Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer and one of the leading causes of cancer-related
death worldwide with a rising annual incidence in numerous
countries except the USA (1).
Conventional therapies including surgery, chemotherapy or
radiotherapy have been shown to be relatively ineffective (2). The main reasons for the lack of
efficacy of these therapies are regional lymph node and/or distal
metastasis of CRC cells (3).
Approximately one quarter of CRC patients present with metastatic
disease at the time of diagnosis, and their 5-year survival rate is
near 10% (4). Therefore,
elucidating the mechanisms involved in the metastatic cascade
underlying this malignancy is a key goal for developing therapeutic
agents to reduce CRC-associated mortality.
Lymphangiogenesis or the growth of lymphatic vessels
(LVs), is an important step in tumor progression that supports
tumor growth and metastatic dissemination (3). Lymphangiogenesis and early regional
metastasis frequently occurs in several types of malignant tumors
including CRC (5). The density of
LVs in or surrounding tumors is closely associated with lymph node
metastasis and prognosis of CRC patients (3). Therefore, metastasis to regional lymph
nodes is generally believed to be the first indication that a tumor
has progressed to metastatic competence (6). Thus, tumor lymphangiogenesis may be a
crucial target of antimetastatic agents.
In the past decade, there has been a dramatic
increase in the number of studies examining mechanisms of
tumor-associated lymphangiogenesis and lymphatic metastasis.
Vascular endothelial growth factor-C (VEGF-C), a member of the VEGF
family, was the first lymphangiogenic growth factor to be
identified (5). Mechanistically,
the binding of VEGF-C to its cognate receptor VEGFR-3, which is
expressed on human lymphatic endothelial cells (HLECs) (6), promotes proliferation and migration of
HLECs as well as the formation of LVs (7,8). Thus,
upregulation of VEGF-C expression has been implicated in induction
of tumor lymphangiogenesis and lymphatic invasion (9). Therefore, targeting VEGF-C could be a
novel anti-lymphangiogenic therapy.
Genomic instability and heterogeneity of tumor cell
populations can lead to a shift in the expression of angiogenic
factors during tumor progression. Various tumors at their advanced
stage express multiple angiogenic factors (10). During this late stage, anti-VEGF-C
therapy is predicted to be ineffective or even encounters drug
resistance. Indeed, it is possible that anti-VEGF-C therapy may
even allow tumors to switch on other angiogenic pathways for
lymphangiogenesis. As a result, tumors can escape from anti-VEGF-C
therapy after a relative long-term treatment. Additionally, drugs
used in anti-VEGF-C therapy can lead to an increase in side-effects
such as rash, diarrhea, hypertension, gastrointestinal perforation
and arterial thromobembolic events (11). These problems highlight the urgent
need for the development of more effective anticancer agents.
Pien Tze Huang (PZH) is a well-known traditional
Chinese formulation that was first prescribed 450 years ago by a
royal physician in the Ming Dynasty. The main active ingredients of
PZH include: Moschus, Calculus Bovis, Snake Gall and Radix
Notoginseng. These ingredients together confer PZH properties of
heat-clearing, detoxification, promotion of blood circulation and
removal of blood stasis (12).
Since in the Chinese medicine system accumulation of toxic dampness
and heat is one of the major causative factors in the pathogenesis
of cancers, PZH is thought to be an effective anticancer agent. In
fact, PZH has long been used as an alternative treatment strategy
for cancers in China and Southeast Asia. Previously, we reported
that PZH can inhibit CRC growth in vivo and in vitro
via promotion of apoptosis and suppression of proliferation of
cancer stem cells (CSCs) as well as inhibition of angiogenesis and
reversal of multidrug resistance through multiple pathways while
causing minimal side-effects (13–17).
Recently, we found that PZH also prevents metastasis by inhibiting
the progression of epithelial-mesenchymal transition (EMT)
(18,19), but the underlying mechanism of the
antimetastasis effects of PZH are still unknown. To further
elucidate the mechanism underlying the anticancer activity of PZH,
in the present study, we evaluated the effects of PZH on tumor
metastasis and lymphangiogenesis using different CRC cell lines and
the VEGF-C-stimulated HLEC model.
Materials and methods
Materials and reagents
Roswell Park Memorial Institute (RPMI)-1640 medium
(C11875500BT), Dulbecco's modified Eagle's medium (DMEM) with high
glucose (C11995500BT), fetal bovine serum (FBS; #10099-141),
penicillin-streptomycin (SV30010), 0.25% trypsin-EDTA (#25200-072),
Pierce RIPA buffer (#89901), Pierce BCA Protein Assay kit (#23227)
and SuperSignal™ West Pico Chemiluminescent Substrate (#34080) were
all purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). CellTiter 96® AQueous Non-Radioactive Cell
Proliferation Assay (MTS; G5430) was provided by Promega
Corporation (Madison, WI, USA). The exogenous VEGF-C (CYT-527) was
purchased from ProSpec-Tany TechnoGene Ltd. (East Brunswick, NJ,
USA). Annexin V-FITC apoptosis detection kit (KGA108) was obtained
from KeyGen Biotech Co., Ltd. (Jiangsu, China). Transwell chambers
(8.0 µm; #3422) were obtained from Corning Life Sciences (Corning,
NY, USA). The In Vitro Angiogenesis assay kit (ECM625) and
nitrocellulose (NC) membrane (0.45 µm; HATF00010) were purchased
from Millipore (Billerica, MA, USA). Rabbit polyclonal antibody
against VEGF-C (#2445), VEGFR-3 (#2485) and β-actin (#4967) were
obtained from Cell Signaling Technology (Beverly, MA, USA). Rabbit
polyclonal antibody against MMP-2 (ab37150) and MMP-9 (ab38898)
were purchased from Abcam (Cambridge, MA, USA). HRP-conjugated goat
anti-rabbit secondary antibody (E030120) was purchased from EarthOx
Life Science (Millbrae, CA, USA). Culture flask and plates were
purchase from NEST Biotechnology Co., Ltd. (Wuxi, Jiangsu, China).
All the other chemicals used, unless otherwise stated, were
obtained from Sigma Chemicals (St. Louis, MO, USA).
Preparation of PZH
PZH was obtained from and authenticated by Zhangzhou
Pien Tze Huang Pharmaceutical Co., Ltd. (Zhangzhou, China) (Chinese
FDA approval no. Z35020242, lot no. 1009039). Stock solutions of
PZH were prepared just before use by dissolving the PZH powder in
phosphate-buffered saline (PBS) to a concentration of 20 mg/ml. The
working concentrations of PZH were made by diluting the stock
solution in the culture medium.
Cell culture
Human metastatic CRC cell lines HCT-8, HCT-116 and
SW620 were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The HLEC was purchased from the JENNIO
Biological Technology Co., Ltd. (Guangzhou, China). HCT-8, HCT-116
cells and HLECs were grown in RPMI-1640; SW620 cells were grown in
DMEM. All cell media were supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin, and cultured at 37°C with 5%
CO2 in a humidified incubator (Forma 3110; Thermo Fisher
Scientific, Inc.).
