Despite the decline in the morbidity of gastric
cancer (GC) in recent years, GC remains the fourth most common
cancer and the second-leading cause of cancer-related death
globally with >700,000 human deaths per year (1,2). The
occurrence of depression challenges cancer treatment and acts as an
underlying indicator of advanced stages and poor prognoses of
cancer patients (3). Depression
care for cancer patients can improve the clinical benefit,
including the treatment response (4,5).
Nevertheless, there are currently no clear theories to explain the
molecular mechanism by which depression is associated with poor
prognoses among cancer patients. As an insult to the human body,
depression causes oxidative stress (OS) (6), which subsequently leads to the
excessive generation of reactive oxygen species (ROS) (7). Gastric tissues are particularly
vulnerable to ROS (8). Excessive
ROS that exceed the scavenging ability of human body trigger
carcinogenesis by activating diverse proto-oncogenes, including ABL
proto-oncogene 1 (ABL1) (9,10).
ABL1 is a non-receptor tyrosine kinase that consists
of the N-terminal Src homology 3 (SH3) domain, Src homology 2 (SH2)
domain, kinase domains, and the C-terminal actin-binding domain
(ABD) (11). ABL1 was upregulated
in GC and colorectal cancer patients with depression, and patients
with major depressive disorder (MDD) (12,13).
Activated ABL1 can regulate nuclear factor (erythroid-derived
2)-like 2 (NRF2) to function in an adaptive manner to react to OS
(14). Additionally, activated ABL1
can consequently lead to dysfunctions of its downstream targets and
corresponding signaling pathways, e.g., the mitogen-activated
protein kinase (MAPK) and mechanistic target of rapamycin (mTOR),
which contributes to the promotion of the development of GC and
results in poor prognosis (14–17).
Therefore, we will collect the evidence that ROS-activated ABL1 is
responsible for the poor prognoses of GC patients with depression.
Because inhibitors of ABL1, such as vandetanib, are widely used in
clinical tumor treatment, clearly understanding the ABL1 in the
process of GC will provide new methods to improve the prognoses of
GC patients with depression.
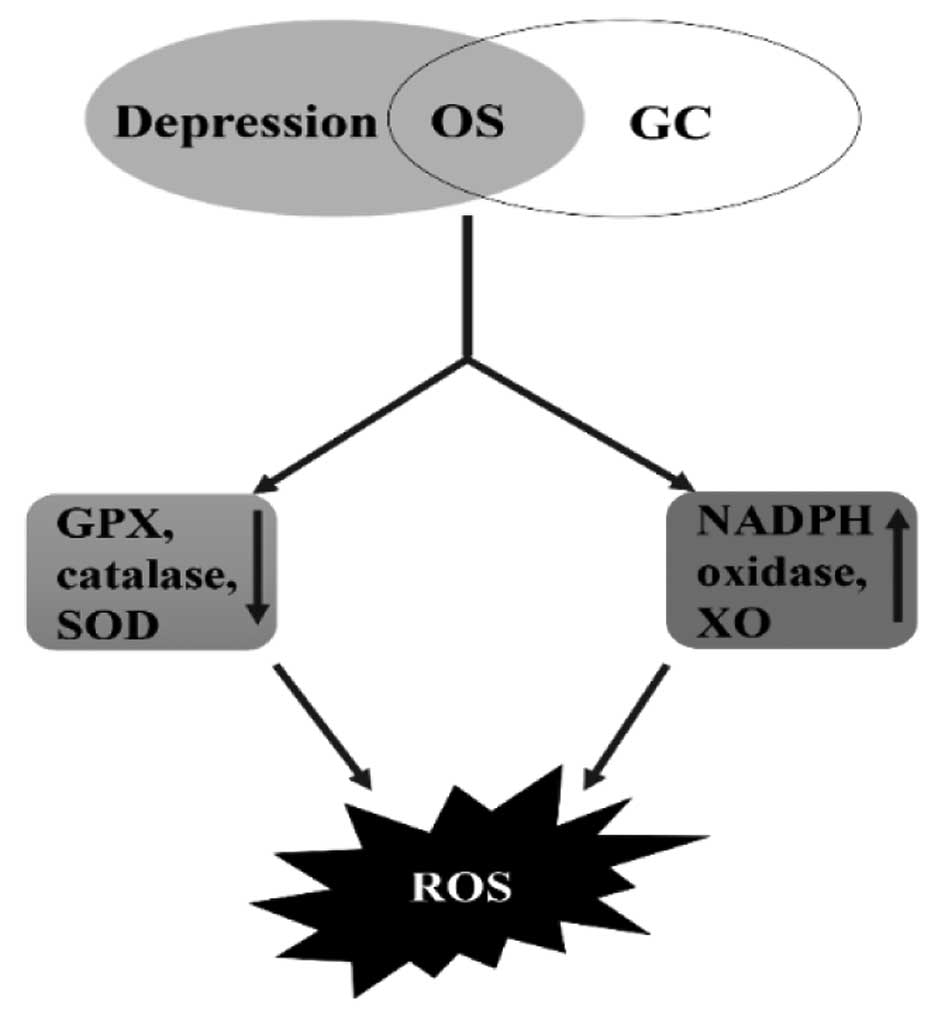
Emerging evidence suggests that OS works as primary
crosslink between depression and GC. OS is primarily caused by the
imbalance between oxidation and antioxidation in vivo. The
recognized marker of OS 8-hydroxydeoxyguano-sine (8-OHdG) is
positively correlated with Hospital Anxiety and Depression Scale
(HADS)-D scores (30). Under OS,
ROS levels are particularly raised among patients with depression.
Decreased antioxidants and elevated oxidants enhance the generation
of ROS in patients with depression. Clinical studies have revealed
that depressive patients exhibit decreased levels of antioxidants,
including glutathione peroxidase (GPX), catalase and superoxide
dismutase (SOD), and these antioxidants exhibit ROS scavenging
activities (31–33). Patients with depression also exhibit
elevated levels of oxidants, such as nicotinamide adenine
dinucleotide phosphate (NADPH) oxidase and xanthine oxidase (XO),
which leads to the production of ROS (34–37).
Indeed, antidepressant treatment has been reported to reduce MDA
(6). Our team discovered that
8-OHdG is significantly elevated in the sera of cancer patients,
including GC patients, with depression compared with those without
depression (13,21). All of the above changes could lead
to the excessive generation of ROS (Fig. 1).
