Introduction
Capers are a common ingredient in Mediterranean
cuisine, particularly Cypriot, Italian and Maltese recipes.
Capparis spinosa L. (CSE), the caper bush, also called
cishangan, Euphorbia lathyris L. and Capparis
masaikai Levl., is a perennial plant that bears round, fleshy
leaves and large white to pinkish-white flowers. CSE is found in
the wild in the Mediterranean, East Africa, Madagascar,
Southwestern and Central Asia, Himalayas, the Pacific Islands,
Indomalaya and Australia. The plant is best known for the edible
flower buds (capers), often used as a seasoning, and the fruit
(caper berries), both of which are usually consumed pickled. The
salted and pickled caper bud (called simply a caper) is often used
as a seasoning or garnish. The mature fruit of the caper shrub are
prepared similarly and marketed as caper berries. The buds are
picked, then pickled in salt, or a salt and vinegar solution, and
drained. Other parts of the Capparis plants are used in the
manufacture of medicines and cosmetics. The shrubby plant is
many-branched, with alternate leaves, thick and shiny, round to
ovate. The flowers are complete, sweetly fragrant and showy, with
four sepals and four white to pinkish-white petals. Intense flavor
is developed as mustard oil is released from each caper bud. This
enzymatic reaction leads to the formation of rutin, often seen as
crystallized white spots on the surfaces of individual caper buds.
In Greek popular medicine, a herbal tea made of caper root and
young shoots is considered beneficial against rheumatism (1–6).
CSE contains glucosinolates (glucocapparin,
glucocleomin, glucoiberin, glucopangulin and singrin), flavonoids
and choline, coumarins, saponins and tannins. Instruction exists on
the use of sprouts, roots, leaves and seeds in the treatment of
strangury and inflammation. Different flavonoids have been
identified in the caper bush and capers: rutin (quercetin
3-rutinoside), quercetin 7-rutinoside, quercetin
3-glucoside-7-rhamnoside, kaempferol 3-rutinoside, astragalin and
kaempferol 3-rhamnorutinoside. Capers contain more quercetin/weight
than any other plant (7). Selenium
is present in capers at high concentrations in comparison with
other vegetable products. Furthermore, CSE extract was reported to
be rich in flavonoids such as kaempferol, rutin, quercetin and
quercetin derivatives, which are known to have anti-allergic,
anti-inflammatory, antioxidant, anti-fungal, anti-bacterial
anti-hepatotoxic, anti-diabetic, antiproliferative and antitumor
properties. Recently, the effect of plant extracts on melanogenesis
has been reported and compounds, such as glycyrrhizin, quercetin
and scoparone, were found to stimulate melanogenesis (8–19).
Doxorubicin (DOX) is a potent broad-spectrum
chemotherapeutic agent that is highly effective in treating
patients with acute lymphoblastic leukemia, Hodgkins lymphoma,
aggressive non-Hodgkins lymphomas, breast and ovarian carcinoma,
and many solid tumors. The therapeutic activity of DOX is achieved
through the processes of intercalating into DNA, inhibiting
topoisomerase II, and preventing DNA and RNA synthesis.
Unfortunately, its clinical chemotherapeutic use is limited by its
severe toxicity on the heart when the accumulative dose reaches a
threshold. The cardiotoxicity particularly subchronic and delayed
cardiotoxicity is manifested by dose-dependent cardiomyopathy and
refractory congestive heart failure with the unique pathological
changes being distention of the endoplasmic reticulum, swelling of
mitochondria, cytoplasmic vacuolization and myofibrillar disarray,
and loss (sarcopenia) in cardiomyocytes as well as apoptosis. A
great deal of research has been carried out to investigate the
molecular mechanisms by which DOX selectively impairs the heart. As
a result, a number of mechanisms were proposed although most of
them are attributable to the basis that DOX increases the
production of ROS in cardiomyocytes (20–25).
It was found in previous experiments that ethyl acetate extract of
CSE has antioxidant activity and improves the oxidative damage
symptoms observed by morphological changes of pathology and
hematoxylin and eosin (H&E) staining. The major mechanism of
heart dysfunction induced by oxidative stress (free radical damage)
that is responsible for DOX cardiotoxicity is the formation of ROS,
which can harm membrane lipids and other cellular components,
leading to cardiomyocyte apoptosis and death. In order to further
research this issue, the present study investigated blood
biochemical indices, injury, energy metabolism, oxidative damage
and mitochondrial membrane potential (Δψm) level of cardiac cells
to study the effect of CSE on DOX-induced cardiac toxicity. Thus,
the first part of the study was designed to investigate whether CSE
has any protective effect on oxidative damage induced by DOX and to
explore whether or not CSE can be used as an adjuvant therapy for
the long-term clinical use of DOX. Our theory basis can make a
foundation for the following research.
In a previous study, we demonstrated that CSE
inhibited tumor cell growth and the main constituent responsible
for the antitumor activity is alkaloid (26). The quaternary ammonium hydroxide of
Capparis spinosa L. (CSQAH) is one of the water-soluble
alkaloids which is obtained by means of ammonium reineckate. It
also inhibited tumor cell growth and induced cell apoptosis.
However, the molecular mechanisms associated with the apoptosis of
human hepatocellular carcinoma HepG2 cells by CSQAH is not clear
and systematically understood. In the second part of the study, we
therefore utilized flow cytometry (FCM) and laser scanning confocal
microscopy (LSCM) to detect Ca2+ concentrations,
Ca2+-Mg2+-ATP enzyme activity and ROS, Bcl-2
and Bax levels to investigate the regulatory mechanism of HepG2
cell apoptosis induced by CSQAH.
In summary, the present study demonstrated the
antioxidant and antitumor activities of CSE which may suppress
tumor growth and alleviate the side-effects of DOX, which may
facilitate tumor treatment in a dual manner.
Materials and methods
Antioxidant activity of CSE and its
protective effect on oxidative damage induced by DOX
Animals and treatment
The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Ethics Committee of the Animal
Experiments of the Heilongjiang University of Chinese Medicine
(permit no. 2013–004). All surgery was performed under sodium
pentobarbital anesthesia, and all efforts were made to minimize
suffering of the animals.
Kunming mice (animal certificate no. POO101009;
provided by the Animal Experiment of Heilongjiang University of
Chinese Medicine), half male and female, were randomly divided into
five groups (n=12/group): normal, DOX and CSE groups; CSE-L, CSE-M
and CSE-H (40, 80 and 120 mg/kg). The numbers of each group fit the
statistical requirements. Food and water were provided freely and
the mice were sacrificed by cervical dislocation in accordance with
ethical requirements. Each mouse was weighed before administration.
The normal group was orally administered normal saline (0.01 ml/g,
daily), and the DOX groups were intraperitoneal injected with DOX
hydrochloride (15 mg/kg) once on day 5. The CSE groups received
different doses of CSE extract orally for five consecutive days and
were intraperitoneally injected with a single dose of DOX
hydrochloridex (15 mg/kg) 2 h after the CSE extract treatment on
day 5, and then CSE was continued to be administered for 2
days.
Screening of antioxidant fraction by EPPH
The fruits of CSE (5 kg) were extracted with 95%
ethanol. Evaporation of the solvent under reduced pressure provided
the condensed ethanol extract, which was then extracted by
petroleum ether, chloroform, ethyl acetate and n-butyl
alcohol in turn. The five factions were assessed for their
antioxidant activities by EPPH method (27).
Effect of CSE on injury and energy metabolism
induced by DOX
The serum of all test groups was prepared. The
levels of lactic dehydrogenase (LDH) and creatine kinase (CK) were
measured by ultraviolet spectrophotometric method according to the
instruction manual of the reagent (28). Then, the cardiac tissue of all test
groups was prepared. The ATPase activities were measured by
ultraviolet spectrophotometric method according to the instruction
manual of the reagent (29).
Effect of CSE on oxidative damage induced by
DOX
The cardiac tissues of all test groups were
prepared. The level of malondialdehyde (MDA) was determinated by
ultraviolet spectrophotometric method according to the instruction
manual of the reagent (30).
Secondly, determination of intracellular ROS levels was performed
by measuring a fluorescent product formed by DCFHDA (10 µmol/l).
The samples were incubated in an incubator at 37°C for 40 min, and
then washed with phosphate-buffered saline (PBS) three times. The
cells were made into a suspension using 300 ml PBS and put through
a 200 mesh screen. The relative amount of fluorescent product was
monitored by FCM at 48 and 535 nm (31). Finally, antioxidation enzyme
indicators in the serum were measured. The mice were sacrificed
under the influence of anesthesia. The heart was excised
immediately, rinsed in ice-cold normal saline, blotted between two
filter papers, and weighed. Heart tissue (0.5 g) was triturated and
dropped into 10% tissue homogenate according to the volume and
weight ratio. The cell debris was removed using centrifugation at
3,000 rpm for 15 min. The cell supernatants were collected, and
then diluted with normal saline in proportion to the required
concentration of tissue homogenate. The protein content of the cell
lysates was determined using the Bradford assay. T-AOC of blood
measurement, superoxide dismutase (SOD), glutathione (GSH) and
catalase (CAT) of heart tissue homogenate assay were carried out
according to the kit illustrations using ultraviolet
spectrophotometer.
