Introduction
Renal cell carcinoma (RCC) is the most common
malignant kidney tumor with a rising annual incidence rate, and
currently accounts for ~2–4% of all malignant adult diseases
worldwide (1). It is categorized
according to histological subtypes: clear cell, papillary,
chromophobe and collecting duct RCC (2). Clear cell RCC (cc-RCC) accounts for
>90% of all RCC cases and the loss of the von Hippel-Lindau
(VHL) tumor-suppressor gene in the biallellic loci occurs in 50–60%
of RCC cases (3). Currently, there
are several therapeutic approaches for RCC, including radical
nephrectomy, conventional chemotherapy and immunotherapy. However,
~40% of patients are resistant to conventional chemotherapy and
irradiation. Such patients may develop systemic recurrence and
together with the resultant high toxicity and low response therapy
failure is inevitable. Thus, the 2–5-year survival rates are less
than 20% (4–6). Novel therapeutic strategies may be
available, but they are prone to side-effects such as fatigue,
nausea, hypertension, proteinuria and neutropenia which need to be
managed. Therefore, an effective and tolerable therapy for RCC is
urgently needed.
Plant extracts have been used as traditional
medicines since antiquity (7,8). They
continue to be used by traditional medicine practitioners and are
being explored in clinical research as potential anticancer agents,
which offer the additional advantage of being less expensive than
conventional drugs. The evaluation of certain naturally occurring
phytochemicals that can reduce the risk and inhibit the progression
of cancer would be useful as it may lead to the identification of
certain candidate plant extracts with the potential for development
as therapeutic cancer drugs. For example, the saponin products
isolated from the seeds of the horse chestnut Aesculus
hippocastanum (Europe), Aesculus turbinata Blume (Japan)
and Aesculus chinensis var. wilsonii (Rehder) (China)
which are known as escin and exist in either α or β form, were
found to have potential application in cancer treatments (9–11).
β-aescin or escin (Fig. 1A) is a
pentacyclic triterpenoid compound with anti-edematous (e.g.,
postoperative edema) (12),
anti-chronic venous insufficiency (CVI) and anti-hemorrhoid
(13,14) properties.
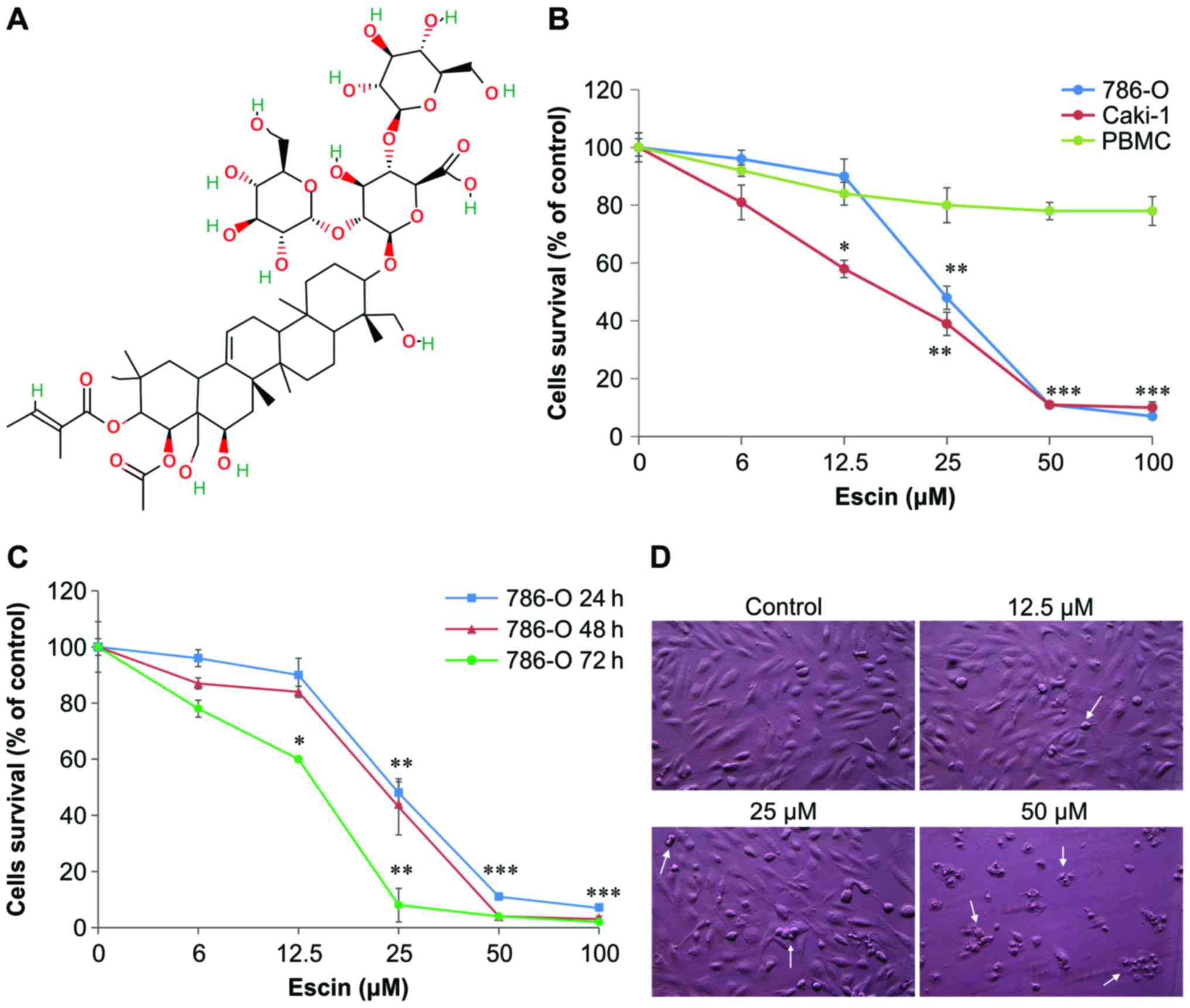 | Figure 1.Effect of escin on the cell survival
of human renal cancer 786-O and Caki-1 cells and normal human
peripheral blood mononuclear cells (PBMCs). (A) The chemical
structure of escin. (B) 786-O, Caki-1 and PBMCs were seeded at a
density of 2×104 cells/well and then treated with escin
(0, 6, 12.5, 25, 50 and 100 µM) or a vehicle-only control for 24 h.
The MTT assay (described in ‘Materials and methods’) was used to
quantify cell viability. (C) 786-O cells were also treated with the
same concentrations of escin for 24, 48 and 72 h, and then cell
viability was assessed by the MTT assay. In (B) and (C), data
points and error bars represent the mean and standard deviation
(SD) of three experiments. Statistical significance *p<0.05,
**p<0.01, ***p<0.001 as compared with control. (D) Effect of
escin on the morphology of 786-O cells. 786-O cells were treated
with control (vehicle only), 12.5, 25 and 50 µM escin for 24 h.
