Introduction
With its incidence rate of almost 50%, glioma is the
most common form among CNS gliomas. Although new therapeutic
approaches have been developed, there are still many common
problems that need to be resolved including therapy resistance to
improve long-term survival. The origin of gliomas is largely
unknown but there is increasing speculation that they might arise
from glioma stem cells (GSCs), which might consist of transformed
neural stem cells (1–4). Recent advances in our understanding of
the biological features of glioma offer opportunities to the design
of a new therapeutic strategy based on targeting essential
signaling pathways.
MicroRNAs (miRNAs) are a family of endogenous small
non-coding RNAs that regulate gene expression via the
sequence-specific base pairing on the 3′-untranslated regions
(3UTRs) of target mRNAs, leading to mRNA cleavage or translation
inhibition (5). A great number of
the human miRNAs function either as oncogenes or as tumor
suppressors. Thus, their function acting as tumor-suppressor or
carcinogenic miRNAs may vary depending on their targets (6–8),
resulting in influencing glioma formation and growth. The miRNAs
have been reported to be aberrantly overexpressed or downregulated
during glioma progression, including miR-20a and miR-106a (9), miR-29a (10), miR-145 (11), miR-656 (12), miR-300 (13), miR-16 (14), miR-34a (15), miR-503 (16), miR-203 (17) miR-100 (18), miR-26a (19), miR-23b (20) and miR-218 (21). These miRNAs play oncogenic or
tumor-suppressive roles in the regulation of cell growth, migration
and invasion by repressing their target genes.
miRNA 30a (miR-30a) is a member of the miR-30
family, which consists of six distinct mature miRNA sequences.
There is considerable evidence suggesting that the dysregulation of
miR-30a is correlated with several types of malignant tumors,
including breast, lung, thyroid, gastric cancer and leukemia
(22). Metadherin (23), SNAIL1 (24), Beclin-1 (25) and PIK3CD (26) are potential targeted genes of
miR-30a, which promotes glioma progression. However, there are
relatively few studies available that report a role for miR-30a in
tumorigenesis and progression of glioma. In the present study, we
investigated the potential involvement of miR-30a by examining its
expression and its effects on tumorigenesis, cell growth, cell
cycle distribution, colony formation, migration, invasion and stem
cell-like properties in glioma. Thus, mechanistic investigation
revealed that miR-30a was shown to control Wnt5a expression in
progression of glioma as a tumor suppressor.
Materials and methods
Clinical samples
Primary glioma tissue samples and normal samples
were obtained from the patients in the First Hospital of Lanzhou
University (Lanzhou, China). None of the patients had received
either radiotherapy or chemotherapy. Both tumor and normal tissues
were histologically confirmed by H&E (hematoxylin and eosin)
staining. The tumor tissues derived from 16 cases with glioma, and
the normal tissues derived from patients with brain injury.
Informed consent was obtained from each patient and the research
protocols were approved by the Ethics Committee of Lanzhou
University Hospital.
Cell culture
Human glioma cells T98G, SHG44, U251, U87 and U373
were obtained from the American Type Culture Collection (ATCC;
Manassas, VA, USA) were cultured in Dulbeccos modified Eagles
medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 µg/ml streptomycin. All cells were cultured
at 37°C in a humidified atmosphere containing 5% CO2.
Human normal cells HA (ScienCell Research Laboratories, Carlsbad,
CA, USA) isolated from human brain (cerebral cortex) were cultured
in microglia medium with 10% FBS.
Oligonucleotide synthesis and
lentiviral transduction
The oligonucleotide of mature miR-30a antagomir was
chemosynthesized, amplified and cloned into GV232-Puro Vectors by
Shanghai Genechem, Co., Ltd. (Shanghai, China). The correct
sequences and insertions were confirmed by DNA sequencing. Cells
were lentivirus-transfected with either the GV232-Puro-miR-30a
recombined vector (LV-miR-30a) or emptyGV232-Puro vector (negative
control, miR-control) using Lipofectamine 2000 transfection reagent
(Invitrogen, Carlsbad, CA, USA). The transduced cells with a cell
density of over 40% confluency were exposed to puromycin
dihydrochloride (1 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) for
resistance selection. Stable cell lines were selected with 0.5
mg/ml puromycin in the first round of selection.
Lentivirus-mediated silencing of miR-30a was verified by
quantitative reverse transcription-PCR (qRT-PCR) and western blot
analysis.
miRNA target validation
A fragment of Wnt5a 3UTR (untranslated regions) was
amplified by PCR and cloned downstream of the firefly luciferase
gene in pGL4 vector (Promega, Madison, WI, USA). The vector was
named wild-type 3UTR. Site-directed mutagenesis of the miR-30a
binding site in Wnt5a 3UTR was performed using GeneTailor
Site-Directed Mutagenesis system (Invitrogen) and named mutant
3UTR.
RNA isolation and real-time
RT-PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen) following the instructions. Briefly, the cells were
lysed in TRIzol and then mixed with chloroform. The lysate was
centrifuged to separate RNA, DNA and protein, the total RNA
recovered was precipitated with isopropanol, washed in 75% ethanol
to remove impurities before dissolved in water. Subsequently, 2 µg
of RNA was treated with DNase to remove contaminating DNA prior to
reverse transcription to cDNA using SYBR® PCR kit
(Takara Bio, Shiga, Japan). With the primers purchased from
Invitrogen, real-time RT-PCR was performed using a sequence
detector (ABI Prism; Applied Biosystems, Foster City, CA, USA) to
measure mRNA expression. The relative expression levels were
quantified by comparing Ct values of the samples with those of the
reference, the data were normalized to the internal control
GAPDH.
Cell viability assay
To detect the growth of glioma cells and the growth
curve, cell viability was assessed by MTT assay. Logarithmic phase
cells were seeded in 96-well culture plates at a density of
5×103 cells/well [the edge wells of the plate are filled
with aseptic phosphate-buffered saline buffer (PBS)]. The cells
were incubated at 37°C, 5% CO2 until cells covered the
bottom of the well. A total of 20 µl of the MTT solution was added
to each well (5 mg/ml, 0.5% MTT) and the cells were cultured for 4
h at 37°C. After the incubation, the supernatant was discarded and
150 µl dimethyl sulfoxide was added to each well. Afterwards, the
culture plate was shaken at low speed for 10 min until the crystals
dissolved completely. The ELISA reader was used to measure the
absorbance at 570 nm.
