Introduction
The immune system plays a critical role in the
prevention of tumour development. The sophisticated bidirectional
interaction between cancer cells and the immune system is defined
as cancer immunoediting (1).
Unfortunately, during this dynamic process, malignant cells may
acquire various molecular and cellular mechanisms enabling them to
escape immune-mediated control and manifest as clinically apparent
cancer. Various immune evasion mechanisms can be grouped into
immunoselection (antigen loss on cancer cell surface) and
immunosubversion (tumour-driven active creation of local and
systemic immunosuppressive milieu) (2). Due to impaired and disbalanced
antitumour immunity in cancer patients (3,4),
immune response-modulating strategies, known as tumour
immunotherapy, have been introduced into clinical practice. Among
the various types of cancer immunotherapy (5), therapeutic cancer vaccination is one
of the most promising approaches, especially when combined with
other immunotherapeutic strategies as well as cancer chemotherapy,
radiation therapy and targeted therapies (6).
Therapeutic cancer vaccination aims at inducing
and/or augmenting cytotoxic cellular immune responses that are able
to quantitatively and qualitatively overwhelm the cancer-driven
immunosuppressive arm of antitumour immune response (7). Therapeutic vaccines exploit mostly
dendritic cells (DCs) that are the main initiators and
orchestrators of adaptive immune responses (8). DCs can be targeted either in
situ or generated ex vivo and reinjected back into the
same patient to achieve their therapeutic effect. Selection of
proper tumour-associated antigens (TAAs) and induction of optimal
DC maturation are the critical steps in therapeutic cancer
vaccination (9). Based on antigen
selection strategy, therapeutic vaccines can be mono- or
oligovalent (using one or several defined TAAs) and polyvalent (a
variety of undefined TAAs is used). In theory, polyvalent vaccines
should be superior to mono- or oligovalent vaccines, since the
former mobilize the immune system to target more than just one or
few TAA(s), which could be potentially lost in individual cases
(10). Moreover, tumours are known
to be heterogeneous (11);
therefore targeting one antigen may target only a portion of the
whole tumour cell spectrum.
Tumour cell lysate (TCL) contains a mixture of
proteins resulting from induced lysis of tumour cells, which
ensures a broad spectrum of target antigens (12). Furthermore, the TCL approach to
vaccination does not require a priori knowledge of relevant
TAAs and targets also potentially unknown TAAs (13). The ease of manufacturing and
storage, lack of limitations dictated by host-specificity and no
obligation to know specific TAAs make TCL an appealing vaccine
candidate. On the other hand, TCL contains not only immunogenic
TAAs, but also various immunosuppressants naturally occurring in
cancer cells, such as hyaluronan, known for inducing tolerogenic
rather than immunogenic maturation of DCs and macrophages (14). Agents, such as Fas ligand (15) or transforming growth factor-β
(16), inducing the apoptosis of
immune cells are also thought to be present in TCL (17). However, the negative effects of
immunosuppressive components present within TCL may potentially be
compensated by properly selected immunostimulatory adjuvants that
are otherwise used for the induction of DC maturation (9).
Adjuvants used in therapeutic cancer vaccination
include Toll-like receptor (TLR) agonists [e.g. imiquimod,
resiquimod and lypopolysaccharide (LPS)], cytokines (e.g.
granulocyte-macrophage colony-stimulating factor, interferons and
interleukins), prostaglandin E2 and their combinations
(18). It was demonstrated that
proper combinations of various TLR agonists are needed for adequate
induction of DC maturation (19).
Hence the use of natural sources of multiple adjuvants may optimize
therapeutic cancer vaccination strategies. Indeed, it was
demonstrated that parts of, or whole, inactivated, pathogens have a
positive impact on the effectiveness of the TCL-derived vaccines.
Experiments on animals and human trials were performed with viral
particles per se (20), but
also as an antigen delivery system, i.e. virosomes. Virosomes are
spherical nanoparticles consisting of a non-viral component: a
phospholipid bilayer, with an embedded viral component on the
surface: influenza virus hemagglutinin and neuraminidase are the
most popular choice. The viral components enable virosomes to fuse
with antigen presenting cells (APCs) and release their contents
directly into the APC cytoplasm, triggering immune response against
the antigen in question (21).
Virosomes also possess immunostimulatory properties on their own
(22), and virosomal vaccines are
capable of eliciting both Th and CTL responses (23). Their efficacy has been demonstrated
in many animal models (24) and in
clinical trials, e.g. where virosomal vaccine containing Her2/neu
peptides induced specific antibody production and a decrease in
circulating regulatory T-lymphocytes in metastatic breast cancer
patients (25).
A possible downside to virosomes is the fact that,
due to the presence of only selected viral proteins, they only have
a fraction of viral full immunostimulatory potential. The rather
limited capacity of a single virosome is also considered a
drawback. In this regard, bacterial ghosts (BGs), empty and intact
non-living bacterial cell envelopes, comprise a platform that can
serve both as a source of multiple adjuvants and a system for
antigen delivery to DCs. BGs are generated by controlled expression
of bacteriophage-cloned protein E-inducing lysis of Gram-negative
bacteria (26,27). The product of E protein-specific
lysis is the intact shell of the bacteria with a conserved surface
and periplasmic molecules that serve as danger signals and immune
potentiators (26,28,29)
and an empty inside, which can be loaded with the desired antigens.
Such a construct is ready to use as a compound delivery system
(30), an anti-bacterial vaccine on
its own (31,32) or a system for the induction of DC
maturation and loading them with antigens, such as TAAs, in a
single-step process (33). BGs
represent a novel approach towards vaccination, immunomodulation
and drug delivery boasting various product advantages. They are
stable at room temperature (RT), non-living and carry almost no
residual DNA. Having the external immunological properties of
living bacteria, BGs act as a natural adjuvant (34).
Bacillus subtilis (B. subtilis) is
known to have anticancer properties, both on its own, as well as
its metabolites. B. subtilis protein metabolites retrieved
from culture medium filtrate have been described as lectins
(35) and have been investigated as
potential anticancer agents due to their cytotoxic properties
(36). Their application as
adjuvant has been first tested in a murine sarcoma model, in
combination with probiotics (36,37),
interferon-γ (38) or on its own
(39), where tumour growth
inhibition, survival benefit, as well as stimulation of macrophages
and spleen mononuclear cells were reported. Lewis lung carcinoma
(LLC) model in C57BL/6 mice was also used with promising results,
regarding tumour inhibition index (40,41),
activity of antitumor effector cells (40–42)
and mouse survival (41,42). B. subtilis is also known to
have immunostimulatory properties on DCs; both on its own (43), as well as its metabolites (44).
In the present study, we investigated the
immunostimulatory potential of E. coli Nissle 1917 BGs (as a
source of multiple immune potentiators) and B. subtilis
B-7025 70 kDa (B.s. B-7025) protein isolates as candidate
adjuvants for TCL-based therapeutic cancer vaccine in a murine lung
cancer model.
