Introduction
Th17 lymphocytes are a T helper cell population that
play a crucial role in the immune response. The name of these cells
originates from the secretion of cytokine IL-17 by these cells
(1). They diverge from innate
CD4+ T cells in the presence of transforming growth
factor-β (TGF-β) and IL-6 (1).
IL-17A and IL-17F are the major cytokines secreted by Th17 cells.
They are highly homogeneous, bind to the same receptor and have
similar biological activities (2).
These cytokines are also involved in the development of
antibacterial and anti-inflammatory responses (2). In contrast, IL-17F is a weak inducer
of the expression of proinflammatory cytokines and it is produced
by a wide spectrum of immune cells (2).
Th17 lymphocytes also secrete interleukin (IL)-21
and IL-22. IL-21 enhances the expression of perforin, granzyme B,
IFN-γ, CXCR3 peripheral NK cells and CD8+ lymphocytes,
which reinforces an antitumor response (3). The expression of IL-22 inhibits TGF-β
(4,5). The protective role of IL-22 in
inflammatory diseases is associated with the induction of the
expression of β-defensins and lipocalin-2 (6,7).
In the present study, we assessed the proportion of
Th17 cells secreting IL-17A, IL-17F, IL-21 and IL-22 in peripheral
blood and in the microenvironment of benign, borderline (BOT) and
malignant epithelial ovarian tumors. We examined the relationship
between the percentage of
CD4+/IL-17A+F+,
CD4+/IL-21+ and
CD4+/IL-22+ Th17 cells in the peripheral
blood and ovarian tissues and the IL-17A serum concentrations. In
the present study, we investigated not only two isoforms, IL-17A
and IL-17F, but also IL-21 and IL-22. To clarify the percentage of
Th17 cells we conducted a study to determinate whether Th17 cells
and IL-17A may be applied as prognosticators in patients affected
by ovarian carcinomas.
Materials and methods
Research subjects and samples
The study group consisted of 60 women. The patients
were subdivided into 3 groups: a group of 24 women with malignant
epithelial ovarian tumors (cystadenocarcinoma), 25 women with
benign ovarian tumors (cystadenoma), and 11 women with serous
borderline tumors (BOTs). The control group consisted of 20 women
without ovarian pathology, undergoing surgery due to urinary
incontinence. All women with ovarian cancer had stage III or IV
tumors according to FIGO (International Journal of Gynecology and
Obstetrics, January, 2014). The study samples included blood serum,
peripheral blood, ovarian tissues without pathology and tissues of
malignant and benign tumors of the ovary. The study was approved by
the Bioethics Committee of the Medical University of Lublin
(KE-0254/90/2011). The patients gave their written consent before
they participated in the present study.
Isolation of mononuclear cells from
peripheral blood (PBMCs)
Immediately after bloood was taken from the
anticubital vein, peripheral blood mononuclear cells (PBMCs) were
isolated by density gradient centrifugation using Gradisol L
formulation at a specific density of 1.077 g/ml (Aqua Medica, Łódź,
Poland) for 20 min at 700 × g. The pellet containing the PBMCs was
washed twice in phosphate-buffered saline (PBS) and evaluated for
the number (using Neubauer chamber) and viability (trypan blue
staining-0.4% trypan blue solution; Sigma-Aldrich, Munich,
Germany). Viability of <95% was a disqualifying criteria for
conducting further research.
Isolation of mononuclear cells
infiltrating tumor and healthy tissues
During surgery, fragments of ovarian tumor not
containing necrotic areas (the size of 1 cm3) or healthy
ovarian tissue were collected and minced with a scalpel. The minced
tissue was suspended in 30 ml of RPMI-1640 medium (Biochrom,
Holliston, MA, USA) and subjected to digestion in a mixture
containing 1 mg/ml collagenase type IA, 1 mg/ml DNase type I, 0.1
mg/ml hyaluronidase (all from Sigma-Aldrich) at 37̊C for 60 min,
and constantly vortexed. After digestion, the suspension was
filtered through a strainer (70 µm; BD Biosciences, San Jose, CA,
USA) and centrifuged for 5 min at 700 × g. The cell suspension was
washed twice in RPMI-1640 medium.
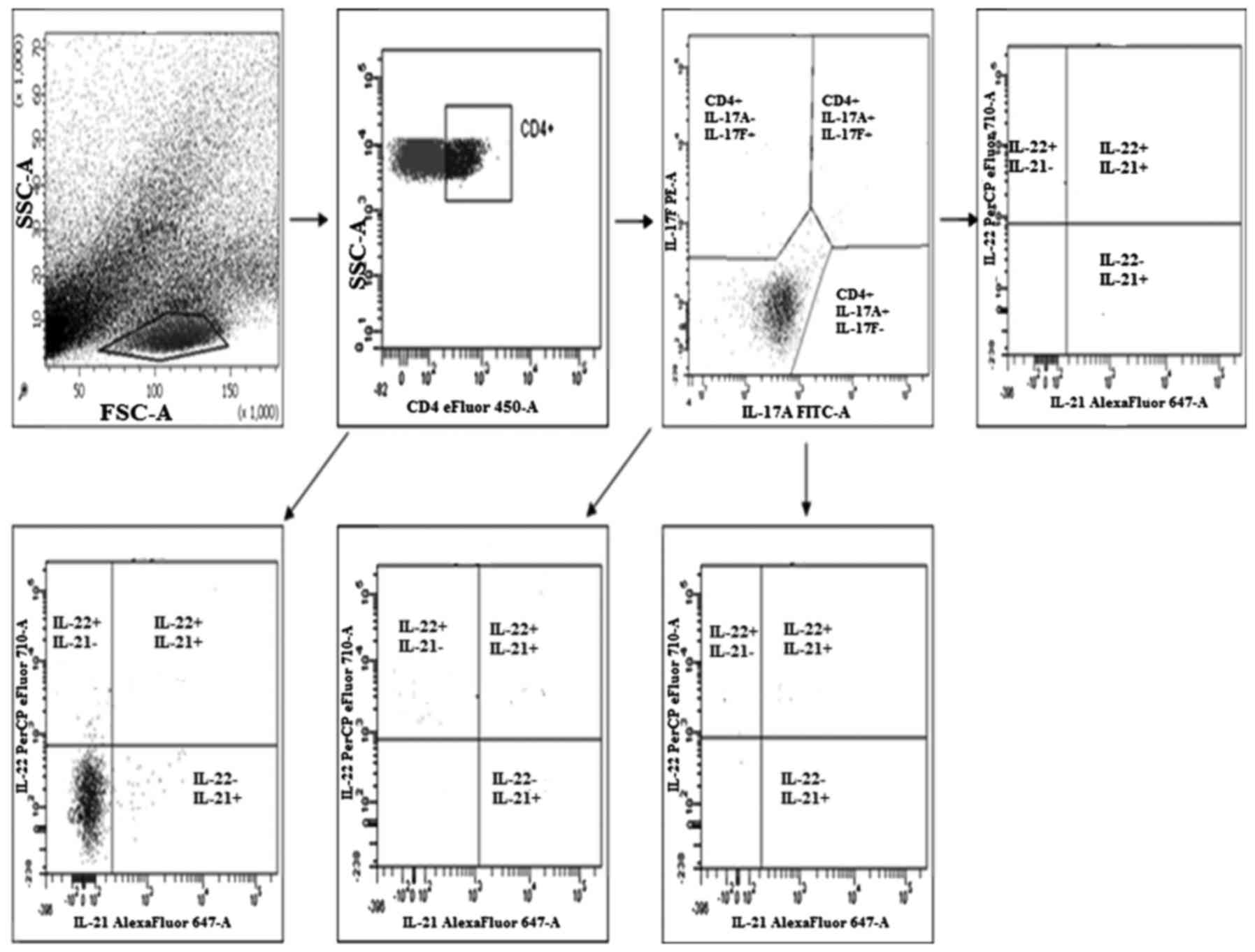
Evaluation of the percentage of Th17
cells
The evaluation of the percentage of Th17 cells
(secreting IL-17A, IL-17F, IL-21 and IL-22) in the PBMCs, healthy
tissue and tumor tissue was performed by flow cytometry using a
Th17 cytokine staining panel according to the manufacturer's
recommendations using a FACSCanto (both from BD Biosciences)
(Fig. 1).
