Introduction
Oral squamous cell carcinoma (OSCC) constitutes more
than 90% of all malignant neoplasms in the oral cavity, with an
increased number of new OSCC cases worldwide and varied
geographical distribution (1,2). OSCC
is prevalent in Southern Asia and Latin America, and is the most
common cancer among the male population (3,4). In
the high-risk areas, betel quid (BQ) chewing, tobacco and alcohol
consumption have been well documented as etiologic factors for OSCC
(4,5). A causal relationship between BQ
chewing and the high incidence of oral cancer has been established,
particularly its correlation with early oral premalignancies such
as oral submucous fibrosis (OSF) (6,7).
Despite the recent rapid improvement in multimodality treatment,
the overall 5-year survival rate of OSCC remains poor. Thus,
elucidation of molecular events during OSCC development and
progression is needed for the early detection and therapy of
OSCC.
Overactivation of Wnt/β-catenin signaling has been
identified in the tumorigenesis of multiple cancers including OSCC,
and plays critical roles in cell proliferation, differentiation,
adhesion and stemness (7,8). Wnt ligands, antagonists and mutations
of APC and β-catenin have been well-defined for its activation. The
dickkopf (DKK) family as Wnt antagonists, contains five members,
DKK1, DKK2, DKK3, DKK4 and DKKL1, with different impacts on
Wnt/β-catenin signaling (9,10). However, the underlying mechanisms
remain elusive. Notably, the secreted Wnt antagonist
dickkopf-related protein 3 (DKK3) is emerging as a crucial
regulator of human cancers (9).
DKK3 is frequently reduced by promoter methylation in multiple
solid and hematological cancers, acting as a functional tumor
suppressor by affecting apoptosis and proliferation.
Re-introduction of DKK3 expression in cancer cells may ba a
potential biomarker and effective therapeutic approach.
There are several contradictory studies of DKK3 in
head and neck squamous cell carcinomas (HNSCC) and OSCC. Its tumor
suppressive and oncogenic functions have been reported in OSCC
(11–15). However, the expression pattern of
DKK3 in the malignancy progression of OSF has not been addressed.
In the present study, we examined DKK3 expression at the protein
and mRNA levels in normal oral mucosa, OSF and OSCC tissues, as
well as the correlation with clinicopathological features. We also
analyzed its potential function in the pathogenesis of OSCC.
Materials and methods
Tissue specimens
OSCC (n=55), OSF (n=45), and normal oral mucosa
(n=15) tissue specimens were obtained at the time of surgical
resection at Xiangya Second Hospital and Xiangya Hospital, Central
South University (Changsha, China) and Shanghai Ninth People's
Hospital, Shanghai Jiaotong University School of Medicine
(Shanghai, China) from January 2013 to June 2014. The patient
informed consents were obtained under a protocol reviewed and
approved by the Institutional Review Boards of the Xiangya School
of Medicine or Shanghai Jiaotong University School of Medicine. The
clinical diagnosis and pathologic stage of the OSF were determined
in terms of the Pingborg criteria (16). OSCC was diagnosed according to the
World Health Organization criteria of 2005. Fifteen normal
specimens were obtained from healthy oral mucosa. Forty-five cases
of OSF were incident, newly diagnosed without OSCC or neoplastic
disease. OSF was classified into 3 grades: early stage (E, n=15),
moderately advanced stage (M, n=15) and advanced stage (A, n=15).
All collected tissues were divided into two parts, one of which was
frozen immediately at −80°C after careful removal of the tumor
mass, OSF tissue in the epithelium layer and grossly normal-looking
epithelium, and the other part was fixed in 4% buffered formalin
solution for pathologic diagnosis and immunohistochemical staining.
Clinicopathologic staging of OSCC was determined by the TNM
classification of the International Union Against Cancer in 2009.
The results of the immunostained formalin-fixed, paraffin-embedded
(FFPE) sections were evaluated separately by two pathologists (C.Z.
and Z.Y. at the Department of Oral Pathology of the respective
affiliate hospital).
Immunohistochemistry
Immunohistochemical staining was performed on 4-µm
serial sections from FFPE specimens. After deparaffinization and
hydration, the slides were treated with endogeneous peroxidase in
3% H2O2 for 20 min. The sections were then
blocked for 30 min at 37°C with 1.5% blocking serum in
phosphate-buffered saline (PBS) before reacting with DKK3 (1:100
dilution; #10365-1-AP; ProteinΤech) at 4°C in a moist chamber
overnight. Negative control slides were duplicate sections in the
absence of primary antibodies. For evaluating DKK3 expression, a
scoring method was used. A mean percentage of positive cells was
determined by the examination of 500 cells in at least 5 areas at a
magnification of ×400. Cells were assigned to 1 of the 5 following
categories according to the percentage of positive cells (PP): 0,
<5%; 1, 5–24%; 2, 25–49%; 3, 50–75%; and 4, >75%. The
intensity of the DKK3 staining (SI) was then scored as
follows: 0, negative (−); 1, weak (+); 2, moderate (++); and 3,
intense (+++). The final immunoreactive score (IRS = SI + PP) was
as follows: -, 0 or 1; +, 2 or 3; ++, 4 or 5; and +++, 6 or 7. The
stained tissues were scored blindly regarding clinical patient
data. Statistical analyses were performed with SPSS 19.0 software.
Statistical significance was evaluated by the Chi-square test
(χ2). The significance level was set at 0.05.
Reverse-transcription polymerase chain
reaction (RT-PCR)
TRIzol reagent (Invitrogen Life Technologies,
Karlsruhe, Germany) was used to extract total RNAs. Reverse
transcription polymerase chain reaction (RT-PCR) was performed
using a kit from Promega (Madison, WI, USA). Real-time PCR was
performed to detect DKK3 expression, according to the
manufacturer's protocol (HT7500 system; Applied Biosystems).