Materials and methods
Mice and cell lines
Eight- to 12-week-old female C57BL/6 mice were
obtained from the State Research Institute Centre for Innovative
Medicine (Vilnius, Lithuania). Mice were housed in plastic cages
(10 mice/cage) under normal daylight conditions with water and food
ad libitum. All animal procedures were carried out in
accordance with the Directive of the European Parliament and of the
Council on the Protection of Animals Used for Scientific Purposes
along with approval of the Lithuania State Food and Veterinary
Service.
Murine metastatic Lewis lung carcinoma LLC1 (LLC)
cell line was a kind gift from R.E. Kavestky Institute of
Experimental Pathology, Oncology and Radiobiology (Kyiv, Ukraine).
Cells were cultivated in Dulbeccos modified Eagles medium (DMEM)
(Lonza, Verviers, Belgium) containing 2 mM L-glutamine, 10% foetal
bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (both
from Gibco, Paisley, UK) in humidified atmosphere containing 5%
CO2 at 37°C. LLC cells were used for tumour implantation
and for preparation of autologous tumour lysate.
BG preparation
Probiotic Gram-negative strain E. coli Nissle
1917 was used for the generation of BGs. BGs were produced by the
controlled expression of the phage-derived lysis protein E, as we
have described previously (45).
Due to the safety reasons, the BG preparation was treated with
β-propiolactone (Ferak, Berlin, Germany) to fully inactivate all
residual non-lysed viable bacterial cells and DNA present in BG
suspension, followed by extensive washing via tangential flow
filtration method (45). Aliquots
of washed BGs were frozen at −80°C and lyophilized. Dry-powdered
product was stored at RT until further use.
B. subtilis B-7025 protein
preparation
The B. subtilis B-7025 protein with molecular
mass of 70 kDa was retrieved from culture filtrate on day 10 by
protein precipitation with ammonium sulfate (Alpharus NVP,
Ukraine), followed by chromatographic separation on a Sephacryl
column S-200 (GE Healthcare Bio-Sciences AB, Uppsala, Sweden), as
described elsewhere (46,47). Acquired protein mix is not
structural bacterial protein, but rather lectin metabolite of B.
subtilis B-7025 (35,37,46,47).
The protein concentration was determined by the Lowry method
(48). The protein metabolite
purity was verified by sodium dodecylsulphate polyacrylamide gel
electrophoresis. The protein concentrate was sterilised using
0.20-µm filters (Sigma, St. Louis, MO, USA) and stored at
−20°С.
Preparation of autologous tumour
lysate
To prepare autologous tumour lysate, LLC cells were
collected via trypsin digestion (Gibco), washed with
phosphate-buffered saline (PBS) (Lonza) 3 times and resuspended in
1 ml of PBS. Cells were treated with 6 freeze-thaw lysing cycles
using liquid nitrogen and a 37°C water bath in an alternating
manner. Cells were centrifuged at 4,000 × g for 10 min, and the
supernatant was collected and passed through a 0.2-µm syringe
filter (Corning, Inc., Corning, NY, USA). Protein concentration was
determined by the the Lowry method (48). Protein concentration in TCL was 150
µg/100 µl of PBS/dose.
Formulations of therapeutic
vaccines
The first vaccine was generated by mixing 150 µg of
LLC lysate with 0.1 mg (2×108 particles) of BGs in 100
µl of PBS per dose (‘LLC+BGs’ vaccine). The second vaccine was
generated by mixing 150 µg of LLC lysate with 150 µg of B.
subtilis B-7025 70 kDa protein mix in 100 µl of PBS per dose
(‘LLC+B.s.B-7025’ vaccine). The third vaccine consisted of
LLC lysate alone, i.e. without adjuvant. Injection of PBS alone
served as a negative control (control group).
Tumour challenge and therapeutic
vaccination
Mice received a subcutaneous (s.c.) injection of
3×105 LLC cells in the left hind-foot on day 0. On day
14, primary tumours were surgically removed by amputating the foot.
Mice with the primary tumour removed were subsequently treated with
either LLC+BGs vaccine (n=13), or LLC+B.s.B-7025 vaccine
(n=13), or LLC lysate alone (n=11). Tumour-challenged, but
untreated mice (n=13) served as a control group. Mice in the
treated groups were vaccinated according to the same scheme; each
vaccine was injected s.c. into the nape of the neck on days 17, 20
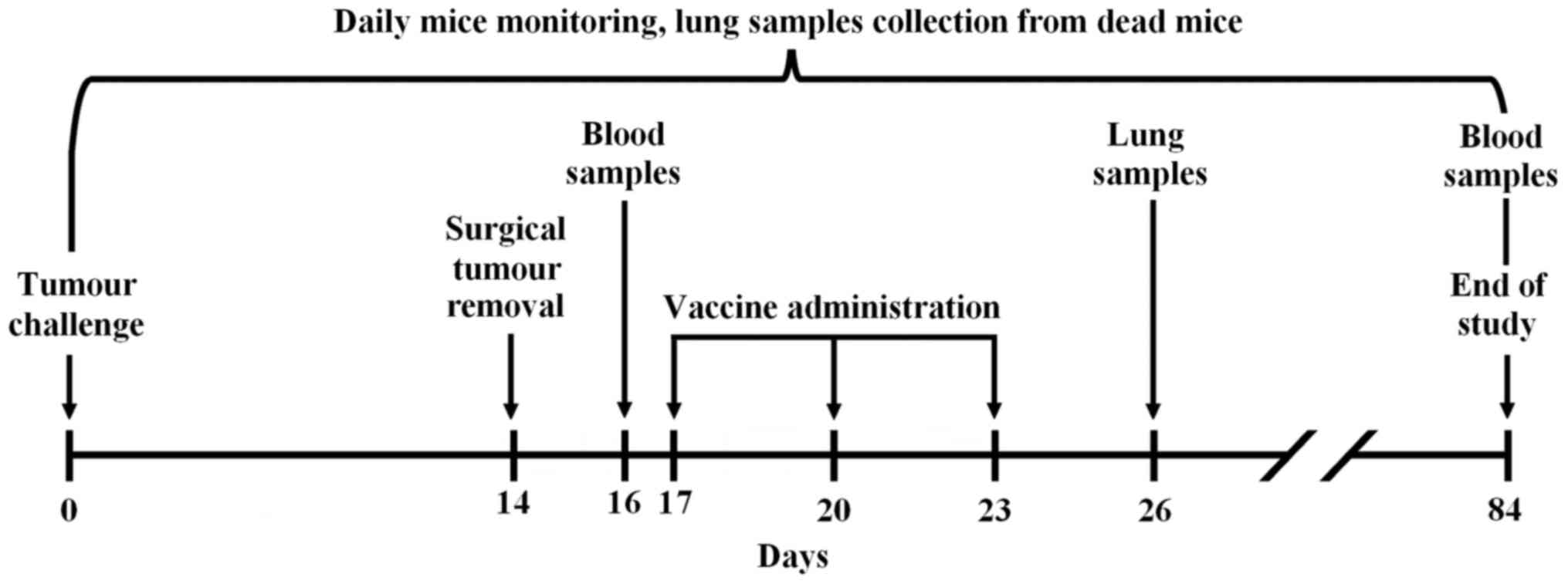
and 23. See vaccination scheme in Fig.
