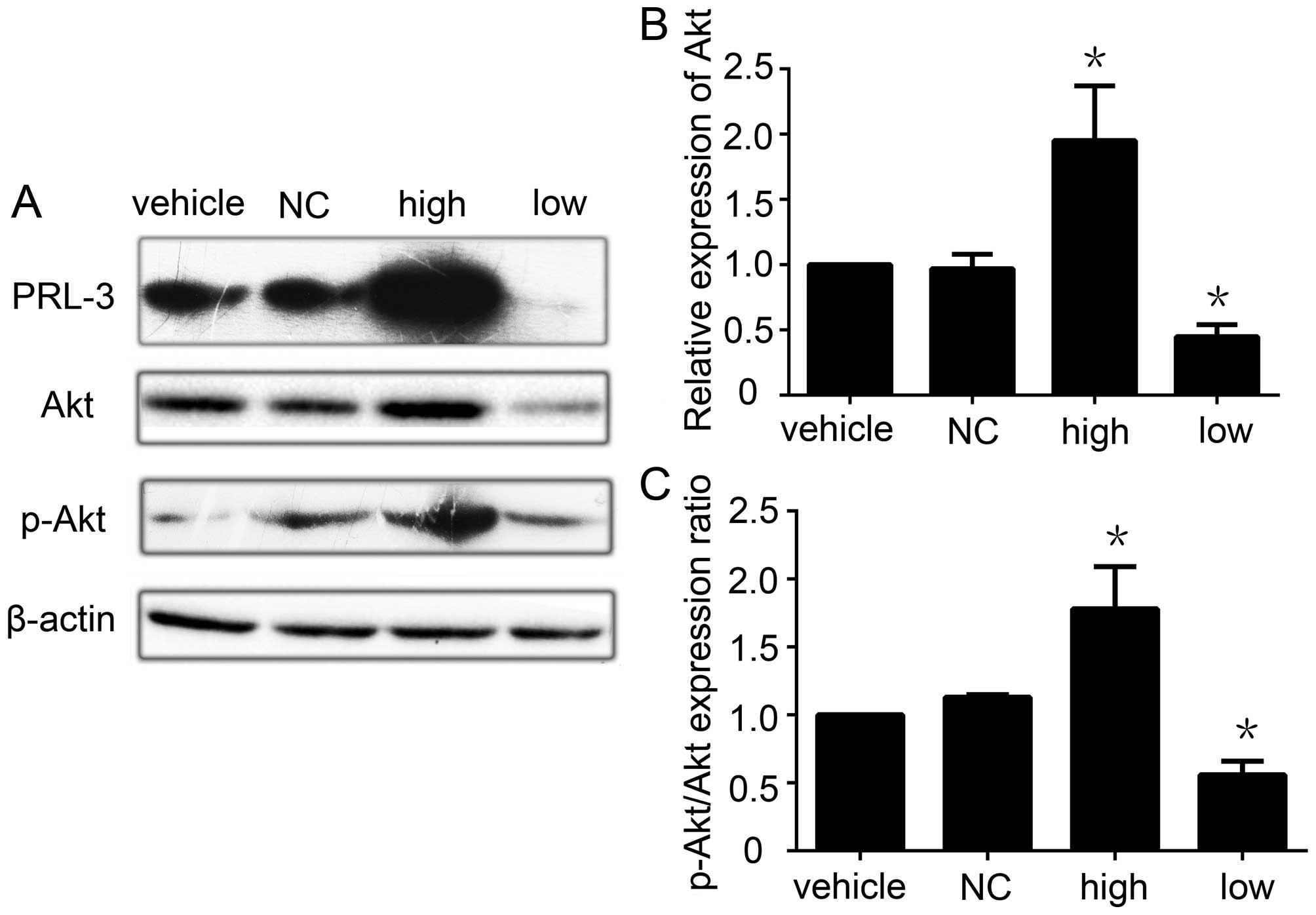
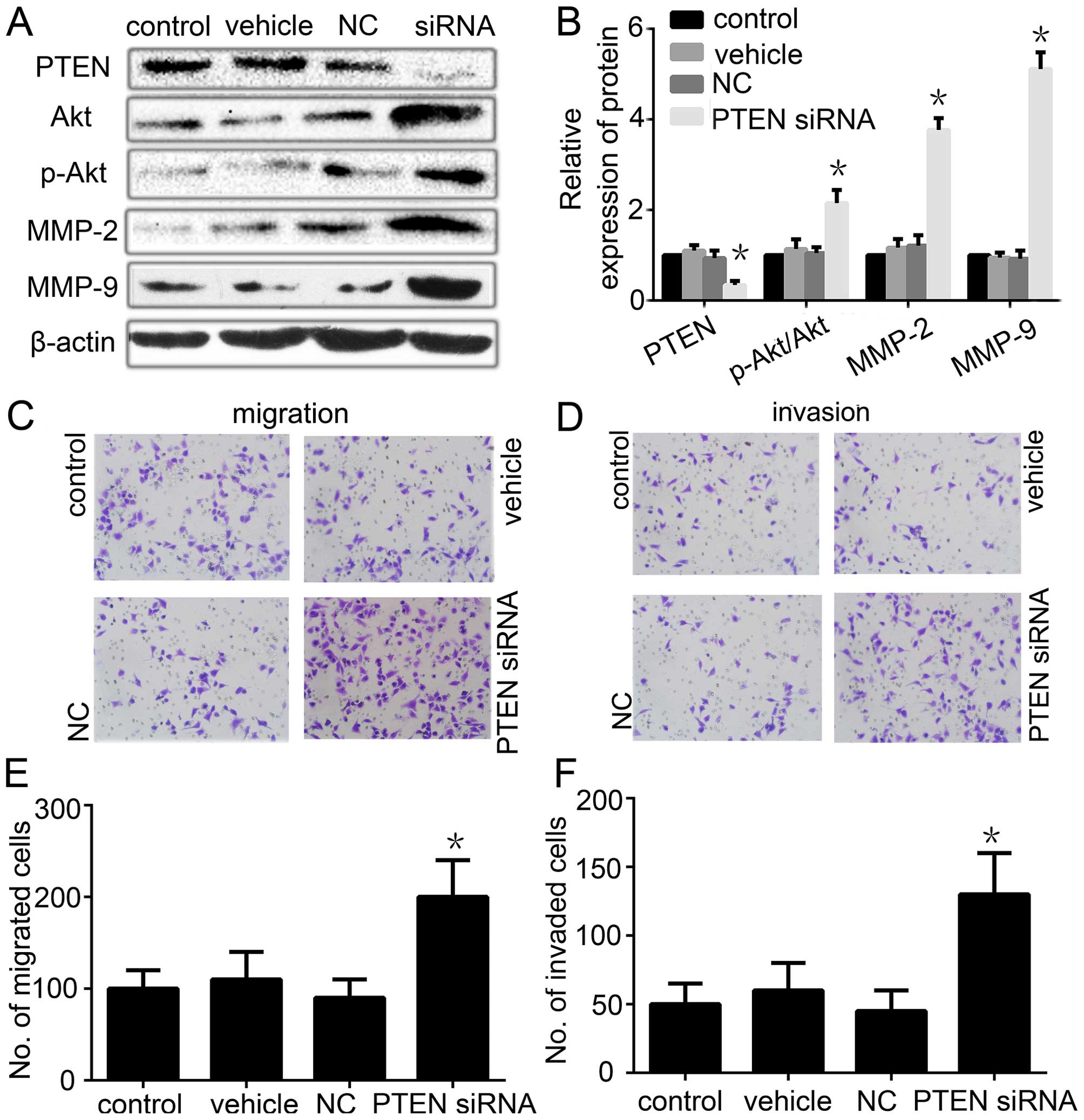
|
1
|
Globocan: 2012, Estimated Cancer
Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
Accessed March 31, 2014.
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Vita F, Vecchione L, Galizia G, Di
Martino N, Fabozzi T, Catalano G, Ciardiello F and Orditura M:
Perspectives in adjuvant therapy of gastric cancer. Oncology.
77(Suppl 1): S38–S42. 2009. View Article : Google Scholar
|
|
4
|
Kagawa S, Shigeyasu K, Ishida M, Watanabe
M, Tazawa H, Nagasaka T, Shirakawa Y and Fujiwara T: Molecular
diagnosis and therapy for occult peritoneal metastasis in gastric
cancer patients. World J Gastroenterol. 20:17796–17803.
