Introduction
Hepatocellular carcinoma (HCC), a common aggressive
carcinoma of the liver, ranks as the third contributor for
tumor-associated death around the world (1,2).
Despite recent advances, there is still an annual incidence of
>560, 000 deaths and a dismal 10% five-year overall survival
rate (2,3). The precise molecular mechanisms
underlying the pathological progression of HCC remain poorly
elucidated.
During the past few years, increasing evidence has
identified HCC as a highly vascularized tumor with high invasion
and metastasis, which contributes to tumor recurrence and poor
survival of HCC patients (4,5). It is
widely accepted that angiogenesis is a prerequisite for the
development and metastasis of carcinoma by supplying nutrients and
oxygen (6,7). A great number of factors have been
reported to be involved in tumor angiogenesis, especially the
vascular endothelial growth factor (VEGF) (8,9).
Targeting cancer vasculature to 'starve a tumor to death' has
become a new approach for carcinoma therapy (7,10).
Though many anti-angiogenic drugs have been developed to
investigate their effect on human malignancies, the efficacy is
modest (11). Thus, it is urgent to
develop more effective therapy against HCC.
MicroRNAs (miRNAs) are evolutionarily conserved
noncoding RNAs with 22-nucleotide length and can act as the
negative regulators of target genes by interacting with the
3′-untranslated region (3′UTR). miRNAs have been corroborated to be
associated with a variety of biological processes, including cell
proliferation, invasion, angiogenesis and fat metabolism (12-15).
Recently, emerging evidence has confirmed the deregulated
expression of miRNAs in carcinomas, including HCC (16,17).
Among them, miRNA-451 (miR-451) has drawn increasing interest due
to its prominent function in the development of some cancers, such
as HCC (16). In previous research
reports the decrease of miR-451 in gastric cancer tissues and its
downregulation tends to be positively correlated with lymphatic
metastasis and overall survival of patients (17). A remarkable reduction of miR-451 has
been validated in HCC cells (16).
Additionally, its elevation obviously delay cell growth and
invasion in HCC. However, no report exists addressing its roles in
angiogenesis of HCC.
In the present study, we aimed to investigate the
effects of miR-451 expression on angiogenesis in HCC. Moreover, the
underlying mechanism was explored.
Materials and methods
Antibodies and reagents
Rabbit polyclonal antibodies to IL-6 receptor
(IL-6R) and proliferating cell nuclear antigen (PCNA) were acquired
from Abcam (Cambridge, UK). Antibodies against signal transducer
and activator of transcription 3 (STAT3) and phosphorylated STAT3
(p-STAT3-Tyr705) were obtained from Cell Signaling Technology.
Rabbit polyclonal antibodies against VEGF receptor 2 (VEGFR2) and
phospho-Tyr1175-VEGFR2 were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). The anti-VEGF antibody was from (R&D
Systems, Minneapolis, MN, USA). Antibodies against ERK1/2 and
phospho-T202/Y204-ERK1/2 were from BD Biosciences (Franklin Lakes,
NJ, USA).
Cell culture
The human hepatoma cell lines HepG2 and HEK293T were
purchased from American Type Culture Collection (ATCC; Rockville,
MD, USA). The umbilical vein endothelial cells (HUVECs) were
obtained from AllCells (Shanghai, China). HepG2 and HEK293T cells
were incubated with Dulbecco's modified Eagle's medium (DMEM)
(Invitrogen Corp., Grand Island, NY, USA) containing 10% fetal
bovine serum. HUVECs were cultured in RPMI-1640 medium (Gibco BRL,
Gaithersburg, MD, USA) supplemented with 10% fetal calf serum, 100
U/ml penicillin and 100 g/ml streptomycin. All cells were incubated
in a humidified atmosphere at 37°C with 5% CO2.
Oligonucleotide transfection
Lentivirus-based plasmids for constitutive
expression of miR-451 or scrambled miRNA (miR-con, used as NC) and
the virus packaging kit were obtained from GeneCopoeia (Rockville,
MD, USA). Following co-transfection into HEK293T using EndoFectin
Lenti transfection reagent according to the manufacturer's
instructions About 48 h later, virus titers were collected and
evaluated by the p24 ELISA kit (Cell Biolabs, Inc., San Diego, CA,
USA). Then, the obtained lentiviral particles were transduced into
HepG2 cells for 24 h. The stable miR-451-overexpressing cells were
selected using puromycin for additional 3 days.
Collection of the tumor-conditioned
medium (TCM)
HepG2 cells with miR-451 were preconditioning with
pcDNA-IL-6R lacking 3′UTR, pIRES-STAT3β [a dominant-negative STAT3
(STAT3D)] or vehicle (GeneChem, Shanghai, China). About 12 h later,
cells were incubated in DMEM medium for further 14 h. Then, the TCM
was centrifuged sequentially at 500 g to discard the detached
cells, followed by 12,000 g centrifugation to remove cell debris at
4°C for 15 min. The supernatant was then gathered and stored at
−80°C for the subsequent experiments.
Luciferase reporter assay
The wild-type (wt) and mutant (mut) 3′UTR of IL-6R
predicted to interact with miR-451 were constructed and cloned to
the firefly luciferase-expressing vector psiCHECK™ (Promega,
Madison, WI, USA). HEK293T cells were seeded into a 96-well plate
and co-transfected with wt-IL-6R or mut-IL-6R 3′UTR reporter
vector, 5 ng of pRL-TK, and miR-451 or miR-con with the help of
Lipofectamine 2000 reagent (Invitrogen Corp., Carlsbad, CA, USA).
After 48 h incubation, luciferase activities were detected by
Dual-Luciferase Reporter system (Promega).
RNA extraction and quantitative real-time
polymerase chain reaction (qRT-PCR)
After treatment under various conditions, the HepG2
cells were lysed with TRI reagent (Sigma) to extract total RNA.
Then, the reverse transcription was performed to synthesize the
cDNA using a High Capacity cDNA Archive kit (Applied Biosystems,
Foster City, CA, USA). The subsequent qRT-PCR was carried out to
evaluate the relative expression of mRNA using the miScript
SYBR®-Green PCR kit (Qiagen, China) and SYBR-Green I
(Molecular Probes, Invitrogen Corp.) for miR-451 and other
molecules. All reaction conditions and processes were implemented
according to the manufacturer's instructions. The specific primers
for miR-451, IL-6R and VEGF were used as previously published
(8,18). The expression levels were normalized
using U6 for miR-451 and β-actin for other genes. All data were
analyzed using the 2−ΔΔCt equation.