Effect of CSE on Δψm levels of cardiac cells
induced by DOX
The purification of cardiac cells was carried out by
counting with a hemocytometer. Cardiac cells (lx106)
were placed in dimethyl sulfoxide (DMSO), and incubated in an
incubator at 37°C for 40 min with Rh123 for testing Δψm, and then
washed with PBS three times. The 300 ml PBS suspension was
filtrated through 200 mesh size screen. Cells (104) were
collected and measured with FCM, excitation and maximum absorption
at 488 and 530 nm (32).
Antitumor activity of CSQAH and its
apoptosis induction on HepG2 cells
Cell culture
Human gastric carcinoma cell line SGC-7901 was
cultured in RPMI-1640 medium with 10% calf serum at 37°C in a 5%
CO2 incubator.
Measurement of Ca2+ by LSCM
HepG2 cells in a logarithmic growth phase were
inoculated in 6-well plates. After 24 h, 1 ml of drugs was added to
each hole. CSQAH was added at concentrations of 100, 200 and 400
µg/ml, respectively. The final concentration of the positive
control group (HCPT) was 5 µg/ml. The control group received the
same volume of RPMI-1640 culture media. After 48 h, the cell
suspension was collected. The cells were washed with PBS and fixed
with 4 µg/ml Fluo-3/AM 200 ml. Then, the cells were incubated for 1
h at 37°C in the dark. The cells were washed one time and suspended
with 400 µl PBS, and then the cells were detected with LSCM
(33).
Analysis of Ca2+-Mg2+-ATP
enzyme by adenosine triphosphatase assay kit
HepG2 cells in the logarithmic growth phase were
inoculated in a 5-ml culture bottle. After incubating for 24 h, the
cells were treated with 1 ml of drugs to each bottle (the doses
same as above). After 24 h, the cells were digested with trypsin
and washed with PBS two times. This operation is carried out
according to the adenosine triphosphatase assay kit manual.
Absorbance was measured with a microplate reader at 636 nm, zeroed
with distilled water (34).
Detection of ROS using FCM
HepG2 cells in the logarithmic growth phase were
inoculated in 6-well plates. After 24 h, 1 ml of the drugs was
added to each hole (the doses same as above). After 48 h, the cells
were collected and suspended in DCFH-DA (diluted 1:1,000 with
RPMI-1640 without serum, the final concentration was 10 µmol/l).
Then, the cells were incubated for 1 h at 37°C and the cells were
washed three times with RPMI-1640 without serum. After filtration
with 300 mesh strainer, the cells were analyzed by FCM (35).
Analysis of Bcl-2 and Bax protein by FCM
HepG2 cells in the logarithmic growth phase were
inoculated in a culture bottle. After incubating for 24 h, the
cells were treated with 1 ml of drugs in each bottle (the doses
same as above). After 24 h, the cells were collected and fixed with
40 g/l paraformaldehyde 2 ml for 40 min. Then, the cells were
washed twice with PBS and treated with 0.1% Triton X-100 1 ml for
15 min, washed twice with PBS again, closed with 1% of BSA 1 ml for
1 h, and then centrifuged. The cells were treated with primary
antibodies specific for Bcl-2 or Bax (diluted 1:200), respectively.
Then, incubation was carried out for 1 h at 37°C. The mixture was
centrifuged and the supernatant was removed and washed with PBS,
and then treated with the secondary antibodies (diluted 1:50). The
cells were incubated at room temperature for 30 min in the dark and
centrifuged. The supernatant was removed, while the cells were
suspended in 800 µl of PBS. After being filtrated with 300 mesh
strainer, the cells were analyzed by FCM (36–38).
Statistical analysis
Differences in proliferation between different
groups were analyzed using one-way ANOVA. Statistical analysis was
performed using SPSS 19.0 software. P<0.01 was considered to
indicate a statistically significant difference. The results are
expressed as mean ± SD.
Results
Antioxidant activity of CSE and its
protective effect on oxidative damage induced by DOX
The antioxidant fraction of CSE
From the EPPH method, the most antioxidant activity
of CSE was the ethyl acetate fraction shown in Table I.
 | Table I.Effect of the extracts on DPPH-free
radical scavenging. |
Table I.
Effect of the extracts on DPPH-free
radical scavenging.
| Extracts | 50% Effective
concentration | Antiradical
efficiency/(ml/mg) |
|---|
| Petroleum
ether | 0.99 | 1.98 |
| Chloroform | 1.06 | 2.13 |
| Ethyl acetate | 3.13 | 6.25 |
| N-butyl
alcohol | 1.17 | 2.34 |
| Water | 1.51 | 3.02 |
Effect of CSE on injury and energy metabolism
induced by DOX
The DOX group exhibited significantly an increased
LDH value (P<0.01). Compared with the DOX group, CSE groups
exhibited decreased levels of LDH (P<0.01). Compared with the
normal group, the CSE groups had increased levels of LDH
(P<0.01) (Table II). The DOX
group exhibited a significantly increased CK value compared with
the normal group (P<0.01). Compared with the DOX group, the CSE
groups exhibited decreased levels of CK (P<0.01). The CSE groups
exhibited increased levels of CK, compared with the normal group
(P<0.01).
 | Table II.Effect of CSE on LDH and CK values in
blood (n=10, mean ± SD). |
Table II.
Effect of CSE on LDH and CK values in
blood (n=10, mean ± SD).
| Group | Dose (mg/kg) | LDH (U/l) | CK (U/ml) |
|---|
| Control | 0 |
1,887.82±147.65 | 419.13±32.43 |
| DOX | 15 |
3,898.03±128.72a |
809.04±34.33a |
| CSE-L | 40 |
3,374.61±116.66a,b |
574.65±30.75a,b |
| CSE-M | 80 |
2,754.62±103.81a,b |
496.80±22.95a,b |
| CSE-H | 120 |
2,187.48±132.51a,b |
504.30±28.91a,b |
The DOX group exhibited decreased levels of
Na+K+-ATPase and Ca2+-ATPase
compared with the normal group (P<0.01). The CSE groups showed
an increase in the level of Na+K+-ATPase when
compared with the DOX alone treated mice. No significant difference
in Na+K+-ATPase was observed between the
CSE-L group and the DOX group (P>0.05), while the levels of
Na+K+-ATPase in the CSE-M and CSE-M groups
were significantly increased compared with the DOX group
(P<0.05). The CSE groups showed an increase in the level of
Ca2+-ATPase when compared with the DOX group. A
significant difference was observed in the CSE-L group when
compared to the DOX group (P<0.05), and significant differences
were also observed in the CSE-M and CSE-H groups (P<0.01). The
level of Ca2+-ATPase in the CSE-L group was
significantly lower compared with the normal group (P<0.01). No
significant difference was observed in CSE-M and CSE-H groups when
compared with the normal group (P>0.01; Table III).
 | Table III.Effect of CSE on ATP activities in
cardiac tissue (n=10) mean ± SD. |
Table III.
Effect of CSE on ATP activities in
cardiac tissue (n=10) mean ± SD.
| Group | Dose (mg/kg) |
Na+K+-ATPase
(µmolPi/mgprot/h) |
Ca2+-TPase
(µmolPi/mgprot/h) |
|---|
| Control | 0 | 6.92±0.51 | 5.66±0.39 |
| DOX | 15 |
5.88±0.60a |
4.68±0.52a |
| CSE-L | 40 |
6.25±0.63a |
5.12±0.42a,b |
| CSE-M | 80 |
6.41±0.46b |
5.38±0.44c |
| CSE-H | 120 |
6.49±0.63b |
5.49±0.41c |
Effect of CSE on oxidative damage induced by
DOX
MDA levels in the cardiac tissues showed that the
MDA level in the DOX group was increased, compared with the normal
group. There was a significant differences(P<0.01). The CSE
groups showed a significant decrease in the level of MDA
(P<0.01) when compared with the DOX alone treated mice. The
levels of MDA in the CSE groups were significantly increased
compared with the normal group (P<0.01; Table IV).
 | Table IV.Effect of CSE on MDA levels in
cardiac tissue (n=10, mean ± SD). |
Table IV.
