Introduction
Colorectal cancer (CRC) is the most common digestive
malignant and devastating primary tumor. Based on global estimates,
it is the third most commonly diagnosed cancer in males and the
second in females (1). Despite
early diagnosis and treatment such as surgery and chemotherapy,
colon cancer can reappear at a later time, even if the cancer was
entirely removed during the initial treatment. Therefore, the
current challenge is to identify new effective less toxic
chemotherapeutic agents that are need in treatment of colon
cancer.
Targeting programmed cell death (PCD) has become a
promising approach in the fight against cancer, which mainly
includes modulation of apoptosis and autophagy (2,3). Type
I PCD, apoptosis, is a biological process with a crucial role in
normal development and tissue homeostasis (4). Type II PCD, autophagic cell death, is
a highly conserved cellular degradation process characterized by
the presence of abundant intracellular autophagic vacuoles termed
autophagosomes. Autophagosomes participate in the recycling of
cellular components by sequestering damaged organelles and
misfolded proteins, targeting them for lysosomal degradation
(5–7). Apoptosis and autophagy are two
distinct processes, coordinately regulating cell survival and cell
death, and occur simultaneously in cancers (8,9).
Accumulated evidence has shown that apoptosis and autophagy is a
response to various anticancer therapies in many kinds of cancer
cells (10–12). In addition, the apoptosis and
autophagy mechanisms are involved in CRC and play an important role
in the multifactorial etiology of CRC (13). So the modulation of apoptosis and
autophagy might be applied in a potential cancer therapy for the
treatment of colon cancer cells.
Rhodiola rosea L, also known as ‘golden
root’, is a perennial herbaceous plant of the Crassulaceae
family, widely distributed at high-altitudes regions (14). It has long been used as adaptogen
traditional Chinese medicine (15).
Reports on the anticancer effect of Rhodiola extracts have
been published (16,17). Salidroside, a major component of
Rhodiola rosea, has been reported to have significant
antitumor effects, such as inhibiting cell proliferation, arresting
cell cycle, and promoting apoptosis of the human bladder, breast,
lung or liver cancer cells 16,18–21.
The existing evidence indicates that salidroside plays antitumor
role by inhibiting tumor metastasis, reducing new angiogenesis and
changing the tumor microenvironment (22–24).
In addition, it is also reported that it can inhibit proliferation,
decrease the migration and invasion of colon carcinoma SW1116 cells
in JAK2/STAT3-dependent pathway (25). However, the relative molecular
mechanisms still need to be studied.
Studies have found that salidroside could decrease
the growth of bladder cancer cell lines via inhibition of the mTOR
pathway and induction of autophagy (16). In addition, mTOR has emerged as an
effective target for colorectal cancer therapy (26). Increasing evidence demonstrates that
PI3K/Akt/mTOR signaling plays a key role in regulation of apoptosis
and autophagy, and targeting PI3K/Akt/mTOR signaling has been
proposed to be a promising strategy for cancer treatment (27–29).
Thus, this study aimed to investigate whether salidroside modulates
apoptosis and autophagy in HT29 human colon cancer cells and to
further elucidate the role of the PI3K/Akt/mTOR signaling in
regulation of cell death.
Materials and methods
Materials
Salidroside (purity >99%) was purchased from
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). RPMI-1640 was purchased from Gibco
(Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased
from Sijiqing (Hangzhou, China). MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide,
trypsin, Acridine orange (AO), LY294002, 3-methyladenine,
Bafilomycin A1, Hoechst 33342, antibodies for the detection of LC3
(#L7543) and Beclin-1 (#B6186) was purchased from Sigma (St. Louis,
MO, USA). Bcl-2 (#15071), Bax (#2772), PI3K (#4292), p-PI3K at
Tyr458 (#4228), Akt (#9272), p-Akt at Ser473 (#9271), mTOR (#2972),
p-mTOR at Ser2448 (#2971) and GAPDH (#5174) were purchased from
Cell Signaling Technology (Beverly, MA, USA). HRP-labeled goat
anti-rat IgG(H+L) (#A0192), HRP-labeled goat anti-rabbit IgG(H+L)
(#A0208), FITC-labeled goat anti-rabbit IgG (H+L) (#A0562) were
purchased from Beyotime Institution of Biotechnology (Haimen,
China).
Cell culture
The human colon cancer HT-29 cells were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). HT-29 cells were cultured in RPMI-1640 containing 10%
heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 mg/ml
streptomycin, and were kept at 37°C in a humidified atmosphere
composed of 5% CO2 and 95% air. Salidroside was diluted
in cell culture medium and regulated to final concentrations of
0.5, 1 and 2 mM, and cultured for the indicated time periods. To
investigate the mechanisms for salidroside-induced apoptosis and
autophagy, cells were pre-treated with the 10 µM LY294002 (a PI3K
inhibitor), 10 nM BA (an autophagy-lysosomal inhibitor) or 10 mM of
3-MA (an autophagy inhibitor) for 30 min, then co-treated with 2 mM
salidroside for further 48 h. Cells were treated with fresh medium
as vehicle control.
Cell proliferation assay
Cell proliferation was assayed by MTT. Briefly, the
cells were seeded in 96-well plates at a density of
1×104 cells/well. After treatment, 0.5 mg/ml MTT was
added to each well and the plates were incubated for another 4 h at
37°C. The formazan crystals were dissolved in dimethyl sulfoxide
(DMSO). Absorbance was determined at 550 nm on an ElX-800
MicroElisa reader (Bio-Tek Inc., Winooski, VT, USA). The cell
viability were expressed as a percentage of the controls.
Hoechst 33342 staining
To quantify and assess nuclear morphology, HT29
cells were cultured on 24-well culture plates. After treatment,
cells were fixed for 20 min with 4% paraformaldehyde in PBS at room
temperature. After staining for 10 min with 10 µg/ml Hoechst 33342,
the cells were visualized and photographed under a DMR fluorescence
microscope (Leica Microsystems, Wetzlar, Germany) with fluorescence
excitation at 340 nm and emission at 510 nm. The apoptotic index
was calculated as: [apoptotic cells number / total cells number] ×
100 (%). At least four different fields from each well were
selected to count ≥500 cells to calculate the rate of
apoptosis.
Immunofluorescence analysis of LC3
distribution
Cells (1×105 cells/cm2 in
24-well plates) were fixed in 4% paraformaldehyde for 30 min at
room temperature. Subsequently, the cells were permeabilized with
0.5% Triton X-100 and blocked with 1% bovine serum albumin in PBS
for 1 h, followed by incubation in anti-LC3 antibody (1:100)
overnight at 4°C, washed and incubated with FITC-labeled goat
anti-rabbit IgG (H+L) (1:500) for 2 h at 37°C, rinsed with PBS, and
counterstained with Hoechst 33342 for 10 min. Images were obtained
using a fluorescence microscope (488-nm filter; Olympus BX51,
Japan).