Western blotting
Cells were solubilized in lysing buffer (Beyotime,
Nantong, China) and the extracted protein concentration was
measured using the BCA assay (Pierce, Rockford, IL, USA). Then,
about 40 µg of proteins was subjected to 12% SDS-PAGE,
followed by the transfer to PVDF membrane (Millipore, Bedford, MA,
USA). After blocking with 5% non-fat milk, the membrane was probed
with primary antibodies against human IL-6R, PCNA, VEGF, STAT3,
p-STAT3, VEGFR2, p-VEGFR2, ERK and p-ERK. Then, HRP-conjugated
secondary antibodies were added for further incubation of 1 h. To
visualize the bound antibodies, the LumiGLO reagent (Pierce) was
introduced. The β-actin was used as protein loading control. All
band intensities were quantified using a Gel Doc™ XR imaging system
and Quantity One (Bio-Rad, USA).
Cell viability assay
Cell viability was monitored by Cell Counting kit
(CCK)-8 (Dojindo, Kumamoto, Japan). Briefly, HUVECs were seeded
onto 96-well plates at the density of 5×103 cells/well.
Then, cells were incubated with the TCM collected from different
background of miR-451 expression for the indicated times (12, 24
and 36 h). Subsequently, 10 µl of CCK8 reagents were added
for further 2 h incubation at 37°C. The absorbance at 450 nm was
measured to assess the number of viable cells by a Safire 2
microplate reader (Tecan, Switzerland). Relative cell viability was
shown as the absorbance percentage of the treatment group to the
control group.
In vitro migration assay
Cell migration was evaluated using the scratch wound
assay. After seeding in 24-well plates, HUVECs were cultured with
various TCM. Then, a single scratch wound was formed by scraping
the cell layer using a tip of 200 µl pipette. About 24 h
later, the scratch wounds were visualized by an inverted
microscope. The scratch wound width was quantified to assess cell
migration ability of HUVECs using the ImageJ software.
Tube formation analysis in vitro
The 96-well culture plates were precoated with
Matrigel (BD Pharmingen, San Jose, CA, USA) overnight. Then, HUVECs
were seeded into the plate at the density of 1×104
cells/well and cultured in the absence or presence of various TCM
from HepG2 cells. The formation of capillary-like structures were
then analyzed at 24-post incubation and photographed under an
inverted microscope. Tube formation was evaluated by counting
branch points in five random fields per well.
Xenograft model of HCC in nude mice
For xenograft implantation experiments, male BALB/c
nude mice aged 4–6 weeks were used and obtained from the Hunan Slac
Jingda Laboratory Animal Co., Ltd. (Changsha, China). All animals
were housed under specific pathogen-free conditions and used
according to the guidelines of the National Institutes of Health
Guide for the Care and Use of Laboratory Animals. Animal care and
procedures were approved by the Institutional Animal Care and Use
Committee of the First Affiliated Hospital of Xi'an Jiaotong
University. HepG2 cells (5×106) transfected with miR-451
or control were subcutaneously injected into mice to establish
xenograft models. Tumor size was detected every 4 days and the
tumor volumes (10 animals/group) were calculated with the following
formula: Tumor volume = (largest diameter x perpendicular
height2)/2. Five weeks later, the animals were
euthanized using sodium pentobarbital and tumors were removed.
Angiogenesis assay in vivo
Tumors from mice were collected and fixed in
formalin. Then, the specimens were processed for paraffin embedding
and subsequently cut into standard 6-µm sections. To
evaluate angiogenesis, immunofluorescence was performed. After
blocking endogenous peroxidase activity and non-specific bind, the
primary antibodies against CD31 (eBioscience) were added. Then, the
samples were incubated with biotin-linked donkey anti-rat and Texas
Red Streptavidin (Jackson ImmunoResearch, West Grove, PA, USA),
followed by the counterstain with DAPI (Sigma). The specimens were
photographed under a Zeiss LSM 510 confocal microscope and blood
vessel areas were calculated by the following formula: % Area =
total red signal/total DAPI signal.
ELISA
The serum from the mice and TCM were collected.
Then, the equal volume of samples was subjected to ELISA to
determine the VEGF concentration using a commercial VEGF ELISA kit
(R&D Systems). All procedures were performed according to the
manufacturer's instructions.
Statistical analysis
Data were analyzed by SPSS 11.0 and shown as means ±
standard deviation (SD). All experiments were performed at least
three times. Comparisons among different groups were analyzed based
on Student's t-test and ANOVA. P<0.05 was considered as
statistically significant.
