Introduction
Colorectal cancer is the third most common
malignancy in males and the second most common in females, with
significant variations in the worldwide distribution. In veterinary
medicine it was described in sheep and dog with a different
incidence (1). A more than 10-fold
variation in the colorectal cancer incidence rate across countries
and the rapid increase in incidence rates in countries experiencing
industrialization suggest a strong link with lifestyle factors. The
highest incidence rates are found in economically developed
countries, whereas the lowest rates are noted in Africa and
South-Central Asia (2). However,
recent 'perturbations' in colorectal cancer incidence trends were
observed probably resulting from a combination of risk factors,
including obesity, sedentary lifestyle, increased prevalence of
smoking, excessive alcohol consumption and 'westernization' in
dietary habits - a diet rich in red and processed meat and low
intake of fruits and vegetables (3,4).
The possibility that fruit and vegetables may help
to reduce the risk for various types of cancer raised great
interest already in the 1970s, when studies conducted to assess
differences in cancer rates and diet between countries, suggested
that various dietary factors may have important effects on cancer
risk (5,6). Several years later, a joint report by
the World Cancer Research Fund together with the American Institute
of Cancer Research found 'convincing' evidence that a high fruit
and vegetable intake would reduce cancer of the colon and rectum
(4,7).
It is known that plants may be used both as a
medicine and a food and it is difficult to draw a line between
these two groups: food may be medicine, and vice versa. In
traditional societies, plants are often used multi-contextually,
for example, as food and for medicine (8). Particularly interesting are the
pharmacological properties of these plants and of the constituents
isolated from them.
Epidemiologic studies suggest that the consumption
of natural compounds lowers the risk of cardiovascular disease,
diabetes, arthritis and cancer (4,9).
The genus Celtis (Ulmaceae) includes about 70
species of shrubs or trees, primarily distributed in the temperate
and tropical regions of the Northern Hemisphere (10). Celtis aetnensis (Tornab.)
Strobl is a bushy shrub present on Mount Etna (Sicily) (11). It is a woody plant, whose leaves are
heart-shaped and slightly crenate on the edge. Some species of
Celtis are used in traditional medicine for the treatment of
low back pain, upset stomach, abdominal pain, as well as for their
astringent and soothing in case of diarrhea, enteritis and
inflammation of the oral cavity. These properties are primarily
attributable to the active principles contained in the leaves, such
as tannins, saponins and flavonoids.
In previous phytochemical reports on species of the
genus Celtis, the presence of betulin, gallic acid,
3,3′-di-O-methy-lellagic acid, moretenol and two triterpene esters,
3β-trans-sinapoyloxylup-20(29)-en-28-ol and
3β-trans-feruloyloxy-16β-hydroxylup-20(29)-ene and five known triterpenes,
3β-O-(E)-feruloylbetulin, 3β-O-(E)-coumaroylbetulin, betulin,
20-epibryonolic acid, and ursolic acid, were isolated from the
twigs of C. philippinensis (10,12).
Antioxidant and cytotoxic activities were reported
for other Celtis species e.g., Celtis philippinensis
Blanco, Celtis africana Burm. f., and Celtis iguanae
(Jacq.) Sarg. (10,13–16),
however, as far as we know, no previous biological and
phytochemical investigations on Celtis aetnensis (Tornab.)
Strobl have been reported.
Many antioxidant compounds have been investigated
for their potential usefulness as cancer chemopreventive agents
(17–19). Since there is an increasing interest
in the in vivo protective effects of natural compounds
contained in plants against oxidative damage involved in several
human diseases such as cancer, the present study investigated the
effects of Celtis aetnensis (Tornab.) Strobl twig extract on
the viability of the human carcinoma Caco2 cell line. In addition,
in order to elucidate the mechanisms of action of this extract, LDH
release, caspase expression, thiol groups, ROS levels, HO-1 and
γ-GCS protein expression were also evaluated.
Materials and methods
Chemicals
3-(4,5-Dimethylthiazol-2-yl)2,5-diphenyltetrazolium
bromide (MTT) and 2′,7′-dichlorofluorescein diacetate (DCFH-DA)
were obtained from Sigma Aldrich Co. (St. Louis, MO, USA). All
other chemicals were purchased from Gibco-BRL Life Technologies
(Grand Island, NY, USA). Polyclonal γ-glutamylcysteine synthetase
(γ-GCS) and caspase antibodies were from Abcam (Victoria, BC,
Canada). Secondary horseradish peroxidase-conjugated anti-rabbit
antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA,
USA). The enhanced chemiluminescence system for developing
immunoblots and nitrocellulose membranes was purchased from
Amersham (Milano, Italy). The ELISA kit, used to measure heme
oxygenase-1 (HO-1) protein concentration, was from Stressgen
Biotechnologies (Victoria, BC, Canada).
Plant material and preparation of the
extract
Twigs of Celtis aetnensis (Tornab.) Strobl
(Ulmaceae) were collected in the area around Linguaglossa (Catania,
Italy) in June 2015. The specimens were obtained thanks to the
Regional Forest Corps Detachment of Catania-Nicolosi and
authenticated by botanist Professor S. Ragusa, Department of Health
Sciences, University of Catanzaro (Italy). A voucher specimen of
the plant was deposited in the herbarium of the same
department.
Twigs of Celtis aetnensis (Tornab.) Strobl
were air dried for 10 days, and then washed free from soil,
powdered and stored in airtight containers at room temperature
until extraction. One fraction of 50 g of the powdered twigs was
exhaustively extracted by maceration with MeOH (w/v ratio of 1:5)
at room temperature for 48 h. The filtrate was dried under vacuum
using a rotary evaporator, and the residue that dissolved in water
was extracted with hexane. The aqueous solution was further
extracted with chloroform. Finally the chloroform solution was
brought to dryness (residual weight 133.6 mg).
Cell culture and treatments
Human colon carcinoma cells (Caco2), obtained from
the American Type Culture Collection (ATCC; Rockville, MD, USA),
were cultured in Dulbecco's modified essential medium (Gibco-BRL
Life Technologies) supplemented with 10% foetal calf serum, 1
mmol/l sodium pyruvate, 2 mmol/l L-glutamine, streptomycin (50
mg/ml) and penicillin (50 U/ml).
The cells were plated at a constant density to
obtain identical experimental conditions in the different tests,
thus to achieve a high accuracy of the measurements. After a 24-h
incubation at 37°C under a humidified 5% carbon dioxide atmosphere
to allow cell attachment, the cells were treated with different
concentrations of the chloroformic extract of twigs from Celtis
aetnensis (Tornab.) Strobl (5, 50, 100, 250 or 500
µg/ml), and incubated for 72 h under the same conditions.
