Introduction
Among patients with bladder cancer, ~75% are
diagnosed with non-muscle invasive bladder cancer (NMIBC) which has
a much more favorable prognosis than muscle-invasive bladder cancer
(1,2). Transurethral resection (TUR) has been
shown to be an efficient and safe method for the treatment of
NMIBC. However, NMIBC recurs in ~70% of patients post-TUR, and
requires lifelong treatment (3–6).
Although, the recurrence rate of NMIBC is significantly decreased
by TUR in combination with intravesical chemotherapy, the rate of
cancer progression is not diminished (7–12).
Preventing recurrence of NMIBC after TUR remains challenging
(13–15).
Over recent decades, there has been an increasing
interest in the employment of hyperthemia at 40̊C and above in the
treatment of malignant tumors (13,16,18–20).
Hyperthermia may increase chemotherapeutic drug uptake, influence
the intracellular distribution and metabolism of drugs, and/or
inhibit repair of DNA damage in neoplastic cells, thus, improving
the effect of chemotherapeutic agents (13,16,20).
Mitomycin C (MMC) as an alkylating cytostatic agent is commonly
used to treat NMIBC. Local administration to the bladder can be
effective without causing high concentrations in the serum, and
thus, causing only mild side-effects (8,12,14,16,17).
Numerous clinical trials have shown that combining MMC
administration with hypothermia can improve tumor ablation and
prevent recurrence post-TUR. Numerous studies have demonstrated
that the thermal enhancement of MMC killing was greater in hypoxic
cells than in aerobic cells. As bladder cancer can be hypoxic, this
provides a very strong rationale for the use of MMC in combination
with hyperthermia (21).
Bladder intracavitary hyperthermic perfusion
chemotherapy (BHPC), the combination of local hyperthermia with
chemotherapy, can significantly improve the effect of intracavity
chemotherapy, and is reported to have an additive or supra-additive
effect, preventing NMIBC recurrence post-TUR (13–16,18–20).
However, these previous conventional trials were based on hot
water-, microwave- or radiofrequency-induced local hyperthermia,
either individually or in combination with cytostatic agents, which
could maintain continuous and stable treatment temperatures, but
lack effective temperature control or regulation, and cannot
provide uniform distribution of heat to the bladder. These
inadequate devices for local heat delivery lack appropriate methods
for monitoring temperature at the targets, limiting the application
of hyperthermia enhanced therapy for cancer in clinical practice.
Therefore, it is necessary to both continuously and stably deliver
heat to bladder neoplastic sites while avoiding causing damage to
surrounding normal tissues. In addition, the pharmacokinetics of
chemotherapeutic agents during thermochemotherapy is also not well
understood.
Over the past 6 years, we performed continuous
circulatory hyperthermic intraperitoneal perfusion chemotherapy for
malignant ascites using a novel ‘BR-TRG-I type high-precision
hyperthermic intraperitoneal perfusion treatment system’ which can
precisely maintain the temperature and perfusion rate of the drug
solution, with satisfactory outcomes (22–25).
However, conductive BHPC for bladder cancer has been rarely
reported. As the bladder wall can be easily heated by irrigation
with a hot isotonic fluid, bladder tumors are particularly suitable
for treatment with perfusion hyperthemia (16,18,19).
In this prospectively randomized controlled study we performed
conductive BHPC for NMIBC after TUR and evaluated its efficacy and
safety.
Materials and methods
Patients
Patients with NMIBC who received TUR were
prospectively recruited between December 2006 and December 2014 at
the Intracelom Hyperthermic Perfusion Therapy Center of Guangzhou
Medical University Cancer Hospital (Guangzhou, China). Inclusion
criteria were: i) age ≥18 years; ii) with single or double NMIBC
sites; iii) having not received radiation therapy in the 4 weeks
preceding enrollment; iv) having not received intravesical
chemotherapy in the 4 weeks preceding enrollment. Exclusion
criteria were: i) known or possible bladder tumors metastasized
from other organs; ii) known or possible malignant bladder tumors
expanding through the serosa, invading locally or metastasizing
into other internal organs; iii) T0 and Tis
stage bladder cancer, determined by urinary cystoscopic
observation; iv) with NMIBC unsuitable for TUR; v) known or
potential pregnancy; vi) active inflammation or infection; vii)
other tumors. The present study was approved by the Medical Ethics
Committee of the Affiliated Cancer Hospital and Institute of
Guangzhou Medical University (Guangzhou, China) (no.
GZMCY20080825). Written informed consent was obtained from all
patients.
The clinical stages of NMIBC were assessed by
cystoscopic observation of the resected tumor biopsies and
determined according to the location, size and invasion depth of
tumors using the TNM classification of malignant tumors (26). Bladder tumor expansion through the
serosa or distal metastasis was ascertained by chest x-ray,
computerized tomography (CT) or magnetic resonance imaging (MRI)
examination.
TUR
Under epidural anaesthesia, the bladder tumors of
the patients were examined by cystoscopic observation, and then
underwent TUR. The tumor profile and safe margin were initially
defined, and resection was then performed carefully, avoiding
bladder perforation and excessive bladder wall distention. After
removal of the bulging mass, hemostasis was achieved in the base
lesions and surrounding mucosa, carefully with grasp and bite as
previously reported (4–6).
After TUR, 24 F 3- or 2-way Foley catheters were
introduced into the bladder cavity for BHPC or intravesical
chemotherapy alone, respectively and warm saline was then injected
into the sac to fix the catheter in the bladder cavity. Resected
specimens were reviewed by a pathologist to evaluate the depth of
resection, and the histological characteristics of specimens were
assessed following hematoxylin and eosin (H&E) staining
post-treatment.