Effect of CSE on MDA levels in
cardiac tissue (n=10, mean ± SD).
| Group | Dose (mg/kg) | MDA
(nmol/mgprot) |
|---|
| Control | 0 | 4.82±0.19 |
| DOX | 15 |
9.19±0.37a |
| CSE-L | 40 |
7.34±0.50a,b |
| CSE-M | 80 |
6.67±0.20a,b |
| CSE-H | 120 |
6.10±0.23a,b |
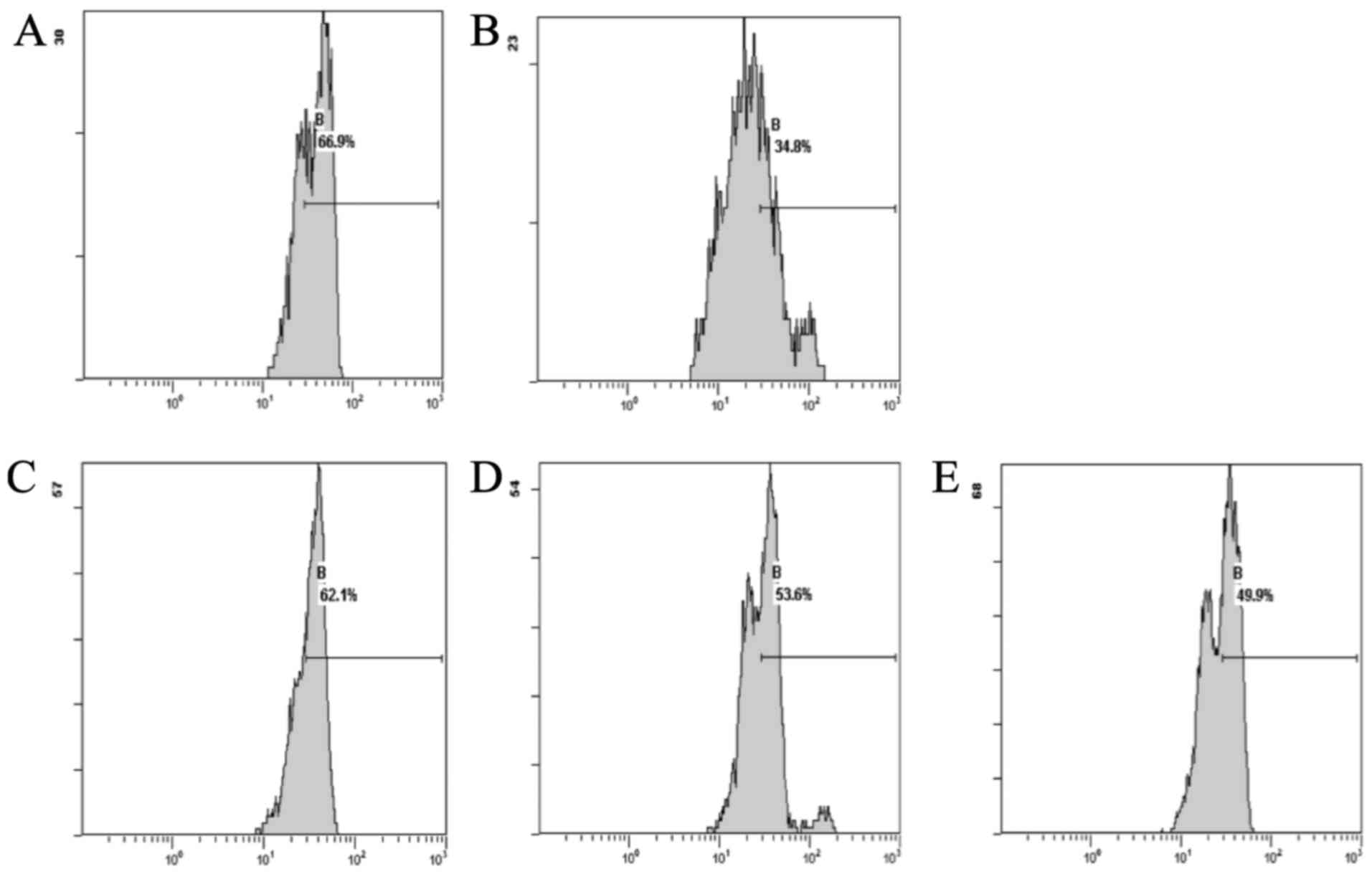
The results showed that the DOX group, compared with
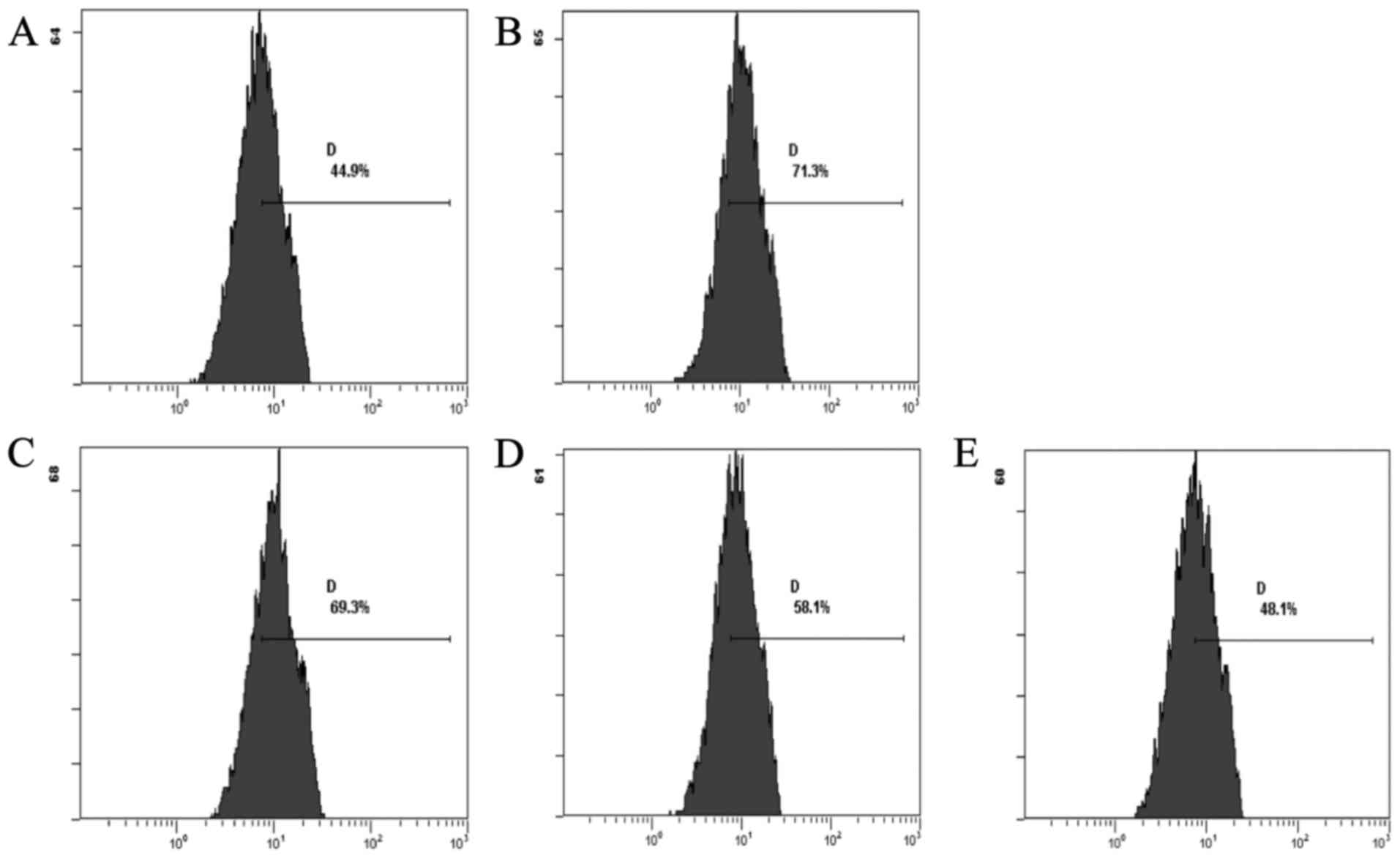
the normal group, exhibited decreased levels of ROS. The CSE groups
showed a decrease in the levels of ROS when compared with the DOX
group. The CSE groups showed an increase in the level of ROS when
compared with the normal group (Fig.
1).
In these tests, compared with normal group, the DOX
group exhibited a significantly decreased T-AOC value (P<0.01).
CSE groups, compared with the DOX group, exhibited significantly
increased T-AOC values (P<0.01). T-AOC values in the CSE groups
were significantly decreased compared with the normal group
(P<0.01). Mice treated with DOX alone showed a significant
(P<0.01) decrease in the activity of SOD in the heart as
compared to the normal control mice. The CSE groups showed a
significant (P<0.05) increase in the activity of SOD when
compared with the DOX alone treated mouse. The SOD level in the
CSE-H group was significantly decreased compared with the normal
group (P<0.05). No significant difference was observed in the
CSE-L and CSE-M groups when compared with the normal group
(P>0.05). GSH levels in the cardiac tissues showed that the DOX
group, compared with the normal group, exhibited a decreased level
of GSH. There was a significant difference (P<0.01). The CSE
groups showed a significant (P<0.01) increase in the levels of
GSH when compared with the DOX alone treated mouse. The CSE groups
compared with normal group had a significant difference
(P<0.01). Mice treated with DOX alone showed a significant
(P<0.01) decrease in the activity of CAT in the heart as
compared to the normal control mice. CSE groups showed a
significant (P<0.01) increase in the activity of CAT when
compared with the DOX alone treated mouse. The CSE-L group showed a
significant difference when compared with the normal group
(P<0.01). No significant differences were observed in the CSE-M
and CSE-H groups when compared with the normal group (P>0.05;
Table V).
 | Table V.Effect of CSE on T-AOC, SOD, CAT and
GSH-Px (n=10, mean ± SD). |
Table V.