Establishment of PBMC culture and
ovarian tissues (tumor or control) and stimulation with
ionomycin
A 24-h culture of the PBMCs and ovarian tissue was
set-up. The medium was prepared consisting of 97% RPMI-1640
(Biochrom) supplemented with 2mM L-glutamine, 2% human albumin (ZLB
Bioplasma, Bern, Switzerland), and antibiotics in an amount of 100
U/ml penicillin and 100 mg/ml streptomycin (both from
Sigma-Aldrich). Cultivation was carried out in 6-well plates in a
5-ml culture medium. For each patient, two cultures were
established, one from the PBMCs and the other from the tumor cells
or normal ovarian tissue. The culture was conducted in an incubator
under standard conditions (5% CO2, 95% humidity, 37̊C)
for 4 h. To individual wells, ionomycin at a concentration of 1
µg/ml and PMA at a concentration of 25 ng/ml were added to
stimulate cells for the production of cytokines and brefeldin at a
concentration 10 µg/ml (both from Sigma-Aldrich) in order to
inhibit the activity of the endoplasmic reticulum, leading to
retain the cytokines within the cell.
Determination of intracellular
cytokines
The 24-h cultures were moved from the culture plate
to two properly signed tubes and were washed twice in 2 ml of Flow
Cytometry Staining Buffer (eBioscience, San Diego, CA, USA) after
vortexing. The constant parameters used during each rinsing in this
procedure consisted of: run time 5 min, 700 × g. After removal of
the supernatant, 5 µl of anti-CD4 (eFluor 450; eBioscience) was
added to each tube. The mixture was incubated for 20 min in
darkness. After washing, the excess of antibody in 2 ml of Flow
Cytometry Staining Buffer, 100 µl IC Fixation Buffer (both from
eBioscience) was added to each tube in order to consolidate.
Mixing/vortexing, this mixture was also incubated for 20 min in
darkness. Then, it was washed twice with 2 ml of the
permeabilization buffer (eBioscience). Preparing the buffer
involved a 10-fold dilution with PBS. After removing the
supernatant, the cells were resuspended in 100 µl of
permeabilization buffer and separated to previously prepared
cytometry tubes into control and test samples. Thereafter, 5 µl of
the antibodies was added to the test samples: anti-IL-17A (FITC),
anti-IL-17F (PE), anti-IL-21 (eFluor 660), anti-IL-22 (PerCP-eFluor
710). After vortexing, all samples were incubated for 20 min in
darkness. They were washed twice. After the cells were suspended in
the Flow Cytometry Staining Buffer, cytometric analysis was carried
out. Analysis was performed using flow cytometric 8-channel BD
FACSCanto II (BD Biosciences, San Diego, CA, USA). Measurements
were performed using BD FACSDiva software.
Evaluation of the IL-17A cytokine
level
The concentration of IL-17A was determined by ELISA.
Quantikine® Human IL-17 ELISA kit (cat. no. D1700;
R&D Systems, Minneapolis, MN, USA) was applied. The procedure
was performed according to the manufacturer's instructions, and an
automatic Victor3 reader (PerkinElmer, Inc., Waltham, MA, USA) was
utilized.
Statistical analysis
Non-parametric and Kruskal-Wallis tests were used to
verify the differences between the studied groups, post hoc (Dunn)
to assess the internal differences between 2 groups, Wilcoxon
matched pairs signed-rank for comparing the value of the parameter
pairs and Spearman's rank correlation coefficient and its validity
to assess the correlation between the two parameters. Kaplan-Meier
analysis was used to compare survival curves depending on the range
of the percentage of CD4+/IL-17+ and
concentration of IL-17A. Overall survival was defined as the
interval between the date of surgery and the last follow-up or date
of death. P-values <0.05 were considered significant.
Statistical analysis was performed using 10.0 PL Statistics for
Windows (StatSoft, Inc., Tulsa, OK, USA).
Results
Study group
There was no differences in age (p=0.4), body mass
index (BMI) (p=0.053), gravidity (p=0.46) or the concentration of
leukocytes in the peripheral blood (p=0.29) of all the study groups
(Table I).
 | Table I.Demographic and clinical
characteristics of the study groups. |
Table I.
Demographic and clinical
characteristics of the study groups.
| Group of
patients | Controls group
(n=20) | Benign tumors
(n=25) | Borderline tumors
(n=11) | Ovarian cancers
(n=24) | P-value |
|---|
| Age (years) | 50 (37–78) | 55 (32–85) | 47 (35–77) | 55 (44–80) | 0.400 |
| BMI
(kg/m2) | 28 (21–36.7) | 26 (11.9–46.7) | 31.6
(17.9–45.7) | 25.6 (25–35.2) | 0.053 |
| Gravidity (n) | 2 (0–4) | 2 (0–7) | 2 (0–5) | 2 (0–6) | 0.460 |
| Leukocytes
103 cells/µl | 7.2
(4.17–11.8) | 5.8 (3–21) | 7.2 (5–10.8) | 7.3
(3.58–13.5) | 0.290 |
Assessment of Th17 lymphocyte
subpopulation in the peripheral blood
The number of Th17 cells in peripheral blood was
decreased in the ovarian cancer patients as compared to the other
study groups. We found no significant differences in the percentage
of CD4+/IL-17A+/IL-17F− (p=0.5)
CD4+/IL-17A−/IL-F+ (p=0.8),
CD4+/IL-17A+/IL-F+ (p=0.32) and
CD4+/IL-17+* (p=0.4) Th17 cells in the
peripheral blood among the study groups (Table II).