Results
miR-451 evidently antagonizes
proliferation, migration and tube formation of HUVECs
To investigate the biological significance of
miR-451 in HCC angiogenesis, its effect on cell proliferation,
migration and tube formation of HUVECs were explored. As shown in
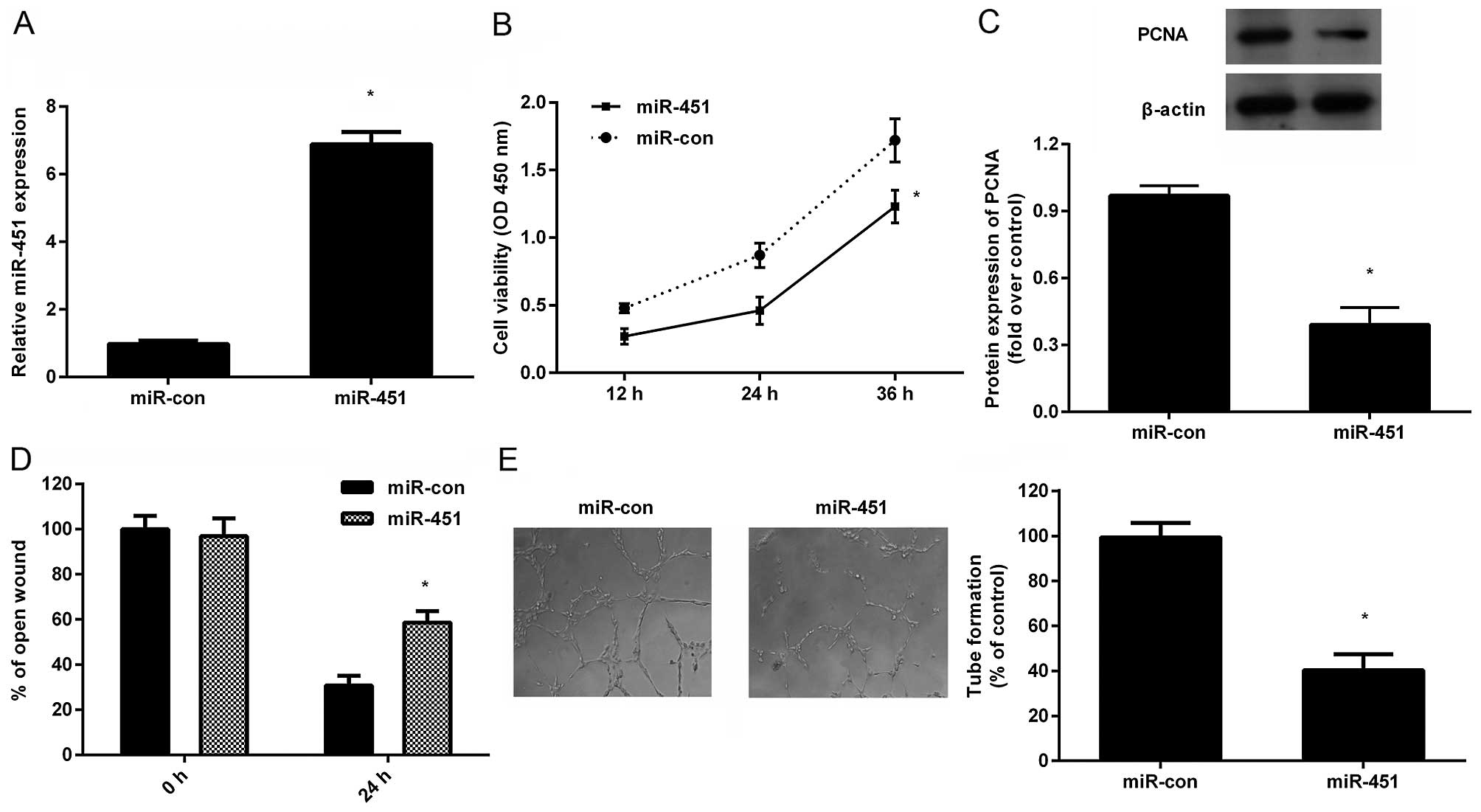
Fig. 1A, a pronounced increase of
miR-451 was validated in HepG2 cells after transfection with
miR-451. Further analysis demonstrated that incubation with TCM
from miR-451-overexpressed HepG2 cells strikingly decreased HUVEC
proliferation in a time-dependent manner (Fig. 1B). Consistently, the expression of
PCNA, a marker for cell proliferation, was also downregulated in
HUVECs when treated with the TCM (Fig.
1C). Moreover, TCM from miR-451-transfected HepG2 cells notably
mitigated cell recruitment of HUVECs (Fig. 1D). Importantly, a remarkable
inhibition in capillary tube formation of HUVECs was substantiated
when HUVECs were grown in TCM obtained from miR-451-elevated HepG2
cells. Accordingly, these data suggested that miR-451 upregulation
in HCC cells might inhibit angiogenesis of HUVECs in
vitro.
Ectopic expression of miR-451 mitigates
tumor growth and angiogenesis in vivo
To further elucidate the function of miR-451 on the
development of carcinoma, HepG2 cells stably expressing miR-451 or
control were subcutaneously injected into BALB/c nude mice.
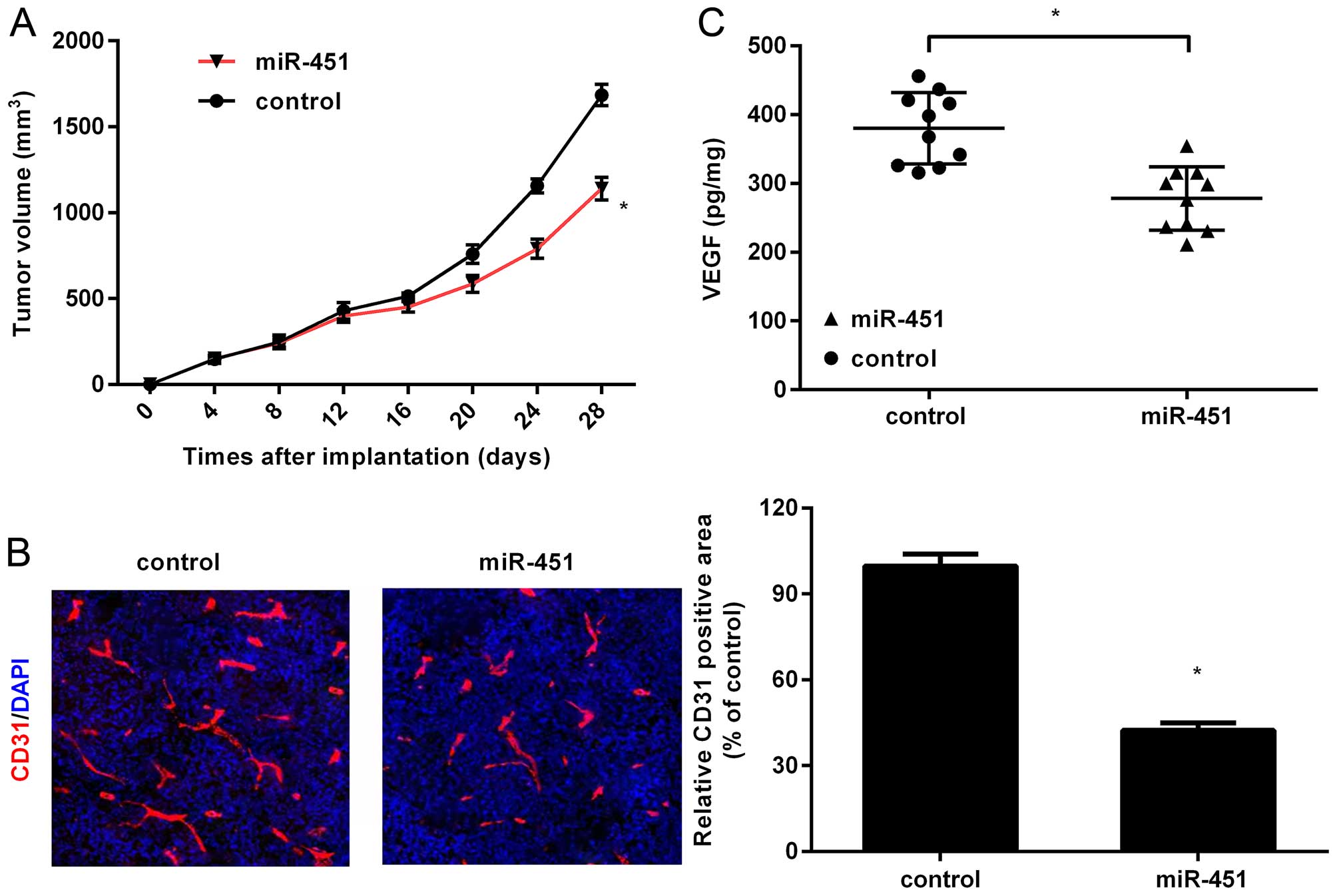
Interestingly, a noticeable decrease in tumor volume was observed
in HepG2-miR-451 tumors in contrast to control groups (Fig. 2A), indicating that miR-451 could
suppress tumor growth in vivo. The obvious decrease in blood
vessel density was corroborated in HepG2-miR-451 groups by
detecting the levels of CD31 (Fig.