ABL1 is well known for its characteristics related
to chronic myelogenous leukemia (CML) in the form of BCR-ABL1,
which mediates abnormal myeloproliferation and leads to the
development of CML (38). As a
non-receptor tyrosine kinase, ABL1 is expressed in the cytoplasm
and the nucleus and is involved in the processes of cell
differentiation, proliferation, adhesion, and stress responses
(39). Similar to other protein
kinases, ABL1 is activated by a ligand and functions through
interactions with downstream targets. Vitally, the expression of
ABL1 is context-dependent and is upregulated in response to OS and
DNA damage (10,14,40).
In diverse cancers with OS that are accompanied by DNA damage,
including CML, acute lymphoblastic leukemia (ALL), kidney, breast,
ovarian and colorectal cancers, as well as GC, ABL1 is highly
expressed (14,38,41–45).
Thus, this system is a perfect model to probe the activation and
the activities of ABL1 in fumarate hydratase-deficient kidney
cancer. Fumarate hydratase deficiency results in the accumulation
of fumarate, which subsequently leads to elevated NADPH oxidase
levels and enhanced generation of ROS (10,14).
ROS levels are positively associated with the expression of ABL1.
Hydrogen peroxide, a type of ROS, promotes ABL1 expression, and the
ROS scavenger N-acetylcysteine (NAC) could inhibit ABL1
expression. Therefore, the metabolic maladjustment-associated
accumulation of ROS is the primary cause of the activation of ABL1.
Indeed, in some types of cancer, ABL1 has been found to be
associated with the level of ROS. ABL1 expression in these cancers
is ROS-dependent. OS and excessive ROS generation are
well-documented contributors to carcinogenesis in GC (33,46).
In GC, ABL1 and c-Src tyrosine kinase (CSK) kinases phosphorylate
cytotoxin-associated gene A (CagA), which is vital in the initial
and development of gastric carcinogenesis (47). The activity of ABL1 is enhanced in
the GTL-16 cell line (48).
Therefore, the ROS generated in GC development might lead to the
activation of ABL1. ROS can regulate the expression of ABL1;
however, the potential mechanism is unknown.
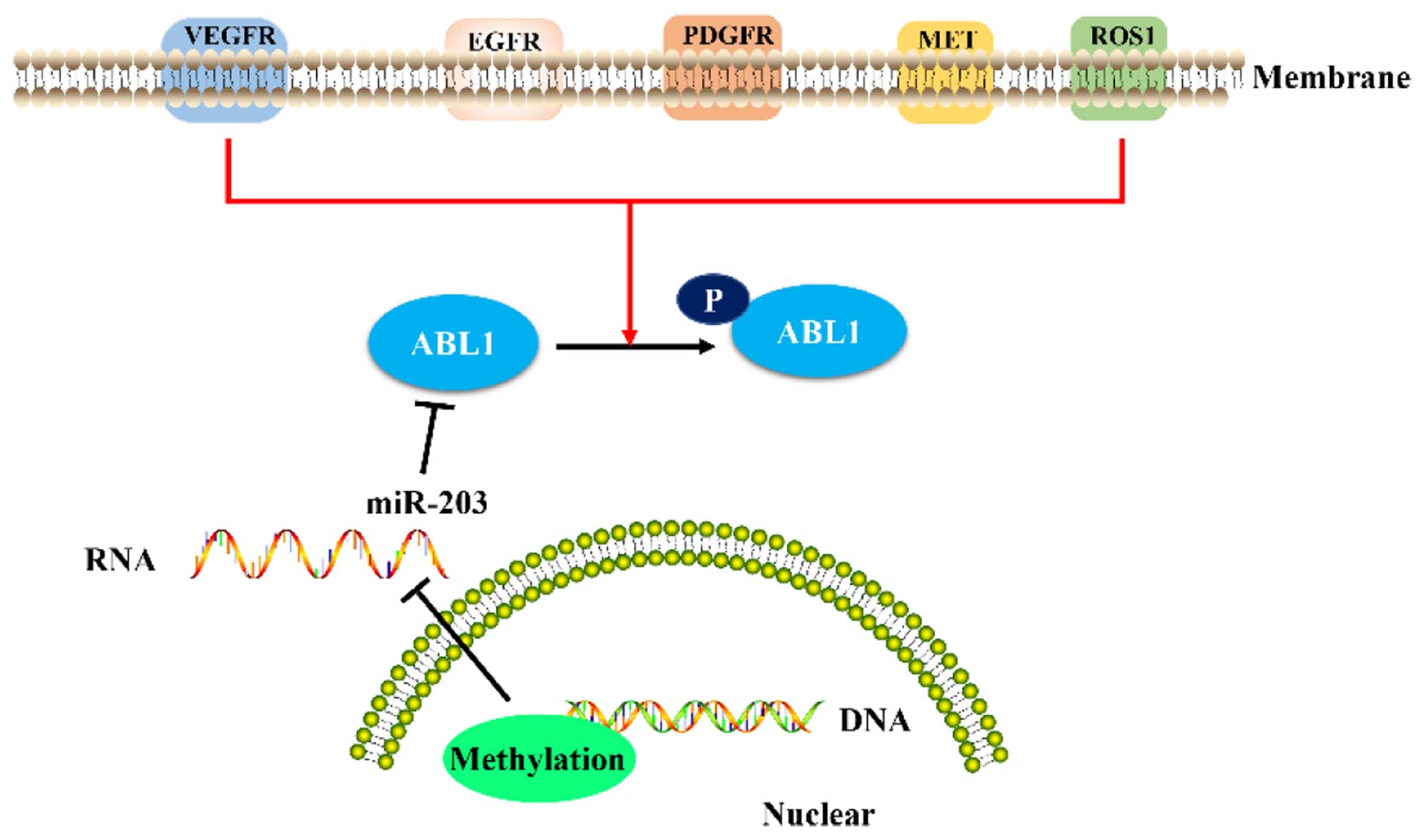
The regulation of gene expression is a complex
process, and only one report has discussed expression of ABL1 in GC
as a target of miR-203. Epigenetically silenced miR-203 releases
the expression of ABL1 to promote gastric lymphomagenesis (45). Furthermore, the increased expression
of ABL1 by epigenetically silenced miR-203 has also been confirmed
in CML (49,50). In Helicobacter pylori
(Hp)-induced GC, miR-203 has been confirmed to be downregulated.