Primers for amplifying DKK3 mRNA sequences were synthesized
as previously described. The 161-bp mRNA of DKK3 was
amplified by PCR with forward primer, 5′-CACCCTCAATGAGATGTTCC and
reverse primer, 5′-TGGTCTCATTGTGATAGCTG. GAPDH as a control
for all the samples was shown in our previous studies (17,18).
PCR amplification was performed with denaturation at 94°C for 30
sec, annealing at 55°C for 30 sec, and extension at 72°C for 30 sec
in 32 cycles. The PCR products were visualized on 2% agarose gels
under ultraviolet transillumination.
Results
Increased expression levels of DKK3 in
OSCC and HNSCC
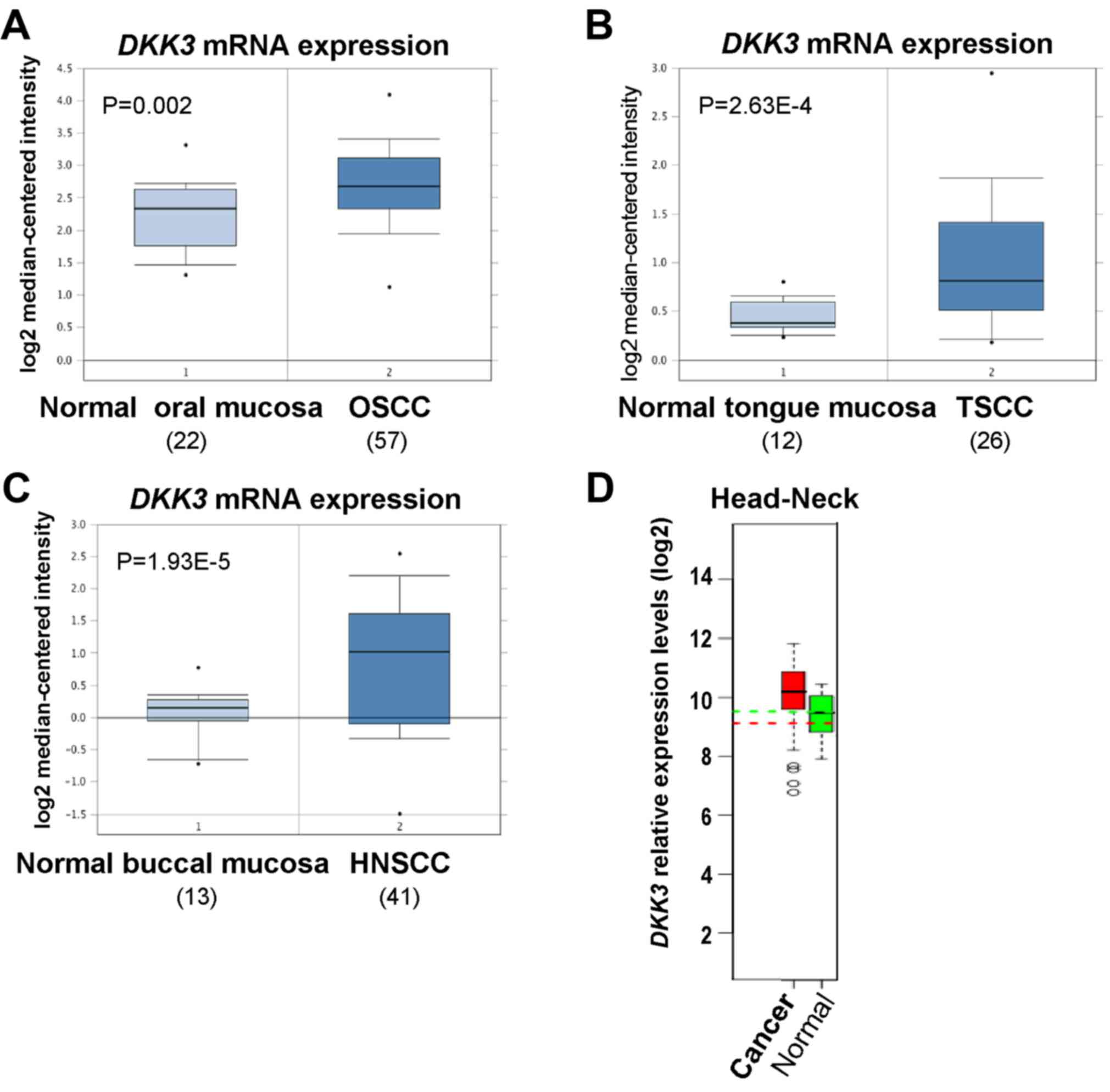
We firstly investigated DKK3 expression in
OSCC and HNSCC using the open access cancer microarray database
Oncomine (https://www.oncomine.org/resource/login.html). Through
analysis of studies by Peng et al, Ye et al and Ginos
et al, the results revealed that DKK3 expression at
the mRNA level was 1.377-fold higher in OSCC than that in normal
oral mucosa tissues (P=0.002) (19), 1.433-fold higher in tongue squamous
cell carcinoma (TSCC) tissues than that in normal tongue mucosa
tissues (P=2.63E-4) (20),
1.831-fold higher in HNSCC tissues than that in normal buccal
mucosa (P=1.93E-5) (21) (Fig. 1A-C). By analyzing microarray data
from the GENT dataset (http://medical-genome.kribb.re.kr/GENT/search/search.php),
DKK3 was found to be overexpressed in HNSCC tissues, compared with
the level in the corresponding normal oral mucosa tissues (Fig. 1D). These results suggest that DKK3
upregulation may play a crucial role in OSCC development.
