Introduction
Endoplasmic reticulum has multiple physiological
functions, including involvement in the processes of protein
synthesis, protein folding, post-translational modifications,
protein transportation, and protein secretion (1–3). There
are various network structures of endoplasmic reticulum, including
tubular, paliform, and sheet structure (3,4).
Several studies have reported the use of the Connectivity Map
database to identify over 20 kinds of small molecule compounds that
induce reorganization of endoplasmic reticulum membrane, indicating
that endoplasmic reticulum undergoes different degrees of dynamic
changes under stress stimulation. The reorganization of endoplasmic
reticulum membrane is a general form of cellular response to stress
(5). In an investigation by
Varadarajan and colleagues, the endoplasmic reticulum of tumor
cells treated with the pan-Bcl-2 inhibitor apogossypol underwent a
dramatic and unique morphological change. This phenomenon, known as
endoplasmic reticulum membrane shrinkage, is seen under an electron
microscope as massive aggregation of the proteins in the
endoplasmic reticulum membrane and was defined by Varadarajan and
colleagues as the reorganization of endoplasmic reticulum membrane.
Apogossypol induces a variety of different cell lines to generate
the same morphological change in endoplasmic reticulum membrane;
these include Jurkat T lymphocytes, HeLa cells, mouse embryonic
fibroblasts (MEFs), Chinese hamster ovarian carcinoma cells (CHOs),
Saccharomycetes, and Schizosaccharomyces pombe
(5). This shows that the
reorganization of endoplasmic reticulum membrane is conservative in
nature, and maybe a new form of cellular stress response.
Since this stress response shows conservatism and
universality, it may be closely related to the mechanism of
cellular damage and anti-damage. Explanation of this mechanism may
provide further insight into the toxicity and side effects of
drugs, and the mechanism of action of antitumor drugs. Because this
dynamic change precedes the unfolded protein response (UPR) of
endoplasmic reticulum, the relationship of the reorganization of
endoplasmic reticulum membrane and the UPR is very interesting.
It is widely accepted that Bcl-2 family proteins are
located in the endoplasmic reticulum. Combined with the
aforementioned results of pan-Bcl-2 inhibitor studies, some
researchers have speculated that Bcl-2 family proteins may have the
function of regulating morphology of endoplasmic reticulum and may
be involved in the reorganization of endoplasmic reticulum
membrane. Some researchers have reported that Bcl-XL induces
endoplasmic reticulum lumen swelling through interacting with BAK,
leading to cell death in 293T cells co-expressing Bcl-XL and BAK
(6). In addition, the Bcl-2 family
protein member BH3-only protein, BNIP, has an effect on the
morphology of endoplasmic reticulum by connecting with adhesion
protein receptor syntaxin-18, and is located in endoplasmic
reticulum (7). Although this
investigation provides evidence that Bcl-2 family proteins affect
the morphological structure of endoplasmic reticulum, strong
evidence of morphology and function is lacking.
In our previous study, we found that the Bcl-2 small
molecular inhibitor S1 was different from other BH3-only mimetics,
and can inhibit high expression of the anti-apoptotic protein Mcl-1
in various tumors. Both S1 and ABT-737 induced autophagy through
interfering with the interaction of Bcl-2 and Beclin-1 (8,9). In
our follow-up study we found that both S1 and ABT-737 induced
slight reorganization of endoplasmic reticulum membrane.
Furthermore, S1 combined with ABT-737 induced severe reorganization
of endoplasmic reticulum membrane. Thus, we speculate that Bcl-2
inhibitors may cause autophagy through inducing the reorganization
of endoplasmic reticulum membrane. This suggests that autophagy was
induced by Bcl-2 inhibitors and may be related to the elimination
of damaged organelles. We found that endoplasmic reticulum partly
disappeared in the presence of persistent membrane reorganization
stress. It has yet to be determined whether reorganization of
endoplasmic reticulum membrane is a self-healing function of
endoplasmic reticulum, or the endoplasmic reticulum portion of
morphology change occurs through elimination, and if it is through
a clearing process, how it is removed (10).
There is evidence that autophagy is responsible for
eliminating long-life proteins, protein aggregation, or damaged
organelles such as endoplasmic reticulum and mitochondria. For
example, in adult myocardial cells with high expression of BNIP3,
the degradation of mitochondria increased by autophagy (11). After knocking down αSNAP in human
epithelial cells the function of Golgi apparatus is lost, and the
Golgi apparatus with loss of function is eliminated by autophagy
(12). Therefore, exploring the
relationship of autophagy and the reconstruction of endoplasmic
reticulum membrane may identify the mechanism of the reorganization
of endoplasmic reticulum membrane.
In this study, we used Bap31 and calnexin as markers
of endoplasmic reticulum membrane, this method has been widely used
and marks the endoplasmic reticulum membrane location. Using RNAi
technology and higher special inhibitor, we explored the
relationship of endoplasmic reticulum and unfolded protein
response, to assess whether autophagy is involved in morphological
changes of endoplasmic reticulum part removal. This is important
for determining the function of autophagy under new cellular stress
forms, at the same time our study also provides important evidence
for the involvement of autophagy in regulating organelle
morphology.
Materials and methods
Reagents and antibodies
Fetal bovine serum (FBS) and Roswell Park Memorial
Institute IMDM culture medium were purchased from Invitrogen.
3-Methyladenine (3-MA) and Chloroquine (CQ) were purchased from
Sigma. The BH3 mimetic S1 was supplied by Professor Zhichao Zhang
and dissolved in dimethyl sulfoxide (DMSO). FITC/Texas
Red-conjugated secondary antibodies were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). Enhanced
chemiluminescence (ECL) reagents were purchased from Thermo
Scientific. Antibodies anti-Bap31, anti-calnexin, anti-CHOP,
anti-Bip, anti-eIF2a, anti-p-eIF2a, anti-LC3, anti-Beclin-1 and
anti-Atg12 were purchased from Santa Cruz Biotechnology Inc., and
horseradish peroxidase-conjugated anti-mouse and anti-rabbit
immunoglobulins were purchased from Proteintech (Chicago, IL,
USA).
Cell culture
Human cervical cancer HeLa cells were cultured at
37°C under 5% CO2 in IMDM culture medium supplemented
with 10% FBS. The cultures were passaged by 0.4% trypsinization,
and fresh medium was changed for 2 days.