Acridine orange staining
Acridine orange staining was used to detect
autophagy induction. After seeding, HT-29 cells were washed with
phosphate-buffered saline (PBS), stained with 1 µg/ml acridine
orange for 15 min at 37°C. Photographs were obtained with a
fluorescence microscope (Axioscop, Carl Zeiss, Thomwood, NY, USA)
equipped with a mercury 100-W lamp, 490-nm band-pass blue
excitation filters, a 500-nm dichroic mirror and a 515-nm long-pass
barrier filter. Autophagic lysosomes appeared as orange/red
fluorescent cytoplasmic vesicles according to their acidity, while
the nuclei were stained green.
Western blot assay
Proteins (35 µg/sample) were separated by SDS-PAGE
and transferred onto nitrocellulose membranes (Millipore, Bedford,
MA, USA). Membranes were blocked with 5% non-fat milk for 1 h and
incubated with the following antibodies: LC3, Beclin-1, Bcl-2, Bax,
PI3K, p-PI3K at Tyr458, Akt, p-Akt at Ser473, mTOR, p-mTOR at
Ser2448 and GAPDH at 1:1,000 overnight at 4°C. The membranes were
washed with TBS/T (TBS with 0.05% Tween-20) and then incubated with
HRP-labeled goat anti-rat IgG(H+L) (1:3,000) or HRP-labeled goat
anti-rabbit IgG(H+L) (1:1,000) at room temperature for 1 h. The
reaction was visualized using ECL and detected using a Luminescent
Image Analyzer LAS-4000 mini. The images were quantified with Multi
Gauge. For each sample, band intensities were normalized to
GAPDH.
Statistical analysis
Statistical differences were evaluated by GraphPad
Prism 5.0 (San Diego, CA, USA). Statistical significance was
determined by one-way analysis of variance (ANOVA) and subsequent
Tukey's test. Differences were considered significant at p<0.05.
The data are expressed as mean ± SD of three independent
experiments.
Results
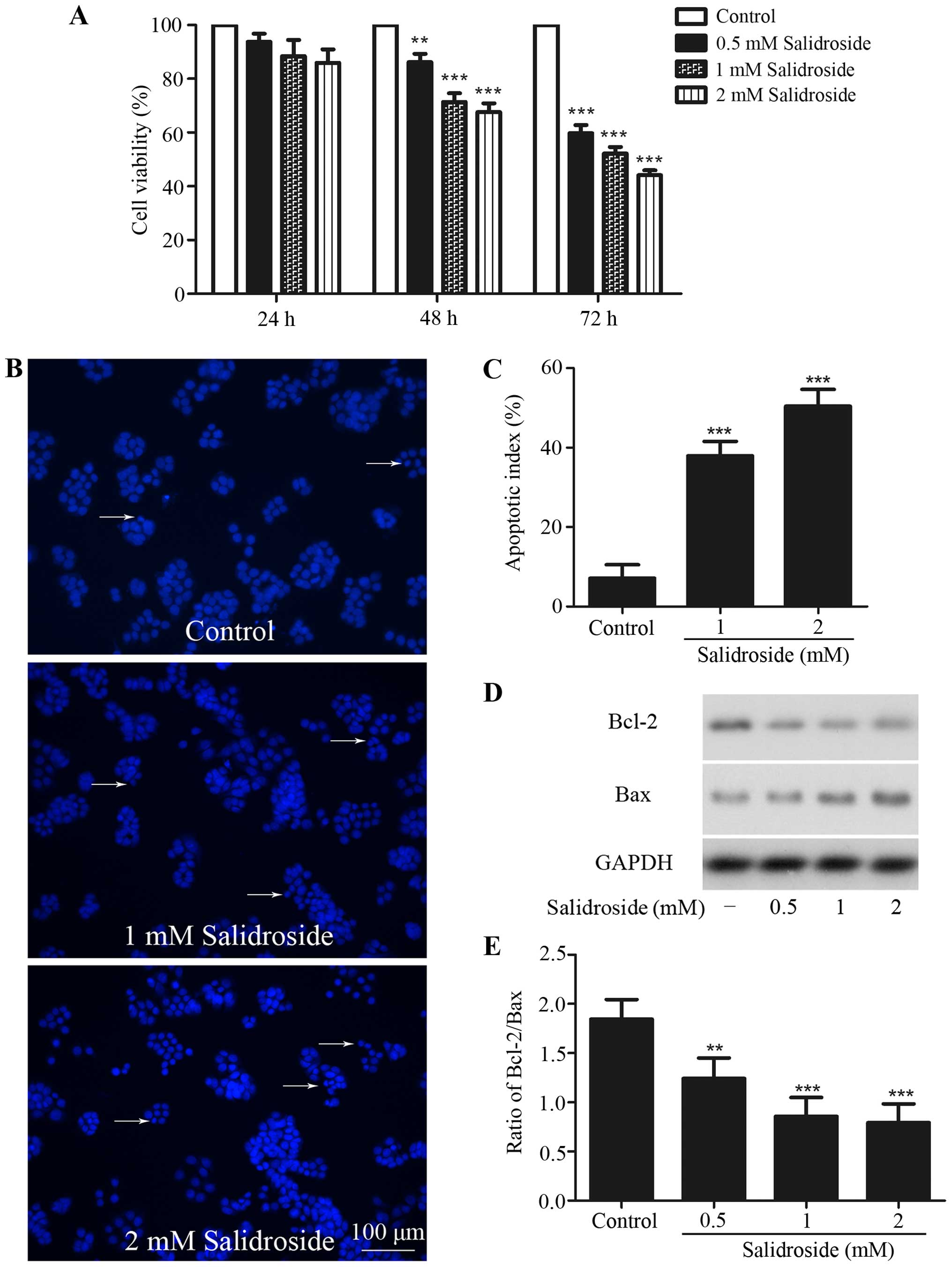
Salidroside inhibits growth and
induces apoptosis in HT-29 colon cancer cells
HT-29 colon cancer cells were treated in indicated
time periods with various concentrations of salidroside to
investigate the cytotoxic activity of salidroside against these
cells. Cell viability was then assessed using MTT assay. MTT assay
revealed a dose-dependent and time-dependent cytotoxic of
salidroside on these cells (Fig.
1A). Compared with control group, treatment with salidroside
(0.5, 1 and 2 mM) for 48 h significantly inhibited cell viability
to 86.17±6.28, 71.38±6.48 and 67.65±6.39%, respectively, and 0.5, 1
and 2 mM salidroside treatment for 72 h inhibited cell viability to
59.81±5.94, 52.23±4.86 and 44.12±3.71%, respectively. However,
treatment with salidroside for 24 h had no inhibitory effect. In
subsequent experiments, 1 and 2 mM salidroside treatment for 48 h
was used to observe the effects of salidroside on HT-29 colon
cancer cells.