1. Mice were observed until day 84.
Sampling
In order to assess the anticancer effectiveness of
the vaccines, mouse lung and blood samples were obtained (Fig. 1). Lungs were analysed for metastasis
since they are the preferred metastatic location for the LLC cell
line (49). Blood samples were
collected from the hip vein before vaccination onset (day 16) and
from the surviving mice of each experimental group at the end of
the experiment (day 84). Three days after the completion of the
therapeutic vaccination course (day 26), 3 mice from each group
were sacrificed to determine the presence of micrometastases. Mice
found dead during the follow-up period underwent histological
analysis as well. The survival of mice was observed daily
throughout the experiment, with the exception of mice sacrificed on
day 26, which were not taken into account.
Analysis of metastasis
In each case, lung tissue was fixed in 10% neutral
buffered formalin, dehydrated in alcohol baths (70% for 12 h/90%
for 12 h/100% for 24 h) and embedded in paraffin. The formed
paraffin blocks were cut with a microtome into slices 3-µm thick.
The sections were deparaffinised, rehydrated and stained with
hematoxylin and eosin morphological stain. Each section was
examined with a light microscope in order to identify the
tumour-infiltrated areas. Images were captured with an automated
Leica DM50000 B microscope equipped with a Leica DFC420 C digital
camera (Leica, Wetzlar, Germany). Images were processed using the
‘Sharpen’ option in the ‘ImageJ’ image analysis program (50). The area of all tumour nodules that
were found in lung tissue was estimated by ‘Freehand selection
tool’ in ‘ImageJ’, and measurements were expressed in
mm2.
Flow cytometry
Blood samples were collected and analysed by flow
cytometry for CD8a surface expression at two different time-points
(days 26 and 84). One hundred microliters of whole blood was
stained with PE-Cy7-conjugated rat anti-mouse CD8a antibody (cat
552877), according to the manufacturer's instructions and lysed
with FACS lysing solution (both from BD Pharmingen, San Diego, CA,
USA). Samples were acquired with LSR II flow cytometer (BD
Pharmingen), using 488 nm excitation laser and 780/60 band pass
filter. At least 1×105 cells were analysed with FACSDiva
software (BD Pharmingen). Singlets and alive cells were identified
based on forward (FSC) and side (SSC) scatter profile.
Statistical analysis
All statistical analyses were performed using
Statistica 10 software (StatSoft, Tulsa, OK, USA). Survival was
evaluated by Kaplan-Meier method and differences in survival
distributions were assessed using ANOVA. Weighted ANOVA variant was
used to take into account the differences in the mouse count in the
groups. Post-hoc analysis was performed using the Dunnett's test.
Differences between tumour areas in lung histochemistry slides were
assessed using the Mann-Whitney U-test. Flow cytometry data were
analysed with the unpaired, two-tailed Student's t-test.
Differences were considered statistically significant for P-values
<0.05.
Results
Survival analysis
The differences in the survival between the groups
were analysed using several statistical models. Kaplan-Meier
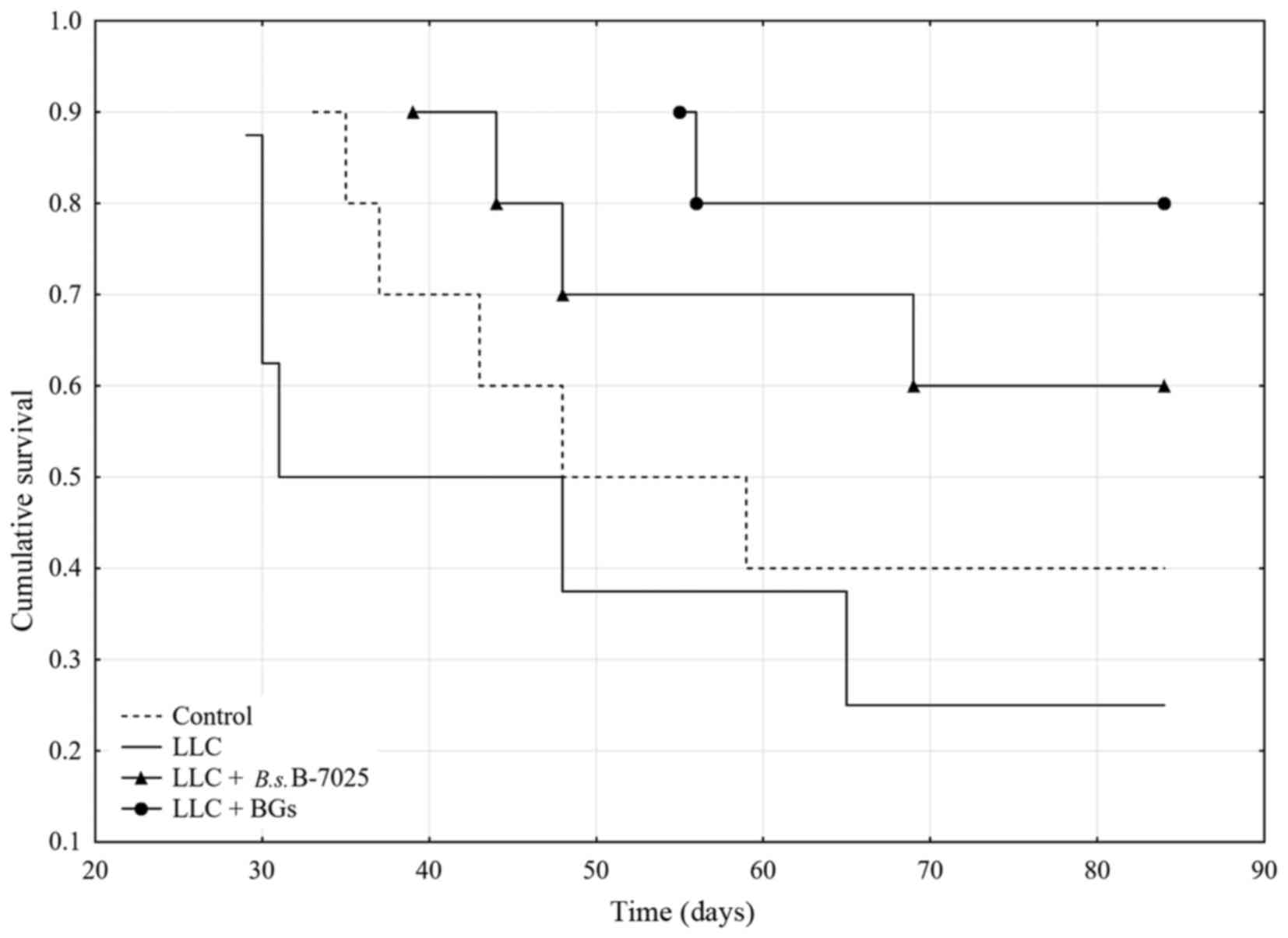
survival curves are displayed in Fig.