Treatment of PZH and exogenous
VEGF-C
CRC cells were seeded into 6-well plates for 12 h,
which was followed by PZH treatment for the indicated period of
time. HLECs were first grown in complete RPMI-1640 (10% FBS) until
~60% confluency, and then continuously cultured in FBS-free medium
overnight. The medium was replaced with RPMI-1640 with 2% FBS and
cells were treated with 5 ng/ml VEGF-C and/or various
concentrations of PZH for the indicated period of time.
Observation of morphological
changes
Morphology of HLECs was observed using a
phase-contrast microscope (FMIL/DFC295; Leica, Wetzlar, German)
after PZH and/or exogenous VEGF-C treatment for 24 h. The images of
five randomly selected views were captured at a magnification of
×200.
Cell viability evaluation
Cell viability was assessed using MTS assay. CRC
cells and HLECs were seeded in 96-well plates. After PZH and/or
exogenous VEGF-C treatment for 24 or 48 h, 10 µl MTS was added to
each well after which the samples were incubated for 1 h at 37°C.
The resulting absorbance was measured at 490 nm using an ELISA
reader (Model ELx800; BioTek, Winooski, VT, USA).
Detection of apoptosis
After incubation with various concentrations of PZH
and/or exogenous VEGF-C for 24 h, apoptosis of HLECs was determined
by FACSCalibur and Annexin V-FITC apoptosis detection kit, as
previously described (14).
Staining was performed according to the manufacturer's
instructions. In the present study, Annexin V/PI double-negative
population (labeled as LL in the FACS diagram) indicates viable
cells; Annexin V-positive/PI-negative or Annexin V/PI
double-positive population (labeled as LR or UR in the FACS
diagram) represents cells undergoing early or late apoptosis,
respectively.
Migration assays
Migration ability of the CRC cell lines was
evaluated by wound-healing, as previously described (15). Briefly, CRC cells were seeded into
6-well plates (106 cells/well in 2 ml culture medium).
After 12 h of incubation, cells were scraped away vertically in
each well using a P200 pipette tip. Five randomly selected views
along the scraped line were photographed from each well using a
phase-contrast inverted microscope at a magnification of ×100.
Cells were then treated with the indicated concentrations of PZH
for 24 h and another set of images were captured in the same way. A
reduction in the scraped area indicates migration of cells. For
HLECs, the migration assay was performed using Transwell cell
culture chambers as previously described (18). The inserts were placed within a
24-well chamber containing 0.7 ml RPMI-1640 with 10% FBS to serve
as a chemoattractant. As before, HLECs were seeded into 6-well
plates and treated with different concentrations of PZH and/or
exogenous VEGF-C for 24 h. Cells (5×104 cells) were
seeded into the inserts suspended in 0.2 ml of serum-free
RPMI-1640. The cells were incubated at 37°C with 5% CO2
for 12 h before the upper surface of the filter was scraped to
remove nonmigratory cells. Migrated cells were then fixed and
stained with crystal violet. For quantification, the average number
of migrating cells/field was assessed by counting five random
fields under a phase-contrast microscope at a magnification of
×200.
Tube formation assays
The HLEC tube formation was assessed using the In
Vitro Angiogenesis assay kit as previously described (15). Briefly, HLECs were treated with PZH
and/or exogenous VEGF-C for 24 h, harvested and diluted
2×105 cells/well in 200 µl medium individually, were
seeded into 1:1 ECMatix gel (v/v) coated 48-well plate, and
incubated for 8 h. The series of tube-like structures were
photographed using a phase-contrast microscope at a magnification
of ×40.
Western blot analysis
Cells were treated with PZH and/or exogenous VEGF-C
for 24 h after which they were lysed using Pierce RIPA buffer
containing protease inhibitor and phosphatase inhibitor cocktails.
The lysates were then centrifuged at 14,000 rpm for 20 min, and the
resulting protein concentrations were determined using BCA protein
assay reagent kit. A total of 50 µg of protein for each sample was
loaded onto a 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and resolved using 20 V for 10 min→ 80 V
for 30 min→ 120 V for 1 h, then transferred onto nitrocellulose
membranes. Following blocking with 5% non-fat dry milk, the
membranes were incubated with antibodies against VEGF-C, VEGFR-3,
MMP-2, MMP-9 and/or β-actin (1:1,000 dilution) overnight at 4°C and
subsequently incubated with HRP-conjugated anti-rabbit secondary
antibodies (1:5,000 dilution) for 1 h at room temperature. The
membranes were then exposed with enhanced chemiluminescence (ECL)
detection using SuperSignal™ West Pico Chemiluminescent Substrate.
Image Lab™ Software (version 3.0) was used for densitometric
analysis and quantification of western blot analyses.
Statistical analysis
All data were collected based on the mean of three
experiments. Statistical analysis was performed using the SPSS
software (version 17.0) for Windows (SPSS, Inc., Chicago, IL, USA)
using one-way ANOVA. P<0.05 was considered to indicate a
statistically significant result.
Results
PZH reduces viability and migration of
CRC cell lines
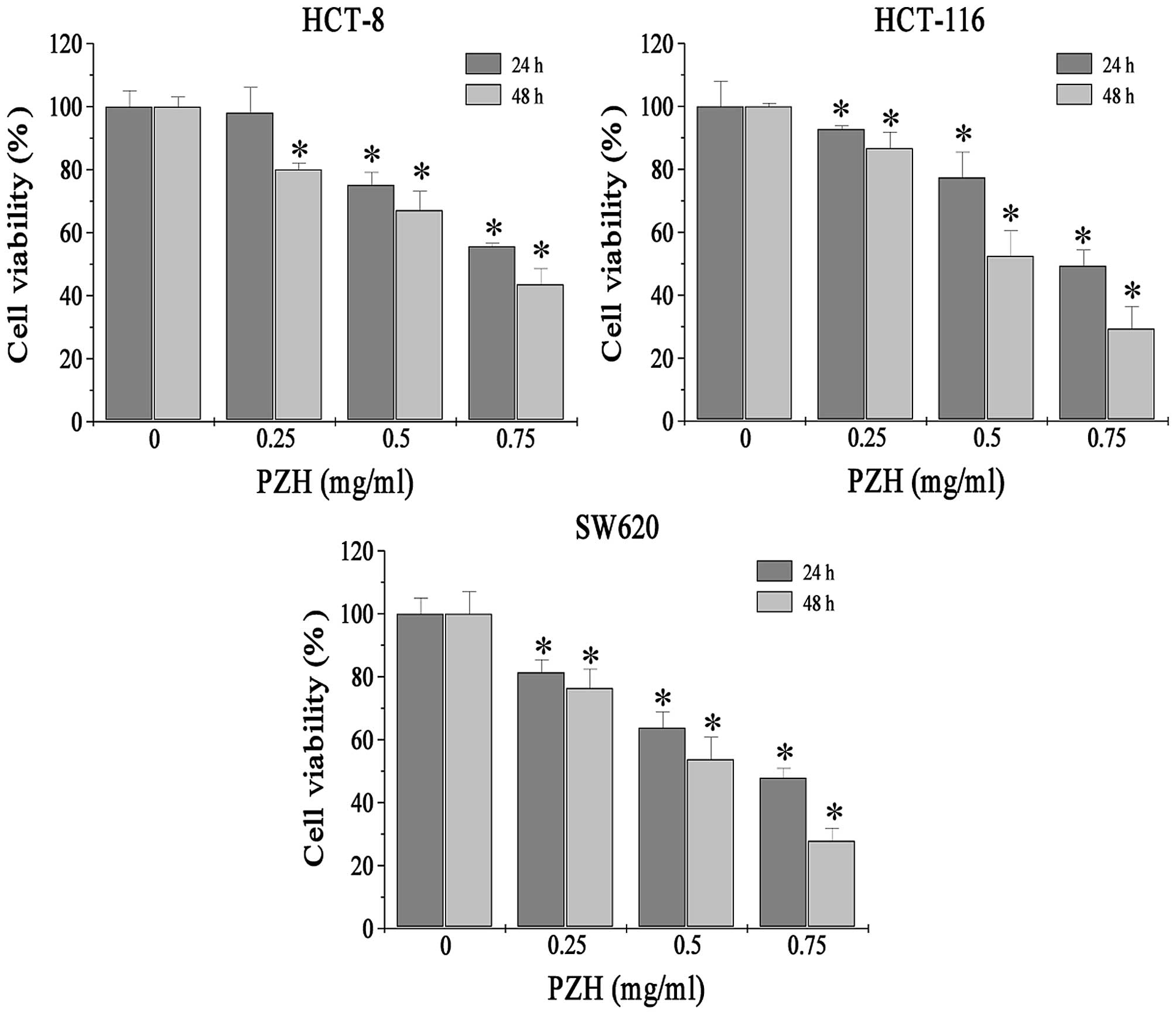
We first determined the effect of PZH on the
viability of various CRC cell lines using MTS assay. PZH treatment
at 0.25, 0.5 and 0.75 mg/ml for 24 or 48 h significantly reduced
cell viability in the HCT-8, HCT-116 and SW620 cells (P<0.05) in
a dose- and time-dependent manner (Fig.