Therefore, miR-203 may be a reason for the high level of ABL1 in
GC. As mentioned above, the expression of ABL1 is ROS-dependent,
and ROS can influence methylation status by sustaining the
stability of hypoxia-inducible factor 1α (HIF-1α) (51), which increases the expression of the
DNMT enzymes DNMT1 and DNMT3B, which keep gene silenced by
hyper-methylation (52).
Interestingly, HIF-1α can also result in the hypo-methylation of
genes by regulating the MAT1A/MAT2A switch (53). Given the role of ROS in the
epigenetic regulation of gene expression, the expression of ABL1
may be mediated by the ROS-related hyper-methylation of miR-203.
Moreover, although the methylation of ABL1 in GC has not been
clarified, in CML, dynamic methylation changes in the ABL1 promoter
have been clearly identified (54,55).
ROS might supervise the expression of ABL1 by directly influencing
the methylation status of ABL1. Additionally, the activity of ABL1
can be triggered by receptor tyrosine kinases (RTKs), including
epidermal growth factor receptor (EGFR), platelet-derived growth
factor receptor (PDGFR), erb-b2 receptor tyrosine kinase 2 (ERBB2),
vascular endothelial growth factor receptor (VEGFR), MET, c-ros
oncogene 1, and receptor tyrosine kinase (ROS1), and their
substrates (9,19,20).
Importantly, EGFR, PDGFR, VEGFR, MET, and ROS1 have been proven to
be elevated in GC (56–60). Moreover, some RTKs, such as EGFR,
are regulated by ROS, and MET has been confirmed to interact with
ABL1 (48,61). Therefore, ROS may affect the
expression of ABL1 through a combination of a variety of mechanisms
(Fig. 2). Additional studies should
be designed to identify the detailed mechanism of the regulation of
the expression of ABL1 by ROS in GC.
The function of ABL1 most strongly depends on its
biological structure. As a tyrosine kinase, the N-terminal SH2
regulates the activation of kinase domains via contact with the
SH2/N-lobe interface (62,63). The N-terminal SH3 is regulated by
Ras and Rab interactor 1 (RIN1) and also influences the activation
of ABL1 (64), and the kinase
domain is involved its major activities. Based on its structure,
ABL1 activates its substrates and associated signaling pathway to
promote gastric tumorigenesis.
Secondly, GC cells exhibit features of the enduring
activation of the Kirsten rat sarcoma viral oncogene homolog
(K-ras)/extracellular signal-regulated kinase (ERK) signaling
pathway (71). The ERK signaling
pathway can directly induce the proliferation of cancer cell
(72). Additionally, ERK also
mediates the expression of miR-21, and increased in miR-21 decrease
programmed cell death 4 (PDCD4) levels, which subsequently leads to
anti-apoptosis and transformation effect on GC cells (73,74).
Additionally, PTEN is also a target of miR-21, and decreased levels
of PTEN account for the proliferation of GC cells (75). Indeed, studies have revealed that
ABL1 can directly interact with RAS and activate the RAS/ERK
signaling pathway to promote carcinogenesis of GC (76). Hence, ROS-dependent ABL1 triggers
the RAS/ERK signaling pathway to promote the proliferation of GC
cells directly or by affecting miR-21.
Third, in GC, hypoxia leads to the generation of ROS
that prevent the degradation of HIF-1α (51). As a transcription factor, the
accumulation of HIF-1α can modulate the expression of genes that
are important in gastric tumorigenesis. For example, HIF-1α
attenuates caveolin-1 (Cav-1) and leads to the
epithelial-mesenchymal transition (EMT) in GC by regulating
E-cadherin (77). HIF-1α activates
the vascular endothelial growth factor (VEGF) pathway to enhance
angiogenesis in GC (78). Crosstalk
between HIF-1α and the tumor suppressor gene p53 has also been
discovered, and the expressions of both of these factors are
correlated with ROS (79,80). HIF-1α also induces miR-328 to
inhibit the activity of PTEN, which then releases the suppression
of the mTOR signaling pathway to promote GC cell survival (81). Notably, ROS-dependent ABL1 directly
communicates with p53 in the GC cell line GTL-16 to promote gastric
tumorigenesis (48). Moreover,
there is also evidence that ABL1 can enhance the activity of HIF-1α
via the mTOR signaling pathway (14). Therefore, ABL1 participates in parts
of the mechanism of gastric carcinogenesis by affecting HIF-1α.
Finally, as discussed above, ABL1 can be activated
by RTK, and RTK can be activated and act as a substrate of ABL1.
One thoroughly investigated factor in GC is CSK (82). Humar et al found that CSK was
activated in mesenchyme-like cancer cells and that CSK activation
is a central event that is required for the development of early
diffuse GC. More importantly, the activation of CSK is mediated by
ABL1 via the activation of the Src homology 2 domain-containing 1
(SHC1) of CSK (15).
Phosphatidylinositol 3-kinase (PI3K) is also a substrate of ABL1,
and the induction of PI3K can trigger the PI3K/v-akt murine thymoma
viral oncogene homolog 1 (AKt)/mTOR signaling pathway, which can
result in the development of GC (1,15).