*CD4+/IL-17+ is the sum of the percentages of
CD4+/IL-17A+/IL-17F−,
CD4+/IL-17A−/IL-17F+ and
CD4+/IL-17A+/IL-17F+ Th17
cells.
 | Table II.Percentage of CD4+ Th17
cells in the peripheral blood of the study groups. |
Table II.
Percentage of CD4+ Th17
cells in the peripheral blood of the study groups.
| Groups | n |
IL-17A+/IL-17F− |
IL-17A−/IL-17F+ |
IL-17A+/IL-17F+ |
|---|
| Control group | 20 | 0.5 (0–2.6) | 0.6 (0–3.3) | 0.6 (0,20.0) |
| Benign tumors | 25 | 0.5 (0–8.7) | 0.45 (0–8.7) | 0.75 (0–30.4) |
| Borderline
tumors | 11 | 0.55 (0.2–2.0) | 0.4 (0–0.8) | 0.65
(0,1-10.1) |
| Ovarian
cancers | 24 | 0.4 (0–1.7) | 0.2 (0–14.1) | 0.2 (0–4.0) |
Percentage of Th17 cells in the
ovarian tumor tissue
The amount of Th17 cells in ovarian tissue was
slightly increased in the ovarian cancer patients compared to that
noted in the other groups. There were no significant differences in
the percentage of
CD4+/IL-17A+/IL-F− (p=0.2),
CD4+/IL-17A−/IL-F+ (p=0.2),
CD4+/IL-17A+/IL-F+ (p=0.7) and
CD4+/IL17+* (p=0.5) among the group of
patients (Table III).
*CD4+/IL-17+ is the sum of the percentages of
CD4+/IL-17A+/IL-17F−,
CD4+/IL-17A−/IL-17F+ and
CD4+/IL-17A+/IL-17F+ Th17
cells.
 | Table III.Percentage of Th17 cells in ovarian
tissues of the study groups. |
Table III.
Percentage of Th17 cells in ovarian
tissues of the study groups.
| Groups | n |
IL-17A+/IL-17F− |
IL-17A−/IL-17F+ |
IL-17A+/IL-17F+ |
|---|
| Control group | 20 | 0.15 (0–4.2) | 0 (0–3.3) | 21.9
(0.1–52.5) |
| Benign tumors | 25 | 1.3 (0–7.0) | 0.25 (0–11.8) | 13.2
(0.2–64.9) |
| Borderline