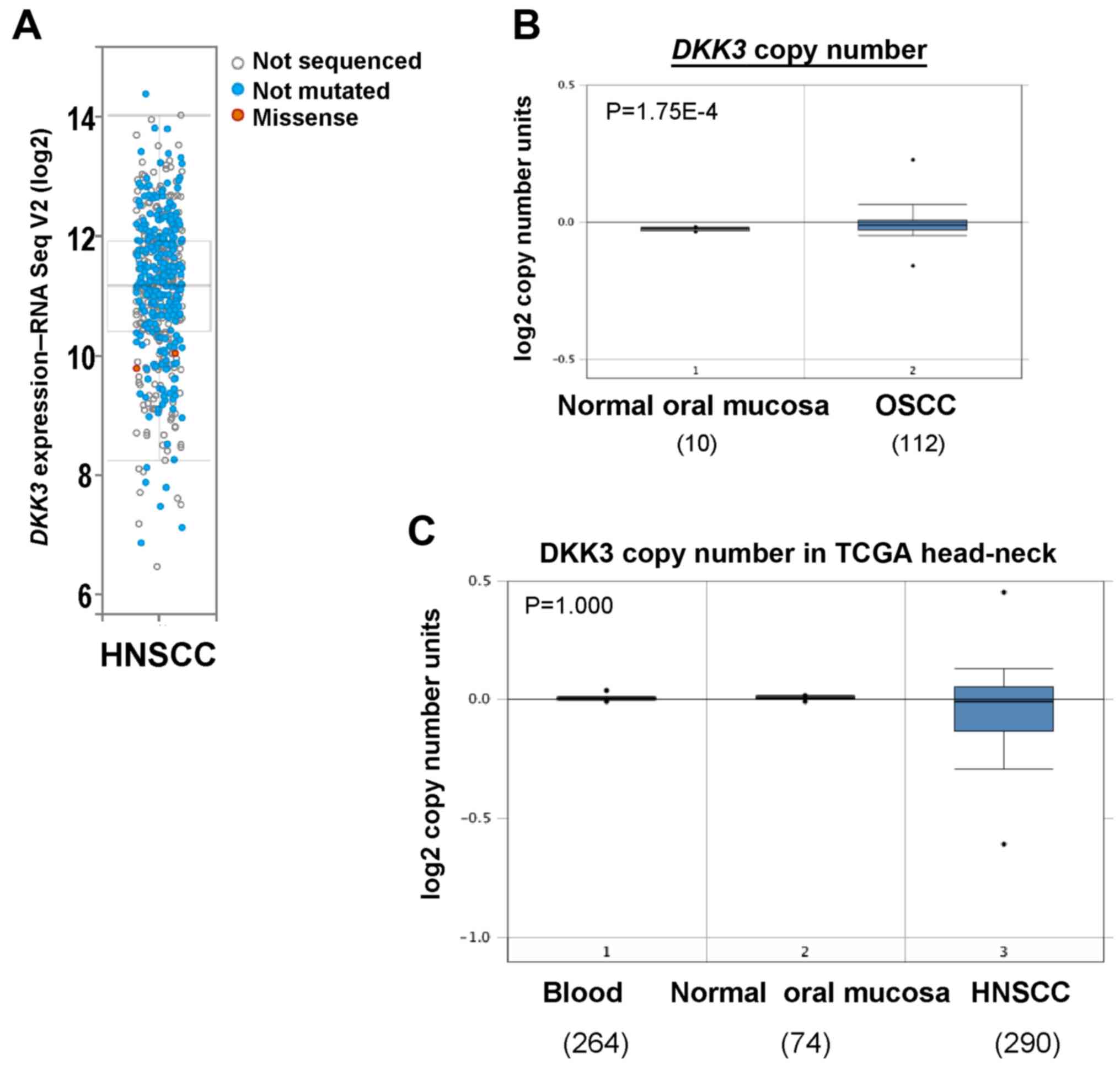
Genetic alteration of DKK3 in
OSCC
We next assessed genetic alterations of DKK3 in
HNSCC including OSCC using The Cancer Genome Atlas (TCGA) database.
DKK3 was overexpressed in two TCGA HNSCC cohorts, with a rare
mutation (Fig. 2A). Copy number
analysis using Oncomine database showed an increased DKK3
gene copy number in OSCC tissues (P=1.75E-4), compared with that
noted in normal oral mucosa tissues (Fig. 2B). Although deletion of DKK3
was observed in the HNSCC TCGA cohort, there was no statistically
significant difference (Fig. 2C).
These results suggest that gain of DKK3 gene copy number may
lead to increased DKK3 expression level in OSCC.