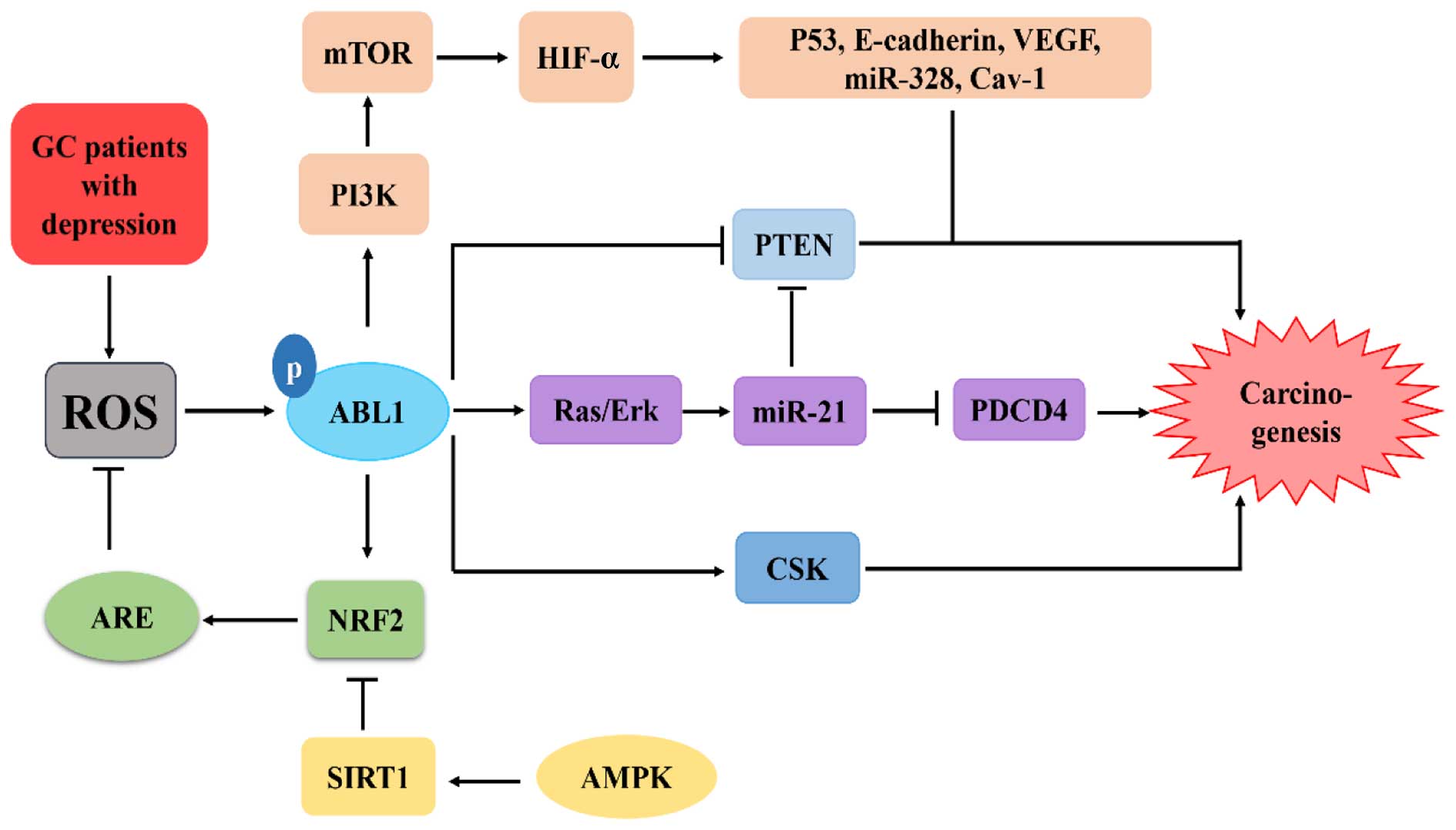
For GC patients, excessive ROS are produced due to
low levels of antioxidants and high levels of oxidants (83). Moreover, the occurrence of
depression among GC patients enhances the production of ROS via a
similar mechanism (13,84). ROS act to directly increase the
expression of ABL1 (9,14,85).
Additionally, ROS also trigger tyrosine kinase inhibitor (TKI) to
increase the activity of ABL1 (34,61).
Importantly, in Hp infection-associated GC, ABL1 functions as a
proto-oncogene by phosphorylating CagA to promote tumorigenesis
(47,86). Taken together, these findings
indicate that ABL1 contributes to the protection of cells from ROS
by inducing NRF2 and enhancing the activity of HIF-1α to catalyze
its substrate and corresponding substrates to promote
carcinogenesis of GC patients with depression (Fig. 4).
ABL1 regulates cell proliferation in Hp-induced
gastric B-cell lymphomas in mucosa-associated lymphoid tissue
(MALT) lymphoma, and blocking the activity of ABL1 with imatinib
can inhibit cell proliferation (45). GTL16 is a subclone of MKN45 that is
characterized as 'Met-addicted' (87). In GTL16, ABL1 has been proven to be
activated by Met, and the activation of ABL1 then mediates the
phosphorylation of p53 by p38-MAPKs, which results in the
overexpression of MDM2, which in turn promotes cell proliferation.
Similarly, the effect of ABL1 in GTL16 can be interfered with by
imatinib and shRNA interference (48). As discussed above, GC patients with
depression have high levels of ROS, and ABL1 may be directly
regulated by ROS-related methylation or miR-203, or the
ROS-activated ligands of ABL1 may act to indirectly promote the
activation of ABL1. Therefore, treatments with TKIs may be
clinically beneficial for GC patients with depression.
Collectively, considering the above clinical
results, depression is a world-class problem and is frequent among
cancer patients. Depression in GC patients is associated with
shorter survival times. Worse, there are no ideal drugs for
clinical depression. Therefore, the probing of the underlying
molecular changes associated with depression in cancer patients
represents a new option. We focus our studies on the role of OS and
its product ROS in GC and depression and conclude that ROS serve as
a crosslink for both; i.e., ROS mediate a mutual promotion process
for depression and GC. In this review, we elucidated the molecular
mechanism of the poor prognosis of GC patients with depression and
found that ABL1, which is a non-receptor tyrosine kinase, may
function as a crucial factor in the development of GC and the poor
prognoses of GC patients with depression. High ROS levels in GC
patients lead to the activation of ABL1, and ABL1 then induces NRF2
to protect the cells from ROS injury. ABL1 results in tumorigenesis
in GC by phosphorylating its substrates and activating signaling
pathways. Considering that inhibitors of ABL1 have been widely used
in the treatment of CML, it is possible to assess the value of ABL1
in the treatment of GC patients with depression.
However, because ABL1 is widely expressed in the
human body and is required for the maintenance of normal
physiological functions, e.g., tissue development and immune
function, it is difficult to avoid the side-effects of ABL1
inhibitors that are related to these physiological functions.
Furthermore, the activity of ABL1 is tissue-specific and
context-dependent; thus, future explorations of ABL1 should
primarily concentrate on research into the biological processes in
which ABL1 functions rather than employing old gene-centric styles.