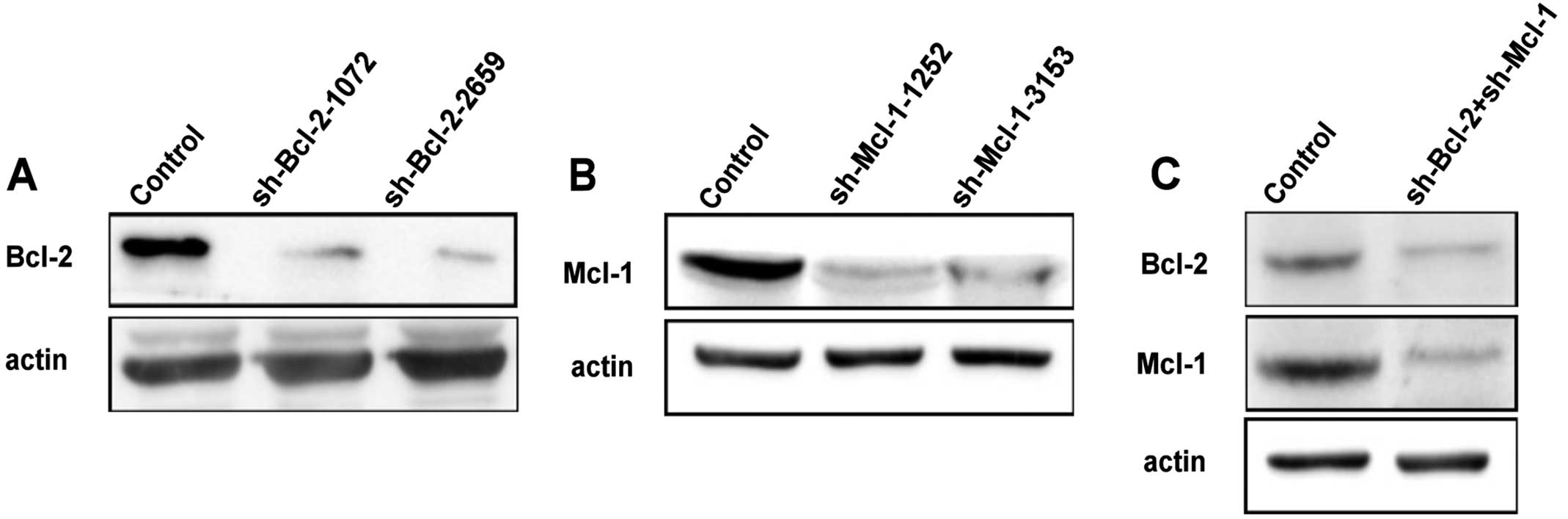
RNA interference
Bcl-2 and Mcl-1 shRNA plasmids were purchased from
Shanghai GenePharma Co., Ltd. (Shanghai, China). The sh-RNA plasmid
insert sequence was used as follows: sh-Bcl-2-1072:
CCGGGAGATAGTGATGAAGTA; sh-Bcl-2-2659: CGCCCTGTGGATGACTGAGTA;
sh-Mcl-1-1252: GCACACCTGGATCCAGGATAA; sh-Mcl-1-3153:
CCGCATTTAATTCATGGTATT; Scr: CCTGTGGAACG TGTCACGCTT.
For transformation using DH5α Escherichia
coli competent cells, the competent cells and the
transformation protocol were prepared according to a modified
procedure based on that of Zhang and colleagues (13).
Stable transfected cell lines were developed using
sh-RNA plasmids, which contain a neomycin resistance marker for the
selection of stably transfected cells. HeLa cells were transfected
with the sh-Bcl-2-1072/2659, sh-Mcl-1-1252/3153 and Scr in a 6-well
plate with Lipofectamine 2000 transfection reagent. All procedures
were performed according to the reagent supplier's guidance. A
selective medium was added containing G418 (600 µg/ml) as the
selective antibiotic pressure. Every 2–3 days, the medium was
replaced with fresh medium. The selection was continued until all
of the non-transfected cells had died. The surviving cells were
then split into a lower density in a 12-well plate. After
transfection, cells were cultured for 48 h before being harvested
or treated as indicated.
Western blot analysis
Cells were washed with phosphate-buffered saline
(PBS) twice and harvested by scraping into 300 µl of RIPA lysis
buffer. Total cell lysates were lysed for 15 min on ice, and at 4°C
for 45 min, and after centrifugation at 12,000 × g for 10 min at
4°C, the supernatant was collected. Protein concentrations in the
supernatants were determined by the Bio-Rad reagent (Hercules, CA,
USA). Equal amounts of proteins (30 µg) were subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto PVDF membrane (Millipore, Billerica, MA, USA).
Transfer efficiency was checked with Ponceau staining. The blots
were blocked in Tris-buffered saline containing nonfat dry milk 5%
(w/v), probed with specific primary antibodies overnight at 4°C.
After washing with PBS containing 0.05% Tween-20 (PBST), the
membrane was incubated with a peroxidase-conjugated secondary
antibody for 2 h at room temperature. Finally, each membrane was
probed to detect β-actin. The final dilutions and incubation times
suggested by the manufacturer were used for each antibody.
Immunodetection was performed using the ECL solution and images
captured by Syngene Bio Imaging (Synoptics, Cambridge, UK).
Densitometry quantitation of the bands was also performed using
equipment from Syngene Bio Imaging.
Immunofluorescence staining and
confocal laser microscopy
Cells were cultured onto coverslips in 24-well
plates overnight, treated with 8 µmol/l S1 and 15 µmol/l ABT-737
for different times, and fixed with 4% paraformaldehyde for 30 min.
After permeabilization with 0.1% Triton X-100 for 5 min, followed
by washing 3 times with PBS, the cells were sealed with bovine
serum albumin for 1 h and incubated with a primary antibody against
Bap31, calnexin, Atg12, Beclin-1 or LC-3 (1:100 dilution) overnight
at 4°C. The cells were rinsed and incubated with FITC/Texas
Red-conjugated secondary antibodies (1:400 dilution; Santa Cruz
Biotechnology Inc.) for 1 h at room temperature, washed with PBS
three times, and examined using the Olympus FV1000 confocal laser
microscope. Line profiles were carried out using Image Tool
software (Image Pro Plus6.0) to quantify the quality of
immunofluorescence for some images. The line profile command
displays a two-dimensional graph that represents the intensities of
pixels along a line within an image. This provides a graphical
representation of background and immunofluorescence signal.
Statistical analysis
Results are expressed as the mean ± standard
deviation (SD) of repeated experiments, as indicated in the figure
legends. Data are representative of three independent experiments
performed in triplicate. Statistical differences were evaluated
using the paired two-tailed Student's t-test. Differences were
considered statistically significant for values of P<0.05.