Colony formation assay
Cells in logarithmic growth phase were digested in
0.5% trypsin/0.04% EDTA and single cell suspension was prepared.
Then, these cells were added to 6-well plates (200 cells/well)
followed by incubation at 37°C in a humidified incubator containing
5% CO2 for 24 h. Non-adherent cells were removed. After
culture for 10–14 days, colonies were present. These cells were
seeded into 96-well plates followed by incubation at 37°C in an
environment with saturated humidity and 5% CO2. The
colony formation efficiency and the morphology of colonies were
photographed using a microscope. The colony size and cells in each
colony were measured.
Cell cycle assay
Total cells were collected by trypsinization. Bovine
pancreatic RNAse (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) was added at a final concentration of 2 µg/ml, incubated at
37°C for 30 min, then 20 µg/ml propidium iodide (PI) was added and
incubated for 20 min at 25°C. Cells (5×104) were
analyzed by FACSCalibur flow cytometer (Becton-Dickinson, Franklin
Lakes, NJ, USA) in each group.
Western blot analysis
Total cells were lysed using the RIPA (Bio-Rad
Laboratories, Inc., Philadelphia, PA, USA) buffer (50 mM Tris-HCl,
pH 8.0, 150 mM NaCl, 1 mM dithiothreitol, 0.1% SDS). Proteins were
separated by SDS-PAGE and transferred to polyvinylidene difluoride
(PVDF) membranes (Millipore, Bedford, MA, USA) at 55 V for 4 h at
4°C. After blocking, the membranes were incubated with primary
antibodies overnight at 4°C, washed three times with TBS Tween-20,
and followed by secondary antibodies conjugated with horseradish
peroxidase at 1:5,000 dilution in TBS for 1 h at 25°C. Western
blotting were visualized on X-ray film by an automated
chemiluminescence system.
Luciferase assay
For reporter assays, wild-type or mutation 3UTR
vector and the control vector pRL-CMV (cytomegalovirus; coding for
Renilla luciferase; Promega) were cotransfected. Luciferase
activity was measured 36 h after transfection. Firefly and
Renilla luciferase reporter activity was measured using
Luc-Pair Duo-luciferase Assay kit 2.0 as per manufacturers
instructions.
Migration assay
In order to evaluate the effect of metastatic
properties, Transwell migration assays was conducted. Glioma cells
(1×105) were placed in the top chamber onto the
non-coated membrane and allowed to migrate toward DMEM medium
containing fetal calf serum (FCS) in the lower chamber. After
incubation with methanol, cells were fixed for 24 h and stained
with 0.1% crystal violet (Sigma-Aldrich). The number of cells was
counted using a light microscope.
Invasion assay
In order to evaluate the effect of metastatic
properties, invasion assay was conducted. Cells (1×105)
were placed in the top chamber onto the Matrigel coated membrane.
Each well was coated freshly with Matrigel (60 µg; BD Biosciences,
San Jose, CA, USA). Cells were placed in serum-free medium or
growth factors, and medium containing serum was used as a
chemoattractant in the lower chamber. After incubating for 48 h,
cells that did invade via the pores were removed by a cotton swab.
Cancer stem cells (CSCs) on the lower surface of the membrane were
fixed in methanol and then stained with crystal violet. The number
of cells was counted using a light microscope (27).
Tumor sphere assay
Sphere formation assay (27) was performed as described. In brief,
cells were plated in 6-well ultralow attachment plates (Corning
Inc., Corning, NY, USA) at a density of 1×103 cells/ml
in DMEM supplemented with 1% N2 supplement (Invitrogen), 2% B27
supplement (Invitrogen), 20 ng/ml human platelet growth factor
(Sigma-Aldrich), 100 ng/ml epidermal growth factor (Invitrogen) and
1% antibiotic-antimycotic (Invitrogen) at 37°C in a humidified
atmosphere containing 5% CO2. Sphere were collected
after 7 days and dissociated with accutase (Innovative Cell
Technologies, Inc., San Diego, CA, USA). The cells obtained from
dissociation were sieved through a 40-µm filter, and counted by
coulter counter using trypan blue dye.
Mouse xenograft models
The mice (6-weeks-old) were purchased from Beijing
Weitongli, Co., Ltd. (Beijing, China). A total of
1.0×106 cells, either stably expressing glioma cells
LV-miR-30a or LV-miR control and LV-miR-30a with Wnt5a
overexpression or LV-miR control with Wnt5a overexpression, were
injected subcutaneously into the abdomen of each mouse,
respectively. After the tumors were ~100 mm3, mice were
examined for the effects of tumor burden and tumor growth, every
two days and tumor measurements were performed weekly. Tumor volume
was calculated using the formula: Tumor volume = [length ×
width2]/2 as previously reported. Approximately 3 weeks
after inoculation, the mice were euthanized by subcutaneous
injection with sodium pentobarbital (40 mg/kg) and the tumors were
weighed, all mice were handled according to the protocol approved
by the Committee on the Ethics of Animal Experiments of the
hospital. All tumors were dissected, and sizes and weights were
measured and recorded (28).
Statistical analysis
All data were expressed as the mean ± SD of at least
three independent experiments. Differences between the groups were
analyzed by one- or two-way ANOVA, followed by Bonferonis multiple
comparison tests using PRISM statistical analysis software (GrafPad
Software, Inc., San Diego, CA, USA). Differences at P<0.05 were
considered as significance level.