Nevertheless, probing the mediation of the ABL1-dependent
phosphorylation of GC should elucidate some of the reasons for
GC-related cell death. Given the accumulated evidence, the
FDA-approved status of TKIs, and the experience of patients with
other cancers, including CML, AML, lung and breast cancer,
following exposure to these drugs, ABL represents a prospective
target for future treatments for GC patients with depression.
This project was supported by the Natural Science
Foundation of Shaanxi Province (no. 99SM50), the National Natural
Science Foundation of China (nos. 81171288 and 81673033) for the
research of OS and depression, and the Fundamental Research Funds
for the Central Universities (no. 0601-08143036).
|
1
|
Riquelme I, Saavedra K, Espinoza JA, Weber
H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC and Bizama C:
Molecular classification of gastric cancer: Towards a
pathway-driven targeted therapy. Oncotarget. 6:24750–24779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong JS and Tian J: Prevalence of anxiety
and depression and their risk factors in Chinese cancer patients.
Support Care Cancer. 22:453–459. 2014. View Article : Google Scholar
|
|
3
|
Delgado-Guay M, Ferrer J, Rieber AG,
Rhondali W, Tayjasanant S, Ochoa J, Cantu H, Chisholm G, Williams
J, Frisbee-Hume S, et al: Financial distress and its associations
with physical and emotional symptoms and quality of life among
advanced cancer patients. Oncologist. 20:1092–1098. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walker J, Hansen CH, Martin P, Symeonides
S, Gourley C, Wall L, Weller D, Murray G and Sharpe M; SMaRT
(Symptom Management Research Trials) Oncology-3 Team: Integrated
collaborative care for major depression comorbid with a poor
prognosis cancer (SMaRT Oncology-3): A multicentre randomised
controlled trial in patients with lung cancer. Lancet Oncol.
15:1168–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andersen BL, DeRubeis RJ, Berman BS,
Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K,
Somerfield MR, et al American Society of Clinical Oncology:
Screening, assessment, and care of anxiety and depressive symptoms
in adults with cancer: An American Society of Clinical Oncology
guideline adaptation. J Clin Oncol. 32:1605–1619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiménez-Fernández S, Gurpegui M,
Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M and Correll CU:
Oxidative stress and antioxidant parameters in patients with major
depressive disorder compared to healthy controls before and after
antidepressant treatment: Results from a meta-analysis. J Clin
Psychiatry. 76:1658–1667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hermes-Lima M, Moreira DC, Rivera-Ingraham
GA, Giraud-Billoud M, Genaro-Mattos TC and Campos EG: Preparation
for oxidative stress under hypoxia and metabolic depression:
Revisiting the proposal two decades later. Free Radic Biol Med.
89:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaturvedi R, de Sablet T, Asim M,
Piazuelo MB, Barry DP, Verriere TG, Sierra JC, Hardbower DM,
Delgado AG, Schneider BG, et al: Increased Helicobacter
pylori-associated gastric cancer risk in the Andean region of
Colombia is mediated by spermine oxidase. Oncogene. 34:3429–3440.
2015. View Article : Google Scholar :
|
|
9
|
Warsch W, Grundschober E, Berger A, Gille
L, Cerny-Reiterer S, Tigan AS, Hoelbl-Kovacic A, Valent P, Moriggl
R and Sexl V: STAT5 triggers BCR-ABL1 mutation by mediating ROS
production in chronic myeloid leukaemia. Oncotarget. 3:1669–1687.
2012. View Article : Google Scholar
|
|
10
|
No authors listed. FH-deficient cancers
depend on ABL1-mediated metabolic adaptation. Cancer Discov.
5:OF92015. View Article : Google Scholar
|
|
11
|
Wang JY: The capable ABL: What is its
biological function? Mol Cell Biol. 34:1188–1197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yi Z, Li Z, Yu S, Yuan C, Hong W, Wang Z,
Cui J, Shi T and Fang Y: Blood-based gene expression profiles
models for classification of subsyndromal symptomatic depression
and major depressive disorder. PLoS One. 7:e312832012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei YC, Zhou FL, He DL, Bai JR, Hui LY,
Wang XY and Nan KJ: The level of oxidative stress and the
expression of genes involved in DNA-damage signaling pathways in
depressive patients with colorectal carcinoma. J Psychosom Res.
66:259–266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sourbier C, Ricketts CJ, Matsumoto S,
Crooks DR, Liao PJ, Mannes PZ, Yang Y, Wei MH, Srivastava G, Ghosh
S, et al: Targeting ABL1-mediated oxidative stress adaptation in
fumarate hydratase-deficient cancer. Cancer Cell. 26:840–850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolch W and Pitt A: Functional proteomics
to dissect tyrosine kinase signalling pathways in cancer. Nat Rev
Cancer. 10:618–629. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv B, Song C, Wu L, Zhang Q, Hou D, Chen
P, Yu S, Wang Z, Chu Y, Zhang J, et al: Netrin-4 as a biomarker
promotes cell proliferation and invasion in gastric cancer.
Oncotarget. 6:9794–9806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Demitrack ES, Gifford GB, Keeley TM,
Carulli AJ, VanDussen KL, Thomas D, Giordano TJ, Liu Z, Kopan R and
Samuelson LC: Notch signaling regulates gastric antral LGR5 stem
cell function. EMBO J. 34:2522–2536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tavoli A, Mohagheghi MA, Montazeri A,
Roshan R, Tavoli Z and Omidvari S: Anxiety and depression in
patients with gastrointestinal cancer: Does knowledge of cancer
diagnosis matter? BMC Gastroenterol. 7:282007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi M, Liu D, Duan H, Han C, Wei B, Qian
L, Chen C, Guo L, Hu M, Yu M, et al: Catecholamine up-regulates
MMP-7 expression by activating AP-1 and STAT3 in gastric cancer.