tumors | 11 | 0.1 (0- 3.9) | 1.0 (0–4.5) | 7.7 (0.4–31.2) |
| Ovarian
cancers | 24 | 0.8 (0–10) | 1.5 (0–14.3) | 14.1
(0.2–46.2) |
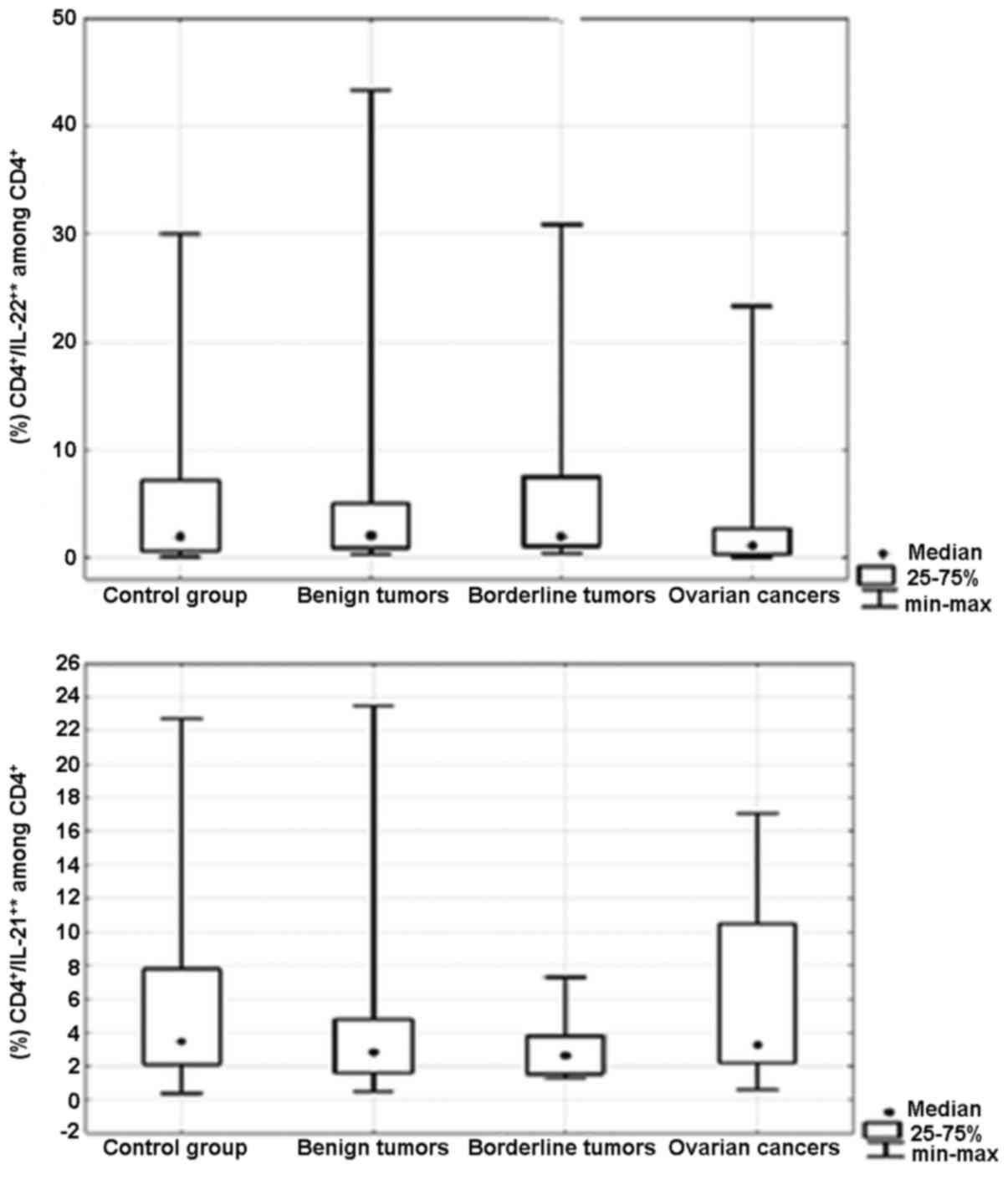
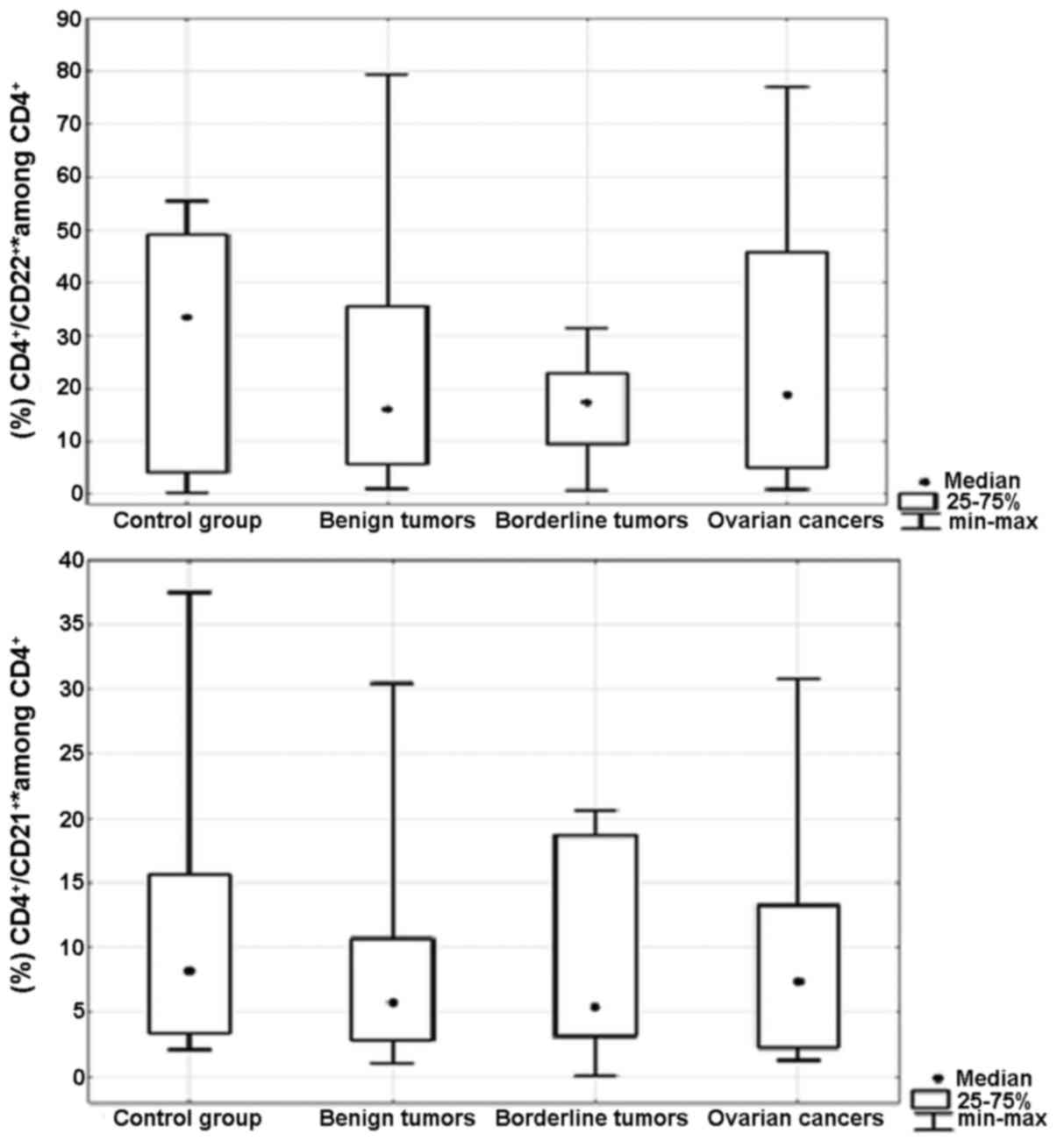
Percentage of Th17 cells secreting
IL-21 and/or IL-22 in the peripheral blood and ovarian tumor
tissues
There were no significant differences in the
percentage of CD4+/IL-21+* (p=0.5) and
CD4+/IL-22+* (p=0.5) Th17 cells in the
peripheral blood between the groups of patients (Fig. 2). Moreover, we did not detect
significant differences in the percentages of
CD4+/IL-21+* (p=0.7) and
CD4+/IL-22+* (p=0.8) Th17 cells in the
ovarian tissue between the groups of patients (Fig. 3). *CD4+/IL-21+
is the sum of the percentages of
CD4+/IL-21+/IL-22− and
CD4+/IL-21+/IL-22+ Th17 cells.
*CD4+/IL-22+ is the sum of the percentages of
the CD4+/IL-21−/IL-22+ and
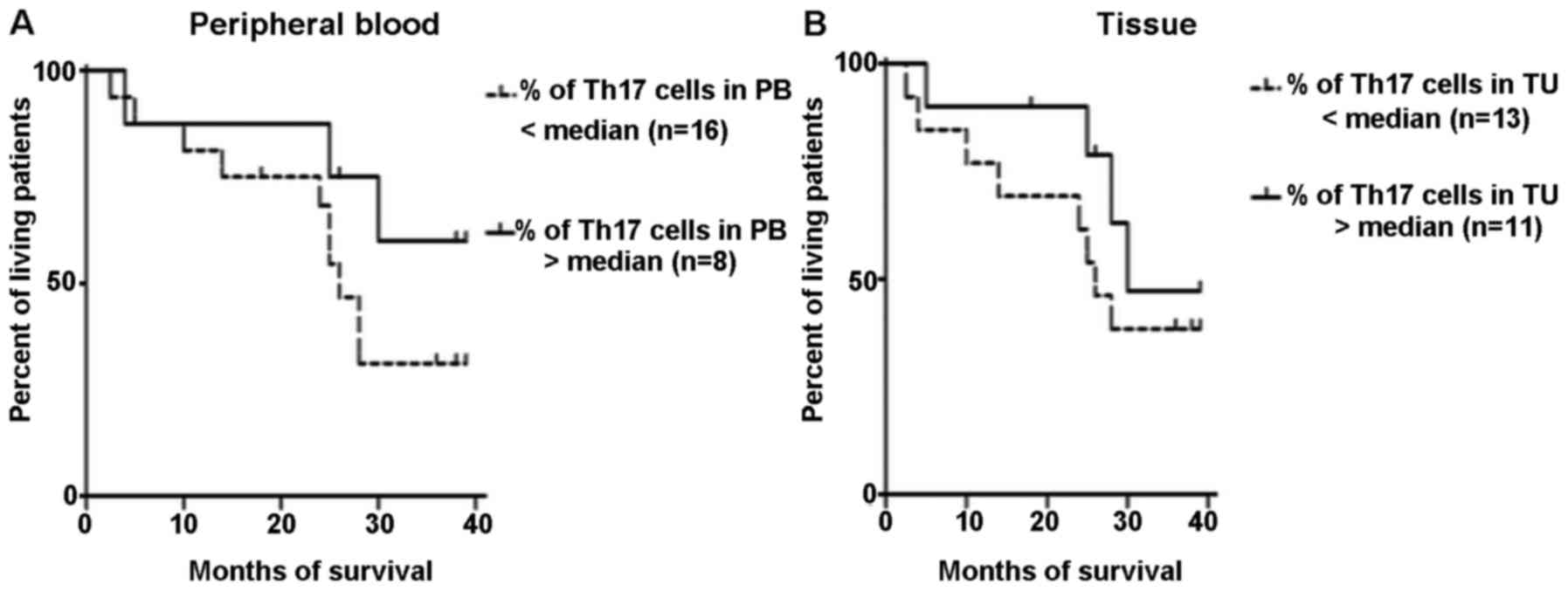
CD4+/IL-21+/IL-22+ Th17 cells. The
percentage of Th17 lymphocytes in peripheral blood and ovarian
cancer tissues was next evaluated as a prognostic factor. The
Kaplan-Meier survival analysis was performed in a group of patients
with ovarian cancer. The patients were subdivided into 2 groups
according to the median specificity and sensitivity: >1.6 or
<1.6% in the peripheral blood, and >16.3 and <16.3% in
ovarian cancer tissue. The result of the Mantel-Cox test was
insignificant in regards to patient survival as dependent on the
percentage of Th17 cells in the peripheral blood (p=0.19) and in
tissue ovarian cancer (p=0.35) (Fig. 4A
and B).
