Introduction
Osteosarcoma is a malignant bone cancer suffered by
adolescents or children under the age of 20 years (1). It is a commonly observed pediatric
malignant bone tumor and accounts for approximately 5% of all
pediatric tumors (2). Osteosarcoma
originates from mesenchymal tissues. Due to its high grade of
malignancy and invasive ability, osteosarcoma presents with lung
metastasis at an early stage. Therefore, its prognosis is poor and
survival rates are low. The 5-year survival rate after amputation
is only 5–15% (3). More and more
studies have confirmed that oncotherapy for malignant cancer,
particularly solid tumors includes comprehensive therapy consisting
of chemotherapy, radiotherapy and molecular-targeted treatment
(4). The introduction and
development of molecular-targeted treatment brings good news to
osteosarcoma patients (5,6).
Apoptosis is a self-destruction mechanism in cells.
In this process, organisms can scavenge aging and abnormal cells
(7). The major strategy of
molecular-targeted treatment is to induce cancer cells to deform
and initiate programmed cell death to scavenge tumor cells
(8).
The occurrence and progression of osteosarcoma are
not only the result of uncontrolled cell proliferation and abnormal
differentiation, but are also related with the unbalance of cell
apoptosis (9). During the
initiation process, apoptosis is inhibited, cell cycle regulation
is destroyed and neoplasm occurs.
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is an important activating agent for cell apoptosis
(10). It can selectively induce
osteosarcoma cells to apoptosis by combining with its ligands while
it is not sensitive to normal cells. This is because its regulation
to induce apoptosis is realized by distribution levels of
receptors. TRAIL receptors include death receptors and decoy
receptors (9). Death receptors are
widely distributed on cell surfaces, including cancer cells and
normal cells. Decoy receptors are not expressed in most tumor cell
surfaces, but are selectively expressed on normal cell surfaces
(11). Differences in the
expression levels of surface receptors in tumor cells and normal
cells is an essential cause of the various lethality of TRAIL. Only
by this regulation of expression levels, TRAIL confers its specific
antitumor functions (12).
TRAIL (TNF-related apoptosis-inducing ligand) is a
newly found family member of the tumor necrosis factor (13). It can selectively induce various
tumor cells to apoptosis while it does not affect the growth and
differentiation of normal cells. It can rapidly induce apoptosis of
specific receptors (14). In
addition, cysteine aspastic acid-specific protease 3 (caspase-3)
and caspase-8 are important initiation factors of the caspase
family and play a fundamental role in cell apoptosis (15).
Triptolide, a diterpenoid epoxide found in the
Thunder God Vine Tripterygium wilfordii has various
pharmacological actions such as anti-inflammation,
immunosuppression, antitumor and anti-fertility and is widely as
rheumatic arthritis, rheumatoid arthritis, nephritis, asthma,
systemic lupus erythematosus and dermatosis (16,17).
Its active ingredients consist of epoxyditerpenes, triterpenes and
alkaloid. As a diterpene monomer, triptolide is the main active
ingredient extracted from Thunder God Vine (16,18).
In addition, the present study explores the possible mechanisms of
triptolide on osteosarcoma, in order to set the basis for novel
strategies in other cancer.
Materials and methods
Cell culture and reagents
The MG-63 human osteosarcoma cell line was obtained
from Shanghai Cell Bank (Shanghai, China) and was maintained in
Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine
serum (FBS) (both from Invitrogen, Carlsbad, CA, USA) and 2 mM
L-glutamine at 37°C with 5% CO2.
MTT assay
MG-63 cells (1×104 cell/well) grown in
96-well culture plates were treated either with various doses of
triptolide (50, 100 or 200 nM) or DMSO for 6, 12 and 24 h. MTT was
added at a final concentration of 0.5 mg/ml and incubated at 37°C
for 4 h. DMSO (Invitrogen) was added to each well to dissolve the
formazan crystals. Cell viability was measured using a microplate
reader (Model 550; Bio-Rad, Hercules, CA, USA) at 570 nm
wavelength.
Cell apoptosis assays
MG-63 cells (1×106 cell/well) grown in
6-well culture plates were treated either with various doses of
triptolide (50, 100 or 200 nM) or DMSO for 12 h. MG-63 cells were
washed once with ice-cold PBS and incubated using the Annexin
V-fluorescein isothiocyanate (FITC) kit (Sigma-Aldrich). Cell
apoptosis was detected using flow cytometry according to the
manufacturer's instructions.
Detection of caspase-3, caspase-8 and
caspase-9 activity
MG-63 cells (1×104 cell/well) grown in
96-well culture plates were treated either with various doses of
triptolide (50, 100 or 200 nM) or DMSO for 12 h. Caspase-Glo
reagent (100 µl) (Ac-DEVD-pNA for caspase-3, Ac-IETD-pNA for
caspase-8, Ac-LEHD-pNA for caspase-9) was added to each well in
culture medium for 45 min at room temperature. Caspase-3, caspase-8
and caspase-9 activity was measured using a micro-plate reader
(Model 550; Bio-Rad) at 405 nm wavelength.
Western blot analysis
MG-63 cells (1×106 cell/well) grown in
6-well culture plates were treated either with various doses of
triptolide (50, 100 or 200 nM) or DMSO for 12 h. MG-63 cells were
lysed in ice-cold lysing buffer consisting of 50 mM Trizma base (pH
7.4; Sigma-Aldrich) and centrifuged at 13,000 × g for 10 min at
4°C. The protein expression levels were measured using the BCA
assay (Beyotime Biotechnology, Jiangsu, China). Proteins (50
µg/lane) were separated on 12% SDS-PAGE gels and then
transferred to polyvinylidene difluoride membranes (Bio-Rad). After
electro-transfer, the membranes were blocked with 5% nonfat milk in
TBST buffer. Next, the membranes were probed with primary mouse
monoclonal anti-human antibodies for death receptor 5 (DR-5), p53,
BAX, pro-caspase-8, c-FLIP, lysosomal, cathepsin B and β-actin
diluted in blocking buffer to a concentration of 1:2,000 at 4°C
overnight. Blots were incubated with horseradish (HRP)-conjugated
secondary antibodies, detected with enhanced chemiluminescence
reagent (Sangon Biotech Co., Ltd., Shanghai, China). The protein
expression was analyzed using TotalLab software (Nonlinear
Dynamics).
Statistical analysis
The results are expressed as the mean ± SD.