BHPC procedure
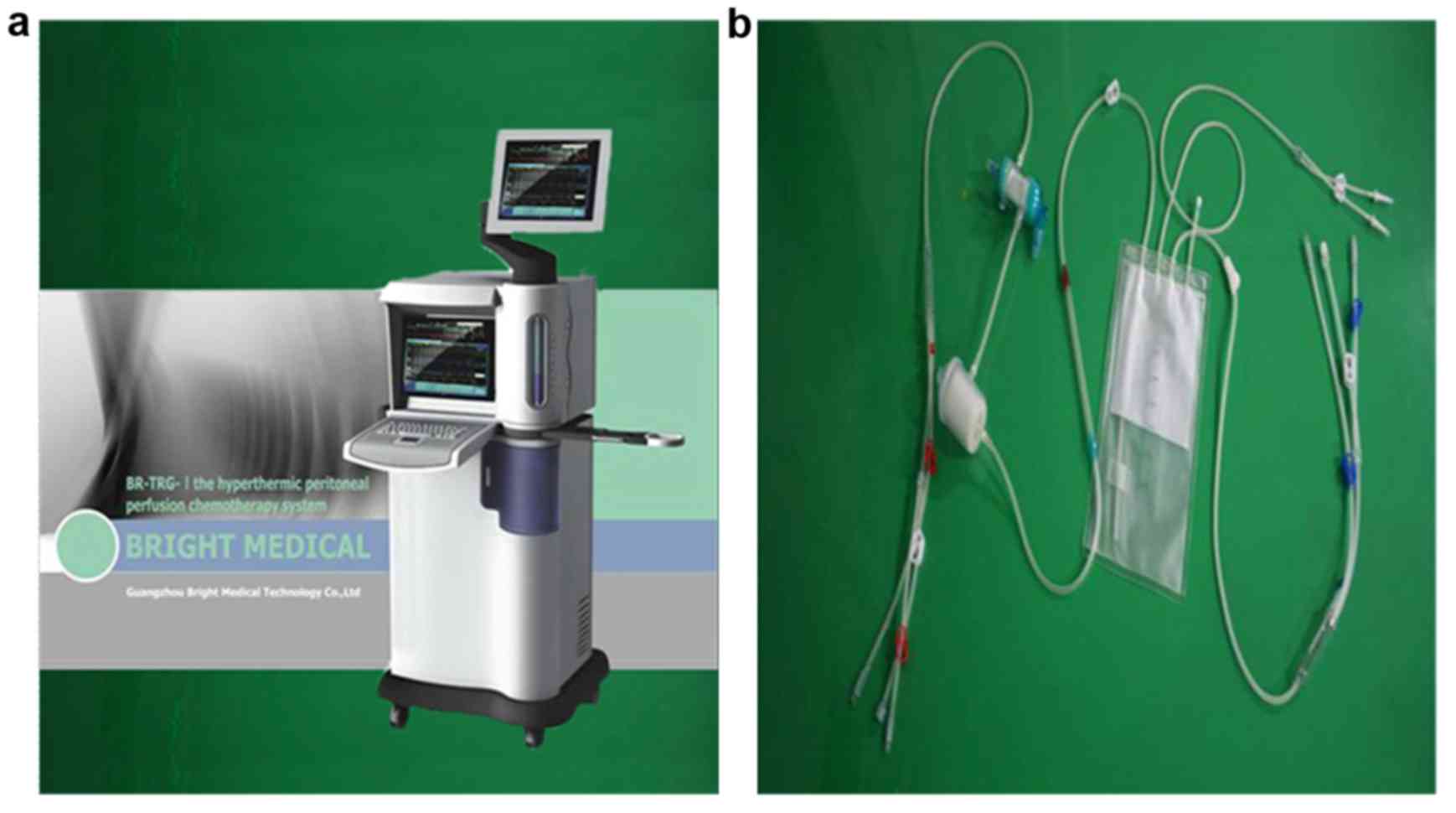
BHPC was performed using our novel ‘BR-TRG-I type
high-precision hyperthermic intraperitoneal perfusion treatment
system’ (Guangzhou Bright Medical Technology Co., Ltd. Guangzhou,
China), in which adaptive fuzzy control technology is principally
used for precise control of the drug solution perfusion temperature
(±0.10̊C) and rate (±5%) (23–26).
The device, shown in Fig. 1, has
been approved by the Chinese Food Drug Administration (approval no.
2009-3260924).
BHPC was carried out 1–2 days after TUR when gross
hematuria was arrested. The ‘BR-TRG-I type high-precision
hyperthermic intraperitoneal perfusion treatment system’ was
connected to pipeline systems (Guangzhou Bright Medical Technology
Co., Ltd.), with a storage bag containing mitomycin C (MMC) in
sterile saline. The perfusion rate was set and the therapeutic
pipeline path was opened to form a circulating system. The
instrument was debugged, and the treatment temperature and time
were set according to the inputted clinical data of the patients
(e.g., 45̊C for 60 min). The treatment temperature during BHPC was
assessed by the ‘BR-TRG-I type high-precision hyperthermic
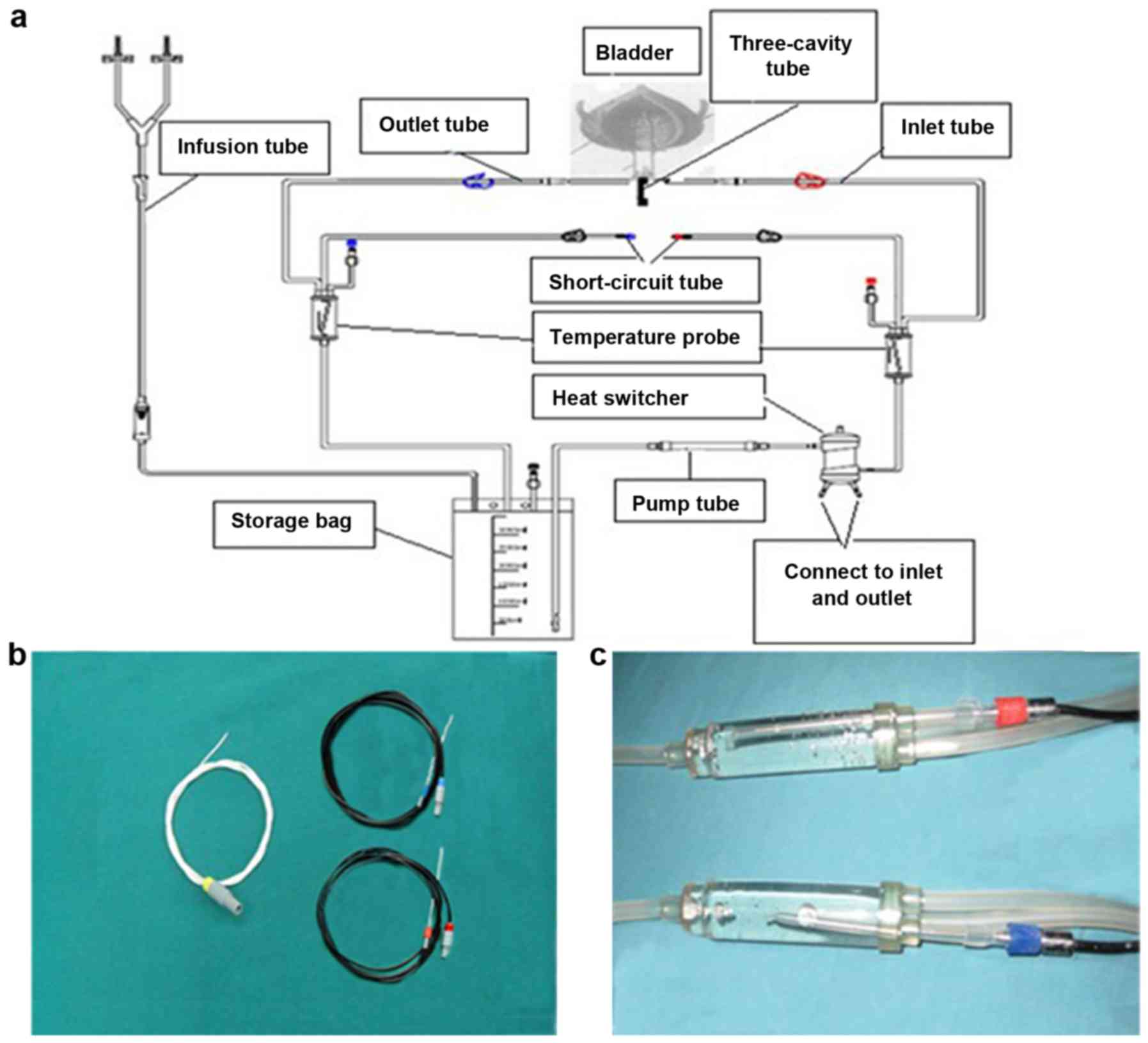
intraperitoneal perfusion treatment system’ with the temperature
probe inserted in a blind pipe within an inflated water sac, linked
to an infusion tube near an infusion pipeline or in a blind pipe
within an inflated water sac, linked to an outlet pipeline near a
24 F 3-way Foley catheter (shown in Fig. 2), which was connected to an infusion
pipeline and an outlet pipeline as shown in Fig. 2.