The nuclear Hoechst 33342 staining assay was used to
detect apoptosis of HT29 cells. Pretreatment with 1 and 2 mM
salidroside for 48 h displayed typical morphological features of
apoptosis including chromatin condensation, nuclear shrinkage, and
the formation of a few apoptotic bodies, and the percentage of
nuclear condensation increased to 37.9±3.7 and 50.4±4.2% from
7.1±3.4%, compared with the control group (Fig. 1B and C).
Western blot analysis was used to evaluate the
expression of Bcl-2 and Bax. A decreased ratio of Bcl-2/Bax was
found after salidroside treatment (Fig.
1D and E). These results revealed that salidroside could induce
apoptosis in HT-29 colon cancer cells.
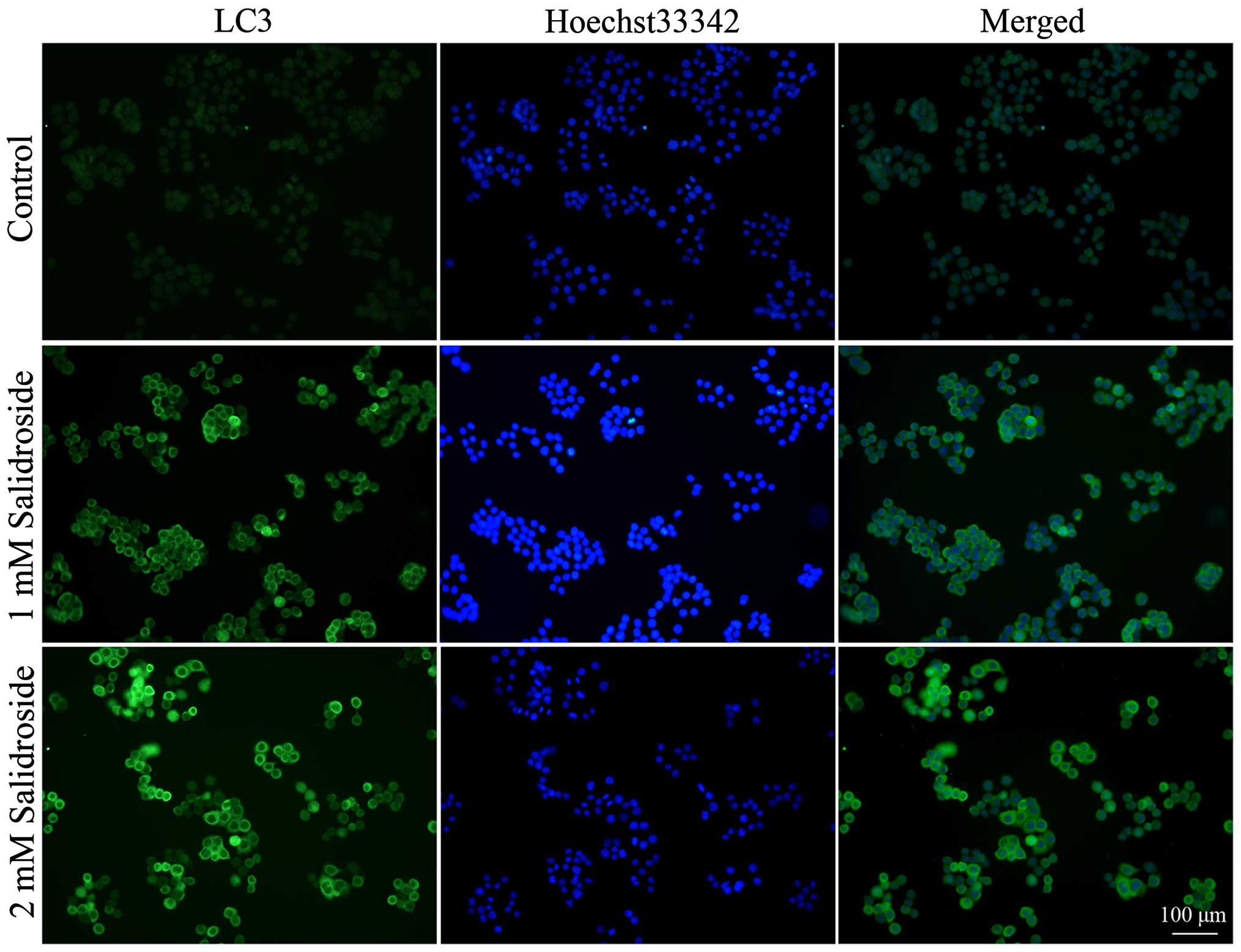
Salidroside induces autophagy in HT-29
colon cancer cells
To determine whether salidroside induces autophagy
in colon cancer cells, we used immunofluorescence to examine the
intracellular distribution of LC3, an autophagy marker (30). Results showed that control HT29
colon cancer cells exhibited weak and diffuse cytoplasmic staining
with LC3-associated green fluorescence, whereas those treated with
salidroside exhibited an increase in LC3 staining intensity, which
is a typical feature of LC3 distribution within autophagosomes
(LC3-II) (Fig. 2).