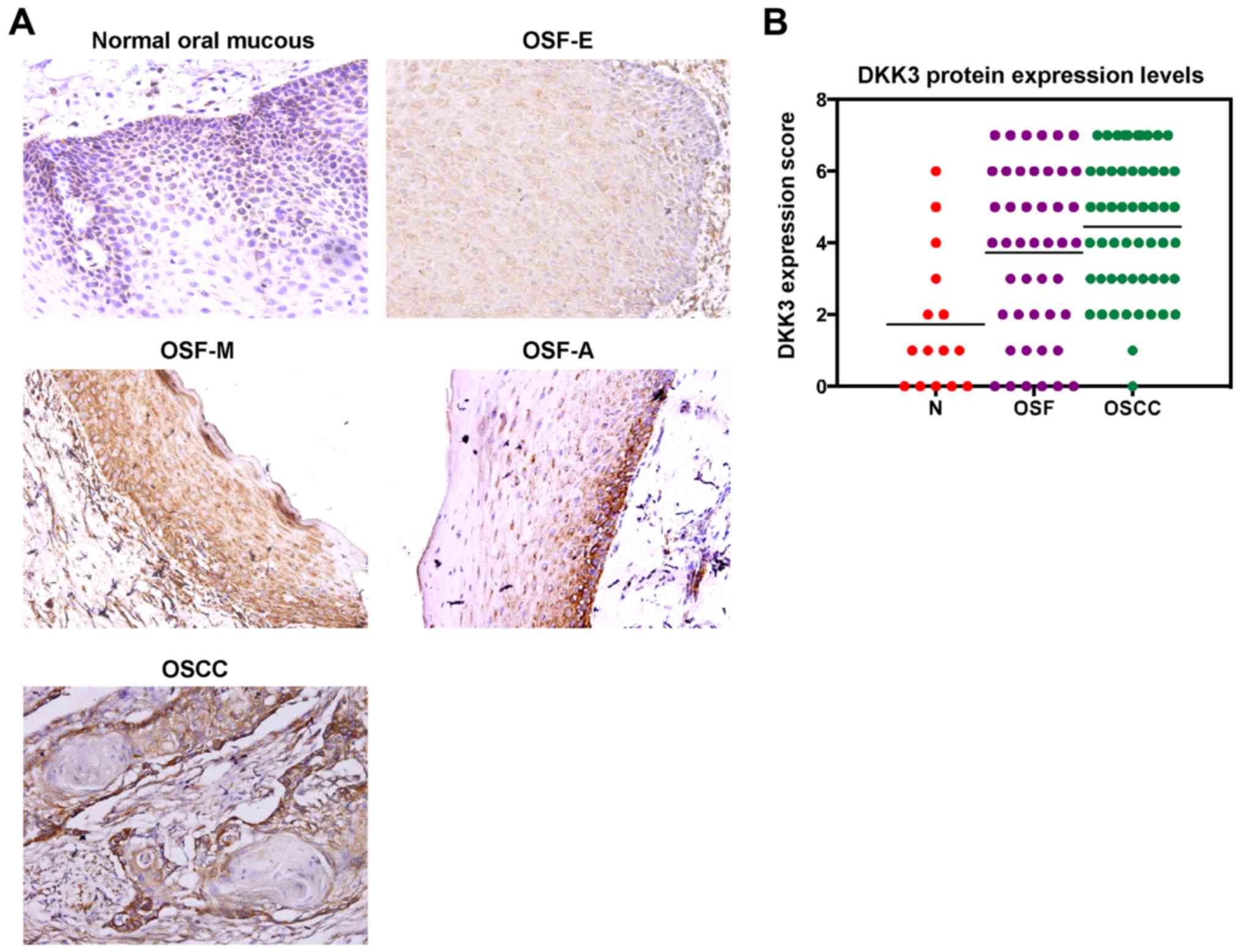
DKK3 upregulation at the protein level
in the carcinogenesis of OSF
To evaluate DKK3 protein expression in malignant
progression of OSF, we firstly performed immunohistochemical
staining using a DKK3-specific antibody in normal oral mucous, OSF
and OSCC tissues. Six of 15 (33.3%) normal oral mucous cases showed
DKK3 positivity in the cytoplasm, and 35 of 45 (77.8%) OSF tissues
showed cytoplasmic DKK3 expression, including tissues from 11 of 15
(73.3%) early stage, 12 of 15 (80.0%) moderately advanced stage and
12 of 15 (80.0%) advanced stage, as well as 53 of 55 (96.4%) OSCC
(Fig. 3A). The average values of
DKK3 expression varied in the different tissue samples, including
the mean score of 1.73 in normal oral mucosa tissues, 3.73 in OSF
tissues and 4.45 in OSCC tissues (Fig.
3B; Table I). DKK3 expression
level was gradually increased in normal oral, OSF and OSCC tissues
(P<0.05).
 | Table I.DKK3 expression in the carcinogenesis
of OSF. |
Table I.
DKK3 expression in the carcinogenesis
of OSF.
|
|
| DKK3 |
|
|
|---|
|
|
|
|
|
|
|---|
| Group | n | − | + | ++ | +++ | DKK3 expression
(%) | Mean DKK3
score |
|---|
| Normal | 15 | 9 | 3 | 2 | 1 | 33.33 | 1.73 |
| OSF | 45 | 10 | 12 | 12 | 11 | 77.78 | 3.73 |
| E | 15 | 4 | 3 | 4 | 4 | 73.33 | 3.53 |
| M | 15 | 3 | 4 | 4 | 4 | 80.00 | 3.67 |
| A | 15 | 3 | 2 | 5 | 5 | 80.00 | 4.00 |
| OSCC | 55 | 2 | 17 | 17 | 19 | 96.36 | 4.45 |
The correlation between DKK3 expression and the
clinicopathological features of the OSCC cases was analyzed,
including age, gender, tumor site, primary tumor, TNM stage and
differentiation grade. DKK3 expression between male patients and
female patients was statistically significant (Table II). These data suggest that DKK3 is
upregulated at the protein level in the carcinogenesis of OSF.
 | Table II.Correlation of DKK3 expression and
the clinicopathological features of the OSCC cases. |
Table II.
Correlation of DKK3 expression and
the clinicopathological features of the OSCC cases.