2B), a common marker for vascular formation. Concomitantly,
ectopic expression of miR-451 in HepG2 tumors also triggered an
analogous downregulation of VEGF concentration in serum in
comparison with HepG2-control groups (Fig. 2C). These results indicated that
miR-451 might act as a critical suppressor of angiogenesis in
HCC.
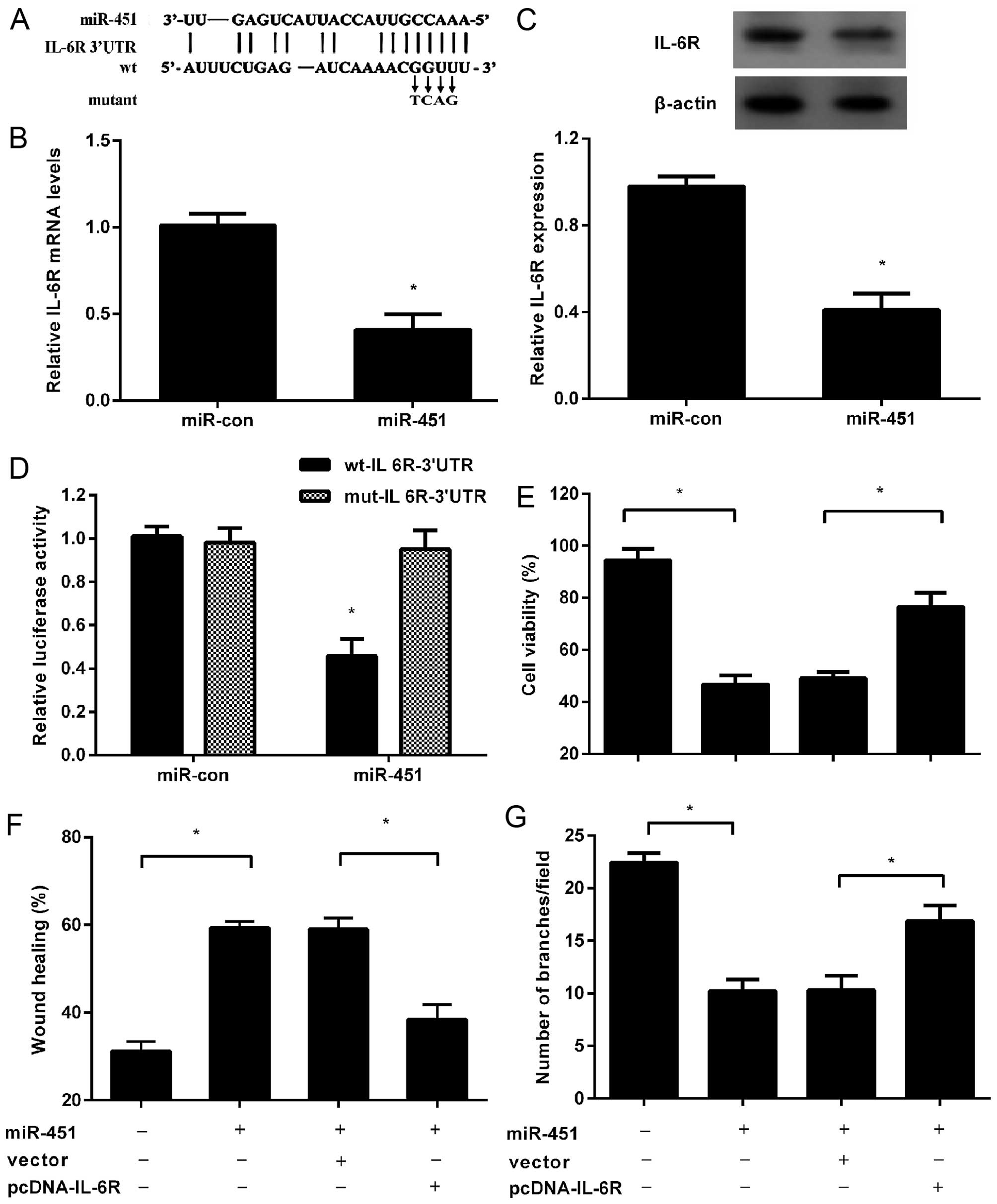
IL-6R is a direct target of miR-451
Analysis was performed to predict the potential
target of miR-451 using publicly available algorithms (TargetScan,
PicTar and microRNA.org). Among these genes, IL-6R
was identified as a potential target based on a predicted binding
site of miR-451 at its 3′UTR (Fig.
