Introduction
Angiogenesis is the formation of new blood vessels.
It is essential for the delivery of nutrients and oxygen to tumor
cells that are distant from existing blood vessels. Angiogenesis is
involved in the pathogenesis of many diseases such as cancer,
atherosclerosis, and diabetic retinopathy (1–3). It
plays an especially important role in tumor growth, metastasis, and
invasion. The development of angiogenesis is associated with a poor
tumor prognosis (4). To initiate
tumor angiogenesis, angiogenic growth factors, including vascular
endothelial growth factors (VEGFs), fibroblast growth factors, and
platelet-derived growth factors, must be secreted (5). Among angiogenic growth factors, VEGF
is highly specific and plays a crucial role in the angiogenesis of
tumors (6). VEGF is induced by
various pathophysiological conditions, including low oxygen tension
(hypoxia), and several growth factors, such as epidermal growth
factor (EGF), transforming growth factor α and β, and insulin-like
growth factor 1 (7). Hypoxia, the
prime stimulus of angiogenesis, enhances stabilization of
hypoxia-inducible factors (HIFs) and their binding to VEGF promoter
elements. Activated HIF induces VEGF transcription (8).
HIF-1α is an oxygen-dependent transcription factor
that plays an important role in cellular adaptation to hypoxia and
in tumor progression. It is composed of the HIF-1α subunit and the
HIF-1β subunit (9). HIF-1α
expression is regulated by oxygen pressure, whereas HIF-1β
expression is unaffected by changes in oxygen tension (3). In addition, HIF-1α is overexpressed in
more than 70% of solid human tumors (10,11).
Oxygen-dependent prolyl hydroxylases catalyze polyubiquitinylation
by the von Hippel-Lindau (VHL) protein E3 ligase complex and
consequent HIF-1α degradation in normoxic conditions (12). In hypoxia, HIF-1α is not degraded
due to a block in prolyl hydroxylation. Stabilized HIF-1α forms an
active complex with HIF-1β in the nucleus and activates the
transcription of target genes such as VEGF by binding to the
promoter region of hypoxia-response elements (HREs) (13).
In addition to hypoxia, HIF-1α expression is induced
by cytokines, hormones, and growth factors that stimulate tyrosine
kinase receptors (5). One of these
cytokines, EGF, also increases the HIF-1α level by activating EGF
receptor (EGFR) signaling in normoxic conditions. EGF-stimulated
HIF-1α expression is regulated by various signal transduction
pathways, such as the phosphatidylinositol-3-kinase (PI3K)/Akt
signaling pathway and the p38, c-Jun N-terminal protein kinase, and
extracellular signal-regulated protein kinase (ERK), which all
belong to the mitogen-activated protein kinase (MAPK) family
signaling pathway (14,15). It is widely known that EGF-induced
phosphorylation of mammalian target of rapamycin (mTOR) leads to
HIF-1α protein synthesis through regulation of p70S6 kinase 1
(p70S6K) and eukaryotic initiation factor 4E-binding protein 1
(4E-BP1) (10).
Delphinidin, a polyphenol that belongs to the group
of anthocyanidins, is abundant in many pigmented fruits (berries
and dark grapes) and vegetables (eggplants and tomatoes).
Delphinidin has anti-inflammatory, antioxidant, anti-proliferative,
and antitumor activities in a variety of cancer cells (16,17).
It was recently reported to have inhibitory effects on angiogenesis
(18) and to prevent
hypoxia-induced activation of nuclear factor-κB (NF-κB), and Akt
inhibition (19). Delphinidin also
decreases downstream signaling cascades crucial for endothelial
cell tube formation through VEGFR-2 inhibition (20). However, there is no information
concerning the molecular mechanisms underlying the VEGF-related
anti-angiogenic effects of delphinidin in lung cancer cells.
In this study, we investigated the inhibitory
effects of delphinidin on HIF-1α and VEGF expression in human lung
adenocarcinoma A549 cells. We found that delphinidin specifically
inhibits EGF-induced VEGF expression by blocking HIF-1α protein
expression, without regulating HIF-1α mRNA expression.
Additionally, delphinidin appeared to reduce HIF-1α protein
synthesis by inhibiting the ERK and Akt/mTOR/p70S6K signaling
pathways.
Materials and methods
Cell culture and materials
A549 (human lung adenocarcinoma cells), NCI-H460
(human lung adenocarcinoma cells, MCF-7 (human breast carcinoma
cells), and PC3M (human prostate cancer cells) were obtained from
the American Type Culture Collection (ATCC, Manassas, VA, USA).
A549 cells were cultured in RPMI-1640 medium (Gibco, Grand Island,
NY, USA) supplemented with 10% fetal bovine serum (FBS) and
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Delphinidin was obtained from Cayman Chemical Co.
(Ann Arbor, MI, USA).
Cell viability assay
Cells were plated in 96-well culture plates at a
density of 1×104 cells/well in RPMI-1640 culture medium
and allowed to attach for 24 h. The media were then discarded and
replaced with 100 µl of new medium containing various
concentrations of delphinidin and cultured for 24 h. A WST-1 assay
kit (Cayman Chemical Co.) was added to each well. The amount of
formazan deposits was quantified according to the supplier's
protocol after 4 h of incubation with WST-1 test solution at 37°C
in a 5% CO2 incubator.
Western blot analysis
A549 cells were pre-incubated for 24 h, then
stimulated with CoCl2 (200 µM) and EGF (20 ng/ml) in the
presence of delphinidin. After incubation, the cells were collected
and washed twice with cold PBS. The cells were lysed in a lysis
buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 2 mM
EDTA, 1 mM EGTA, 1 mM NaVO3, 10 mM NaF, 1 mM
dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 25 µg/ml
aprotinin, and 25 µg/ml leupeptin] and maintained on ice for 30
min. The cell lysates were obtained via centrifugation, and the
protein concentrations were determined using a protein assay kit.
Aliquots of the lysates were separated on 12% SDS-polyacrylamide
gel and transferred onto a nitrocellulose transfer membrane
(Whatman, Dassel, Germany) with a glycine transfer buffer [192 mM
glycine, 25 mM Tris-HCl (pH 8.8), 20% methanol (v/v)]. After
blocking the nonspecific site with 1% bovine serum albumin (BSA),
the membrane was incubated overnight with a specific primary
antibody at 4°C. The membrane was then incubated for an additional
1 h with a peroxidase-conjugated secondary antibody (1:1,000;
Vector Laboratories, Inc., Burlingame, CA, USA) at room
temperature. The immunoactive proteins were detected using an
enhanced chemiluminescence (ECL) western blot analysis detection
kit (Advansta, Inc., Menlo Park, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was
carried out using a commercial kit (SuperScript II RNase H-reverse
transcriptase; Invitrogen) and total RNA (1 µg) from A549 cells,
according to the manufacturer's instructions. The sequences of the
primers were as follows: for HIF-1α, 5′-CTCAAAGTCGGACAGCCTCA-3′
(sense) and 5′-AATGAGCCACCAGTGTCCAA-3′ (antisense); for VEGF,
5′-CTACCTCCACCATGCCAAGT- 3′ (sense) and 5′-TCTCTCCTATGTGCTGGCCT-3′
(antisense); for β-actin, 5′-GCCATCGTCACCAACTGGGAC-3′ (sense) and
5′-CGATTTCCCGCTCGGCCGTGG-3′ (antisense). PCR products were
visualized using 1% agarose gel electrophoresis with ethidium
bromide staining.