|
|
| DKK3 |
|---|
|
|
|
|
|---|
| Clinicopathological
features | Total (n) | + | − | P-value |
|---|
| Age (years) |
|
|
| >0.05 |
|
<50 | 43 | 42 | 1 |
|
|
≥50 | 12 | 11 | 1 |
|
| Gender |
|
|
| <0.01 |
|
Male | 52 | 52 | 0 |
|
|
Female | 3 | 1 | 2 |
|
| Tumor site |
|
|
| >0.05 |
|
Tongue | 40 | 39 | 1 |
|
|
Others | 15 | 13 | 2 |
|
| Primary tumor |
|
|
| >0.05 |
|
T1+T2 | 12 | 11 | 1 |
|
|
T3+T4 | 43 | 42 | 1 |
|
| TNM stage |
|
|
| >0.05 |
|
I+II | 12 | 11 | 1 |
|
|
III+IV | 43 | 42 | 1 |
|
| Differentiation
grade |
|
|
| >0.05 |
|
Well | 20 | 19 | 1 |
|
|
Moderate-poor | 35 | 34 | 1 |
|
DKK3 mRNA expression is elevated in
the carcinogenesis of OSF
DKK3 expression at the mRNA level was
examined in normal oral mucosa and OSF tissues, as well as OSCC and
their paired adjacent tissues by semi-quantitative RT-PCR. We found
that DKK3 was expressed in normal oral mucosa (Fig. 4A) and OSF tissues in different
stages (Fig. 4B) with varied
levels. DKK3 expression was detected in OSCC and their
adjacent OSF tissues. Results showed that DKK3 expression
levels were higher in OSCC tissues, than their adjacent OSF tissues
(Fig. 4C). Moreover, the
DKK3 expression level was upregulated in OSCC tissues,
compared with the paired adjacent normal tissues (Fig. 4D). Increased mRNA expression level
of DKK3 in OSCC tissues was further confirmed by real-time
RT-PCR, compared with their adjacent normal or OSF tissues
(Fig. 4E). Therefore, the
DKK3 mRNA expression level is increased in the
carcinogenesis of OSF.
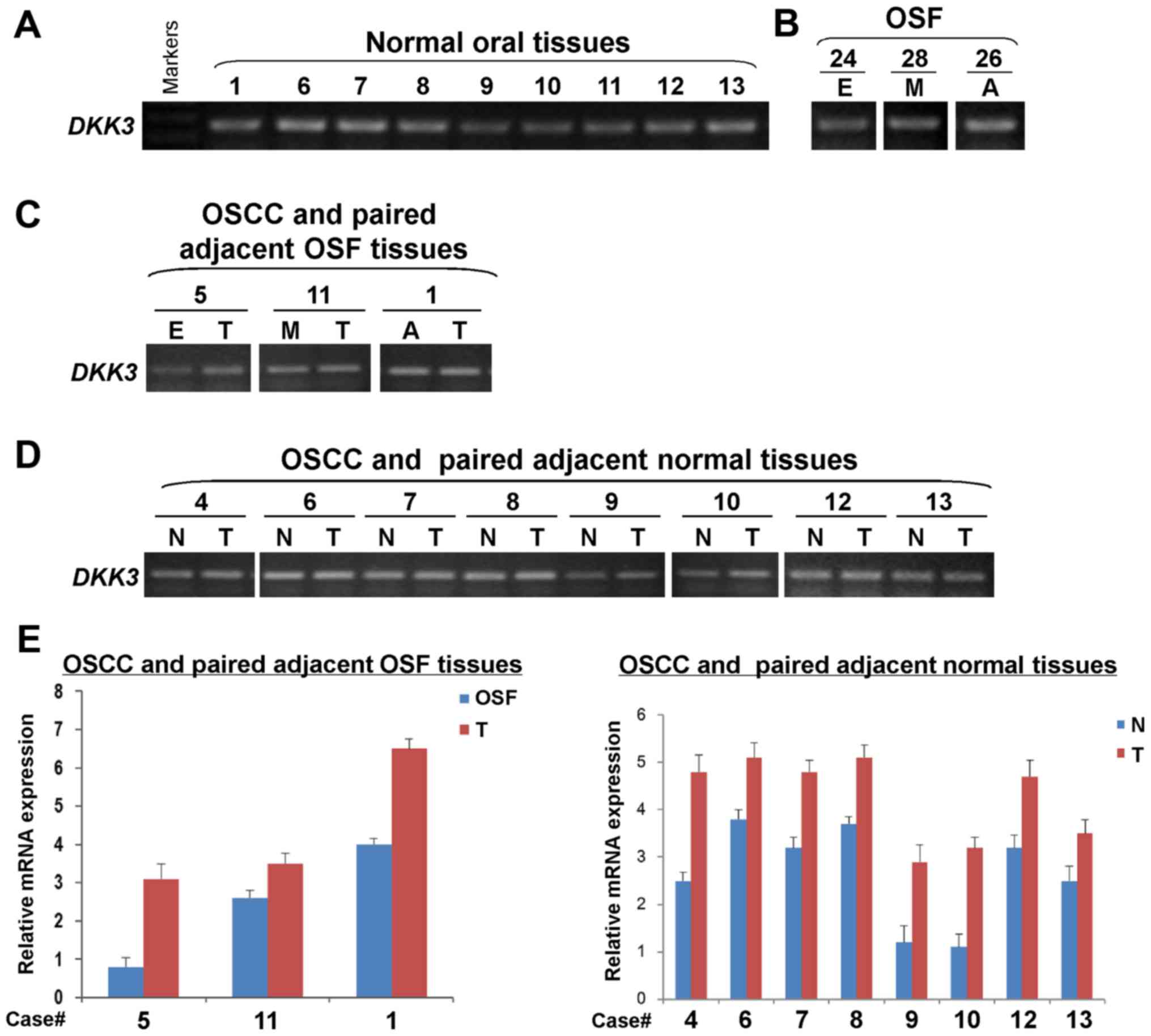 | Figure 4.Detection of DKK3 mRNA
expression in normal oral mucosa, OSF and OSCC tissues.