Before BHPC, the perfusion liquid was adjusted to
45̊C within the bladder according to the perfusion pressure and the
patient's subjective experience. The amount of perfusion fluid
within the bladder cavity was increased or decreased accordingly,
and the temperature was monitored closely using the temperature
probe during the perfusion procedure. At the early stage of
employing BHPC treatment using our self-developed ‘BR-TRG-I type
high-precision hyperthermic intraperitoneal perfusion treatment
system’, to assure the safety of the treatment and avoid any
adverse effects of hyperthermia and high pressure in the bladder,
the vital signs of the patients (including blood pressure, heart
and respiratory rate, and blood oxygen saturation) were monitored
using a G3HJ20025 multi-parameter patient monitor (Mindray
Bio-Medical Electronics Co. Ltd., Shenzhen, China). BHPC treatment
was terminated when the clinical characteristics of the patients
deteriorated. BHPC was performed once a week for a total of 3
rounds for each patient. The 24 F 3-way Foley catheter was retained
for 3–5 days, thus, bleeding had to be monitored, but removed after
the second or third session.
Intravesical chemotherapy
For patients in the chemotherapy group, a 24 F 2-way
Foley catheter was introduced into the bladder cavity for
intravesical chemotherapy after TUR. At room temperature, MMC in
sterile saline was delivered to the bladder through a catheter
injection syringe by way of the 24 F 2-way Foley catheter. The
catheter was then closed. During the treatment, patients frequently
moved, transitioning from lying in the supine or prone position, to
lying on the left or right side, and sitting upright to evenly
distribute the therapeutic agent within the bladder cavity. After 1
h, the MMC solution was discharged. Intravesical chemotherapy was
performed once a week, for a total of 3 times for each patient, and
the 24 F 2-way Foley catheter was retained or removed as described
for the BHPC group.
Pharmacokinetics of MMC
Venous blood was sampled from venous indwelling
needles. Perfusion liquid samples were collected from the perfusion
system short circuit outflow catheters in the BHPC group, or
directly from the 24 F 2-way Foley catheter in the chemotherapy
group. Perfusion liquid, serum or MMC standard samples (200 µl,
degassed prior to use) were mixed with an equal volume of
acetonitrile containing 20 µg diazepam as an internal standard by
vortexing for 1 min. The mixture was then centrifuged at 15,000 rpm
for 15 min and the supernatant was collected. Aliquots of 10 µl of
supernatant were subjected to LC-20AB high-performance liquid
chromatography (HPLC; Shimazdu, Kyoto, Japan) with Zorbax RP and
C18 (250×4.6 mm; particle size 5 µm) packed columns in
the stationary phase. The analysis protocol was carried out as
previously described (17,27,28),
with some modifications. The mobile phase consisted of 50 mM
potassium dihydrogen phosphate buffer solution, acetonitrile and
methanol at a ratio of 60:20:20 (%, volume) at pH 3.0, and was
filtered through a 0.22-µm membrane filter (Millipore, Billerica,
MA, USA). The flow rate of the injection of samples was 1.2 ml/min,
and the absorption wavelength for detection was 210 nm. The column
oven temperature was maintained at 35°C. The MMC concentration of
perfusion liquid and serum were calculated according to standard
curves within the linear range of 40–60 ng/ml for the perfusion
liquid and 0.5–15 µg/ml for the serum.
Clinical follow-up and efficacy
assessment
For the presence of bleeding and edema at the wound
surface after the TURBT, it was difficult to see whether there were
any residual tumors which were determined as the presence of the
tumor remnant in the primary tumor sites under the cystoscopic
observation, and thus, it could not be assured that tumors were
totally resected. When the residual tumors after 3 rounds of BHPC
or intravesical chemotherapy were found, a second resection was
carried out, followed by the histological examination of the
resected biopsies to observe the histological changes of the
residual tumors after the intravesical treatment. Tumor biopsies
taken before and post-treatment were stained using H&E and
graded by cystoscopic observation as previously described (4–6).
Follow-up consisted of urinary cystoscopic observation at 1 month
after TUR, and then quarterly thereafter for up to 1 year. Patients
received abdominal and pelvic CT scans after 3, 6 and 12 months,
for up to 1 year, or when were clinically indicated. One year
later, follow-up was carried out every 6 months or less frequently,
when the patients exhibited no signs of recurrence. Primary end
points were the recurrence of tumors, disease-free survival (DFS)
period and rate during the follow-up. Toxic effects of anticancer
drugs were graded according to the Common Toxicity Criteria of
National Cancer Institute (NCI) for Adverse Events (29).
Statistical analysis
Data were analyzed using SPSS version 19.0 (SPSS,
Inc., Chicago, IL, USA). All continuous data were presented as the
mean ± standard deviation. Student's t-test was used for
between-group comparisons, and paired sample t-test was used to
compare results at multiple time-points between groups. DFS was
analyzed using the Kaplan-Meier curve method. A P-value of <0.05
was considered to indicate a statistically significant result.
Results
Patient demographic and clinical
characteristics
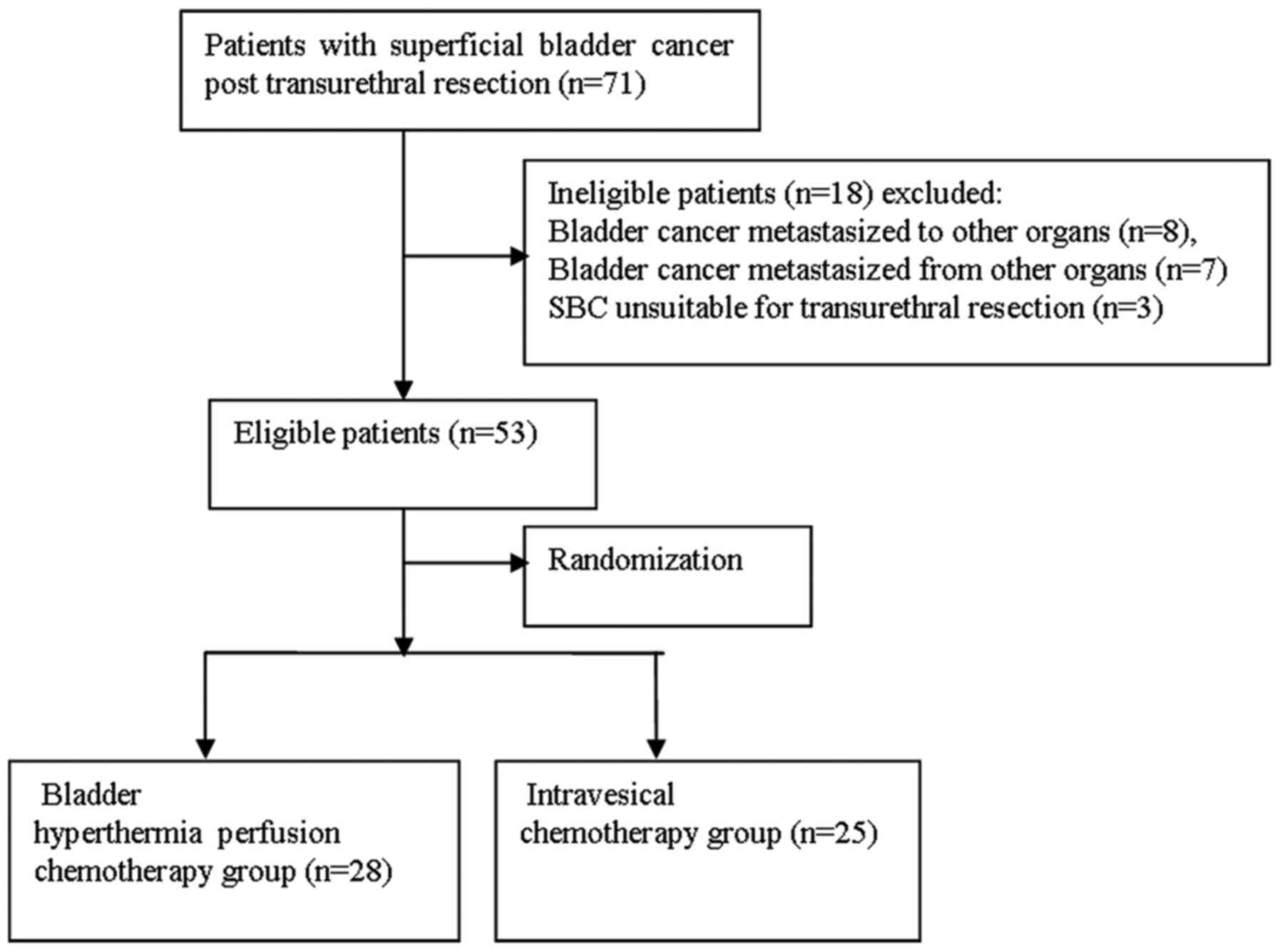
As indicated in Fig.