Enzyme-linked immunosorbent assay
(ELISA)
Cells were plated in 6-well culture plates at
2×105 cells/well in RPMI-1640 culture medium and treated
with various concentrations of delphinidin for 12 h in the presence
of EGF. The VEGF levels in the culture supernatant were determined
by ELISA using the VEGF ELISA development kit (R&D Systems,
Inc., Minneapolis, MN, USA) according to the manufacturer's
instructions.
Luciferase reporter gene assay
The ability of delphinidin to inhibit HIF-1α
transcription was determined by the reporter gene assay dependent
on the HRE. In brief, at 50–80% confluency, A549 cells were
co-transfected with pGL3-HRE-luciferase, which contained six copies
of HRE derived from the human VEGF gene, and pRL-CMV (Promega
Corp., Madison, WI, USA), which encoded Renilla luciferase
(Rluc) under the control of a constitutive promoter, using
Lipofectamine Plus reagent (Invitrogen) according to the
manufacturer's instructions.
Real-time polymerase chain reaction
(PCR)
Total RNA was extracted from the cells with TRIzol
reagent (Invitrogen), and reverse-transcriptase reactions were
performed with a commercial kit (SuperScript II RNase H-reverse
transcriptase; Invitrogen) using 50 µg/ml RNA. Quantitative PCRs
were conducted on a real-time PCR system (LightCycler; Roche
Diagnostics, Basel, Switzerland) with a commercial kit (SYBR-Green
with low ROX; Enzynomics, Daejeon, Korea) in a reaction mixture
containing diluted cDNA (1/5) as a template and the real-time PCR
primers. The sequences of the primers were as follows: for HIF-1α,
5′-CTCAAAGTCGGACAGCCTCA-3′ (sense) and 5′-AATGAGCCACCAGTGTCCAA-3′
(antisense); for VEGF, 5′-CTACCTCCACCATGCCAAGT-3′ (sense) and
5′-TCTCTCCTATGTGCTGGCCT-3′ (antisense); for β-actin,
5′-ACAGGAAGTCCCTTGCCATC-3′ (sense) and 5′-AGGGAGACCAAAAGCCTTCA-3′
(antisense). The relative mRNA expression levels were normalized to
the value of β-actin for each reaction.
Matrigel plug assay
C57BL/6N mice (male, 6 weeks) were purchased from
Samtako (Osan, Korea) and maintained in pathogen-free conditions.
A549 cells at subconfluence were harvested, washed with PBS, and
re-suspended in serum-free medium. Aliquots of cells
(3×106) were mixed with 0.5 ml of Matrigel in the
presence or absence of EGF (200 ng/ml) and delphinidin.
Immediately, the mixture was subcutaneously injected into mice. The
mice were sacrificed when tumors were visible, and the Matrigel
plugs were carefully separated from adjacent tissue and
photographed.
Hemoglobin concentration
measurement
The separated Matrigel plugs were excised, and then
were placed in cold PBS at 4°C overnight to liquefy the Matrigel.
Specimens were subjected to centrifugation at 14,000 rpm and the
supernatants were collected. The hemoglobin content was determined
using Drabkin's reagent kit (Sigma Chemical Co., St. Louis, MO,