Effect of CSE on T-AOC, SOD, CAT and
GSH-Px (n=10, mean ± SD).
| Group | Dose (mg/kg) | T-AOC | SOD (U/mg
prot) | CAT (U/mg
prot) | GSH-Px (mg/g
prot) |
|---|
| Control | 0 | 294.17±10.97 | 181.20±13.88 | 381.64±23.46 | 11.88±1.26 |
| DOX | 15 |
200.75±6.67b |
162.20±14.54b |
317.71±23.38b |
8.31±0.99b |
| CSE-L | 40 |
239.56±7.91b,d |
176.91±11.96c |
351.31±28.66b,d |
9.58±0.62b,d |
| CSE-M | 80 |
253.31±6.46b,d |
178.53±13.51c |
360.67±24.55d |
10.16±0.63b,d |
| CSE-H | 120 |
250.60±6.84b,d |
166.25±13.62c,a |
369.82±23.58d |
9.50±0.68b,d |
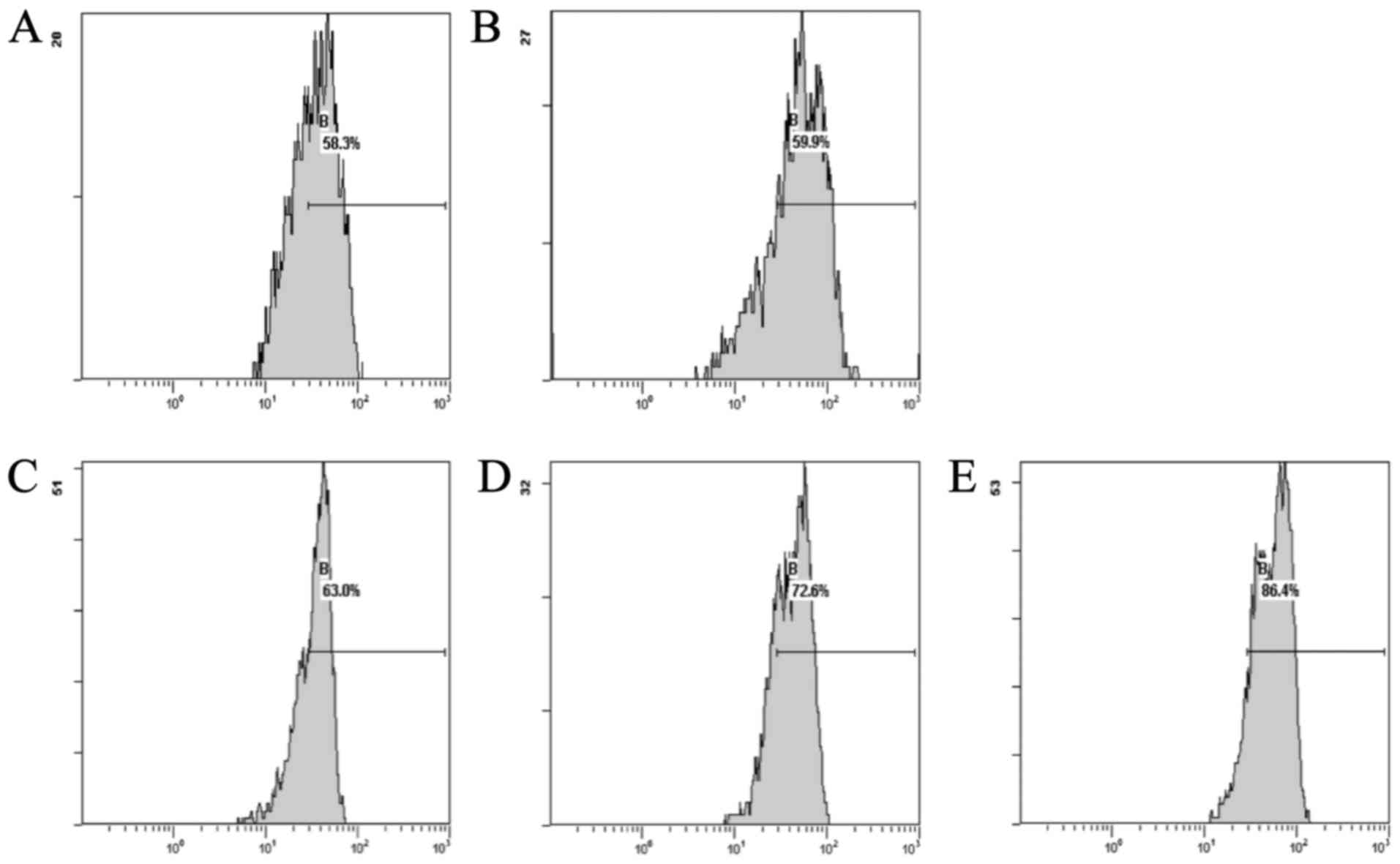
Effect of CSE on the Δψm levels in cardiac cells
induced by DOX
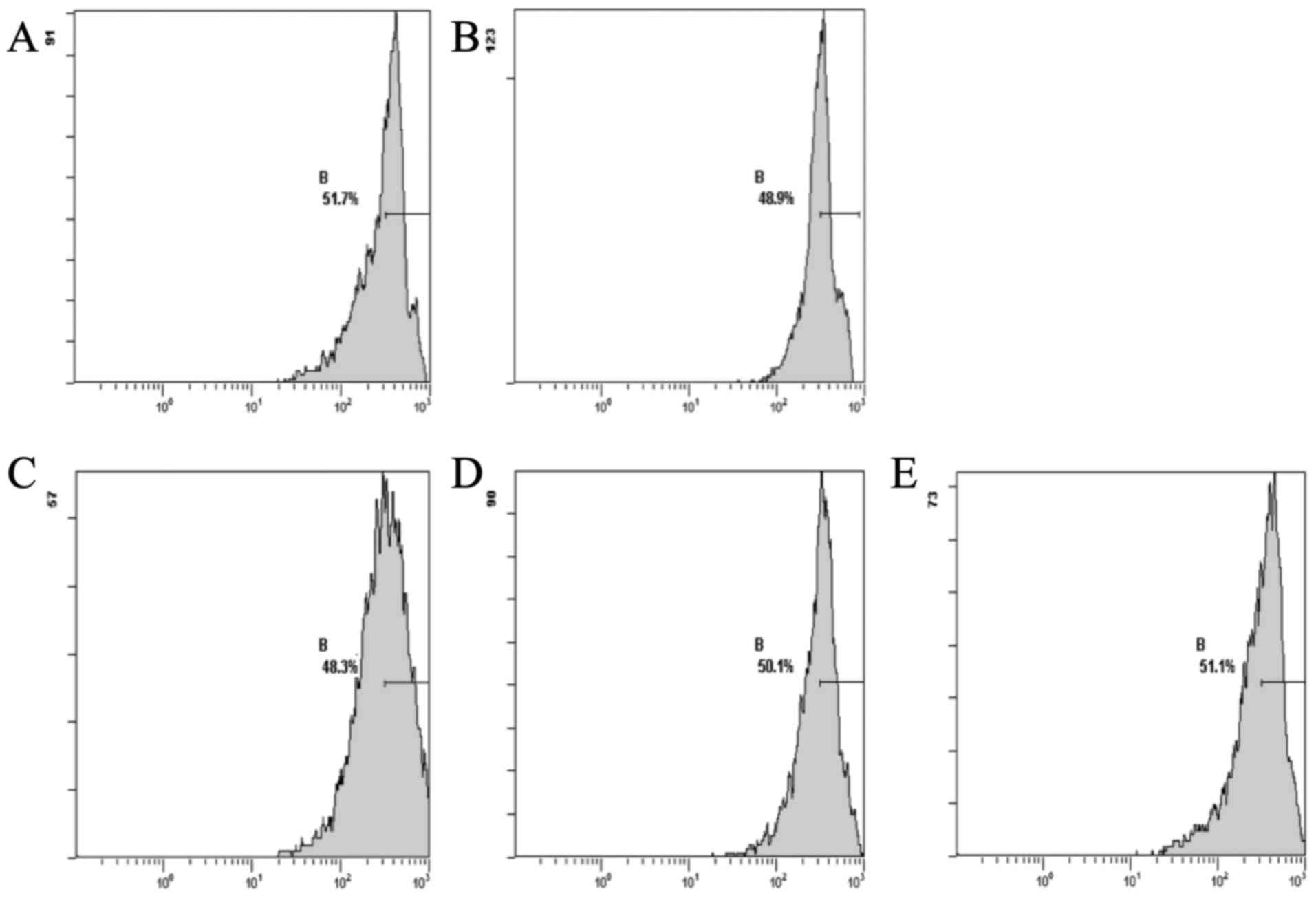
The results showed that the DOX group, compared with
the normal group, exhibited a decreased level of Δψm. The CSE
groups showed an increase in the levels of Δψm when compared with
the DOX group. The CSE groups showed an increase in the level of
Δψm when compared with the normal group (Fig. 2).