2. By the end of the observation period (day 84) the survival
rate in the LLC+BGs group, LLC+B.s.B-7025 group, LLC group
and control group was 80, 60, 25 and 40%, respectively.
ANOVA survival analysis [F(2,25)=5.0116, P=0.01478] indicated
statistically significant differences in survival times between the
groups (Table I). Post-hoc analysis
showed that mice in the LLC+BGs group, but neither in the
LLC+B.s.B-7025 group nor the LLC group, survived
significantly longer compared with the untreated control. This
indicated that treatment with LLC+B.s.B-7025 and
non-adjuvanted LLC lysate had no therapeutic effect in this
experimental setting. There was no significant survival difference
between mice treated with LLC+BGs and LLC+B.s.B-7025
vaccines, but the group treated with LLC lysate alone had
statistically lower survival than the groups treated with LLC+BGs
or LLC+B.s.B-7025 vaccines (Table II).
 | Table I.Survival of the C57BL/6 mice after
removal of LLC tumour and subsequent therapeutic vaccination with
various formulations of syngeneic LLC lysate. |
Table I.
Survival of the C57BL/6 mice after
removal of LLC tumour and subsequent therapeutic vaccination with
various formulations of syngeneic LLC lysate.
| Group | Days (median) | Days (mean) | Days
(Std.Err.) | Days (−95.00%) | Days (+95.00%) | n (total=28) |
|---|
| Control | 53.5 | 59 | 7 | 43 | 75 | 10 |
| LLC | 39.5 | 50 | 9 | 30 | 70 | 8 |
|
LLC+B.s.B-7025 | 84.0 | 70 | 6 | 57 | 84 | 10 |
| LLC+BGs | 84.0 | 78 | 4 | 70 | 87 | 10 |
 | Table II.ANOVA post-hoc analysis of survival
(Dunnetts method). |
Table II.
ANOVA post-hoc analysis of survival
(Dunnetts method).
| Group | P-value |
|---|
| Control | – | 0.3486 | 0.9834 | 0.9989 |
| LLC | 0.9621 | – | 0.9989 | 1.0000 |
|
LLC+B.s.B-7025 | 0.2296 | 0.0485 | – | 0.9559 |
| LLC+BGs | 0.0475 | 0.0070 | 0.3750 | – |
Analysis of metastasis
Three days after the last vaccination, no metastatic
foci were found in the sacrificed mice in any of the groups. On the
other hand, morphological analysis of the mice that died during the
follow-up period revealed development of pulmonary metastatic foci
in 100% of the analysed control and LLC samples. In contrast, only
33% of the analysed lung samples of both the LLC+B.s.B-7025
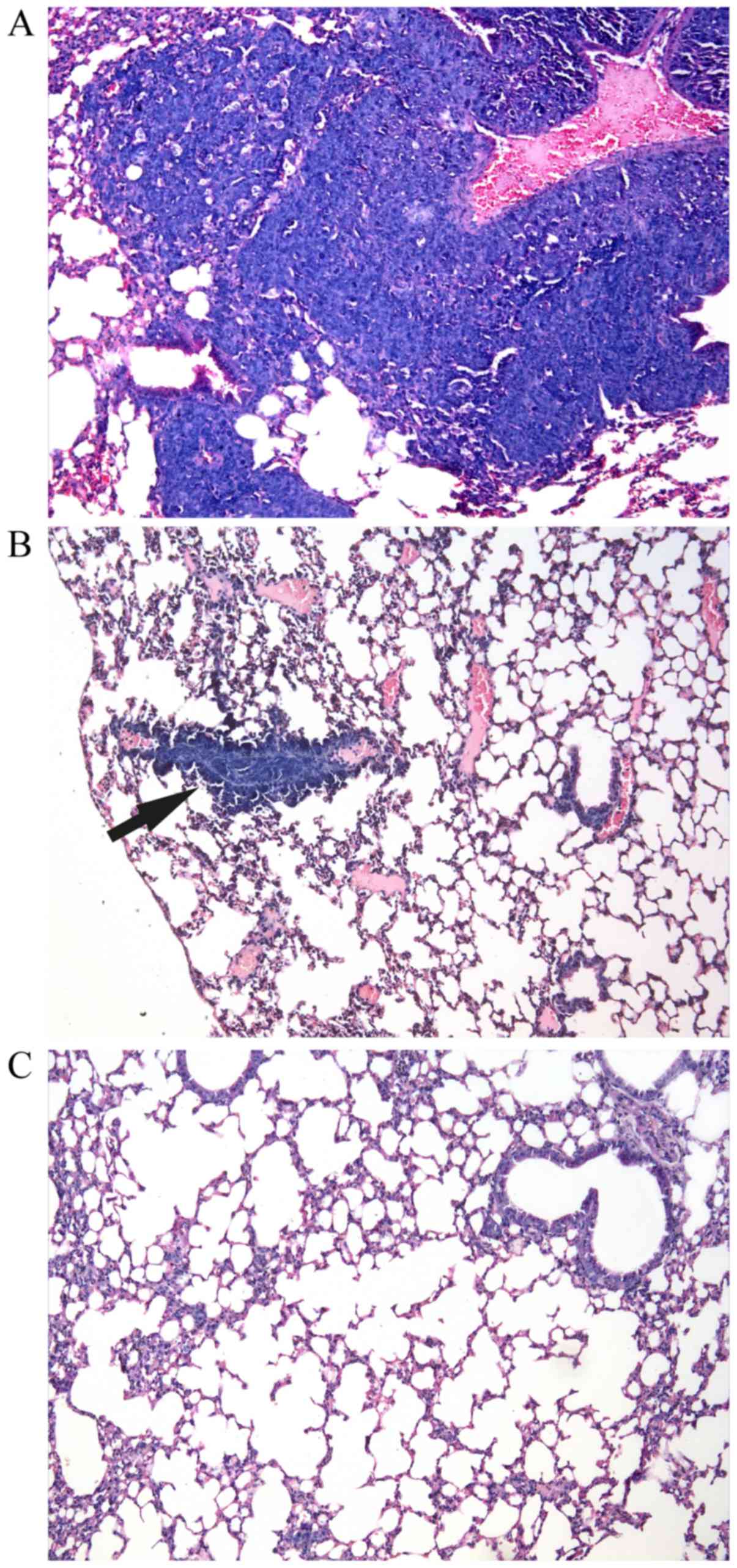
and LLC+BGs groups displayed signs of metastasis. Fig. 3 shows representative lung slices
presenting various spreads of metastasis in the investigated
groups. The mean size of the metastatic tumours in the LLC group
was 0.452±0.3 mm2, whereas in the LLC+B.s.B-7025
group it was 0.016±0.013 mm2 and in the LLC+BGs group
only 0.006±0.0001 mm2. Mann-Whitney U-test confirmed
that the LLC+BGs metastasis size was significantly smaller then
that of the LLC group (P=0.019), unlike the LLC+B.s.B-7025
group (P=0.514).
