD, Nemenoff RA, Merrick
DT, et al: Fibroblast growth factor (FGF) and FGF receptor-mediated
autocrine signaling in non-small-cell lung cancer cells. Mol
Pharmacol. 75:196–207. 2009. View Article : Google Scholar :
|
|
30
|
Ishiwata T, Matsuda Y, Yamamoto T, Uchida
E, Korc M and Naito Z: Enhanced expression of fibroblast growth
factor receptor 2 IIIc promotes human pancreatic cancer cell
proliferation. Am J Pathol. 180:1928–1941. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shrikhande SV, Kleeff J, Reiser C, Weitz
J, Hinz U, Esposito I, Schmidt J, Friess H and Büchler MW:
Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann
Surg Oncol. 14:118–127. 2007. View Article : Google Scholar
|
|
32
|
Friedl P and Alexander S: Cancer invasion
and the microenvironment: Plasticity and reciprocity. Cell.
147:992–1009. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: Regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shah N, Jin K, Cruz LA, Park S, Sadik H,
Cho S, Goswami CP, Nakshatri H, Gupta R, Chang HY, et al: HOXB13
mediates tamoxifen resistance and invasiveness in human breast
cancer by suppressing ERα and inducing IL-6 expression. Cancer Res.
73:5449–5458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao W, Wang W, Xiong XG, Cao C, Yan TD,
Chen G, Chen H, Yin W, Liu J, Gu Y, et al: Prognostic impact of
MMP-2 and MMP-9 expression in pathologic stage IA non-small cell
lung cancer. J Surg Oncol. 104:841–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim HS, Kim MJ, Kim EJ, Yang Y, Lee MS and
Lim JS: Berberine-induced AMPK activation inhibits the metastatic
potential of melanoma cells via reduction of ERK activity and COX-2
protein expression. Biochem Pharmacol. 83:385–394. 2012. View Article : Google Scholar
|
|
38
|
Guo J, Xu Y, Ji W, Song L, Dai C and Zhan
L: Effects of exposure to benzo[a]pyrene on metastasis of breast
cancer are mediated through ROS-ERK-MMP9 axis signaling. Toxicol
Lett. 234:201–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhong HM, Ding QH, Chen WP and Luo RB:
Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities
through inhibition of iNOS and MMP expression, p38 and ERK
phosphorylation and blocking NF-κB nuclear translocation. Int
Immunopharmacol. 17:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Longnecker DS and Terhune PG: What is the
true rate of K-ras mutation in carcinoma of the pancreas? Pancreas.
17:323–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fedorov YV, Rosenthal RS and Olwin BB:
Oncogenic Ras-induced proliferation requires autocrine fibroblast
growth factor 2 signaling in skeletal muscle cells. J Cell Biol.
152:1301–1305. 2001. View Article : Google Scholar : PubMed/NCBI
|