Semi-quantitative RT-PCR assessed DKK3 expression in (A)
normal oral mucosa tissues, (B) OSF tissues, (C) OSCC and paired
adjacent OSF tissues, as well as (D) OSCC and paired adjacent
normal tissues. GAPDH was used as an internal control. (E)
Quantitative real-time RT-PCR was used to confirm DKK3
expression in samples from OSCC and paired adjacent OSF or normal
tissues. DKK3, dickkopf WNT signaling pathway inhibitor 3. OSF,
oral submucous fibrosis: E, early stage; M, moderately advanced
stage; A, advanced stage. OSCC, oral squamous cell carcinoma (T);
N, normal mucosa tissue. |
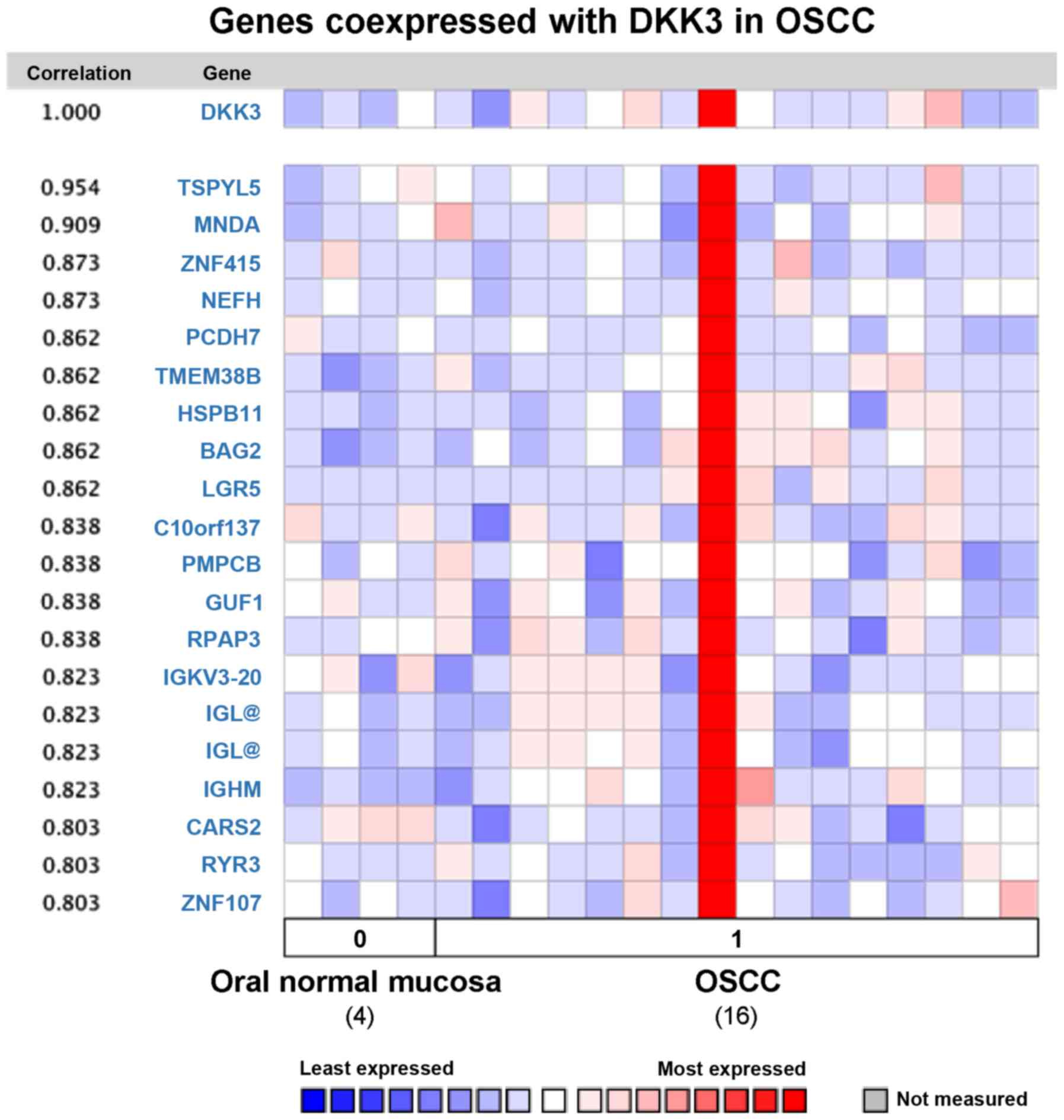
Analysis of co-expressed genes with
DKK3 in OSCC
To further investigate the potential function of
DKK3 in OSCC, average linkage hierarchical clustering was applied
to identify the co-expressed gene set with DKK3 in the Oncomine
database. One study comparing DKK3 expression data for normal oral
mucosa and OSCC tissues was selected for analysis, by using human
genome U133A array with 12,624 genes measured. Nineteen genes were
co-expressed with DKK3 in OSCC with correlation rate from 0.803 to
0.954 (Fig. 5): TSPYL5, MNDA,
ZNF415, NEFH, PCDH7, TMEM38B, HSPB11, BAG2, LGR5, co10orf137,
PMPCB, GUF1, RPAP3, IGKV3-20, IGL, IGHM, CARS2, RYR3 and ZNF107.
Several of these genes are related to Wnt and p53 signaling,
apoptosis, Ca2+ and mitochondrial signaling
pathways.
Discussion
Cumulative genetic and epigenetic aberrations in
cancer genes contribute to OSCC tumorigenesis. Thus, identification
of key genes involved in OSCC initiation and progression,
particularly malignant progression of precancerous OSF lesions, is
vital. As one of the Wnt antagonists, DKK family members antagonize
the Wnt signaling pathway by interacting with the Wnt co-receptors
LRP5 and LRP6, further preventing their interaction with Wnt
ligands (10,22,23).
Unlike other family members, only DKK3 and DKKL1 contain sgy-domain
thus are divergent members of the DKK family (10), suggesting possible Wnt-independent
function of DKK3 other than as a Wnt antagonist in
tumorigenesis.
DKK3 downregulation by promoter CpG methylation has
been reported in multiple types of cancer, including thyroid
(24), lung (25,26),
gastric (27), colon (27), hepatocellular (27,28),
breast (29,30), ovarian (31), cervical (32) and renal (33,34)
cancers, as well as acute myeloid leukemia (35) and glioma (36). Various studies found that DKK3 is a
putative Wnt signaling inhibitor. In OSCC, DKK3 possesses
tumor-suppressive function or oncogenic function. DKK3 was found to
be epigenetically inactivated in oral cancer, along with other Wnt
antagonists WIF1 and SFRPs (12).