Antitumor activity of CSQAH and its
apoptosis induction on HepG2 cells
Observation of CSQAH-induced change in
[Ca2+] in the HepG2 cells
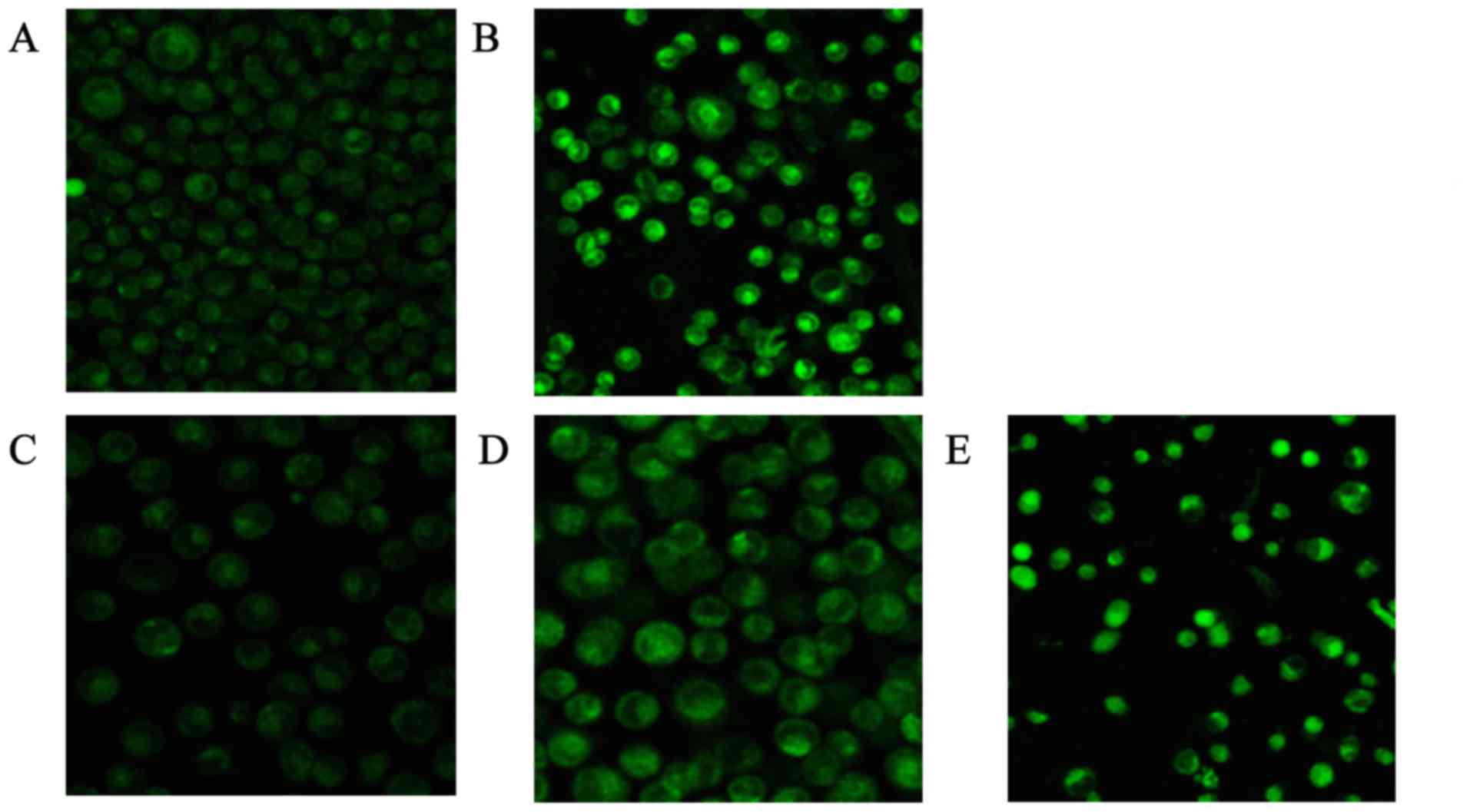
The brightness of the green fluorescence was greater
with increasing concentrations of CSQAH. Different concentrations
of CSQAH increased the concentration of Ca2+ in the
cytoplasm in a dosage-dependent manner. HepG2 cells exhibited an
increase in Ca2+ levels compared with the blank control
group (P<0.01). As shown in Table
VI and Fig. 3, the result
showed that the concentration of Ca2+ increased with the
increase in the concentration of CSQAH. After 48 h, the green
fluorescence of HCPT was significantly enhanced.
 | Table VI.Effect of CSQAH on activity of
Ca2+-Mg2+-ATPase. |
Table VI.
Effect of CSQAH on activity of
Ca2+-Mg2+-ATPase.
| Group | Dose (µg/ml) |
Ca2+-Mg2+-ATPase |
|---|
| Control | 0 | 0.104±0.020 |
| HCPT | 5 |
0.067±0.024b |
| CSQAH-L | 100 |
0.079±0.003a |
| CSQAH-M | 200 |
0.065±0.004b |
| CSQAH-H | 400 |
0.037±0.002b |
Effect of CSQAH on activity of
Ca2+-Mg2+-ATPase
With the increasing concentration of CSQAH, the
optical density (OD) was decreased. Thus, the
Ca2+-Mg2+-ATPase enzyme activity in the HepG2
cells was decreased. The result is shown in Table VII. Each increasing concentration
of CSQAH decreased the activity of
Ca2+-Mg2+-ATPase enzyme. When the CSQAH
groups were compared with the control group, there was a
significantly difference (P<0.05 and P<0.01). The
Ca2+-Mg2+-ATPase enzyme activity of the HCPT
group was lower than that noted in the control group
(P<0.01).
 | Table VII.Effect of CSQAH on variation of
[Ca2+] in the HepG-2 cells. |
Table VII.
Effect of CSQAH on variation of
[Ca2+] in the HepG-2 cells.
| Group | Dose (µg/ml) | FI of
[Ca2+] |
|---|
| Control | 0 | 39.31±2.16 |
| HCPT | 5 |
49.84±2.89a |
| CSQAH-L | 100 |
60.37±3.91b |
| CSQAH-M | 200 |
79.84±5.54b |
| CSQAH-H | 400 |
108.52±5.69b |
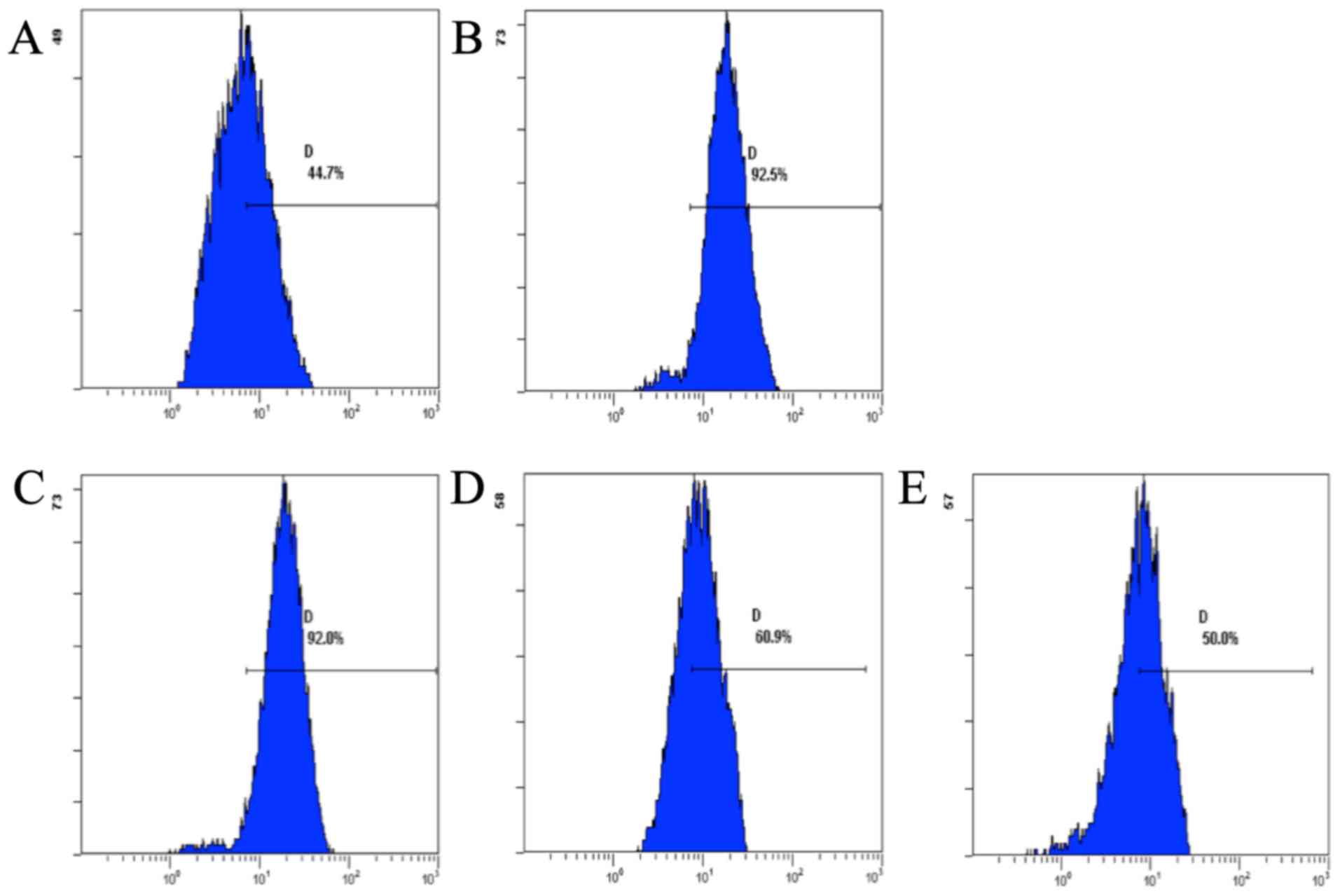
Effect of CSQAH on the level of ROS
Flow cytometric analysis showed that CSQAH increased
the levels of ROS in te HepG2 cells. The production of ROS was
decreased in a dose-dependent manner (Fig. 4).