Cells were viewed by phase contrast microscopy and photographed at
a magnification of ×200. The white arrows indicate apoptotic
cells. |
Moreover, escin also displays anti-inflammatory
activity (15,16) (e.g., carrageenan-induced hind paw
edema in an animal model), ex vivo rat aortic disk
angiogenesis, as well as endothelial cell proliferation, migration
and apoptosis (17), hypoglycemic
(18), anti-obesity (19) and anti-secretory effects in a rat
model (20). Due to its versatile
properties, escin has been considered as a potential candidate
chemotheraputic agent for the treatment of cancer (9–11).
Escin has been shown to induce apoptosis in several types of human
tumor cells, such as pancreatic cancer cells (21), acute leukemia Jurkat T cells
(22), leukemia HL-60 cells
(23), chronic myeloid leukemia
K562 cells (24),
cholangiocarcinoma cells (25),
hepatoma cells (26) and colon
cancer cells (27). In addition,
escin was reported to induce apoptosis in some of these tumor cells
(hepatoma and colon cancer cells) by causing cell cycle arrest at
the G1/S phase mediated by p21WAF1/CIP1 upregulation,
which is associated with the reduced level of cyclin E/Cdk2
(26,27). However, the molecular mechanisms by
which escin induces apoptosis in tumor cells remain to be
defined.
In the present study, we demonstrate for the first
time that escin effectively induces apoptosis in human renal cancer
cells. We also provide evidence to suggest that the
intrinsic-mitochondrial apoptosis pathway is mainly responsible for
escin-induced apoptosis in these tumor cells.
Materials and methods
Cell culture and reagents
Human renal cancer cell lines (786-O and Caki-1)
were purchased from Bioresource Collection and Research Center
(BCRC; Hsinchu, Taiwan) and American Type Culture Collection (ATCC;
Manassas, VA, USA), respectively. The cell lines were cultured in
RPMI or McCoy's medium and supplemented with 10% fetal bovine serum
(FBS) (Gibco, Gaithersburg, MD, USA) and 1% antibiotic antimycotic
solution. Cells were incubated at 37°C with 5% CO2.
Escin was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Escin was prepared by dissolving the lyophilized powder in dimethyl
sulfoxide (DMSO) to a final concentration of 100 mmol/l. The stock
solution was stored at −20°C until use.
Cytotoxicity assay
The cytotoxic effects of escin on renal cancer cell
lines were assessed with the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich Co.). The cell lines were seeded onto 24-well
plates and treated with various concentrations of escin for 24, 48,
and 72 h. After incubation for the indicated periods, the medium
was removed, and 200 µl of 1X MTT solution was added to each well
for 4 h. The medium was aspirated and the formazan product in the
cells was solubilized by adding DMSO. An aliquot of 150 µl was
measured using a microplate autoreader (L225-0137; PerkinElmer,
Taipei, Taiwan) at the wavelength of 540 nm. The IC50
values with dose-dependent curves were calculated by linear
interpolation and carried out in triplicates.
Cell cycle analysis
Propidium iodide (PI) staining and flow cytometry
were used to perform cell cycle analysis. First, 1×106
786-O cells were seeded on 10 cm dishes for 24 h. The cells were
collected after trypsinization and washed with ice-cold
phosphate-buffered saline (PBS), fixed and permeabilized with 70%
ethanol at −20°C overnight. The next day, after the cells were
washed with ice-cold PBS, they were incubated with PI staining
solution (0.2 mg/ml RNase, 20 µg/ml PI and 0.1% Triton X-100) for
30 min at room temperature in the dark. Data were collected using a
flow cytometer (BD FACSCalibur; BD Biosciences, San Jose, CA, USA)
and data were analyzed with WinMDI software (version 2.9). All
experiments were performed in triplicate and 10,000 events were
counted for each sample.
Annexin V assay
The BioVision Annexin V-FITC apoptosis detection kit
was used for the apoptosis assay (BioVision Inc., Milpitas, CA,
USA). First, 786-O cells were seeded onto 10-cm dishes for 24 h and
then exposed to different doses of escin for 16 h. Cells were
harvested by trypsinization, washed twice with PBS, and resuspended
in 500 µl of binding buffer. Cell suspensions were then incubated
with 5 µl of Annexin V-FITC and 5 µl of PI for 10 min at room
temperature in the dark. The cells were immediately evaluated by
flow cytometry (FACSCalibur; Becton-Dickinson, San Jose, CA,
USA).
Western blot analysis
Renal cancer cells (1×106) were seeded in
10-cm culture dishes overnight and treated with the indicated
concentrations of escin for 24 h. The cells were harvested, washed
twice in PBS, and lysed for 30 min at 4°C with ice-cold RIPA buffer
(1% NP-40 in 150 mM NaCl), 50 mM Tris (pH 7.5) and 2 mM EDTA.
Protein concentrations were measured using the Bradford protein
assay. Equal amounts of protein were loaded on 10–15% sodium
dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
gels, transferred to polyvinylidene difluoride (PVDF) membranes,
and blocked with 5% non-fat milk in Tris-buffered saline with 0.5%
Tween-20 (TBST) buffer (20 mM Tris-HCl, 120 mM NaCl, 0.1% Tween-20)
for 1 h. The membranes were incubated with various primary
antibodies against cdc-2 (GeneTex, Inc., Irvine, CA, USA), Bcl-2,
Bcl-xL, Bax, caspase-3 and −9, poly(ADP-ribose) polymerase (PARP)
(1:1,000; Cell Signaling Technology, Boston, MA, USA) and cyclin
B1, caspase-8 (p18), Fas, FasL, FADD, β-actin and GAPDH (1:1,000;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight.
After washing, the blots were incubated with HRP-labelled secondary
antibodies for 2 h. The signals of the blots were then developed
using the enhanced chemiluminescence (ECL) system and analyzed with
the LAS3000 system (Fujifilm, Tokyo, Japan).
Mitochondrial membrane potential
assay
Mitochondrial-specific cationic dye JC-1
(Invitrogen, Carlsbad, CA, USA), which undergoes
potential-dependent accumulation in the mitochondria, was used.
When the mitochondrial membrane potential (ΔΨm) is below 120 mV,
JC-1 is monomeric and emits green light (540 nm) following
excitation with blue light (490 nm). At membrane potentials >120
mV, JC-1 monomer aggregates and emits red light (590 nm) following
excitation with green light (540 nm). For the assay, the cells were
seeded onto 6-well plates and treated with various concentrations
of escin for 24 h, followed by staining with 5 µM JC-1 for 30 min
at 37°C. Fluorescence was monitored with a fluorescence plate
reader at wavelengths of 490 nm (excitation)/540 nm (emission) and
540 nm (excitation)/590 nm (emission). Changes in the ΔΨm were
indicated by changes in the ratio of intensities between the
measurements at test wavelengths of 590 nm (red) and 540 nm
(green).