Results
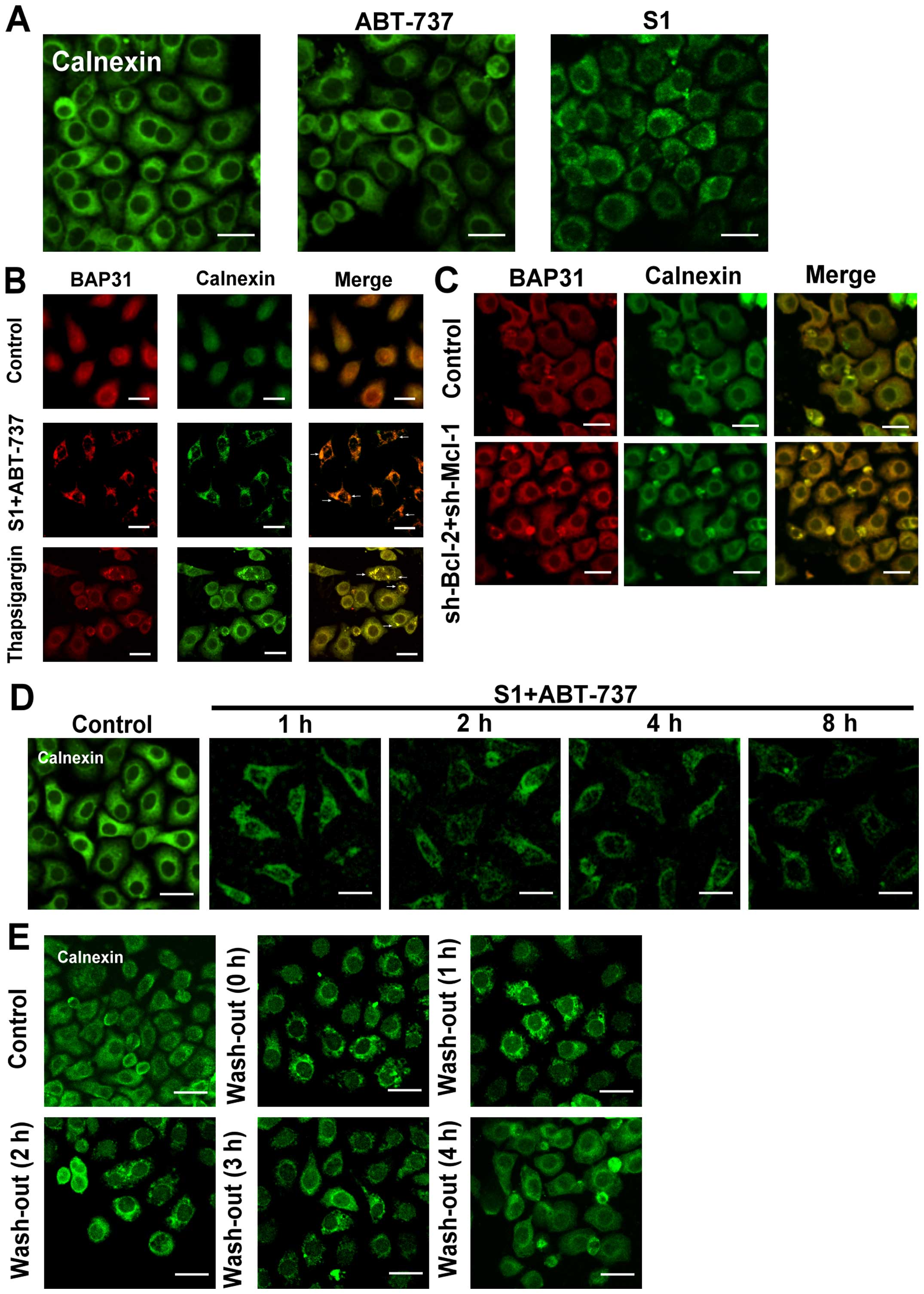
S1 combined with ABT-737 induces ER
membrane remodeling in HeLa cells
Although initially identified as central regulators
of apoptosis at the mitochondrial level, the importance of Bcl-2
proteins at the endoplasmic reticulum is now well established
(14). In our study, we used
fluorescence microscopy to monitor endoplasmic reticulum membrane
aggregation (5), and found that
either 10 µmol/l ABT-737, which inhibits Bcl-2 but not Mcl-1, or 8
µmol/l S1, which inhibits Mcl-1, induced a lower rate of
endoplasmic reticulum reorganization after 8 h treatment (Fig. 1A). However, when the two inhibitors
were applied together, the endoplasmic reticulum membrane
reorganization was increased at 4 h. A clustering of endoplasmic
reticulum membrane proteins (Bap31/calnexin) was observed in cells
treated with 8 µmol/l S1 combined with 15 µmol/l ABT-737 (Fig. 1B). When applied together, S1 and
ABT-737 induced a profound aggregation of membranous structures
resembling those induced by 5 µmol/l thapsigargin (Tg) (Fig. 1B). These results suggested that
Bcl-2 family proteins might play an important role in endoplasmic
reticulum membrane reorganization.
We also observed endoplasmic reticulum membrane
reorganization in HeLa cells which knock down Bcl-2 and Mcl-1 with
small interfering RNA (Figs. 1C and
2). In addition, calnexin clusters
were seen at 1 h in HeLa cells treated with S1 combined with
ABT-737, gradually becoming more serious with prolonged exposure
time (Fig. 1D). Rapid and complete
dispersal of endoplasmic reticulum membrane reorganization was
observed when S1 and ABT-737 were washed out (Fig. 1E), indicating the reorganization of
the endoplasmic reticulum membranes is reversible.
S1 combined with ABT-737 induces
endoplasmic reticulum membrane remodeling earlier than UPR-related
changes in HeLa cells
We investigated whether the inhibition of Bcl-2 and
Mcl-1 by 8 µmol/l S1 and 15 µmol/l ABT-737 could trigger canonical
endoplasmic reticulum stress. Results showed that expression of
Bip, eIF2a, and CHOP was upregulated following treatment with S1
combined with ABT-737 in HeLa cells, however, S1 combined with
ABT-737 in HeLa cells had little effect on the expression of
p-eIF2a. This results showed that the UPR-related changes were
detected after 8 h, whereas the reorganization of the endoplasmic
reticulum membranes occurred before 8 h (Fig. 3A and B, *P<0.05, **P<0.01).
Furthermore, 500 µmol/l tauroursodeoxycholate (TUDCA), an
endoplasmic reticulum stress inhibitor, failed to abolish
endoplasmic reticulum membrane reorganization induced by S1
combined with ABT-737 in HeLa cells (Fig. 3C).