3, 71 eligible patients with NMIBC post-TUR were primarily
recruited, and 53 were eventually enrolled in the present study.
The enrolled participants included 39 male and 14 female patients,
with a mean age of 51.3±2.4 years (ranging from 37 to 66 years).
There were 7 patients with stage T1 and 46 with stage T2 tumors. As
non-muscle invasive T1 and T2 bladder cancer can be easily resected
by TURBT, which is required for BHPC treatment, T1 and T2 patients
were enrolled in the present study. Usually, NMIBC can be totally
resected by TURBT, with a similar efficacy to cystectomy but with
less complications. BHPC following TURBT effectively prevented the
postoperative recurrence of tumors. Therefore, intravesical
treatment was used. According to the computer-based random
distribution, patients were randomly assigned into the BHPC group
(28 patients) or chemotherapy group (25 patients) (Fig. 3). In all patients gross hematuria
was arrested 2 days post-TUR, but slight hematuria persisted for up
to one week following the first treatment. Age, gender, disease
course and tumor location, stage and size, did not significantly
differ between the 2 groups (all P>0.05; Table I).
 | Table I.Characteristics of the patients with
superficial bladder cancer post-transurethral resection. |
Table I.
Characteristics of the patients with
superficial bladder cancer post-transurethral resection.
|
| BHPC group
(n=28) | Chemotherapy group
(n=25) | P-value |
|---|
| Age in years, mean
(range) | 50.7±1.9
(37–66) | 51.6±2.3
(39–66) | >0.05 |
| Gender n (%) |
|
Male | 26 (92.9%) | 23 (92.0%) | >0.05 |
|
Female | 2 (7.1%) | 2 (8.0%) | >0.05 |
| Disease courses
(days) | 11.35±1.3 | 11.27±1.6 | >0.05 |
| Tumor location, n
(%) |
| Side
wall | 6 (21.5) | 6 (24.0) | >0.05 |
|
Posterior wall | 5 (17.9) | 4 (16.0) | >0.05 |
| Top
area | 3 (10.7) | 2 (8.0) | >0.05 |
|
Triangle area | 8 (28.6) | 8 (32.0) | >0.05 |
| Double
tumors | 6 (21.5) | 5 (20.0) | >0.05 |
| Tumor size (cm), n
(%) |
|
≥0.5 | 16 (57.1) | 13 (52.0) | >0.05 |
|
<0.5 | 12 (50.0) | 12 (48.0) | >0.05 |
| Tumor stage, n
(%) |
| T1 | 4 (14.3) | 3 (12.0) | >0.05 |
| T2 | 24 (85.7) | 22 (88.0) | >0.05 |
Gross outcomes and adverse
effects
BHPC and intravesical chemotherapy were successful.
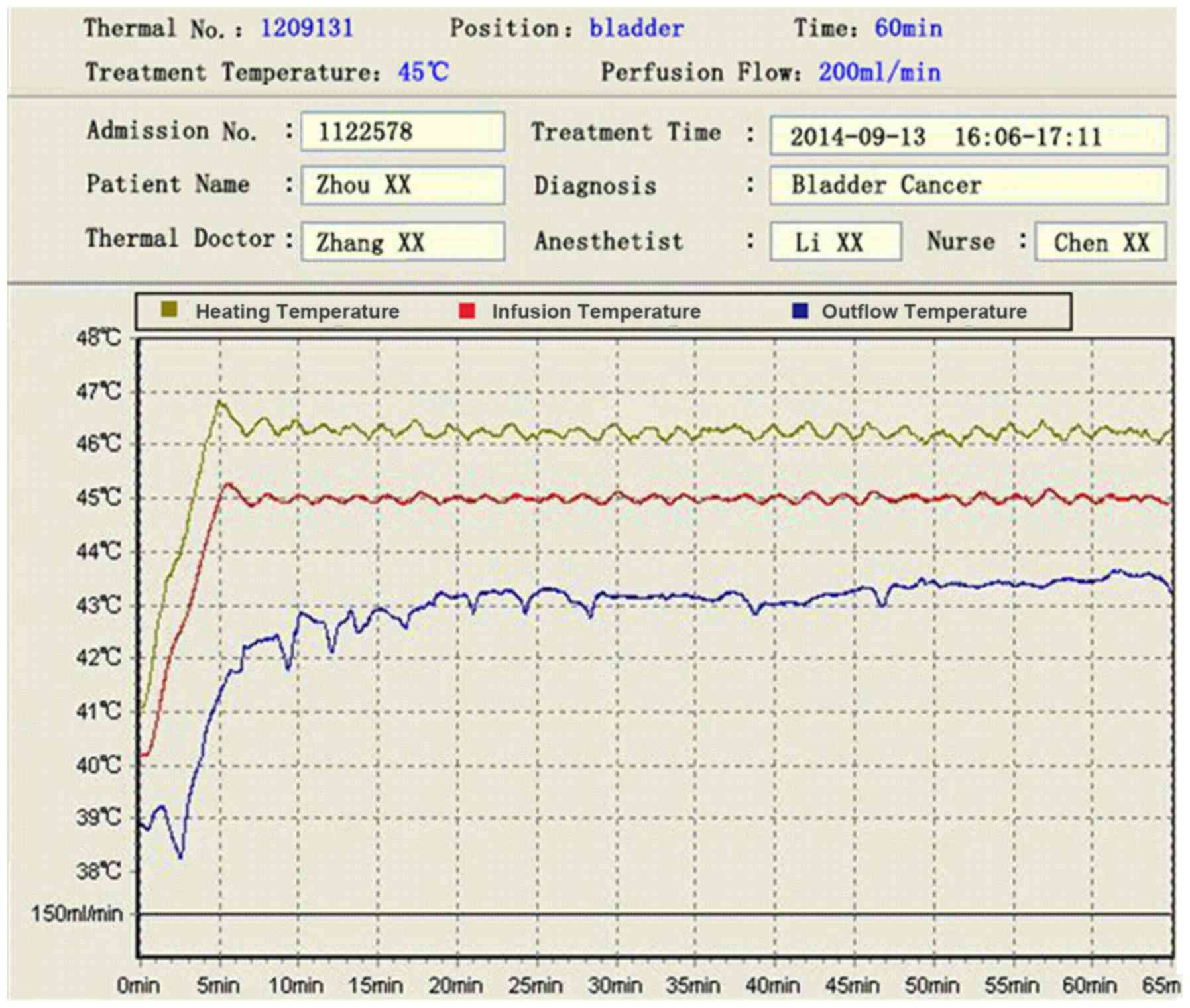
During BHPC the temperature of the infusion tube was stably
controlled at ~45̊C, an outlet pipeline at ~43̊C, and the
temperature in the bladder cavity at ~44̊C (Fig. 4). All patients tolerated all 3 BHPC
or intravesical chemotherapy treatments, except for one case where
treatment was interrupted due to severe chemical cystitis.