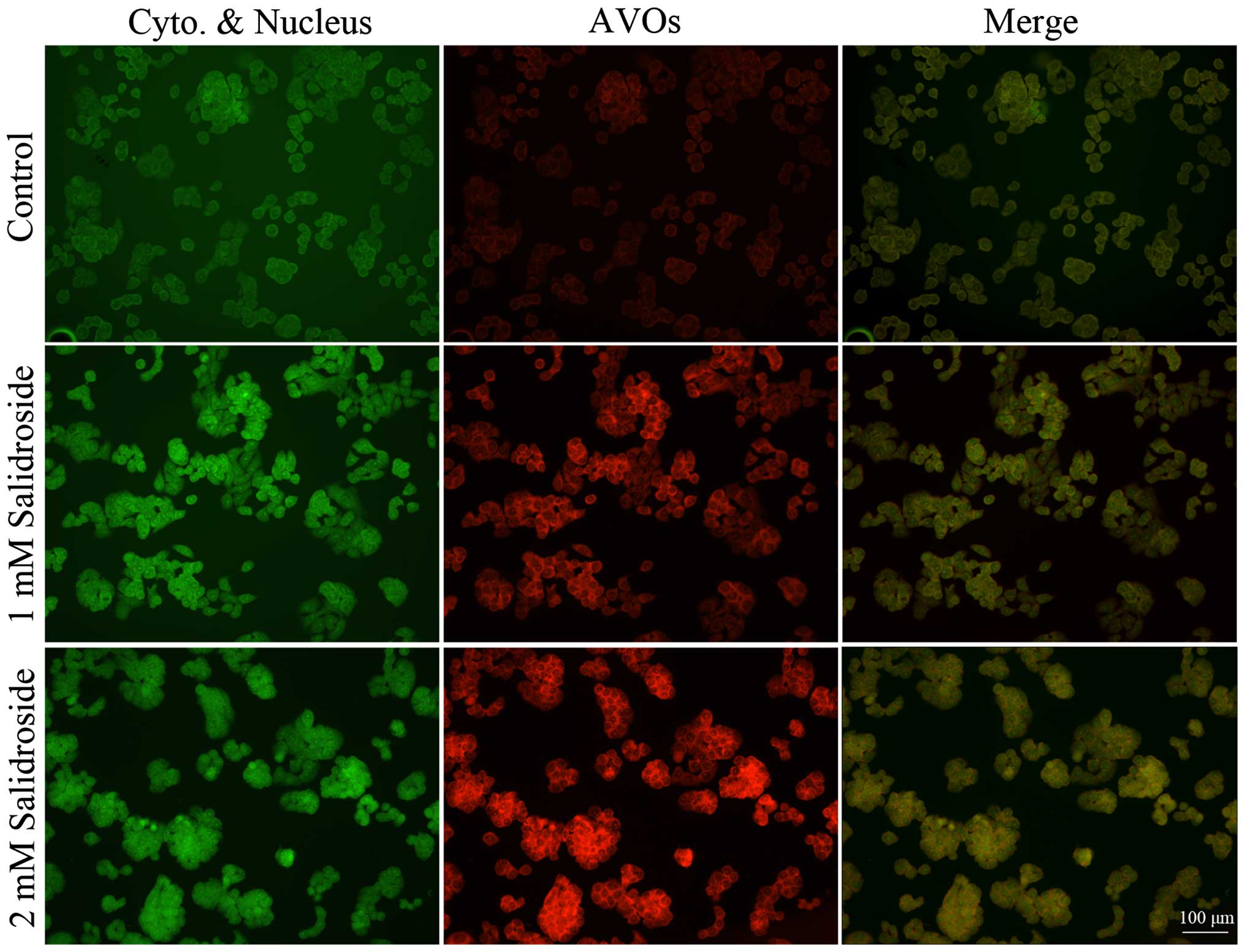
To further determine the effect of salidroside on
autophagy, we analyzed the accumulation of acidic vesicular
organelles. Vital staining of HT-29 colon cancer cells with
acridine orange revealed the appearance of acidic vesicular
organelles with bright red fluorescence after salidroside treatment
(Fig. 3). Conversely, the majority
of control cells exhibited only minimal red fluorescence.
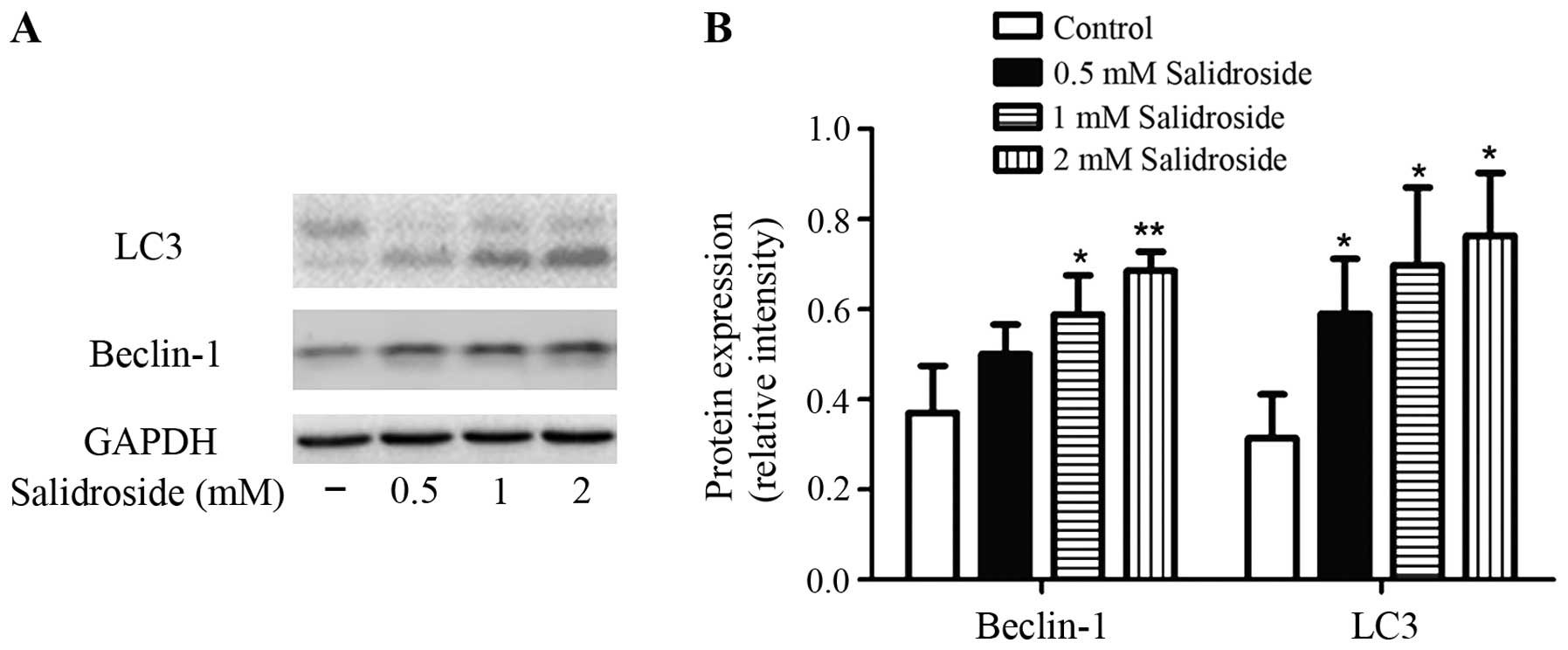
To clarify the mechanisms underlying the
salidroside-induced autophagy on colon cancer cells, we examined
the effect of salidroside treatment on LC3 and Beclin-1 expression.
It is well known that LC3-II/−I ratio directly correlates with the
formation of autophagosomes (31).
Lysates of cells were subjected to western blot analysis. As shown
in Fig. 4, the ratio of LC3-II to
LC3-I was increased by treatment with salidroside (0.5, 1 and 2 mM)
for 48 h compared with control. Salidroside also increased the
expression of Beclin-1 compared with control.