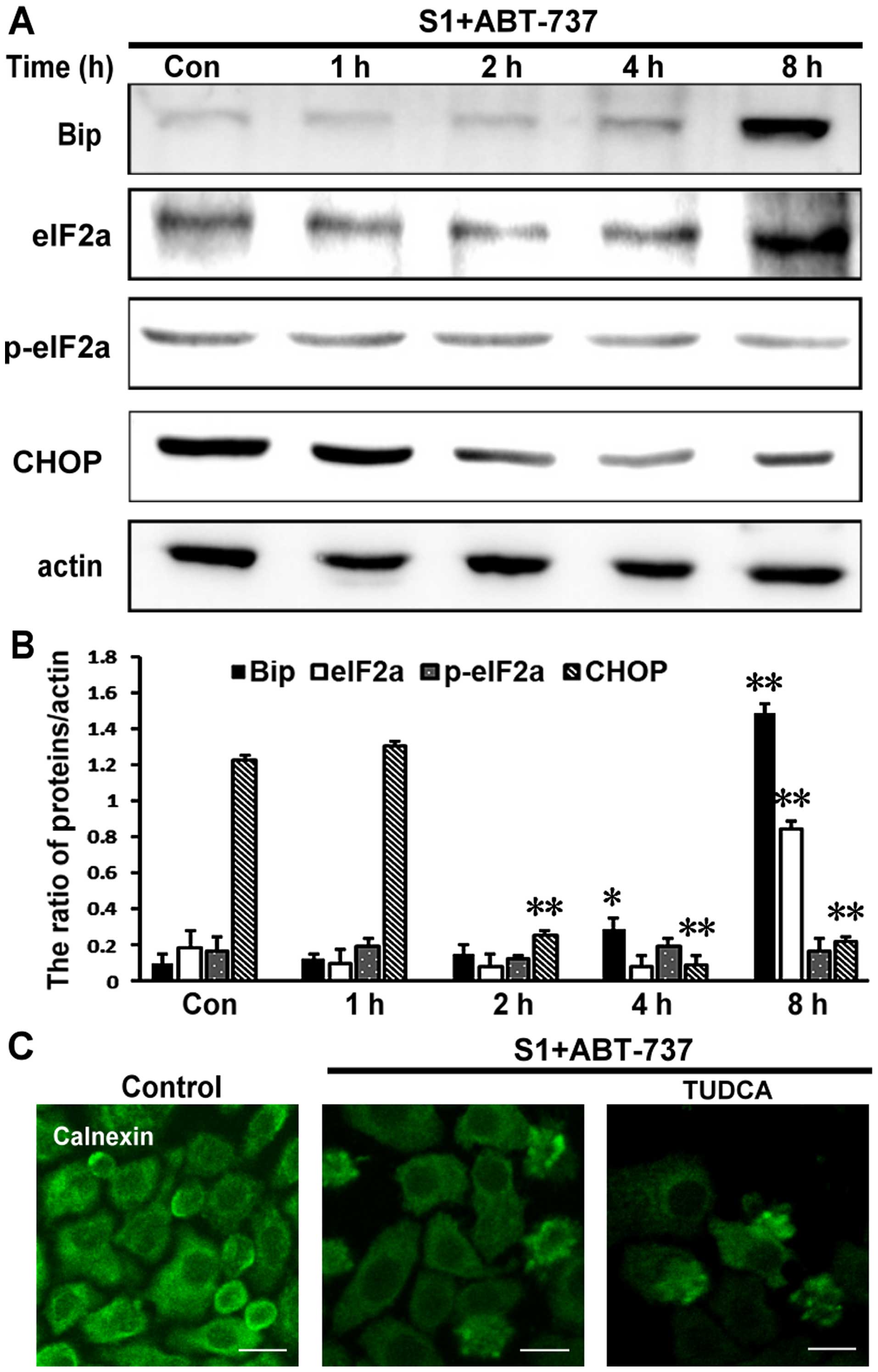 | Figure 3.The reorganization of endoplasmic
reticulum membrane earlier than the changes of the expression of
UPR-related proteins. (A) Western blot analysis for the expression
of Bip, eIF2a, p-eIF2a and CHOP in HeLa cells treated with 15
µmol/l ABT-737 and 8 µmol/l S1 for various times (0, 1, 2, 4, 8 h).
(B) Quantitative analysis of Bip, eIF2a, p-eIF2a and CHOP protein
levels from (A). Data are presented as mean ± SD, n=3, *P<0.05,
**P<0.01 versus control group. (C) Confocal microscopy of
calnexin in the cytoplasm of HeLa cells treated with 15 µmol/l
ABT-737 and 8 µmol/l S1 or combined with 500 µmol/l TUDCA in HeLa
cells for 4 h (scale bar, 20 µm). |
S1 combined with ABT-737 downregulates
endoplasmic reticulum membrane proteins
Of note, in this study we found that S1 combined
with ABT-737 markedly decreased the expression of the endoplasmic
reticulum proteins calnexin and Bap31 as early as 4 h (Fig. 4A and C, *P<0.05). Protein
degradation is a fundamental cellular process that is executed by
the separately regulated autophagy-lysosomal and
ubiquitin-proteasome systems (15).
As expected, degradation of ER membrane proteins was blocked by 10
mM 3-MA (Fig. 4B and D, *P<0.05,
#P<0.05). These results suggested that autophagy
might be associated with decrease of endoplasmic reticulum membrane
proteins.
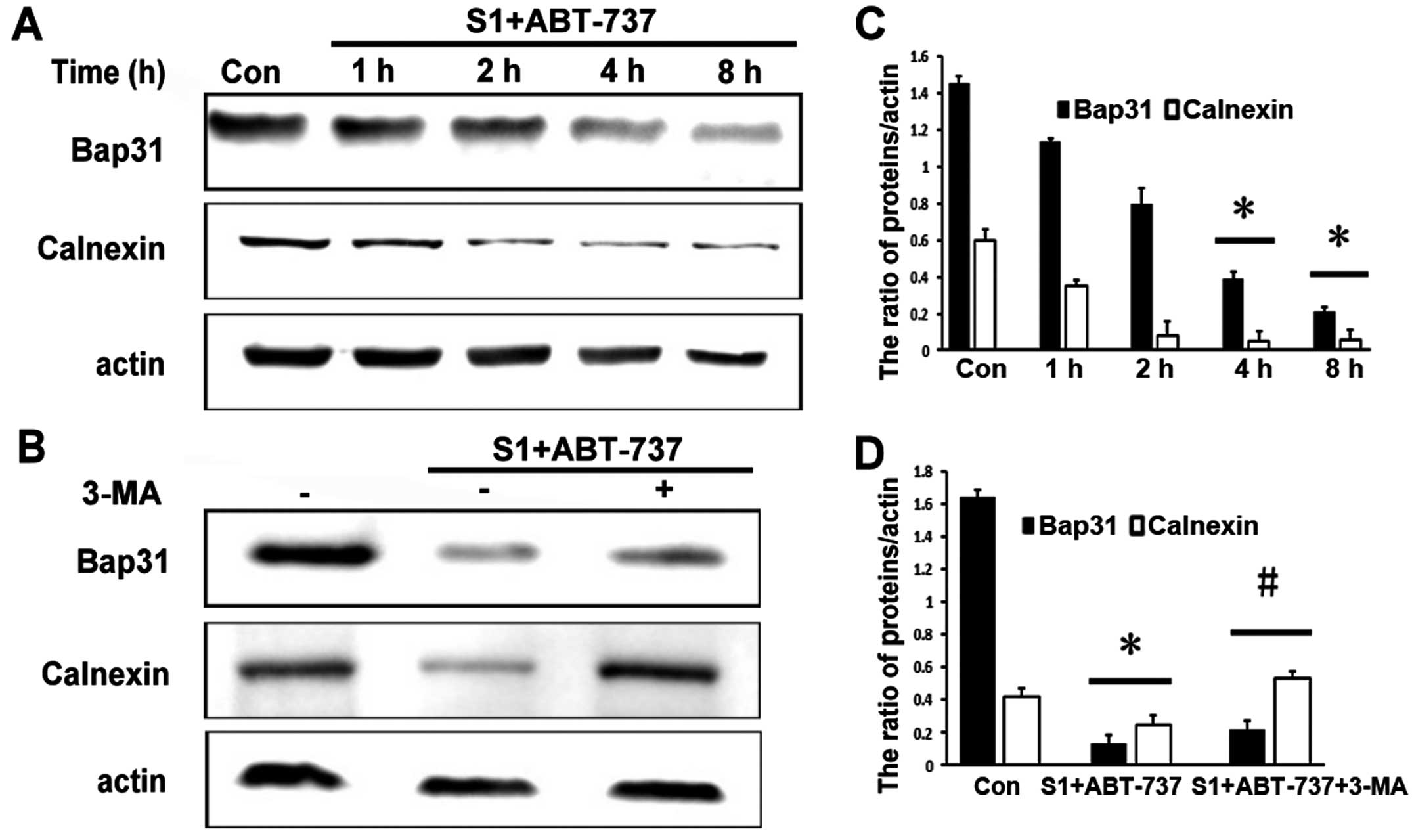 | Figure 4.Degradation of ER membrane proteins is
blocked by 3-MA. (A) Western blot analysis for the expression of