Effect of CSQAH on the expression levels of Bcl-2
and Bax
When HepG2 cells were treated with CSQAH for 48 h,
Bcl-2 and Bax were detected by FCM. The results are shown in
Figs. 5 and 6. Significant changes in Bax and Bcl-2
expression (an increase in Bax and a decrease in Bcl-2) were
observed in the CSQAH-treated HepG2 cells in a dose-dependent
manner.
Discussion
Antioxidant activity of CSE and its
protective effect on oxidative damage induced by DOX
After determination of the activity of lactic
dehydrogenase (LDH) and creatine kinase (CK) in serum, the
doxorubicin (DOX) group exhibited significantly increased LDH and
CK values compared with the normal group (P<0.01). In the
Capparis spinosa L. (CSE) groups, the level of LDH and CK
were lower than the levels in the DOX group (P<0.01). These
results indicated that DOX decreased the level of myocardial injury
(LDH and CK); thus, CSE protected against DOX-induced myocardial
injury. Previous pathology experimental observation found that DOX
causes serious tissue and cell damage. After tissues and cells are
damaged, enzymes are released into the bloodstream (39,40).
DOX can alter the norms of blood biochemistry, and CSE can
significantly inhibit DOX-induced increases in serum activity of
LDH and CK. According to the results of the present study, it can
be concluded that CSE protects myocardial tissue and cells in
mice.
The damage of free radicals can reduce the activity
of Na+K+-ATpase and Ca2+-ATPase
leading to abnormal energy metabolism. The activity of
Na+K+-ATPase and Ca2+-ATPase was
reduced in the DOX group, while DOX initiated membrane lipid
peroxidation (41). MDA is the
metabolite of lipid peroxidation products, which could interfere
with metabolic to cellular acidosis. An increase in
Na+-Ca2+ exchange, also accelerates the
Ca2+ internal flow, then induces intracellular calcium
overload. DOX can induce calcium overload, so the mitochondria need
to consume a large amount of ATP to intake excessive-free
Ca2+ to reduce the levels of ATP in the cell. Reduction
of ATP also causes sarcoplasmic reticulum
Ca2+ATPase-power shortage, which reduces the ability to
absorb Ca2+. After further aggravation of calcium
overload, a vicious circle may occur in the cell. CSE can increase
the activity of ATPase and maintain the stability of the biofilm
structure, protecting the biological membrane from oxidative
damage. MDA is a sign of lipid peroxidation. MDA not only reflects
the degree of free radicals produced, but also reflects the degree
of lipid peroxidation (42,43). The MDA levels in the CSE groups were
significantly lower than the corresponding values in the DOX group,
which indicated that CSE could significantly guard against lipid
peroxidation. CSE has a protective effect on rat myocardial
tissue.
In the present study, the level of intracellular ROS
production was monitored by flow cytometry (FCM) (44,45).
The results showed that the DOX group exhibited increased
production of ROS compared with the normal group, whereas treatment
with CSE significantly decreased the generation of ROS. These
results suggested that CSE can balance ROS production and
neutralization and inhibit DOX-induced intracellular ROS generation
and protect cells from damage.
Through determination of the full blood total
antioxidation ability (T-AOC), the DOX group exhibited
significantly decreased T-AOC values (P<0.01). The CSE groups
exhibited significantly increased T-AOC values compared with the
DOX group (P<0.01). The results indicated that administration of
CSE can improve body total antioxidation ability. Administration of
DOX to rats significantly altered the cardiac activities of CAT,
SOD and GSH-peroxidase (GSH-Px), which reflect the changes in free
radicals in myocardial tissues. The mechanism of DOX-induced
cardiotoxicity involves the generation of ROS (46,47).
For increasing production of ROS by oxidative stress, the activity
of the cardiac antioxidant enzyme SOD is significantly reduced. The
main cellular damages caused by ROS included lipid peroxidation and
MDA were formed in hearts. CSE has obvious ability to scavenge free
radicals, which can be attributed to the enhancment of the activity
of antioxidant enzymes (CAT, SOD and GSH-Px). DOX-induced
cardiotoxicity caused the activity of cardiac antioxidant enzymes
CAT, SOD and GSH-Px which were significantly reduced.
Administration of CSE to the mice significantly increased the
cardiac activities of CAT, SOD, and GSH-Px as compared to the DOX
group, indicating the protective effect of CSE. The antioxidant
effect of CSE has been shown to decrease DOX-induced cardiotoxicity
as indicated by the ability to inhibit the production of free
radicals, thus accelerating free radical consumption and reducing
the consumption of antioxidant enzymes.
The formation of ROS which could harm membrane
lipids, reduces the fluidity of membrane and increases the cell
membrane permeability, causing Δψm decrease (48–50).
This could cause oxidative damage to proteins, resulting in protein
denaturation and crosslink, and a change in enzyme activity
(51). The level of Δψm was
monitored by FCM. The results showed that the DOX group exhibited a
decreased level of Δψm compared with the normal group. The CSE
groups exhibited decreased levels of Δψm compared with the DOX
group. CSE prevented ROS-mediated peroxidative damage to the
mitochondrial membrane and opened MPTP, and increased mitochondrial
membrane fluidity.
Antitumor activity of CSQAH and its
apoptosis induction in HepG2 cells
Ca2+ plays a pivotal role in the
physiology and biochemistry of organisms and cells. They play an
important role in signal transduction pathways, where they act as a
secondary messenger. Ca2+ make their entrance into the
cytoplasm either from outside the cell through the cell membrane
via calcium channels or from some internal calcium storages.
Ca2+ could damage cells when they enter in excessive
numbers. Excessive entry of calcium into a cell may damage it or
even cause it to undergo apoptosis, or death by necrosis (52,53).
In this experiment, the Ca2+-specific molecular probe
Fluo-3/AM was used to carry CSQAH at different concentrations to
treat HepG2 cells, and LCSM was used to observe changes in
[Ca2+] in the cells. The result of the research showed
that the strength of green fluorescence increased with the increase
in the concentration of CSQAH. The HepG2 cells exhibited increase
Ca2+ levels compared with the control group,
significantly (P<0.01). This shows that the concentration of
Ca2+ may increase with the increase in the concentration
of CSQAH. On the one hand, this suggests that CSQAH can effect the
calcium channels in HepG2 cells, which in turn leads to the rise of
the concentration of Ca2+ in the cell. On the other
hand, it could be that CSQAH activates endoplasmic reticulum, which
is due to the stimulation of a specific Ca2+ release
(54,55). When the concentration of
Ca2+ is increased, endogenous nuclease is activated,
cutting the DNA chain, inducing apoptosis of cells. Thereby, we
indicate that the mechanism of apoptosis which was induced by CSQAH
is possibly related to Ca2+ release. The result of the
microplate reader showed that the OD value was decreased with the
increasing dose, which indicated that the amount of inorganic
phosphorus declined. It also demonstrated that the activity of
Ca2+-Mg2+-ATPase enzyme was indirectly
decreased. Experimental data showed that each group of CSQAH could
decrease the activity of Ca2+-Mg2+-ATPase
enzyme. There was a significantly difference in the CSQAH groups
compared with the control group (P<0.05). The decrease in
Ca2+-Mg2+-ATPase enzyme activity confirmed
that the increase in Ca2+ concentration was inevitable,
while Ca2+ overload induced apoptosis.
ROS are chemically reactive molecules containing
oxygen. Examples include oxygen ions and peroxides. ROS are formed
as a natural byproduct of the normal metabolism of oxygen and have
important roles in cell signaling and homeostasis. ROS are
constantly generated and eliminated in the biological system and
are required to drive regulatory pathways. Under normal physiologic
conditions, cells control ROS levels by balancing the generation of
ROS with their elimination by a scavenging system. However, under
oxidative stress conditions, excessive ROS may damage cellular
proteins, lipids and DNA, leading to fatal lesions in cell that
contribute to carcinogenesis. Ultra-ROS could damage DNA, RNA, and
proteins. This may result in significant damage to cell structures.