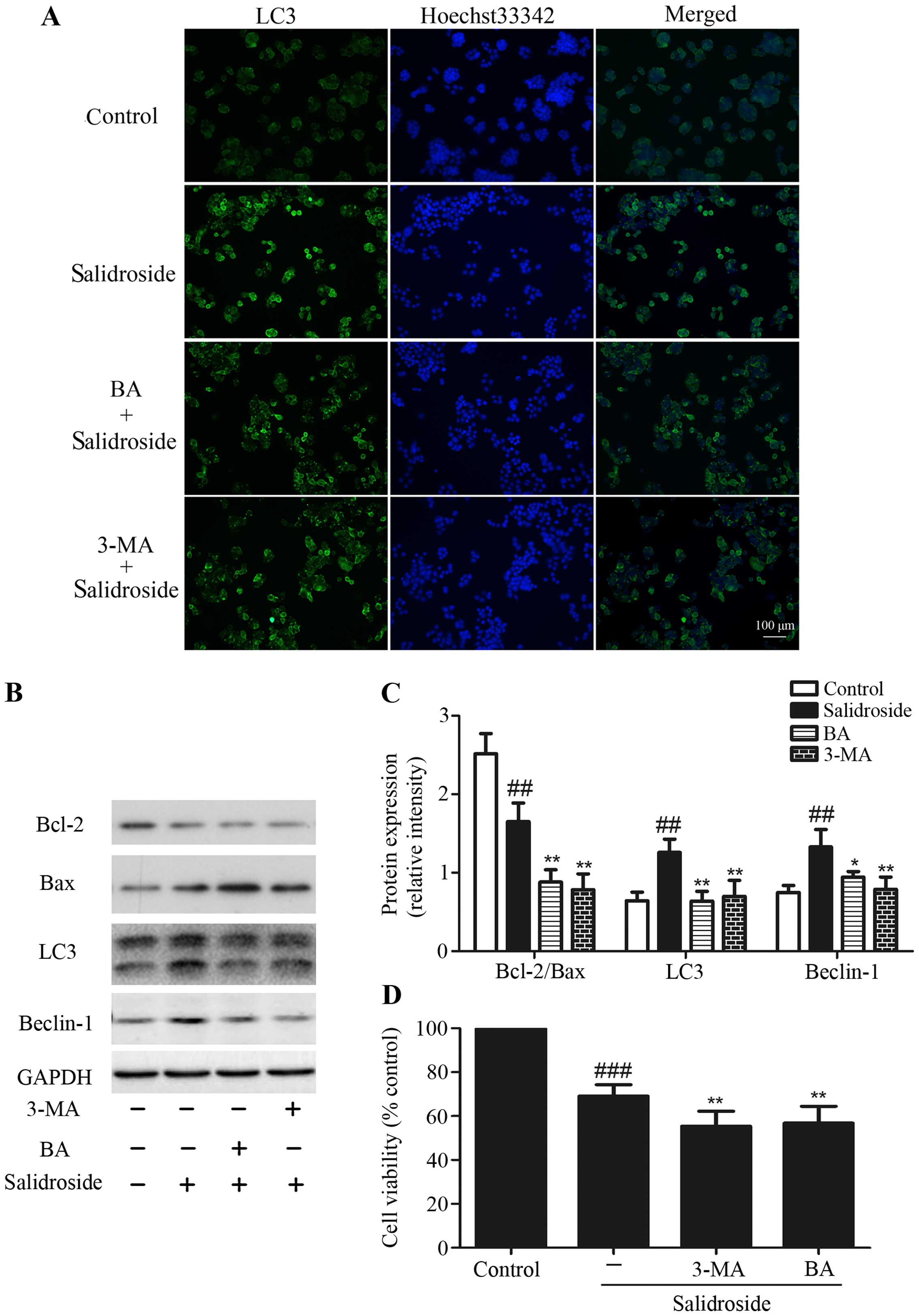
Inhibition of autophagy enhances
salidroside-induced apoptosis
As described above, we found that salidroside
exhibited increased apoptosis and autophagy in HT29 cells. Then we
used 3-MA (an inhibitor of autophagy) and BA (an
autophagy-lysosomal inhibitor) to determine the inter-relationship
between apoptosis and autophagy after treating HT29 cells with
salidroside. HT29 colon cancer cells treated with salidroside (2
mM) combine with 3-MA (10 mM) or BA (10 nM) decreased the formation
of LC3+ autophagic vacuoles compared with salidroside
alone (Fig. 5A). In addition, the
increase of LC3-II/−I ratio and Beclin-1 protein expression by
salidroside were considerably decreased by pre-treatment with 3-MA
or BA, suggesting that 3-MA and BA blocked autophagy induction by
salidroside. We also found that treating HT29 cells with
salidroside decreased the radio of Bcl-2/Bax, which was augmented
when salidroside was combined with 3-MA or BA (Fig. 5B and C). MTT assays revealed that
treatment of HT29 cells with salidroside and 3-MA or BA decreased
the cell viability more than treatment with salidroside alone
(Fig. 5D). These results indicated
that suppression of autophagy could enhance the salidroside-induced
apoptosis.