3A). It has been reported that IL-6/IL-6R signaling is involved
in tumor angiogenesis (8). To
clarify the underlying mechanism related to miR-451-mediated
inhibitory effect on angiogenesis in tumors, the expression of
IL-6R was assessed. As expected, overexpression of miR-451 markedly
reduced the mRNA levels of IL-6R in HepG2 cells (Fig. 3B). Simultaneously, a similar
decrease of IL-6R protein was also demonstrated following miR-451
transfection (Fig. 3C). Noticeably,
the luciferase activity was markedly diminished following
co-transfection of miR-451 with wt-IL-6R-3′UTR vector, but not in
mut-IL-6R-3′UTR groups (Fig. 3D),
indicating that IL-6R was a direct target of miR-451.
Overexpression of IL-6R attenuates the
inhibitory effect of miR-451 on HUVEC proliferation, migration and
tube formation
Based on the target relationship between miR-451 and
IL-6R, we further explored whether miR-451 elicits its inhibitory
role in angiogenesis of HUVECs by directly targeting IL-6R.
Following transfection with pCDNA-IL-6R lacking 3′UTR, the
inhibitory effect of TCM from miR-451-transfected HepG2 cells on
HUVEC proliferation was obviously ameliorated (Fig. 3E). Consistently, the decreased
migration of HUVECs triggered by miR-451 elevation was also
attenuated in the above culture medium (Fig. 3F). Notably, a similar increase in
capillary tube formation of HUVECs was also observed when HUVECs
were incubated with TCM from HepG2 cells co-transfected with
miR-451 and IL-6R. The above data confirmed that miR-451 could
suppress angiogenesis of HUVECs in vitro by targeting
IL-6R.
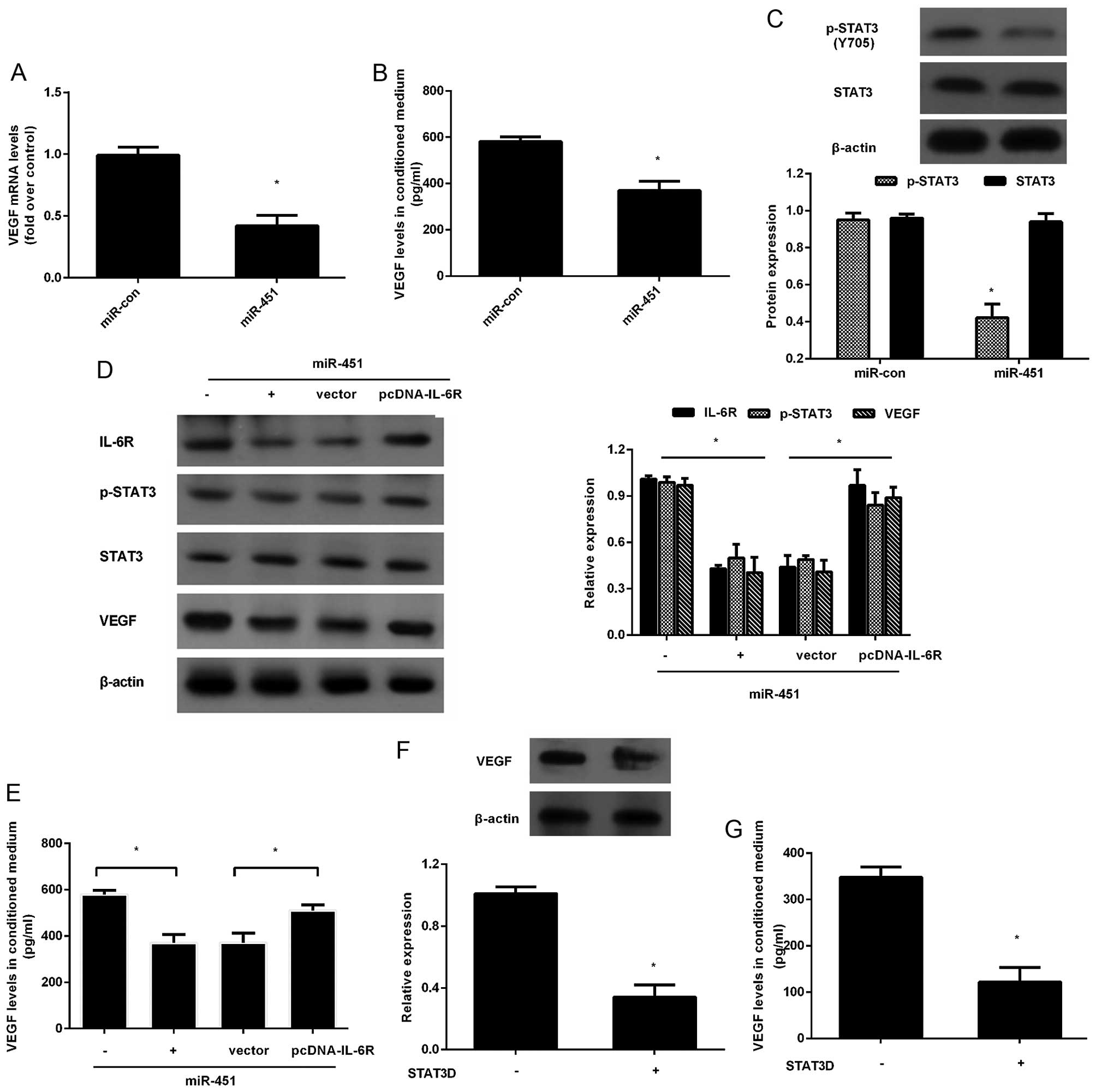
miR-451 suppresses VEGF production by
blocking IL-6R-STAT3 signaling
VEGF is widely accepted as a vital regulator for
angiogenesis. Accumulation evidence corroborates that IL-6/IL-6R
exerts an important role in angiogenesis by activating STAT3-VEGF
signaling (19,20). To further illustrate the underlying
mechanism involved in miR-451-trigged inhibition on tumor
angiogenesis, we investigated the expression of VEGF. Consistent
with our hypothesis, elevation of miR-451 noticeably abrogated the
mRNA level of VEGF (Fig. 4A).