Detection of reactive oxygen species
(ROS)
A flow cytometric assay of intracellular ROS, which
can trigger apoptosis, using dihydroethidium (DHE) (Setareh Biotech
LLC, Eugene, OR, USA), a fluorescent superoxide indicator, was
previously described (28–31). In the present study, 786-O cells
were treated with 0–100 µM escin for 24 h. Subsequently, these
cells were incubated with 2 µM DHE in serum-free medium at 37°C for
15 min, washed once with serum-free medium, and then centrifuged at
450 × g to remove extracellular DHE. Finally, the cells were
analyzed by flow cytometry.
Statistical analysis
Experiments were performed three times and the data
are presented as mean ± SD. Student's t-test was carried out to
assess the statistical differences. p<0.05 was considered to
indicate a statistically significant result.
Results
Escin exhibits cytotoxic effects on
human renal cancer 786-O and Caki-1 cells
To determine the cytotoxic effect of escin on human
renal cancer cells, 786-O and Caki-1 cells were treated with
several concentrations of escin (12.5–100 µM) for 24, 48 and 72 h.
Normal human peripheral blood mononuclear cells (PBMCs) (as normal
control cells) were treated with the indicated concentrations of
escin for 24 h. Cell viability of these treated cells was then
determined using the MTT assay. As shown in Fig. 1B and C, after 24 h, escin markedly
reduced the viability of the 786-O and Caki-1 cells in a
concentration-dependent manner with IC50 values of
40.6±1.2 and 35.0±0.8 µM, respectively. The IC50 values
of escin were 40.6±1.2, 35.4±0.5 and 26.2±0.5 µM in the 786-O cells
at 24, 48 and 72 h, respectively. In contrast, PBMCs treated with
escin for 24 h exhibited >75% cell survival (Fig. 1B). At the concentration of 50 µM
escin, marked morphological changes such as shrinkage and rounding
were noted in the 786-O cells (Fig.
1D). This corroborated the results from the MTT assay (Fig. 1B and C). Taken together, these
results suggest that escin induces cell death or apoptosis.
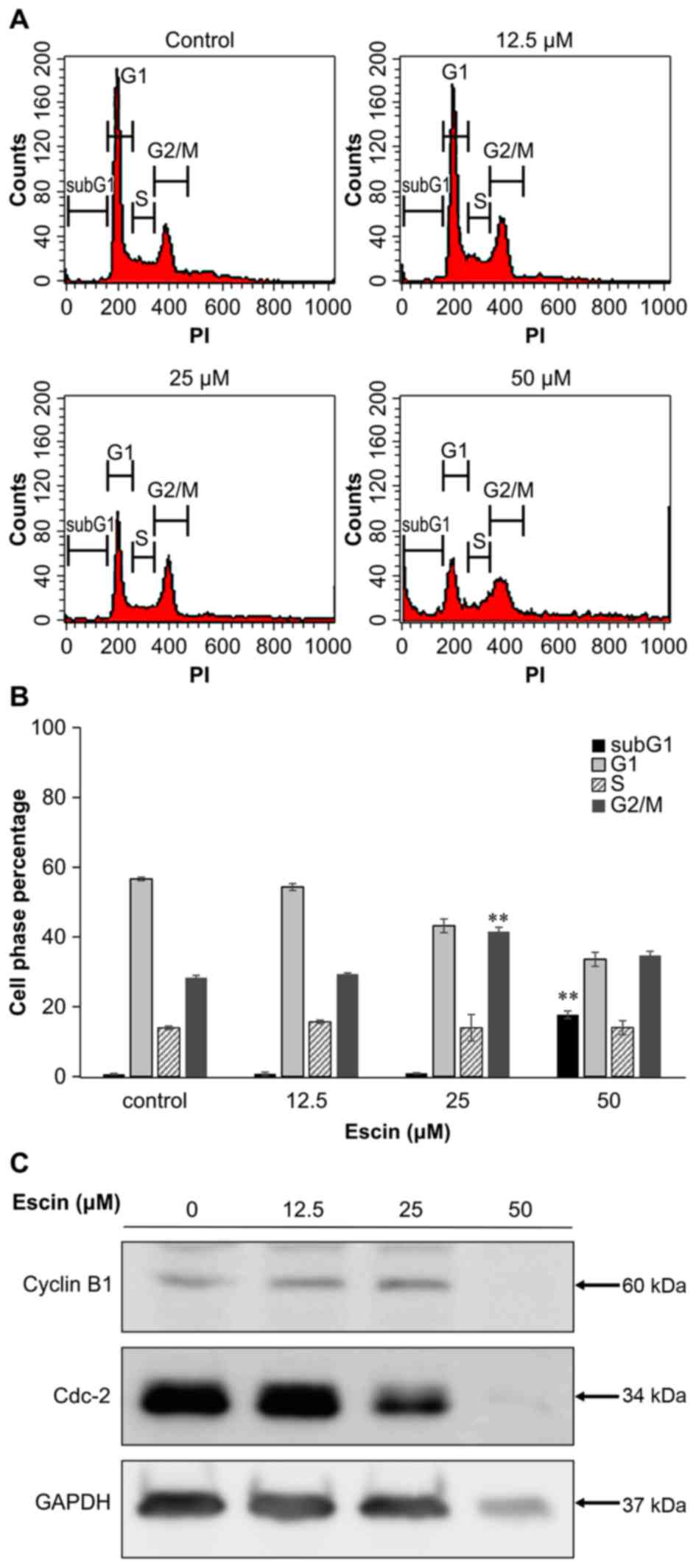
Escin induces cell cycle G2/M arrest
in 786-O cells
To elucidate the underlying mechanism of
escin-induced cell death, we analyzed the distribution of the cell
cycle in 786-O cells treated with several concentrations of escin
for 24 h. As shown in Fig. 2A and
B, the mean percentage of sub-G1 cells, which is an indicator
of cell death, in 786-O cells treated with the higher tested
concentration (50 µM) of escin (17.7±0.7%) was significantly higher
than that in the untreated cells (0.7±0.02%) (p<0.01). In
addition, the percentage of cells in the G2/M transition was
significantly higher in 786-O cells treated with 25 µM escin
(41.6±2%) than in untreated cells (28.3±1.1%) (p<0.01). The
levels of cdc-2 protein were decreased relative to the internal
control of GAPDH in the cells treated with 25 µM escin, but there
were no significant changes in cyclin B1 protein levels following
treatment with 25 µM escin (Fig.