Cumulatively, this is known as oxidative stress (56–58).
From the results obtained, with the increase in CSQAH
concentration, the level of ROS was increased when compared with
the control group (P<0.01). This illustrates that CSQAH could
increase the level of ROS in HepG2 cells. The result indicates the
accumulation of ROS, which play an important role in the
mitochondrial control of apoptosis induced by CSQAH.
Apoptosis regulator Bcl-2 is a family of
evolutionarily related proteins. These proteins govern
mitochondrial outer membrane permeabilization (MOMP) and could be
either pro-apoptotic (Bax, BAD, Bak and and Bok) or anti-apoptotic
(including Bcl-2, Bcl-xL and Bcl-w). There are a number of theories
concerning how the Bcl-2 gene family exerts their pro-apoptotic or
anti-apoptotic effect. An important theory states that this is
achieved by activation or inactivation of an inner mitochondrial
permeability transition pore, which is involved in the regulation
of matrix Ca2+, pH and voltage. It is also believed that
certain Bcl-2 family proteins induce or inhibit the release of
Cyt-c into the cytosol, which activate caspase-9 and −3, leading to
apoptosis (59,60). When HepG2 cells were treated with
CSQAH for 48 h, FCM analysis showed that Bcl-2 expression levels in
the CSQAH-treated groups were downregulated, while Bax expression
levels were upregulated, and the effects were dosage-dependent. The
result showed that CSQAH could downregulate anti-apoptotic
proteins.
In conclusion, the present study revealed the
antioxidant and antitumor activities of CSE. On the one hand, the
ethyl acetate extract of CSE showed antioxidant activity using the
DPPH method to determine free radical elimination ability. CSE had
protective effects on cardiac toxicity of DOX, decreasing the
activity of LDH and CK. CSE improved the ability of myocardial
tissue to scavenge free radicals, inhibited the lipid peroxidation,
recovered activity of antioxidant enzymes, adjusted the energy
metabolism of myocardial tissue, inhibited the generation of a
large number of ROS in the cells, raised the level of Δψm, and
improved the metabolism of free radicals. CSE had protective
effects on DOX-induced myocardial damage. Moreover, the quaternary
ammonium hydroxide of Capparis spinosa L. (CSQAH) induced
HepG2 cells apoptosis by increasing Ca2+ concentrations
and ROS levels, decreasing the
Ca2+-Mg2+-ATPase activity in HepG2 cells, and
downregulating anti-apoptotic Bcl-2 expression while upregulating
apoptotic Bax expression. In summary, the present study
demonstrated the antioxidant and antitumor activities of CSE which
may suppress tumor growth and alleviate the side-effects of DOX,
which may facilitate tumor treatment in a dual manner.
Acknowledgements
The present study was supported in part by the Open
Research Program for Key Laboratory of College of Heilongjiang
Province (China) (CPAT-2012003), the Natural Science Project of
Department of Education of Heilongjiang Province (China)
(12541205), the Innovation Talents Project of Science and
Technology of Harbin City (China) (2014RFQXJ154), the Doctoral
Research Project of Harbin University of Commerce (12DL008), and
the Graduate Students Innovative Research Project of Harbin
University of Commerce (YJSCX2015-390HSD).
References
|
1
|
Germanò MP, De Pasquale R, D'Angelo V,
Catania S, Silvari V and Costa C: Evaluation of extracts and
isolated fraction from Capparis spinosa L. buds as an antioxidant
source. J Agric Food Chem. 50:1168–1171. 2002.
|
|
2
|
Orphanos PI: Germination of caper
(Capparis spinosa L.) seeds. J Hortic Sci. 58:267–270. 1983.
View Article : Google Scholar
|
|
3
|
Higton RN and Akeroyd JR: Variation in
Capparis spinosa L. in Europe. Bot J Linn Soc. 106:104–112.
1991.
|
|
4
|
Castro Ramos RD and Nosti Vega M: The
caper (Capparis spinosa L.). Grasas Aceites. 38:183–186. 1987.
|
|
5
|
Aytaç Z and Kınaci Ceylan GA: Yield and
some morphological characteristics of caper (Capparis spinosa L.)
population cultivated at various slopes in Aegean ecological
conditions. Pak J Bot. 2:591–596. 2009.
|
|
6
|
Kulisic-Bilusic T, Schmöller I, Schnäbele
K, Siracusa L and Ruberto G: The anticancerogenic potential of
essential oil and aqueous infusion from caper (Capparis spinosa
L.). Food Chem. 1:261–267. 2012. View Article : Google Scholar
|
|
7
|
Tlili N, Khaldi A, Triki S and Munné-Bosch
S: Phenolic compounds and vitamin antioxidants of caper (Capparis
spinosa). Plant Foods Hum Nutr. 65:260–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonina F, Puglia C, Ventura D, Aquino R,
Tortora S, Sacchi A, Saija A, Tomaino A, Pellegrino ML and de
Caprariis P: In vitro antioxidant and in vivo photoprotective
effects of a lyophilized extract of Capparis spinosa L buds. J
Cosmet Sci. 53:321–335. 2002.PubMed/NCBI
|
|
9
|
Siracusa L, Kulisic-Bilusic T, Politeo O,
Krause I, Dejanovic B and Ruberto G: Phenolic composition and
antioxidant activity of aqueous infusions from Capparis spinosa L.
and Crithmum maritimum L. before and after submission to a two-step
in vitro digestion model. J Agric Food Chem. 59:12453–12459.
2011.
|
|
10
|
Abraham SV Issac, Palani A, Ramaswamy BR,
Shunmugiah KP and Arumugam VR: Antiquorum sensing and antibiofilm
potential of Capparis spinosa. Arch Med Res. 42:658–668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
al-Said MS, Abdelsattar EA, Khalifa SI and
el-Feraly FS: Isolation and identification of an anti-inflammatory
principle from Capparis spinosa. Pharmazie. 43:640–641.
1988.PubMed/NCBI
|
|
12
|
Boga C, Forlani L, Calienni R, Hindley T,
Hochkoeppler A, Tozzi S and Zanna N: On the antibacterial activity
of roots of Capparis spinosa L. Nat Prod Res. 25:417–421. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gadgoli C and Mishra SH: Antihepatotoxic
activity of p-methoxy benzoic acid from Capparis spinosa. J
Ethnopharmacol. 66:187–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huseini HF, Hasani-Rnjbar S, Nayebi N,
Heshmat R, Sigaroodi FK, Ahvazi M, Alaei BA and Kianbakht S:
Capparis spinosa L. (Caper) fruit extract in treatment of type 2
diabetic patients: A randomized double-blind placebo-controlled
clinical trial. Complement Ther Med. 21:447–452. 2013.
|
|
15
|
Lam SK and Ng TB: A protein with
antiproliferative, antifungal and HIV-1 reverse transcriptase
inhibitory activities from caper (Capparis spinosa) seeds.
Phytomedicine. 16:444–450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eddouks M, Lemhadri A and Michel JB:
Hypolipidemic activity of aqueous extract of Capparis spinosa L. in
normal and diabetic rats. J Ethnopharmacol. 98:345–350. 2005.
View Article : Google Scholar
|
|
17
|
Ali-Shtayeh MS and Abu Ghdeib SI:
Antifungal activity of plant extracts against dermatophytes.
Mycoses. 42:665–672. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou HF, Xie C, Jian R, Kang J, Li Y,
Zhuang CL, Yang F, Zhang LL, Lai L, Wu T, et al: Biflavonoids from
caper (Capparis spinosa L.) fruits and their effects in inhibiting
NF-kappa B activation. J Agric Food Chem. 59:3060–3065. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ziyyat A, Legssyer A, Mekhfi H, Dassouli
A, Serhrouchni M and Benjelloun W: Phytotherapy of hypertension and
diabetes in oriental Morocco. J Ethnopharmacol. 58:45–54. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Houghton PJ, Hylands PJ, Mensah AY, Hensel
A and Deters AM: In vitro tests and ethnopharmacological
investigations: Wound healing as an example. J Ethnopharmacol.
100:100–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu JB, Ye SF, Liang CL, Li YC, Yu YJ, Lai
JM, Lin H, Zheng J and Zhou JY: Qi-Dan Fang ameliorates
adriamycin-induced nephrotic syndrome rat model by enhancing renal
function and inhibiting podocyte injury. J Ethnopharmacol.
151:1124–1132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Unverferth DV, Magorien RD, Leier CV and
Balcerzak SP: Doxorubicin cardiotoxicity. Cancer Treat Rev.
9:149–164. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alexander J, Dainiak N, Berger HJ, Goldman
L, Johnstone D, Reduto L, Duffy T, Schwartz P, Gottschalk A, Zaret
BL, et al: Serial assessment of doxorubicin cardiotoxicity with
quantitative radionuclide angiocardiography. N Engl J Med.
300:278–283. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Legha SS, Benjamin RS, Mackay B, Ewer M,
Wallace S, Valdivieso M, Rasmussen SL, Blumenschein GR and
Freireich EJ: Reduction of doxorubicin cardiotoxicity by prolonged
continuous intravenous infusion. Ann Intern Med. 96:133–139. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arola OJ, Saraste A, Pulkki K, Kallajoki
M, Parvinen M and Voipio-Pulkki LM: Acute doxorubicin
cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res.