Chloroformic extract was dissolved in dimethyl sulfoxide (DMSO) and
diluited with medium to give final concentrations of total extract
ranging from 5 to 500 µg/ml. Four replicates were performed
for each sample.
At the end of the treatment, the cells were scraped,
washed with PBS and immediately utilized for analysis.
MTT bioassay
MTT assay was performed to monitor cell viability,
measuring the conversion of tetrazolium salt to yield colored
formazan, the amount of which is proportional to the number of
living cells. For this assay, the cells were set up
(8×103 cells/well) in a 96-multiwell flat-bottomed 200
µl microplate (20). The
optical density of each well was measured with a microplate
spectrophotometer reader (Titertek Multiskan; Flow Laboratories,
Helsinki, Finland) at λ=570 nm.
Lactic dehydrogenase release
Lactic dehydrogenase (LDH) release was measured to
evaluate cell necrosis as a result of cell membrane disruption. LDH
activity was measured spectrophotometrically in the culture medium
and in the cellular lysates, at λ=340 nm by analyzing NADH
reduction (21). The percentage of
LDH release was calculated as the percentage of the total amount,
considered as the sum of the enzymatic activity present in the
cellular lysate and that in the culture medium.
Thiol group determination
Thiol groups (RSH) were measured using a
spectrophotometric assay as previously described (22). Results are expressed as
µmol/mg protein. Protein concentration was measured
according to Bradford (23).
Reactive oxygen species assay
Determination of reactive oxygen species (ROS) was
performed using a fluorescent probe 2′,7′-dichlorofluorescein
diacetate (DCFH-DA), as previously described (24). The fluorescence (corresponding to
the oxidized radical species 2′,7′-dichlorofluorescein, DCF) was
monitored spectrofluorometrically (excitation, λ=488 nm; emission,
λ=525 nm). The total protein content was evaluated for each sample,
and the results are reported as the percentage increase in
fluorescence intensity/mg protein with respect to the control
(untreated) cells.
HO-1 protein expression
A commercially available enzyme-linked immunosorbent
assay (ELISA) kit was used to measure HO-1 protein concentration in
the cellular lysates. The assay was performed in accordance with
the protocol provided by the manufacturer. Absorbance at λ=450 nm
was measured and HO-1 concentration was calculated from a standard
curve generated with purified HO-1 (25). The limits of detection provided by
the manufacturer were 0.78–25 ng/ml. Results are expressed as ng/mg
protein.
Western blottings
Caco2 cells were harvested using cell lysis buffer.
The lysate was collected for western blot analysis and protein
levels were visualized by immunoblotting with antibodies against
γ-GCS and caspase-3 as previously described (21).
Statistical analysis
One-way analysis of variance (ANOVA) followed by
Bonferroni's t-test was performed in order to estimate significant
differences among groups. Data are reported as mean values ± SD,
and differences between groups were considered to be significant at
P<0.005.
Results
Effects of Celtis aetnensis (Tornab.)
Strobl on Caco2 cell viability
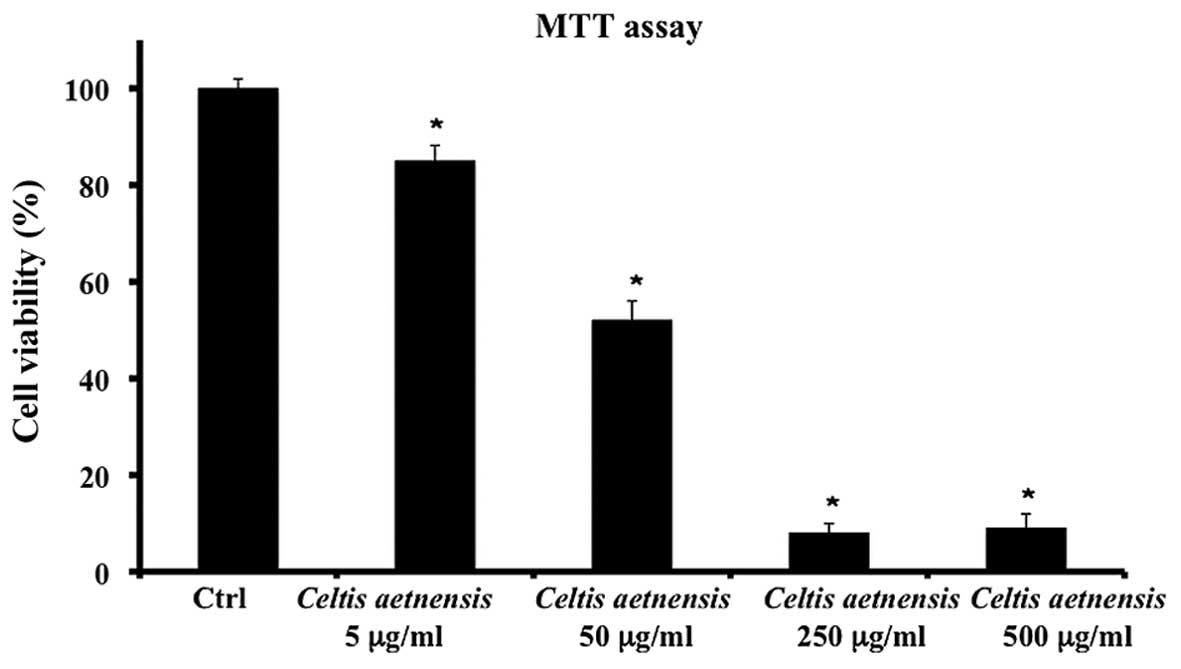
The treatment of Caco2 cells with 5, 50, 250 or 500
µg/ml of chloroformic extract of Celtis aetnensis
(Tornab.) Strobl for 72 h resulted in a significant and
dose-dependent reduction in cell viability (Fig. 1); since 250 and 500 µg/ml of
chloroformic extract showed similar effects in other experiments we
used 250 µg/ml of the extract.