Results
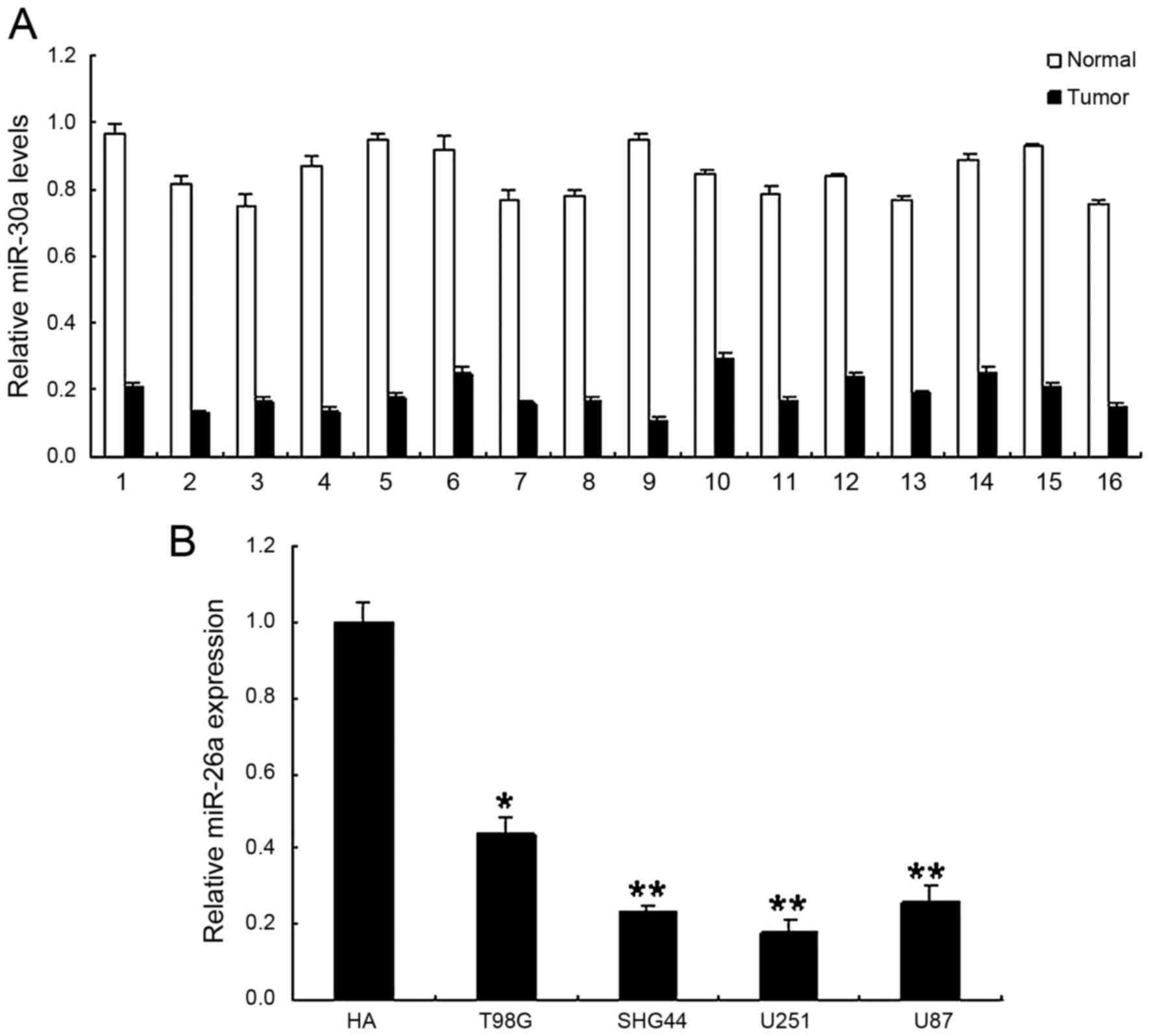
Low expression of miR-30a in human
glioma
To explore the possible action of miR-30a in glioma,
the expression of miR-30a was examined and quantified by real-time
RT-PCR at both tissue and cell levels. We examined the expression
of miR-30a in 16 cases with glioma and their compared normal
tissues. As shown in Fig. 1A, the
expression levels of miR-30a in glioma samples were lower than
those in normal samples. Similarly, miR-30a in human glioma cells
was shown significantly reduced comparing with control cells
(Fig. 1B). It is suggested that
miR-30a may function as a tumor suppressor in the progression of
human glioma.
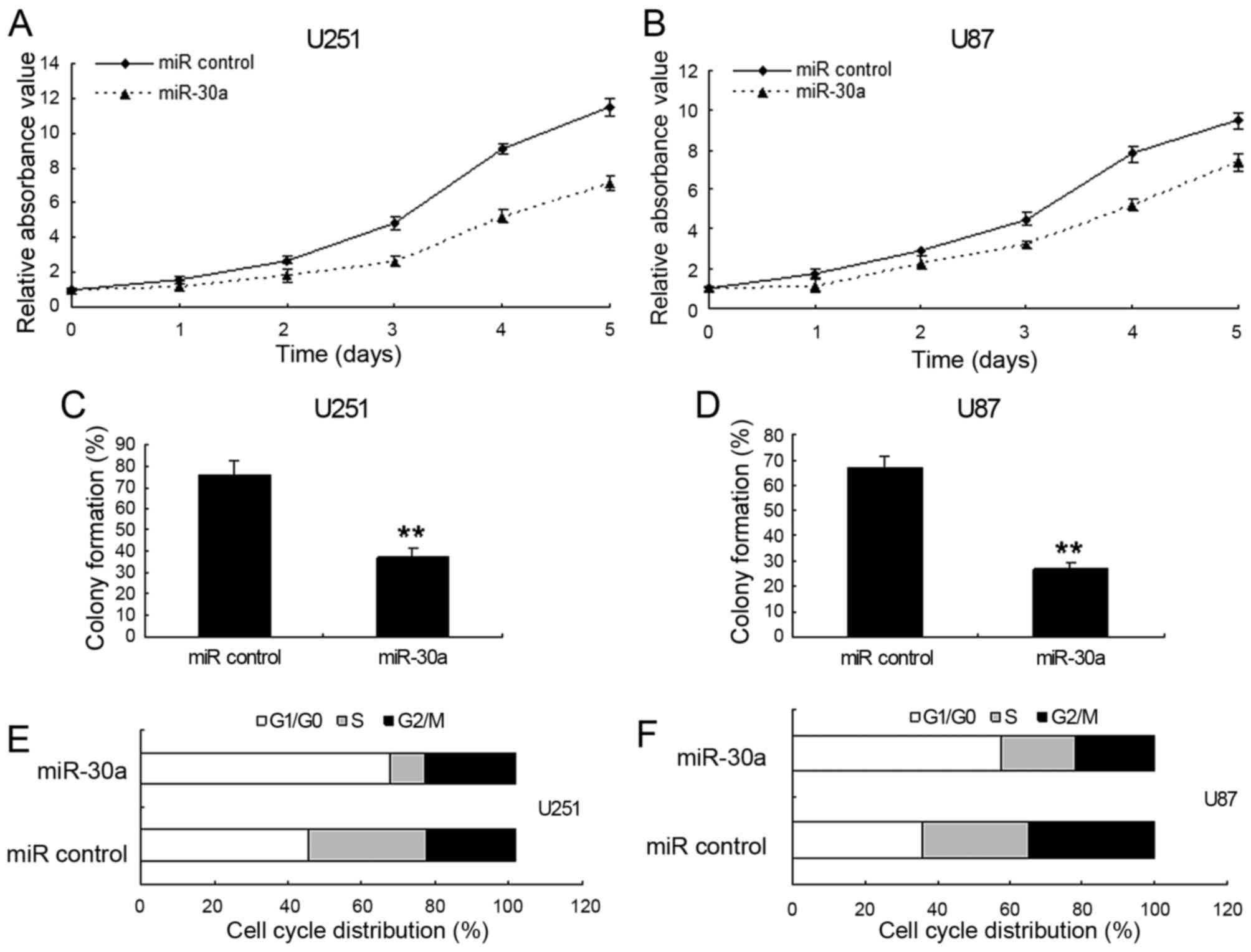
miR-30a suppresses proliferation and
mediates the accumulation of G1-phase glioma cells
To investigate the effect of miR-30a on cell
proliferation, U87 and U251 cells were transfected with
LV-miR-control or LV-miR-30a, respectively. MTT and colony
formation assay showed that miR-30a inhibited cell proliferation in
U251 cells (Fig. 2A and C) and U87
cells (Fig. 2B and D). In addition,