60:1789–1792. 2000.PubMed/NCBI
|
|
26
|
Ji YB and Yu L: N-butanol extract of
Capparis spinosa L. induces apoptosis primarily through a
mitochondrial pathway involving mPTP open, cytochrome C release and
caspase activation. Asian Pac J Cancer Prev. 15:9153–9157.
2014.
|
|
27
|
Hossain MA and Rahma SMM: Total phenolics
flavonoids and antioxidant activity of tropical fruit pineapple.
Food Res Int. 3:672–676. 2011. View Article : Google Scholar
|
|
28
|
Wallimann T, Wyss M, Brdiczka D, Nicolay K
and Eppenberger HM: Intracellular compartmentation, structure and
function of creatine kinase isoenzymes in tissues with high and
fluctuating energy demands: The ‘phosphocreatine circuit’ for
cellular energy homeostasis. Biochem J. 281:21–40. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Obradovic M, Bjelogrlic P, Rizzo M,
Katsiki N, Haidara M, Stewart AJ, Jovanovic A and Isenovic ER:
Effects of obesity and estradiol on
Na+/K+-ATPase and their relevance to
cardiovascular diseases. J Endocrinol. 218:R13–R23. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pryor WA and Stanley JP: Letter: A
suggested mechanism for the production of malonaldehyde during the
autoxidation of polyunsaturated fatty acids. Nonenzymatic
production of prostaglandin endoperoxides during autoxidation. J
Org Chem. 40:3615–3617. 1975.
|
|
31
|
Bani S, Gautam M, Sheikh FA, Khan B, Satti
NK, Suri KA, Qazi GN and Patwardhan B: Selective Th1 up-regulating
activity of Withania somnifera aqueous extract in an experimental
system using flow cytometry. J Ethnopharmacol. 107:107–115. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jaiswal PK, Gupta J, Shahni S and Thakur
IS: NADPH oxidase-mediated superoxide production by intermediary
bacterial metabolites of dibenzofuran: A potential cause for
trans-mitochondrial membrane potential (ΔΨm) collapse in human
hepatoma cells. Toxicol Sci. 147:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hoffman A, Goetz M, Vieth M, Galle PR,
Neurath MF and Kiesslich R: Confocal laser endomicroscopy:
Technical status and current indications. Endoscopy. 38:1275–1283.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fernando KC and Barritt GJ: Pinocytosis in
2,5-di-tert-butylhydroquinone-stimulated hepatocytes and evaluation
of its role in Ca2+ inflow. Mol Cell Biochem. 162:23–29.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Devasagayam TPA, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: Current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.PubMed/NCBI
|
|
36
|
Lo K, Brinkman RR and Gottardo R:
Automated gating of flow cytometry data via robust model-based
clustering. Cytometry A. 73:321–332. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Julius MH, Masuda T and Herzenberg LA:
Demonstration that antigen-binding cells are precursors of
antibody-producing cells after purification with a
fluorescence-activated cell sorter. Proc Natl Acad Sci USA.
69:1934–1938. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Basiji DA, Ortyn WE, Liang L,
Venkatachalam V and Morrissey P: Cellular image analysis and
imaging by flow cytometry. Clin Lab Med. 27653–670. (viii)2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schlattner U, Tokarska-Schlattner M and
Wallimann T: Mitochondrial creatine kinase in human health and
disease. Biochim Biophys Acta. 1762:164–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vlkovicová J, Javorková V, Mézesová L,
Pechánová O, Andriantsitohaina R and Vrbjar N: Dual effect of
polyphenolic compounds on cardiac
Na+/K+-ATPase during development and
persistence of hypertension in rats. Can J Physiol Pharmacol.
87:1046–1054. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ketzer LA, Arruda AP, Carvalho DP and de
Meis L: Cardiac sarcoplasmic reticulum Ca2+-ATPase: Heat
production and phospholamban alterations promoted by cold exposure
and thyroid hormone. Am J Physiol Heart Circ Physiol.
297:H556–H563. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marnett LJ: Lipid peroxidation-DNA damage
by malondialdehyde. Mutat Res. 424:83–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Buranrat B and Connor JR: Cytoprotective
effects of ferritin on doxorubicin-induced breast cancer cell
death. Oncol Rep. 34:2790–2796. 2015.PubMed/NCBI
|
|
45
|
Han D, Williams E and Cadenas E:
Mitochondrial respiratory chain-dependent generation of superoxide
anion and its release into the intermembrane space. Biochem J.
353:411–416. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sheng B, Xu G, Chen D, Chen J, Gao W, Gao
W, Li X, Yang J, Liu F, Gao Y, et al: TT-AOC, MDA, SOD, CAT and
IL-6 levels in rat pulmonary edema induced by hypobaric hypoxia. J
Third Mil Med Univ. 23:2364–2367. 2012.
|
|
47
|
Kirby AJ and Schmidt RJ: The antioxidant
activity of Chinese herbs for eczema and of placebo herbs - I. J
Ethnopharmacol. 56:103–108. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Szliszka E and Krol W: Soy isoflavones
augment the effect of TRAIL-mediated apoptotic death in prostate
cancer cells. Oncol Rep. 26:533–541. 2011.PubMed/NCBI
|
|
49
|
Fernandez-Sanz C, Ruiz-Meana M, Castellano
J, Miro-Casas E, Nuñez E, Inserte J, Vázquez J and Garcia-Dorado D:
Altered FoF1 ATP synthase and susceptibility to mitochondrial
permeability transition pore during ischaemia and reperfusion in
aging cardiomyocytes. Thromb Haemost. 113:441–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Watanabe T, Saotome M, Nobuhara M,
Sakamoto A, Urushida T, Katoh H, Satoh H, Funaki M and Hayashi H:
Roles of mitochondrial fragmentation and reactive oxygen species in
mitochondrial dysfunction and myocardial insulin resistance. Exp
Cell Res. 323:314–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gauthier LD, Greenstein JL, Cortassa S,
O'Rourke B and Winslow RL: A computational model of reactive oxygen
species and redox balance in cardiac mitochondria. Biophys J.
105:1045–1056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Slinchenko NM: Effect of fluoroaluminate
on the ATP-hydrolysing and Ca2+-transporting activity of
the purified Ca2+, Mg2+-ATPase of myometrium
cell plasma membranes. Ukr Biokhim Zh. 75:95–98. 2003.(In
Ukrainian).
|
|
53
|
Toledo-Maciel A, Gonçalves-Gomes S, de
Gouveia Castex M and Vieyra A: Progressive inactivation of plasma
membrane (Ca2++Mg2+)ATPase by Cd2+
in the absence of ATP and reversible inhibition during catalysis.
Biochemistry. 37:15261–15265. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Monaco G, Decrock E, Arbel N, van Vliet
AR, La Rovere RM, De Smedt H, Parys JB, Agostinis P, Leybaert L,
Shoshan-Barmatz V, et al: The BH4 domain of anti-apoptotic Bcl-XL,
but not that of the related Bcl-2, limits the voltage-dependent
anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic
Ca2+ signals to mitochondria. J Biol Chem.
290:9150–9161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Barrezueta LF, Oshima CT, Lima FO, De
Oliveira Costa H, Gomes TS, Neto RA and De Franco MF: The intrinsic
apoptotic signaling pathway in gastric adenocarcinomas of Brazilian
patients: Immunoexpression of the Bcl-2 family (Bcl-2, Bcl-x, Bak,
Bax, Bad) determined by tissue microarray analysis. Mol Med Rep.
3:261–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rada B and Leto TL: Oxidative innate
immune defenses by Nox/Duox family NADPH oxidases. Contrib
Microbiol. 15:164–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sumiyoshi H, Matsushita A, Nakamura Y,
Matsuda Y, Ishiwata T, Naito Z and Uchida E: Suppression of STAT5b
in pancreatic cancer cells leads to attenuated gemcitabine
chemoresistance, adhesion and invasion. Oncol Rep. 35:3216–3226.
2016.PubMed/NCBI
|
|
58
|
Park SY, Kim JY, Lee SM, Jun CH, Cho SB,
Park CH, Joo YE, Kim HS, Choi SK and Rew JS: Capsaicin induces
apoptosis and modulates MAPK signaling in human gastric cancer
cells. Mol Med Rep. 9:499–502. 2014.PubMed/NCBI
|
|
59
|
Muchmore SW, Sattler M, Liang H, Meadows
RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong
SL, et al: X-ray and NMR structure of human Bcl-xL, an
inhibitor of programmed cell death. Nature. 381:335–341. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sucha L, Hroch M, Rezacova M, Rudolf E,
Havelek R, Sispera L, Cmielova J, Kohlerova R, Bezrouk A and Tomsik
P: The cytotoxic effect of α-tomatine in MCF-7 human adenocarcinoma
breast cancer cells depends on its interaction with cholesterol in
incubation media and does not involve apoptosis induction. Oncol
Rep. 30:2593–2602. 2013.PubMed/NCBI
|