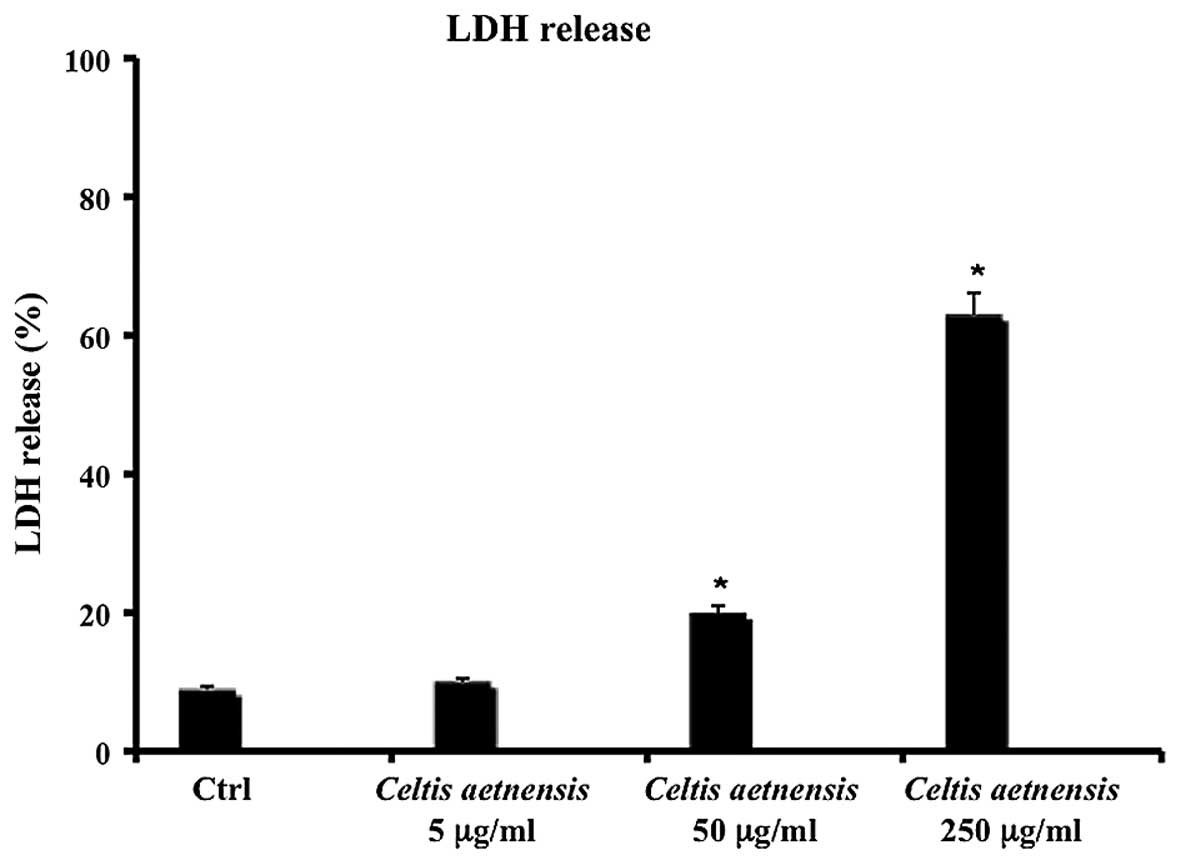
LDH release
Necrosis results in distruption of the cytoplasmic
membrane with release of cytoplasmic LDH and other cytotoxic
substances by necrotic cells into the medium. Thus, the existence
of LDH in culture medium represents an indirect index of the
membrane permeability of the treated cells. As shown in Fig. 2, 72 h of incubation with a
chloroformic extract of Celtis aetnensis (Tornab.) Strobl (5
µg/ml) did not cause LDH release, while a statistically
significant increase in LDH release was observed in the Caco2 cells
treated with 50 and 250 µg/ml of extract (Fig. 2).
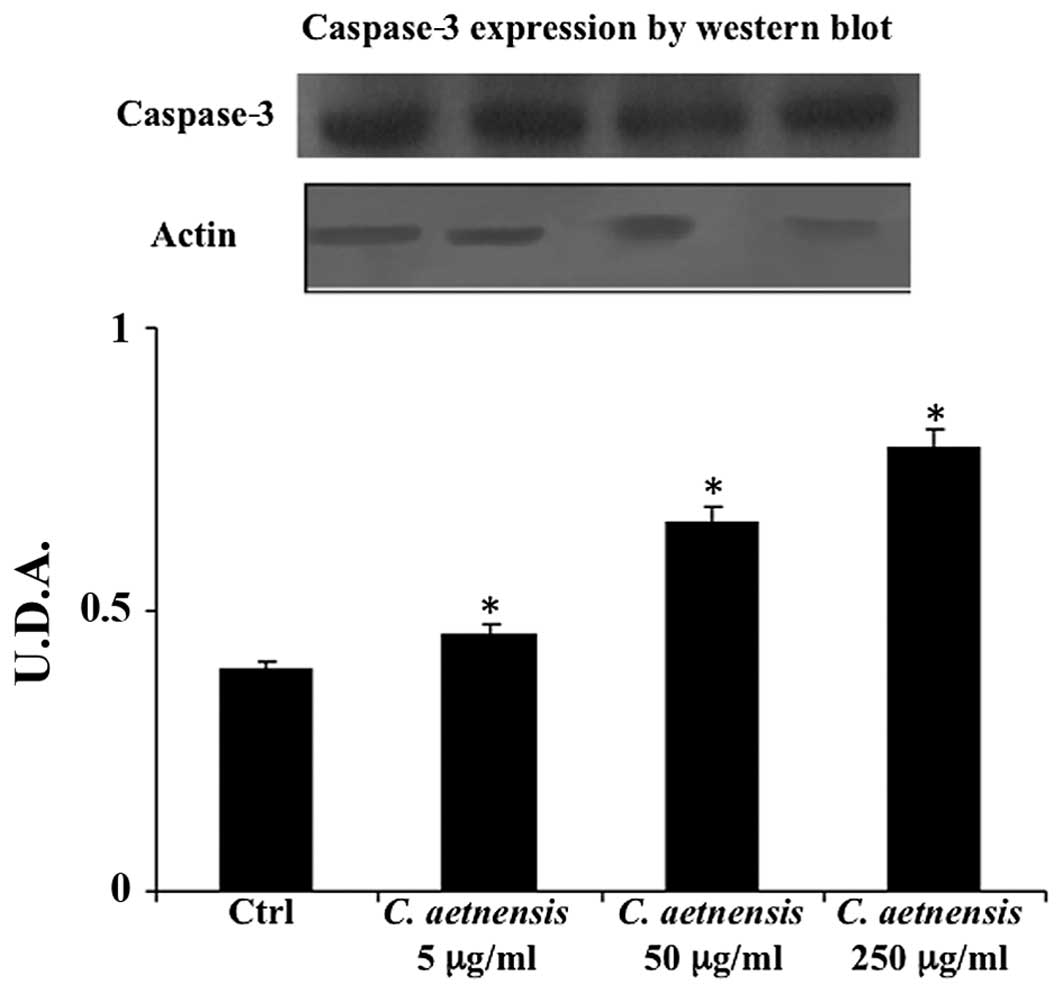
Caspase determination
Western blot analysis of caspase-3 is considered a
good marker of apoptosis. As shown in Fig. 3, the treatment of Caco2 cells with a
chloroformic extract of Celtis aetnensis (Tornab) Strobl
(5-50-250 µg/ml) induced apoptotic cell death. This effect
was also evident at the lowest dosage (5 µg/ml).