Bap31, calnexin in HeLa cells treated with 15 µmol/l ABT-737 and 8
µmol/l S1 for various times (0, 1, 2, 4, 8 h). (B) Western blot
analysis for the expression of Bap31, calnexin in HeLa cells
treated with 15 µmol/l ABT-737 and 8 µmol/l S1 or combined with 10
mM 3-MA in HeLa cells for 4 h. (C) Quantitative analysis of Bap31,
calnexin protein levels from (A). Data are presented as mean ± SD,
n=3, *P<0.05 versus control group. (D) Quantitative analysis of
Bap31, calnexin protein levels from (B). Data are presented as mean
± SD, n=3, *P<0.05 versus control group, #P<0.05
versus S1 combined with ABT-737 group. |
S1 combined with ABT-737 induces
autophagy in HeLa cells
In addition to regulating apoptosis, it has been
reported that Bcl-2 protein has a function in autophagy (16). Moreover, research has shown that
autophagy plays an important role in cellular quality control and
is responsible for removing protein aggregates and dysfunctional
organelles (17). In addition, our
previous study reported that S1 induced autophagy through
inhibiting the interaction of Bcl-2 and Beclin1, and then inducing
the release of autophagy initiation protein Beclin1, finally
inducing autophagy in U251 cells (8) and the mechanism of ABT-737 induces
autophagy similarly to S1 (9).
Therefore, we further explored whether S1 combined with ABT-737
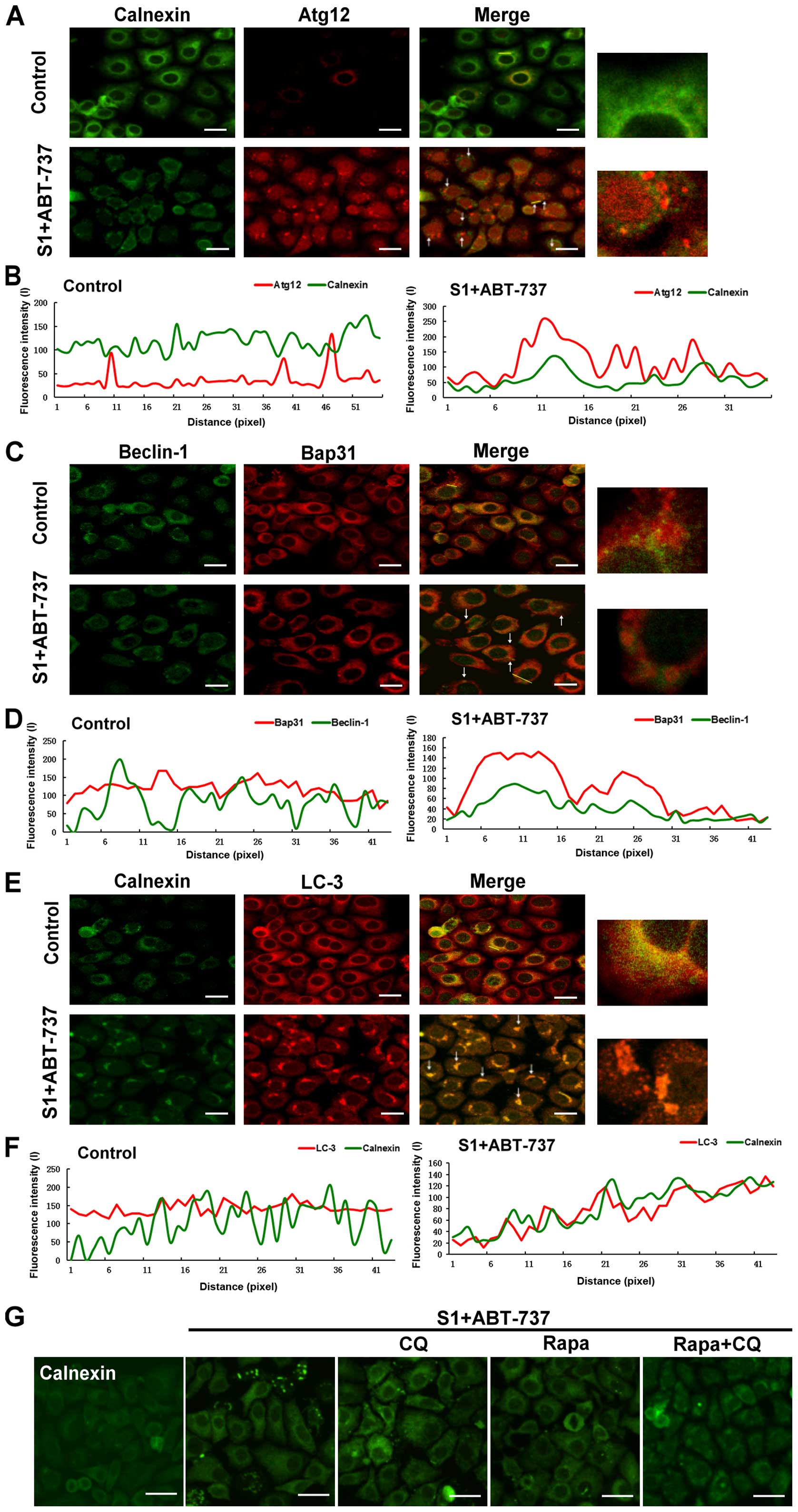
could indeed induce autophagy in HeLa. Atg12-Atg5 and Atg8 (LC3),
the two ubiquitin-like conjugation systems, are required for the
initiation and expansion of autophagosomal membranes (17).