Qualitative data were analyzed using the χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Triptolide suppresses the viability of
osteosarcoma cells
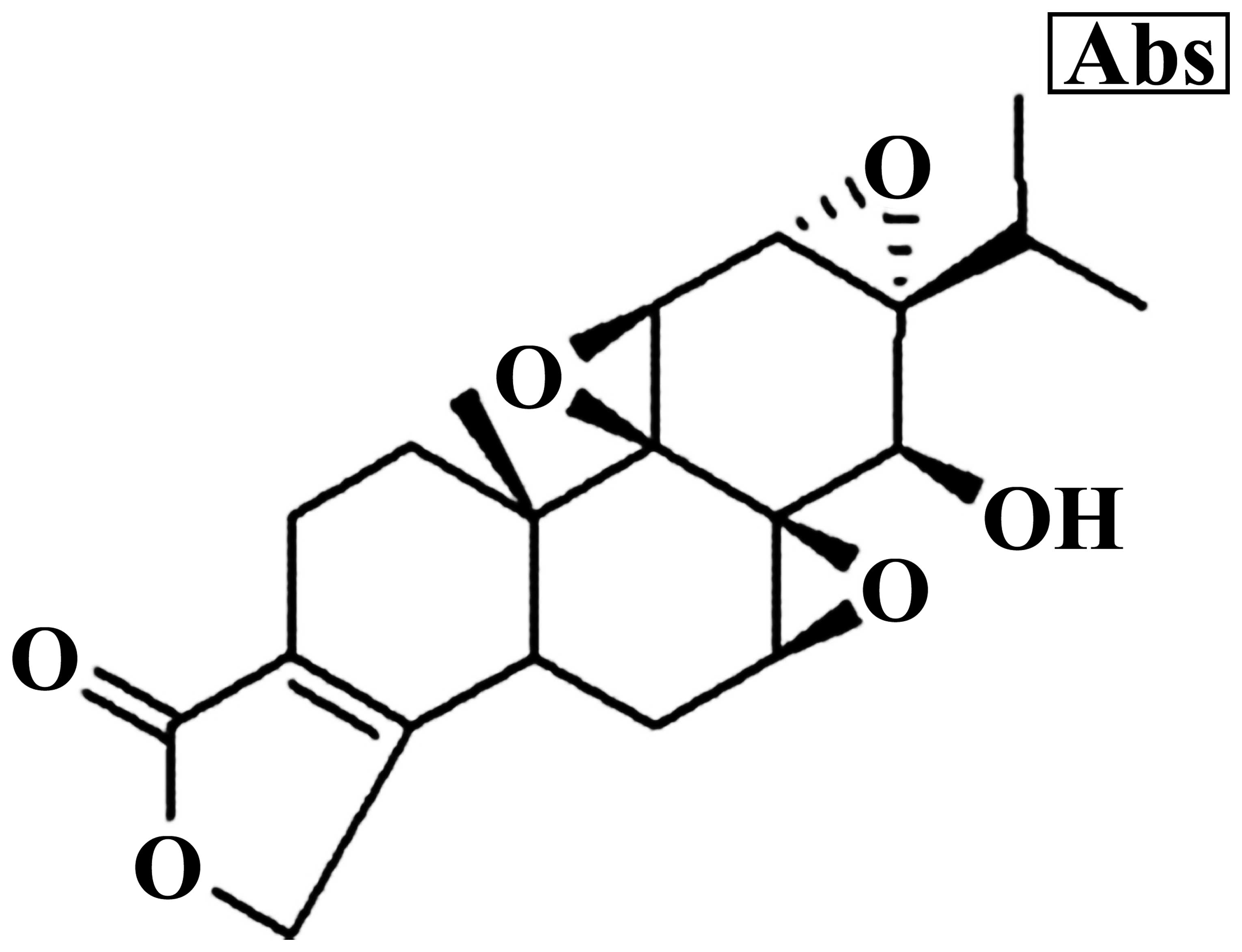
The chemical structure of triptolide is showed in
Fig. 1. MG-63 cells were treated
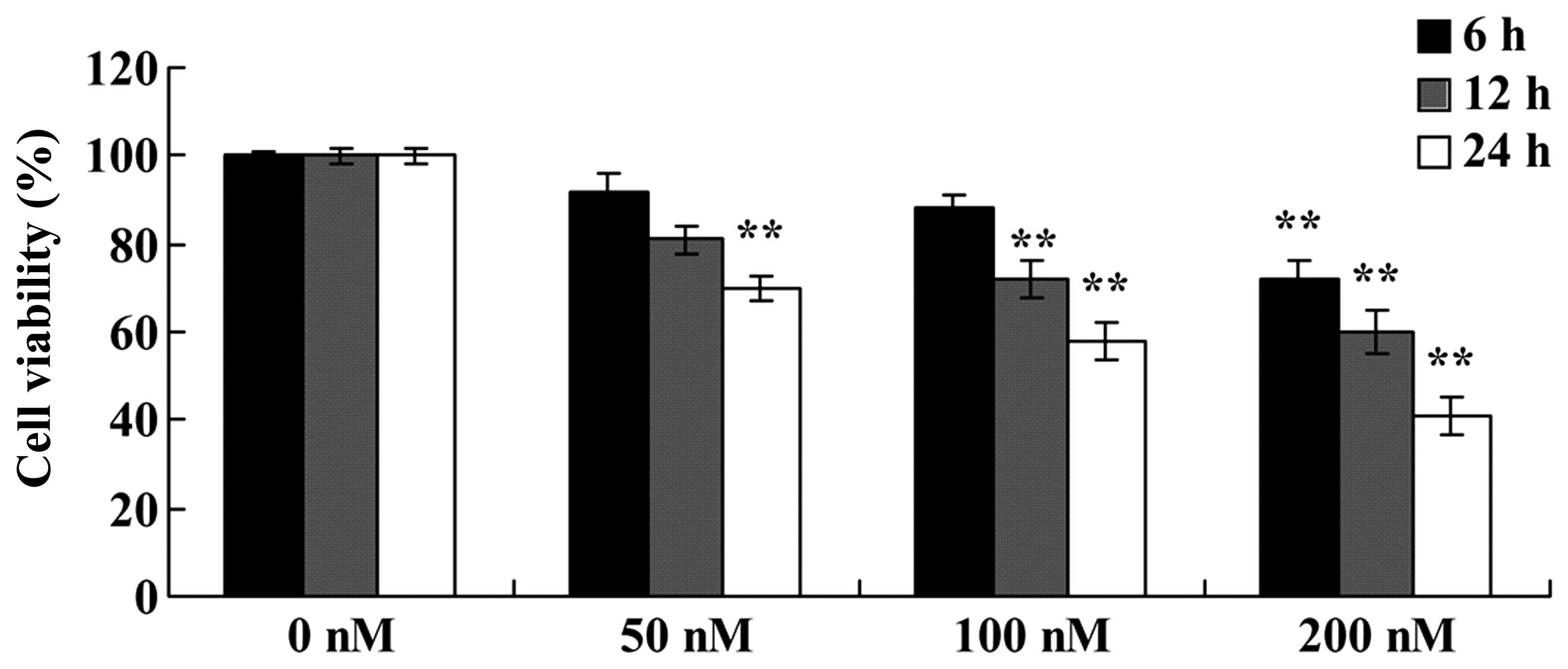
with increasing doses of triptolide (50, 100 or 200 nM) for 24 h.
As shown in Fig. 2, triptolide
inhibited the viability of the MG-63 cells in a dose- and
time-dependent manner. The cell viability was significantly reduced
at 50 nM for 24 h; 100 nM for 12 or 24 h; and 100 nM for 6, 12 or
24 h (Fig. 2). These results
indicate that triptolide suppressed the viability of the MG-63
cells, which may contribute to a cure for osteosarcoma.
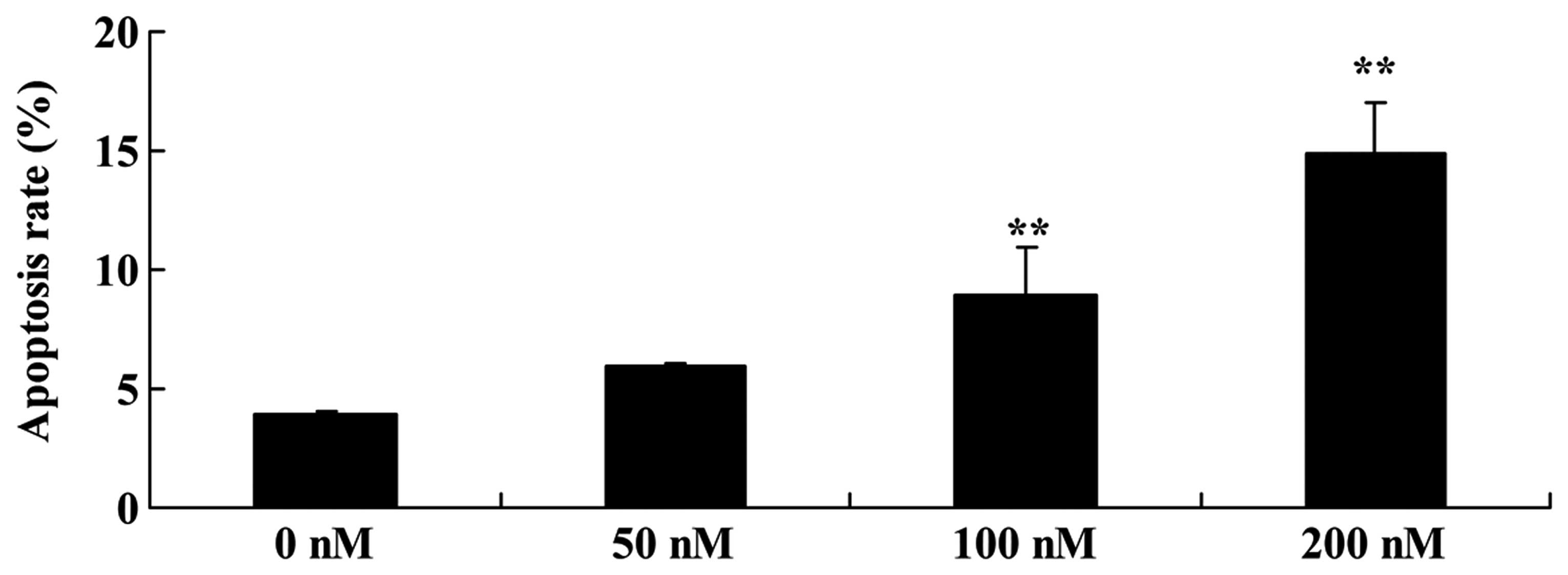
Triptolide induces the cell apoptosis of
osteosarcoma
The effect of triptolide on the apoptosis of
osteosarcoma cells was investigated. We found that the cell
apoptosis of MG-63 cells was significantly induced in a
dose-dependent manner following treatment with 100 or 200 nM of
triptolide for 12 h (Fig. 3).