2C). These results suggest that escin induces cell cycle G2/M
arrest in 786-O cells.
To elucidate the type of cell death caused by escin,
we stained escin-treated 786-O cells with Annexin V-FITC and PI to
define apoptosis and necrosis, respectively. The percentage of
early and late apoptotic, and necrotic cells in the 786-O cell line
increased in a concentration-dependent manner (Fig. 3A). At the highest tested
concentration of escin, early apoptosis occurred in 77.5% of the
786-O cells compared to the internal control (4.5%) in a
concentration-dependent manner (Fig.
3A). Thus, the percentages of live, early apoptotic and late
apoptotic cells were calculated and subjected to statistical
analysis (Fig. 3B). These results
indicate that escin induced apoptosis in the 786-O cells.
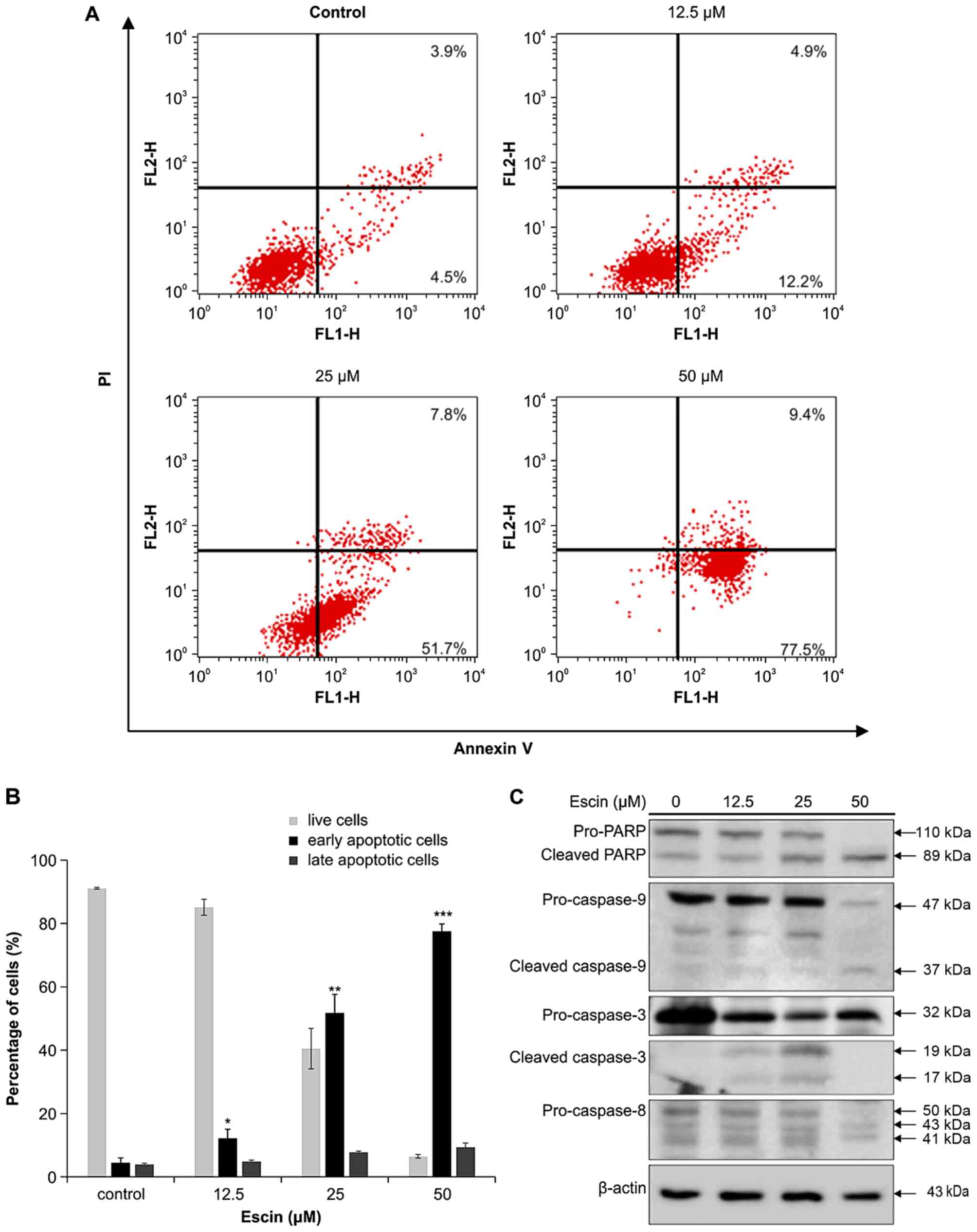 | Figure 3.Flow cytometry analysis of
escin-induced apoptosis in 786-O cells. (A) Effect of escin on
Annexin V binding in 786-O cells. Cells were treated with 0, 12.5,
25 and 50 µM escin for 16 h. Subsequently, the treated cells were
labelled with Annexin V-fluorescein isothiocyanate and PI. In each
flow cytometry plot, the lower right quadrant (Annexin
V+/PI−) shows early apoptotic cells, while
the upper right quadrant (Annexin V+/PI+)
depicts late apoptotic and necrotic cells. (B) The percentages of
live, early apoptotic and late apoptotic cells were calculated and
subjected to statistical analysis. Data are representative of three
independent experiments. Statistical significance *p<0.05,
**p<0.01, ***p<0.001 as compared with control. (C) Effect of
escin on caspase activation in the 786-O cells. 786-O cells were
treated with 0, 12.5, 25, and 50 µM escin for 24 h. Total cell
lysates were resolved by sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) and immunoblotted with antibodies
against the cleaved forms of caspase-9, −8 and −3, poly(ADP-ribose)
polymerase (PARP) and β-actin. Data are representative of three
independent experiments. |
Escin induces caspase-dependent
apoptosis in 786-O cells
Caspases, such as caspase-9 −8 and −3/7, can be
activated by either an intrinsic mitochondrial-mediated pathway or
an extrinsic death receptor-mediated pathway (32). Activated caspases cause cleavage of
PARP, which is a marker of apoptosis. Thus, to determine whether
escin induces apoptosis through the intrinsic or extrinsic pathway,
we performed western blot analysis of the pro-form and cleaved
forms of caspase-8, −9 and −3, and PARP. As illustrated in Fig. 3C, 786-O cells treated with indicated
concentrations of escin for 24 h expressed elevated levels of the
cleaved forms of caspase-9 and −3, and PARP. It is important to
note that the protein level of caspase-3 in 50 µM escin-treated
786-O cells was not detected. We reasoned that many cells treated
with 50 µM escin may be dead and detach from the wells. In
addition, the cleaved caspase-3 protein (17 kDa), which is the
smallest proteins among all caspase-cleaved protein molecules, is
easily further degraded and may not be detected by western blot
analysis. However, the cleaved caspase-8 protein level (molecular
mass; 18 kDa) was not detected among the concentrations of escin
used to treat the cells (Fig. 3C).