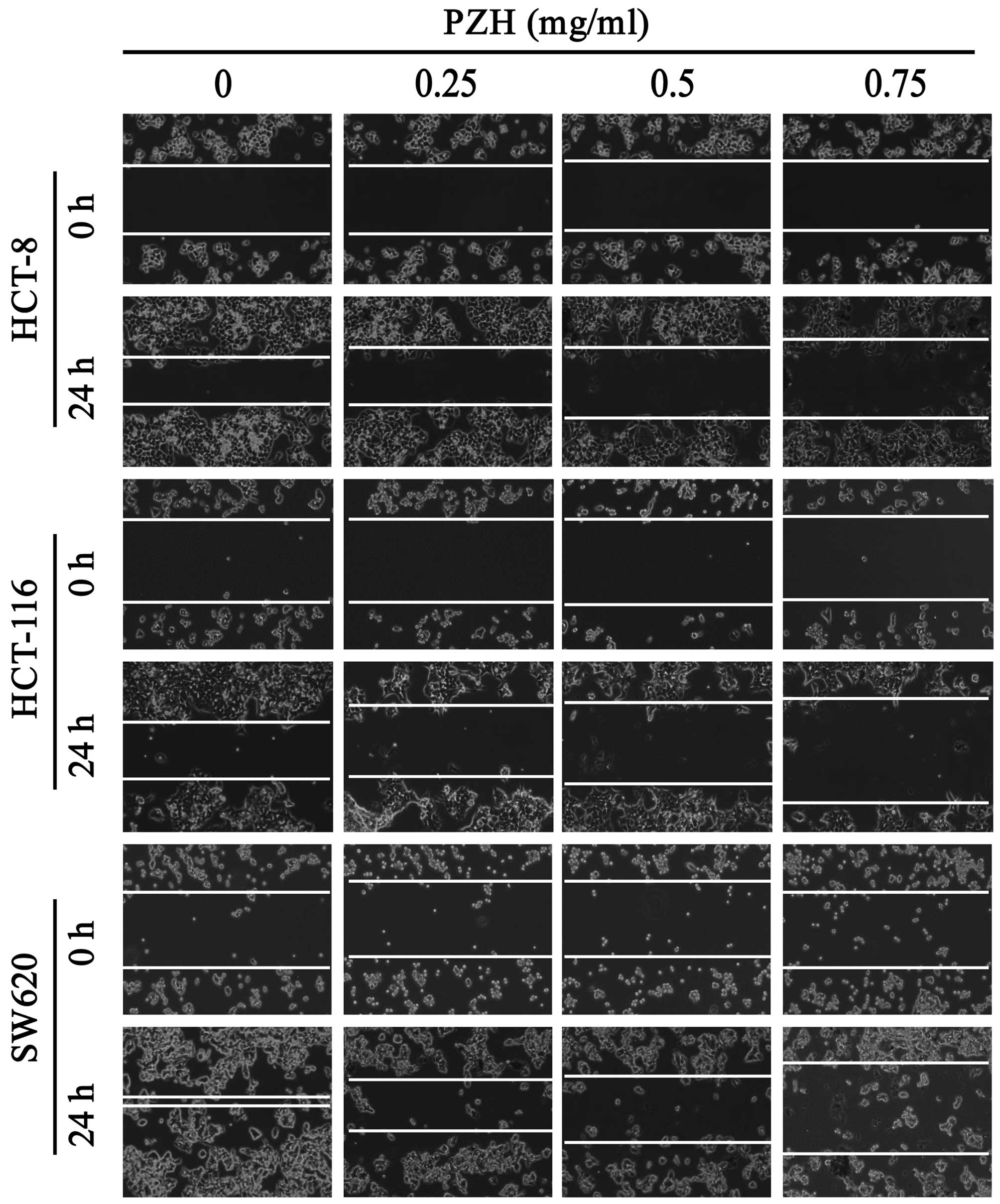
1). We next evaluated the effect of PZH on migration of various
CRC cell lines using a wound-healing assay. Twenty-four hours
post-wounding, untreated HCT-8, HCT-116 and SW620 cells migrated
into the wounded (clear) area of the cell monolayer, whereas PZH
treatment dose-dependently inhibited migration of all these three
cell lines (Fig. 2).