During BHPC or intravesical chemotherapy, patients
exhibited no adverse effects except for transient fever and
abdominal distension. No gastrointestinal events or bone marrow
suppression were observed, and laboratory tests revealed no
significant changes in blood markers, electrolyte levels or liver
and kidney function after treatment in either group.
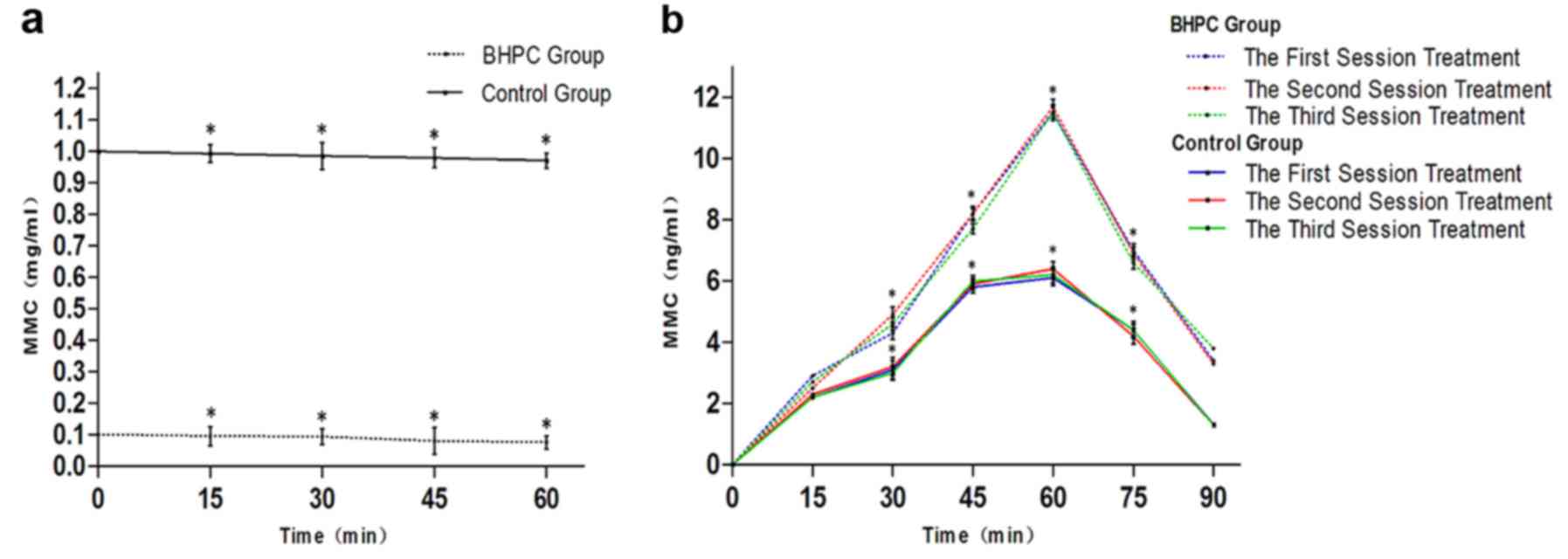
Pharmacokinetics of MMC
Although HPLC analysis indicated that the bladder
perfusion MMC concentration differed significantly between the 2
groups, the MMC concentration gradually decreased during treatment
in both groups, and the rate of decrease in concentration did not
significantly differ between the 2 groups (Fig. 5a). The MMC concentration of the BHPC
perfusion liquid group was significantly lower locally than that of
the chemotherapy group (P>0.05; Fig.
5a). However, serum MMC concentration in the BHPC group during
administration at a 45̊C temperature for 1 h was significantly
higher than that of the chemotherapy group (P<0.05; Fig. 5b). During the first treatment
session, serum MMC concentration in the BHPC group of patients did
not significantly differ from the serum MMC concentration during
the second or third treatment sessions in either the BHPC or
chemotherapy groups, and there was no significant difference in the
MMC concentration of the perfusion liquid and serum between the 2
groups (P>0.05; Fig. 5b).
Cystoscopic and histological
observation
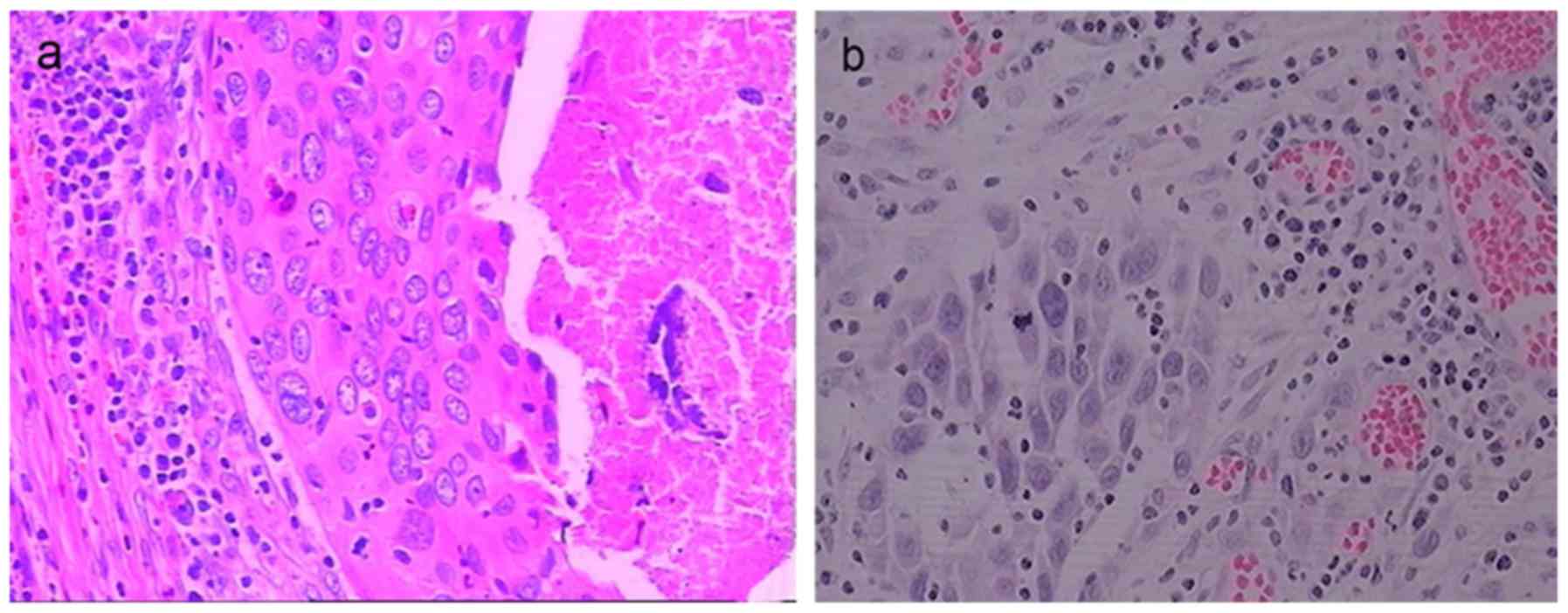
In the BHPC group of patients, cystoscopic
observation revealed that no viable tumors remained in most
patients, although, tumors remained in 3 patients. Residual tumors
were observed to be covered by a grey-white slough, which extended
across the bladder mucosa, and associated with congestion and edema
at the mucosal surface. Inflammatory cells (sometimes including
numerous eosinophils) were observed infiltrating the lamina
propria, and complete necrosis was accompanied by local vascular
changes (such as necrosis and thrombosis) in the small vessels of
tumors, and hemorrhage into the stroma (Fig. 6a). In the chemotherapy group of
patients, residual bladder tumors were observed in only 2 patients,
and only congestion and edema of the bladder mucosa except for one
case complicated with severe chemical cystitis was noted. In both
patients with residual bladder tumors, the bladder mucosa appeared
healthy around the residual tumors. Inflammatory cells (sometimes
including numerous eosinophils) were observed to infiltrate the
lamina propria, but in the absence of local vascular changes or
hemorrhage they were observed to infiltrate the stroma (Fig. 6b).