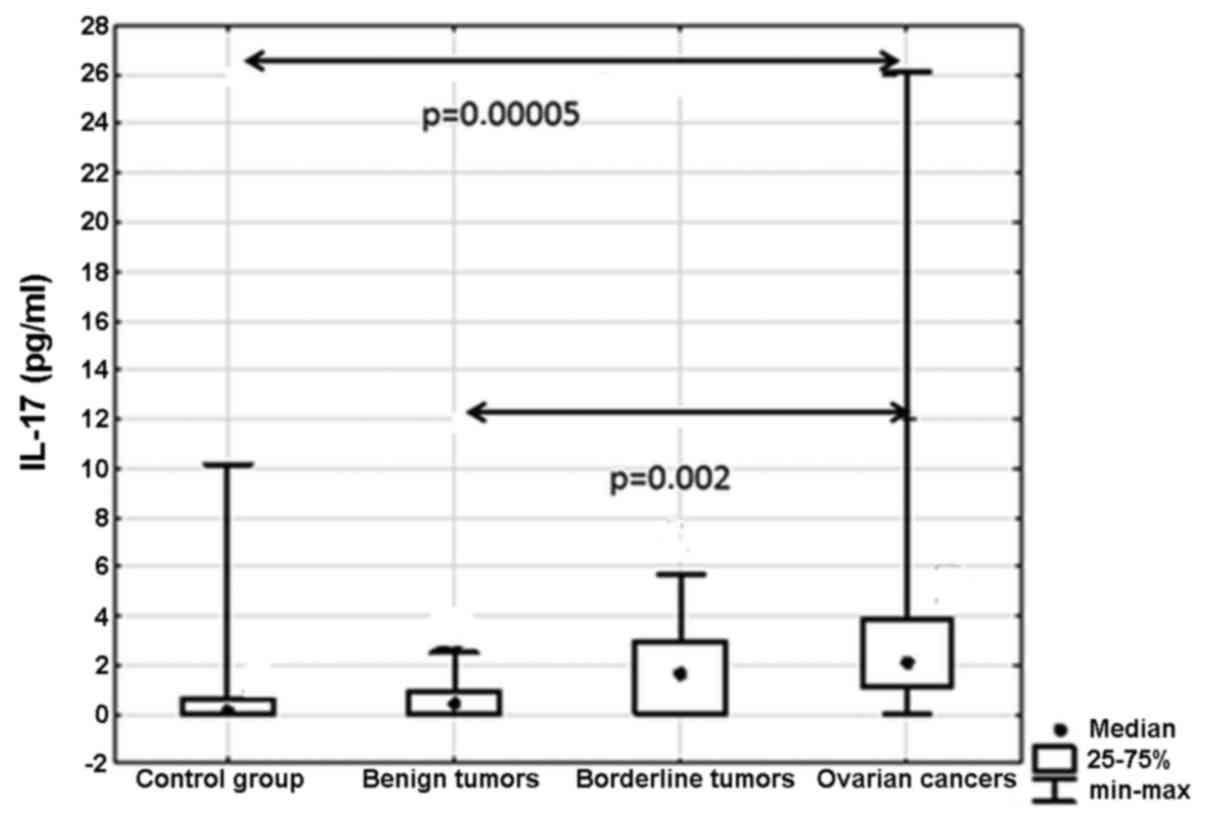
Concentration of interleukin-17A
(IL-17A)
Our results showed statistically significant
differences between the analyzed groups (p=0.001) (Fig. 5). Post hoc analysis showed
significantly higher concentrations of IL-17A in women with ovarian
cancer compared to the group without ovarian pathology (p=0.00005)
as well as that between patients with malignant and benign ovarian
tumors (p=0.002).
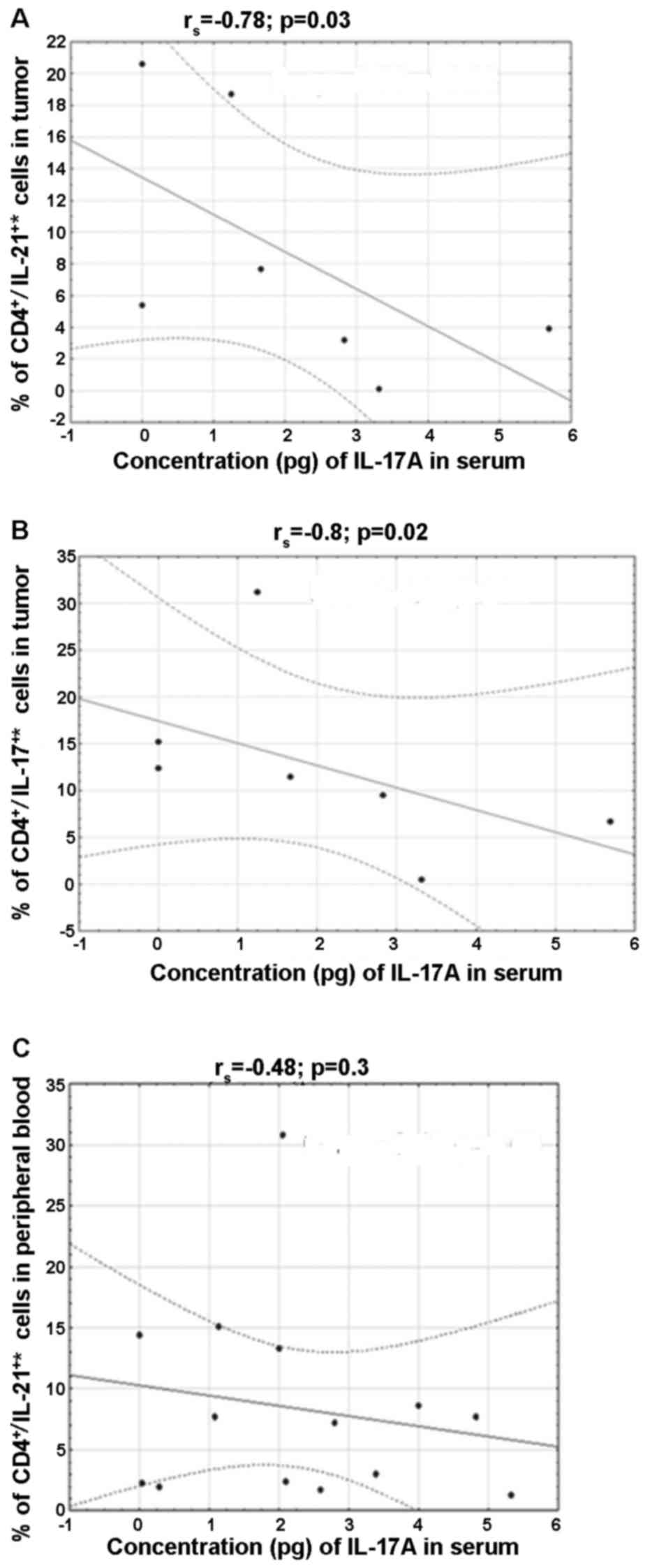
Relationship between IL-17A and Th17
cell subpopulations secreting CD4+/IL-17+*,
CD4+/IL-21+ and
CD4+/IL-22+ in peripheral blood and in the
tissues of ovarian tumors
A negative correlation was found in the percentage
of CD4+/IL-21+* (rs=−0.8, p=0.02)
and CD4+/IL-17+* (rs=−0.78,
p=0.03) in the tissue and IL-17A in blood serum of patients with
borderline tumors. Moreover, a negative correlation was shown
between IL-17A and the percentage of
CD4+/IL-21+* in peripheral blood
(rs=−0.48, p=0.03) in the group of patients with ovarian
cancer (Fig. 6).