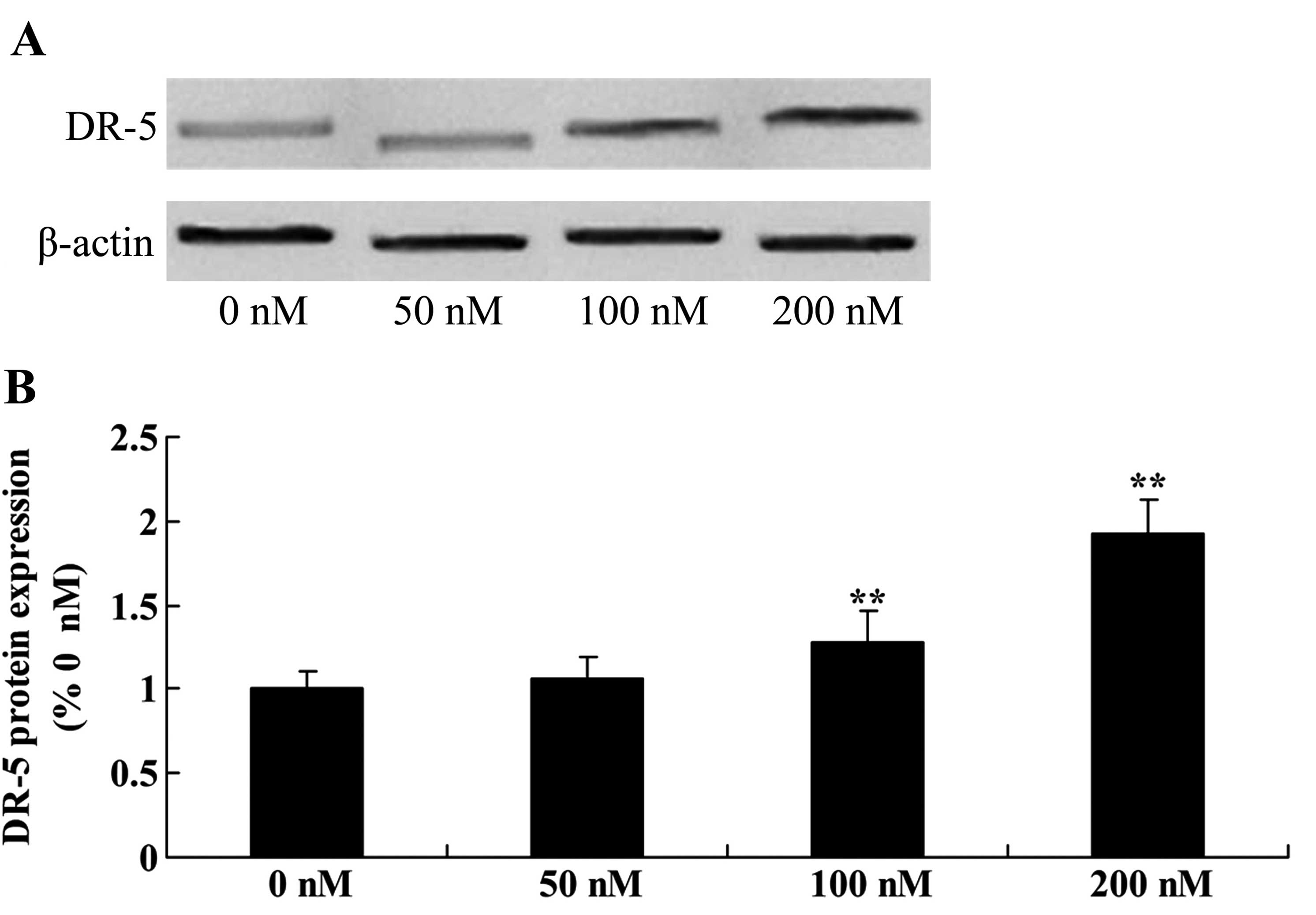
Triptolide increases DR-5 protein in
osteosarcoma cells
The role of triptolide in the DR-5 signaling pathway
was examined. DR-5 protein expression was detected using western
blotting in the MG-63 cells treated with 50, 100 or 200 nM
triptolide. As shown in Fig. 4,
treatment with 100 or 200 nM of triptolide for 12 h significantly
increased DR-5 protein expression in a dose-dependent manner,
compared with the level in cells treated with DMSO.
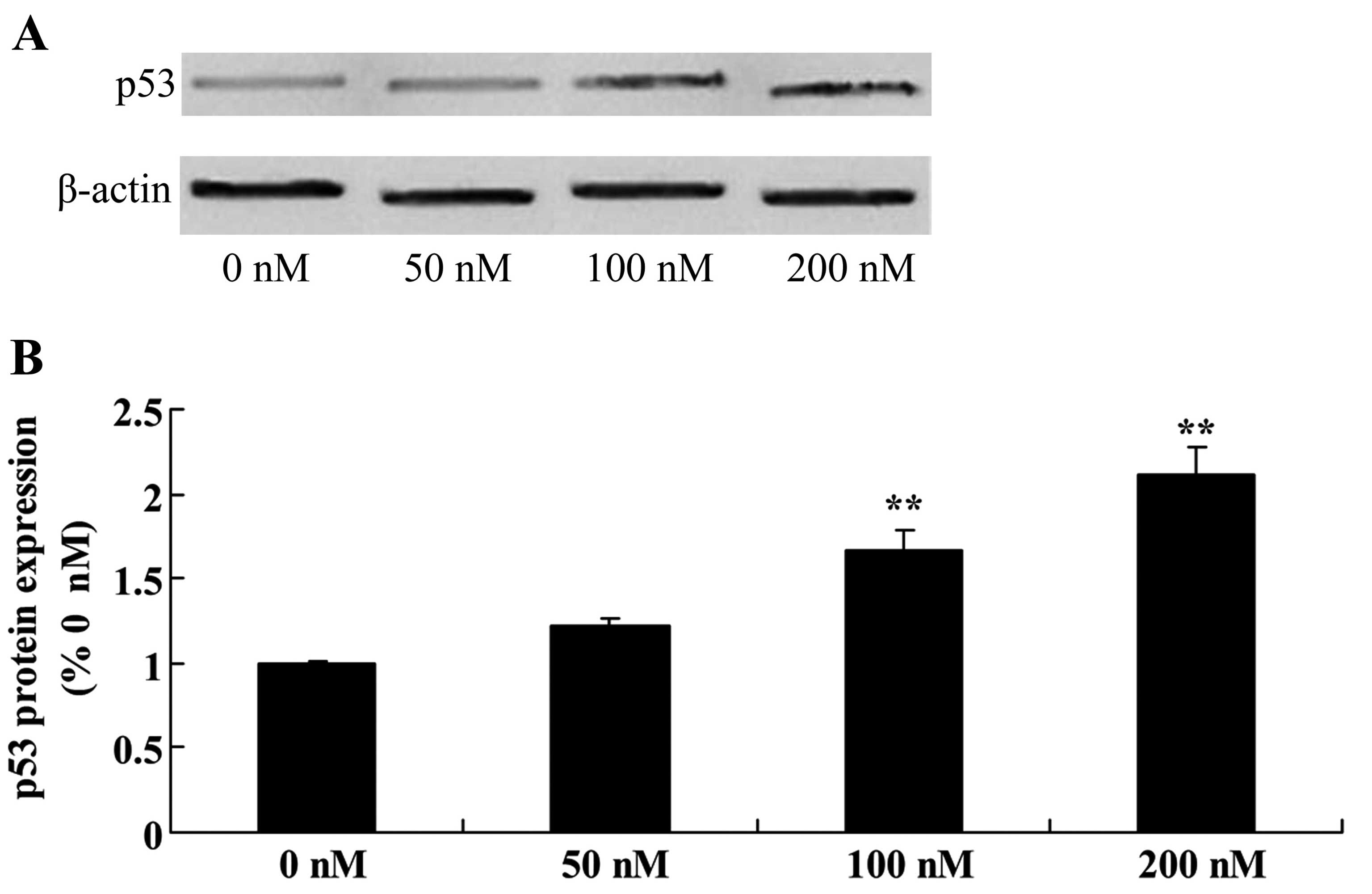
Triptolide increases p53 protein in
osteosarcoma cells
Given that MG-63 cells are resistant to triptolide
treatment, we focused on the p53 protein in the following study.
There was a significant increase in p53 protein expression in the
MG-63 cells following treatment of triptolide at 100 or 200 nM,
compared with the level in cells treated with DMSO (Fig. 5).