Mol Cancer. 9:2692010.PubMed/NCBI
|
|
20
|
Audier AG: Determination of a
constitutional neuroendocrine factor probably influencing tumor
development in man: Prophylactic and therapeutic aspects. Cancer
Detect Prev. 11:203–208. 1988.PubMed/NCBI
|
|
21
|
Wei YC, Zhou FL, He DL, Bai JR, Ding H,
Wang XY and Nan KJ: Oxidative stress in depressive patients with
gastric adenocarcinoma. Int J Neuropsychopharmacol. 12:1089–1096.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nordin K, Berglund G, Glimelius B and
Sjödén PO: Predicting anxiety and depression among cancer patients:
A clinical model. Eur J Cancer. 37:376–384. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayakawa Y, Ariyama H, Stancikova J,
Sakitani K, Asfaha S, Renz BW, Dubeykovskaya ZA, Shibata W, Wang H,
Westphalen CB, et al: Mist1 expressing gastric stem cells maintain
the normal and neoplastic gastric epithelium and are supported by a
perivascular stem cell niche. Cancer Cell. 28:800–814. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lim K, Hyun YM, Lambert-Emo K, Capece T,
Bae S, Miller R, Topham DJ and Kim M: Neutrophil trails guide
influenza-specific CD8+ T cells in the airways. Science.
349:aaa43522015. View Article : Google Scholar
|
|
25
|
Finisguerra V, Di Conza G, Di Matteo M,
Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H,
Granot Z, et al: MET is required for the recruitment of
anti-tumoural neutrophils. Nature. 522:349–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harker JA, Lewis GM, Mack L and Zuniga EI:
Late interleukin-6 escalates T follicular helper cell responses and
controls a chronic viral infection. Science. 334:825–829. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hodes GE, Kana V, Menard C, Merad M and
Russo SJ: Neuroimmune mechanisms of depression. Nat Neurosci.
18:1386–1393. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi Q, Smith TG, Michonski JD, Stein KD,
Kaw C and Cleeland CS: Symptom burden in cancer survivors 1 year
after diagnosis: A report from the American Cancer Society's
Studies of Cancer Survivors. Cancer. 117:2779–2790. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soubeyran P, Fonck M, Blanc-Bisson C,
Blanc JF, Ceccaldi J, Mertens C, Imbert Y, Cany L, Vogt L, Dauba J,
et al: Predictors of early death risk in older patients treated
with first-line chemotherapy for cancer. J Clin Oncol.
30:1829–1834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Zhang J, Yan J, Wang Y and Li Y:
Leucocyte telomere shortening in relation to newly diagnosed type 2
diabetic patients with depression. Oxid Med Cell Longev.
2014:6739592014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rybka J, Kędziora-Kornatowska K,
Banaś-Leżańska P, Majsterek I, Carvalho LA, Cattaneo A, Anacker C
and Kędziora J: Interplay between the pro-oxidant and antioxidant
systems and proinflammatory cytokine levels, in relation to iron
metabolism and the erythron in depression. Free Radic Biol Med.
63:187–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang SL, Yu PL and Chung KR: The
glutathione peroxidase-mediated reactive oxygen species resistance,
fungicide sensitivity and cell wall construction in the citrus
fungal pathogen Alternaria alternata. Environ Microbiol.
18:923–935. 2016. View Article : Google Scholar
|
|
33
|
Bhattacharyya A, Chattopadhyay R, Mitra S
and Crowe SE: Oxidative stress: An essential factor in the
pathogenesis of gastrointestinal mucosal diseases. Physiol Rev.
94:329–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan EC, Jiang F, Peshavariya HM and
Dusting GJ: Regulation of cell proliferation by NADPH
oxidase-mediated signaling: Potential roles in tissue repair,
regenerative medicine and tissue engineering. Pharmacol Ther.
122:97–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Ma L, Li Y and Cho CH: Exposure to
cigarette smoke increases apoptosis in the rat gastric mucosa
through a reactive oxygen species-mediated and p53-independent
pathway. Free Radic Biol Med. 28:1125–1131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seo JS, Park JY, Choi J, Kim TK, Shin JH,
Lee JK and Han PL: NADPH oxidase mediates depressive behavior
induced by chronic stress in mice. J Neurosci. 32:9690–9699. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maes M, Galecki P, Chang YS and Berk M: A
review on the oxidative and nitrosative stress (O&NS) pathways
in major depression and their possible contribution to the
(neuro)degenerative processes in that illness. Prog
Neuropsychopharmacol Biol Psychiatry. 35:676–692. 2011. View Article : Google Scholar
|
|
38
|
Holm F, Hellqvist E, Mason CN, Ali SA,
Delos-Santos N, Barrett CL, Chun HJ, Minden MD, Moore RA, Marra MA,
et al: Reversion to an embryonic alternative splicing program
enhances leukemia stem cell self-renewal. Proc Natl Acad Sci USA.
112:15444–15449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Colicelli J: ABL tyrosine kinases:
Evolution of function, regulation, and specificity. Sci Signal.
3:re62010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Keersmaecker K, Rocnik JL, Bernad R,
Lee BH, Leeman D, Gielen O, Verachtert H, Folens C, Munck S,
Marynen P, et al: Kinase activation and transformation by
NUP214-ABL1 is dependent on the context of the nuclear pore. Mol
Cell. 31:134–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jabbour E, Kantarjian H, Ravandi F, Thomas
D, Huang X, Faderl S, Pemmaraju N, Daver N, Garcia-Manero G, Sasaki
K, et al: Combination of hyper-CVAD with ponatinib as first-line
therapy for patients with Philadelphia chromosome-positive acute
lymphoblastic leukaemia: A single-centre, phase 2 study. Lancet
Oncol. 16:1547–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Greuber EK, Smith-Pearson P, Wang J and
Pendergast AM: Role of ABL family kinases in cancer: From leukaemia
to solid tumours. Nat Rev Cancer. 13:559–571. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Deb S, Wong SQ, Li J, Do H, Weiss J, Byrne
D, Chakrabarti A, Bosma T, Fellowes A, Dobrovic A, et al kConFab
Investigators: Mutational profiling of familial male breast cancers
reveals similarities with luminal A female breast cancer with rare
TP53 mutations. Br J Cancer. 111:2351–2360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cunningham JM, Vierkant RA, Sellers TA,
Phelan C, Rider DN, Liebow M, Schildkraut J, Berchuck A, Couch FJ,
Wang X, et al Ovarian Cancer Association Consortium: Cell cycle
genes and ovarian cancer susceptibility: A tagSNP analysis. Br J
Cancer. 101:1461–1468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Craig VJ, Cogliatti SB, Rehrauer H,
Wündisch T and Müller A: Epigenetic silencing of microRNA-203
dysregulates ABL1 expression and drives Helicobacter-associated
gastric lymphomagenesis. Cancer Res. 71:3616–3624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chiang YT, Yen YW and Lo CL: Reactive
oxygen species and glutathione dual redox-responsive micelles for
selective cytotoxicity of cancer. Biomaterials. 61:150–161. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Müller A: Multistep activation of the
Helicobacter pylori effector CagA. J Clin Invest. 122:1192–1195.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Furlan A, Stagni V, Hussain A, Richelme S,
Conti F, Prodosmo A, Destro A, Roncalli M, Barilà D and Maina F:
Abl interconnects oncogenic Met and p53 core pathways in cancer
cells. Cell Death Differ. 18:1608–1616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bueno MJ, Pérez de Castro I, Gómez de
Cedrón M, Santos J, Calin GA, Cigudosa JC, Croce CM,
Fernández-Piqueras J and Malumbres M: Genetic and epigenetic
silencing of microRNA-203 enhances ABL1 and BCR-ABL1 oncogene
expression. Cancer Cell. 13:496–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Faber J, Gregory RI and Armstrong SA:
Linking miRNA regulation to BCR-ABL expression: The next dimension.