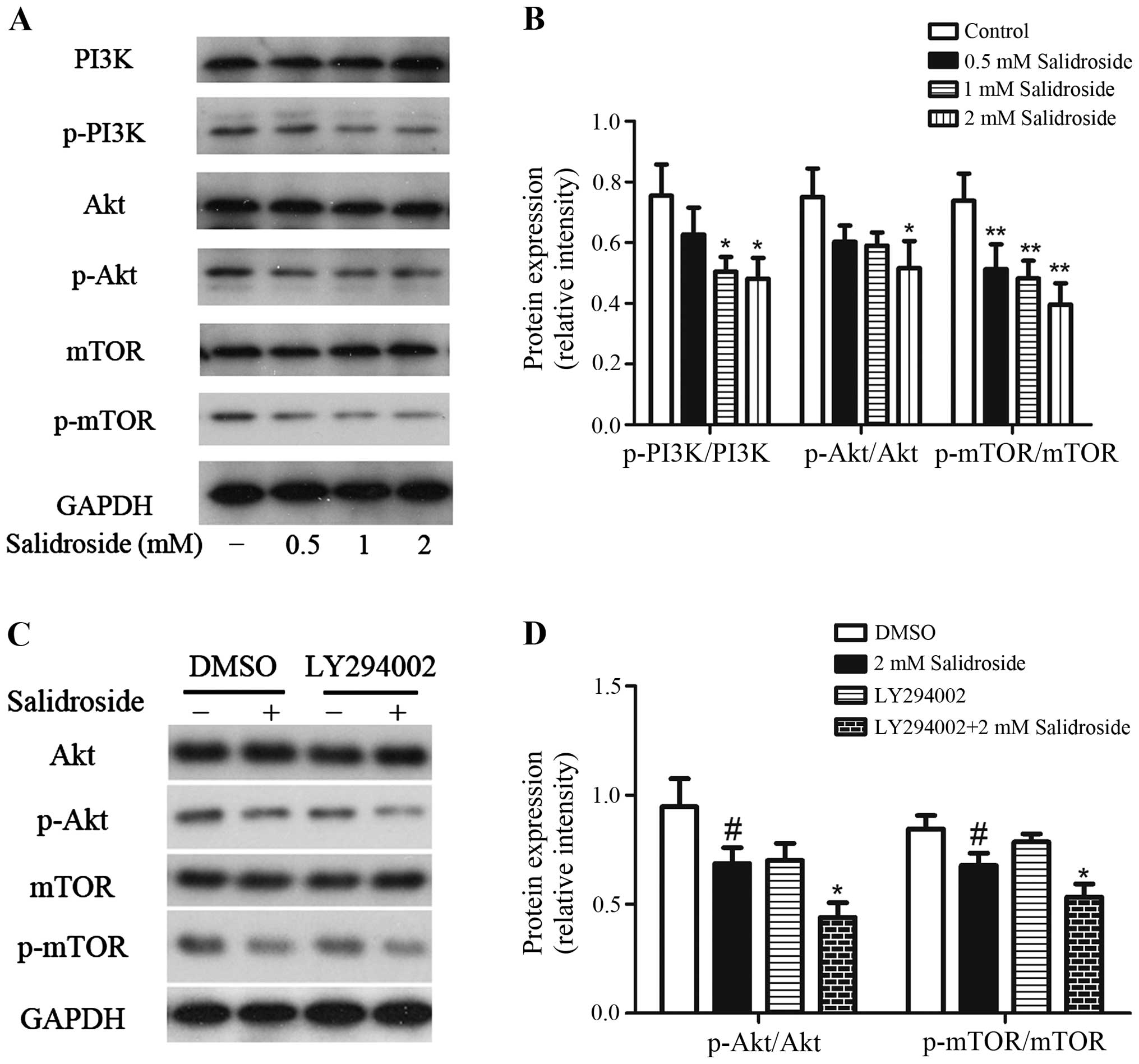
Salidroside inhibits the activation of
PI3K/Akt/mTOR signaling pathway
Since we have observed that salidroside could induce
apoptosis and autophagy in HT29 colon cancer cells, we further
investigated the possible mechanisms. The PI3K/Akt/mTOR signaling
pathway is a key pathway related to cell survival/death (9,32),
then we investigated if this pathway plays a central role in
salidroside-mediated cell death. As shown in Fig. 6A and B, salidroside treatment causes
significant decrease in the phosphorylation levels of PI3K, Akt,
and mTOR, and there was no change observed in the total PI3K, Akt,
and mTOR protein level. There was a 16.9, 33.2 and 36.2% decline in
the ratio of p-PI3K over PI3K, 19.6, 21.3 and 31.2% decrease in the
ratio of p-Akt over Akt, and 30.5, 34.7 and 46.3% reduction in the
ratio of p-mTOR over mTOR in HT29 colon cancer cells when treated
with salidroside at 0.5, 1 and 2 mM, respectively. In addition,
treatment of HT29 cells with 10 µM LY294002 plus 2 mM salidroside
decreased the ratio of p-Akt/Akt and p-mTOR/mTOR more than
treatment with salidroside alone (Fig.
6C and D). Thus, these data suggested that the salidroside
inhibited the activation of PI3K/Akt/mTOR signaling pathway.
Discussion
In our research, we found that salidroside inhibited
the growth of HT-29 human colorectal cancer cells concentration-
and time-dependently, which is consistent with the results that
salidroside inhibited proliferation, decreased the migration and
invasion of SW1116 cells (25).
However, whether the anticancer effect of salidroside is related
with apoptosis and autophagy have not been elucidated.
Apoptosis is an active process in which apoptotic
cells undergo chromatin condensation and fragmentation followed by
the formation of apoptotic bodies (33). Several genes have been shown to
regulate apoptosis. The proteins of the Bcl-2 family represent a
critical checkpoint in major apoptotic signal transduction cascades
(34). In addition, apoptotic cell
death is typically determined by the ratio of Bcl-2/Bax (35–37).
In this study, we found that salidroside induced cell apoptosis,
accompanied by an increase of chromatin condensation and nuclear
fragmentation, and a decrease of Bcl-2/Bax expression ratio. We
thus examined whether salidroside induced autophagy.
Autophagy in cancer has begun to be investigated and
has been suggested as a novel potential target for improved
anticancer therapies (38–40). Autophagy is characterized by
engulfment of cytoplasm and organelles into double-membrane bound
structures, autophagosomes, and delivery to and subsequent
degradation in lysosomes (41).
Autophagy allows degradation of the cytoplasmic contents under
certain stress conditions such as oxidative stress, nutrient
starvation, misfolded protein accumulation, and irradiation is a
temporary survival mechanism. Recent studies shown that autophagy
is needed for cancer survival and tumorigenesis (42,43).
Autophagy can serve as a mechanism of self-defense by recycling
essential molecules, and by contributing to therapy resistance
(44–46). Conversely, autophagy can inhibit
tumor progression (45). Extensive
autophagy can also result in destruction of vital cell
constituents, committing the cell to death (47). LC3 and ATG12, two ubiquitin-like
protein systems, play an important role for the autophagosomal
membrane formation and expansion (48). LC3 consists of two forms, LC3-I and
its cleavage form, LC3-II. The conversion of soluble LC3-I to the
membrane bound LC3 II is considered as one of the makers of
autophagy induction in the cells. Detecting LC3-II by
immunoblotting or immunofluorescence is a reliable method for
monitoring autophagosome formation (30,48,49).
Beclin-1 has been well demonstrated to initiate autophagosome
formation during autophagy (50).
Autophagy is characterized morphologically by the formation of
LC3+ autophagic vacuoles and accumulation acidic
vesicular organelles (30,51). Our data showed that salidroside
treatment increased the formation of LC3+ autophagic
vacuoles and the accumulation of acidic vesicular organelles.
Western blot analysis found that salidroside remarkably increased
the ratio of LC3-II/LC3-I and Beclin-1 protein expression in a
dose-dependent manner. It is obviously that salidroside induced
autophagy in HT29 colon cancer cells.
3-MA is a popular inhibitor of the autophagic agent.
It has been reported to inhibit the activity of PI3-kinase (a
kinase that is essential for vesicle nucleation, the first phase of
autophagosome formation) and blocks the formation of
preautophagosome, autophagosome, and autophagic vacuoles (52). BA, a known inhibitor of the late
phase of autophagy, prevents maturation of autophagic vacuoles by
inhibiting fusion between autophagosomes and lysosomes (53). Addition of 3-MA or BA attenuated the
formation of LC3+ autophagic vacuoles and inhibited the
increase of LC3-II/−I ratio and Beclin-1 protein expression induced
by salidroside in HT29 colon cancer cells. Moreover, pre-treatment
with 3-MA or BA augmented the inhibitory effects of salidoside on
the expression of Bcl-2/Bax ratio and the cell viability.