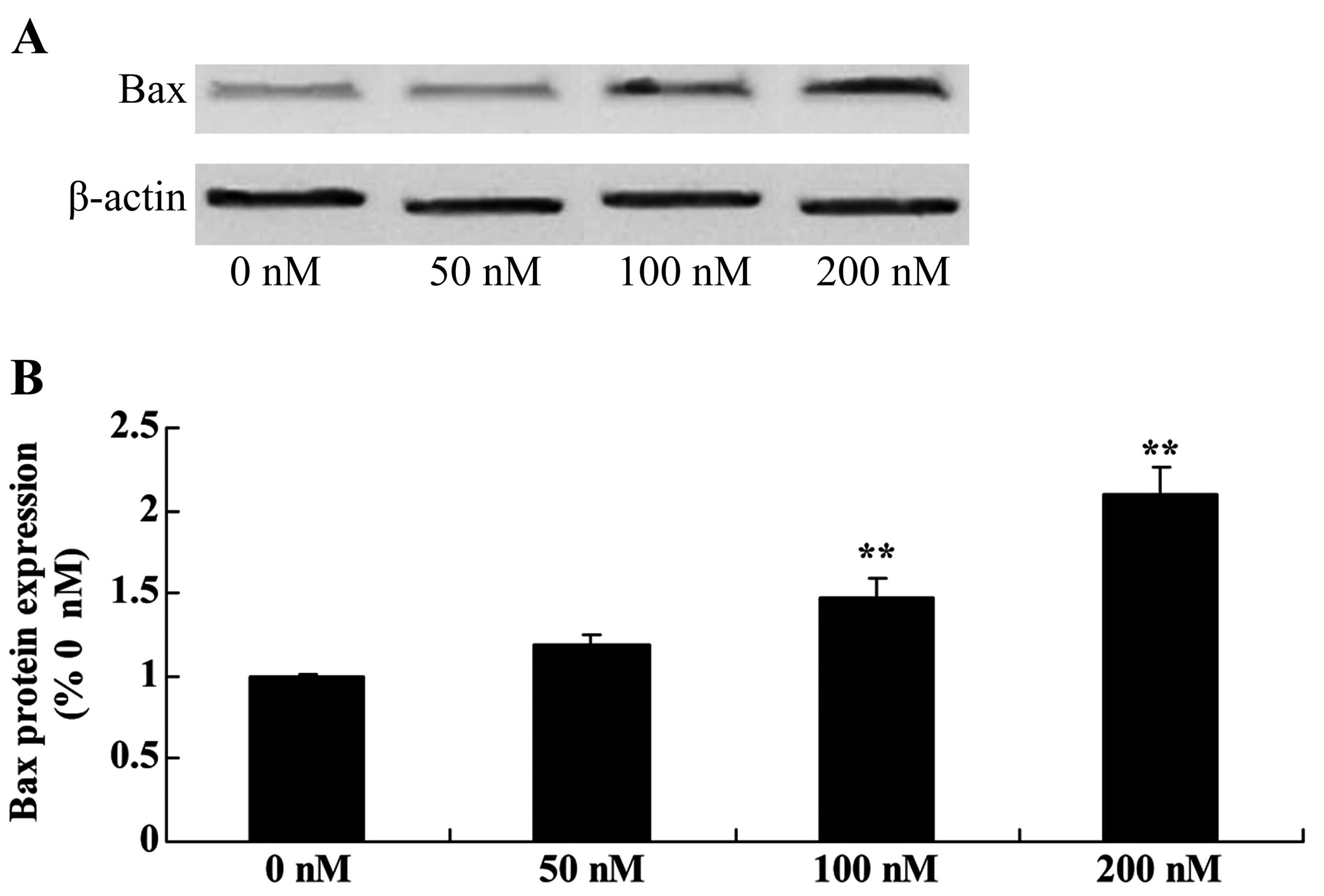
Ttriptolide increases Bax protein in
osteosarcoma cells
To assess triptolide/TRAIL-induced MG-63 cell
apoptosis, the Bax signaling pathway of apoptosis was analyzed.
Triptolide (100 or 200 nM) significantly activated Bax protein
expression in the MG-63 cells, compared with the level in cells
treated with DMSO (Fig. 6).
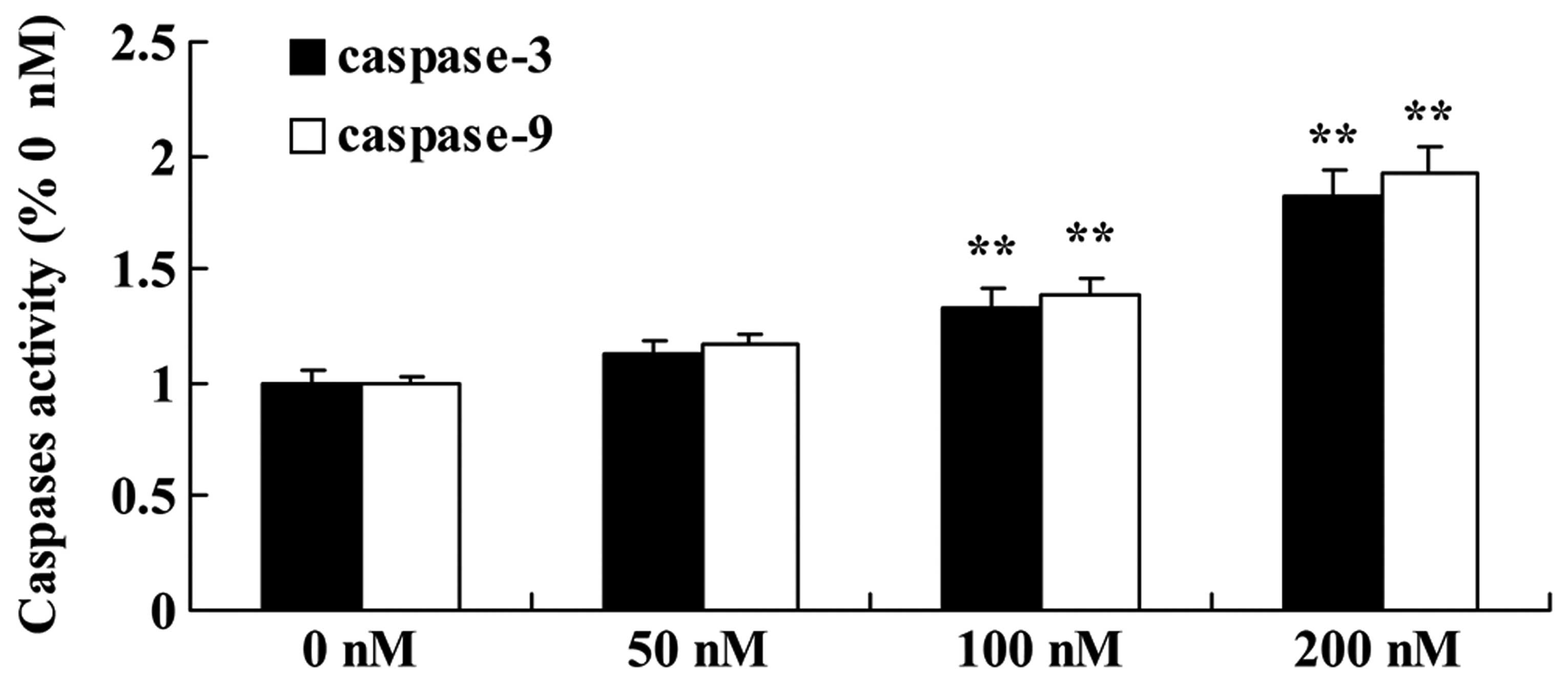
Triptolide increases caspase-9/caspase-3
activity in osteosarcoma cells
MG-63 cells were incubated with triptolide (50, 100
or 200 nM) for 12 h. Incubation of the MG-63 cells in culture
medium containing 100 or 200 nM triptolide resulted in a
statistically significant increase in caspase-9/-3 activity in a
dose-dependent manner, compared with the activity noted in the
cells treated with DMSO (Fig.
7).
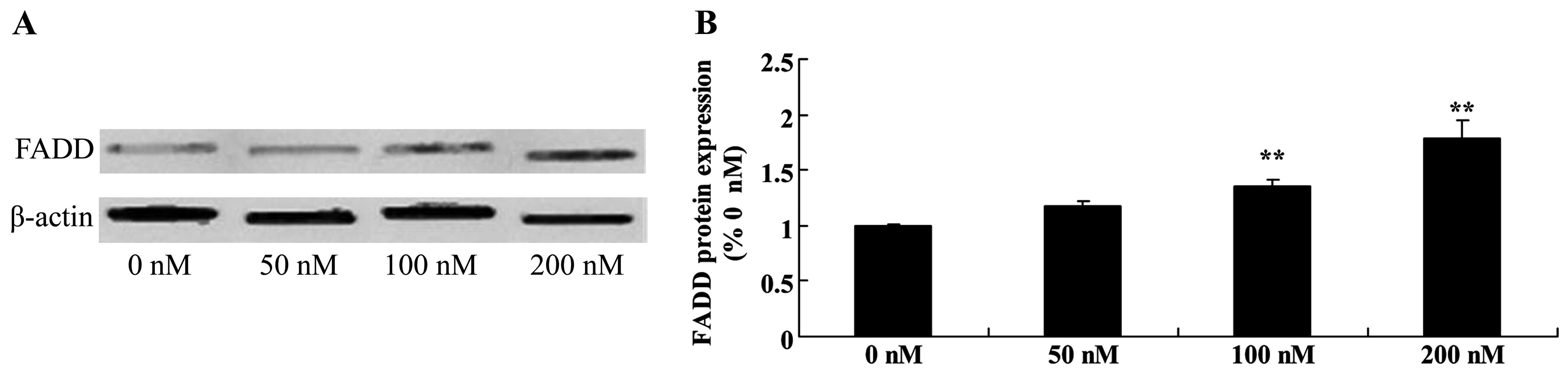
Triptolide increases FDAA protein in
osteosarcoma cells
In general, TRAIL triggers apoptosis through binding
to the FDAA apoptosis signaling pathway. Triptolide (100 or 200 nM)
treatment markedly increased the expression of FDAA protein in a
dose-dependent manner in the MG-63 cells (Fig. 8).