Moreover, the concentration of VEGF in conditioned medium of HepG2
cells was also reduced (Fig. 4B).
Additionally, miR-451 upregulation significantly inhibited the
STAT3 phosphorylation (Fig. 4C).
Interestingly, IL-6R upregulation drastically antagonized the
reduction of p-STAT3 and VEGF expression trigged by miR-451
overexpression (Fig. 4D).
Furthermore, the increased expression of IL-6R obviously
upregulated the concentration of VEGF in TCM collected from
miR-451-transfected HepG2 cells (Fig.
4E). Concomitantly, blocking STAT3 signaling with STAT3D
noticeably suppressed VEGF expression (Fig. 4F) and concentration (Fig. 4G), implying that miR-451 could
dampen VEGF production secreted by HCC cells through IL-6R-STAT3
signaling.
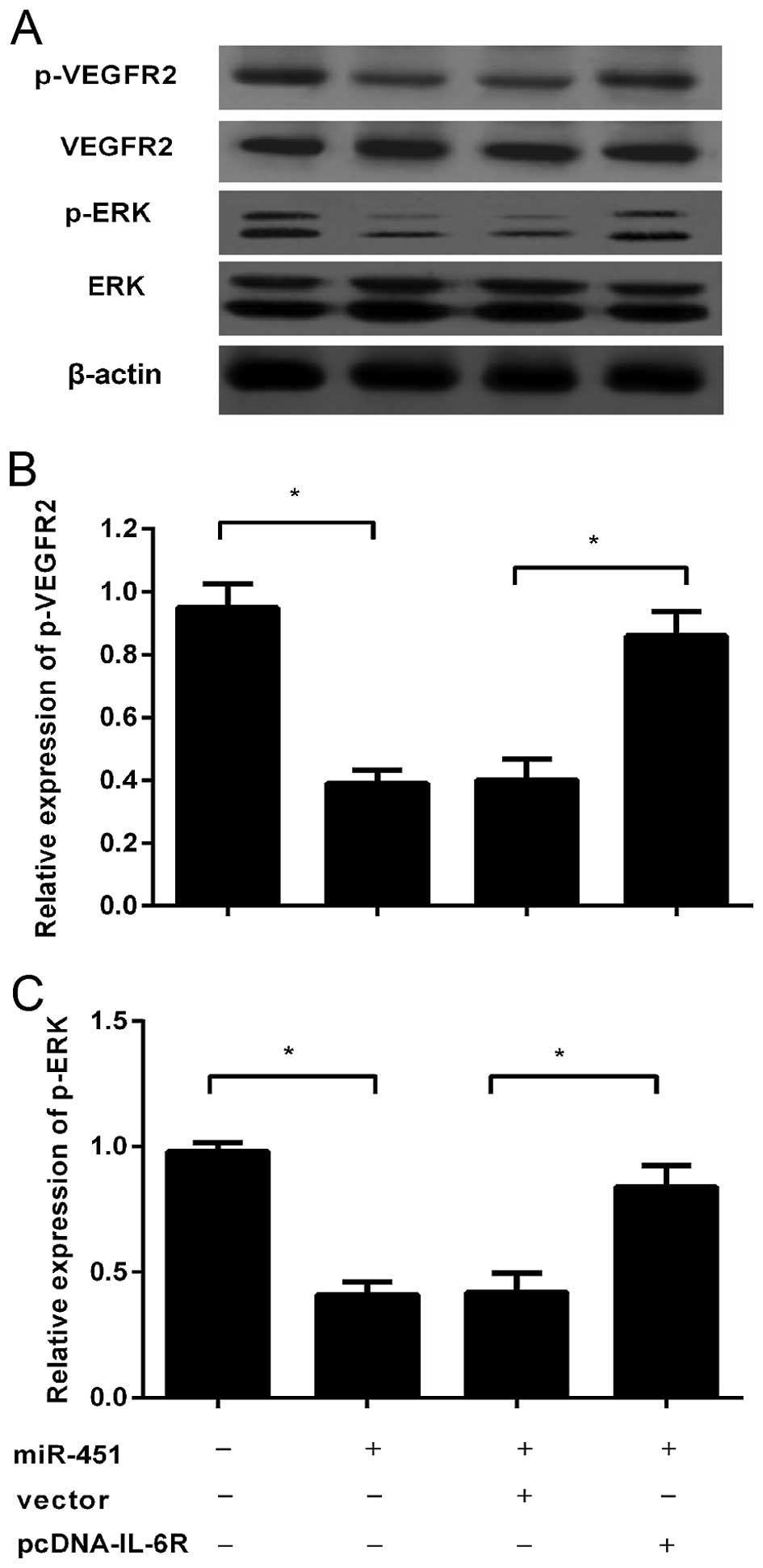
miR-451 inhibits VEGFR2 signaling in
HUVECs
Convincing evidence indicates that tumor
cell-produced VEGF can induce endothelial cell proliferation,
migration and angiogenesis by activating VEGFR2, which then
phosphorylates its down-stream ERK and subsequently induces
angiogenesis (21). We further
assess whether miR-451-decreased VEGF production by IL-6R-STAT3
signaling can abolish the VEGFR2 pathway in HEVECs. Western
blotting confirmed the obvious downregulation of p-VEGFR2 and p-ERK
in HUVECs incubated with TCM of HepG2 cells that stably
overexpressed miR-451 (Fig. 5A and
B). Surprisingly, overexpression of IL-6R could remarkably
restore the reduction of VEGF levels in TCM from
miR-451-transfected HCC cells, which then ameliorated the
inhibitory effect on the phosphorylation of VEGFR2 and p-ERK in
HUVECs (Fig. 5C). Together, these
results demonstrated that miR-451 could block the VEGFR2 pathway in
HUVECs, which will lead to reduction in angiogenesis.
Discussion
Angiogenesis has an indispensable role in
facilitating the development and progression of carcinoma (22,23).
miR-451 is frequently decreased in various tumor types, including
HCC (16). Substantial research has
identified miR-451 as a tumor suppressor by exerting its restrained
effect on cell proliferation, invasion and migration (16,17).