Follow-up and prognosis
Patients were followed-up for 47±2.3 months (range
8–78 months). The follow-up period did not significantly differ
between the 2 groups (BHPC group 47±2.7 months vs. chemotherapy
group 46±1.9 months; P>0.05). All patients were reported to be
living well. In patients of the BHPC group, cystoscopic observation
revealed recurrence of tumors in the bladder cavity of 3 patients
at 14, 17 and 19 months post-BHPC. The tumor recurrence rate was
10.7% (3 out of 28 patients), the mean DFS period was 37±1.2 months
(ranging from 8 to 78 months), and the DFS rate was 92.9% during
the follow-up (Table II). In
contrast, in the chemotherapy group, cystoscopic observation
revealed recurrence of the tumors in the bladder cavity of 7
patients at 6, 13, 13, 15, 16, 21 and 27 months after chemotherapy.
The tumor recurrence rate was 28.0% (7 out of 25 patients), the
mean DFS period was 19±0.9 months (ranging from 6 to 54 months),
and the DFS rate was 72.0% during follow-up (Table II). The tumor recurrence rate was
significantly lower while the DFS period and DFS rate were
significantly higher in the BHPC group than in the chemotherapy
group (all P<0.05; Table
II).
 | Table II.Clinical efficacy in patients with
superficial bladder cancer post-transurethral resection treated by
BHPC or intravesical chemotherapy alone. |
Table II.
Clinical efficacy in patients with
superficial bladder cancer post-transurethral resection treated by
BHPC or intravesical chemotherapy alone.
|
| Tumor recurrence
rate % (n/total) | DFS period
(months) | DFS rate (%) | Complications
n |
|---|
| BHPC group
(n=28) | 10.7 (3/28) | 37±1.2 | 92.9 | 0 |
| Chemotherapy group
(n=25) | 28.0 (7/25) | 19±0.9 | 72.0 | 1 |
| P-value | 0.02 | 0.001 | <0.001 |
|
Discussion
Due to the unique location and anatomic structure of
the urinary bladder, bladder tumors are highly suitable for local
hyperthermia combined with intravesical chemotherapy (11,12,14–16,18,19).
Thermochemotherapy combining the direct antitumorigenic effect of
local hyperthermia with a potentiated antitumorigenic cytostatic
agent improves chemotherapy outcomes (19,30).
However, conventional thermochemotherapy procedures do not allow
precise and stable temperature control at the lesion sites, which
hampers treatment efficacy (31–34).
We developed a ‘BR-TRG-I type high-precision hyperthermic
intraperitoneal perfusion treatment system’, which allows precise
automatic control of the temperature and perfusion rate of the drug
solution. This system includes an automatic cooling function and
adaptive fuzzy control technology for precise control of the
perfusion temperature and rate, and thus, control of the local
temperature in the bladder cavity of NMIBC patients (22–25).
To effectively kill the residual cancer cells within the bladder as
well as to kill the cancer cells dropped from the bladder cavity
and adhered to the bladder wall, BHPC using this device was
performed immediately after the TUR of bladder tumors instead of
weeks later. In the present study, we demonstrated that BHPC was
successfully carried out (33,34),
and the rate of side-effects was similar to previously published
studies (31–34). This could effectively help to stop
bleeding at the wound surface and prevent the tumor recurrence
after TURBT.
In the present study, we performed a randomized
controlled trial to evaluate the treatment efficacy of BHPC. Since
BHPC involves perfusion chemotherapy in addition to local
hyperthermia, we used intravesical chemotherapy instead of simple
conventional systematic chemotherapy as the control. BHPC
significantly decreased tumor recurrence and increased the DFS
period and rate for NMIBC patients post-TUR when compared with
intravesical chemotherapy alone. This result is consistent with a
previous study that indicates the advantage of conventional
intravesical thermochemotherapy over intravesical chemotherapy
alone (31). We attribute this
effect to the synergistic effect of bladder hyperthermia and
chemotherapy. In this technique, the bladder mucosa is fully
extended by a large volume of BHPC, which allows chemotherapeutics
better access to the remnant bladder tumor.
MMC can be systemically absorbed via intravesical
administration. Numerous studies in humans have indicated that
plasma concentrations after normal doses do not reach a critical
toxic value (21). In the present
study, both BHPC and intravesical chemotherapy achieved high local
drug concentrations in the perfusion liquid, thus, at the
intravesical cavity of the bladder, but serum concentrations of MMC
were maintained below those associated with systematic
administration of the drug. Although, the serum MMC concentrations
in the BHPC group were significantly higher than those in the
intravesical perfusion chemotherapy group in the present study, it
did not reach sufficiently high levels to cause serious adverse
effects (21,34,35).
The advantages of BHPC over single intravesical chemotherapy can be
attributed to the synergy of local hyperthermia treatment and
chemotherapy.
In the present study, the perfusion fluid MMC
concentration gradually decreased during BHPC or chemotherapy
treatment, perhaps due to absorption or the dilution by urine
during treatment. As indicated by the higher serum concentration,
patients in the BHPC group absorbed more MCC, indicating that
thermal treatment may increase the absorption of chemotherapeutic
drugs, however, MMC was diluted more significantly with urine in
the intravesical chemotherapy group, and overall the decrease in
perfusion liquid MCC concentration did not differ significantly
between the 2 groups.
HPLC analysis indicated that the BHPC group
perfusion liquid contained a significantly lower MMC concentration
than that of the chemotherapy group. However, serum MMC
concentration in the BHPC group was significantly higher than in
the chemotherapy group when administered at 45̊C for 1 h. This may
be attributable to the fact that local hyperthermia induced by BHPC
increases the chemotherapeutic drug intake of tumor cells, drug
intracellular distribution and metabolism, which may in turn
increase drug release into the serum from tumor cells. This may
also be associated with the full extension of the bladder wall
(induced by the large volume of thermal perfusion liquid)
increasing the absorptive area of bladder mucosa. In the present
study, the MMC concentration of the perfusion liquid and serum did
not differ significantly between the 2 groups (P>0.05), or
between the first and second, or third BHPC treatment. This result
suggested that bladder mucosal wound by TUR of bladder carcinoma
did not increase the absorption of chemotherapeutic drugs. In
addition, the plasma half-life of MMC is 30–50 min (34,35),
and we found that the serum MMC concentration remained stable
during BHPC, but rapidly declined after BHPC. We hypothesized that
absorption of chemotherapeutic drugs compensated for metabolism of
chemotherapeutic drugs during BHPC
The aim of BHPC treatment post-TUR is to cause
maximal killing of residual tumors, with minimal damage to normal
tissues. In the present study, the whole bladder mucosa was covered
by a grey-white slough post-BHPC, with congestion and edema at the
mucosa surface and with inflammatory cell (e.g. eosinophils)
infiltration in the lamina propria. However these features resolved
2 months post-BHPC. This result revealed that BHPC is safe, targets
tumor cells precisely, and causes minimal damage to the normal
bladder mucosa. Tumor cell necrosis causes a shedding on the
surface bladder wall, and has no significant adverse impact on the
normal bladder mucosa. In previous studies, histological changes
were observed after the local hyperthermia treatment, including
nuclear vacuolation and pyknosis, cytoplasmic vacuolation and
decrease of epithelial adhesion (19,20,30).