there was no difference on cell proliferation between cells with
the LV-miR-control and the control cells (data not shown). To
further ascertain miR-30a mediating growth inhibition, cells with
LV-miR-30a and cell cycle distribution were examined. U251 cells
infected with LV-miR-30a had an increased percentage of cells in
G1 phase but fewer cells in S phase comparing with the
control (Fig. 2E and F). As shown
in Fig. 2, there was a correlation
between the growth-suppressive effect of miR-30a and the
G0/G1 phase arrest. Therefore, the
accumulation of G1-phase glioma cells mediated by
miR-30a is as a direct cause of the cell proliferation
inhibition.
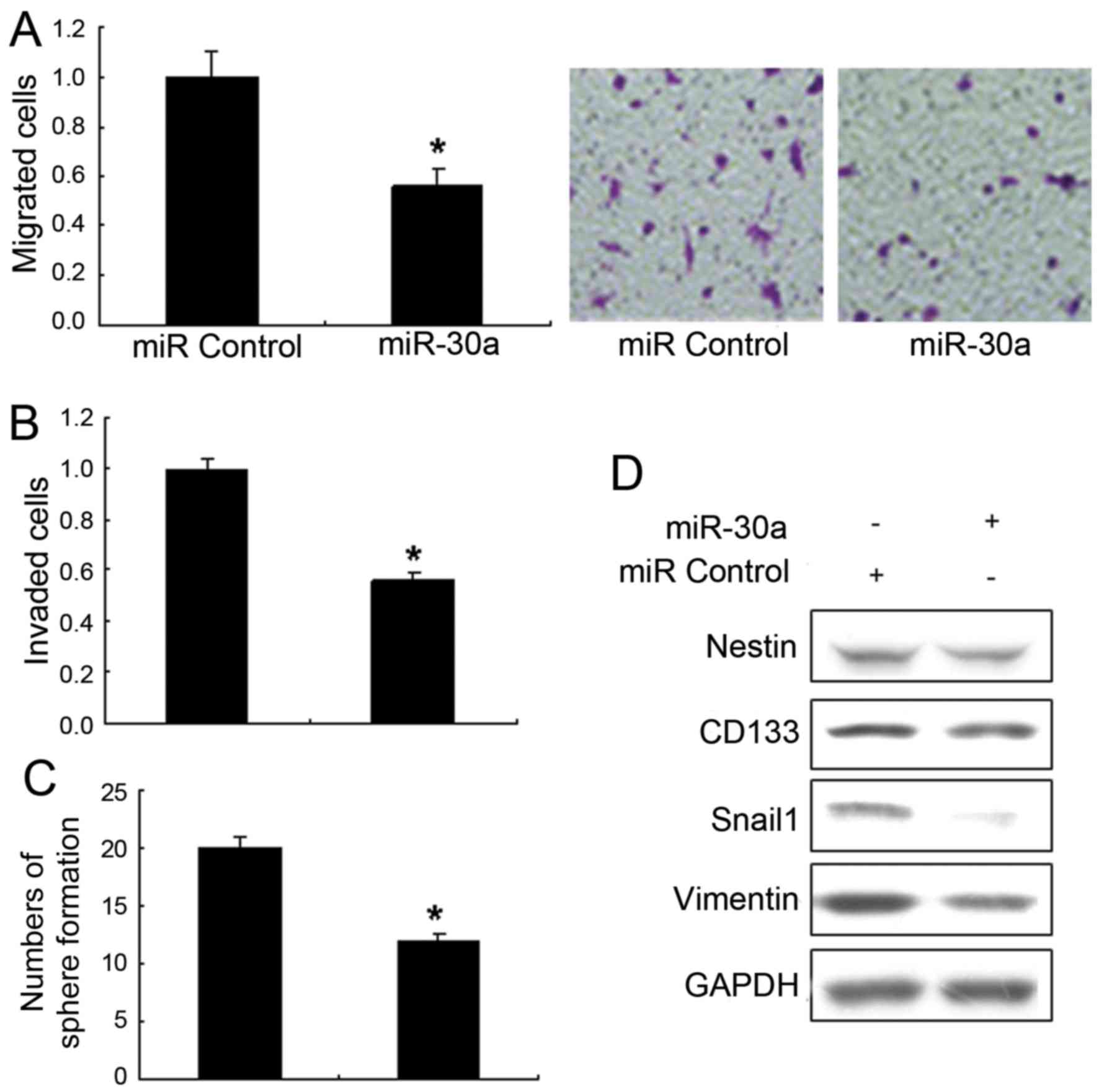
miR-30a inhibits stem cell-like
properties in glioma
To investigate the effects of miR-30a on glioma
metastasis and stem cell-like properties, the Transwell migration
assays and invasion assays of U251 cells with LV-miR-30a were
performed. It is shown that upregulation of miR-30a significantly
decreased migration (Fig. 3A) and
invasion (Fig. 3B) exposed to
TGF-β. The sphere formation of U251 cells with LV-miR-30a was much
lower than the control (Fig. 3C).