USA), as previously described (21). All surgical and experimental
procedures used in this study were approved by the Institutional
Review Board Committee at Daegu Catholic University Medical Center
which conforms to the US National Institutes of Health Guidelines
for the Care and Use of Laboratory Animals.
Statistical analysis
All results are representative of at least three
independent experiments performed in triplicate. The statistical
significance between experimental and control values was calculated
using a one-way ANOVA test.
Results
Delphinidin inhibits CoCl2-
and EGF-induced HIF-1 expression in various cancer cells
HIF-1 is a master regulator of numerous genes
involved in cell survival, adaptation to hypoxia, metabolism, and
angiogenesis (22). Before
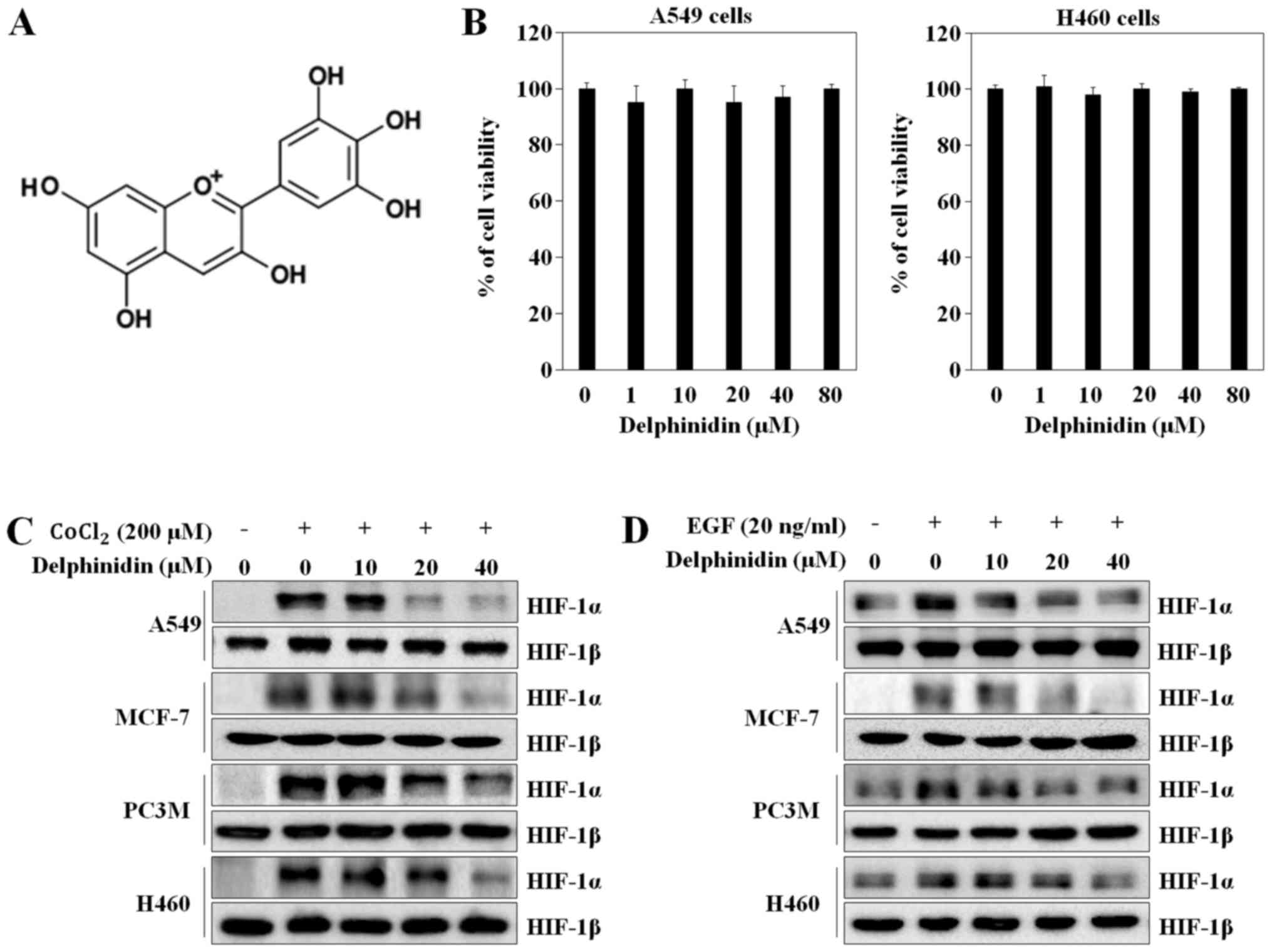
investigating the inhibitory potential of delphinidin (Fig. 1A), the cytotoxic effects of
delphinidin were examined by the WST-1 assay. Delphinidin did not
significantly affect the viability of A549 or NCI-H460 lung cancer
cells at the indicated concentrations (Fig. 1B). Delphinidin did not show any
cytotoxic effects at concentrations of up to 40 µM; therefore, 10,
20 and 40 µM delphinidin was used in the subsequent experiments. To
determine if delphinidin inhibits HIF-1α expression, we
investigated the HIF-1α and HIF-1β protein levels under various
conditions using western blot analysis. CoCl2 (200 µM)
and EGF (20 ng/ml) treatment greatly induced HIF-1α protein
expression levels in the various cancer cells. Delphinidin
dose-dependently decreased CoCl2-stimulated HIF-1α
protein expression in the A549 (lung carcinoma), NCI-H460 (lung
carcinoma), MCF-7 (breast carcinoma), and PC3M (prostate cancer)
cells. In particular, at a concentration of 40 µM, delphinidin
completely abrogated HIF-1α protein expression. However,
CoCl2 and delphinidin did not affect HIF-1β protein
expression (Fig. 1C). Under the
EGF-induced conditions, delphinidin decreased HIF-1α protein
expression without affecting HIF-1β protein expression (Fig. 1D). As the inhibitory effects of
delphinidin on CoCl2- and EGF-stimulated HIF-1α protein
expression in A549 cells was higher than that in the other cell
lines, A549 cells were used in the subsequent experiments.
Delphinidin inhibits CoCl2-
and EGF-induced VEGF transcription levels and HRE promoter
activity
VEGF, the main target gene of HIF-1α, directly
participates in angiogenesis (23).
To determine if the decrease in HIF-1α expression by delphinidin
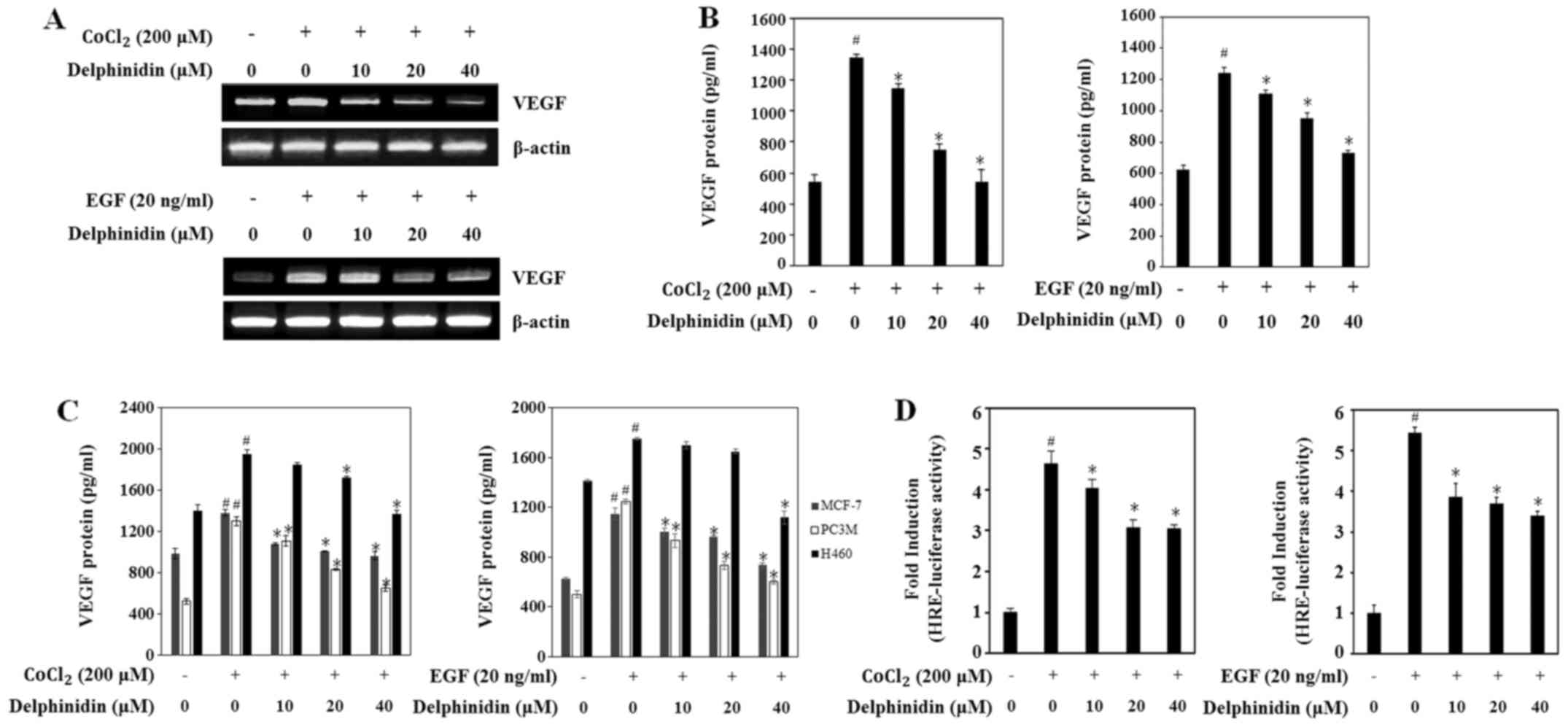
affects VEGF expression, the VEGF mRNA levels were evaluated using
RT-PCR. Delphinidin dose-dependently decreased the VEGF mRNA levels
under the CoCl2- and EGF-stimulated conditions (Fig. 2A). Moreover, treatment with 40 µM
delphinidin dramatically reduced the VEGF mRNA levels in both
conditions.