*CD4+/IL-21+ is the sum of the percentages of
CD4+/IL-21+/IL-22− and
CD4+/IL-21+/IL-22+ Th17 cells.
*CD4+/IL-17+ is the sum of the percentages of
CD4+/IL-17A+/IL-17F−,
CD4+/IL-17A−/IL-17F+ and
CD4+/IL-17A+/IL-17F+ Th 17
cells
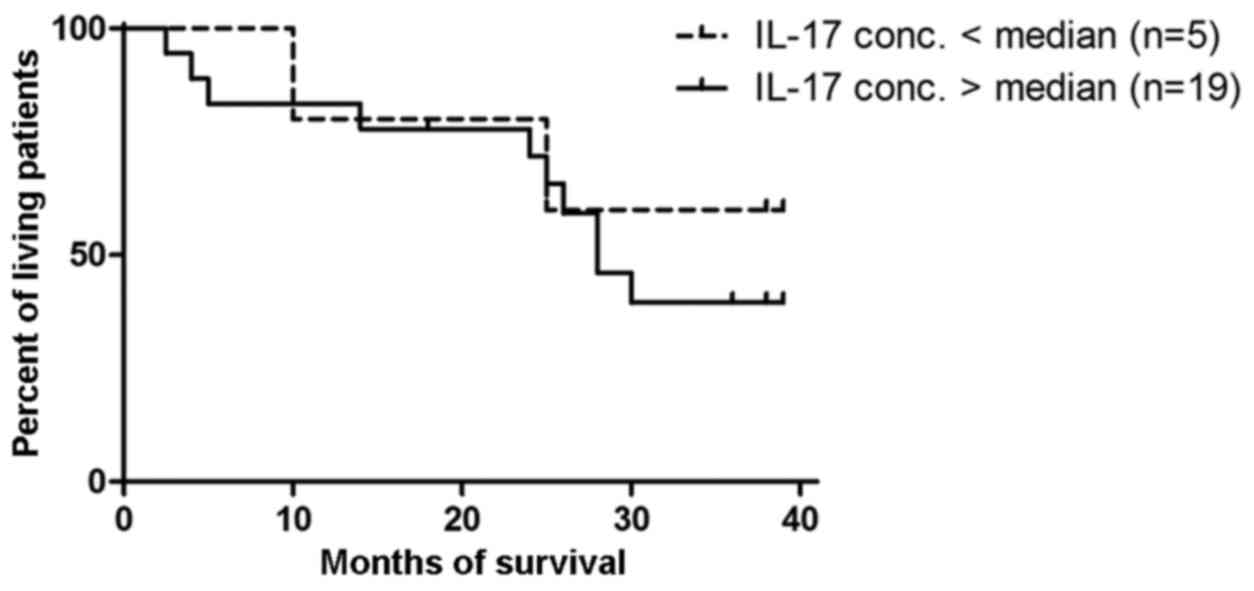
Prognostic value of IL-17A in ovarian
cancer patients
The patients were divided into 2 groups according to
the median value of IL-17A (5 women with >0.87 pg/ml and 19
women with <0.87 pg/ml). The result of the Mantel-Cox test was
not significant (p=0.6) (Fig.
7).
Discussion
The multiparameter analysis of cytokines secreted by
Th17 cells has not been previously performed. Currently we focused
on the determination of the capacity of these cells to secrete
IL-17 with separation into isoforms. Moreover, we assessed not only
two isoforms of IL-17 (A and F), but also IL-21 and IL-22.
The phenotypes, distribution in tissues and profile
of the secreted cytokines in ovarian tumors have not yet been fully
explored. We found that the distribution of the different
subpopulations of Th17 lymphocytes
(CD4+/IL-17A+/IL-F−;
CD4+/17A−/IL-F+,
CD4+/IL-17A+/IL-F+,
CD4+/IL-17+,
CD4+/IL-21+ and
CD4+/IL-22+) in peripheral blood in
non-malignant, borderline (BOT) and malignant tumor tissues were
not significantly different in women compared to patients without
ovarian pathology. There were no significant differences between
these parameters in terms of Th17 cell proportion, although, a
higher concentration of Th17 cells in the tissue was observed,
similarly to Kryczek et al (9). The authors demonstrated that the
percentage of Th17 cells in the CD4 lymphocytes in tumor tissue was
significantly higher compared to peripheral blood. The increased
concentration of Th17 in a tissue and their migration to the tumor
may be associated with a high expression of CXCR4, CCR6 and CD161
(9). Moreover, it is important to
know the functions and interactions beetwen IL-17F and IL-17A in
the neoplastic microenviroment at both the molecular and cellular
levels. Understanding these relationships may provide a new
strategy for systemic anticancer therapy, particularly for ovarian
cancer patients.
Kryczek et al (9) also analyzed the relationship between
the percentage of Th17 cells and other immune cell subpopulations.
They observed an inverse correlation between Th17 and Treg
lymphocytes. Tregs have high expression of CD39 (ectonucleotidase),
which converts ATP into adenosine. They suggested that the
development of Th17 cells is inhibited by Tregs through the
adenosinergic pathway. Ye et al (10) noted that the cells from each line of
development may be interconverted in the tumor microenvironment by
acquiring different functions. Th17 lymphocytes that transform into
IFN-γ+FOXP3+ T cells acquire potent
immunosuppressive properties. This conversion is possibly part of
the tumor escape strategy against cells of the immune system.