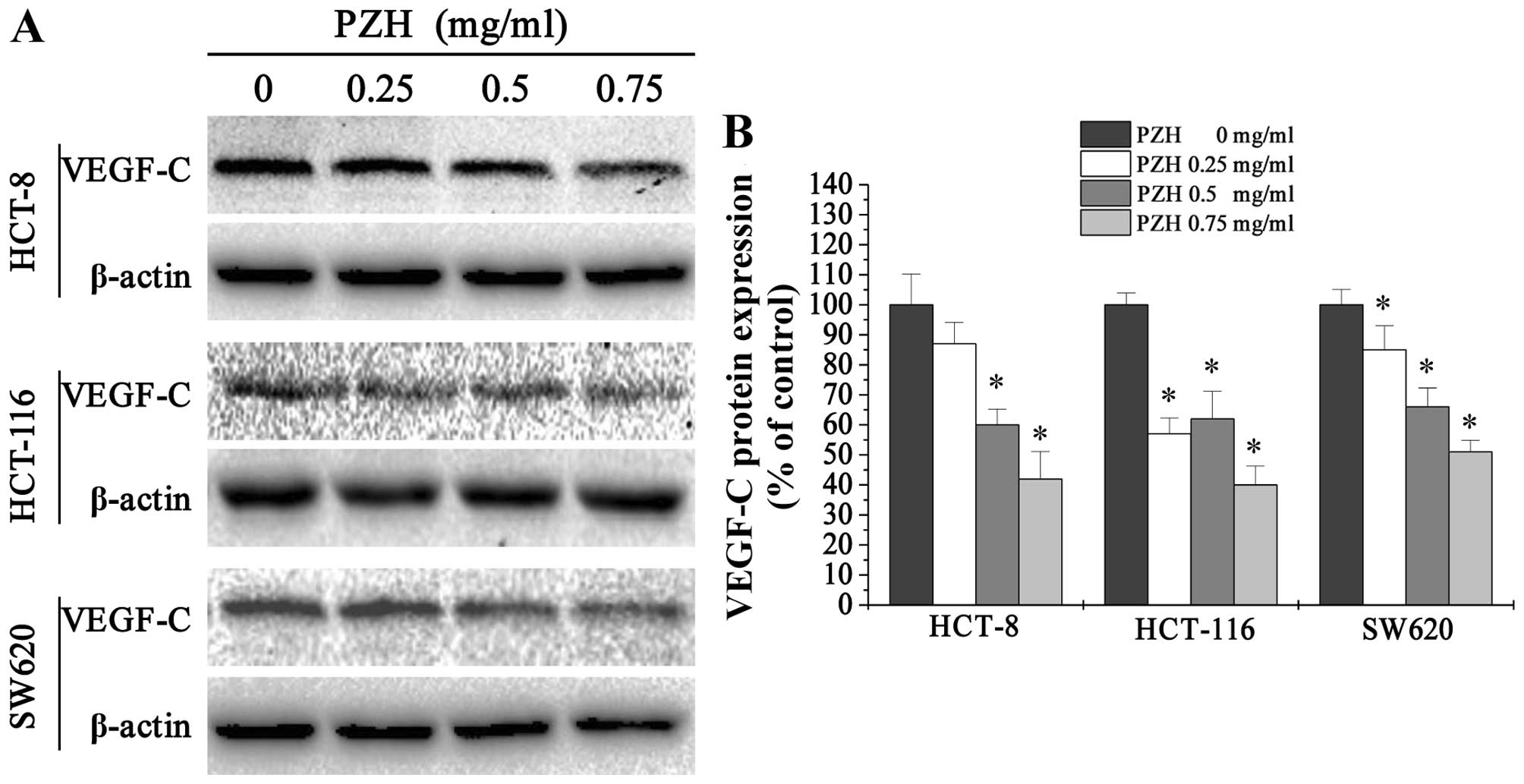
PZH downregulates the expression of
VEGF-C in CRC cell lines
To explore a potential mechanism underlying the
antimetastasis activity of PZH, we performed western blotting to
examine the protein expression of VEGF-C in HCT-8, HCT-116 and
SW620 cells. We found that PZH treatment profoundly and
dose-dependently reduced the expression of VEGF-C (Fig. 3).
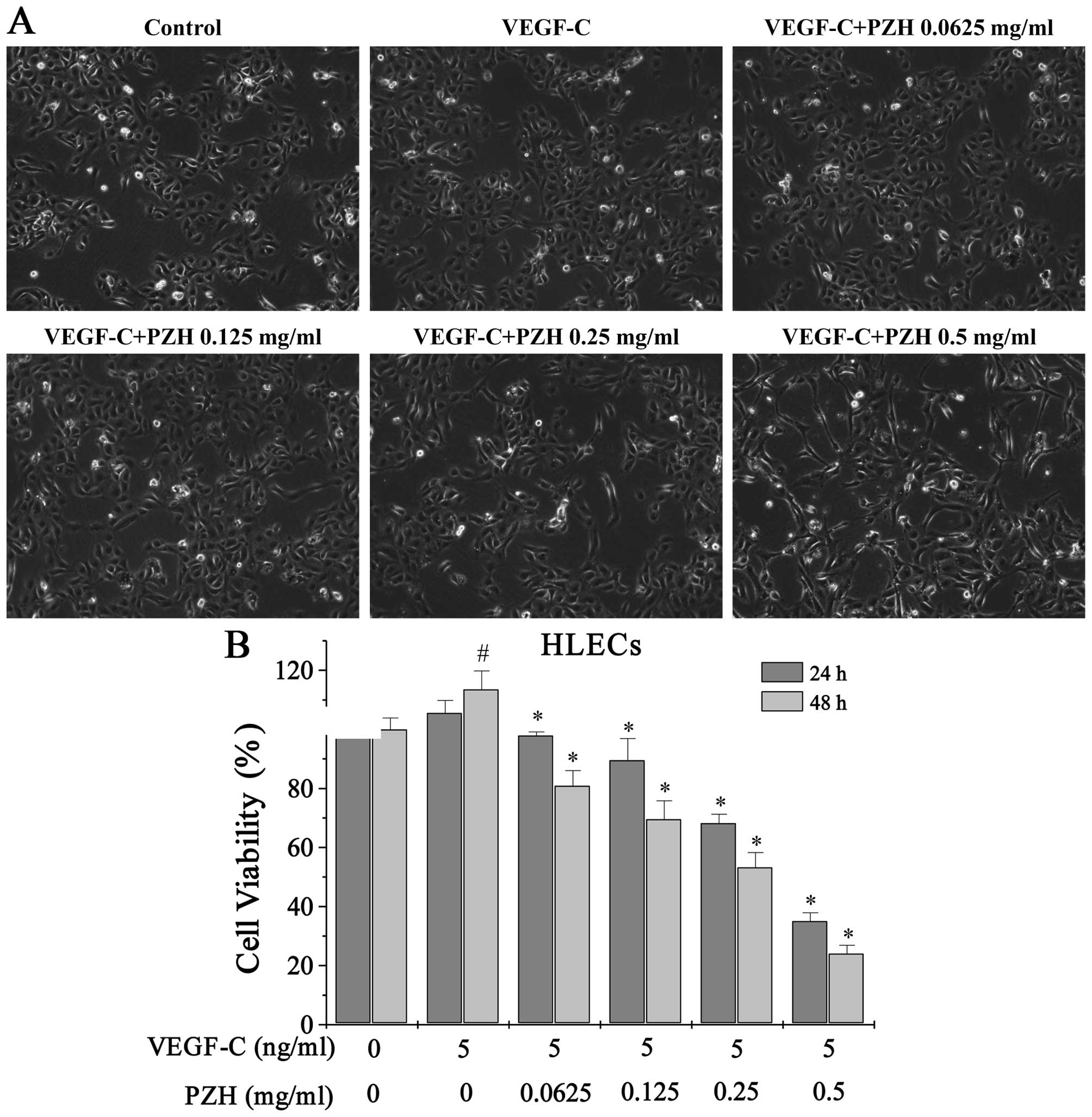
PZH reduces cell confluency and
viability of VEGF-C-stimulated HLECs
As seen from the cell morphology, VEGF-C stimulation
increased the confluency of HLECs, while PZH treatment decreased
cell confluency in a dose-dependent manner (Fig. 4A). Moreover, we found that cell
viability of HLECs increased to 105.56% at 24 h and 113.56% by 48 h
(P<0.05) after VEGF-C stimulation compared to control cells that
did not receive VEGF-C, using an MTS assay. Treatment with
0.0625–0.5 mg/ml of PZH for 24 h decreased the viability of
VEGF-C-stimulated cells from 97.94 to 35.07% and from 81.01 to
24.10% after 48 h (P<0.05 vs. PZH-untreated cells) (Fig. 4B).