DKK3 deletion was detected in HNSCC samples using Oncomine and TCGA
databases, although no statistical significance and significantly
prolonged overall survival were observed, suggesting that DKK3 may
function as a tumor-suppressor gene (TSG) in OSCC. Recent studies
provide strong evidence that DKK3 plays an oncogenic role in OSCC.
DKK3 protein expression was found to be increased in dysplasia and
further in OSCC, and was found to be correlated with OSCC
metastasis and poor prognosis, independent of the Wnt signaling
pathway (13–15).
In the present study, we found that DKK3 was
statistically significantly upregulated in OSCC development by
bioinformatic analysis. A rare genetic mutation of DKK3 was found
in OSCC. DKK3 gene copy number was increased in OSCC,
compared to normal oral mucosa, thus further causing DKK3
upregulation in OSCC. We also found that DKK3 either at the protein
level or at the mRNA level was expressed in normal oral mucosal
tissues, and the levels were gradually increased in the different
stages of OSF and OSCC tissues with BQ chewing, which is consistent
with other reports. By analyzing co-expressed genes with DKK3,
potential biological functions and molecular mechanisms of DKK3 in
OSCC pathogenesis were shown. For example, DKK3 activates the Wnt
signaling pathway by binding to and activating LGR5 (37,38);
DKK3 was found to inhibit the p53 signaling pathway by modulating
TSPYL5 (39) and ZNF415 (40); DKK3 also suppressed apoptosis
through the mitochondrial signaling pathway.
In summary, we provide evidence that the DKK3
expression level is increased in the carcinogenesis of OSF. DKK3
copy number gains are responsible for its upregulation in OSCC. The
present study revealed the oncogenic role of DKK3 in the malignant
progression of OSF, and sheds light on the development of a
valuable tumor biomarker for the early detection of OSCC.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81202133) and the Cross
Research Fund of Biomedical Engineering of Shanghai Jiaotong
University (YG2016MS04).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braakhuis BJ, Brakenhoff RH and Leemans
CR: Head and neck cancer: Molecular carcinogenesis. Ann Oncol.
16:(Suppl 2). ii249–ii250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leemans CR, Braakhuis BJ and Brakenhoff
RH: The molecular biology of head and neck cancer. Nat Rev Cancer.
11:9–22. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pindborg JJ, Murti PR, Bhonsle RB, Gupta
PC, Daftary DK and Mehta FS: Oral submucous fibrosis as a
precancerous condition. Scand J Dent Res. 92:224–229.
1984.PubMed/NCBI
|
|
6
|
Tilakaratne WM, Klinikowski MF, Saku T,
Peters TJ and Warnakulasuriya S: Oral submucous fibrosis: Review on
aetiology and pathogenesis. Oral Oncol. 42:561–568. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wollina U, Verma SB, Ali FM and Patil K:
Oral submucous fibrosis: An update. Clin Cosmet Investig Dermatol.
8:193–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:42012. View Article : Google Scholar
|
|
9
|
Veeck J and Dahl E: Targeting the Wnt
pathway in cancer: The emerging role of Dickkopf-3. Biochim Biophys
Acta. 1825:18–28. 2012.PubMed/NCBI
|
|
10
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katase N, Gunduz M, Beder L, Gunduz E,
Lefeuvre M, Hatipoglu OF, Borkosky SS, Tamamura R, Tominaga S,
Yamanaka N, et al: Deletion at Dickkopf (dkk)-3 locus (11p15.2) is
related with lower lymph node metastasis and better prognosis in
head and neck squamous cell carcinomas. Oncol Res. 17:273–282.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pannone G, Bufo P, Santoro A, Franco R,
Aquino G, Longo F, Botti G, Serpico R, Cafarelli B, Abbruzzese A,
et al: WNT pathway in oral cancer: Epigenetic inactivation of
WNT-inhibitors. Oncol Rep. 24:1035–1041. 2010.PubMed/NCBI
|
|
13
|
Fujii M, Katase N, Lefeuvre M, Gunduz M,
Buery RR, Tamamura R, Tsujigiwa H and Nagatsuka H: Dickkopf (Dkk)-3
and β-catenin expressions increased in the transition from normal
oral mucosal to oral squamous cell carcinoma. J Mol Histol.
42:499–504. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kataoka K, Du G, Maehara N, Murata H,
Sakaguchi M and Huh N: Expression pattern of REIC/Dkk-3 in mouse
squamous epithelia. Clin Exp Dermatol. 37:428–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Katase N, Lefeuvre M, Tsujigiwa H, Fujii
M, Ito S, Tamamura R, Buery RR, Gunduz M and Nagatsuka H: Knockdown
of Dkk-3 decreases cancer cell migration and invasion independently
of the Wnt pathways in oral squamous cell carcinoma-derived cells.
Oncol Rep. 29:1349–1355. 2013.PubMed/NCBI
|
|
16
|
Gupta PC, Sinor PN, Bhonsle RB, Pawar VS
and Mehta HC: Oral submucous fibrosis in India: A new epidemic?
Natl Med J India. 11:113–116. 1998.PubMed/NCBI
|
|
17
|
Zhou S, Chen L, Mashrah M, Zhu Y, He Z, Hu
Y, Xiang T, Yao Z, Guo F and Zhang C: Expression and promoter
methylation of Wnt inhibitory factor-1 in the development of oral
submucous fibrosis. Oncol Rep. 34:2636–2642. 2015.PubMed/NCBI
|
|
18
|
Zhou S, Chen L, Mashrah M, Zhu Y, Liu J,
Yang X, He Z, Wang L, Xiang T, Yao Z, et al: Deregulation of
secreted frizzled-related proteins is associated with aberrant
β-catenin activation in the carcinogenesis of oral submucous
fibrosis. Onco Targets Ther. 8:2923–2931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng CH, Liao CT, Peng SC, Chen YJ, Cheng
AJ, Juang JL, Tsai CY, Chen TC, Chuang YJ, Tang CY, et al: A novel
molecular signature identified by systems genetics approach
predicts prognosis in oral squamous cell carcinoma. PLoS One.