Fialová et al (11) found an
increased recruitment of Th17 cells in women at the early clinical
stages of ovarian cancer, while the decreased migration of Tregs
was observed in patients affected by advanced disease.
Miyahara et al (12) suggested that a low concentration of
Th17 cells, and a high concentration of Tregs is associated with
elevated concentrations of TGF-β cytokines in the tumor. They found
that tumor cells secrete large amount of the latent form of TGF-β
(inactive). However, the level of the active form of TGF was very
low due to its short half-life. In addition, TGF-β may be present
in the free form and in the form of membrane-bound Tregs. Increased
percentage of Tregs and reduced Th17 cells in the tumor supported
the view that these cells mutually regulate the presence of other
cytokines in the tumor microenvironment (8,11).
However, the molecular mechanisms underlying the formation and
mutual regulation of Treg and Th17 cells in the tumor
microenvironment remains unknown.
There is increasing evidence suggesting that Th17
cells protect against cancer development in several manners.
Firstly, tumor-infiltrating Th17 cells express several effector
cytokines, similar to those detected in patients affected by
infectious diseases. This suggests that Th17 cells infiltrating the
tumor may be functionally similar to T effector cells. In
accordance with this possibility, Th17 cells are negatively
correlated with the presence of Tregs and are positively correlated
with effector cells secreting IFN-γ, cytotoxic CD8+ and
NK cells, in the same tumor microenvironment. Secondly, Muranski
et al reported a protective role of Th17 cells during
carcinogenesis (13). Transgenic T
cells with a Th17 phenotype after treatment with TGF-β and IL-6
induced tumor eradication in mice. Furthermore, mice deficient in
IL-17 showed accelerated tumor growth and lung metastases in
several cancer models. Tumor-infiltrating Th17 lymphocytes do not
produce granzyme B and perforin; therefore, Th17 cells do not act
as intermediaries in direct cytotoxic activity against tumor cells
(3). Instead, Th17 cells recruit
other effector cells of the immune system. According to this
hypothesis, IL-17 and INF-γ derived from Th17 cells synergistically
induce the production of CXCL9 and the chemokine CXCL10 by tumor
cells, which in turn promotes the migration of effector T
lymphocytes into the compartment (3). CXCL9 and CXCL10 levels were found to
be directly correlated with the number of CD8+
tumor-infiltrating and NK cells. Th17 lymphocytes stimulate tumor
cells to secrete CCL20, which recruits dendritic cells into the
tumor microenvironment. These data strongly support the view that
Th17 cells play an indirect role in antitumor immunity through the
promotion of effector T cells, NK and dendritic cells (7).
There is strong evidence demonstrating the role of
IL-17 secreted by Th17 cells in the promotion of carcinogenesis.
IL-17 induces IL-6 production by tumor cells and stromal cells.
IL-6 activates STAT3, which increases the level of genes
facilitating tumor progression and the development of metastasis
(7). Tartour et al (14) reported that transfection with IL-17
of human cervical cancer cells increased tumor growth when
transplanted into nude mice. In addition, mice lacking IL-17
demonstrated reduced tumor growth of B16 melanoma and MB49 bladder
cancer, suggesting a role of IL-17 in promoting tumor growth
(14).
Kryczek et al (9) also demonstrated a correlation between
the percentage of Th17 cells and patient survival time. In patients
whose peritoneal fluid contained high concentrations of IL-17, the
average survival time was 78 months. In turn, patients with
decreased levels of IL-17 in peritoneal fluid lived significantly
shorter (27 months). Opposite data were published by Lan et
al (15) who found that high
IL-17 expression was correlated with improved progression-free
survival in advanced stage ovarian cancer patients. No significant
difference was observed in overall survival between the high and
low IL-17 expression groups (15).
In the present study, the decreased percentage of Th17 cells
(CD4+/IL-17+*) in the tumor microenvironment
did not correlate with a reduced survival time of patients affected
by ovarian cancer, probably due to advanced stage of the ovarian
cancer group.
The next step of the research was to assess the
proportion of Th17 lymphocytes secreting IL-21 and IL-22. The
percentages of CD4+/IL-21+/IL-22−,
CD4+/IL-21−/IL-22+,
CD4+/IL-21+/IL-22+,
CD4+/IL-21+ and
CD4+/IL-22+ Th17 cells in peripheral blood
and tissue did not significantly differ among the groups. Moreover,
a significant correlation was found between
CD4+/IL-21+ in peripheral blood and IL-17A in
the group of women with ovarian cancer. IL-21 is produced
predominantly by Th17 and NKT cells. In cooperation with TGF-β it
induces the differentiation of T cells towards the Th17 phenotype.
It also participates in the mutual regulation of Th17 and Treg
cells. In certain circumstances, IL-21 may exert anti-inflammatory
effects due to its ability to inhibit dendritic cell maturation and
stimulation of IL-10 (15). In this
context, IL-21 stimulates an immune response against tumor cells
and promotes a CD8+ T cell response against viruses
(16,17). Immunostimulatory activity of IL-21
may be possibly applied as a potential immunotherapeutic agent for
the treatment of human cancers. Neutralization of IL-22 may reduce
metastasis, chemoresistance, and inflammation associated with
cancer. Given that IL-2 BP is a specific natural antagonist of
IL-22, it may be a prime candidate as an anti-IL-22 therapy.
Anti-TNF drugs such as adalimumab, etanercept and infliximab
transiently decrease IL-22 expression, likely since Th22 cells
depend on TNF for differentiation. The anti-IL-6 antibody
toclizumab may also suppress the differentiation of both Th17 and
Th22 cells. A neutralizing antibody against IL-12p40, ustekinumab,
is able to target both IL-12 and IL-23 and therefore prevent the
differentiation of Th1, Th17 and Th22 cells, eliminating sources of
IL-22 (18–20). However, the nature and clinical
relevance of IL-22+ cells is poorly defined in patients
affected by ovarian cancer.
Xiang et al (21) reported that IL-17 contributed to
ovarian cancer malignancy by promoting the self-renewal of
CD133+ cancer stem cells and IL-17. They showed by
recombinant human IL-17 stimulation and IL-17 transfection that the
growth and sphere formation capacities of CD133+ ovarian
cancer stem cells were significantly enhanced in a dose-dependent
manner increasing the tumorigenesis capacity in nude mice. These
data suggest that the IL-17 signaling pathway may serve as a
therapeutic target for patients with ovarian cancer. Kryczek et
al (22) also revealed a
relationship between a key transcription factor (STAT3) and an
important epigenetic marker (H3K79) in determining cancer stemness
and tumorigenesis.