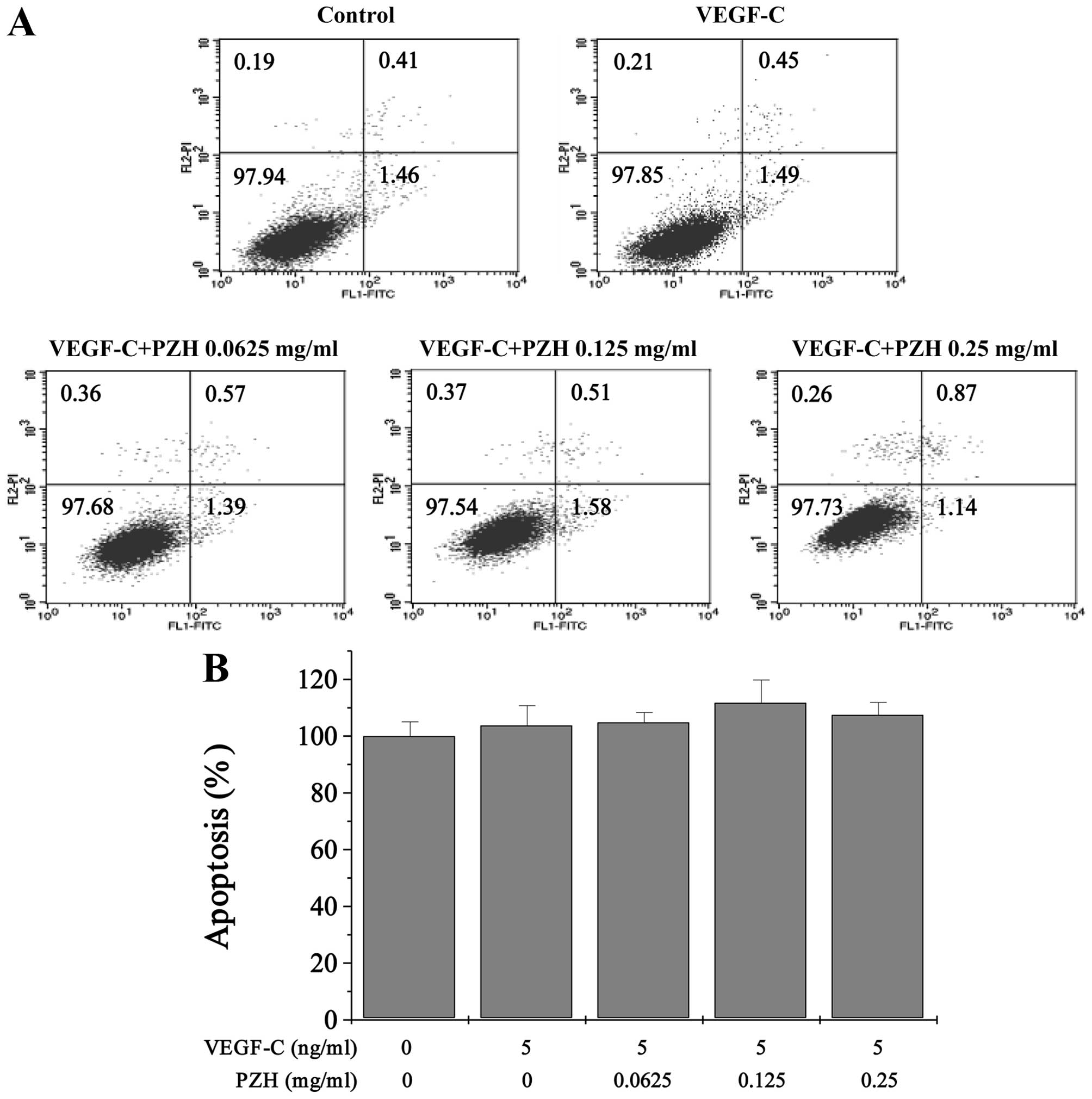
PZH does not affect the cell apoptosis
of VEGF-C-stimulated HLECs
The effect of PZH on apoptosis in the
VEGF-C-stimulated HLECs was determined by Annexin V/PI staining
followed by FACS analysis. The results indicated that the
percentage of cells undergoing apoptosis after treatment with
0.0625, 0.125 or 0.25 mg/ml of PZH (including the early and late
apoptotic cells) was not significantly different when compared with
the control cells that did not receive PZH treatment (Fig. 5).
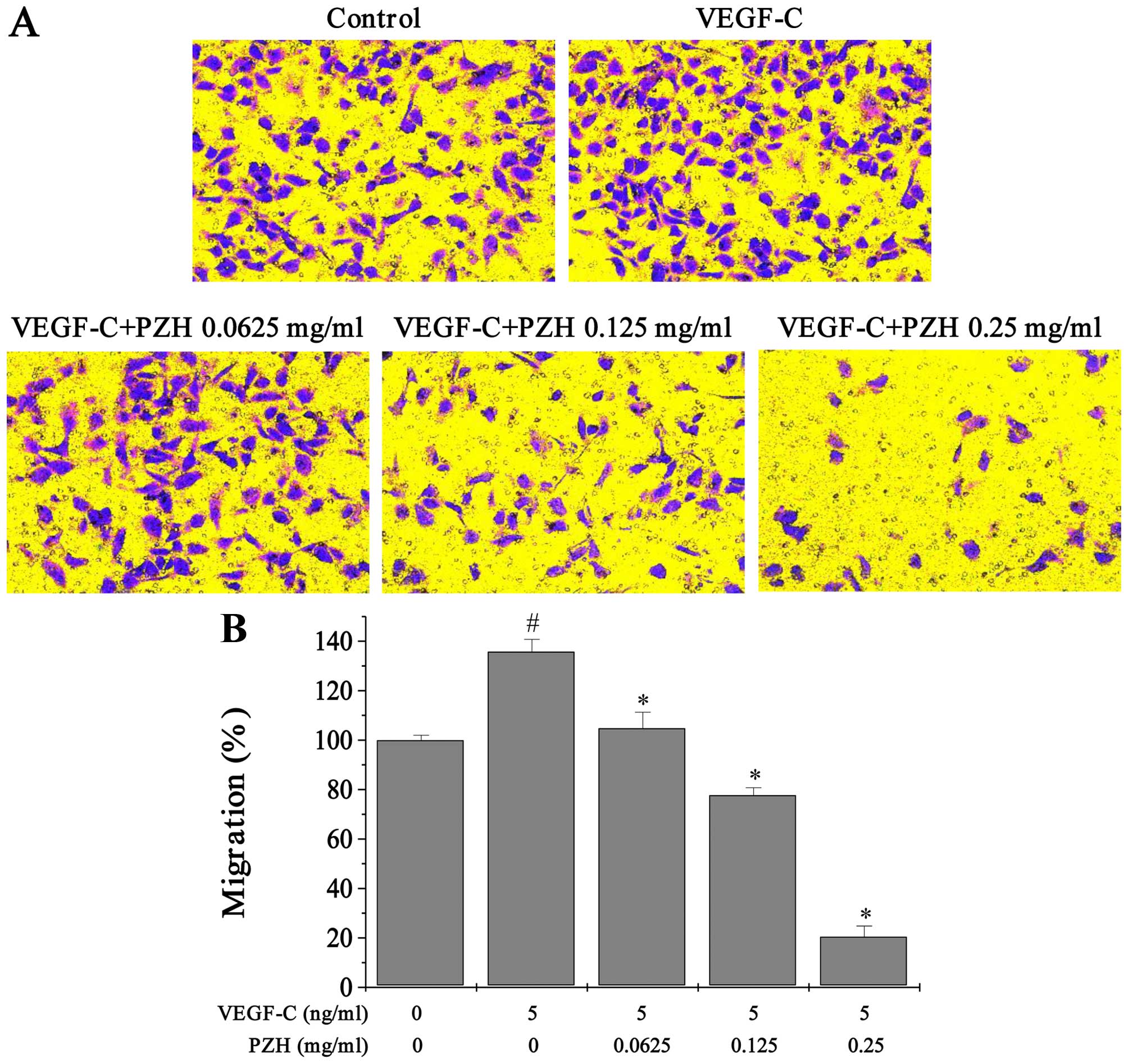
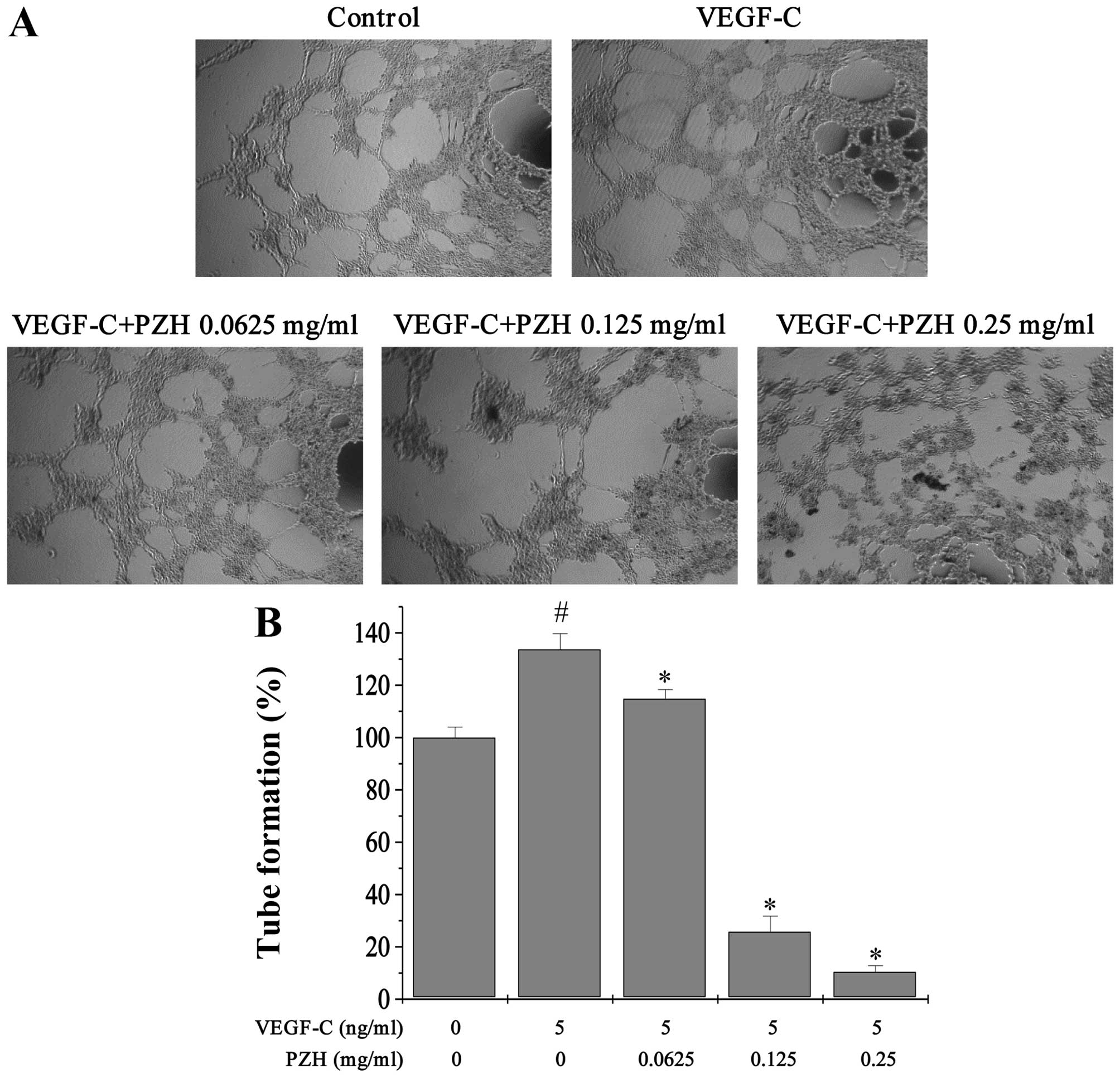
PZH attenuates cell migration and tube
formation ability of VEGF-C-stimulated HLECs
Transwell assays were performed to determine the
effects of PZH on migration of HLECs. VEGF-C stimulated cells had
enhanced migratory capacity by ~35.74% (P<0.0327). Treatment
with 0.0625–0.25 mg/ml of PZH dose-dependently reduced the cell
migratory ability by 104.81–20.49% (P<0.05) (Fig. 6). Similarly, exogenous VEGF-C
stimulation led to a 33.69% (P<0.0218) increase in tube
formation of HLECs, compared to control cells that did not receive
VEGF-C, which in turn was decreased by PZH treatment by
114.81–10.49% (P<0.05) (Fig.
7).