Cancer Cell. 13:467–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park JH, Kim TY, Jong HS, Kim TY, Chun YS,
Park JW, Lee CT, Jung HC, Kim NK and Bang YJ: Gastric epithelial
reactive oxygen species prevent normoxic degradation of
hypoxia-inducible factor-1alpha in gastric cancer cells. Clin
Cancer Res. 9:433–440. 2003.PubMed/NCBI
|
|
52
|
Watson CJ, Collier P, Tea I, Neary R,
Watson JA, Robinson C, Phelan D, Ledwidge MT, McDonald KM, McCann
A, et al: Hypoxia-induced epigenetic modifications are associated
with cardiac tissue fibrosis and the development of a
myofibroblast-like phenotype. Hum Mol Genet. 23:2176–2188. 2014.
View Article : Google Scholar
|
|
53
|
Frau M, Feo F and Pascale RM: Pleiotropic
effects of methionine adenosyltransferases deregulation as
determinants of liver cancer progression and prognosis. J Hepatol.
59:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Asimakopoulos FA, Shteper PJ, Krichevsky
S, Fibach E, Polliack A, Rachmilewitz E, Ben-Neriah Y and
Ben-Yehuda D: ABL1 methylation is a distinct molecular event
associated with clonal evolution of chronic myeloid leukemia.
Blood. 94:2452–2460. 1999.PubMed/NCBI
|
|
55
|
Sun B, Jiang G, Zaydan MA, La Russa VF,
Safah H and Ehrlich M: ABL1 promoter methylation can exist
independently of BCR-ABL transcription in chronic myeloid leukemia
hematopoietic progenitors. Cancer Res. 61:6931–6937.
2001.PubMed/NCBI
|
|
56
|
Hayashi Y, Bardsley MR, Toyomasu Y,
Milosavljevic S, Gajdos GB, Choi KM, Reid-Lombardo KM, Kendrick ML,
Bingener-Casey J, Tang CM, et al: Platelet-derived growth factor
receptor-α regulates proliferation of gastrointestinal stromal
tumor cells with mutations in KIT by stabilizing ETV1.
Gastroenterology. 149:420–32.e16. 2015. View Article : Google Scholar
|
|
57
|
Wu Z, Zhang Z, Ge X, Lin Y, Dai C, Chang
J, Liu X, Geng R, Wang C, Chen H, et al: Identification of
short-form RON as a novel intrinsic resistance mechanism for
anti-MET therapy in MET-positive gastric cancer. Oncotarget.
6:40519–40534. 2015.PubMed/NCBI
|
|
58
|
Javle M, Smyth EC and Chau I: Ramucirumab:
Successfully targeting angiogenesis in gastric cancer. Clin Cancer
Res. 20:5875–5881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kuboki Y, Yamashita S, Niwa T, Ushijima T,
Nagatsuma A, Kuwata T, Yoshino T, Doi T, Ochiai A and Ohtsu A:
Comprehensive analyses using next-generation sequencing and
immunohistochemistry enable precise treatment in advanced gastric
cancer. Ann Oncol. 27:127–133. 2016. View Article : Google Scholar
|
|
60
|
Lee J, Lee SE, Kang SY, Do IG, Lee S, Ha
SY, Cho J, Kang WK, Jang J, Ou SH, et al: Identification of ROS1
rearrangement in gastric adenocarcinoma. Cancer. 119:1627–1635.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Höcker M, Rosenberg I, Xavier R, Henihan
RJ, Wiedenmann B, Rosewicz S, Podolsky DK and Wang TC: Oxidative
stress activates the human histidine decarboxylase promoter in AGS
gastric cancer cells. J Biol Chem. 273:23046–23054. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lorenz S, Deng P, Hantschel O,
Superti-Furga G and Kuriyan J: Crystal structure of an SH2-kinase
construct of c-Abl and effect of the SH2 domain on kinase activity.
Biochem J. 468:283–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dölker N, Górna MW, Sutto L, Torralba AS,
Superti-Furga G and Gervasio FL: The SH2 domain regulates c-Abl
kinase activation by a cyclin-like mechanism and remodulation of
the hinge motion. PLOS Comput Biol. 10:e10038632014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Thai M, Ting PY, McLaughlin J, Cheng D,
Müschen M, Witte ON and Colicelli J: ABL fusion oncogene
transformation and inhibitor sensitivity are mediated by the
cellular regulator RIN1. Leukemia. 25:290–300. 2011. View Article : Google Scholar :
|
|
65
|
Chung KS, Han G, Kim BK, Kim HM, Yang JS,
Ahn J, Lee K, Song KB and Won M: A novel antitumor piperazine alkyl
compound causes apoptosis by inducing RhoB expression via ROS
mediated c Abl/p38 MAPK signaling. Cancer Chemother Pharmacol.
72:1315–1324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chen CC, Chu CB, Liu KJ, Huang CY, Chang
JY, Pan WY, Chen HH, Cheng YH, Lee KD, Chen MF, et al: Gene
expression profiling for analysis acquired oxaliplatin resistant
factors in human gastric carcinoma TSGH-S3 cells: The role of IL-6
signaling and Nrf2/AKR1C axis identification. Biochem Pharmacol.