These histological changes reflected damage to the bladder cancer
caused by hyperthermia treatment. In the present study, cystoscopic
and histological observations revealed grey-white slough covering
the whole bladder mucosa around residual tumors in the BHPC group
of patients, while in contrast in the intravesical chemotherapy
group of patients, the NMIBC mucosa appeared healthy in all but one
patient complicated with severe chemical cystitis.
Previous studies showed that dermal vessels were far
more responsive to temperature changes than normal epithelial
cells, and that severe and persistent vascular reactions were often
elicited by prolonged episodes of low intensity hyperthermia that
fail to harm the epidermis (13,16,20).
Thermal effects upon blood vessels of the mucosa led to exudation
of plasma and sometimes interstitial hemorrhage. These vascular
changes may exacerbate direct thermal injury of the lamina propria,
sometimes resulting in areas of necrosis with exfoliation of
epithelium and subsequent formation of hypervascular inflamed
granulation tissue (13,16,20).
Our results indicate complete necrosis accompanied by local
vascular changes (such as necrosis and thrombosis) in the small
vessels of tumors, and hemorrhage into the stroma in the BHPC
group. While in the intravesical chemotherapy group, no local
vascular changes were observed in the small vessels of tumors, and
also no hemorrhage into the stroma were observed. These vascular
changes may be partially responsible for preventing NMIBC
recurrence since tumor vessels are more susceptible to thermal
injury than normal tissue vessels after hyperthermia. These
histological changes suggest that BHPC may be more effective in
killing tumor cells in residual tumors, inhibiting tumor
angiogenesis and blocking mucosal blood supply of residual tumors
in bladder cancer recurrence post-TUR than intravesical
chemotherapy. This result is consistent with some similar studies
(13,20).
BHPC using the device ‘BR-TRG-I type high-precision
hyperthermic intraperitoneal perfusion treatment system’ in the
present study was first developed by us. According to our previous
experience 3 rounds of HIPEC was used to treat abdominal tumors in
order to get good outcomes (22–25),
consequently, BHPC was also conducted for 3 rounds in the present
study. Nevertheless, for our self-developed device-based BHPC, the
chemotherapeutic types of drugs and dosages involved, the treatment
time and temperature are still being explored.
As ‘BR-TRG-I type high-precision hyperthermic
intraperitoneal perfusion treatment system’ was first used in BHPC
during the study period, it was not easy to recruit numerous
patients who were willing to receive BHPC using this device in the
randomized controlled study. Therefore, a limitation of the present
study was that the sample size was relatively small. In the future
we will try to enroll more patients to validate these results.
Meanwhile, since the bladder wall became thinner during the
perfusion of liquid, it was not practical to detect the temperature
in the bladder wall or within the bladder tissue, consequently we
were only able to detect the internal temperature of the infusion
pipeline and outflow pipeline from the bladder cavity, which cannot
guarantee an accurate reading of the temperature of the bladder
mucosa due to the fact that heat conduction and undermucosal blood
flow may promote loss of heat. Thus, the temperature of the bladder
wall in contact with the mucosa and the mucosa itself was likely
lower than the temperature in the infusion pipeline and bladder
cavity. From Fig. 4 displaying the
temperature of the perfusion fluid during BHPC, it can be seen that
the intravesical temperature ranged from 43 to 45°C. In a pilot
animal study (data not shown), we demonstrated that during BHPC in
pig and rabbits, the temperature (42–43°C) at the serosal surface,
outmost layer of the bladder wall, was a bit lower (2–3°C) than
that (44–44.5°C) in the bladder cavity. Therefore, the temperature
reaching the bladder wall and tissues was still high enough to
reach the treatment temperature level (over 40°C). Based on the
animal study, we set the perfusion temperature at 45°C, and
similarly, in the bladder wall and tissues a temperature still
higher than 40°C would be reached thus effectively killing the
residual cancer cells establishing an effective treatment
temperature for tumor hyperthermic therapy.
During hyperthermic intraperitoneal chemotherapy,
widely employed in the treatment of ovarian cancer and malignant
ascites with satisfactory outcomes (22–25),
the temperature probes sensed the temperature of the perfusion
liquid or within the abdominal cavity instead of the tumor tissues
directly. However, this did not hamper hyperthermia in the cancer
tissues. Similarly, during BHPC for patients in the present study,
although the temperatures in the bladder wall and tissues were not
directly assessed, the perfusion temperature of 45°C reaching the
bladder wall and cancer tissues was estimated to be over 40°C, i.e.
the treatment temperature level, although there was some heat loss
during conduction and heat removal by blood vessels. Therefore,
this treatment was defined as bladder intracavitary hyperthermia
and the bladder wall received a hyperthermic temperature.
In conclusion, BHPC is a feasible, safe and
promising approach for the treatment of patients with NMIBC who
undergo TUR, capable of decreasing the NMIBC recurrence rate, and
prolonging the NMIBC recurrence interval time and increasing the
DFS rate and period.
Acknowledgements
The present study was supported by the Breakthroughs
in Key Areas of Guangdong and Hong Kong Projects (no.
2006Z1-E6041), the Guangdong Provincial Science and Technological
Programs (no. 2009A030301013), and the Guangdong Province Natural
Science Foundation Programs (no. 2014A030313494).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hızlı F, Argun G, Güney I, Güven O, Arık
AI, Başay S, Günaydın H, Başar H and Köşüş A: Obturator nerve block
transurethral surgery for bladder cancer: Comparison of inguinal
and intravesical approaches: Prospective randomized trial. Ir J Med
Sci. 185:555–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aziz A, Gierth M, Rink M, Schmid M, Chun
FK, Dahlem R, Roghmann F, Palisaar RJ, Noldus J, Ellinger J, et al:
PROMETRICS 2011 Research Group: Optimizing outcome reporting after
radical cystectomy for organ-confined urothelial carcinoma of the
bladder using oncological trifecta and pentafecta. World J Urol.
33:1945–1950. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hermann GG, Mogensen K, Lindvold LR, Haak
CS and Haedersdal M: Office-based transurethral devascularisation
of low grade non-invasive urothelial cancer using diode laser. A
feasibility study. Lasers Surg Med. 47:620–625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1,054 patients. J Clin Oncol.
19:666–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takai T, Inamoto T, Komura K, Yoshikawa Y,
Uchimoto T, Saito K, Tanda N, Kouno J, Minami K, Uehara H, et al:
Feasibility of photodynamic diagnosis for challenging TUR-Bt cases
including muscle invasive bladder cancer, BCG failure or 2nd-TUR.
Asian Pac J Cancer Prev. 16:2297–2301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lamm DL, Riggs DR, Traynelis CL and Nseyo
UO: Apparent failure of current intravesical chemotherapy
prophylaxis to influence the long-term course of superficial
transitional cell carcinoma of the bladder. J Urol. 153:1444–1450.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Newling D: Intravesical therapy in the
management of superficial transitional cell carcinoma of the
bladder: The experience of the EORTC GU Group. Br J Cancer.