Metastasis associated markers were also detected by western blot
analysis and the results showed that vimentin and SNAIL1 decreased
in the U251 cells with LV-miR-30a (Fig.
3D). The above indicated that miR-30a prevented the glioma cell
from stem-like cells.
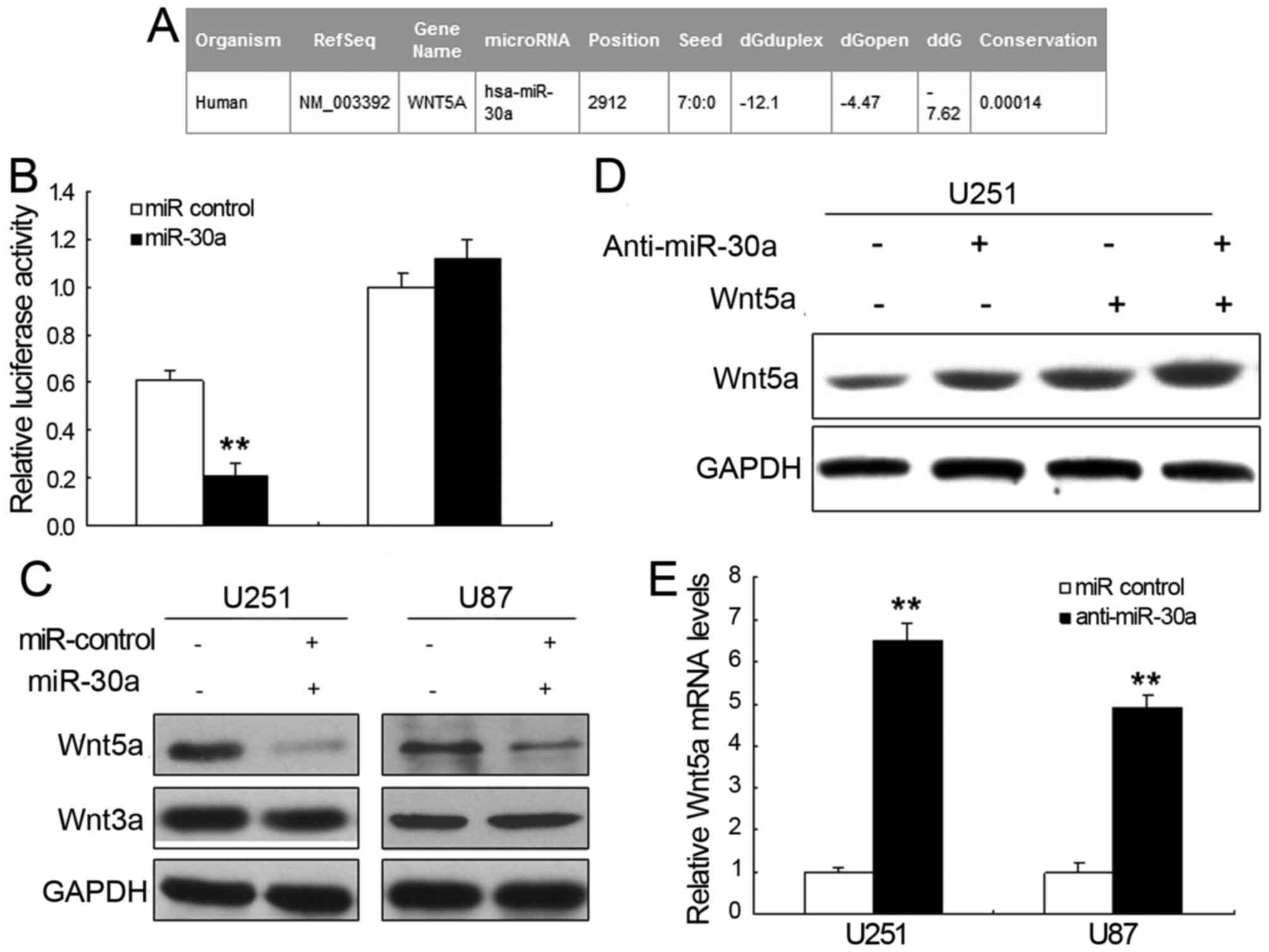
Wnt5a is a target gene for
miR-30a
To further explore the possible molecular mechanisms
of miR-30a-mediated glioma progress inhibition, we applied the
bioinformatic analysis to search the potential targets of miR-30a.
It is shown that Wnt5a is considered to be directly suppressed by
miR-30a (Fig. 4A). The luciferase
activity of pGL4-Wnt5α-WT in U251 cells was much lower than in
control cells (Fig. 4B). Moreover,
pGL4-Wnt5a-Mut luciferase activity was rescued. Furthermore, we
examined whether miR-30a could regulate the expression of
endogenous Wnt5a in U251 cells. Comparing with the control, the
mRNA levels of endogenous Wn5a (Fig.
4C) were downregulated when cells were transected with miR-30a.
The expression of Wnt5a increased in the cells with anti-miR-30a
(Fig. 4D and E). These data
indicated that Wnt5a acted as a new target gene for miR-30a.