Collectively, these results indicated that inhibition of autophagy
decreased cell viability and increased apoptosis, which revealed
that autophagy provided a protective mechanism against
salidroside-induced apoptosis.
Increasing evidence suggests that cross-talk between
apoptosis and autophagy is made especially complicated by the fact
that they share many common regulatory molecules, such as Bcl-2 and
the PI3K/Akt/mTOR signaling pathway (33,54).
PI3K activates the downstream serine/threonine kinase Akt, which in
turn, through a cascade of regulators, triggers the phosphorylation
and activation of the serine/threonine kinase mTOR (55). PI3K/Akt/mTOR, a major intracellular
signaling pathway, has received much attention in recent years
given its potential role in cancer (8,56). As
a convergence point for a multitude of upstream signals, this
critical pathway stimulates the activity of numerous downstream
effectors and mediates enhanced cellular survival, growth, protein
synthesis, motility, and other functions of pro-tumorigenic impact
(57). Inhibition of PI3K/Akt/mTOR
signaling pathway causes cell death associated with apoptosis
and/or autophagy (58,59). As shown by our western blot
analysis, salidroside significantly decreased the activation of
PI3K, Akt, and mTOR in HT-29 human colorectal cancer cells. In
addition, PI3K inhibitor LY294002 could further inhibit the
activation of Akt and mTOR induced by salidroside treatment. Thus,
the inhibition of the PI3K/Akt/mTOR signaling pathway contributes,
at least in part, to the apoptosis-inducing and autophagy-inducing
effect of salidroside in HT-29 human colorectal cancer cells.
In this study, we demonstrated the cell growth
inhibitory effect of salidroside on HT-29 human colorectal cancer
cells. We elucidated the underlying mechanism that involves
cross-talk between apoptosis and autophagy via inhibition of
PI3K/Akt/mTOR signaling pathways. In conclusion, we have provided a
basis for molecular mechanism of salidroside in colon cancer
treatment. The potential application of salidroside in inhibiting
colon cancer cell proliferation makes it an attractive agent for
colorectal cancer research, and possibly treatment.
Acknowledgements
This study was supported by the Technological
Innovation and Demonstration of Social Undertakings Projects
(HS2014049) of Nantong, Jiangsu, China.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaabane W, User SD, El-Gazzah M, Jaksik
R, Sajjadi E, Rzeszowska-Wolny J and Los MJ: Autophagy, apoptosis,
mitoptosis and necrosis: Interdependence between those pathways and
effects on cancer. Arch Immunol Ther Exp (Warsz). 61:43–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su M, Mei Y and Sinha S: Role of the
crosstalk between autophagy and apoptosis in cancer. J Oncol.
2013:102735–102748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Woodle ES and Kulkarni S: Programmed cell
death. Transplantation. 66:681–691. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Periyasamy-Thandavan S, Jiang M,
Schoenlein P and Dong Z: Autophagy: Molecular machinery,
regulation, and implications for renal pathophysiology. Am J
Physiol Renal Physiol. 297:F244–F256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li JP, Yang YX, Liu QL, Pan ST, He ZX,
Zhang X, Yang T, Chen XW, Wang D, Qiu JX, et al: The
investigational Aurora kinase A inhibitor alisertib (MLN8237)
induces cell cycle G2/M arrest, apoptosis, and autophagy via p38
MAPK and Akt/mTOR signaling pathways in human breast cancer cells.
Drug Des Devel Ther. 9:1627–1652. 2015.PubMed/NCBI
|
|
9
|
Pan ST, Qin Y, Zhou ZW, He ZX, Zhang X,
Yang T, Yang YX, Wang D, Qiu JX and Zhou SF: Plumbagin induces G2/M
arrest, apoptosis, and autophagy via p38 MAPK- and
PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell
carcinoma cells. Drug Des Devel Ther. 9:1601–1626. 2015.PubMed/NCBI
|
|
10
|
Liu YL, Yang PM, Shun CT, Wu MS, Weng JR
and Chen CC: Autophagy potentiates the anti-cancer effects of the
histone deacetylase inhibitors in hepatocellular carcinoma.
Autophagy. 6:1057–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang Q, Li F, Shi K, Yang Y and Xu C:
Sodium selenite-induced activation of DAPK promotes autophagy in
human leukemia HL60 cells. BMB Rep. 45:194–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Chen LX, Ouyang L, Cheng Y and
Liu B: Plant natural compounds: Targeting pathways of autophagy as
anti-cancer therapeutic agents. Cell Prolif. 45:466–476. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burada F, Nicoli ER, Ciurea ME, Uscatu DC,
Ioana M and Gheonea DI: Autophagy in colorectal cancer: An
important switch from physiology to pathology. World J Gastrointest
Oncol. 7:271–284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panossian A, Wikman G and Sarris J:
Rosenroot (Rhodiola rosea): Traditional use, chemical composition,
pharmacology and clinical efficacy. Phytomedicine. 17:481–493.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hung SK, Perry R and Ernst E: The
effectiveness and efficacy of Rhodiola rosea L.: A systematic
review of randomized clinical trials. Phytomedicine. 18:235–244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Li X, Simoneau AR, Jafari M and Zi
X: Rhodiola rosea extracts and salidroside decrease the growth of
bladder cancer cell lines via inhibition of the mTOR pathway and
induction of autophagy. Mol Carcinog. 51:257–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu Y, Roberts L, Shetty K and Schneider
SS: Rhodiola crenulata induces death and inhibits growth of breast
cancer cell lines. J Med Food. 11:413–423. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Lin S, Yu D, Qiu S, Zhang X and Mei
R: A preliminary study: The anti-proliferation effect of
salidroside on different human cancer cell lines. Cell Biol
Toxicol. 26:499–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu X, Zhang X, Qiu S, Yu D and Lin S:
Salidroside induces cell-cycle arrest and apoptosis in human breast
cancer cells. Biochem Biophys Res Commun. 398:62–67. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Peng X, Hu Z, Zhao Q, He J, Li J
and Zhong X: Effects of over-expression of ANXA10 gene on
proliferation and apoptosis of hepatocellular carcinoma cell line
HepG2. J Huazhong Univ Sci Technolog Med Sci. 32:669–674. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014.PubMed/NCBI
|
|
22
|
Skopińska-Rózewska E, Malinowski M,
Wasiutyński A, Sommer E, Furmanowa M, Mazurkiewicz M and Siwicki
AK: The influence of Rhodiola quadrifida 50% hydro-alcoholic
extract and salidroside on tumor-induced angiogenesis in mice. Pol
J Vet Sci. 11:97–104. 2008.PubMed/NCBI
|
|
23
|
Sun C, Wang Z, Zheng Q and Zhang H:
Salidroside inhibits migration and invasion of human fibrosarcoma
HT1080 cells. Phytomedicine. 19:355–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Yao Y, Wang H, Guo Y, Zhang H and
Chen L: Effects of salidroside on glioma formation and growth
inhibition together with improvement of tumor microenvironment.