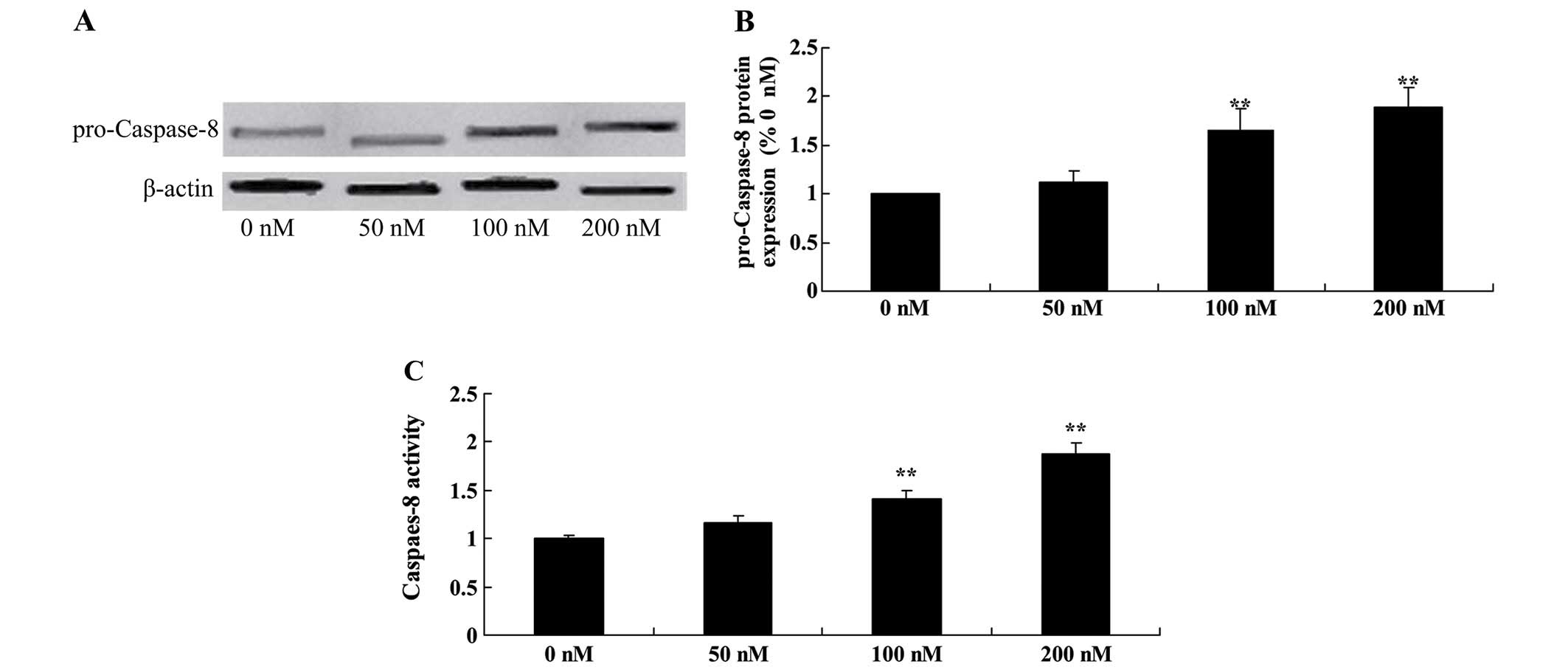
Triptolide increases pro-caspase-8
protein and caspase-8 activity in osteosarcoma cells
To explore the underlying mechanism that may be
responsible for the anticancer effect of triptolide on apoptosis,
we examined pro-caspase-8 protein and caspase-8 activity in the
MG-63 cells. Treatement of triptolide at 100 and 200 nM
significantly increased pro-caspase-8 protein expression and
caspase-8 activity in the MG-63 cells (Fig. 9).
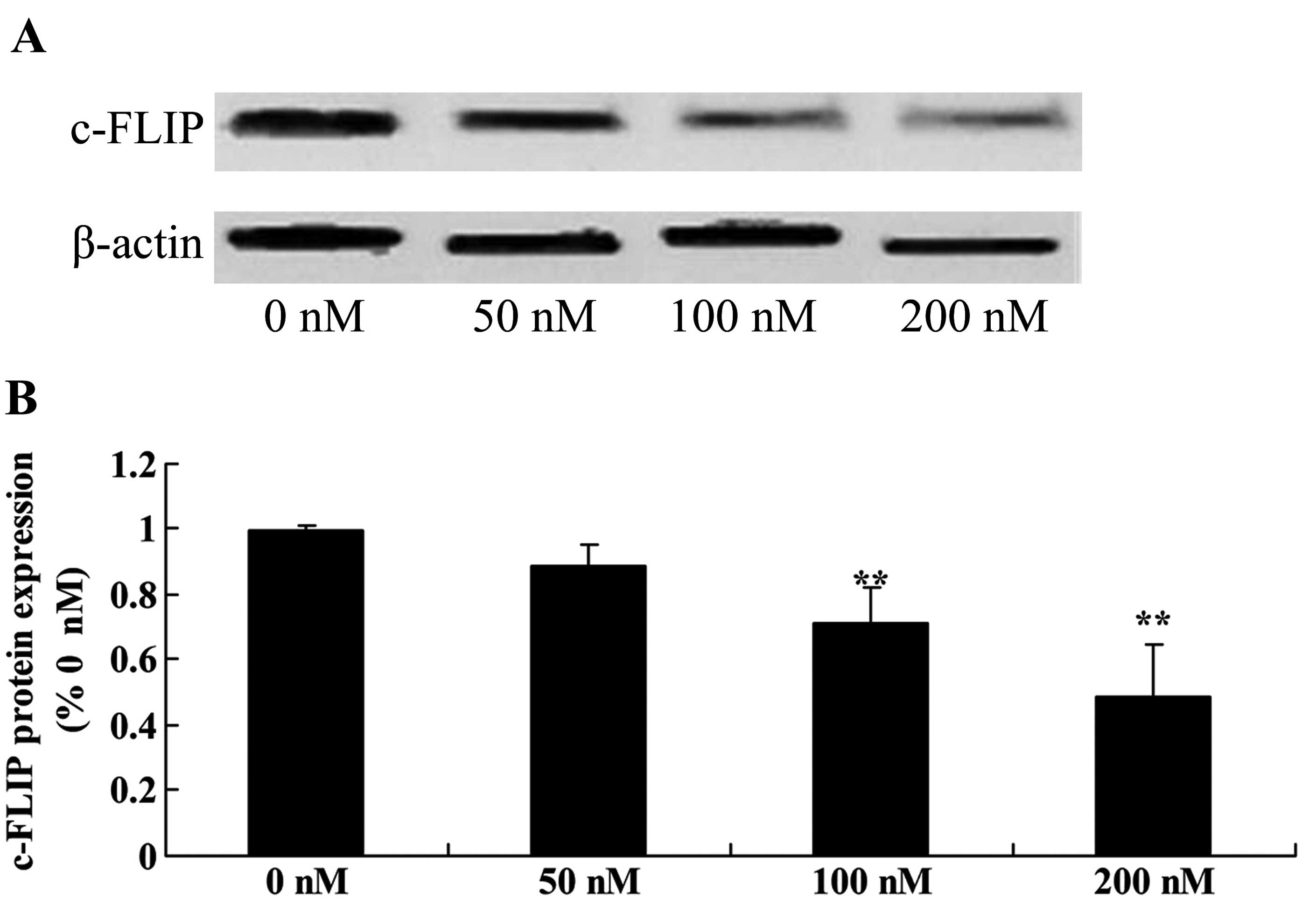
Triptolide suppresses c-FLIP protein in
osteosarcoma cells
To verify whether c-FLIP upregulation is responsible
for the anticancer effect of triptolide on the apoptosis of
osteosarcoma cells, we measured c-FLIP protein expression using
western blot analysis. As shown in Fig. 10, c-FLIP protein expression was
significantly suppressed in the MG-63 cells following treatment
with triptolide (100 or 200 nM) (Fig.
10).