miR-30a inhibits metastasis and sphere
formation by targeting Wnt5a signal pathway in glioma cells
In view of the fact that Wnt5a was the latent target
of miR-30a, over-expression of Wnt5a was performed to test whether
miR-30a regulates metastasis and sphere formation by targeting
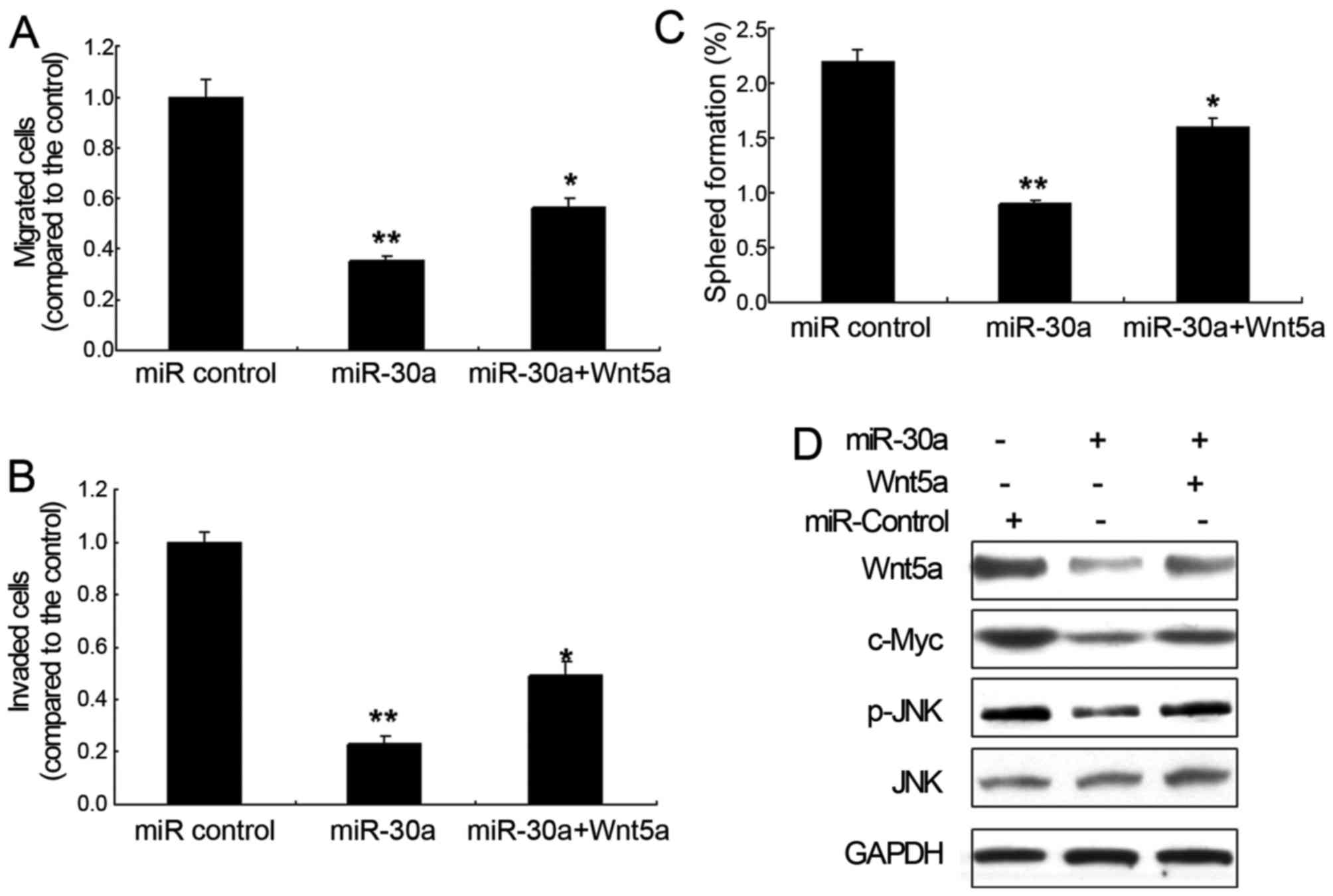
Wnt5a signal pathway in glioma cells. It is suggested that miR-30a
in U251 cells inhibited cell migration and invasion with Wnt5a
overexpression (Fig. 5A and B).
Sphere formation assay also demonstrated that miR-30a inhibited the
self-renewal ability of glioma cells (Fig. 5C). Since Wnt signaling pathway is an
important pathway involved in primary glioma, we then detected
several downstream proteins in Wnt signaling pathway. The
protooncogene cMyc, downstream of the Wnt singling pathway, was
decreased by miR-30a overexpression. A similar result was obtained
for phosphorylated JNK, an important downstream protein of Wnt5a
(Fig. 5D).
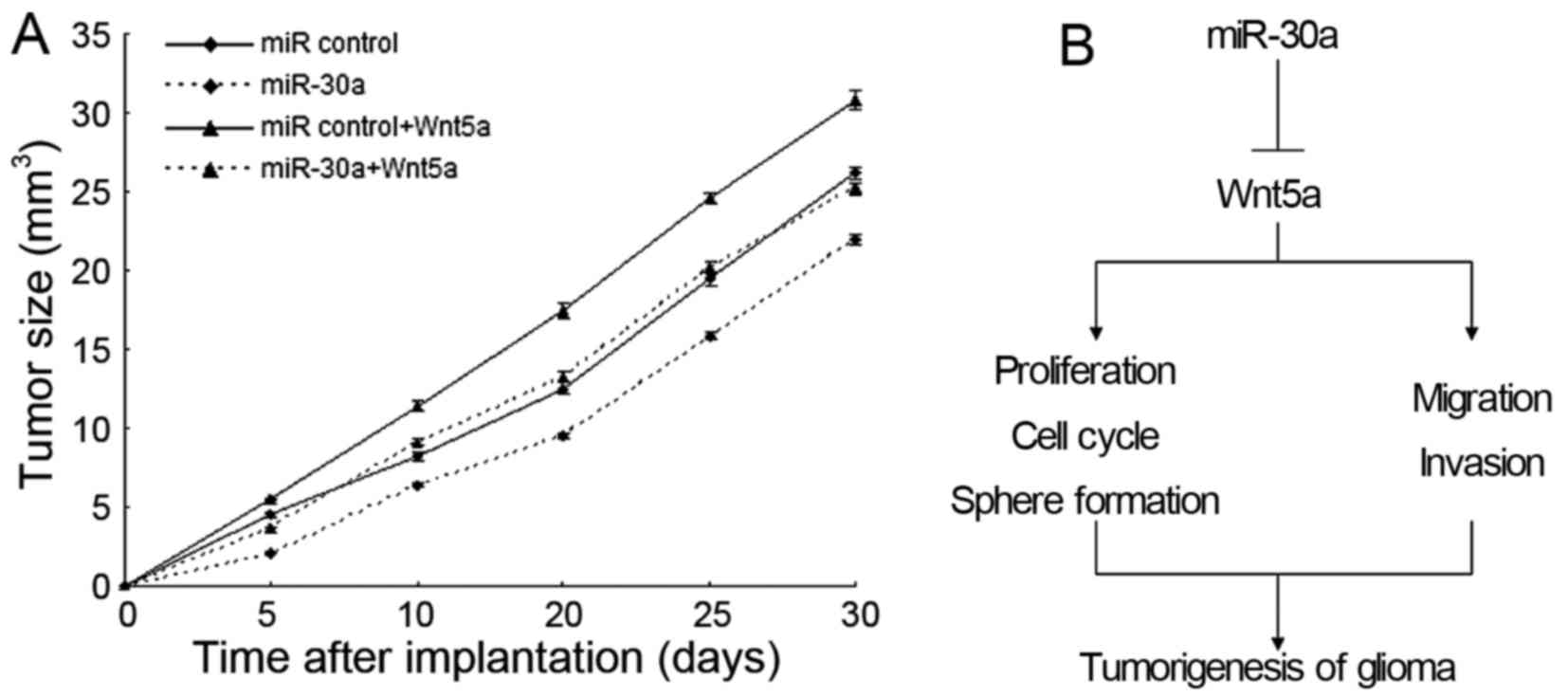
miR-30a inhibits glioma growth by
targeting Wnt5a signal pathway in vivo
Based on the above effect of miR-30a observed in
in vitro experiments, we further investigate miR-30a
mediating growth inhibition in vivo. The xenograft model of
glioma in nude mice was applied using U251-miR-30a and U251-miR
control. Next, we measured the tumor size every 5 days and plotted
the growth curve against the average tumor size. The results showed
that miR-30a suppressed glioma growth in vivo (Fig. 6A).