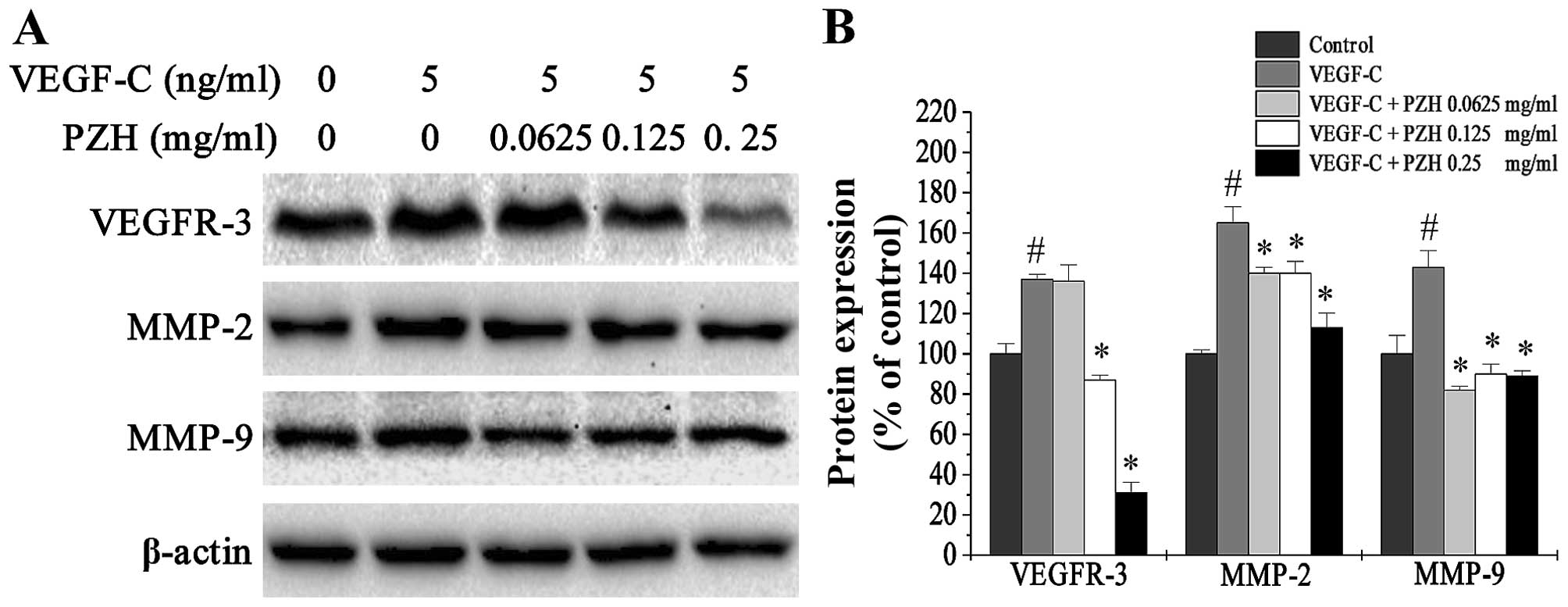
PZH downregulates the expression of
VEGFR-3, MMP-2 and MMP-9 in VEGF-C-stimulated HLECs
VEGFR-3 is the cognate receptor of VEGF-C that is
expressed in HLECs. Binding of VEGF-C to VEGFR-3 results in
proliferation of HLECs as well as the formation of LVs. Moreover,
activation of the VEGF-C/VEGFR-3 signaling pathway can lead to the
upregulation of matrix metalloproteinases (MMPs). Expression of
MMP-2 and MMP-9 has been associated with the migration ability of
VEGF-C-stimulated cells and lymphangiogenesis (20). To further explore the
anti-lymphangiogenesis properties of PZH, we used western blot
analysis to determine the relative expression of these proteins
after PZH treatment. We found that VEGFR-3, MMP-2 and MMP-9 were
upregulated after VEGF-C stimulation and downregulated with PZH
treatment (Fig. 8).
Discussion
The growth and metastatic spread of malignant tumors
is critically dependent on the development of new vasculature.
Lymphangiogenesis is strongly associated with tumor metastasis and
has therefore been evaluated in various types of tumor including
colon malignancies (5), esophageal
carcinoma (21) and breast cancer
(22). Recently, numerous studies
have cast new light on the process of lymphangiogenesis and
potential molecular mechanisms underlying tumor regional lymph node
metastasis. One such mechanism is tumor-induced lymphangiogenesis.
Evidence of intratumoral LVs has raised the possibility that tumor
cells themselves contribute to lymphatic metastasis through the
induction of lymphangiogenic processes (23). It is well known that tumor cells
enter the lymphatic vasculature by eliciting lymphangiogenesis via
growth factor production. Moreover, lymphangiogenic growth factors
produced by tumor cells stimulate growth and dilation of the
tumor-induced LVs as well as facilitate tumor regional lymph node
metastasis.
VEGF-C, a lymphangiogenic growth factor, is a key
regulator of lymphangiogenesis and tumor metastasis (24). Early studies have suggested that
VEGF-C can promote the growth of new LVs and regional metastasis by
binding to their receptor tyrosine kinase VEGFR-3, which was found
to be densely expressed in lymphatic endothelial cells (25,26).
Studies with human or animal tumor models have demonstrated that
malignant tumor cells themselves can secrete high levels of VEGF-C
(27), and this overexpression of
tumor-derived VEGF-C may play an important role in the occurrence
of intratumoral lymphangiogenesis leading to the dissemination of
tumor cells to regional lymph nodes (28–30).
Targeting lymphangiogenesis and VEGF-C may be a new
treatment strategy for CRC. However, gene therapy targeting VEGF-C
or the use of VEGFR-3 receptor antagonist has many disadvantages in
clinical practice and can lead to drug-resistance or other
side-effects. For this reason, the advantage of traditional Chinese
medicine (TCM) has been fully recognized. TCM formulas consist of a
combination of many natural products, each of which contains
numerous chemical compounds. Therefore, TCM prescription is often
considered to have multi-component and multi-target effect. Due to
the broad range of therapeutic functions of TCM, it has long been
used to treat various diseases. One such prescription is Pien Tze
Huang (PZH), which exhibits specific anticancer activities.
In the present study, we demonstrated that PZH
significantly reduced viability and migration in different CRC cell
lines, which indicated that PZH possesses markedly antimetastasis
ability (Figs. 1 and 2). To better understand its underlying
mechanism, we determined the expression of VEGF-C in different CRC
cell lines and found that PZH markedly downregulated VEGF-C
expression. This suggests a potential mechanism, that the
inhibition of VEGF-C expression is associated with the
lymphangiogenesis-suppressive effects of PZH (Fig. 3). Since VEGF-C secreted from CRC
cells can lead to the growth of HLECs and the subsequent formation
of lymph vessels, we used exogenous VEGF-C stimulation of HLECs to
establish a model of these processes in vitro. As shown in
the results, 5 ng/ml of exogenous VEGF-C stimulation significantly
enhanced cell confluency, viability and the ability of migration
and tube formation, while PZH treatment attenuated all of these
effects (Figs. 4 and 6). Notably, we found no change in cell
apoptosis in response to PZH treatment (Fig. 5), which suggests that PZH suppresses
lymphangiogenesis mainly by the inhibition of cell proliferation,
migration and tube formation but not directly by promoting
apoptosis of HLECs. Next, to further investigate the mechanisms of
the anticancer effects of PZH on VEGF-C stimulated HLECs, we
determined its effect on the expression of related proteins.
VEGFR-3 is the cognate receptor to VEGF-C and is expressed in
HLECs. PZH treatment notably downregulated the expression of
VEGFR-3, which was increased by exogenous VEGF-C stimulation.
Moreover, the VEGF-C/VEGFR-3 signaling pathway can lead to the
activation of downstream transcription factors, resulting in the
upregulation of MMPs, which is important for cell migration.
Particularly, the increase in MMP-2 and MMP-9 can lead to the
breakdown of extracellular matrix (ECM) in the surrounding tumor
tissue and allow for the migration of HLECs. However, MMP-2 and
MMP-9 can trigger the release of VEGF-C resulting in the
proliferation of HLECs and the formation or remodeling of LVs
(31,32). We found that PZH prominently
downregulated the expression of MMP-2 and MMP-9, which was
increased by exogenous VEGF-C stimulation. Overall, these results
demonstrate that PZH suppression of lymphangiogenesis via the
downregulation of VEGF-C may be a molecular mechanism by which PZH
inhibits metastasis in CRC.