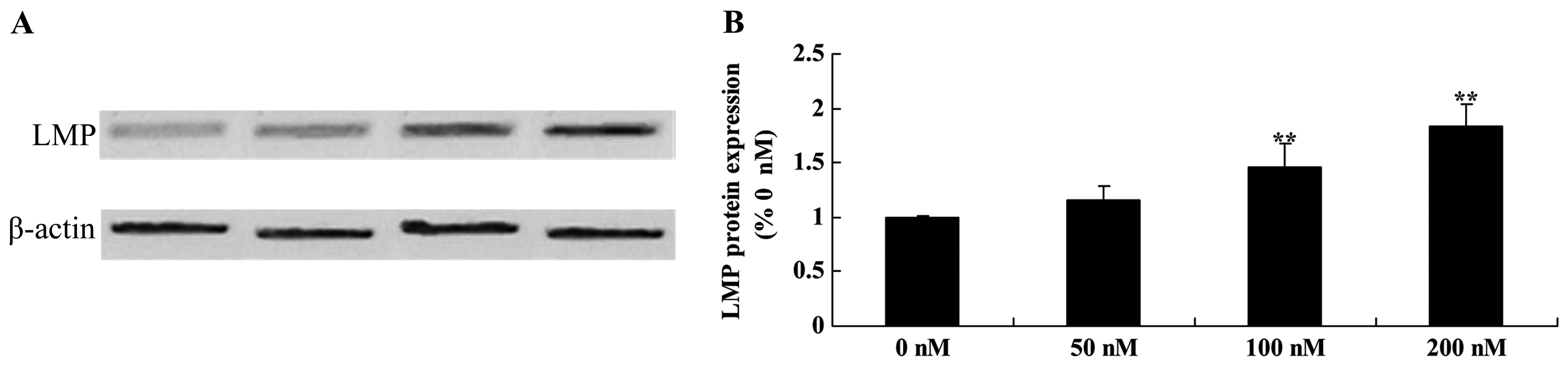
Ttriptolide increases LMP protein in
osteosarcoma cells
We examined whether LMP activation is involved in
the anticancer effects of triptolide on TRAIL-induced apoptosis in
MG-63 cells. The results revealed that 100 and 200 nM of triptolide
significantly promoted the LMP protein expression in MG-63 cells
(Fig. 11).
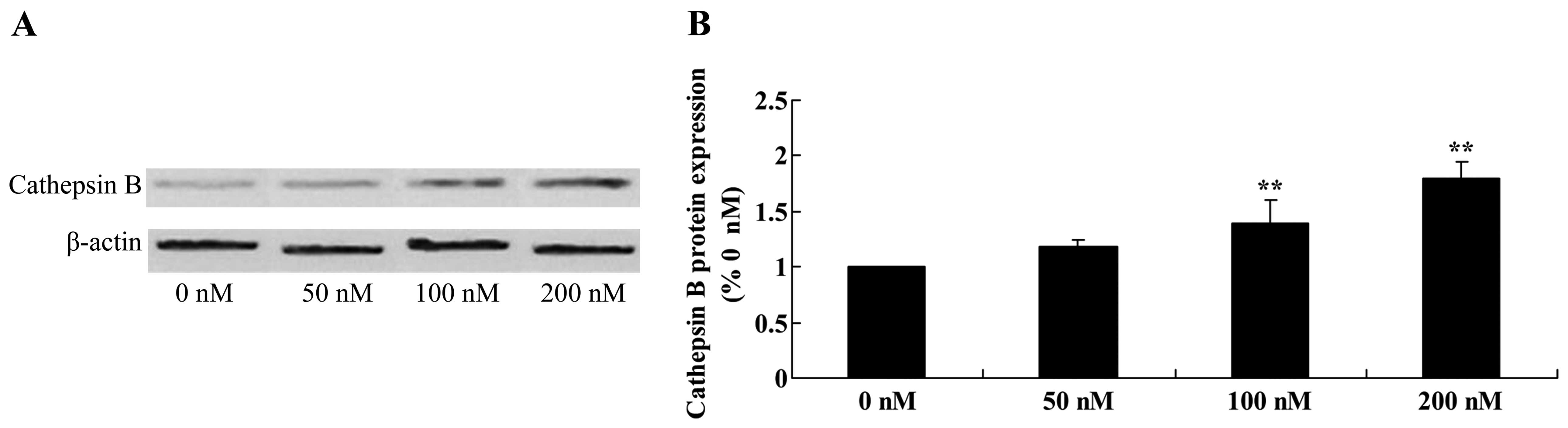
Triptolide increases cathepsin B protein
in osteosarcoma cells
We next examined whether activation of cathepsin B
is involved in the anticancer effects of triptolide on osteosarcoma
cells. Furthermore, the protein expression of cathepsin B was
significantly enhanced following treatment with 100 and 200 nM of
triptolide, compared with the cells treated with DMSO (Fig. 12).
Discussion
Characterized by high malignant potential and
invasive ability, osteosarcoma, a common tumor in adolescents,
exhibits lung metastasis at an early stage (19). Consequently, it is associated with a
poor prognosis and a low survival rate. The pathogenesis of
osteosarcoma is still being researched. It is now realized that
tumors are the result of uncontrolled cell growth and
differentiation as protooncogenes are activated. It is polygenic
and involves multiple factors (20). Tumorigenesis is closely related with
multiple genes (21). More and more
studies confirm that oncotherapy for malignant cancer, particularly
solid tumors includes comprehensive therapy consisting of
chemotherapy, radiotherapy and molecular-targeted treatment
(6). The results obtained in the
present study demonstrated that triptolide significantly suppressed
the cell viability and induced the cell apoptosis of osteosarcoma.
Reno et al reported that triptolide inhibited cell
migration, invasion and metastasis of lung cancer cells (22). Therefore, we hypothesized that
triptolide may reduce the growth of osteosarcoma cells.
Caspase-8 is a cysteine proteinase, distributed in
tissues and cell lines, such as bones and cartilages (23). With FADD-like death effector domain,
it participates in cell apoptosis mechanisms and can combine with
FADD through death effector domain (24). Caspase-8 participates in cell
apoptosis through forming a death-inducing signaling complex
(25). The caspase protease family
occupies the central role in the apoptosis process and participates
directly in early initiation, signal transmission and late
apoptotic effects (26). Caspase-8
is at the peak of the cascade reaction. Its expression not only
reflects apoptosis levels but also reflects the existence of
apoptosis initiators, suggesting that the occurrence of
osteosarcoma is related with low expression of caspase-8 (27). However, the expression of caspase-8
in malignant cancer is still controversial. Some scholars have
found that upregulated expression of caspase-8 indicates poor
prognosis. This may be due to the fact that in highly proliferative
tissues, apoptosis may be facilitated, which reflects the
complexity of apoptosis and proliferation (28). During the pathological processes of
osteosarcoma, inhibition of apoptosis due to the inactivation of
cancer-suppressor genes is the main cause, therefore, caspase-8 has
low expression (29). These data
together indicate that triptolide significantly increased
pro-caspase-8 protein expression and caspase-8 activity in the
MG-63 cells. Zhao et al reported that triptolide induced
growth inhibition and apoptosis through caspase-8/caspase-9, and
p53 expression in human laryngocarcinoma cells (30).
Studies suggest that DR-5 is expressed in normal
cells, such as foetal livers and lungs as well as adult livers,
lungs, lymphocytes, ovary and spleens (31). Particularly, it has higher
expression in tumor tissues of lung cancer, breast carcinoma,
ovarian cancer, rectal cancer and cervical cancer (31,32).
DR-5 is an important target protein of the effects of anticancer
drugs. Under normal conditions, DR-5 has higher activity, which is
possibly related with its stability (11). Overexpression of DR-5 can directly
induce cells to undergo exogenous apop-tosis. In the present study,
triptolide increased DR-5, p53 and Bax protein expression, and
promoted caspase-9/-3 activity in the osteosarcoma cells. Carter
et al reported that triptolide sensitized acute myeloid
leukemia (AML) cells (33).
c-FLIP is an important anti-apoptotic protein in the
exogenous apoptosis signaling pathway. Studies have confirmed that
compared with normal cells, c-FLIP is upregulated in various types
of tumors (34). High expression of
c-FLIP is related with tumor metastasis and poor prognosis. It was
reported that c-FLIP contains three alternative splice
variants-c-FLIPt, c-FLIPs and c-FLIPr (35). c-FLIP contains two DED structural
domains. c-FLIPs has about 20 amino acids and these amino acids are
important for the ubiquitination and degradation of c-FLIPs
(35). In comparison to c-FLIPs,
c-FLIP has a lack of amino acid sequence at C. c-FLIP has a longer
c-terminus and is similar with pro-caspase-8 in structure (29). Additionally, c-FLIP has a cleavage
site of caspase-8 and can be incised at the Asp-376 (LEVD) site.
After incision, it can produce fragments with enzymatic activities:
p43-c-FLlp. c-FLIPL-pro-caspase-8/caspase-10 heterodimer is
probably more stable than pro-caspase-8/caspase-10 homodimer
(36). The present results
demonstrated that triptolide significantly suppressed c-FLIP
protein expression in the MG-63 cells. Chen et al reported
that triptolide sensitized pancreatic cancer cells through
suppression of c-FLIP protein expression (37).
Studies have found that as an upstream molecule of
the osteogenesis signal pathway, LMP-1 can accumulate many
osteoblastic genes to participate in osteogenic differentiation and
osteogenesis (38). Three types of
spliceosomes of LMP are LMP-1, LMP-2 and LMP-3 (39). It was suggested that LMP-1 could
inhibit malignant phenotypes of osteosarcoma through facilitating
osteogenic differentiation of osteosarcoma cells (40). The present results showed that
triptolide increased LMP protein in the osteosarcoma cells. Owa
et al reported that triptolide induced lysosomal-mediated
programmed cell death in MCF-7 breast cancer cells through LMP
(16).