2014.PubMed/NCBI
|
|
5
|
Bando E, Yonemura Y, Takeshita Y,
Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T,
Nishimura G and Miwa K: Intraoperative lavage for cytological
examination in 1,297 patients with GC. Am J Surg. 178:256–262.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Turaga KK, Gamblin TC and Pappas S:
Surgical treatment of peritoneal carcinomatosis from gastric
cancer. Int J Surg Oncol. 2012:4056522012.PubMed/NCBI
|
|
7
|
Bessette DC, Qiu D and Pallen CJ: PRL
PTPs: Mediators and markers of cancer progression. Cancer
Metastasis Rev. 27:231–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong Q, Ding X, Chang B, Wang H and Wang
A: PRL-3 promotes migration and invasion and is associated with
poor prognosis in salivary adenoid cystic carcinoma. J Oral Pathol
Med. 45:111–118. 2016. View Article : Google Scholar
|
|
9
|
Kato H, Semba S, Miskad UA, Seo Y, Kasuga
M and Yokozaki H: High expression of PRL-3 promotes cancer cell
motility and liver metastasis in human colorectal cancer: A
predictive molecular marker of metachronous liver and lung
metastases. Clin Cancer Res. 10:7318–7328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo K, Li J, Tang JP, Koh V, Gan BQ and
Zeng Q: Catalytic domain of PRL-3 plays an essential role in tumor
metastasis: Formation of PRL-3 tumors inside the blood vessels.
Cancer Biol Ther. 3:945–951. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Zeng H, Zhang X, Zhao Y, Sha H, Ge
X, Zhang M, Gao X and Xu Q: Phosphatase of regenerating liver-3
promotes motility and metastasis of mouse melanoma cells. Am J
Pathol. 164:2039–2054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai N, Lu AP, Shou CC and Li JY:
Expression of phosphatase regenerating liver 3 is an independent
prognostic indicator for gastric cancer. World J Gastroenterol.
15:1499–1505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng Q, Dong JM, Guo K, Li J, Tan HX, Koh
V, Pallen CJ, Manser E and Hong W: PRL-3 and PRL-1 promote cell
migration, invasion, and metastasis. Cancer Res. 63:2716–2722.
2003.PubMed/NCBI
|
|
14
|
Li ZR, Wang Z, Zhu BH, He YL, Peng JS, Cai
SR, Ma JP and Zhan WH: Association of tyrosine PRL-3 phosphatase
protein expression with peritoneal metastasis of GC and prognosis.
Surg Today. 37:646–651. 2007. View Article : Google Scholar
|
|
15
|
Xing X, Lian S, Hu Y, Li Z, Zhang L, Wen
X, Du H, Jia Y, Zheng Z, Meng L, et al: Phosphatase of regenerating
liver-3 (PRL-3) is associated with metastasis and poor prognosis in
GC. J Transl Med. 11:309. 2013. View Article : Google Scholar
|
|
16
|
Miskad UA, Semba S, Kato H and Yokozaki H:
Expression of PRL-3 phosphatase in human gastric carcinomas: Close
correlation with invasion and metastasis. Pathobiology. 71:176–184.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Cai SR, He YL, Zhan WH, Chen CQ,
Cui J, Wu WH, Wu H, Song W, Zhang CH, et al: High expression of
PRL-3 can promote growth of gastric cancer and exhibits a poor
prognostic impact on patients. Ann Surg Oncol. 16:208–219. 2009.
View Article : Google Scholar
|
|
18
|
Li Z, Cao Y, Jie Z, Liu Y, Li Y, Li J, Zhu
G, Liu Z, Tu Y, Peng G, et al: miR-495 and miR-551a inhibit the
migration and invasion of human gastric cancer cells by directly
interacting with PRL-3. Cancer Lett. 323:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Waite KA and Eng C: Protean PTEN: Form and
function. Am J Hum Genet. 70:829–844. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bermúdez Brito M, Goulielmaki E and
Papakonstanti EA: Focus on PTEN regulation. Front Oncol. 5:1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye B, Jiang LL, Xu HT, Zhou DW and Li ZS:
Expression of PI3K/AKT pathway in gastric cancer and its blockade
suppresses tumor growth and metastasis. Int J Immunopathol
Pharmacol. 25:627–636. 2012.PubMed/NCBI
|
|
22
|
Myers MP, Pass I, Batty IH, Van der Kaay
J, Stolarov JP, Hemmings BA, Wigler MH, Downes CP and Tonks NK: The
lipid phosphatase activity of PTEN is critical for its tumor
supressor function. Proc Natl Acad Sci USA. 95:13513–13518. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stambolic V, Suzuki A, de la Pompa JL,
Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM,
Siderovski DP and Mak TW: Negative regulation of PKB/Akt-dependent
cell survival by the tumor suppressor PTEN. Cell. 95:29–39. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: The PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li NA, Wang W, Xu B and Gong H: miR-196b
regulates gastric cancer cell proliferation and invasion via
PI3K/AKT/mTOR signaling pathway. Oncol Lett. 11:1745–1749.