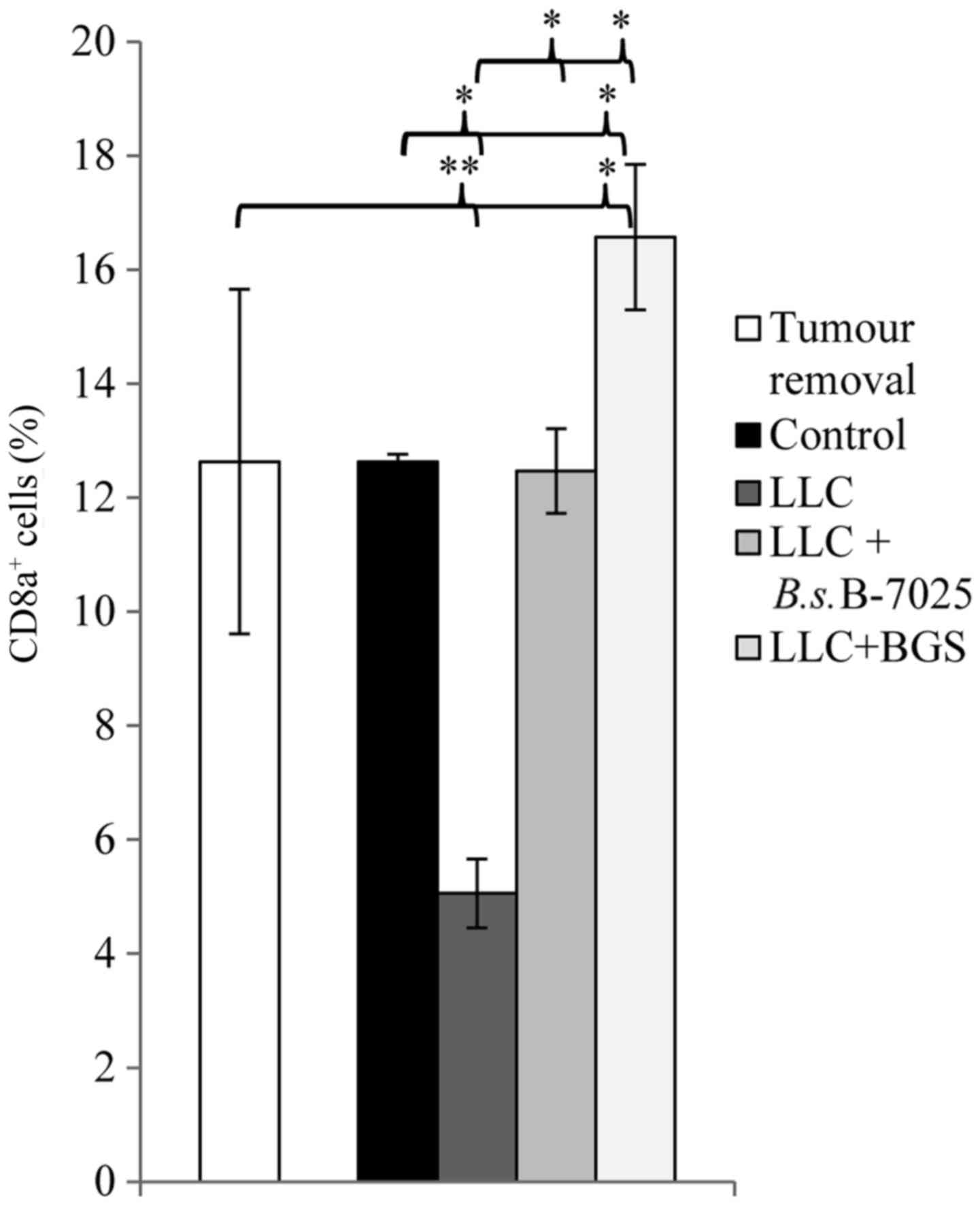
Flow cytometry
Whole blood samples were analysed for CD8a surface
antigen expression at two different time-points (before the
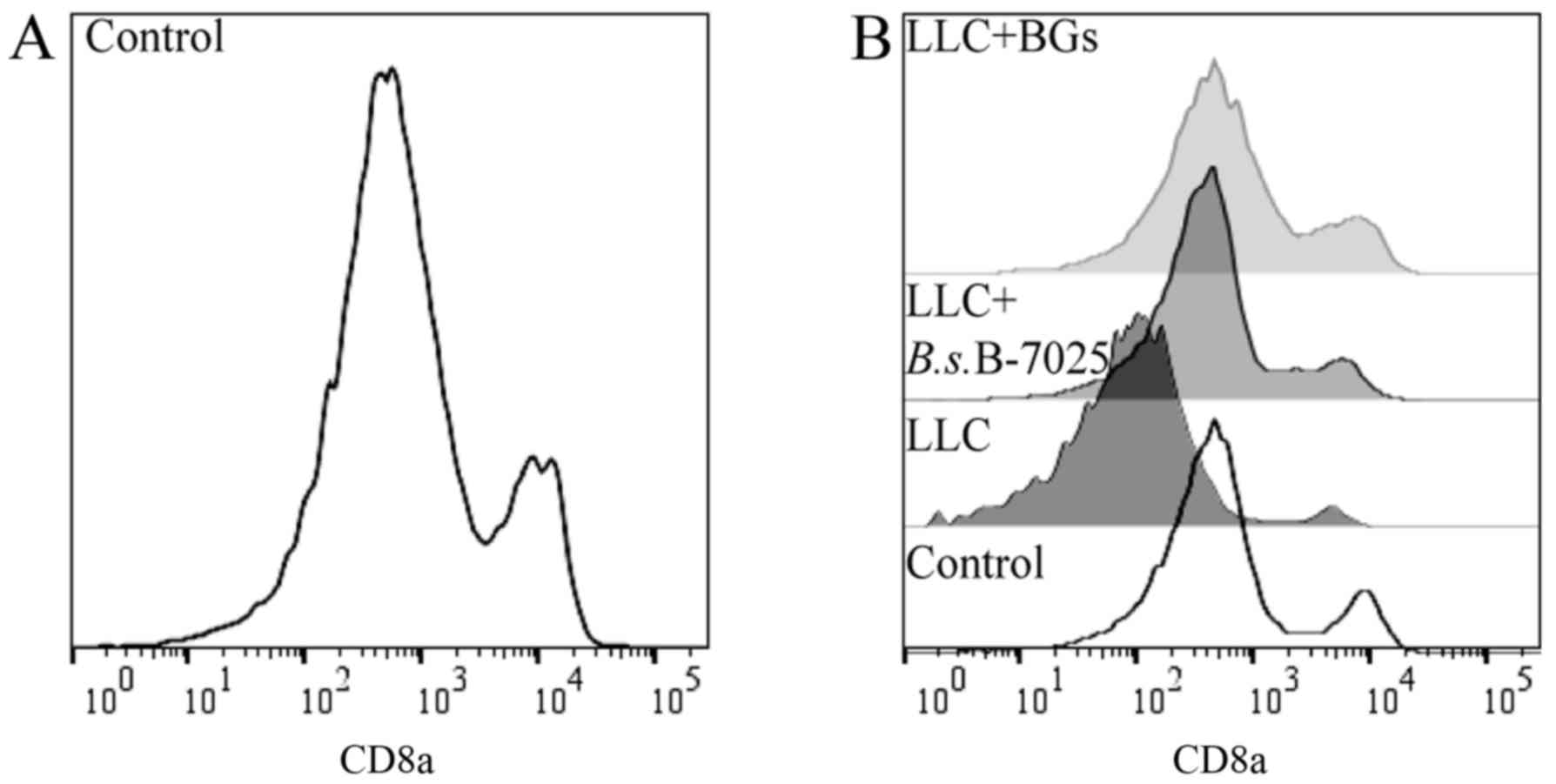
vaccination and at the end of the study). Representative graphs are
shown in Fig. 4. Summarised results
are presented in Fig. 5. The number
of cells expressing CD8a remained at the same level before and
after vaccination in both the control and LLC+B.s.B-7025
groups. On the other hand, compared to the measurement taken before
the vaccinations, the mice treated with non-adjuvanted LLC lysate
had a significantly lower CD8a count in the blood at the end of the
study (P<0.001), whereas the mice in the LLC+BGs group had a
significantly higher CD8a+ cell count at the end of the
study (P=0.019). Notably, at the end of the study, CD8a expression
in the LLC+BGs group was significantly higher than that noted in
the control (p=0.047), LLC+B.s.B-7025 (P=0.026) and LLC
(P=0.002) groups. Mice treated with LLC+B.s.B-7025 vaccine
had a higher CD8a+ cell count than that noted in the
mice treated with the LLC lysate alone (P<0.001), but not
statistically different from the control group (P=0.782) (Fig. 5).