Our previous data revealed that the percentage of
Th17 cells in peritoneal fluid (PF) corresponds with the severity
of endometriosis (23). The
percentage of Th17 lymphocytes in PF was significantly higher in
patients with moderate/severe endometriosis compared to patients
with a minimal/mild form of the disease (23). Continuing with our research we
demonstrated a higher percentage of Th17 cells in tissue than in
peripheral blood and a negative correlation between
CD4+/IL-17+ lymphocytes and the concentration
of IL-17A in patients with ovarian cancer, thus that IL-21 could
adjust Th17 differentiation. The generation of Th17 cells in the
conventional manner is attenuated by blocking IL-21. This cytokine
is capable of acting on Th17 cells in an autocrine manner in
response to antigen stimulation (24).
In conclusion, we showed that more Th17 cells
secreted IL-17A and IL-21 in the tissues of borderline ovarian
tumors and less IL-17A in serum. We also observed that in
peripheral blood of patients with ovarian cancer, a higher
percentage of Th17 lymphocytes was negatively correlated with a
lower concentration of IL-17A in serum. An increased percentage of
Th17 cells in ovarian tissue did not influence the survival time of
patients with ovarian cancer.
Acknowledgements
The permission to conduct the present study was
obtained from the Bioethics Committee of the Medical University in
Lublin (no. KE-0254/90/2011). Funding for the present study was
supported by the grant MNsd 410/2011.
Glossary
Abbreviations
Abbreviations:
|
BOT
|
borderline tumor
|
|
CCR6
|
CC chemokine receptor
|
|
CCL20
|
CC chemokine ligand 20
|
|
CXCR3
|
CXC chemokine receptor
|
|
IL
|
interleukin
|
|
NK cells
|
natural killer cells
|
|
TGF-β
|
transforming growth factor-β
|
|
Tregs
|
lymphocyte T regulatory cells
|
|
Th17 lymphocytes
|
T helper 17 lymphocytes
|
References
|
1
|
Romagnani S: Human Th17 cells. Arthritis
Res Ther. 10:2062008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwakura Y, Nakae S, Saijo S and Ishigame
H: The roles of IL-17A in inflammatory immune responses and host
defense against pathogens. Immunol Rev. 226:57–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Søndergaard H and Skak K: IL-21: Roles in
immunopathology and cancer therapy. Tissue Antigens. 74:467–479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geboes L, Dumoutier L, Kelchtermans H,
Schurgers E, Mitera T, Renauld JC and Matthys P: Proinflammatory
role of the Th17 cytokine interleukin-22 in collagen-induced
arthritis in C57BL/6 mice. Arthritis Rheum. 60:390–395. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zenewicz LA and Flavell RA: IL-22 and
inflammation: Leukin' through a glass onion. Eur J Immunol.
38:3265–3268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin-Orozco N, Muranski P, Chung Y, Yang
XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW and Dong C: T
helper 17 cells promote cytotoxic T cell activation in tumor
immunity. Immunity. 31:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Yi T, Kortylewski M, Pardoll DM,
Zeng D and Yu H: IL-17 can promote tumor growth through an
IL-6-Stat3 signaling pathway. J Exp Med. 206:1457–1464. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kryczek I, Banerjee M, Cheng P, Vatan L,
Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, et
al: Phenotype, distribution, generation, and functional and
clinical relevance of Th17 cells in the human tumor environments.
Blood. 114:1141–1149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye J, Su X, Hsueh EC, Zhang Y, Koenig JM,
Hoft DF and Peng G: Human tumor-infiltrating Th17 cells have the
capacity to differentiate into IFN-γ+ and
FOXP3+ T cells with potent suppressive function. Eur J
Immunol. 41:936–951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fialová A, Partlová S, Sojka L, Hromádková
H, Brtnický T, Fučíková J, Kocián P, Rob L, Bartůňková J and Spíšek
R: Dynamics of T-cell infiltration during the course of ovarian
cancer: The gradual shift from a Th17 effector cell response to a
predominant infiltration by regulatory T-cells. Int J Cancer.
132:1070–1079. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyahara Y, Odunsi K, Chen W, Peng G,
Matsuzaki J and Wang RF: Generation and regulation of human
CD4+ IL-17-producing T cells in ovarian cancer. Proc
Natl Acad Sci USA. 105:15505–15510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muranski P, Boni A, Antony PA, Cassard L,
Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K,
et al: Tumor-specific Th17-polarized cells eradicate large
established melanoma. Blood. 112:362–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tartour E, Fossiez F, Joyeux I, Galinha A,
Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V,
et al: Interleukin 17, a T-cell-derived cytokine, promotes
tumorigenicity of human cervical tumors in nude mice. Cancer Res.
59:3698–3704. 1999.PubMed/NCBI
|
|
15
|
Lan C, Huang X, Lin S, Huang H, Cai Q, Lu
J and Liu J: High density of IL-17-producing cells is associated
with improved prognosis for advanced epithelial ovarian cancer.
Cell Tissue Res. 352:351–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brandt K, Bulfone-Paus S, Foster DC and
Rückert R: Interleukin-21 inhibits dendritic cell activation and
maturation. Blood. 102:4090–4098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veldhoen M, Hirota K, Westendorf AM, Buer
J, Dumoutier L, Renauld JC and Stockinger B: The aryl hydrocarbon
receptor links TH17-cell-mediated autoimmunity to
environmental toxins. Nature. 453:106–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huber S, Gagliani N, Zenewicz LA, Huber
FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, et
al: IL-22BP is regulated by the inflammasome and modulates
tumorigenesis in the intestine. Nature. 491:259–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabat R, Ouyang W and Wolk K: Therapeutic
opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov.
13:21–38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caproni M, Antiga E, Melani L, Volpi W,
Del Bianco E and Fabbri P: Serum levels of IL-17 and IL-22 are
reduced by etanercept, but not by acitretin, in patients with
psoriasis: A randomized-controlled trial. J Clin Immunol.
29:210–214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiang T, Long H, He L, Han X, Lin K, Liang
Z, Zhuo W, Xie R and Zhu B: Interleukin-17 produced by tumor
microenvironment promotes self-renewal of CD133+ cancer
stem-like cells in ovarian cancer. Oncogene. 34:165–176. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kryczek I, Lin Y, Nagarsheth N, Peng D,
Zhao L, Zhao E, Vatan L, Szeliga W, Dou Y, Owens S, et al:
IL-22+CD4+ T cells promote colorectal cancer
stemness via STAT3 transcription factor activation and induction of
the methyltransferase DOT1L. Immunity. 40:772–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gogacz M, Winkler I, Bojarska-Junak A,
Tabarkiewicz J, Semczuk A, Rechberger T and Adamiak A: Increased
percentage of Th17 cells in peritoneal fluid is associated with
severity of endometriosis. J Reprod Immunol. 117:39–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei L, Laurence A, Elias KM and O'Shea JJ:
IL-21 is produced by Th17 cells and drives IL-17 production in a
STAT3-dependent manner. J Biol Chem. 282:34605–34610. 2007.
View Article : Google Scholar : PubMed/NCBI
|