2016.PubMed/NCBI
|
|
27
|
Akrami H, Mahmoodi F, Havasi S and Sharifi
A: PlGF knockdown inhibited tumor survival and migration in gastric
cancer cell via PI3K/Akt and p38MAPK pathways. Cell Biochem Funct.
34:173–180. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun L, Liu L, Liu X, Wang Y, Li M, Yao L,
Yang J, Ji G, Guo C, Pan Y, et al: MGr1-Ag/37LRP induces cell
adhesion-mediated drug resistance through FAK/PI3K and MAPK pathway
in gastric cancer. Cancer Sci. 105:651–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Quah SY, Dong JM, Manser E, Tang
JP and Zeng Q: PRL-3 down-regulates PTEN expression and signals
through PI3K to promote epithelial-mesenchymal transition. Cancer
Res. 67:2922–2926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang H, Vardy LA, Tan CP, Loo JM, Guo K,
Li J, Lim SG, Zhou J, Chng WJ, Ng SB, et al: PCBP1 suppresses the
translation of metastasis-associated PRL-3 phosphatase. Cancer
Cell. 18:52–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang XY, Song R, Chen W, Yang YY, Gu YH,
Shu YQ, Wu XD, Wu XF, Sun Y, Shen Y, et al: PRL-3 promotes the
malignant progression of melanoma via triggering dephosphorylation
and cytoplasmic localization of NHERF1. J Invest Dermatol.
135:2273–2282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: A tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng L, Xing X, Li W, Qu L, Meng L, Lian
S, Jiang B, Wu J and Shou C: PRL-3 promotes the motility, invasion,
and metastasis of LoVo colon cancer cells through PRL-3-integrin
beta1-ERK1/2 and-MMP2 signaling. Mol Cancer. 8:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Z, Al-Aidaroos AQ, Park JE, Yuen HF,
Zhang SD, Gupta A, Lin Y, Shen HM and Zeng Q: PRL-3 activates
mTORC1 in cancer progression. Sci Rep. 5:170462015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li WJ, Xing XF, Qu LK, Meng L and Shou CC:
Construction and expression of PRL-3 plasmid with C104S point
mutation and CAAX deletion. Beijing Da Xue Xue Bao. 41:516–520.
2009.In Chinese. PubMed/NCBI
|
|
37
|
Pascaru M, Tanase C, Vacaru AM, Boeti P,
Neagu E, Popescu I and Szedlacsek SE: Analysis of molecular
determinants of PRL-3. J Cell Mol Med. 13:3141–3150. 2009.
View Article : Google Scholar
|
|
38
|
Kozlov G, Cheng J, Ziomek E, Banville D,
Gehring K and Ekiel I: Structural insights into molecular function
of the metastasis-associated phosphatase PRL-3. J Biol Chem.
279:11882–11889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McParland V, Varsano G, Li X, Thornton J,
Baby J, Aravind A, Meyer C, Pavic K, Rios P and Köhn M: The
metastasis-promoting phosphatase PRL-3 shows activity toward
phosphoinositides. Biochemistry. 50:7579–7590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matter WF, Estridge T, Zhang C, Belagaje
R, Stancato L, Dixon J, Johnson B, Bloem L, Pickard T, Donaghue M,
et al: Role of PRL-3, a human muscle-specific tyrosine phosphatase,
in angiotensin-II signaling. Biochem Biophys Res Commun.
283:1061–1068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fiordalisi JJ, Keller PJ and Cox AD: PRL
tyrosine phosphatases regulate rho family GTPases to promote
invasion and motility. Cancer Res. 66:3153–3161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jian M, Nan L, Guocheng J, Qingfu Z,
Xueshan Q and Enhua W: Downregulating PRL-3 inhibit migration and
invasion of lung cancer cell via RhoA and mDia1. Tumori.