Discussion
In the present study, C57BL/6 mice, inoculated with
metastasizing LLC cells, received adjuvant treatment with
therapeutic vaccines following surgical removal of primary tumour.
The proposed approach is clinically relevant, due to the fact that
management of micrometastatic or unresectable metastatic disease in
patients after primary tumour resection is still a challenge in
modern oncology (51,52). Primary tumour removal was introduced
to focus more on metastasis inhibition rather than on tumour growth
inhibition. Lungs were investigated for metastatic foci due to the
known affinity of LLC cells, which spread preferentially to lung
tissue (49). Syngeneic LLC lysate
was used as a vaccine (source of tumour antigens for immunization).
To counter the potential immunosuppressive characteristics of the
tumour lysate (17) and augment the
evolving antitumour immune responses, two distinct bacterial-based
adjuvants were used with the therapeutic vaccination. These
adjuvants included either B. subtilis B-7025 70 kDa protein
isolates or bacterial ghost generated from probiotic E. coli
Nissle 1917. Whole bacterial-based adjuvant systems gained
attention in oncology with the introduction of bacillus
Calmette-Guérin immunotherapy for the treatment of non-muscle
invasive bladder cancer (53).
Currently this type of cancer immunotherapy was further improved by
manipulating bacteria and loading them with tumour antigens in
order to ensure tumour antigen specificity of the elicited immune
responses. Such innovative approaches include live, attenuated
Listeria monocytogenes bacteria consisting of gene deletions
to diminish their pathogenicity and engineered to express tumour
antigens (54). Recent data have
shown that immunotherapy with mesothelin-expressing, live,
attenuated L. monocytogenes CRS-207 plus chemotherapy
demonstrate encouraging clinical activity in patients with
malignant pleural mesothelioma (55). Our investigated BG platform also
emerges as a very promising immunotherapeutic tool of this kind. It
may even be safer, since it exploits completely non-living and
intact bacterial envelopes rather than attenuated or killed, but
metabolically active, bacteria.
The study results showed that treatment of
tumour-bearing mice with LLC+BGs or LLC+B.s.B-7025 prevented
the formation of lung metastasis in 67% of the mice, while no
protective effect was detected in the mice treated with LLC
lysate-alone and in mice without treatment (control group)
(Fig. 3). Although the median
overall survival of the mice treated either with LLC+BGs and
LLC+B.s.B-7025 was the same (84 days) and increased compared
with the controls (53.5 days), only the treatment with LLC+BGs led
to a statistically significant median overall survival (Fig. 2). Moreover, the survival rate of the
mice from the LLC+BGs treatment group at day 84 was significantly
increased when compared with the survival rate of mice from the
LLC+B.s.B-7025 treatment group (80 vs. 60%). Mice treated
with non-adjuvant LLC lysate had a significantly shorter survival
than those treated with LLC+BGs or LLC+B.s.B-7025
Cytotoxic lymphocytes are thought to be the key
factor in successful anticancer immune response (56). Smaller metastatic foci found in lung
samples (Fig. 3) can explain why
the LLC+BGs vaccine achieved a better survival rate than
LLC+B.s.B-7025. Smaller metastatic foci found in lung
samples of the mice treated with the LLC+BGs vaccine (compared to
lung samples of mice from other treatment groups) along with
improved median overall survival of mice might be associated with
the detected elevated numbers of circulating CD8a+ T
cells at the end of the study. Subcutaneous administration of
vaccine made of LLC+BGs elicited a significantly higher number of
circulating CD8a+ T cells in the tumour-bearing mice
compared to the numbers of total circulating CD8a+ T
cells detected in blood samples obtained from the non-treated mice
as well as from mice treated with the vaccine made of
LLC+B.s.B-7025 and LLC alone. Moreover, only mice treated
with LLC+BGs vaccine, compared to other treatment groups, had at
the end of the study a significantly higher number of circulating
CD8a+ T cells than before the vaccination onset
(Fig. 5). Noteworthy, the standard
deviation of CD8a+ cells in the LLC+BGs group was three
times smaller when measured in the surviving vs. the dead mice at
the end of the study (data not shown). This fact implies that the
surviving mice can be characterised by a successful CTL
mobilisation. This is in line with already published data, where
authors also detected higher CD8a levels only in responder mice,
treated with a TCL-based vaccine (57).
Notably, the level of blood CD8a+ T cells
was significantly decreased in the mice treated with the
non-adjuvant-modified LLC lysate compared with the controls and
mice treated with adjuvanted LLC lysates (Fig. 5). In addition, mice treated with
non-adjuvanted LLC lysate showed the lowest survival rate of only
25% compared with 80, 60 and 40% in the LLC+BGs,
LLC+B.s.B-7025 and control groups, respectively. We assume
that various immunosuppressants present in the tumour lysate were
responsible for the decrease in CD8a+ T cell population
as a result of predominant cancer-associated immunosuppressive
environment. However, coupling of LCC lysate vaccine preparations
with strong immunostimulators, especially with BGs, can modulate
the character of LCC lysate from immunosuppressive to
immunostimulatory by improving both the recognition and
presentation of tumour-associated neo-antigens by professional
antigen-presenting cells capable then of activating and
(re)stimulating effector immune competent cells. Indeed it has been
previously shown that the generation of tolerogenic DCs was induced
by culturing immature DCs from healthy donors with the plasma of
pancreatic cancer patients (58) or
supernatants of various tumour cell lines (14,59).