Our previous study validated the stinking downregulation of miR-451
in HCC cells; its elevation notably suppressed HCC cell growth and
invasion, indicating a potential role as tumor suppressor in HCC
(16). To date, nevertheless, its
effect on angiogenesis in HCC remains undefined. In this study, we
substantiated a finding that ectopic expression of miR-451 in HCC
cells prominently inhibited cell proliferation, migration and
capillary tube formation of HUVECs in vitro. Interestingly,
its overexpression noticeably antagonized tumor growth and
angiogenesis in vivo. Mechanism analysis reinforced that
miR-451 suppressed VEGF production in HCC cells by targeting
IL-6R-STAT3 signaling, as well as inhibiting the VEGFR2 signaling
in HUVECs. Therefore, this research confirmed that miR-451 might
act as a novel tumor suppressor in HCC by antagonizing angiogenesis
through directly targeting IL-6R-STAT3-VEGF pathway.
Here, the IL-6R was identified as a candidate target
of miR-451 using bioinformatics tools. IL-6R is known as a unique
receptor of IL-6. Multiple research has documented the high
expression of IL-6R and IL-6 in some tumors (12,24).
IL-6 has been proved to possess multiple biological function
through IL-6R-mediated STAT3 signaling, such as cell growth and
carcinogenesis (8). Increasing
studies confirm that IL-6R exerts crucial roles in tumor
angiogenesis (8,20). Ablation of IL-6R pronouncedly
reduces oral squamous cell carcinoma (OSCC) growth and tumor
angiogenesis by suppressing STAT3-mediated VEGF signaling,
indicating a therapeutic approach against OSCC (20). It is intriguing to speculate that
miR-451 may elicit its inhibitory effect on tumor angiogenesis by
targeting IL-6R. Consistent with this hypothesis, miR-451
overexpression prominently mitigated IL-6R expression. Further
luciferase activity assay reinforced that IL-6R was the direct
target of miR-451. Elevation of IL-6R drastically attenuated the
inhibitory effect of miR-451 on cell viability and migration of
HUVECs. Importantly, IL-6R upregulation also antagonized the
decrease in tube formation of HUVECs when incubated with TCM from
miR-451-overexpressed HCC cells. Thus, based on these results we
speculate that miR-451 might attenuate angiogenesis in HCC by
targeting IL-6R.
Convincing evidence indicates that angiogenesis is
pivotal for the growth and development of various cancer (19,23).
During this process, tumor cells can secrete VEGF into the
microenvironment to activate the vascular endothelial cells, which
will subsequently facilitate tumor angiogen-esis to meet tumor need
for blood supply. VEGF has been reported to be an indispensable
regulator for angiogenesis by regulating endothelial cell
proliferation, migration and tube formation; blocking VEGF results
in the regression of vascular network, ultimately suppressing tumor
growth and metastasis (25,26). In the present study, miR-451
elevation significantly abrogated the expression of VEGF and
secretion in HCC cells. Previous research has demonstrated that
IL-6R can trigger angiogenesis by activating STAT3-VEGF pathway
(8). STAT3 is constitutively
activated in a variety of cancers and interrupting STAT3 signaling
obviously attenuates tumor angiogenesis by VEGF production
(27,28). Our previous results identified IL-6R
as a direct target of miR-451. Further mechanistic analysis
corroborated that the IL-6R-STAT3-VEGF signaling was notably
restrained in HCC cells after miR-451 transfection. Upregulation of
IL-6R counteracted the decrease of VEGF in miR-451-over-expressed
HCC cells. Similarly to a previous study, blocking STAT3 signaling
with STAT3D significantly decreased VEGF levels (28). Thus, the above data manifested that
miR-451 might antagonize angiogenesis in HCC by targeting
IL-6R-STST3-VEGF pathway.
A novel finding of this research is that miR-451
elevation in HCC cells inhibited VEGF levels in tumor
microenvironment, which in turn suppressed the activation of VEGFR2
signaling in HUVECs. It is widely accepted that tumor-secreted VEGF
can bind to and activate VEGFR2 signaling to promote vascular
endothelial cell proliferation, migration and tube formation via
ERK pathway (21,29). Blocking VEGFR2 can induce vessel
normalization and survival benefit in mice bearing gliomas
(30). Recently, suppressing VEGFR2
signaling has been proposed as a promising strategy for the
clinical treatment of HCC (31).
Accordingly, our research suggested miR-451 could abrogate the
VEGF-VEGFR2 signaling, which finally abolished angiogenesis in
HCC.
In conclusion, elevation of miR-451 in HCC cells
saliently antagonized the viability, migration and tube formation
of HUVECs by targeting IL-6R-STAT3-VEGF signaling. Importantly, its
upregulation reduced tumor growth and angiogenesis of HCC in
vivo. Moreover, overexpression of miR-451 in HCC cells also
impaired VEGFR2 signaling in HUVECs. Therefore, miR-451 may act as
a suppressor for angiogenesis of HCC by targeting IL-6R-STST3-VEGF
signaling, indicating a promising therapeutic agent against
HCC.
Acknowledgments
Financial support was provided by the Science and
Technology Research and Development Program of Shaanxi Province
(2016SF-023) and National Natural Science Foundation of China
(NSFC) (no. 81372582).
References
|
1
|
Yang JD and Roberts LR: Hepatocellular
carcinoma: A global view. Nat Rev Gastroenterol Hepatol. 7:448–458.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Altekruse SF, McGlynn KA and Reichman ME:
Hepatocellular carcinoma incidence, mortality, and survival trends
in the United States from 1975 to 2005. J Clin Oncol. 27:1485–1491.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verslype C, Rosmorduc O and Rougier P;
ESMO Guidelines Working Group: Hepatocellular carcinoma: ESMO-ESDO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 23(Suppl 7): vii41–vii48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J,
Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al: Long noncoding RNA
associated with microvascular invasion in hepatocellular carcinoma
promotes angiogenesis and serves as a predictor for hepatocellular
carcinoma patients' poor recurrence-free survival after
hepatectomy. Hepatology. 56:2231–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C, Wu X, Zhang H, Yang G, Hao M, Sheng
S, Sun Y, Long J, Hu C, Sun X, et al: A Huaier polysaccharide
restrains hepatocellular carcinoma growth and metastasis by
suppression angiogenesis. Int J Biol Macromol. 75:115–120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cuevas I, Layman H, Coussens L and
Boudreau N: Sustained endothelial expression of HoxA5 in vivo
impairs pathological angiogenesis and tumor progression. PLoS One.