These results suggest that escin induced intrinsic-pathway
apoptosis in these renal cancer cells.
Escin induces mitochondrial-mediated
apoptosis in 786-O cells
To confirm that escin induces intrinsic apoptosis,
we determined the effect of escin on the mitochondrial membrane
potential (ΔΨm) in 786-O cells. Loss of ΔΨm is a hallmark of
intrinsic apoptosis, since it is associated with the release of
pro-apoptotic proteins into the cytosol (33). In escin-treated 786-O cells stained
with JC-1 dye, a concentration-dependent decrease in red
fluorescence and an increase in green fluorescence were observed
(Fig. 4B and C). This suggests that
escin reduced ΔΨm. These results support the hypothesis that escin
induces mitochondrial-mediated apoptosis in the renal cancer
cells.
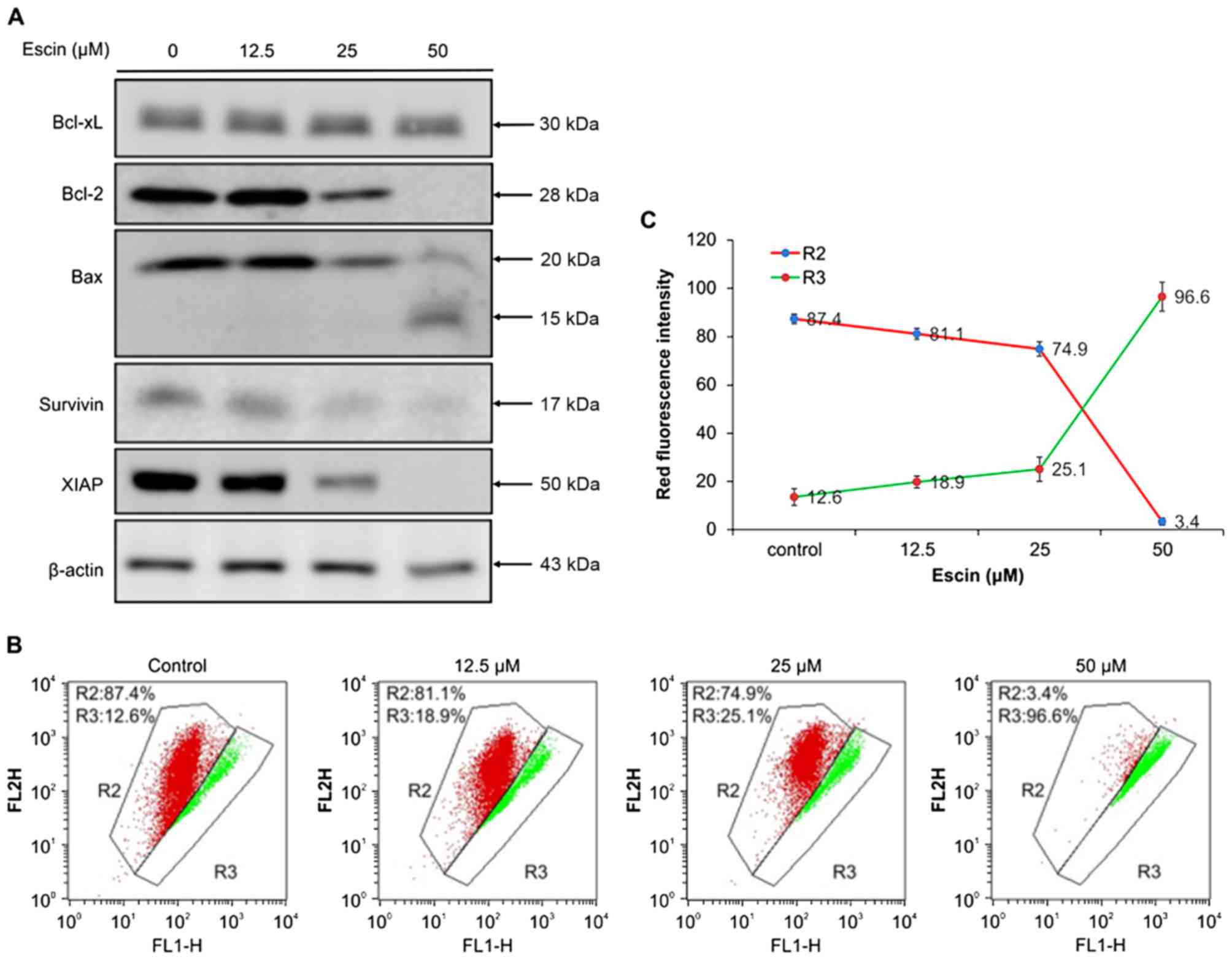 | Figure 4.Effect of escin on
mitochondrial-mediated apoptosis in 786-O cells. (A) Effect of
escin on the expression of Bcl-2 family proteins and IAP protein in
the 786-O cells. 786-O cells were treated with 0, 12.5, 25 and 50
µM escin for 24 h. Cytosolic lysates of attached and floating cells
were resolved by SDS-PAGE and then western blotted with anti-Bcl2,
anti-Bcl-xL, anti-Bax, anti-XIAP, anti-survivin and anti-β-actin.
(B) Effect of escin on the mitochondrial membrane potential. 786-O
cells were treated with 0, 12.5, 25 and 50 µM escin for 24 h, and
then stained with JC-1 dye (R2, aggregated JC-1, red fluorescence;
R3, monomeric JC-1, green fluorescence), and then the red:green
fluorescence ratio, which indicates changes in the mitochondrial
membrane potential, was measured by flow cytometry. (C) Effect of
escin on depolarization of mitochondrial membrane potential. 786-O
cells were treated with several concentrations of escin. JC-1 green
fluorescence intensity relative to the red fluorescence intensity
was measured in the 786-O cells. |
To further elucidate the mechanism of escin-induced
mitochondrial-mediated apoptosis, we determined the effect of escin
on the expression of Bcl-2 family proteins which regulate
cytochrome c release and caspase activity (33,34).
Specifically, we examined three Bcl-2 family proteins, namely Bax,
which promotes cytochrome c release, and Bcl-2 and Bcl-xL,
both of which inhibit cytochrome c release (33). As shown in Fig. 4A, escin (25 and 50 µM) decreased the
expression of Bcl-2 and increased the cleavage fragment (molecular
mass; 15 kDa) of Bax, although the Bcl-xL protein level was
unchanged in 786-O cells, which differed from the observed
expression patterns of the other proteins. Furthermore, the levels
of IAP proteins, including XIAP and survivin, which can bind to
caspases-9/−3/−7 and prevent apoptosis, were significantly
decreased (Fig. 4A). Taken
together, these results suggest that escin induced
mitochondrial-mediated apoptosis in the renal cancer cells by
disrupting anti-apoptotic proteins that regulate the release of
caspases and relieve downstream inhibition of apoptosis.