Our results showed that the expression of the
Atg12-Atg5 complex was increased in HeLa cells treated with S1
combined with ABT-737 (Fig. 5A and
B, *P<0.05, **P<0.01). When autophagy occurs, LC3 protein
appears as dots, and the soluble form of LC3 (LC3-I) changes into
the lipidated and autophagosome-associated form (LC3-II) (18,19).
Compared with the control group, S1 and ABT-737 increased the
expression of LC3-II in HeLa cells (Fig. 5A and B, *P<0.05, **P<0.01).
Autophagy-related protein Beclin-1 is very important for the
formation of autophagosomes. The combination of S1 and ABT-737 also
increased the expression of Beclin-1 after 4 h in HeLa cells
(Fig. 5A and B, *P<0.05,
**P<0.01). In addition, visible LC3 dots in the cytoplasm were
observed in HeLa cells treated with S1 combined with ABT-737
(Fig. 5C). These results indicated
that autophagy does indeed occur in HeLa cells treated with S1
combined with ABT-737.
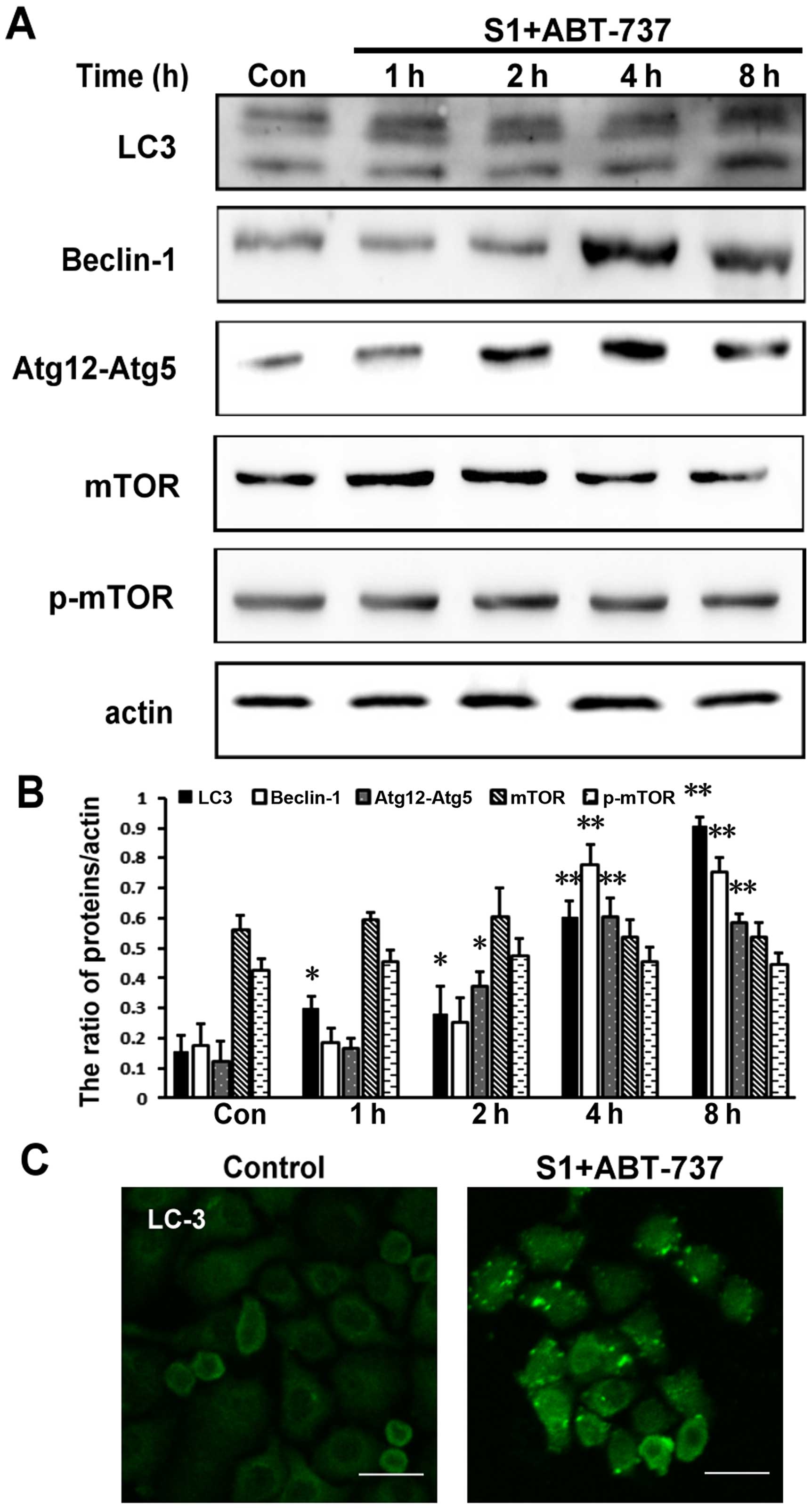 | Figure 5.S1 combined with ABT-737 induces
autophagy in HeLa cells. (A) Western blot analysis for the
expression of LC3, Beclin-1, Atg12-Atg5, mTOR and p-mTOR in HeLa
cells treated with 15 µmol/l ABT-737 and 8 µmol/l S1 for various
times (0, 1, 2, 4, 8 h). (B) Quantitative analysis of LC3,
Beclin-1, Atg12-Atg5, mTOR and p-mTOR protein levels from (A). Data
are presented as mean ± SD, n=3, *P<0.05, **P<0.01 versus
control group. (C) Confocal microscopy of LC3 in the cytoplasm of
HeLa cells treated with 15 µmol/l ABT-737 and 8 µmol/l S1 for the 8
h. |
The mTOR pathway is the classical pathway that is
often inhibited in autophagy (20,21).
Therefore, we tested the expression level of phosphorylated protein
p-mTOR involved in the mTOR pathway. However, in this study, p-mTOR
remained relatively unchanged in HeLa cells treated with S1
combined with ABT-737 (Fig. 5A and
B). Therefore, we speculated that autophagy induced by S1
combined with ABT-737 might occur through reorganization of the
endoplasmic reticulum membranes.
Aggregation of the endoplasmic
reticulum are removed by autophagy
The previous results showed that autophagy might be
associated with decrease of endoplasmic reticulum membrane
proteins. Therefore, we further investigated whether autophagy
promoted the removal of membrane-reorganized endoplasmic reticulum.