In conclusion, PZH exerts its antimetastatic
activities through the suppression of VEGF-C-mediated
lymphangiogenesis, and thus may be developed as a promising
multi-potent anticancer agent for the clinical treatment of
CRC.
Acknowledgements
The present study was sponsored by the Natural
Science Foundation of Fujian Province, China (no. 2015J01337).
Glossary
Abbreviations
Abbreviations:
|
PZH
|
Pien Tze Huang
|
|
CRC
|
colorectal cancer
|
|
HLEC
|
human lymphatic endothelial cell
|
|
LV
|
lymphtic vessel
|
|
VEGF-C
|
vascular endothelial growth
factor-C
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Becouarn Y and Rougier P: Clinical
efficacy of oxaliplatin monotherapy: Phase II trials in advanced
colorectal cancer. Semin Oncol. 25:(Suppl 5). S23–S31. 1998.
|
|
3
|
Ng M, Roy-Chowdhury S, Lum SS, Morgan JW
and Wong JH: The impact of the ratio of positive to total lymph
nodes examined and outcome in colorectal cancer. Am Surg.
75:873–876. 2009.PubMed/NCBI
|
|
4
|
Jemal A, Clegg LX, Ward E, Ries LA, Wu X,
Jamison PM, Wingo PA, Howe HL, Anderson RN and Edwards BK: Annual
report to the nation on the status of cancer, 1975–2001, with a
special feature regarding survival. Cancer. 101:3–27. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sundlisaeter E, Dicko A, Sakariassen PØ,
Sondenaa K, Enger PØ and Bjerkvig R: Lymphangiogenesis in
colorectal cancer - prognostic and therapeutic aspects. Int J
Cancer. 121:1401–1409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onogawa S, Kitadai Y, Tanaka S, Kuwai T,
Kimura S and Chayama K: Expression of VEGF-C and VEGF-D at the
invasive edge correlates with lymph node metastasis and prognosis
of patients with colorectal carcinoma. Cancer Sci. 95:32–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JG, Chae YS, Sohn SK, Cho YY, Moon JH,
Park JY, Jeon SW, Lee IT, Choi GS and Jun SH: Vascular endothelial
growth factor gene polymorphisms associated with prognosis for
patients with colorectal cancer. Clin Cancer Res. 14:62–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parr C and Jiang WG: Quantitative analysis
of lymphangiogenic markers in human colorectal cancer. Int J Oncol.
23:533–539. 2003.PubMed/NCBI
|
|
9
|
Royston D and Jackson DG: Mechanisms of
lymphatic metastasis in human colorectal adenocarcinoma. J Pathol.
217:608–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:(Suppl 16). S15–S18. 2002.
View Article : Google Scholar
|
|
11
|
Hsu JY and Wakelee HA: Monoclonal
antibodies targeting vascular endothelial growth factor: Current
status and future challenges in cancer therapy. BioDrugs.
23:289–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chinese Pharmacopoeia Commission, .
Pharmacopoeia of the People's Republic of China. 1. Chinese Medical
Science and Technology Press; Beijing: pp. 573–575. 2010
|
|
13
|
Shen A, Hong F, Liu L, Lin J, Wei L, Cai
Q, Hong Z and Peng J: Pien Tze Huang inhibits the proliferation of
human colon carcinoma cells by arresting G1/S cell cycle
progression. Oncol Lett. 4:767–770. 2012.PubMed/NCBI
|
|
14
|
Lin JM, Wei LH, Chen YQ, Liu XX, Hong ZF,
Sferra TJ and Peng J: Pien Tze Huang induced apoptosis in human
colon cancer HT-29 cells is associated with regulation of the Bcl-2
family and activation of caspase 3. Chin J Integr Med. 17:685–690.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei L, Chen P, Chen Y, Shen A, Chen H, Lin
W, Hong Z, Sferra TJ and Peng J: Pien Tze Huang suppresses the
stem-like side population in colorectal cancer cells. Mol Med Rep.
9:261–266. 2014.PubMed/NCBI
|
|
17
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 30:1701–1706. 2013.PubMed/NCBI
|
|
18
|
Shen A, Chen H, Chen Y, Lin J, Lin W, Liu
L, Sferra TJ and Peng J: Pien Tze Huang overcomes multidrug
resistance and epithelial-mesenchymal transition in human
colorectal carcinoma cells via suppression of TGF-β pathway. Evid
Based Complement Alternat Med. 2014:6794362014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin W, Zhuang Q, Zheng L, Cao Z, Shen A,
Li Q, Fu C, Feng J and Peng J: Pien Tze Huang inhibits liver
metastasis by targeting TGF-β signaling in an orthotopic model of
colorectal cancer. Oncol Rep. 33:1922–1928. 2015.PubMed/NCBI
|
|
20
|
Grau S: Expression of
lymphangiogenesis-related factors in malignant gliomas. Neurosci
Res. 60:40–49. 2008.PubMed/NCBI
|
|
21
|
Saad RS, Lindner JL, Liu Y and Silverman
JF: Lymphatic vessel density as prognostic marker in esophageal
adenocarcinoma. Am J Clin Pathol. 131:92–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu HT, Ma R, Yang QF, Du G and Zhang CJ:
Lymphangiogenic characteristics of triple negativity in
node-negative breast cancer. Int J Surg Pathol. 17:426–431. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oliver G and Detmar M: The rediscovery of
the lymphatic system: Old and new insights into the development and
biological function of the lymphatic vasculature. Genes Dev.
16:773–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Caunt M, Mak J, Liang WC, Stawicki S, Pan
Q, Tong RK, Kowalski J, Ho C, Reslan HB, Ross J, et al: Blocking
neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell.
13:331–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bahram F and Claesson-Welsh L:
VEGF-mediated signal transduction in lymphatic endothelial cells.
Pathophysiology. 17:253–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Girling JE and Rogers PA: Regulation of
endometrial vascular remodelling: Role of the vascular endothelial
growth factor family and the angiopoietin-TIE signalling system.
Reproduction. 138:883–893. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saintigny P, Kambouchner M, Ly M, Gomes N,
Sainte-Catherine O, Vassy R, Czernichow S, Letoumelin P, Breau JL,
Bernaudin JF, et al: Vascular endothelial growth factor-C and its
receptor VEGFR-3 in non-small-cell lung cancer: Concurrent
expression in cancer cells from primary tumour and metastatic lymph
node. Lung Cancer. 58:205–213. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang L, Kaipainen A and Folkman J:
Lymphangiogenesis new mechanisms. Ann NY Acad Sci. 979:111–119.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Su JL, Chen PS, Chien MH, Chen PB, Chen
YH, Lai CC, Hung MC and Kuo ML: Further evidence for expression and
function of the VEGF-C/VEGFR-3 axis in cancer cells. Cancer Cell.
13:557–560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tammela T and Alitalo K:
Lymphangiogenesis: Molecular mechanisms and future promise. Cell.
140:460–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al:
Matrix metalloproteinase-9 triggers the angiogenic switch during
carcinogenesis. Nat Cell Biol. 2:737–744. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rojiani MV, Alidina J, Esposito N and
Rojiani AM: Expression of MMP-2 correlates with increased
angiogenesis in CNS metastasis of lung carcinoma. Int J Clin Exp
Pathol. 3:775–781. 2010.PubMed/NCBI
|