Cathepsin B is related with the decomposition of
laminin in the extracellular matrix (41). Laminin is the main ingredient of the
basilar membrane which is associated with tumor invasion and
metastasis. In addition, it can degrade fibronectin and collagen
type IV (42). In the present
study, triptolide increased cathepsin B protein in the osteosarcoma
cells. Owa et al reported that triptolide induced
lysosomal-mediated programmed cell death in MCF-7 breast cancer
cells through cathepsin B and caspase-3 (16).
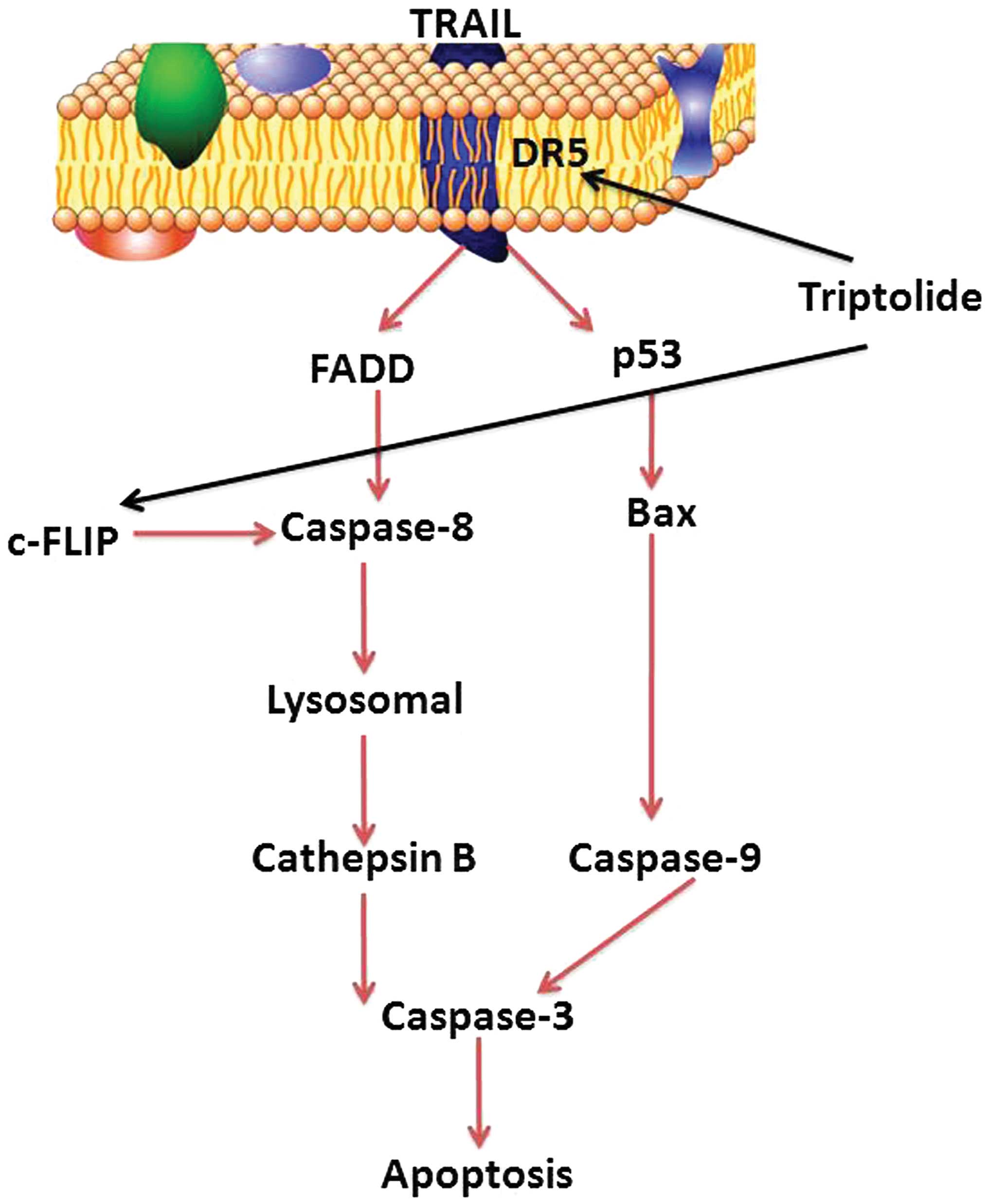
In conclusion, the present study indicated that
triptolide significantly suppressed cell viability, induced cell
apoptosis of osteosarcoma cells in vitro, and evidence was
presented that its anticancer effects may be associated with the
activation of the DR-5/p53/Bax/caspase-9/-3 signaling pathway and
the DR-5/FADD/caspase-8/lysosomal/cathepsin B/caspase-3 signaling
pathway in MG-63 cells (Fig. 13).
However, further in-depth studies are required to investigate the
possible molecular mechanisms of triptolide, and whether triptolide
could be effectively applied in clinical practice.
References
|
1
|
Ferrari S, Meazza C, Palmerini E,
Tamburini A, Fagioli F, Cozza R, Ferraresi V, Bisogno G, Mascarin
M, Cefalo G, et al: Nonmetastatic osteosarcoma of the extremity.
Neoadjuvant chemotherapy with methotrexate, cisplatin, doxorubicin
and ifosfamide An Italian Sarcoma Group study (ISG/OS-Oss). Tumori.
100:612–619. 2014.
|
|
2
|
Zalupski MM, Rankin C, Ryan JR, Lucas DR,
Muler J, Lanier KS, Budd GT, Biermann JS, Meyers FJ and Antman K:
Adjuvant therapy of osteosarcoma - A phase II trial: Southwest
Oncology Group study 9139. Cancer. 100:818–825. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagarajan R, Clohisy DR, Neglia JP, Yasui
Y, Mitby PA, Sklar C, Finklestein JZ, Greenberg M, Reaman GH,
Zeltzer L, et al: Function and quality-of-life of survivors of
pelvic and lower extremity osteosarcoma and Ewing's sarcoma: The
Childhood Cancer Survivor Study. Br J Cancer. 91:1858–1865. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berg J, Gebhardt MC and Rand WM: Effect of
timing of postoperative chemotherapy on survival of dogs with
osteosarcoma. Cancer. 79:1343–1350. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grignani G, Palmerini E, Ferraresi V,
D'Ambrosio L, Bertulli R, Asaftei SD, Tamburini A, Pignochino Y,
Sangiolo D, Marchesi E, et al Italian Sarcoma Group: Sorafenib and
everolimus for patients with unresectable high-grade osteosarcoma
progressing after standard treatment: A non-randomised phase 2
clinical trial. Lancet Oncol. 16:98–107. 2015. View Article : Google Scholar
|
|
6
|
Petrilli AS, de Camargo B, Filho VO,
Bruniera P, Brunetto AL, Jesus-Garcia R, Camargo OP, Pena W,
Péricles P, Davi A, et al Brazilian Osteosarcoma Treatment Group
Studies III and IV: Results of the Brazilian Osteosarcoma Treatment
Group Studies III and IV: Prognostic factors and impact on
survival. J Clin Oncol. 24:1161–1168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan M, Ni J, Song D, Ding M and Huang J:
Activation of unfolded protein response protects osteosarcoma cells
from cisplatin-induced apoptosis through NF-κB pathway. Int J Clin
Exp Pathol. 8:10204–10215. 2015.
|
|
8
|
Yang TM, Qi SN, Zhao N, Yang YJ, Yuan HQ,
Zhang B and Jin S: Induction of apoptosis through
caspase-independent or caspase-9-dependent pathway in mouse and
human osteosarcoma cells by a new nitroxyl spin-labeled derivative
of podophyllotoxin. Apoptosis. 18:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanikoglu F, Cort A, Ozben H, Hanikoglu A
and Ozben T: Epoxomicin sensitizes resistant osteosarcoma cells to
TRAIL induced apoptosis. Anticancer Agents Med Chem. 15:527–533.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotta T, Suzuki H, Nagai S, Yamamoto K,
Imakiire A, Takada E, Itoh M and Mizuguchi J: Chemotherapeutic
agents sensitize sarcoma cell lines to tumor necrosis
factor-related apoptosis-inducing ligand-induced caspase-8
activation, apoptosis and loss of mitochondrial membrane potential.
J Orthop Res. 21:949–957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Locklin RM, Federici E, Espina B, Hulley
PA, Russell RG and Edwards CM: Selective targeting of death
receptor 5 circumvents resistance of MG-63 osteosarcoma cells to
TRAIL-induced apoptosis. Mol Cancer Ther. 6:3219–3228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sonnemann J, Trommer N, Becker S, Wittig
S, Grauel D, Palani CD and Beck JF: Histone deacetylase
inhibitor-mediated sensitization to TRAIL-induced apoptosis in
childhood malignancies is not associated with upregulation of TRAIL
receptor expression, but with potentiated caspase-8 activation.