HeLa cells were stained with an antibody against calnexin or Bap31
to label the endoplasmic reticulum, and with antibodies against
Beclin-1, Atg12, or LC-3 to label the autophagosomes. The cells
analyzed by high-resolution imaging revealed extensive
co-localization between autophagosomes and endoplasmic reticulum of
membrane reorganized induced by the combination of S1 and ABT-737
in HeLa cells (Fig. 6A, C and E). A
pseudo-line scan analysis confirmed that autophagosomes
co-localized with endoplasmic reticulum of membrane reorganized
induced by the combination of S1 and ABT-737 in HeLa cells
(Fig. 6B, D and F). Rapamycin, an
autophagy inducer, relieved endoplasmic reticulum membrane
reorganization induced by S1 combined with ABT-737 in HeLa cells
(Fig. 6G). In contrast, blockage of
autophagy activity by 10 mmol/l CQ could not delay or relieve
endoplasmic reticulum membrane reorganization induced by S1
combined with ABT-737 (Fig. 6G).
These results suggested that collapse/aggregation of the
endoplasmic reticulum might trigger autophagy for the clearance of
aggregated organelles.
Discussion
To date, numerous studies have reported that when
the activities of Bcl-2 family proteins in endoplasmic reticulum
were changed, endoplasmic reticulum underwent certain morphological
changes, including lumen swelling, membrane fusion, reorganization,
and permeabilization. Wang and colleagues used the YFP fluorescent
protein to mark the endoplasmic reticulum protein PDI, and the
overexpression of BAK and BAX in endoplasmic reticulum membrane
induced the release of PDI to the cytoplasm, suggesting that BAK
and BAK can increase endoplasmic reticulum membrane
permeabilization (22). Hetz and
colleagues found that cells treated with thapsigargin undergo
endoplasmic reticulum membrane reorganization involving the massive
aggregation of endoplasmic reticulum membrane, and that this
reorganization was completely inhibited in cells with double
knock-down BAX and BAK, confirming that Bcl-2 family proteins could
regulate the structure and dynamic changes of endoplasmic reticulum
(23).
In this study, we also discovered a new kind of cell
stress response that occurred after treatment with a Bcl-2
inhibitor. The main feature of this response was the acute and
recoverable bulk aggregation of the endoplasmic reticulum membrane,
which preceded the changes in the expression of the endoplasmic
reticulum stress response-related marker proteins GRP78 and CHOP.
We discovered similar endoplasmic reticulum reorganization,
including massive aggregation of endoplasmic reticulum membrane in
HeLa cells with knockdown Bcl-2 and Mcl-1, which indicated that
Bcl-2 family proteins played an important role in the structure and
dynamics of the endoplasmic reticulum membrane. The morphological
change of the endoplasmic reticulum membrane induced by inhibition
of Bcl-2 family proteins is similar to the reorganization of
endoplasmic reticulum membrane induced by thapsigargin. Therefore,
both of the above results suggest that Bcl-2 family proteins play a
pivotal role in the structure and function of the endoplasmic
reticulum.
It is worth noting that the period during which this
cell stress response occurs is different from the occurrence of
UPR. Several studies suggested that S1 and ABT-737 could
up-regulate the expression of the endoplasmic reticulum stress
protein Bip, and obviously increased the expression of the
endoplasmic reticulum stress-related apoptotic proteins CHOP and
Caspase-4, this evidence showed that S1 and ABT-737 induced
apoptosis through endoplasmic reticulum stress (8,24).
However, we discovered that the reorganization of endoplasmic
reticulum membrane preceded the changes in the expression of
UPR-related proteins. We further confirmed the relationship between
the time of endoplasmic reticulum membrane aggregation and the
occurrence of UPR using the endoplasmic reticulum stress inhibitor
TUDCA. Our results showed that TUDCA had no effect on the
reorganization of endoplasmic reticulum membrane induced by S1
combined with ABT-737.
Autophagy is a process of eliminating partly damaged
organelles, and in several studies electron microscopy showed
endoplasmic reticulum and mitochondria in autophagic vacuoles
(25,26). Our previous work showed that Bcl-2
inhibitors could induce autophagy, and that the inhibition of
autophagy can increase apoptosis. We then explored whether S1
combined with ABT-737 induced autophagy. Our results showed that
the expression of the autophagy-related proteins Beclin-1, Atg12,
and LC3-II increased in HeLa cells treated with S1 combined with
ABT-737, in addition, we observed dot aggregation of LC-3. These
results confirmed that S1 combined with ABT-737 induced autophagy
in HeLa cells.
The above results indicated that the Bcl-2
inhibitors S1 and ABT-737 not only induce ER membrane
reorganization but also induce autophagy, furthermore, the ER
membrane reorganization occurs earlier than autophagy induced by S1
and ABT-737. When the ER membrane reorganization induced by S1 and
ABT-737 is at high level, it will induce autophagy and autophagy
will reduce the ER membrane reorganization. Therefore, the Bcl-2
inhibitors eventually reduce ER membrane reorganization possibly
due to enhanced autophagy.
Furthermore, we speculated that Bcl-2 inhibitors
might cause autophagy through inducing the reorganization of the
endoplasmic reticulum membrane. We were interested in whether the
aggregation of endoplasmic reticulum membrane triggers autophagy,
and whether this autophagy would partly eliminate endoplasmic
reticulum aggregated. According to the work of Varadarajan and
colleagues (5), we used the
expression of the endoplasmic reticulum membrane marker proteins
calnexin and Bap31 to represent the contents of endoplasmic
reticulum, and we determined that S1 combined with ABT-737
obviously decreased the expression of endoplasmic reticulum
membrane marker proteins, and the autophagy inhibitor 3-MA
recovered the expression of endoplasmic reticulum membrane marker
proteins in cells that were treated with S1 and ABT-737. These
results suggested that autophagy might be related to the decrease
of endoplasmic reticulum contents.
Several different factors may lead to endoplasmic
reticulum fragments being degraded by sequestration into
double-membrane vesicles, Hayashi-Nishino et al used
electron tomography to show that early autophagic structures as a
subdomain of the endoplasmic reticulum formed a cradle encircling
endoplasmic reticulum in mammalian culture cells (26). Overexpression of a mutant form of
Atg4B caused defects in autophagosome formation. We show a process
by which aggregated portions of endoplasmic reticulum treated with
S1 and ABT-737 were degraded by autophagy in HeLa cells. It is
possible that autophagosomes envelop specialized areas of the
endoplasmic reticulum that are aggregated. This theory is also
corroborated by observations from high-resolution imaging that
revealed extensive co-localization between autophagosomes and the
endoplasmic reticulum of membrane reorganized induced by S1 and
ABT-737 in HeLa cells. Our results showed that inhibition of
autophagy by CQ could not delay or relieve S1 and ABT-737-induced
endoplasmic reticulum membrane reorganization, whereas rapamycin
greatly relieved endoplasmic reticulum membrane reorganization,
suggesting that reorganization may trigger autophagy.