Chin J Cancer Res. 25:520–526. 2013.PubMed/NCBI
|
|
25
|
Sun KX, Xia HW and Xia RL: Anticancer
effect of salidroside on colon cancer through inhibiting JAK2/STAT3
signaling pathway. Int J Clin Exp Pathol. 8:615–621.
2015.PubMed/NCBI
|
|
26
|
Wang XW and Zhang YJ: Targeting mTOR
network in colorectal cancer therapy. World J Gastroenterol.
20:4178–4188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maira SM, Furet P and Stauffer F:
Discovery of novel anticancer therapeutics targeting the
PI3K/Akt/mTOR pathway. Future Med Chem. 1:137–155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu P and Hu YZ: PI3K/Akt/mTOR pathway
inhibitors in cancer: A perspective on clinical progress. Curr Med
Chem. 17:4326–4341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanida I, Minematsu-Ikeguchi N, Ueno T and
Kominami E: Lysosomal turnover, but not a cellular level, of
endogenous LC3 is a marker for autophagy. Autophagy. 1:84–91. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou ZW, Li XX, He ZX, Pan ST, Yang Y,
Zhang X, Chow K, Yang T, Qiu JX, Zhou Q, et al: Induction of
apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated
pathways by plumbagin in human prostate cancer cells. Drug Des
Devel Ther. 9:1511–1554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsai JP, Lee CH, Ying TH, Lin CL, Lin CL,
Hsueh JT and Hsieh YH: Licochalcone A induces autophagy through
PI3K/Akt/mTOR inactivation and autophagy suppression enhances
Licochalcone A-induced apoptosis of human cervical cancer cells.
Oncotarget. 6:28851–28866. 2015.PubMed/NCBI
|
|
34
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korsmeyer SJ: Regulators of cell death.
Trends Genet. 11:101–105. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang E and Korsmeyer SJ: Molecular
thanatopsis: A discourse on the BCL2 family and cell death. Blood.
88:386–401. 1996.PubMed/NCBI
|
|
37
|
Bar-Am O, Weinreb O, Amit T and Youdim MB:
Regulation of Bcl-2 family proteins, neurotrophic factors, and APP
processing in the neurorescue activity of propargylamine. FASEB J.
19:1899–1901. 2005.PubMed/NCBI
|
|
38
|
Hsuan SW, Chyau CC, Hung HY, Chen JH and
Chou FP: The induction of apoptosis and autophagy by Wasabia
japonica extract in colon cancer. Eur J Nutr. 55:491–503. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yim NH, Jung YP, Kim A, Ma CJ, Cho WK and
Ma JY: Oyaksungisan, a traditional herbal formula, inhibits cell
proliferation by induction of autophagy via JNK activation in human
colon cancer cells. Evid Based Complement Alternat Med.
2013:231874–231883. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang S, Wang X, Contino G, Liesa M, Sahin
E, Ying H, Bause A, Li Y, Stommel JM, Dell'antonio G, et al:
Pancreatic cancers require autophagy for tumor growth. Genes Dev.
25:717–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo JY, Chen HY, Mathew R, Fan J,
Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM,
Karantza V, et al: Activated Ras requires autophagy to maintain
oxidative metabolism and tumorigenesis. Genes Dev. 25:460–470.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ellington AA, Berhow MA and Singletary KW:
Inhibition of Akt signaling and enhanced ERK1/2 activity are
involved in induction of macroautophagy by triterpenoid B-group
soyasaponins in colon cancer cells. Carcinogenesis. 27:298–306.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gozuacik D and Kimchi A: Autophagy as a
cell death and tumor suppressor mechanism. Oncogene. 23:2891–2906.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ogier-Denis E and Codogno P: Autophagy: A
barrier or an adaptive response to cancer. Biochim Biophys Acta.
1603:113–128. 2003.PubMed/NCBI
|
|
47
|
Clarke PG: Developmental cell death:
Morphological diversity and multiple mechanisms. Anat Embryol
(Berl). 181:195–213. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kaser A and Blumberg RS: Autophagy,
microbial sensing, endoplasmic reticulum stress, and epithelial
function in inflammatory bowel disease. Gastroenterology.
140:1738–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao Y and Klionsky DJ: Physiological
functions of Atg6/Beclin 1: A unique autophagy-related protein.
Cell Res. 17:839–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
52
|
Petiot A, Ogier-Denis E, Blommaart EF,
Meijer AJ and Codogno P: Distinct classes of phosphatidylinositol
3′-kinases are involved in signaling pathways that control
macroautophagy in HT-29 cells. J Biol Chem. 275:992–998. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yamamoto A, Tagawa Y, Yoshimori T,
Moriyama Y, Masaki R and Tashiro Y: Bafilomycin A1 prevents
maturation of autophagic vacuoles by inhibiting fusion between
autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E
cells. Cell Struct Funct. 23:33–42. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A,
Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX, et al: Arenobufagin,
a natural bufadienolide from toad venom, induces apoptosis and
autophagy in human hepatocellular carcinoma cells through
inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 34:1331–1342.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rodon J, Dienstmann R, Serra V and
Tabernero J: Development of PI3K inhibitors: Lessons learned from
early clinical trials. Nat Rev Clin Oncol. 10:143–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang F, Wang Q, Zhou ZW, Yu SN, Pan ST, He
ZX, Zhang X, Wang D, Yang YX, Yang T, et al: Plumbagin induces cell
cycle arrest and autophagy and suppresses epithelial to mesenchymal
transition involving PI3K/Akt/mTOR-mediated pathway in human
pancreatic cancer cells. Drug Des Devel Ther. 9:537–560.
2015.PubMed/NCBI
|
|
57
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang K, Liu R, Li J, Mao J, Lei Y, Wu J,
Zeng J, Zhang T, Wu H, Chen L, et al: Quercetin induces protective
autophagy in gastric cancer cells: Involvement of Akt-mTOR- and
hypoxia-induced factor 1α-mediated signaling. Autophagy. 7:966–978.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Shrivastava A, Kuzontkoski PM, Groopman JE
and Prasad A: Cannabidiol induces programmed cell death in breast
cancer cells by coordinating the cross-talk between apoptosis and
autophagy. Mol Cancer Ther. 10:1161–1172. 2011. View Article : Google Scholar : PubMed/NCBI
|