86:872–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K,
Yamamoto M, Talalay P and Kensler TW: Sensitivity to carcinogenesis
is increased and chemoprotective efficacy of enzyme inducers is
lost in nrf2 transcription factor-deficient mice. Proc Natl Acad
Sci USA. 98:3410–3415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cai N, Bigdeli TB, Kretzschmar W, Li Y,
Liang J, Song L, Hu J, Li Q, Jin W, Hu Z, et al CONVERGE
consortium: Sparse whole-genome sequencing identifies two loci for
major depressive disorder. Nature. 523:588–591. 2015. View Article : Google Scholar
|
|
69
|
Nabavi SF, Bilotto S, Russo GL, Orhan IE,
Habtemariam S, Daglia M, Devi KP, Loizzo MR, Tundis R and Nabavi
SM: Omega-3 polyunsaturated fatty acids and cancer: Lessons learned
from clinical trials. Cancer Metastasis Rev. 34:359–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cha EJ, Noh SJ, Kwon KS, Kim CY, Park BH,
Park HS, Lee H, Chung MJ, Kang MJ, Lee DG, et al: Expression of
DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Xing R, Li W, Cui J, Zhang J, Kang B, Wang
Y, Wang Z, Liu S and Lu Y: Gastrokine 1 induces senescence through
p16/Rb pathway activation in gastric cancer cells. Gut. 61:43–52.
2012. View Article : Google Scholar
|
|
72
|
Leto SM, Sassi F, Catalano I, Torri V,
Migliardi G, Zanella ER, Throsby M, Bertotti A and Trusolino L:
Sustained inhibition of HER3 and EGFR is necessary to induce
regression of HER2-amplified gastrointestinal carcinomas. Clin
Cancer Res. 21:5519–5531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Tu H, Sun H, Lin Y, Ding J, Nan K, Li Z,
Shen Q and Wei Y: Oxidative stress upregulates PDCD4 expression in
patients with gastric cancer via miR-21. Curr Pharm Des.
20:1917–1923. 2014. View Article : Google Scholar
|
|
74
|
Ling M, Li Y, Xu Y, Pang Y, Shen L, Jiang
R, Zhao Y, Yang X, Zhang J, Zhou J, et al: Regulation of miRNA-21
by reactive oxygen species-activated ERK/NF-κB in arsenite-induced
cell transformation. Free Radic Biol Med. 52:1508–1518. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shi Z, Zhang J, Qian X, Han L, Zhang K,
Chen L, Liu J, Ren Y, Yang M, Zhang A, et al: AC1MMYR2, an
inhibitor of dicer-mediated biogenesis of Oncomir miR-21, reverses
epithelial-mesenchymal transition and suppresses tumor growth and
progression. Cancer Res. 73:5519–5531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Albano F, Zagaria A, Anelli L, Coccaro N,
Impera L, Minervini CF, Minervini A, Rossi AR, Tota G, Casieri P,
et al: Gene expression profiling of chronic myeloid leukemia with
variant t(9;22) reveals a different signature from cases with
classic translocation. Mol Cancer. 12:362013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kannan A, Krishnan A, Ali M, Subramaniam
S, Halagowder D and Sivasithamparam ND: Caveolin-1 promotes gastric
cancer progression by up-regulating epithelial to mesenchymal
transition by crosstalk of signalling mechanisms under hypoxic
condition. Eur J Cancer. 50:204–215. 2014. View Article : Google Scholar
|
|
78
|
Rath S, Das L, Kokate SB, Pratheek BM,
Chattopadhyay S, Goswami C, Chattopadhyay R, Crowe SE and
Bhattacharyya A: Regulation of Noxa-mediated apoptosis in
Helicobacter pylori-infected gastric epithelial cells. FASEB J.
29:796–806. 2015. View Article : Google Scholar
|
|
79
|
Sumiyoshi Y, Kakeji Y, Egashira A,
Mizokami K, Orita H and Maehara Y: Overexpression of
hypoxia-inducible factor 1alpha and p53 is a marker for an
unfavorable prognosis in gastric cancer. Clin Cancer Res.
12:5112–5117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hammond EM and Giaccia AJ:
Hypoxia-inducible factor-1 and p53: Friends, acquaintances, or
strangers? Clin Cancer Res. 12:5007–5009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Seok JK, Lee SH, Kim MJ and Lee YM:
MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the
tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res.
42:8062–8072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Humar B, Fukuzawa R, Blair V, Dunbier A,
More H, Charlton A, Yang HK, Kim WH, Reeve AE, Martin I, et al:
Destabilized adhesion in the gastric proliferative zone and c-Src
kinase activation mark the development of early diffuse gastric
cancer. Cancer Res. 67:2480–2489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bauer G, Bereswill S, Aichele P and
Glocker E: Helicobacter pylori protects oncogenically transformed
cells from reactive oxygen species-mediated intercellular induction
of apoptosis. Carcinogenesis. 35:1582–1591. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mendes-da-Silva RF, Lopes-de-Morais AA,
Bandim-da-Silva ME, Cavalcanti GA, Rodrigues AR, Andrade-da-Costa
BL and Guedes RC: Prooxidant versus antioxidant brain action of
ascorbic acid in well-nourished and malnourished rats as a function
of dose: A cortical spreading depression and malondialdehyde
analysis. Neuropharmacology. 86:155–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bolton-Gillespie E, Schemionek M, Klein
HU, Flis S, Hoser G, Lange T, Nieborowska-Skorska M, Maier J,
Kerstiens L, Koptyra M, et al: Genomic instability may originate
from imatinib-refractory chronic myeloid leukemia stem cells.
Blood. 121:4175–4183. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wei J, O'Brien D, Vilgelm A, Piazuelo MB,
Correa P, Washington MK, El-Rifai W, Peek RM and Zaika A:
Interaction of Helicobacter pylori with gastric epithelial cells is
mediated by the p53 protein family. Gastroenterology.
134:1412–1423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bertotti A, Bracco C, Girolami F, Torti D,
Gastaldi S, Galimi F, Medico E, Elvin P, Comoglio PM and Trusolino
L: Inhibition of Src impairs the growth of met-addicted gastric
tumors. Clin Cancer Res. 16:3933–3943. 2010. View Article : Google Scholar : PubMed/NCBI
|