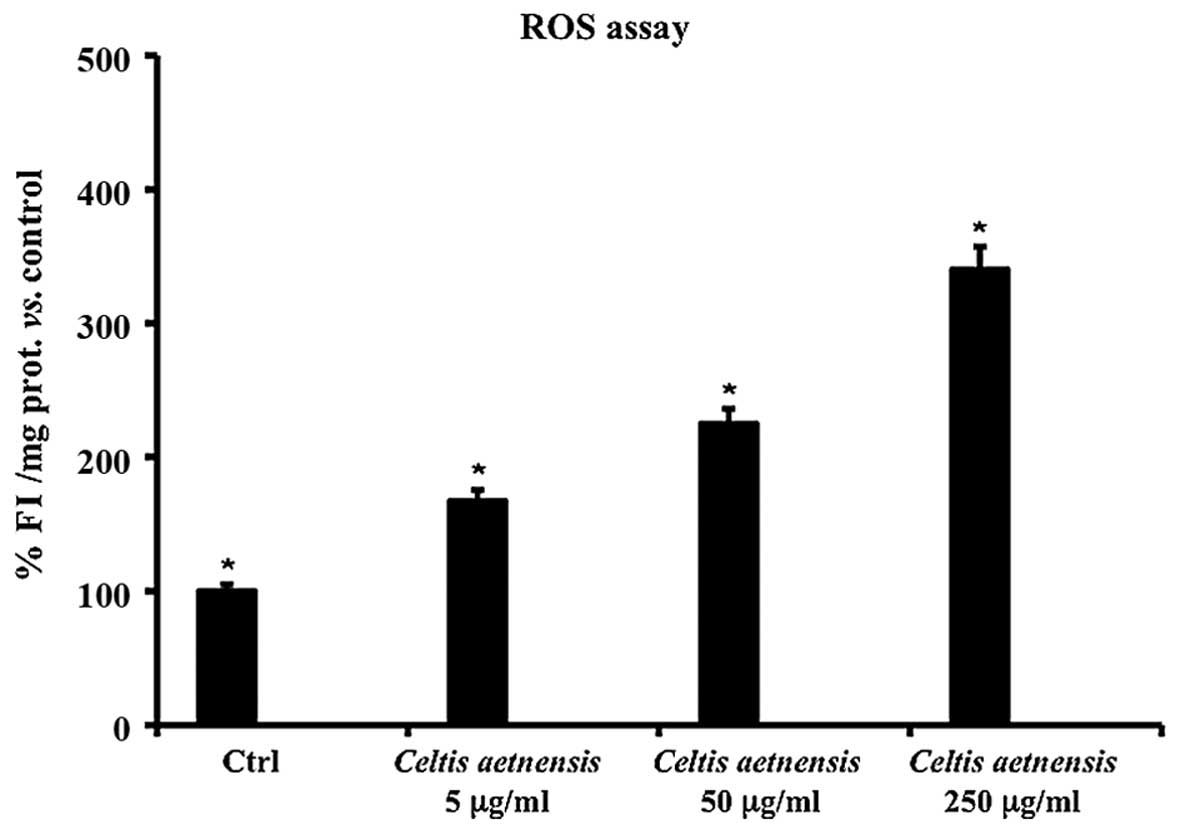
ROS assay
ROS are believed to be involved in cell death
induced by a variety of stimuli and various antitumoral agents. In
order to test the hypothesis that chloroformic extract-induced cell
death may be mediated by an elevation in ROS levels, a fluorescent
probe, DCFH-DA was used for ROS determination. This probe diffuses
into the cells, intracellular esterases hydrolyze the acetate
groups and the resulting DCFH then reacts with intracellular
oxidants resulting in the observed fluorescence.
The intensity of fluorescence (FI) is proportional
to the levels of intracellular oxidant species. As shown in
Fig. 4, the addition of the
chloroformic extract of Celtis aetnensis (Tornab.) Strobl at
5, 50 and 250 µg/ml for 72 h caused a significant increase
in FI with respect to the untreated Caco2 cells.
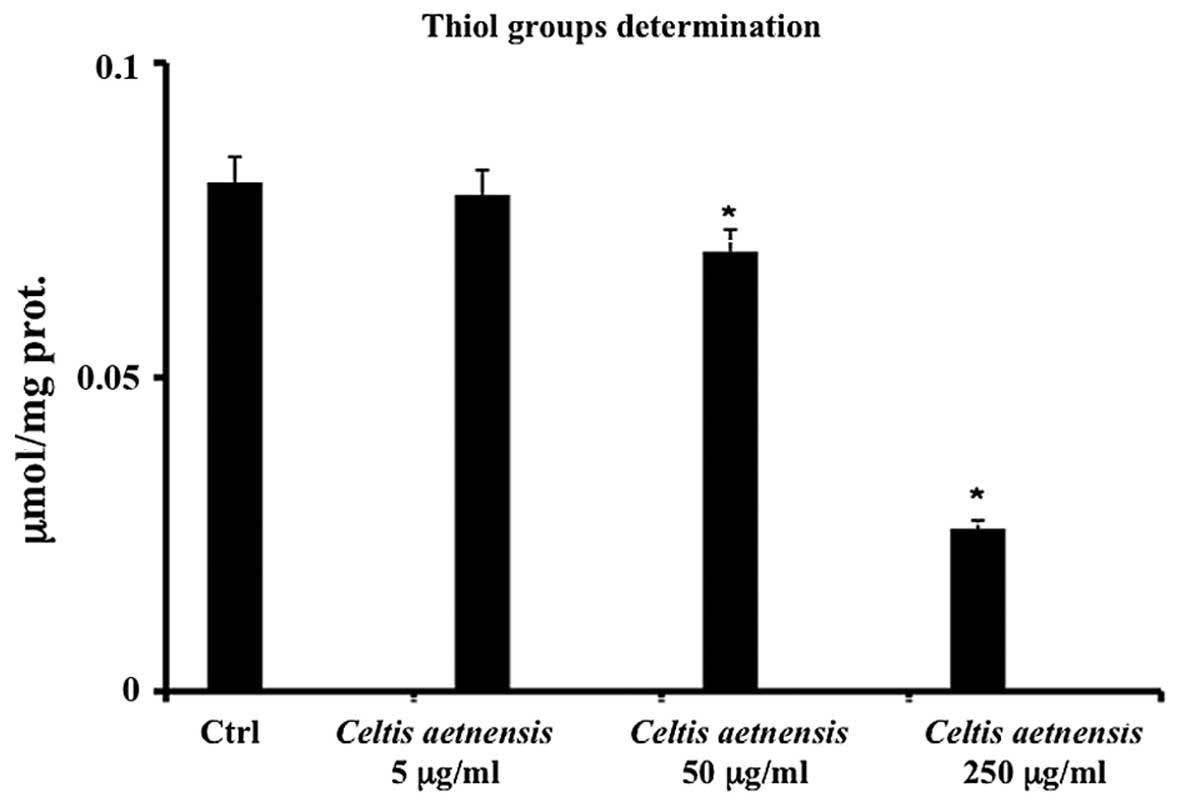
Thiol groups
In order to further confirm the involvement of
radical/oxidative species in the action mechanism of chloroformic
extract of Celtis aetnensis (Tornab.) Strobl, thiol group
levels (RSH) were measured in the Caco2 cells. The treatment of
these cells with 50–250 µg/ml of the chloroformic extract of
Celtis aetnensis (Tornab.) Strobl resulted in a significant
reduction in the levels of RSH (Fig.