Escin-induced apoptosis is associated
with ROS production
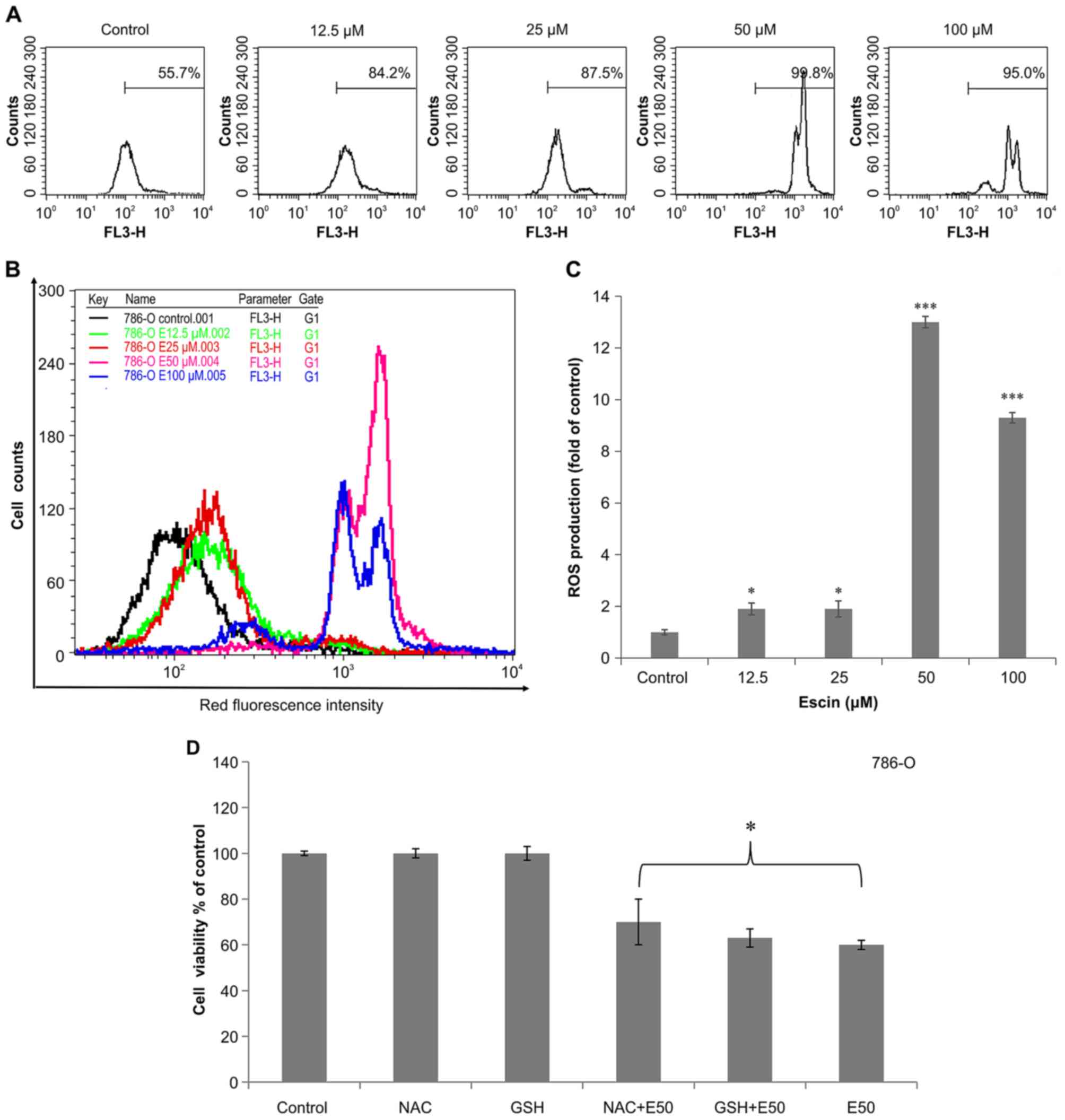
Since ROS can alter the cellular redox state and ΔΨm
(35,36), we investigated whether escin-treated
786-O cells produce ROS. DHE staining revealed that red
fluorescence intensity (Fig. 5A and
B) and ROS production (Fig. 5C)
increased in the 786-O cells treated with escin for 24 h. In
addition, DHE staining showed that the red fluorescence intensity
was significantly increased in the 786-O cells treated at the
indicated concentration (≥12.5 µM) of escin (Fig. 5C). This indicates that ROS play a
crucial role in escin-induced apoptosis in 786-O cells. Moreover,
pretreatment of these cells with two antioxidants, NAC and GSH,
reduced escin-induced cell apoptosis (Fig. 5D). Collectively, these results
suggest that escin-induced mitochondrial-mediated apoptosis is
associated with ROS production in renal cancer cells.
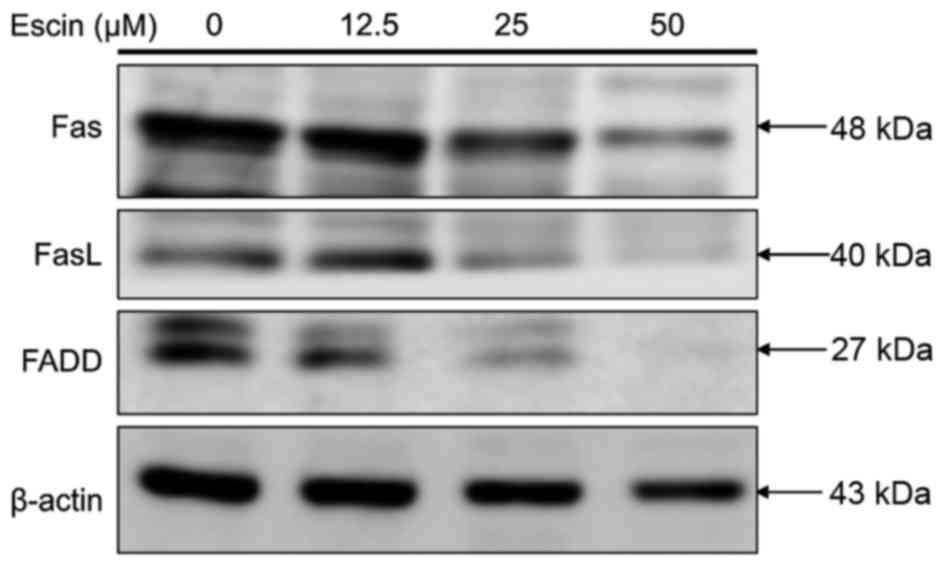
Escin does not induce death
receptor-mediated apoptosis in 786-O cells
To ascertain whether escin induces extrinsic
apoptosis, we determined the effect of escin on the expression of
proteins involved in the death receptor pathway in 786-O cells. As
shown in Fig. 6, treatment of 786-O
cells with >25 µM escin did not increase but decreased
expression of the Fas death receptor, Fas-L and Fas-associated
death domain (FADD) protein levels as compared to those of internal
control β-actin. These results support the hypothesis that escin
does not induce death receptor-mediated apoptosis in renal cancer
cells.