Next, we examined the effects of delphinidin on the
secretion of the VEGF protein in CoCl2- and
EGF-stimulated conditions via ELISA assay. Secretion of VEGF
protein was significantly increased by up to 2-fold in cells
treated with CoCl2 or EGF compared with untreated cells
(Fig. 2B). Delphinidin treatment
dose-dependently decreased the secretion of VEGF protein in the
CoCl2- and EGF-stimulated A549 cells. Because
delphinidin inhibited HIF-1α protein expression in various cancer
cells, we sought to confirm that delphinidin regulated VEGF
secretion in the other cancer cell lines. In MCF-7 and PC3M cells,
CoCl2- and EGF-stimulated VEGF protein secretion was
also decreased by delphinidin in a dose-dependent manner (Fig. 2C). Although non-treated H460 cells
secreted high levels of VEGF protein, treatment with 40 µM
delphinidin decreased CoCl2- and EGF-induced VEGF
protein secretion levels when compared with the non-treated H460
cells. These results suggest that the inhibitory effect of
delphinidin on VEGF protein production is regulated by decreasing
the VEGF mRNA level in CoCl2- and EGF-stimulated
conditions in various cell lines.
The binding of HIF-1 to HRE in the VEGF promoter is
a predominant enhancer of VEGF production (13). Thus, to investigate if delphinidin
suppresses HRE promoter activity by decreasing HIF-1α protein
expression, we performed an HRE promoter reporter gene assay. A549
cells were co-transfected with the pGL3-HRE-luciferase and
β-galactosidase-luciferase plasmids for 24 h and then treated with
the indicated concentration of delphinidin for 12 h. HRE promoter
activity was increased up to 4.5-fold in the
CoCl2-stimulated cells compared with the untreated cells
(Fig. 2D). Delphinidin
dose-dependently decreased CoCl2-stimulated HRE promoter
activity. In addition, EGF-induced HRE promoter activity was
increased up to ~5-fold compared with the untreated cells and was
rapidly decreased after treatment with 10 µM delphinidin. These
results showed that the inhibitory effects of delphinidin on VEGF
transcriptional activity are related to the regulation of HRE
promoter activity by inhibiting HIF-1α expression.
The inhibitory effects of delphinidin
on CoCl2- and EGF-induced HIF-1α expression are not
related to transcriptional levels
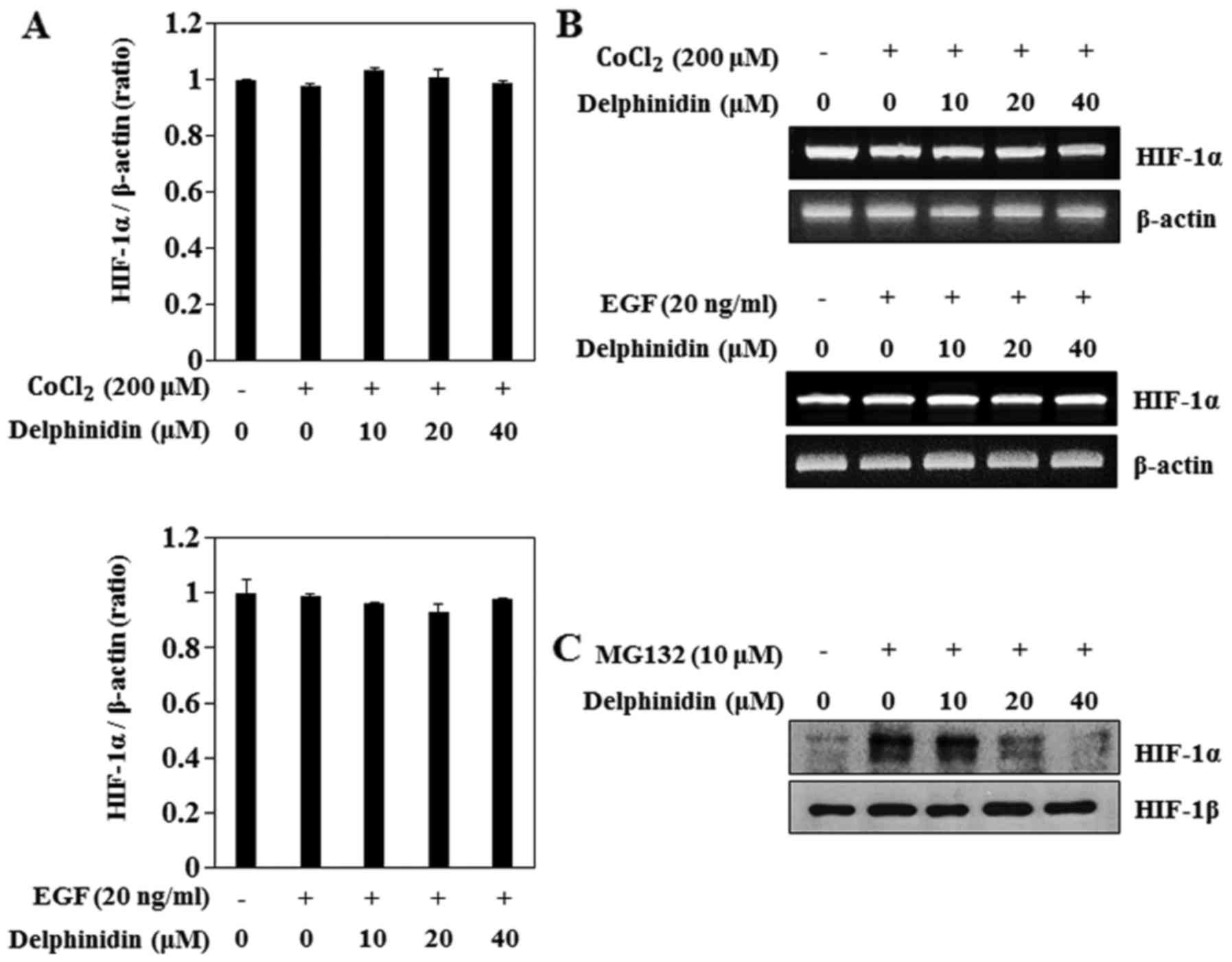
To determine if delphinidin decreased HIF-1α protein
expression by regulating the HIF-1α mRNA levels, we examined the
effects of delphinidin on CoCl2- and EGF-induced HIF-1α
transcriptional activity using real-time PCR. CoCl2 and
EGF treatment did not induce HIF-1α mRNA levels (Fig. 3A). Furthermore, delphinidin
treatment did not change the HIF-1α mRNA level in CoCl2-
or EGF-stimulated A549 cells. In addition, the results of the
RT-PCR assay showed that delphinidin did not affect the HIF-1α mRNA
levels (Fig. 3B). These results
showed that HIF-1α mRNA levels were not responsible for the
inhibition of HIF-1α protein expression by delphinidin in the
CoCl2- and EGF-induced conditions. Next, to determine
the effect of delphinidin on HIF-1α protein proteasomal
degradation, proteasome inhibitor MG132 was used. MG132
significantly increased HIF-1α protein accumulation (Fig. 3C). Delphinidin also inhibited
MG132-induced HIF-1α, which suggests that delphinidin did not
appear to affect the proteasomal degradation of the HIF-1α protein.