6:e234522011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ye H, Yu T, Temam S, Ziober BL, Wang J,
Schwartz JL, Mao L, Wong DT and Zhou X: Transcriptomic dissection
of tongue squamous cell carcinoma. BMC Genomics. 9:692008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ginos MA, Page GP, Michalowicz BS, Patel
KJ, Volker SE, Pambuccian SE, Ondrey FG, Adams GL and Gaffney PM:
Identification of a gene expression signature associated with
recurrent disease in squamous cell carcinoma of the head and neck.
Cancer Res. 64:55–63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holmen SL, Robertson SA, Zylstra CR and
Williams BO: Wnt-independent activation of beta-catenin mediated by
a Dkk1-Fz5 fusion protein. Biochem Biophys Res Commun. 328:533–539.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujii Y, Hoshino T and Kumon H: Molecular
simulation analysis of the structure complex of C2 domains of DKK
family members and β-propeller domains of LRP5/6: Explaining why
DKK3 does not bind to LRP5/6. Acta Med Okayama. 68:63–78.
2014.PubMed/NCBI
|
|
24
|
Yin DT, Wu W, Li M, Wang QE, Li H, Wang Y,
Tang Y and Xing M: DKK3 is a potential tumor suppressor gene in
papillary thyroid carcinoma. Endocr Relat Cancer. 20:507–514. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jung IL, Kang HJ, Kim KC and Kim IG:
Knockdown of the Dickkopf 3 gene induces apoptosis in a lung
adenocarcinoma. Int J Mol Med. 26:33–38. 2010.PubMed/NCBI
|
|
26
|
Kobayashi K, Ouchida M, Tsuji T, Hanafusa
H, Miyazaki M, Namba M, Shimizu N and Shimizu K: Reduced expression
of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor
cells. Gene. 282:151–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato H, Suzuki H, Toyota M, Nojima M,
Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M, et
al: Frequent epigenetic inactivation of DICKKOPF family genes in
human gastrointestinal tumors. Carcinogenesis. 28:2459–2466. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang L, He H, Lv R, Zhang M, Huang H, An
Z and Li S: Preliminary mechanism on the methylation modification
of Dkk-1 and Dkk-3 in hepatocellular carcinoma. Tumour Biol.
36:1245–1250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Veeck J, Bektas N, Hartmann A, Kristiansen
G, Heindrichs U, Knüchel R and Dahl E: Wnt signalling in human
breast cancer: Expression of the putative Wnt inhibitor Dickkopf-3
(DKK3) is frequently suppressed by promoter hypermethylation in
mammary tumours. Breast Cancer Res. 10:R822008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiang T, Li L, Yin X, Zhong L, Peng W, Qiu
Z, Ren G and Tao Q: Epigenetic silencing of the WNT antagonist
Dickkopf 3 disrupts normal Wnt/β-catenin signalling and apoptosis
regulation in breast cancer cells. J Cell Mol Med. 17:1236–1246.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
You A, Fokas E, Wang LF, He H, Kleb B,
Niederacher D, Engenhart-Cabillic R and An HX: Expression of the
Wnt antagonist DKK3 is frequently suppressed in sporadic epithelial
ovarian cancer. J Cancer Res Clin Oncol. 137:621–627. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park
J, Chae M, Zhang W and Lee JH: Dkk3, downregulated in cervical
cancer, functions as a negative regulator of beta-catenin. Int J
Cancer. 124:287–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurose K, Sakaguchi M, Nasu Y, Ebara S,
Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, et al:
Decreased expression of REIC/Dkk-3 in human renal clear cell
carcinoma. J Urol. 171:1314–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ueno K, Hirata H, Majid S, Chen Y, Zaman
MS, Tabatabai ZL, Hinoda Y and Dahiya R: Wnt antagonist DICKKOPF-3
(Dkk-3) induces apoptosis in human renal cell carcinoma. Mol
Carcinog. 50:449–457. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valencia A, Román-Gómez J, Cervera J, Such
E, Barragán E, Bolufer P, Moscardó F, Sanz GF and Sanz MA: Wnt
signaling pathway is epigenetically regulated by methylation of Wnt
antagonists in acute myeloid leukemia. Leukemia. 23:1658–1666.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mizobuchi Y, Matsuzaki K, Kuwayama K,
Kitazato K, Mure H, Kageji T and Nagahiro S: REIC/Dkk-3 induces
cell death in human malignant glioma. Neuro Oncol. 10:244–253.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carmon KS, Lin Q, Gong X, Thomas A and Liu
Q: LGR5 interacts and cointernalizes with Wnt receptors to modulate
Wnt/β-catenin signaling. Mol Cell Biol. 32:2054–2064. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
de Lau W, Barker N, Low TY, Koo BK, Li VS,
Teunissen H, Kujala P, Haegebarth A, Peters PJ, van de Wetering M,
et al: Lgr5 homologues associate with Wnt receptors and mediate
R-spondin signalling. Nature. 476:293–297. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Epping MT, Meijer LA, Krijgsman O, Bos JL,
Pandolfi PP and Bernards R: TSPYL5 suppresses p53 levels and
function by physical interaction with USP7. Nat Cell Biol.
13:102–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng Y, Wang Y, Li Y, Deng Y, Hu J, Mo X,
Li N, Li Y, Luo N, Yuan W, et al: A novel human gene ZNF415 with
five isoforms inhibits AP-1- and p53-mediated transcriptional
activity. Biochem Biophys Res Commun. 351:33–39. 2006. View Article : Google Scholar : PubMed/NCBI
|