Discussion
Escin has been shown to have antitumor effects in
various cancer cells both in vitro and in vivo
(21,37). However, the molecular mechanisms by
which escin exerts its antitumor effects on these cancer cells are
largely unknown. For example, escin has been demonstrated to
exhibit antitumor activities against human acute leukemia Jurkat T,
HL-60, chronic myeloid leukemia K562, cholangiocarcinoma, hepatoma,
colonic cancer, pancreatic and prostate cancer cells (22–27,37,38).
In the present study, we demonstrated for the first time that escin
caused cell death or apoptosis in human renal cancer cells by
inducing the intrinsic-mitochondrial apoptosis pathway involving
G2/M arrest and reactive oxygen species (ROS) generation.
Our results showed that escin was cytotoxic to human
renal cancer cells (786-O and Caki-1) after a 24-h treatment with
IC50 values of 40.6±1.2 and 35.0±0.8 µM, respectively.
However, this effect was more pronounced in the latter cell line
Caki-1 (VHL+). It is known that 786-O (VHL mutant) cell
line is a malignant cancer with the faster growing rate than that
of Caki-1 cells, and the IC50 value of escin for these
cells was similar to that reported for human cholangiocarcinoma
cells (QBC939) (44.36±1.67 µM) (25). In the present study, we demonstrated
that the protein level of cell cycle regulatory protein cdc-2 was
significantly decreased following treatment with 25 µM escin in the
786-O cells. In addition, escin (25 µM) caused cell cycle arrest at
G2/M transition in 786-O cells. However, additional experiments
involving cell cycle regulators, such as cyclin-dependent kinase
Cdc25C, are needed to confirm these findings.
We also established that escin induced
caspase-dependent apoptosis in the 786-O cells, and that the
mitochondrial-mediated pathway is involved. Specifically, we showed
that escin increased the percentage of early apoptotic cells
(Fig. 3A and B), and decreased the
mitochondrial membrane potential (Fig.
4B and C). We further demonstrated that escin increased the
expression levels of several proteins that are important in
apoptosis (32–34); escin concomitantly decreased the
expression of anti-apoptotic proteins, such as Bcl-2, XIAP,
survivin but increased the expression of proapoptotic protein, such
as Bax cleavage (Fig. 4A), as well
as downstream proteins, such as the cleaved forms of PARP and
caspase-9 and −3 (Fig. 3C).
Collectively, these results suggest that the cytotoxicity of escin
to human renal cancer cells is mediated by multi-pronged intrinsic
apoptotic pathways.
Furthermore, we demonstrated that escin-induced
apoptosis in 786-O cells is associated with the production of ROS
(Fig. 5A-C), which can be inhibited
by antioxidants (Fig. 5D). This
finding is consistent with several previous studies showing that
escins induce production of ROS, loss of mitochondrial membrane
potential, and caspase-dependent apoptosis in human cancer cells,
such as cholangiocarcinoma cell lines (QBC939) (26) which is associated with the
mitochondrial pathway. However, it is important to note that the
balance in ROS levels can be markedly affected by many
environmental factors including chemotherapeutic agents (35). ROS production is believed to reduce
mitochondrial membrane potential, which in turn causes the release
of cytochrome c and initiates the apoptotic cascade
(36). It is worth noting that, at
higher concentrations, escin did not increase the expression of
Fas, FasL, FADD or caspase-8, which are the components of the
death-inducing signaling complex (Figs.
3C and 6). These results suggest that the death receptor
pathway is not involved in the mechanisms of escin cytotoxicity.
Another advantage of using escin is that it has low toxicity in
human cells and has been used in folk medicine (39). In the present study, we demonstrated
that escin exhibited no significant toxic effects on normal human
PBMCs (Fig. 1B). Our results
warrant further testing of escin as a chemotherapeutic agent for
the treatment of renal cancer in human patients.
In conclusion, the results of the present study
indicated that escin induced apoptosis in human renal cancer cells,
by a mechanism involving the intrinsic-mitochondrial apoptosis
pathway including G2/M arrest and ROS generation. These findings
also suggest that escin may be a clinically valuable
chemotherapeutic agent for the treatment of human renal cancer.
Acknowledgements
The present study was supported by the Taichung
Veterans General Hospital and Hung Kuang University (grant no.
TCVGH-HK1038003).
References
|
1
|
Znaor A, Lortet-Tieulent J, Laversanne M,
Jemal A and Bray F: International variations and trends in renal
cell carcinoma incidence and mortality. Eur Urol. 67:519–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheville JC, Lohse CM, Zincke H, Weaver AL
and Blute ML: Comparisons of outcome and prognostic features among
histologic subtypes of renal cell carcinoma. Am J Surg Pathol.
27:612–624. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singer EA, Gupta GN and Srinivasan R:
Update on targeted therapies for clear cell renal cell carcinoma.
Curr Opin Oncol. 23:283–289. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janzen NK, Kim HL, Figlin RA and
Belldegrun AS: Surveillance after radical or partial nephrectomy
for localized renal cell carcinoma and management of recurrent
disease. Urol Clin North Am. 30:843–852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amato RJ: Chemotherapy for renal cell
carcinoma. Semin Oncol. 27:177–186. 2000.PubMed/NCBI
|
|
6
|
Hsu RJ, Ho JY, Cha TL, Yu DS, Wu CL, Huang
WP, Chu P, Chen YH, Chen JT and Yu CP: WNT10A plays an oncogenic
role in renal cell carcinoma by activating WNT/β-catenin pathway.
PLoS One. 7:e476492012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gurib-Fakim A: Medicinal plants:
Traditions of yesterday and drugs of tomorrow. Mol Aspects Med.
27:1–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cragg GM and Newman DJ: Natural products:
A continuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tutton PJ and Barkla DH: Influence of
prostaglandin analogues on epithelial cell proliferation and
xenograft growth. Br J Cancer. 41:47–51. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stone OJ: Cancer resistance,
carcinogenesis and ground substance viscosity. Med Hypotheses.
20:117–124. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sirtori CR: Aescin: Pharmacology,
pharmacokinetics and therapeutic profile. Pharmacol Res.