In the light of this preliminary study, BGs emerge
as a novel adjuvant and antigen delivery platform for TCL-based
therapeutic cancer vaccination. This straightforward and
potentially clinically effective immunotherapeutical approach
requires more extensive pre-clinical investigation and warrants
consideration outside the animal models.
Acknowledgements
This study was supported by the Lithuanian Research
Council project ‘Immunomodulating properties of bacteriophage
derived dsRNA of different size and their use as vaccine adjuvants’
no. TAP-LLT-15-028.
References
|
1
|
Vesely MD and Schreiber RD: Cancer
immunoediting: Antigens, mechanisms, and implications to cancer
immunotherapy. Ann NY Acad Sci. 1284:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zitvogel L, Tesniere A and Kroemer G:
Cancer despite immunosurveillance: Immunoselection and
immunosubversion. Nat Rev Immunol. 6:715–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bose A, Chakraborty T, Chakraborty K, Pal
S and Baral R: Dysregulation in immune functions is reflected in
tumor cell cytotoxicity by peripheral blood mononuclear cells from
head and neck squamous cell carcinoma patients. Cancer Immun.
8:102008.PubMed/NCBI
|
|
4
|
Noguchi A, Kaneko T, Naitoh K, Saito M,
Iwai K, Maekawa R, Kamigaki T and Goto S: Impaired and imbalanced
cellular immunological status assessed in advanced cancer patients
and restoration of the T cell immune status by adoptive T-cell
immunotherapy. Int Immunopharmacol. 18:90–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Galluzzi L, Vacchelli E, Bravo-San Pedro
JM, Buqué A, Senovilla L, Baracco EE, Bloy N, Castoldi F, Abastado
JP, Agostinis P, et al: Classification of current anticancer
immunotherapies. Oncotarget. 5:12472–12508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Butterfield LH: Cancer vaccines. BMJ.
350:h9882015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palucka K and Banchereau J: Cancer
immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schijns V, Tartour E, Michalek J,
Stathopoulos A, Dobrovolskienė NT and Strioga MM: Immune adjuvants
as critical guides directing immunity triggered by therapeutic
cancer vaccines. Cytotherapy. 16:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiang CL, Coukos G and Kandalaft LE:
Whole tumor antigen caccines: Where Are We? Vaccines (Basel).
3:344–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerlinger M, Rowan AJ, Horswell S, Larkin
J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A,
Tarpey P, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Srivatsan S, Patel JM, Bozeman EN, Imasuen
IE, He S, Daniels D and Selvaraj P: Allogeneic tumor cell vaccines:
The promise and limitations in clinical trials. Hum Vaccin
Immunother. 10:52–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schnurr M, Galambos P, Scholz C, Then F,
Dauer M, Endres S and Eigler A: Tumor cell lysate-pulsed human
dendritic cells induce a T-cell response against pancreatic
carcinoma cells: An in vitro model for the assessment of tumor
vaccines. Cancer Res. 61:6445–6450. 2001.PubMed/NCBI
|
|
14
|
Kuang DM, Zhao Q, Xu J, Yun JP, Wu C and
Zheng L: Tumor-educated tolerogenic dendritic cells induce
CD3epsilon down-regulation and apoptosis of T cells through
oxygen-dependent pathways. J Immunol. 181:3089–3098. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schuster N and Krieglstein K: Mechanisms
of TGF-beta-mediated apoptosis. Cell Tissue Res. 307:1–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong B, Dai G, Xu L, Zhang Y, Ling L, Sun
L and Lv J: Tumor cell lysate induces the immunosuppression and
apoptosis of mouse immunocytes. Mol Med Rep. 10:2827–2834.
2014.PubMed/NCBI
|
|
18
|
Strioga MM, Felzmann T, Powell DJ Jr,
Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J and Schijns
VE: Therapeutic dendritic cell-based cancer vaccines: The state of
the art. Crit Rev Immunol. 33:489–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beck B, Dörfel D, Lichtenegger FS, Geiger
C, Lindner L, Merk M, Schendel DJ and Subklewe M: Effects of TLR
agonists on maturation and function of 3-day dendritic cells from
AML patients in complete remission. J Transl Med. 9:1512011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawahara M and Takaku H: Intradermal
immunization with combined baculovirus and tumor cell lysate
induces effective antitumor immunity in mice. Int J Oncol.
43:2023–2030. 2013.PubMed/NCBI
|
|
21
|
Temizoz B, Kuroda E and Ishii KJ: Vaccine
adjuvants as potential cancer immunotherapeutics. Int Immunol.
28:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurooka M and Kaneda Y: Inactivated Sendai
virus particles eradicate tumors by inducing immune responses
through blocking regulatory T cells. Cancer Res. 67:227–236. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bungener L, Huckriede A, Wilschut J and
Daemen T: Delivery of protein antigens to the immune system by
fusion-active virosomes: A comparison with liposomes and ISCOMs.
Biosci Rep. 22:323–338. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Tu Z, Feng F, Shi H, Chen K and Xu
X: Virosome, a hybrid vehicle for efficient and safe drug delivery
and its emerging application in cancer treatment. Acta Pharm.
65:105–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wiedermann U, Wiltschke C, Jasinska J,
Kundi M, Zurbriggen R, Garner-Spitzer E, Bartsch R, Steger G,
Pehamberger H, Scheiner O, et al: A virosomal formulated Her-2/neu
multi-peptide vaccine induces Her-2/neu-specific immune responses
in patients with metastatic breast cancer: A phase I study. Breast
Cancer Res Treat. 119:673–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Witte A, Wanner G, Sulzner M and Lubitz W:
Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli.
Arch Microbiol. 157:381–388. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Henrich B, Lubitz W and Plapp R: Lysis of
Escherichia coli by induction of cloned phi X174 genes. Mol Gen
Genet. 185:493–497. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Montanaro J, Inic-Kanada A, Ladurner A,
Stein E, Belij S, Bintner N, Schlacher S, Schuerer N, Mayr UB,
Lubitz W, et al: Escherichia coli Nissle 1917 bacterial ghosts
retain crucial surface properties and express chlamydial antigen:
An imaging study of a delivery system for the ocular surface. Drug
Des Devel Ther. 9:3741–3754. 2015.PubMed/NCBI
|
|
29
|
Eko FO, Mayr UB, Attridge SR and Lubitz W:
Characterization and immunogenicity of Vibrio cholerae ghosts
expressing toxin-coregulated pili. J Biotechnol. 83:115–123. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kudela P, Koller VJ and Lubitz W:
Bacterial ghosts (BGs) - advanced antigen and drug delivery system.
Vaccine. 28:5760–5767. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu W, Yang G, Zhang Y, Yuan J and An L:
Generation of biotechnology-derived Flavobacterium columnare ghosts
by PhiX174 gene E-mediated inactivation and the potential as
vaccine candidates against infection in grass carp. J Biomed
Biotechnol. 2012:7607302012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cai K, Zhang Y, Yang B and Chen S:
Yersinia enterocolitica ghost with msbB mutation provides
protection and reduces proinflammatory cytokines in mice. Vaccine.