In summary, the current study showed that the S1 and
ABT-737 combination triggered endoplasmic reticulum membrane
reorganization in HeLa cells, and importantly autophagy played a
crucial role in the clearance of damaged organelles and can be a
catabolic process. Inhibition of Bcl-2 and Mcl-1 resulted in
endoplasmic reticulum membrane reorganization, and the collapsed
endoplasmic reticulum then activated autophagy for clearance of
aggregated organelles. Abrupt endoplasmic reticulum aggregation
occurs following sequestration. Lastly, the fully formed
autophagosomes acidified their contents, including aggregated
organelles and then degradate them. These events provide new
insight that autophagy can remove the endoplasmic reticulum
membrane reorganization induced by Bcl-2 inhibitors ABT-737 and S1
and it may provide a framework for analyzing autophagy in certain
diseases.
Acknowledgements
The study was supported by the National Natural
Science Foundation of China (grant numbers: 81472419 and
81272876).
References
|
1
|
Kim H, Bhattacharya A and Qi L:
Endoplasmic reticulum quality control in cancer: Friend or foe.
Semin Cancer Biol. 33:25–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dicks N, Gutierrez K, Michalak M,
Bordignon V and Agellon LB: Endoplasmic reticulum stress, genome
damage, and cancer. Front Oncol. 5:112015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin S, Sun S and Hu J: Molecular basis for
sculpting the endoplasmic reticulum membrane. Int J Biochem Cell
Biol. 44:1436–1443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
English AR and Voeltz GK: Endoplasmic
reticulum structure and interconnections with other organelles.
Cold Spring Harb Perspect Biol. 5:a0132272013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varadarajan S, Bampton ET, Smalley JL,
Tanaka K, Caves RE, Butterworth M, Wei J, Pellecchia M, Mitcheson
J, Gant TW, et al: A novel cellular stress response characterised
by a rapid reorganisation of membranes of the endoplasmic
reticulum. Cell Death Differ. 19:1896–1907. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klee M and Pimentel-Muiños FX: Bcl-X(L)
specifically activates Bak to induce swelling and restructuring of
the endoplasmic reticulum. J Cell Biol. 168:723–734. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakajima K, Hirose H, Taniguchi M,
Kurashina H, Arasaki K, Nagahama M, Tani K, Yamamoto A and Tagaya
M: Involvement of BNIP1 in apoptosis and endoplasmic reticulum
membrane fusion. EMBO J. 23:3216–3226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhong JT, Xu Y, Yi HW, Su J, Yu HM, Xiang
XY, Li XN, Zhang ZC and Sun LK: The BH3 mimetic S1 induces
autophagy through ER stress and disruption of Bcl-2/Beclin 1
interaction in human glioma U251 cells. Cancer Lett. 323:180–187.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malik SA, Shen S, Mariño G, BenYounès A,
Maiuri MC and Kroemer G: BH3 mimetics reveal the network properties
of autophagy-regulatory signaling cascades. Autophagy. 7:914–916.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rubinsztein DC, Gestwicki JE, Murphy LO
and Klionsky DJ: Potential therapeutic applications of autophagy.
Nat Rev Drug Discov. 6:304–312. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quinsay MN, Thomas RL, Lee Y and
Gustafsson AB: Bnip3-mediated mitochondrial autophagy is
independent of the mitochondrial permeability transition pore.
Autophagy. 6:855–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naydenov NG, Harris G, Morales V and
Ivanov AI: Loss of a membrane trafficking protein αSNAP induces
non-canonical autophagy in human epithelia. Cell Cycle.
11:4613–4625. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Xie C, Zhong J, Chen H, Zhang H
and Wang X: A549 cell proliferation inhibited by RNAi mediated
silencing of the Nrf2 gene. Biomed Mater Eng. 24:3905–3916.
2014.PubMed/NCBI
|
|
14
|
Heath-Engel HM, Chang NC and Shore GC: The
endoplasmic reticulum in apoptosis and autophagy: Role of the BCL-2
protein family. Oncogene. 27:6419–6433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Long MJ, Gollapalli DR and Hedstrom L:
Inhibitor mediated protein degradation. Chem Biol. 19:629–637.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maejima Y, Kyoi S, Zhai P, Liu T, Li H,
Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, et al: Mst1
inhibits autophagy by promoting the interaction between Beclin1 and
Bcl-2. Nat Med. 19:1478–1488. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanna RA, Quinsay MN, Orogo AM, Giang K,
Rikka S and Gustafsson AB: Microtubule-associated protein 1 light
chain 3 (LC3) interacts with Bnip3 protein to selectively remove
endoplasmic reticulum and mitochondria via autophagy. J Biol Chem.
287:19094–19104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tooze SA, Jefferies HB, Kalie E, Longatti
A, McAlpine FE, McKnight NC, Orsi A, Polson HE, Razi M, Robinson
DJ, et al: Trafficking and signaling in mammalian autophagy. IUBMB
Life. 62:503–508. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paglin S, Lee NY, Nakar C, Fitzgerald M,
Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N, et
al: Rapamycin-sensitive pathway regulates mitochondrial membrane
potential, autophagy, and survival in irradiated MCF-7 cells.
Cancer Res. 65:11061–11070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa
T, Aoki H, Mills GB and Kondo S: Synergistic augmentation of
rapamycin-induced autophagy in malignant glioma cells by
phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer
Res. 65:3336–3346. 2005.PubMed/NCBI
|
|
22
|
Wang X, Olberding KE, White C and Li C:
Bcl-2 proteins regulate ER membrane permeability to luminal
proteins during ER stress-induced apoptosis. Cell Death Differ.
18:38–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hetz C, Bernasconi P, Fisher J, Lee AH,
Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A,
Glimcher LH, et al: Proapoptotic BAX and BAK modulate the unfolded
protein response by a direct interaction with IRE1alpha. Science.
312:572–576. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu N, Xu Y, Sun JT, Su J, Xiang XY, Yi
HW, Zhang ZC and Sun LK: The BH3 mimetic S1 induces endoplasmic
reticulum stress-associated apoptosis in cisplatin-resistant human
ovarian cancer cells although it activates autophagy. Oncol Rep.
30:2677–2684. 2013.PubMed/NCBI
|
|
25
|
Schuck S, Gallagher CM and Walter P:
ER-phagy mediates selective degradation of endoplasmic reticulum
independently of the core autophagy machinery. J Cell Sci.
127:4078–4088. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi-Nishino M, Fujita N, Noda T,
Yamaguchi A, Yoshimori T and Yamamoto A: A subdomain of the
endoplasmic reticulum forms a cradle for autophagosome formation.
Nat Cell Biol. 11:1433–1437. 2009. View
Article : Google Scholar : PubMed/NCBI
|