5); the lowest concentration of the chloroformic extract of
Celtis aetnensis (Tornab.) Strobl did not alter the levels
of thiol groups RSH with respect to the control.
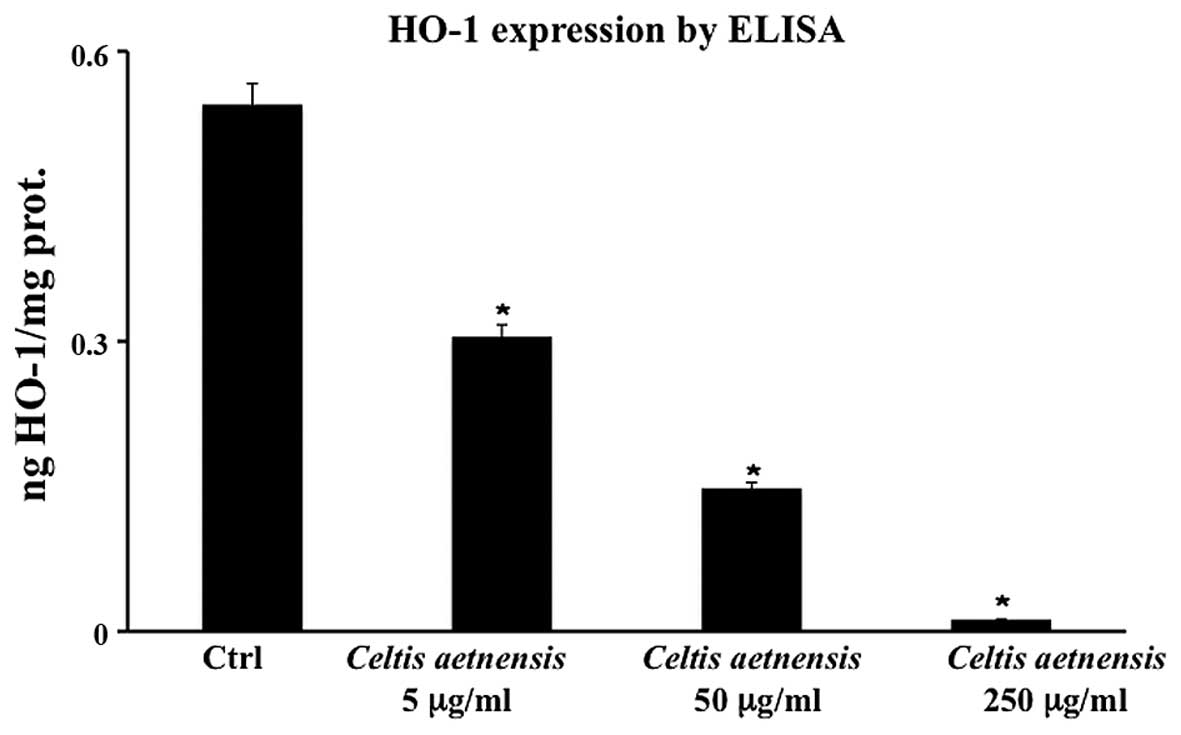
HO-1 by ELISA
Fig. 6 shows that
the addition of 5, 50 and 250 µg/ml of the extract to Caco2
cells, resulted in a significant decrease in HO-1 protein
expression which was undetectable at the highest concentration of
the extract (Fig. 6).