Discussion
Although previous studies have shown that miR-30a
may act as a tumor suppressor or oncogene, the detailed mechanism
of miR-30a involvement in glioma is not well understood. In the
present study, we found that the glioma tissues had a significant
downregulation of the expression of miR-30a comparing with the
normal tissues. From this finding, we aimed to clear the suppressor
role of miR-30a in glioma. Thus, we also discovered that miR-30a
induced G1 arrest of glioma cells and suppressed cell
proliferation. Our finding suggested that miR-30a suppressed the
progression of glioma by targeting Wnt5a for the first time. In
addition, overexpression of miR-30a in U251 cells restrained
oncogenesis in nude mice, which showed that miR-30a acts as a tumor
suppressor in glioma.
Over the past few decades, many studies have focused
on the cancer-related role of miR-30 family. This family is widely
known to be implicated in various cellular processes including cell
differentiation, organ development and substance metabolism
(29,30). Several studies indicated that
miR-30a could regulate breast cancer cell proliferation and
migration and reduce the oncogenic abilities of breast and lung
cancer depending on their targets (31–33).
In order to investigate potential target genes of miR-30a, we used
bioinformatics analyses to search the latent target genes and than
discovered that Wnt5a was a novel potential target gene of miR-30a.
Wnt family members are classified into two groups: Wnt1, Wnt3a and
Wnt7a, activate the β-catenin pathway, and have been shown to be
present in mammals (34,35). Another group including Wnt2, Wnt4,
Wnt5a, Wnt5b, Wnt6 and Wnt11, activate a β-catenin-independent
pathway that primarily regulates cell motility and polarity
(34,35). Moreover, β-catenin-independent
pathway is known to activate various protein kinases including
protein kinase C (PKC), Ca2+/calmodulin-dependent
protein kinase II, Rho-associated kinase and c-jun N-terminal
kinase (JNK). In particular, Wnt5a is a representative of the Wnt
family that activate the β-catenin-independent pathway and distinct
routes (36,37). Previous research showed that Wnt5a
stimulates proliferation and migration in cancer cells. Moreover,
Wnt5a expression is related with the aggressiveness of ocular
melanoma, lung, breast and gastric cancer, indicating that Wnt5a
has oncogenic properties (36,37). A
recent study showed that the glioma cells were found to express
significantly high levels of Wnt5a. It was also suggested that
Wnt5a promotes invasion activities of glial cells at least via the
activation and expression of JNK and matrix metalloproteinase-1
(MMP-1) (37). In this study, we
proved that Wnt5a was a novel target gene of miR-30a that could
inhibit metastasis and sphere formation.
In conclusion, we identified miR-30a as a tumor
suppressor miRNA in glioma, and low miR-30a expression may become
an unfavorable prognostic factor in patients with glioma.
Furthermore, miR-30a functions as the tumor suppressor in human
glioma by targeting Wnt5a. These findings indicated that miR-30a
acts as a potential target for treating glioma and the key function
of miR-30a in glioma oncogenesis. It also has great value in early
diagnosis, and in prognostic diagnosis. This study offers
theoretical basis to better understand the molecular mechanism of
glioma and its potential therapeutic strategies.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Gansu Province (no. 1606RJZA348) and the
Science and Technology Project of Lanzhou city (2015–2-67).
References
|
1
|
Godlewski J, Newton HB, Chiocca EA and
Lawler SE: MicroRNAs and glioblastoma; the stem cell connection.
Cell Death Differ. 17:221–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Wang H, Eyler CE, Hjelmeland AB and
Rich JN: Turning cancer stem cells inside out: An exploration of
glioma stem cell signaling pathways. J Biol Chem. 284:16705–16709.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gladson CL, Prayson RA and Liu WM: The
pathobiology of glioma tumors. Annu Rev Pathol. 5:33–50. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kartha RV and Subramanian S: Competing
endogenous RNAs (ceRNAs): New entrants to the intricacies of gene
regulation. Front Genet. 5:82014.eCollection. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li M, Li J, Liu L, Li W, Yang Y and Yuan
J: MicroRNA in human glioma. Cancers (Basel). 5:1306–1331. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Dutta A and Abounader R: The role
of microRNAs in glioma initiation and progression. Front Biosci
(Landmark Ed). 17:700–712. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong JW: MicroRNA-induced silencing of
glioma progression. J Neurosci. 30:3868–3869. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Wang B, Shi Y, Xu C, Xiao HL, Ma
LN, Xu SL, Yang L, Wang QL, Dang WQ, et al: Oncogenic miR-20a and
miR-106a enhance the invasiveness of human glioma stem cells by
directly targeting TIMP-2. Oncogene. 34:407–419. 2015. View Article : Google Scholar
|
|
10
|
Zhao D, Jiang X, Yao C, Zhang L, Liu H,
Xia H and Wang Y: Heat shock protein 47 regulated by miR-29a to
enhance glioma tumor growth and invasion. J Neurooncol. 118:39–47.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan X, Cheng Q, Peng R, Ma Z, Chen Z, Cao
Y and Jiang B: ROCK1, a novel target of miR-145, promotes glioma
cell invasion. Mol Med Rep. 9:1877–1882. 2014.PubMed/NCBI
|
|
12
|
Guo M, Jiang Z, Zhang X, Lu D, Ha AD, Sun
J, Du W, Wu Z, Hu L, Khadarian K, et al: miR-656 inhibits glioma
tumorigenesis through repression of BMPR1A. Carcinogenesis.