Cancer Biol Ther. 13:417–424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sage EK, Kolluri KK, McNulty K, Lourenco
SS, Kalber TL, Ordidge KL, Davies D, Gary Lee YC, Giangreco A and
Janes SM: Systemic but not topical TRAIL-expressing mesenchymal
stem cells reduce tumour growth in malignant mesothelioma. Thorax.
69:638–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lamothe B and Aggarwal BB: Ectopic
expression of Bcl-2 and Bcl-xL inhibits apoptosis induced by
TNF-related apoptosis-inducing ligand (TRAIL) through suppression
of caspases-8, 7, and 3 and BID cleavage in human acute myelogenous
leukemia cell line HL-60. J Interferon Cytokine Res. 22:269–279.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saggioro FP, Neder L, Stávale JN,
Paixão-Becker AN, Malheiros SM, Soares FA, Pittella JE, Matias CC,
Colli BO, Carlotti CG Jr, et al: Fas, FasL, and cleaved caspases 8
and 3 in glioblastomas: A tissue microarray-based study. Pathol Res
Pract. 210:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owa C, Messina ME Jr and Halaby R:
Triptolide induces lysosomal-mediated programmed cell death in
MCF-7 breast cancer cells. Int J Womens Health. 5:557–569.
2013.PubMed/NCBI
|
|
17
|
Pan J: RNA polymerase - an important
molecular target of triptolide in cancer cells. Cancer Lett.
292:149–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Q: Triptolide and its expanding
multiple pharmacological functions. Int Immunopharmacol.
11:377–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alvarez FJ, Kisseberth W, Hosoya K,
Lara-Garcia A, Kosarek C, Murahari S, Au JL, Wientjes MG, Couto J
and Couto G: Postoperative adjuvant combination therapy with
doxorubicin and noncytotoxic suramin in dogs with appendicular
osteosarcoma. J Am Anim Hosp Assoc. 50:12–18. 2014. View Article : Google Scholar
|
|
20
|
Arndt CA, Koshkina NV, Inwards CY, Hawkins
DS, Krailo MD, Villaluna D, Anderson PM, Goorin AM, Blakely ML,
Bernstein M, et al: Inhaled granulocyte-macrophage colony
stimulating factor for first pulmonary recurrence of osteosarcoma:
Effects on disease-free survival and immunomodulation. A report
from the Children's Oncology Group. Clin Cancer Res. 16:4024–4030.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rakha EA, Tan PH, Shaaban A, Tse GM,
Esteller FC, van Deurzen CH, Purnell D, Stotter A, Chan T,
Yamaguchi R, et al: Do primary mammary osteosarcoma and
chondrosarcoma exist? A review of a large multi-institutional
series of malignant matrix-producing breast tumours. Breast.
22:13–18. 2013. View Article : Google Scholar
|
|
22
|
Reno TA, Kim JY and Raz DJ: Triptolide
inhibits lung cancer cell migration, invasion, and metastasis. Ann
Thorac Surg. 100:1817–1824; discussion 1824–1825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van Raam BJ and Salvesen GS: Proliferative
versus apoptotic functions of caspase-8 Hetero or homo: The
caspase-8 dimer controls cell fate. Biochim Biophys Acta.
1824:113–122. 2012. View Article : Google Scholar
|
|
24
|
Saitoh Y, Hamano A, Mochida K, Kakeya A,
Uno M, Tsuruyama E, Ichikawa H, Tokunaga F, Utsunomiya A, Watanabe
T, et al: A20 targets caspase-8 and FADD to protect HTLV-I-infected
cells. Leukemia. Oct 6–2015, (Epub ahead of print) http://dx.doi.org/10.1038/leu.2015.267.
|
|
25
|
Kang S, Fernandes-Alnemri T, Rogers C,
Mayes L, Wang Y, Dillon C, Roback L, Kaiser W, Oberst A, Sagara J,
et al: Caspase-8 scaffolding function and MLKL regulate NLRP3
inflammasome activation downstream of TLR3. Nat Commun. 6:75152015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng XU, Xia KE, Chen PO, Ali Sheikh MS,
Yang DF, Li SM and Yang TL: Reversion of left ventricle remodeling
in spontaneously hypertensive rats by valsartan is associated with
the inhibition of caspase-3, -8 and -9 activities. Biomed Rep.
3:533–536. 2015.PubMed/NCBI
|
|
27
|
Lin ML, Lu YC, Su HL, Lin HT, Lee CC, Kang
SE, Lai TC, Chung JG and Chen SS: Destabilization of CARP mRNAs by
aloe-emodin contributes to caspase-8-mediated p53-independent
apoptosis of human carcinoma cells. J Cell Biochem. 112:1176–1191.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kaseta MK, Gomatos IP, Khaldi L,
Tzagarakis GP, Alevizos L, Themistocleous GS, Leandros E and
Soucacos PN: Prognostic value of bax, cytochrome C, and caspase-8
protein expression in primary osteosarcoma. Hybridoma (Larchmt).
26:355–362. 2007. View Article : Google Scholar
|
|
29
|
Kataoka T: The caspase-8 modulator c-FLIP.
Crit Rev Immunol. 25:31–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao F, Huang W, Ousman T, Zhang B, Han Y,
Clotaire DZ, Wang C, Chang H, Luo H, Ren X, et al: Triptolide
induces growth inhibition and apoptosis of human laryngocarcinoma
cells by enhancing p53 activities and suppressing E6-mediated p53
degradation. PLoS One. 8:e807842013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JY, Jung KH, Morgan MJ, Kang YR, Lee
HS, Koo GB, Hong SS, Kwon SW and Kim YS: Sensitization of
TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated
DR5 upregulation in human hepatocellular carcinoma cells. Mol
Cancer Ther. 12:274–285. 2013. View Article : Google Scholar
|
|
32
|
Kim EY, Yu JS, Yang M and Kim AK:
Sub-toxic dose of apigenin sensitizes HepG2 cells to TRAIL through
ERK-dependent up-regulation of TRAIL receptor DR5. Mol Cells.
35:32–40. 2013. View Article : Google Scholar
|
|
33
|
Carter BZ, Mak DH, Schober WD, Dietrich
MF, Pinilla C, Vassilev LT, Reed JC and Andreeff M: Triptolide
sensitizes AML cells to TRAIL-induced apoptosis via decrease of
XIAP and p53-mediated increase of DR5. Blood. 111:3742–3750. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gordy C, Liang J, Pua H and He YW: c-FLIP
protects eosinophils from TNF-α-mediated cell death in vivo. PLoS
One. 9:e1077242014. View Article : Google Scholar
|
|
35
|
Haag C, Stadel D, Zhou S, Bachem MG,
Möller P, Debatin KM and Fulda S: Identification of c-FLIP(L) and
c-FLIP(S) as critical regulators of death receptor-induced
apoptosis in pancreatic cancer cells. Gut. 60:225–237. 2011.
View Article : Google Scholar
|
|
36
|
Schleich K, Buchbinder JH, Pietkiewicz S,
Kähne T, Warnken U, Öztürk S, Schnölzer M, Naumann M, Krammer PH
and Lavrik IN: Molecular architecture of the DED chains at the
DISC: regulation of procaspase-8 activation by short DED proteins
c-FLIP and procaspase-8 prodomain. Cell Death Differ. Oct 23–2015,
(Epub ahead of print) http://dx.doi.org/10.1038/cdd.2015.137.
|
|
37
|
Chen Z, Sangwan V, Banerjee S, Chugh R,
Dudeja V, Vickers SM and Saluja AK: Triptolide sensitizes
pancreatic cancer cells to TRAIL-induced activation of the death
receptor pathway. Cancer Lett. 348:156–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang X, Chen Y, Fan X, Zhang H and Kun L:
Osteogenesis and mineralization in a rabbit mandibular distraction
osteogenesis model is promoted by the human LMP-1 gene. J Orthop
Res. 33:521–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ishioka S, Sagae S, Ito E and Kudo R:
Ultrastructural study of benign, low-malignant potential (LMP), and
malignant ovarian tumors. Med Electron Microsc. 37:37–44. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pan H, Li X, Wang J, Zhang K, Yang H, Li
Z, Zheng Z and Liu H: LIM mineralization protein-1 enhances bone
morphogenetic protein-2-mediated osteogenesis through activation of
ERK1/2 MAPK pathway and upregulation of Runx2 transactivity. J Bone
Miner Res. 30:1523–1535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Aisa MC, Rahman S, Senin U, Maggio D and
Russell RG: Cathepsin B activity in normal human osteoblast-like
cells and human osteoblastic osteosarcoma cells (MG-63): Regulation
by interleukin-1 beta and parathyroid hormone. Biochim Biophys
Acta. 1290:29–36. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiao WJ, Xu J, Pan H, Wang TY and Shen Y:
Effect of endothelin-1 in esophageal squamous cell carcinoma
invasion and its correlation with cathepsin B. World J
Gastroenterol. 13:4002–4005. 2007. View Article : Google Scholar : PubMed/NCBI
|