Therefore, we anticipated that the inhibitory effects of
delphinidin on CoCl2- and EGF-induced HIF-1α protein
expression are regulated by a synthesis pathway.
Delphinidin inhibits CoCl2-
and EGF-induced HIF-1α expression by blocking the ERK and
PI3K/Akt/mTOR/p70S6K signaling pathways in A549 cells
The MAPK and Akt/mTOR pathways are associated with
the regulation of HIF-1α protein synthesis at the translational
levels (24,25). Thus, to confirm that MAPK, Akt, and
mTOR pathway activity is related to CoCl2- and
EGF-induced HIF-1α protein expression, A549 cells were exposed to
various kinase inhibitors. PD98059 (a MEK inhibitor), wortmannin (a
PI3K inhibitor), and rapamycin (an mTOR inhibitor) blocked
CoCl2-induced HIF-1α expression, similarly to
delphinidin (Fig. 4A). By contrast,
SB203589 (a p38 inhibitor) did not affect HIF-1α protein expression
in A549 cells. In EGF-stimulated cells PD98059, wortmannin, and
rapamycin also blocked HIF-1α expression (Fig. 4B). These results indicate that
CoCl2- and EGF-induced HIF-1α protein expression is
regulated by the ERK, PI3K and mTOR pathways, whereas the
p38-mediated pathways are not involved.
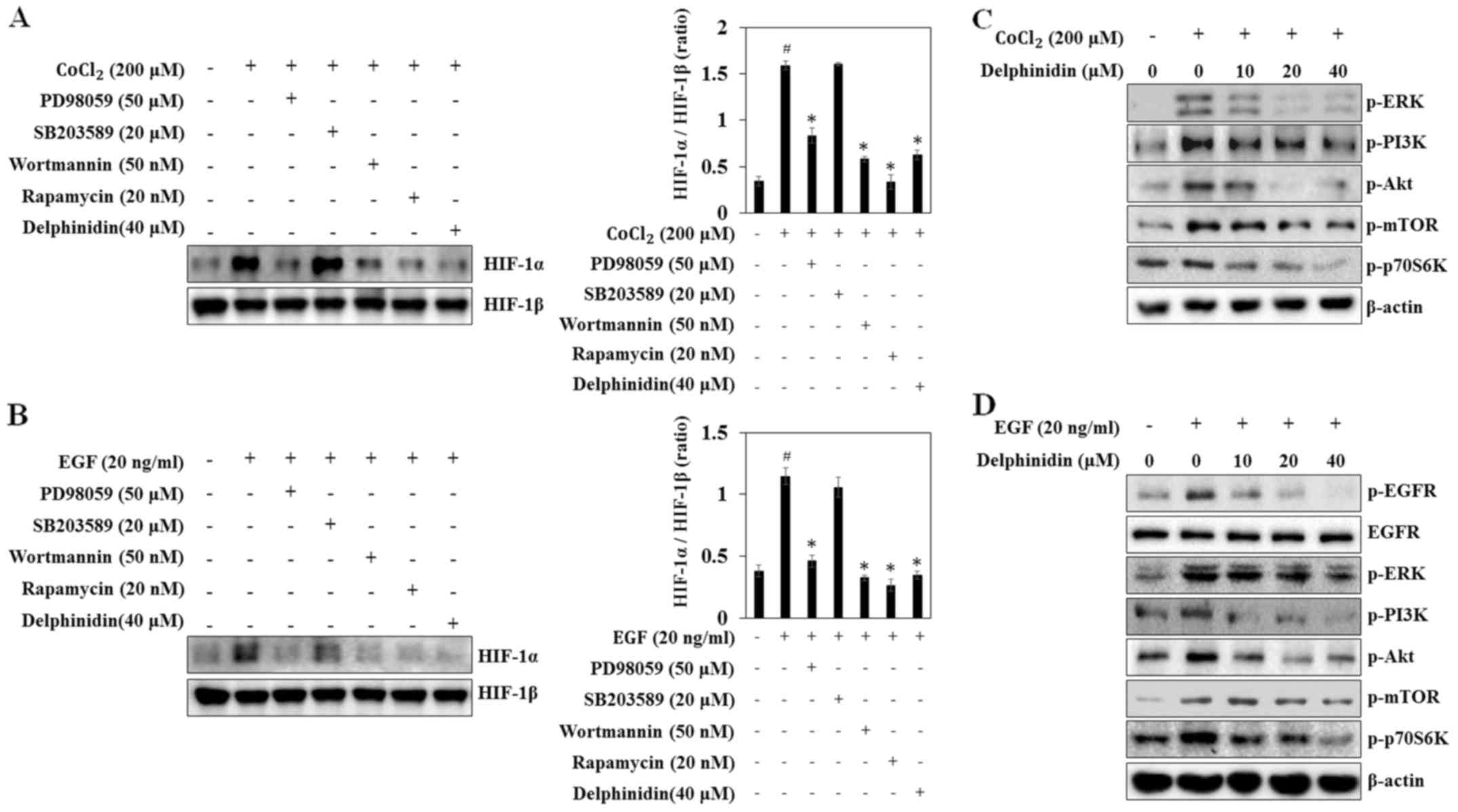 | Figure 4.Inhibitory effects of delphinidin on
CoCl2- or epidermal growth factor (EGF)-induced
phosphorylation of EGFR, extracellular signal-regulated protein
kinase (ERK), phosphatidylinositol-3-kinase (PI3K), Akt, mammalian
target of rapamycin (mTOR), and p70S6 kinase (p70S6K) in A549
cells. (A and B) A549 cells were pretreated with delphinidin,
PD98059, SB203589, wortmannin, or rapamycin for 30 min, and then
induced by CoCl2 or EGF treatment for 6 h. The nuclear
extracts were subjected to western blot analysis using antibodies
against HIF-1α or HIF-1β. (C and D) A549 cells were pretreated with
the indicated concentrations of delphinidin for 1 h, followed by
incubation with CoCl2 or EGF for 10 min. The
phosphorylated levels of EGFR, ERK, PI3K, Akt, mTOR, and p70S6K
were determined by western blot analysis. |
To determine the mechanisms underlying HIF-1α
inhibition by delphinidin, the phosphorylated forms of ERK, PI3K,
Akt and mTOR were detected via western blot analysis. The
phosphorylation of ERK, PI3K, Akt, mTOR and p70S6K were increased
at 10 min after CoCl2- and EGF-treatment, while
delphinidin reduced phosphorylation of ERK, PI3K, Akt, mTOR, and
p70S6K in CoCl2-stimulated A549 cells (Fig. 4C and D). Similarly, delphinidin
dose-dependently inhibited EGF-induced phosphorylation of ERK,
PI3K, Akt, mTOR, and p70S6K. Phosphorylation of tyrosine residue
1068 (Tyr1068) of EGFR is part of the initial activation
process and these phosphotyrosine residues subsequently serve as
docking sites for intracellular signaling molecules (26). Thus, to determine if the inhibition
of HIF-1α protein synthesis was mediated by the downregulation of
the EGFR tyrosine residue, the effect of delphinidin on the
phosphorylation of EGFR (Tyr1068) was determined. The
results showed that delphinidin inhibited EGF-induced EGFR
phosphorylation. These results suggest that delphinidin suppresses
HIF-1α protein synthesis by the inhibiting EGFR-dependent and
-independent activation of the ERK and PI3K/Akt/mTOR/p70S6K
signaling pathways.