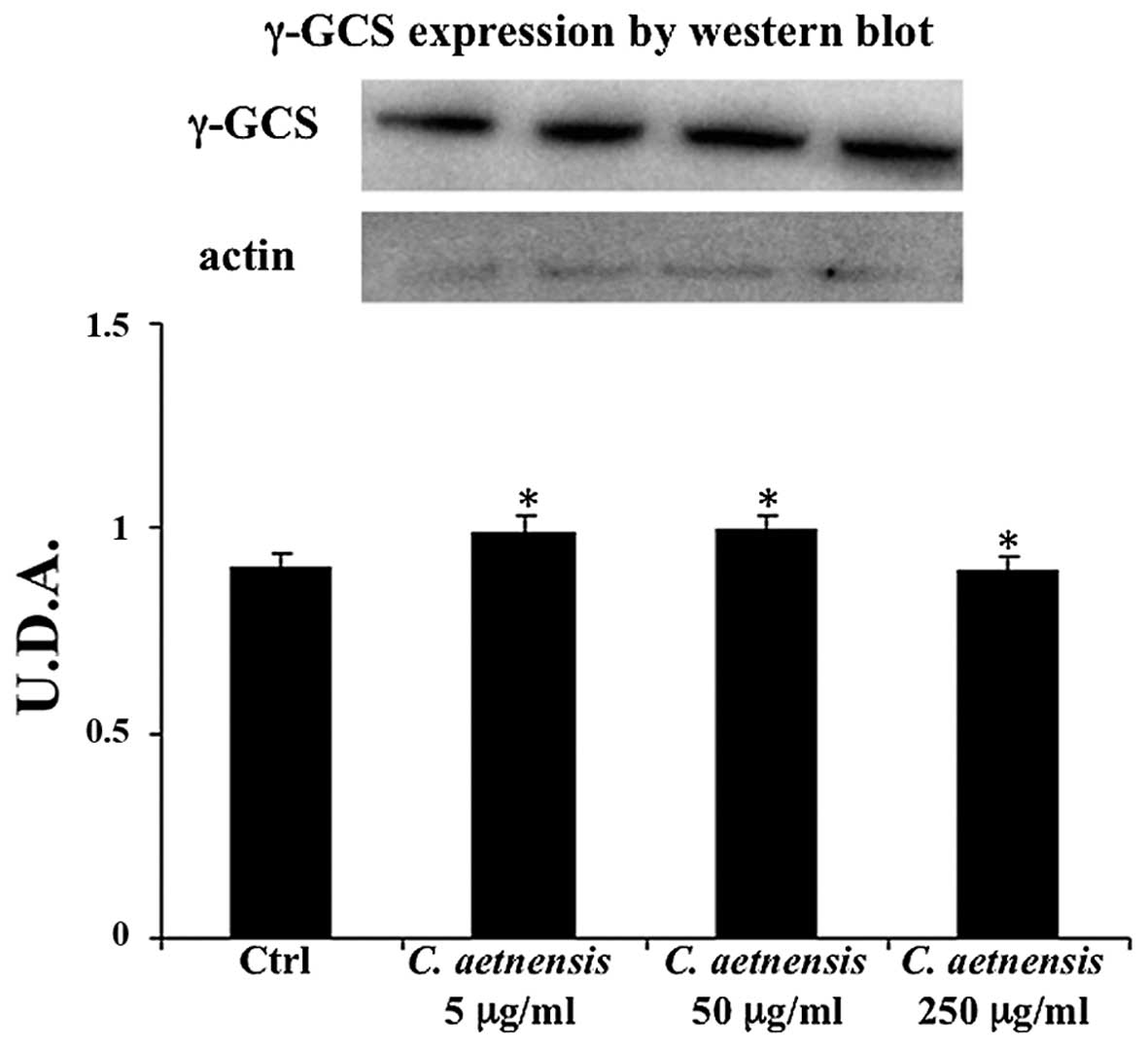
γ-GCS determination
No significant change in γ-GCS expression was
observed in the Caco2 cells treated with the extract of Celtis
aetnensis (Tornab.) Strobl with respect to the untreated cells
(Fig. 7).
Discussion
Cancer, a malignant tumor or a neoplasm, is a
generic term for a broad group of diseases that can affect any part
of the body via failure of regulation of cell mitosis. The
processes of cancer development are i) rapid and abnormal cell
division and growth, ii) formation of malignant tumors, iii)
invasion to nearby adjoining parts of the body, and iv) spread to
other organs through the lymphatic system and/or bloodstream.
Cancer remains one of the most threatening diseases worldwide
affecting human health and quality of life in spite of the many
recent advances in the knowledge of its molecular biology of
induction and progression.
Cancer cells differ from normal cells due to the
following properties: unlimited replication potential, absence of
apoptosis, absence of telomere shortening, angiogenesis and
metastasis. Colorectal carcinogenesis is related to the progressive
loss of homeostatic control of cell proliferation, differentiation
and apoptosis (26,27). The human body exerts protective
effects against tumor development mainly through apoptosis, cell
cycle arrest and immune responses. Apoptosis, also known as
programmed cell death, involves cell blebbing, shrinkage, nuclear
fragmentation, chromatin condensation and chromosomal DNA
fragmentation. It can be triggered by the activation of
tumor-suppressor genes, caspases, apoptosis-inducing factors,
cytotoxic T cells and natural killer (NK) cells via a Fas ligand-
or perforin/granzyme B-dependent pathway (28,29).
Natural compounds have been shown to affect
molecular events involved in the initiation, promotion and
progression of cancer, thereby inhibiting carcinogenesis.
Furthermore, their inhibitory activity may ultimately suppress the
final steps of carcinogenesis as well angiogenesis and
metastasis.
Previous studies on ethnomedicine, together with
extensive laboratory findings, indicate that flavonoids and
triterpenic compounds play important roles in the prevention and
treatment of cancer (10,30–33).
Because of their safety, low toxicity and general acceptance as
dietary supplements, fruits, vegetables, and other dietary elements
(phytochemicals and minerals) are being investigated for the
prevention of cancer. Extensive research over the past several
decades has identified numerous dietary and botanical natural
compounds with chemopreventive potential.
A number of reports have highlighted the important
role of pro-oxidants and/or antioxidants by natural and synthetic
compounds in chemo-prevention for many cancers (34,35).
Antioxidants have been thought to mainly suppress carcinogenesis
during the initiation phase, since most act as radical scavengers,
or inducers or inhibitors of xenobiotic metabolizing enzymes
including phase I and II enzymes (36). In addition, some radical scavengers
may also have pro-oxidative potential because of their conversion
to more reactive or stable radicals after reaction with ROS.
There is a growing interest in flavonoids and
triterpene esters and their synthetic derivatives due to their
possible applications in cancer chemotherapy as anticancer and
anti-inflammatory agents (37).
Recently, it has been reported that a chloroformic extract of C.
philippinensis twigs exhibited cytotoxic activity against the
KB (human oral epidermoid carcinoma) cell line (10).
According to these findings, the results obtained in
the present study showed that a chloroformic extract of Celtis
aetnensis (Tornab.) Strobl significantly reduced the cell
viability of Caco-2 cells and induced apoptotic and/or necrotic
cell death in a concentration-dependent manner (Fig. 1). In particular, a chloroformic
extract of Celtis aetnensis (Tornab.) Strobl induced
apoptosis at the lowest concentration (5 µM), while higher
dosages (50–250 µg/ml) were able to increase LDH release, a
marker of necrotic death (Figs. 2
and 3). Here, the reported data
regarding the MTT assay, LDH release and caspase expression suggest
that the chloroformic extract of Celtis aetnensis (Tornab.)
Strobl penetrated the cancer cells with consequent apoptosis and or
necrosis depending on the concentration.
The involvement of ROS in necrotic/apoptotic cell
death induced by different agents, such as oxidants, toxicants or
drugs, has been suggested (38). It
is interesting to note that many human cancer cell types exist in a
highly oxidative state due to decreased antioxidant protective
enzyme levels compared to their normal tissue counterparts. Thus,
cancer cells may be more sensitive to ROS generation within the
cells. Measurement of the cellular content of ROS after exposure to
different concentrations of chloroformic extract of Celtis
aetnensis (Tornab.) Strobl demostrated a significant
dose-dependent increase in the levels of ROS (Fig. 4).