44:183–193. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu F, Hou Y, Jiang W, Wang R and Liu K:
Escin: Inhibiting inflammation and promoting gastrointestinal
transit to attenuate formation of postoperative adhesions. World J
Surg. 29:1614–1620. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Diehm C, Trampisch HJ, Lange S and Schmidt
C: Comparison of leg compression stocking and oral horse-chestnut
seed extract therapy in patients with chronic venous insufficiency.
Lancet. 347:292–294. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bielanski TE and Piotrowski ZH:
Horse-chestnut seed extract for chronic venous insufficiency. J Fam
Pract. 48:171–172. 1999.PubMed/NCBI
|
|
15
|
Rothkopf M and Vogel G: New findings on
the efficacy and mode of action of the horse chestnut saponin
escin. Arzneimittelforschung. 26:225–235. 1976.(In German).
PubMed/NCBI
|
|
16
|
Matsuda H, Li Y, Murakami T, Ninomiya K,
Yamahara J and Yoshikawa M: Effects of escins Ia, Ib, IIa, and IIb
from horse chestnut, the seeds of Aesculus hippocastanum L., on
acute inflammation in animals. Biol Pharm Bull. 20:1092–1095. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XH, Xu B, Liu JT and Cui JR: Effect
of beta-escin sodium on endothelial cells proliferation, migration
and apoptosis. Vascul Pharmacol. 49:158–165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimura H, Ogawa S, Jisaka M, Kimura Y,
Katsube T and Yokota K: Identification of novel saponins from
edible seeds of Japanese horse chestnut (Aesculus turbinata BLUME)
after treatment with wooden ashes and their nutraceutical activity.
J Pharm Biomed Anal. 41:1657–1665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu JN, Zhu XM, Han LK, Saito M, Sun YS,
Yoshikawa M, Kimura Y and Zheng YN: Anti-obesity effects of escins
extracted from the seeds of Aesculus turbinata BLUME
(Hippocastanaceae). Chem Pharm Bull. 56:12–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marhuenda E, Alarcón de la Lastra C and
Martín MJ: Antisecretory and gastroprotective effects of aescine in
rats. Gen Pharmacol. 25:1213–1219. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YW, Wang SJ, Zhou YN, Pan SH and Sun
B: Escin augments the efficacy of gemcitabine through
down-regulation of nuclear factor-κB and nuclear
factor-κB-regulated gene products in pancreatic cancer both in
vitro and in vivo. J Cancer Res Clin Oncol. 138:785–797. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Gao J, Cai X, Zhao Y, Wang Y, Lu
W, Gu Z, Zhang S and Cao P: Escin sodium induces apoptosis of human
acute leukemia Jurkat T cells. Phytother Res. 25:1747–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu YP, Wu LM, Jiang YL, Wang WX and Li
LD: Beta-escin, a natural triterpenoid saponin from Chinese horse
chestnut seeds, depresses HL-60 human leukaemia cell proliferation
and induces apoptosis. J Pharm Pharmacol. 60:1213–1220. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niu YP, Li LD and Wu LM: Beta-aescin: A
potent natural inhibitor of proliferation and inducer of apoptosis
in human chronic myeloid leukemia K562 cells in vitro. Leuk
Lymphoma. 49:1384–1391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen DY, Kang JH, Song W, Zhang WQ, Li WG,
Zhao Y and Chen QX: Apoptosis of human cholangiocarcinoma cell
lines induced by β-escin through mitochondrial caspase-dependent
pathway. Phytother Res. 25:1519–1526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou XY, Fu FH, Li Z, Dong QJ, He J and
Wang CH: Escin, a natural mixture of triterpene saponins, exhibits
antitumor activity against hepatocellular carcinoma. Planta Med.
75:1580–1585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Patlolla JM, Raju J, Swamy MV and Rao CV:
β-Escin inhibits colonic aberrant crypt foci formation in rats and
regulates the cell cycle growth by inducing p21waf1/cip1
in colon cancer cells. Mol Cancer Ther. 5:1459–1466. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia Z, Bergstrand A, DePierre JW and
Nässberger L: The antidepressants imipramine, clomipramine, and
citalopram induce apoptosis in human acute myeloid leukemia HL-60
cells via caspase-3 activation. J Biochem Mol Toxicol. 13:338–347.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bindokas VP, Jordán J, Lee CC and Miller
RJ: Superoxide production in rat hippocampal neurons: Selective
imaging with hydroethidine. J Neurosci. 16:1324–1336.
1996.PubMed/NCBI
|
|
30
|
Satoh T, Numakawa T, Abiru Y, Yamagata T,
Ishikawa Y, Enokido Y and Hatanaka H: Production of reactive oxygen
species and release of L-glutamate during superoxide anion-induced
cell death of cerebellar granule neurons. J Neurochem. 70:316–324.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan SY, Cheng CL, Ho HC, Wang SS, Chiu
KY, Su CK, Ou YC and Lin CC: Nortriptyline induces mitochondria and
death receptor-mediated apoptosis in bladder cancer cells and
inhibits bladder tumor growth in vivo. Eur J Pharmacol.
761:309–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu CS, Chen YJ, Chen JJ, Shieh JJ, Huang
CH, Lin PS, Chang GC, Chang JT and Lin CC: Terpinen-4-ol induces
apoptosis in human nonsmall cell lung cancer in vitro and in vivo.
Evid Based Complement Alternat Med. 2012:8182612012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng SB, Wu LC, Hsieh YC, Wu CH, Chan YJ,
Chang LH, Chang CM, Hsu SL, Teng CL and Wu CC: Supercritical carbon
dioxide extraction of aromatic turmerone from Curcuma longa Linn.
induces apoptosis through reactive oxygen species-triggered
intrinsic and extrinsic pathways in human hepatocellular carcinoma
HepG2 cells. J Agric Food Chem. 60:9620–9630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Piao S, Kang M, Lee YJ, Choi WS, Chun YS,
Kwak C and Kim HH: Cytotoxic effects of escin on human
castration-resistant prostate cancer cells through the induction of
apoptosis and G2/M cell cycle arrest. Urology. 84:982.e1–982.e7.
2014. View Article : Google Scholar
|
|
38
|
Rimmon A, Vexler A, Berkovich L, Earon G,
Ron I and Lev-Ari S: Escin chemosensitizes human pancreatic cancer
cells and inhibits the nuclear factor-kappaB signaling pathway.
Biochem Res Int. 2013:2517522013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Küçükkurt I, Ince S, Keleş H, Akkol EK,
Avci G, Yeşilada E and Bacak E: Beneficial effects of Aesculus
hippocastanum L. seed extract on the body's own antioxidant defense
system on subacute administration. J Ethnopharmacol. 129:18–22.
2010. View Article : Google Scholar : PubMed/NCBI
|