Delphinidin inhibits tumor
angiogenesis in vivo
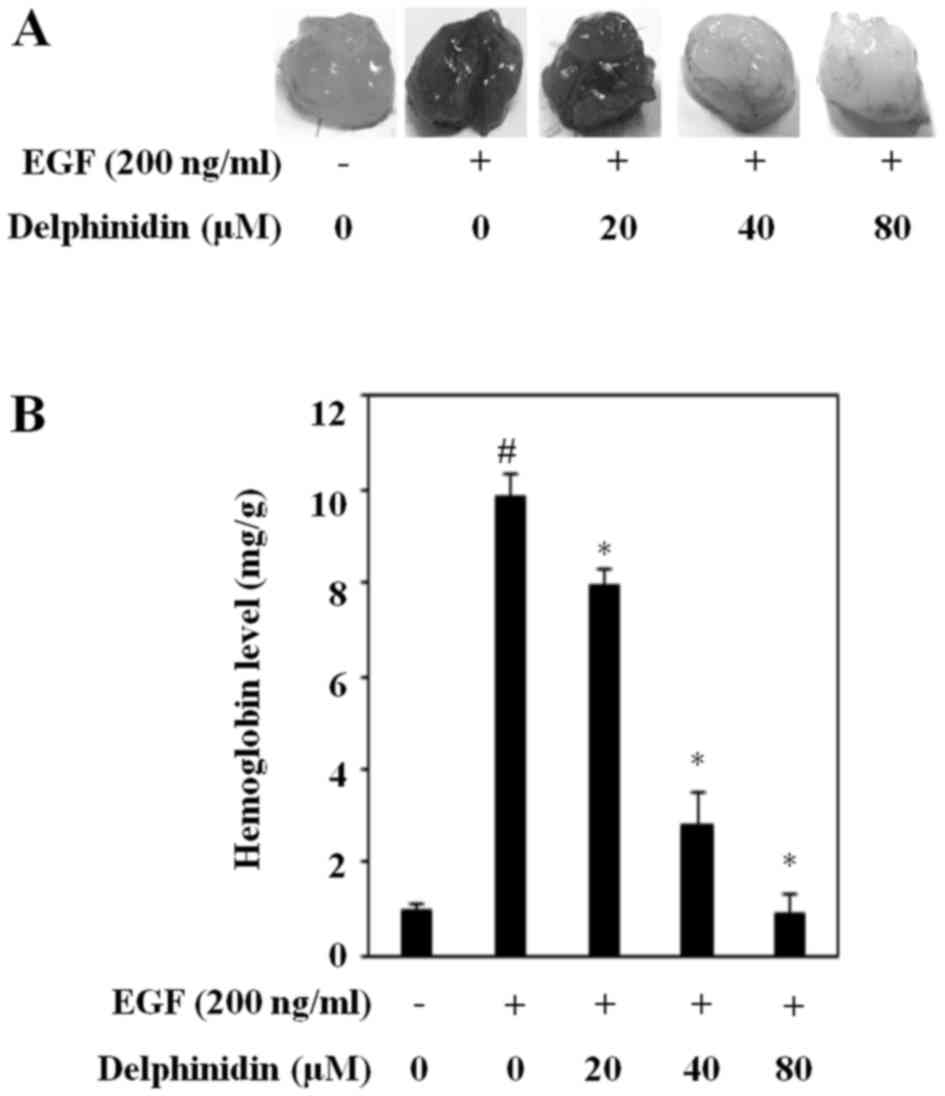
To further determine if delphinidin inhibits
angiogenesis by blocking the expression of HIF-1α and VEGF, we
performed a Matrigel plug assay in vivo. Matrigel plugs
mixed with only cancer cells did not induce blood vessel formation.
New blood vessel formation was induced by EGF-treated cancer cells
(Fig. 5A). Delphinidin considerably
suppressed EGF-induced angiogenesis, and it was almost completely
abrogated upon treatment with 80 µM delphinidin. Moreover,
delphinidin dose-dependently reduced the EGF-increased hemoglobin
content (Fig. 5B). These results
indicate that delphinidin is an angiogenesis inhibitor and an
antitumor therapeutic agent in human lung adenocarcinoma A549
cells.
Discussion
Natural dietary polyphenolic compounds prevent
cancer due to their multitude of biological activities (27). Delphinidin, a polyphenol that
belongs to the group of anthocyanidins, has potent antioxidant,
anti-proliferative, and anti-angiogenic properties in various cells
(28). It was reported that
delphinidin exerts an inhibitory effect on angiogenesis (18). It also inhibits endothelial cell
proliferation and cell cycle progression through transient
activation of ERK1/2 (16).
However, there is no information about its ability to regulate
angiogenesis via HIF-1α-dependent signaling pathways.
Tumor cells promote angiogenesis through an
oxygen-sensing mechanism by regulating angiogenic and
anti-angiogenic factors under hypoxic conditions (29). Cellular adaptation to hypoxia is
associated with several transcriptional factors, such as activator
protein-1, NF-κB, and HIF-1 (3).
Among the transcriptional factors, activation of HIF-1, which is
composed of the HIF-1α and HIF-1β subunits, increases cell
proliferation and invasion in cancers, such as lung and prostate
cancer (30,31). HIF-1α expression is regulated not
only by hypoxia but also by ions. Among ions, CoCl2 has
been used both in vivo (32)
and in vitro (33) to mimic
hypoxic conditions (33). It
stabilizes HIF-1α by inhibiting HIF-1α-specific prolyl hydroxylase,
leading to the impaired binding of the VHL protein to HIF-1α and
prevention of its proteasomal degradation (34,35).
In addition, HIF-1α expression is regulated by EGF, which increases
HIF-1α translation by stimulating EGFR in normoxia. Therefore, we
investigated the inhibitory effects of delphinidin on HIF-1α and
VEGF expression in EGF- and CoCl2-stimulated lung cancer
cells.