These results suggest that in the Caco-2 tumor cell
line the chloroformic extract of Celtis aetnensis (Tornab.)
Strobl acted as a pro-oxidant rather than as an antioxidant. These
observations indicate that this extract may act indirectly and that
its action may be mediated by other intracellular factors, likely
targets of ROS.
These results are in agreement with other authors
who demonstrated that phenolic compounds with high reducing ability
may also act as pro-oxidants, thus generating ROS (39). The pro-oxidant activity of
chloroformic extract of Celtis aetnensis (Tornab.) Strobl in
Caco-2 cells was confirmed by results regarding thiol group
determination; these endogenous antioxidants act concurrently by
scavenging and/or reducing free radicals, breaking the peroxidative
chain and thus allowing the repair of oxidatively damaged molecules
(Fig. 5). The present study
demonstrated that exposure of Caco2 cells to a chloroformic extract
of Celtis aetnensis (Tornab.) Strobl induced a significant
dose-dependent decrease in RSH levels suggesting that the
antioxidant system was not able to buffer the overproduction of ROS
(Fig. 5). The intracellular
decrease in RSH content appears to be a central event in Caco2 cell
death induced by the chloroformic extract of Celtis
aetnensis (Tornab.) Strobl. suggesting an interference of
flavonoid and triterpenic compounds on the oxidant/antioxidant cell
balance with consequent cell damage.
This study also evaluated the expression of HO-1,
one of the most effective mechanisms for cellular protection
against oxidative stress. The expression of HO-1 is increased by
several types of cell stress including redox signals, heme, UV
radiation, heavy metals, cytokines and ROS (40,41).
The role of HO-1 in cancer biology is far from completely
understood. In cancer, HO-1 has been described as a pro-tumoral
molecule due to its anti-apoptotic effects on colon cancer and
hepatoma in murine models and its proangiogenic effects in human
pancreatic cancer (42,43). By contrast, in human tongue cancer,
low HO-1 expression has been associated with an increased risk of
developing lymph node metastasis (44).
In this study, we observed that the extract of
Celtis aetnensis (Tornab.) Strobl decreased the expression
of HO-1 (Fig. 6). Since HO-1
expression represents an important protective endogenous mechanism,
its reduced expression, together with low RSH levels and high ROS
production, may contribute to cell death.
To better understand the actions of the Celtis
aetnensis (Tornab.) Strobl extract, we also evaluated γ-GCS
expression, the rate-limiting enzyme in gluthatione synthesis,
which can be considered as one of the major antioxidant
enzymes.
A shown in Fig. 7
the expression of γ-GCS was not modified in the Celtis
aetnensis-treated Caco-2 cells suggesting that lowered GSH
levels, observed following extract treatment may be due to increase
ROS generation rather than to inhibition of glutathione synthesis.
Thus, the chloroformic extract of Celtis aetnensis (Tornab.)
Strobl, inducing a decrease in antioxidant defenses, acts on Caco2
cells which are susceptible to oxidative damage, as a potentially
powerful promoter of oxidative processes.
It is reasonable to hypothesize that some of the
compounds present in Celtis aetnensis (Tornab.) Strobl
extract, which have been shown to afford considerable protection
against cancer by inhibiting, for example, oxidative stress, may
also exhibit anticancer effects. Thus, our study supports the
growing body of data suggesting the bioactivities of Celtis
aetnensis (Tornab.) Strobl and its potential impact on cancer
therapy and on human health. Identification of natural
chemopreventive compounds is urgently needed to help the further
design and administration of preclinical and clinical trials.
Acknowledgments
The authors would like to thank Dr M. Wilkinson
(Research Assistant) for proofreading the manuscript.
References
|
1
|
Moulton JE: Tumors of alimentary tract.
Tumors in Domestic Animals. Moulton JE III: University of
California Press; Berkeley: pp. 240–272. 1977
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Worldwide variations in colorectal cancer. CA
Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fung TT and Brown LS: Dietary patterns and
the risk of colorectal cancer. Curr Nutr Rep. 2:48–55. 2013.
View Article : Google Scholar :
|
|
5
|
Armstrong B and Doll R: Environmental
factors and cancer incidence and mortality in different countries,
with special reference to dietary practices. Int J Cancer.
15:617–631. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Key TJ: Fruit and vegetables and cancer
risk. Br J Cancer. 104:6–11. 2011. View Article : Google Scholar :
|
|
7
|
World Cancer Research Fund and American
Institute for Cancer Research: WCRF/AICR Systematic Literature
Review Continuous Update Project Report: The Associations between
Food. (Nutrition and Physical Activity and the Risk of Colorectal
Cancer). 2011, http://www.wcrf.org/int/research-we-fund/cancer-prevention-recommendations/pant-foodsurisimplewww.wcrf.org/int/research-we-fund/cancer-prevention-recommendations/pant-foods.
Access date: June 2015.
|
|
8
|
Etkin NL: Medicinal cuisines: Diet and
ethnopharmacology. Int J Pharmacogn. 34:313–326. 1996. View Article : Google Scholar
|
|
9
|
Lee JE: Vitamin D and colorectal cancer
prevention: A review of epidemiologic studies. Curr Nutr Rep.
2:27–36. 2013. View Article : Google Scholar
|
|
10
|
Hwang BY, Chai HB, Kardono LBS, Riswan S,
Farnsworth NR, Cordell GA, Pezzuto JM and Kinghorn AD: Cytotoxic
triterpenes from the twigs of Celtis philippinensis.
Phytochemistry. 62:197–201. 2003. View Article : Google Scholar
|
|
11
|
Pignatti S: Flora d'Italia. Edagricole;
Bologna: pp. 1221997
|
|
12
|
Chari VM, Neelakantan S and Seshadri TR:
Chemical components of Betula utilis and Celtis australis. Indian J
Chem. 6:231–234. 1968.
|
|
13
|
Borges FFV, Machado TC, Cunha KS, Pereira
KC, Costa EA, De Paula JR and Chen-Chen L: Assessment of the
cytotoxic, genotoxic, and antigenotoxic activities of Celtis
iguanaea (Jacq) in mice. An Acad Bras Cienc. 85:955–964. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Santa-Cruz LH, Turner CE, Knapp JE, Schiff
PL Jr and Slatkin DJ: Moretenol and other constituents of Celtis
laevigata. Phytochemistry. 14:2532–2533. 1975. View Article : Google Scholar
|
|
15
|
Adedapo AA, Jimoh FO, Afolayan AJ and
Masika PJ: Antioxidant properties of the methanol extracts of the
leaves and stems of Celtis africana. Rec Nat Prod. 3:23–31.