10:e01217202015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Welti J, Loges S, Dimmeler S and Carmeliet
P: Recent molecular discoveries in angiogenesis and antiangiogenic
therapies in cancer. J Clin Invest. 123:3190–3200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB,
Lee CN and Hsieh CY: Interleukin-6 promotes cervical tumor growth
by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene.
22:1517–1527. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van der Veldt AA, Lubberink M, Bahce I,
Walraven M, de Boer MP, Greuter HN, Hendrikse NH, Eriksson J,
Windhorst AD, Postmus PE, et al: Rapid decrease in delivery of
chemotherapy to tumors after anti-VEGF therapy: Implications for
scheduling of anti-angiogenic drugs. Cancer Cell. 21:82–91. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ge G, Wang A, Yang J, Chen Y, Yang J, Li Y
and Xue Y: Interleukin-37 suppresses tumor growth through
inhibition of angiogenesis in non-small cell lung cancer. J Exp
Clin Cancer Res. 35:132016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar
|
|
12
|
Plummer PN, Freeman R, Taft RJ, Vider J,
Sax M, Umer BA, Gao D, Johns C, Mattick JS, Wilton SD, et al:
MicroRNAs regulate tumor angiogenesis modulated by endothelial
progenitor cells. Cancer Res. 73:341–352. 2013. View Article : Google Scholar
|
|
13
|
Zaravinos A, Radojicic J, Lambrou GI,
Volanis D, Delakas D, Stathopoulos EN and Spandidos DA: Expression
of miRNAs involved in angiogenesis, tumor cell proliferation, tumor
suppressor inhibition, epithelial-mesenchymal transition and
activation of metastasis in bladder cancer. J Urol. 188:615–623.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bo W, Hu Y, Feng X, Zhang H, Tian L and
Liu A: The tumor suppressor role of miR-4782-3p in hepatocellular
carcinoma. Oncol Rep. 35:2107–2112. 2016.PubMed/NCBI
|
|
15
|
Wang W, Zhang E and Lin C: MicroRNAs in
tumor angiogenesis. Life Sci. 136:28–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu X, Zhang X, Xiang J, Lv Y and Shi J:
miR-451: Potential role as tumor suppressor of human hepatoma cell
growth and invasion. Int J Oncol. 45:739–745. 2014.PubMed/NCBI
|
|
17
|
Su Z, Zhao J, Rong Z, Geng W and Wang Z:
MiR-451, a potential prognostic biomarker and tumor suppressor for
gastric cancer. Int J Clin Exp Pathol. 8:9154–9160. 2015.PubMed/NCBI
|
|
18
|
Liu D, Liu C, Wang X, Ingvarsson S and
Chen H: MicroRNA-451 suppresses tumor cell growth by
down-regulating IL6R gene expression. Cancer Epidemiol. 38:85–92.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou Y, Guo CG and Zhang MM: Inhibition of
human hepatocellular carcinoma tumor angiogenesis by siRNA
silencing of VEGF via hepatic artery perfusion. Eur Rev Med
Pharmacol Sci. 19:4751–4761. 2015.
|
|
20
|
Shinriki S, Jono H, Ota K, Ueda M, Kudo M,
Ota T, Oike Y, Endo M, Ibusuki M, Hiraki A, et al: Humanized
anti-interleukin-6 receptor antibody suppresses tumor angiogenesis
and in vivo growth of human oral squamous cell carcinoma. Clin
Cancer Res. 15:5426–5434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim BM, Lee DH, Choi HJ, Lee KH, Kang SJ,
Joe YA, Hong YK and Hong SH: The recombinant kringle domain of
urokinase plasminogen activator inhibits VEGF165-induced
angiogenesis of HUVECs by suppressing VEGFR2 dimerization and
subsequent signal transduction. IUBMB Life. 64:259–265. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plate KH, Scholz A and Dumont DJ: Tumor
angiogenesis and anti-angiogenic therapy in malignant gliomas
revisited. Acta Neuropathol. 124:763–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paauwe M, Heijkants RC, Oudt CH, van Pelt
GW, Cui C, Theuer CP, Hardwick JC, Sier CF and Hawinkels LJ:
Endoglin targeting inhibits tumor angiogenesis and metastatic
spread in breast cancer. Oncogene. Jan 25–2016.Epub ahead of print.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kishimoto T: Interleukin-6: From basic
science to medicine - 40 years in immunology. Annu Rev Immunol.
23:1–21. 2005. View Article : Google Scholar
|
|
25
|
Chekhonin VP, Shein SA, Korchagina AA and
Gurina OI: VEGF in tumor progression and targeted therapy. Curr
Cancer Drug Targets. 13:423–443. 2013. View Article : Google Scholar
|
|
26
|
Wang R, Zhao N, Li S, Fang JH, Chen MX,
Yang J, Jia WH, Yuan Y and Zhuang SM: MicroRNA-195 suppresses
angiogenesis and metastasis of hepatocellular carcinoma by
inhibiting the expression of VEGF, VAV2, and CDC42. Hepatology.
58:642–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL, et al: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bold G, Schnell C, Furet P, McSheehy P,
Brüggen J, Mestan J, Manley PW, Drückes P, Burglin M, Dürler U, et
al: A novel potent oral series of VEGFR2 inhibitors abrogate tumor
growth by inhibiting angiogenesis. J Med Chem. 59:132–146. 2016.
View Article : Google Scholar
|
|
30
|
Chae SS, Kamoun WS, Farrar CT, Kirkpatrick
ND, Niemeyer E, de Graaf AM, Sorensen AG, Munn LL, Jain RK and
Fukumura D: Angiopoietin-2 interferes with anti-VEGFR2-induced
vessel normalization and survival benefit in mice bearing gliomas.
Clin Cancer Res. 16:3618–3627. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ku CY, Wang YR, Lin HY, Lu SC and Lin JY:
Corosolic acid inhibits hepatocellular carcinoma cell migration by
targeting the VEGFR2/Src/FAK pathway. PLoS One. 10:e01267252015.
View Article : Google Scholar : PubMed/NCBI
|