HIF-1α protein expression was effectively increased
in the CoCl2- and EGF-induced conditions. Delphinidin
markedly decreased the stimulated HIF-1α protein expression without
having toxic effects on lung cancer cells (Fig. 1). Previous studies reported that
treatment with delphinidin for 24 h does not have cytotoxic effects
on lung cancer cells or normal cells (17,36),
suggesting that the inhibitory effects of delphinidin on HIF-1α
protein expression are not related to cell cytotoxicity. To
investigate the possible pathway by which delphinidin inhibits
HIF-1α expression, we evaluated the effect of delphinidin on the
phosphorylation of various kinases. HIF-1α protein synthesis is
induced by EGF-induced PI3K/Akt and mTOR signaling pathways
(15). mTOR controls the
translation of various genes, by regulating two important
downstream substrates, p70S6K and 4E-BP1 (37,38).
Phosphorylation of p70S6K induces HIF-1α protein synthesis by
controlling HIF-1α mRNA translation within the 5′ untranslated
region (39). In addition, ERK1
directly phosphorylates the carboxy-terminal domain of HIF-1α in
hypoxia conditions (40).
Therefore, to confirm the mechanism involved in the HIF-1α
regulation by delphinidin, we used the inhibitors of MAPK, PI3K and
mTOR. Similar to the effect of delphinidin, PD98059, wortmannin,
and rapamycin decreased both the CoCl2- and EGF-induced
HIF-1α protein expressions. On the other hand, the treatment of the
cells with SB203589 did not change the HIF-1α protein expression in
the CoCl2-stimulated condition. In the EGF-stimulated
condition, only SB203589 did not influence HIF-1α protein
expression (Fig. 4). These findings
indicate that the CoCl2- and EGF-induced HIF-1α protein
expression levels were regulated through the ERK, Akt, and mTOR
signaling pathways. In addition, we found that delphinidin inhibits
the phosphorylation of ERK, PI3K, Akt, mTOR, and p70S6K. These
results corresponded with the previous findings that delphinidin
inhibits HER2 and ERK1/2 signaling with growth inhibition and
apoptosis in breast cancer cells (41) and that delphinidin inhibits
EGF-induced activation of PI3K and phosphorylation of Akt at
Ser473 (28). In
addition, delphinidin decreased EGF-induced EGFR phosphorylation at
Tyr1068. EGFR phosphorylation (Tyr1068)
generates a motif for Grb2/SH2 domain binding, which initiates ERK
activation (26), suggesting that
delphinidin suppresses EGF-induced ERK by inhibiting EGFR
phosphorylation. However, because delphinidin decreased
CoCl2- as well as EGF-induced HIF-1α protein expression,
regulation of the ERK, PI3K, Akt, mTOR, and p70S6K signaling
pathways plays a major role in the inhibitory effect of delphinidin
on HIF-1α protein synthesis.
VEGF is a major mediator of angiogenesis, which
induces tumor growth, metastasis, and invasion by delivering oxygen
and nutrients to cancer cells (42). As expected, delphinidin
significantly decreased both CoCl2- and EGF-induced VEGF
mRNA expression and VEGF secretion and dose-dependently decreased
CoCl2- and EGF-induced HRE promoter activity (Fig. 2). Additionally, delphinidin markedly
suppressed EGF-stimulated new blood vessel formation. These results
suggest that delphinidin can be clinically used as an angiogenesis
inhibitor in cancer therapy.
In conclusion, this study found for the first time
that delphinidin suppresses HIF-1α protein expression. The
inhibitory effects of delphinidin on VEGF transcriptional activity
and tumor angiogenesis may be related to HIF-1α protein synthesis
by suppression of the ERK, mTOR, and p70S6K pathways in both the
CoCl2- and EGF-induced conditions. Therefore, this study
demonstrated that delphinidin may be useful as a new
anti-angiogenic agent in lung cancer.
Acknowledgements
This study was supported by a grant of Xavier,
Catholic University of Daegu (2015).
Glossary
Abbreviations
Abbreviations:
|
VEGF
|
vascular endothelial growth factor
|
|
EGF
|
epidermal growth factor
|
|
EGFR
|
EGF receptor
|
|
HIF
|
hypoxia-inducible factor
|
|
VHL
|
von Hippel-Lindau
|
|
HREs
|
hypoxia-response elements
|
|
mTOR
|
mammalian target of rapamycin
|
|
4E-BP1
|
eukaryotic initiation factor
4E-binding protein 1
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
p70S6K
|
p70S6 kinase
|
References
|
1
|
Xu X, Mao W, Chen Q, Zhuang Q, Wang L, Dai
J, Wang H and Huang Z: Endostar, a modified recombinant human
endostatin, suppresses angiogenesis through inhibition of
Wnt/β-catenin signaling pathway. PLoS One. 9:e1074632014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin JM, Jeong YJ, Cho HJ, Park KK, Chung
IK, Lee IK, Kwak JY, Chang HW, Kim CH, Moon SK, et al: Melittin
suppresses HIF-1α/VEGF expression through inhibition of ERK and
mTOR/p70S6K pathway in human cervical carcinoma cells. PLoS One.
8:e693802013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jeong JH, Jeong YJ, Cho HJ, Shin JM, Kang
JH, Park KK, Park YY, Chung IK, Chang HW, Magae J, et al:
Ascochlorin inhibits growth factor-induced HIF-1α activation and
tumor-angiogenesis through the suppression of EGFR/ERK/p70S6K
signaling pathway in human cervical carcinoma cells. J Cell
Biochem. 113:1302–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De S, Chen J, Narizhneva NV, Heston W,
Brainard J, Sage EH and Byzova TV: Molecular pathway for cancer
metastasis to bone. J Biol Chem. 278:39044–39050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeong YJ, Cho HJ, Magae J, Lee IK, Park KG
and Chang YC: Ascofuranone suppresses EGF-induced HIF-1α protein
synthesis by inhibition of the Akt/mTOR/p70S6K pathway in
MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol.
273:542–550. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hosseini H, Rajabibazl M, Ebrahimizadeh W
and Dehbidi GR: Inhibiting angiogenesis with human single-chain
variable fragment antibody targeting VEGF. Microvasc Res. 97:13–18.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boddy JL, Fox SB, Han C, Campo L, Turley
H, Kanga S, Malone PR and Harris AL: The androgen receptor is
significantly associated with vascular endothelial growth factor
and hypoxia sensing via hypoxia-inducible factors HIF-1a, HIF-2a,
and the prolyl hydroxylases in human prostate cancer. Clin Cancer
Res. 11:7658–7663. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren W, Mi D, Yang K, Cao N, Tian J, Li Z
and Ma B: The expression of hypoxia-inducible factor-1α and its
clinical significance in lung cancer: A systematic review and
meta-analysis. Swiss Med Wkly. 143:w138552013.PubMed/NCBI
|
|
12
|
Soggia A, Ramond C, Akiyama H, Scharfmann
R and Duvillie B: von Hippel-Lindau gene disruption in mouse
pancreatic progenitors and its consequences on endocrine
differentiation in vivo: Importance of HIF1-α and VEGF-A
upregulation. Diabetologia. 57:2348–2356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu Y, Lucia K, Lange M, Kuhlen D, Stalla
GK and Renner U: Hypoxia inducible factor-1 is involved in growth
factor, glucocorticoid and hypoxia mediated regulation of vascular
endothelial growth factor-A in human meningiomas. J Neurooncol.