98:370–376. 2012.PubMed/NCBI
|
|
43
|
Li Z, Zhan W, Wang Z, Zhu B, He Y, Peng J,
Cai S and Ma J: Inhibition of PRL-3 gene expression in gastric
cancer cell line SGC7901 via microRNA suppressed reduces peritoneal
metastasis. Biochem Biophys Res Commun. 348:229–237. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Zheng P, Liu Y, Ji T, Liu X, Yao S,
Cheng X, Li Y, Chen L, Xiao Z, et al: An epigenetic role for PRL-3
as a regulator of H3K9 methylation in colorectal cancer. Gut.
62:571–581. 2013. View Article : Google Scholar
|
|
45
|
Molleví DG, Aytes A, Padullés L,
Martínez-Iniesta M, Baixeras N, Salazar R, Ramos E, Figueras J,
Capella G and Villanueva A: PRL-3 is essentially overexpressed in
primary colorectal tumours and associates with tumour
aggressiveness. Br J Cancer. 99:1718–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mayinuer A, Yasen M, Mogushi K, Obulhasim
G, Xieraili M, Aihara A, Tanaka S, Mizushima H, Tanaka H and Arii
S: Upregulation of protein tyrosine phosphatase type IVA member 3
(PTP4A3/PRL-3) is associated with tumor differentiation and a poor
prognosis in human hepatocellular carcinoma. Ann Surg Oncol.
20:305–317. 2013. View Article : Google Scholar :
|
|
47
|
Khapare N, Kundu ST, Sehgal L, Sawant M,
Priya R, Gosavi P, Gupta N, Alam H, Karkhanis M, Naik N, et al:
Plakophilin3 loss leads to an increase in PRL3 levels promoting K8
dephosphorylation, which is required for transformation and
metastasis. PLoS One. 7:e385612012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J,
Zhang S, Wu J, Yu K and Han Y: Matrine induces apoptosis in human
acute myeloid leukemia cells via the mitochondrial pathway and Akt
inactivation. PLoS One. 7:e468532012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bessette DC, Wong PC and Pallen CJ: PRL-3:
A metastasis-associated phosphatase in search of a function. Cells
Tissues Organs. 185:232–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu H, Lai W, Zhang Y, Liu L, Luo X, Zeng
Y, Wu H, Lan Q and Chu Z: Tumor-associated macrophage-derived IL-6
and IL-8 enhance invasive activity of LoVo cells induced by PRL-3
in a KCNN4 channel-dependent manner. BMC Cancer. 14:3302014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Miskad UA, Semba S, Kato H, Matsukawa Y,
Kodama Y, Mizuuchi E, Maeda N, Yanagihara K and Yokozaki H: High
PRL-3 expression in human gastric cancer is a marker of metastasis
and grades of malignancies: An in situ hybridization study.
Virchows Archiv. 450:303–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang Z, He YL, Cai SR, Zhan WH, Li ZR, Zhu
BH, Chen CQ, Ma JP, Chen ZX, Li W, et al: Expression and prognostic
impact of PRL-3 in lymph node metastasis of gastric cancer: Its
molecular mechanism was investigated using artificial microRNA
interference. Int J Cancer. 123:1439–1447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xu W, Yang Z, Zhou SF and Lu N:
Posttranslational regulation of phosphatase and tensin homolog
(PTEN) and its functional impact on cancer behaviors. Drug Des
Devel Ther. 8:1745–1751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bononi A and Pinton P: Study of PTEN
subcellular localization. Methods. 77–78:92–103. 2015. View Article : Google Scholar
|
|
55
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F, et al: PTEN: Multiple functions in human malignant
tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Khasigov PZ, Podobed OV, Gracheva TS,
Salbiev KD, Grachev SV and Berezov TT: Role of matrix
metalloproteinases and their inhibitors in tumor invasion and
metastasis. Biochemistry. 68:711–717. 2003.PubMed/NCBI
|
|
58
|
Willems L, Tamburini J, Chapuis N, Lacombe
C, Mayeux P and Bouscary D: PI3K and mTOR signaling pathways in
cancer: New data on targeted therapies. Curr Oncol Rep. 14:129–138.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ortega-Molina A and Serrano M: PTEN in
cancer, metabolism, and aging. Trends Endocrinol Metab. 24:184–189.
2013. View Article : Google Scholar
|
|
60
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|