61:497–499. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rawls WH, Lamm DL, Lowe BA, Crawford ED,
Sarosdy MF, Montie JE, Grossman HB and Scardino PT: Fatal sepsis
following intravesical bacillus Calmette-Guerin administration for
bladder cancer. J Urol. 144:1328–1330. 1990.PubMed/NCBI
|
|
10
|
Hayne D, Stockler M, McCombie SP,
Chalasani V, Long A, Martin A, Sengupta S and Davis ID: BCG+MMC
trial: Adding mitomycin C to BCG as adjuvant intravesical therapy
for high-risk, non-muscle-invasive bladder cancer: A randomised
phase III trial (ANZUP 1301). BMC Cancer. 15:4322015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamat AM, Briggman J, Urbauer DL, Svatek
R, Nogueras González GM, Anderson R, Grossman HB, Prat F and Dinney
CP: Cytokine panel for response to intravesical therapy (CyPRIT):
Nomogram of changes in urinary cytokine levels predicts patient
pesponse to bacillus Calmette-Guérin. Eur Urol. 69:197–200. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferlazzo G, Magno C, Lupo G, Rizzo M,
Iemmo R, Semino C and Melioli G: A phase I study of intravesical
continuous perfusion of recombinant interleukin-2 in patients with
superficial bladder cancer. Am J Clin Oncol. 18:100–104. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colombo R, Da Pozzo LF, Lev A, Freschi M,
Gallus G and Rigatti P: Neoadjuvant combined microwave induced
local hyperthermia and topical chemotherapy versus chemotherapy
alone for superficial bladder cancer. J Urol. 155:1227–1232. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geijsen ED, de Reijke TM, Koning CC,
Vörding Zum Vörde Sive PJ, de la Rosette JJ, Rasch CR, van Os RM
and Crezee J: Combining mitomycin C and regional 70 MHz
hyperthermia in patients with nonmuscle invasive bladder cancer: A
pilot study. J Urol. 194:1202–1208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Colombo R, Salonia A, Da Pozzo LF, Naspro
R, Freschi M, Paroni R, Pavone-Macaluso M and Rigatti P:
Combination of intravesical chemotherapy and hyperthermia for the
treatment of superficial bladder cancer: Preliminary clinical
experience. Crit Rev Oncol Hematol. 47:127–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gofrit ON, Shapiro A, Pode D, Sidi A,
Nativ O, Leib Z, Witjes JA, van der Heijden AG, Naspro R and
Colombo R: Combined local bladder hyperthermia and intravesical
chemotherapy for the treatment of high-grade superficial bladder
cancer. Urology. 63:466–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milla P, Fiorito C, Soria F, Arpicco S,
Cattel L and Gontero P: Intravesical thermo-chemotherapy based on
conductive heat: A first pharmacokinetic study with mitomycin C in
superficial transitional cell carcinoma patients. Cancer Chemother
Pharmacol. 73:503–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uchibayashi T, Nakajima K, Hisazumi H,
Nishino A and Miyoshi N: Hyperthermic intravesical peplomycin
perfusion treatment for bladder cancer. Br J Urol. 72:65–67. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Owusu RA, Abern MR and Inman BA:
Hyperthermia as adjunct to intravesical chemotherapy for bladder
cancer. Biomed Res Int. 2013:2623132013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ekin RG, Akarken I, Cakmak O, Tarhan H,
Celik O, Ilbey YO, Divrik RT and Zorlu F: Results of intravesical
chemo-hyperthermia in high-risk non-muscle invasive bladder cancer.
Asian Pac J Cancer Prev. 16:3241–3245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paroni R, Salonia A, Lev A, Da Pozzo LF,
Cighetti G, Montorsi F, Rigatti P and Colombo R: Effect of local
hyperthermia of the bladder on mitomycin C pharmacokinetics during
intravesical chemotherapy for the treatment of superficial
transitional cell carcinoma. Br J Clin Pharmacol. 52:273–278. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ba M, Long H, Zhang X, Tang Y, Wu Y, Yu F,
Wang S and Cui S: Different sequential approaches of cytoreductive
surgery and hyperthermic intraperitoneal chemotherapy in treating
ovarian cancer with malignant ascites. J Cancer Res Clin Oncol.
140:1497–1506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ba MC, Long H, Cui SZ, Tang YQ, Wu YB,
Zhang XL, Tang HS and Bai SX: Multivariate comparison of
B-ultrasound guided and laparoscopic continuous circulatory
hyperthermic intraperitoneal perfusion chemotherapy for malignant
ascites. Surg Endosc. 27:2735–2743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ba MC, Cui SZ, Lin SQ, Tang YQ, Wu YB,
Wang B and Zhang XL: Chemotherapy with laparoscope-assisted
continuous circulatory hyperthermic intraperitoneal perfusion for
malignant ascites. World J Gastroenterol. 16:1901–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui S, Ba M, Tang Y, Liu J, Wu Y, Wang B,
Zhang X, Tang H and Zhong S: B ultrasound-guided hyperthermic
intraperitoneal perfusion chemotherapy for the treatment of
malignant ascites. Oncol Rep. 28:1325–1331. 2012.PubMed/NCBI
|
|
26
|
Wittekind C: 2010 TNM system: On the 7th
edition of TNM classification of malignant tumors. Pathologe.
31:331–332. 2010.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dwivedi R, Singh M, Kaleekal T, Gupta YK
and Tripathi M: Concentration of antiepileptic drugs in persons
with epilepsy: A comparative study in serum and saliva. Int J
Neurosci. 126:972–978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdelaleem EA, Naguib IA, Zaazaa HE and
Hussein EA: Development and validation of HPLC and HPTLC methods
for determination of cefoperazone and its related impurities. J
Chromatogr Sci. 54:179–186. 2016.PubMed/NCBI
|
|
29
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The Common Terminology Criteria for
Adverse Events Version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lammers RJ, Witjes JA, Inman BA,
Leibovitch I, Laufer M, Nativ O and Colombo R: The role of a
combined regimen with intravesical chemotherapy and hyperthermia in
the management of non-muscle-invasive bladder cancer: A systematic
review. Eur Urol. 60:81–93. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Inman BA, Stauffer PR, Craciunescu OA,
Maccarini PF, Dewhirst MW and Vujaskovic Z: A pilot clinical trial
of intravesical mitomycin-C and external deep pelvic hyperthermia
for non-muscle-invasive bladder cancer. Int J Hyperthermia.
30:171–175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soria F, Milla P, Fiorito C, Pisano F,
Sogni F, Di Marco M, Pagliarulo V, Dosio F and Gontero P: Efficacy
and safety of a new device for intravesical thermochemotherapy in
non-grade 3 BCG recurrent NMIBC: A phase I–II study. World J Urol.
34:189–195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sousa A, Piñeiro I, Rodríguez S, Aparici
V, Monserrat V, Neira P, Carro E, Murias C and Uribarri C:
Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in
intermediate-high-risk non-muscle-invasive bladder cancer. Int J
Hyperthermia. 32:374–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colombo R, Salonia A, Leib Z,
Pavone-Macaluso M and Engelstein D: Long-term outcomes of a
randomized controlled trial comparing thermochemotherapy with
mitomycin-C alone as adjuvant treatment for non-muscle-invasive
bladder cancer (NMIBC). BJU Int. 107:912–918. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van der Heijden AG and Dewhirst MW:
Effects of hyperthermia in neutralising mechanisms of drug
resistance in non-muscle-invasive bladder cancer. Int J
Hyperthermia. 32:434–445. 2016. View Article : Google Scholar : PubMed/NCBI
|