119:263–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan B, Wang YX, Yao T and Zhu YC: p38
Mitogen-activated protein kinase mediates hypoxia-induced vascular
endothelial growth factor release in human endothelial cells. Sheng
Li Xue Bao. 57:13–20. 2005.PubMed/NCBI
|
|
15
|
Déry MA, Michaud MD and Richard DE:
Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic
activators. Int J Biochem Cell Biol. 37:535–540. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martin S, Favot L, Matz R, Lugnier C and
Andriantsitohaina R: Delphinidin inhibits endothelial cell
proliferation and cell cycle progression through a transient
activation of ERK-1/−2. Biochem Pharmacol. 65:669–675. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pal HC, Sharma S, Strickland LR, Agarwal
J, Athar M, Elmets CA and Afaq F: Delphinidin reduces cell
proliferation and induces apoptosis of non-small-cell lung cancer
cells by targeting EGFR/VEGFR2 signaling pathways. PLoS One.
8:e772702013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Favot L, Martin S, Keravis T,
Andriantsitohaina R and Lugnier C: Involvement of cyclin-dependent
pathway in the inhibitory effect of delphinidin on angiogenesis.
Cardiovasc Res. 59:479–487. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seo BN, Ryu JM, Yun SP, Jeon JH, Park SS,
Oh KB, Park JK and Han HJ: Delphinidin prevents hypoxia-induced
mouse embryonic stem cell apoptosis through reduction of
intracellular reactive oxygen species-mediated activation of JNK
and NF-κB, and Akt inhibition. Apoptosis. 18:811–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lamy S, Blanchette M, Michaud-Levesque J,
Lafleur R, Durocher Y, Moghrabi A, Barrette S, Gingras D and
Béliveau R: Delphinidin, a dietary anthocyanidin, inhibits vascular
endothelial growth factor receptor-2 phosphorylation.
Carcinogenesis. 27:989–996. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Passaniti A, Taylor RM, Pili R, Guo Y,
Long PV, Haney JA, Pauly RR, Grant DS and Martin GR: A simple,
quantitative method for assessing angiogenesis and antiangiogenic
agents using reconstituted basement membrane, heparin, and
fibroblast growth factor. Lab Invest. 67:519–528. 1992.PubMed/NCBI
|
|
22
|
Ahn GO, Seita J, Hong BJ, Kim YE, Bok S,
Lee CJ, Kim KS, Lee JC, Leeper NJ, Cooke JP, et al: Transcriptional
activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells
promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci
USA. 111:2698–2703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Shi Y, Du Y, Ning X, Liu N, Huang
D, Liang J, Xue Y and Fan D: Dual-specificity phosphatase DUSP1
protects overactivation of hypoxia-inducible factor 1 through
inactivating ERK MAPK. Exp Cell Res. 309:410–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Semenza G: Signal transduction to
hypoxia-inducible factor 1. Biochem Pharmacol. 64:993–998. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rojas M, Yao S and Lin YZ: Controlling
epidermal growth factor (EGF)-stimulated Ras activation in intact
cells by a cell-permeable peptide mimicking phosphorylated EGF
receptor. J Biol Chem. 271:27456–27461. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin S, Giannone G, Andriantsitohaina R
and Martinez MC: Delphinidin, an active compound of red wine,
inhibits endothelial cell apoptosis via nitric oxide pathway and
regulation of calcium homeostasis. Br J Pharmacol. 139:1095–1102.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Afaq F, Zaman N, Khan N, Syed DN, Sarfaraz
S, Zaid MA and Mukhtar H: Inhibition of epidermal growth factor
receptor signaling pathway by delphinidin, an anthocyanidin in
pigmented fruits and vegetables. Int J Cancer. 123:1508–1515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Semenza GL: HIF-1: mediator of
physiological and pathophysiological responses to hypoxia. J Appl
Physiol (1985). 88:1474–1480. 2000.PubMed/NCBI
|
|
30
|
Tong E, Xu Y, Li G, Zou K and Zou L: The
effects of β-elemene on the expression of mTOR, HIF-1A, survivin in
lung adenocarcinoma A549 cell. Afr J Tradit Complement Altern Med.
10:18–23. 2013.PubMed/NCBI
|
|
31
|
Lv L, Yuan J, Huang T, Zhang C, Zhu Z,
Wang L, Jiang G and Zeng F: Stabilization of Snail by HIF-1α and
TNF-α is required for hypoxia-induced invasion in prostate cancer
PC3 cells. Mol Biol Rep. 41:4573–4582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Badr GA, Zhang JZ, Tang J, Kern TS and
Ismail-Beigi F: Glut1 and glut3 expression, but not capillary
density, is increased by cobalt chloride in rat cerebrum and
retina. Brain Res Mol Brain Res. 64:24–33. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang GL and Semenza GL: Characterization
of hypoxia-inducible factor 1 and regulation of DNA binding
activity by hypoxia. J Biol Chem. 268:21513–21518. 1993.PubMed/NCBI
|
|
34
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, et al: C. elegans EGL-9 and mammalian homologs define a
family of dioxygenases that regulate HIF by prolyl hydroxylation.
Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Afaq F, Zaman N, Khan N, Syed DN, Sarfaraz
S, Zaid MA and Mukhtar H: Inhibition of epidermal growth factor
receptor signaling pathway by delphinidin, an anthocyanidin in
pigmented fruits and vegetables. Int J Cancer. 123:1508–1515. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marhold M, Tomasich E, El-Gazzar A, Heller
G, Spittler A, Horvat R, Krainer M and Horak P: HIF1α regulates
mTOR signaling and viability of prostate cancer stem cells. Mol
Cancer Res. 13:556–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Activation of the mTOR signaling pathway in breast cancer MCF-7
cells by a peptide derived from Porphyra yezoensis. Oncol Rep.
33:19–24. 2014.PubMed/NCBI
|
|
39
|
Jefferies HB, Fumagalli S, Dennis PB,
Reinhard C, Pearson RB and Thomas G: Rapamycin suppresses 5′TOP
mRNA translation through inhibition of p70s6k. EMBO J.
16:3693–3704. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Minet E, Arnould T, Michel G, Roland I,
Mottet D, Raes M, Remacle J and Michiels C: ERK activation upon
hypoxia: Involvement in HIF-1 activation. FEBS Lett. 468:53–58.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ozbay T and Nahta R: Delphinidin inhibits
HER2 and Erk1/2 signaling and suppresses growth of
HER2-overexpressing and triple negative breast cancer cell lines.
Breast Cancer (Auckl). 5:143–154. 2011.PubMed/NCBI
|
|
42
|
Kondo Y, Arii S, Mori A, Furutani M, Chiba
T and Imamura M: Enhancement of angiogenesis, tumor growth, and
metastasis by transfection of vascular endothelial growth factor
into LoVo human colon cancer cell line. Clin Cancer Res. 6:622–630.
2000.PubMed/NCBI
|