2009.
|
|
16
|
El-Alfy TS, El-Gohary HM, Sokkar NM, Hosny
M and Al-Mahdy DA: A new flavonoid C-Glycoside from Celtis
australis L. and Celtis occidentalis L. leaves and potential
antioxidant and cytotoxic activities. Sci Pharm. 79:963–975. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weisburger JH: Antimutagenesis and
anticarcinogenesis, from the past to the future. Mutat Res.
480–481:23–35. 2001. View Article : Google Scholar
|
|
18
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohshima H, Tatemichi M and Sawa T:
Chemical basis of inflammation-induced carcinogenesis. Arch Biochem
Biophys. 417:3–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Acquaviva R, Campisi A, Murabito P, Raciti
G, Avola R, Mangiameli S, Musumeci I, Barcellona ML, Vanella A and
Li Volti G: Propofol attenuates peroxynitrite-mediated DNA damage
and apoptosis in cultured astrocytes: An alternative protective
mechanism. Anesthesiology. 101:1363–1371. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Acquaviva R, Di Giacomo C, Sorrenti V,
Galvano F, Santangelo R, Cardile V, Gangia S, D'Orazio N, Abraham
NG and Vanella L: Antiproliferative effect of oleuropein in
prostate cell lines. Int J Oncol. 41:31–38. 2012.PubMed/NCBI
|
|
22
|
Di Giacomo C, Acquaviva R, Sorrenti V,
Vanella A, Grasso S, Barcellona ML, Galvano F, Vanella L and Renis
M: Oxidative and antioxidant status in plasma of runners: effect of
oral supplementation with natural antioxidants. J Med Food.
12:145–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russo A, Borrelli F, Campisi A, Acquaviva
R, Raciti G and Vanella A: nitric oxide-related toxicity in
cultured astrocytes: Effect of Bacopa monniera. Life Sci.
73:1517–1526. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Volti G, Galvano F, Frigiola A,
Guccione S, Di Giacomo C, Forte S, Tringali G, Caruso M, Adekoya OA
and Gazzolo D: Potential immunoregulatory role of heme oxygenase-1
in human milk: A combined biochemical and molecular modeling
approach. J Nutr Biochem. 21:865–871. 2010. View Article : Google Scholar
|
|
26
|
Bastide P, Darido C, Pannequin J, Kist R,
Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande
F, et al: Sox9 regulates cell proliferation and is required for
Paneth cell differentiation in the intestinal epithelium. J Cell
Biol. 178:635–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Panza A, Pazienza V, Ripoli M, Benegiamo
G, Gentile A, Valvano MR, Augello B, Merla G, Prattichizzo C,
Tavano F, et al: Interplay between SOX9, β-catenin and PPARγ
activation in colorectal cancer. Biochim Biophys Acta.
1833:1853–1865. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang WM, Chan E, Kwok CY, Lee YK, Wu JH,
Wan CW, Chan RYK, Yu PHF and Chan SW: A review of the anticancer
and immunomodulatory effects of Lycium barbarum fruit.
Inflammopharmacology. 20:307–314. 2012. View Article : Google Scholar
|
|
30
|
Sarkar FH, Li Y, Wang Z and Kong D: Novel
targets for prostate cancer chemoprevention. Endocr Relat Cancer.
17:R195–R212. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reyes FJ, Centelles JJ, Lupiáñez JA and
Cascante M: (2Alpha,3beta)-2,3-dihydroxyolean-12-en-28-oic acid, a
new natural triterpene from Olea europea, induces caspase dependent
apoptosis selectively in colon adenocarcinoma cells. FEBS Lett.
580:6302–6310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murakami S, Takashima H, Sato-Watanabe M,
Chonan S, Yamamoto K, Saitoh M, Saito S, Yoshimura H, Sugawara K,
Yang J, et al: Ursolic acid, an antagonist for transforming growth
factor (TGF)-beta1. FEBS Lett. 566:55–59. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conner EM and Grisham MB: Inflammation,
free radicals, and antioxidants. Nutrition. 12:274–277. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang SY and Jiao H: Scavenging capacity of
berry crops on superoxide radicals, hydrogen peroxide, hydroxyl
radicals, and singlet oxygen. J Agric Food Chem. 48:5677–5684.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hirano T, Abe K, Gotoh M and Oka K: Citrus
flavone tangeretin inhibits leukaemic HL-60 cell growth partially
through induction of apoptosis with less cytotoxicity on normal
lymphocytes. Br J Cancer. 72:1380–1388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
World Cancer Research Fund and American
Institute for Cancer Research: Patterns of diet and cancer. Food,
Nutrition and the Prevention of Cancer: A Global Perspective.
American Institute for Cancer Research; Washington, DC: pp.
430–471. 1997
|
|
39
|
Wlodek L and Steven HZ: Antioxidants,
programmed cell death, and cancer. Nutr Res. 21:295–307. 2001.
View Article : Google Scholar
|
|
40
|
Choi AM and Alam J: Heme oxygenase-1:
Function, regulation, and implication of a novel stress-inducible
protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol.
15:9–19. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Camhi SL, Alam J, Wiegand GW, Chin BY and
Choi AM: Transcriptional activation of the HO-1 gene by
lipopolysaccharide is mediated by 5′ distal enhancers: Role of
reactive oxygen intermediates and AP-1. Am J Respir Cell Mol Biol.
18:226–234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sunamura M, Duda DG, Ghattas MH, Lozonschi
L, Motoi F, Yamauchi J, Matsuno S, Shibahara S and Abraham NG: Heme
oxygenase-1 accelerates tumor angiogenesis of human pancreatic
cancer. Angiogenesis. 6:15–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hill M, Pereira V, Chauveau C, Zagani R,
Remy S, Tesson L, Mazal D, Ubillos L, Brion R, Asghar K, et al:
Heme oxygenase-1 inhibits rat and human breast cancer cell
proliferation: Mutual cross inhibition with indoleamine
2,3-dioxygenase. FASEB J. 19:1957–1968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yanagawa T, Omura K, Harada H, Nakaso K,
Iwasa S, Koyama Y, Onizawa K, Yusa H and Yoshida H: Heme
oxygenase-1 expression predicts cervical lymph node metastasis of
tongue squamous cell carcinomas. Oral Oncol. 40:21–27. 2004.
View Article : Google Scholar
|