31:334–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Riedmann EM, Kyd JM, Cripps AW and Lubitz
W: Bacterial ghosts as adjuvant particles. Expert Rev Vaccines.
6:241–253. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Muhammad A, Champeimont J, Mayr UB, Lubitz
W and Kudela P: Bacterial ghosts as carriers of protein subunit and
DNA-encoded antigens for vaccine applications. Expert Rev Vaccines.
11:97–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Podgorskiĭ VS, Kovalenko EA, Getman EI,
Potebnia GP and Tanasienko OA: Lectin activity of antitumor
substances synthesized by Bacillus subtilis B-7025. Mikrobiol Z.
64:10–17. 2002.(In Russian). PubMed/NCBI
|
|
36
|
Tanasienko OA, Cheremshenko NL, Titova GP,
Potebnya MG, Gavrilenko MM, Nagorna SS and Kovalenko NK: Elevation
of the efficacy of antitumor vaccine prepared on the base of
lectines from B. subtilis B-7025 upon its combined application with
probiotics in vivo. Exp Oncol. 27:336–338. 2005.
|
|
37
|
Potebnia HP, Safronova LA, Cheremshenko
NL, Lisovenko HS, Sorokulova IB, Prykhodko VO, Trokhymenko NV,
Tanasiienko OA and Bombin AV: Influence of probiotic subalin on
efficiency of antitumor vaccine. Mikrobiol Zh. 68:51–58. 2006.(In
Ukrainian).
|
|
38
|
Potebnya GP, Lisovenko GS, Trokhimenko NV,
Cheremshenko NL, Didenko GV, Reder AS and Andronati SA: Elevation
of efficacy of cancer vaccine combined with interferon and inducer
of endogeneous interferon synthesis amixin. Exp Oncol. 30:319–323.
2008.PubMed/NCBI
|
|
39
|
Tanasienko OA, Rudyk MP, Pozur VV and
Potebnya GP: Influence of bacterial lectins on some reactions of
nonspecific immunity in sarcoma 37 transplanted mice. Exp Oncol.
32:254–257. 2010.PubMed/NCBI
|
|
40
|
Potebnya G, Cheremshenko N, Lisovenko G,
Voyekova I, Bazas' V, Todor I and Chekhun V: Antitumor efficacy of
autovaccines prepared from chemoresistant tumor cells with the use
of lectin OF B. subtilis B-7025. Exp Oncol. 29:277–280. 2007.
|
|
41
|
Potebnya GP, Kudryavets YY, Lisovenko GS,
Cheremshenko NL, Voeykova IM, Trokhimenko NV, Symchich TV and
Evstrateyva LM: Experimental study of the efficacy of combined use
of cancer vaccine and interferon. Exp Oncol. 29:102–105.
2007.PubMed/NCBI
|
|
42
|
Potebnya GP, Voeykova IM, Lisovenko GS,
Cheremshenko NL, Todor IM, Yudina OY, Kovtonyuk OV and Chekhun VF:
Antitumor and antimetastatic activities of vaccine prepared from
cisplatin-resistant lewis lung carcinoma. Exp Oncol. 31:226–230.
2009.PubMed/NCBI
|
|
43
|
Xu X, Huang Q, Mao Y, Cui Z, Li Y, Huang
Y, Rajput IR, Yu D and Li W: Immunomodulatory effects of Bacillus
subtilis (natto) B4 spores on murine macrophages. Microbiol
Immunol. 56:817–824. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee SW, Park HJ, Park SH, Kim N and Hong
S: Immunomodulatory effect of poly-γ-glutamic acid derived from
Bacillus subtilis on natural killer dendritic cells. Biochem
Biophys Res Commun. 443:413–421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Langemann T, Koller VJ, Muhammad A, Kudela
P, Mayr UB and Lubitz W: The Bacterial ghost platform system:
Production and applications. Bioeng Bugs. 1:326–336. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Didenko GV, Dvorschenko OS, Lisovenko GS,
Kovalenko NG, Potebnya GP, Kikot VV, Pozur VK and Golub AA: The
modification of cancer vaccine prepared on the base of metabolic
products of B. subtilis 7025 with the use of sorbents and
automacrophages. Exp Oncol. 25:116–118. 2003.
|
|
47
|
Didenko GV, Kuzmenko AP, Shpak EG,
Tawrovska IA, Nadirashvili NA, Blum IO and Potebnya GP:
Optimization of the methods of isolation, electrophoretic
characterization, and antitumor efficacy of cytotoxic metabolites
from the culture filtrate Bacillus subtilis B-7025. Dop. NAS
Ukraine. 7:185–190. 2012.
|
|
48
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
49
|
Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY
and Deng J: Cardamonin inhibits metastasis of Lewis lung carcinoma
cells by decreasing mTOR activity. PLoS One. 10:e01277782015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schirrmacher V, Fournier P and Schlag P:
Autologous tumor cell vaccines for post-operative active-specific
immunotherapy of colorectal carcinoma: Long-term patient survival
and mechanism of function. Expert Rev Vaccines. 13:117–130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Laufer I, Iorgulescu JB, Chapman T, Lis E,
Shi W, Zhang Z, Cox BW, Yamada Y and Bilsky MH: Local disease
control for spinal metastases following ‘separation surgery’ and
adjuvant hypofractionated or high-dose single-fraction stereotactic
radiosurgery: Outcome analysis in 186 patients. J Neurosurg Spine.
18:207–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Brandau S and Suttmann H: Thirty years of
BCG immunotherapy for non-muscle invasive bladder cancer: A success
story with room for improvement. Biomed Pharmacother. 61:299–305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rothman J and Paterson Y: Live-attenuated
Listeria-based immunotherapy. Expert Rev Vaccines. 12:493–504.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jahan T, Hassan R, Alley E, Kindler H,
Antonia S, Whiting C, Coussens L, Murphy AL, Thomas A and
Brockstedt DG: 208O_PR: CRS-207 with chemotherapy (chemo) in
malignant pleural mesothelioma (MPM): results from a phase 1b
trial. J Thorac Oncol. 11:(4 Suppl). S1562016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Becht E, Giraldo NA, Dieu-Nosjean MC,
Sautès-Fridman C and Fridman WH: Cancer immune contexture and
immunotherapy. Curr Opin Immunol. 39:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kawahara M and Takaku H: A tumor lysate is
an effective vaccine antigen for the stimulation of CD4(+) T-cell
function and subsequent induction of antitumor immunity mediated by
CD8(+) T cells. Cancer Biol Ther. 16:1616–1625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tjomsland V, Spångeus A, Sandström P,
Borch K, Messmer D and Larsson M: Semi mature blood dendritic cells
exist in patients with ductal pancreatic adenocarcinoma owing to
inflammatory factors released from the tumor. PLoS One.
5:e134412010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kúdela P, Schwarczová Z, Sedlák J and
Bizik J: Conditioned medium from HeLa cells enhances motility of
human monocyte-derived dendritic cells but abrogates their
maturation and endocytic activity. Neoplasma. 48:382–388.
2001.PubMed/NCBI
|