35:1698–1706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang D, Yang G, Chen X, Li C, Wang L, Liu
Y, Han D, Liu H, Hou X, Zhang W, et al: mir-300 promotes
self-renewal and inhibits the differentiation of glioma stem-like
cells. J Mol Neurosci. 53:637–644. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 105:265–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li SZ, Hu YY, Zhao J, Zhao YB, Sun JD,
Yang YF, Ji CC, Liu ZB, Cao WD, Qu Y, et al: MicroRNA-34a induces
apoptosis in the human glioma cell line, A172, through enhanced ROS
production and NOX2 expression. Biochem Biophys Res Commun.
444:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Chen X, Lian H, Liu J, Zhou B,
Han S, Peng B, Yin J, Liu W and He X: MicroRNA-503 acts as a tumor
suppressor in glioblastoma for multiple antitumor effects by
targeting IGF-1R. Oncol Rep. 31:1445–1452. 2014.PubMed/NCBI
|
|
17
|
Chen Z, Li D, Cheng Q, Ma Z, Jiang B, Peng
R, Chen R, Cao Y and Wan X: MicroRNA-203 inhibits the proliferation
and invasion of U251 glioblastoma cells by directly targeting PLD2.
Mol Med Rep. 9:503–508. 2014.PubMed/NCBI
|
|
18
|
Alrfaei BM, Vemuganti R and Kuo JS:
microRNA-100 targets SMRT/NCOR2, reduces proliferation, and
improves survival in glioblastoma animal models. PLoS One.
8:e808652013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo P, Nie Q, Lan J, Ge J, Qiu Y and Mao
Q: C-Myc negatively controls the tumor suppressor PTEN by
upregulating miR-26a in glioblastoma multiforme cells. Biochem
Biophys Res Commun. 441:186–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang J, Yang J, Wang Z, Wu G and Liu F:
TFAM is directly regulated by miR-23b in glioma. Oncol Rep.
30:2105–2110. 2013.PubMed/NCBI
|
|
21
|
Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin
W and Zhang Y: MicroRNA-218 inhibits glioma invasion, migration,
proliferation, and cancer stem-like cell self-renewal by targeting
the polycomb group gene Bmi1. Cancer Res. 73:6046–6055. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu
Z, Hu G and Yang Q: MicroRNA-30a suppresses breast tumor growth and
metastasis by targeting metadherin. Oncogene. 33:3119–3128. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu H, Wu H, Liu X, Li B, Chen Y, Ren X,
Liu CG and Yang JM: Regulation of autophagy by a beclin 1-targeted
microRNA, miR-30a, in cancer cells. Autophagy. 5:816–823. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhong M, Bian Z and Wu Z: miR-30a
suppresses cell migration and invasion through downregulation of
PIK3CD in colorectal carcinoma. Cell Physiol Biochem. 31:209–218.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ouzounova M, Vuong T, Ancey PB, Ferrand M,
Durand G, Kelm Le-Calvez F, Croce C, Matar C, Herceg Z and
Hernandez-Vargas H: MicroRNA miR-30 family regulates non-attachment
growth of breast cancer cells. BMC Genomics. 14:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao S, Ye X, Xiao L, Lian X, Feng Y, Li F
and Li L: MiR-26a inhibits prostate cancer progression by
repression of Wnt5a. Tumour Biol. 35:9725–9733. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu Y, Dong L, Bao S, Wang M, Yun Y and Zhu
R: FK228 augmented temozolomide sensitivity in human glioma cells
by blocking PI3K/AKT/mTOR signal pathways. Biomed Pharmacother.
84:462–469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shivdasani RA: MicroRNAs: Regulators of
gene expression and cell differentiation. Blood. 108:3646–3653.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen S: MicroRNAs in animal development.
Editorial. Semin Cell Dev Biol. 21:7272010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou Z, Ni M, Zhang J, Chen Y, Ma H, Qian
S, Tang L, Tang J, Yao H, Zhao C, et al: MiR-30a can inhibit DNA
replication by targeting RPA1 thus slowing cancer cell
proliferation. Biochem J. 473:2131–2139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumarswamy R, Mudduluru G, Ceppi P,
Muppala S, Kozlowski M, Niklinski J, Papotti M and Allgayer H:
MicroRNA-30a inhibits epithelial-to-mesenchymal transition by
targeting Snai1 and is downregulated in non-small cell lung cancer.
Int J Cancer. 130:2044–2053. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng CW1, Wang HW, Chang CW, Chu HW, Chen
CY, Yu JC, Chao JI, Liu HF, Ding SL and Shen CY: MicroRNA-30a
inhibits cell migration and invasion by downregulating vimentin
expression and is a potential prognostic marker in breast cancer.
Breast Cancer Res Treat. 134:1081–1093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kikuchi A, Yamamoto H, Sato A and
Matsumoto S: Wnt5a: Its signalling, functions and implication in
diseases. Acta Physiol (Oxf). 204:17–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nishita M, Enomoto M, Yamagata K and
Minami Y: Cell/tissue-tropic functions of Wnt5a signaling in normal
and cancer cells. Trends Cell Biol. 20:346–354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McDonald SL and Silver A: The opposing
roles of Wnt-5a in cancer. Br J Cancer. 101:209–214. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Katoh M: WNT signaling in stem cell
biology and regenerative medicine. Curr Drug Targets. 9:565–570.
2008. View Article : Google Scholar : PubMed/NCBI
|