Introduction
Lung cancer is the leading cause of cancer-related
death worldwide, and non-small cell lung cancer (NSCLC) accounts
for 80% of all lung cancer cases (1). Although huge progress has been
achieved in the diagnosis and treatment of NSCLC, more than 70% of
the patients lose the opportunity of surgery due to advanced tumor
stage. In addition, the 5-year survival rate for NSCLC patients
with this stage is less than 15% (2). Therefore, it is important to explore
specific biomarkers for the diagnosis of early stage NSCLC. As a
group of endogenous non-coding small RNAs (18–23 nt), microRNAs
(miRNAs) are known to regulate the translation of mRNAs as target
genes through directly binding to their 3′-untranslated region
(3-UTR). Many studies have shown that dysfunction of miRNAs promote
the development of a variety of malignant tumors, including lung
cancer.
miR-367, an miRNA located on chromosome 4 q25,
belongs to the miRNA-302/367 cluster (3). Previous studies have mainly
investigated the functions of miR-367 in embryonic stem cell
self-renewal and the maintain of pluripotency (4). Recent studies revealed that miR-367
participates in the tumorigenesis of various types of cancers, such
as hepatocellular carcinoma (5),
medulloblastoma (6) and pancreatic
cancer (7). In contrast, in the
study of gastric carcinoma, miR-367 inhibited cell migration and
invasion, and its expression in cancer tissues was much lower than
that in normal stomach tissues (8).
This implied that miR-367 may play different roles in various
tumors. In the study of NSCLC, miR-367 was expressed higher in
cancer tissues than that in normal lung tissue, and its high
expression was closely related to the poor prognosis of the
patients (9). However, its
mechanisms in the tumorigenesis of NSCLC are still unknown.
F-box/WD-40 domain protein 7 (FBXW7), one of the
best studied F-box proteins, participates in ubiquitination and
degradation of target proteins, such as c-Myc, c-Jun and cyclin E
(10). Most of its target proteins
are oncoproteins, and low expression of FBXW7 is correlated with
the poor prognosis of cancer patients; thus, it was recognized as a
powerful tumor suppressor. FBXW7 takes part in multiple signaling
pathways, including cell proliferation, apoptosis, migration and
metastasis. Previous studies have shown that the expression of
Fbxw7 is regulated by multiple transcription factors, among which
miRNA is an important post-transcriptional regulatory factor. For
instance, miR-223 (11) and miR-25
(12) promote proliferation and
inhibit apoptosis in gastric cancer by directly suppressing
FBXW7.
In the present study, we found that the level of
miR-367 was higher in NSCLC tissues and cells than levels in normal
lung tissues and cells, and its high expression was indicative of a
poor patient prognosis. However, the levels of miR-367 and FBXW7
were negative correlated in NSCLC tissues. In addition, we
demonstrated that miR-367 promoted cell proliferation, cell cycle
progression and inhibited apoptosis by directly binding to and
inhibiting FBXW7 in vivo and in vitro. Therefore,
miR-367 may be a crucial target for the early diagnosis and
treatment of NSCLC.
Materials and methods
Patients and clinical specimens
Sixty NSCLC patients who underwent pulmonary surgery
in our department from January 2013 to January 2016 were included
in the present study. None of the patients had received neoadjuvant
radiotherapy/chemotherapy before surgery. All patients with NSCLC
were confirmed by histopathologic evaluation. Written informed
consent was obtained from the patients in accordance with the
Declaration of Helsinki before sample collection. Immediately after
resection, NSCLC and matched normal adjacent tissues (not less than
50 mm away from the NSCLC) specimens were stored at −80°C until
required. The present study was approved by the Ethics Committee of
The First Affiliated Hospital of Xi'an Jiaotong University.
Clinicopathological parameters are listed in Table I.
 | Table I.Correlation between the expression of
miR-367 and the clinicopathological characteristics of the NSCLC
patients. |
Table I.
Correlation between the expression of
miR-367 and the clinicopathological characteristics of the NSCLC
patients.
|
|
| miR-367 expression
level |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | No. of cases
(n=60) | High (n=35) | Low (n=25) | P-value |
|---|
| Age (years) |
|
|
| 0.124 |
|
<60 | 38 | 25 | 13 |
|
|
≥60 | 22 | 10 | 12 |
|
| Sex |
|
|
| 0.853 |
|
Male | 40 | 23 | 17 |
|
|
Female | 20 | 12 | 8 |
|
| Tumor size
(cm) |
|
|
| 0.003b |
| ≤3 | 25 | 9 | 16 |
|
|
>3 | 35 | 26 | 9 |
|
| TNM tumor
stage |
|
|
| 0.034a |
|
I+II | 35 | 16 | 17 |
|
|
III+IV | 25 | 19 | 6 |
|
| Histology |
|
|
| 0.582 |
|
Adenocarcinoma Squamous
cell | 40 | 25 | 15 |
|
|
Carcinoma | 13 | 7 | 6 |
|
|
Other | 7 | 3 | 4 |
|
| Degree of
differentiation |
|
|
| 0.001b |
| Well
and moderate | 24 | 8 | 16 |
|
|
Poor | 36 | 27 | 9 |
|
| Lymph node
metastasis |
|
|
| 0.193 |
|
Yes | 37 | 24 | 13 |
|
| No | 23 | 11 | 12 |
|
| Smoking
history |
|
|
| 0.430 |
|
Yes | 44 | 27 | 17 |
|
| No | 16 | 8 | 8 |
|
Cell culture and transfection
Human bronchial epithelial cells (Beas-2B) and three
NSCLC cell lines (A549, H460 and H1299) were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA), while
the 95D cell line was obtained from the China Center for Type
Culture Collection (CCTCC; Wuhan, China). All cell lines were
incubated at 37°C in a humidified atmosphere with 5%
CO2. Beas-2B, A549, H460 and 95D cells were grown in
Dulbeccos modified Eagles medium (DMEM) and H1299 was grown in
Roswell Park Memorial Institute (RPMI)-1640 medium (both from
HyClone, Logan, UT, USA), supplemented with 10% heat inactivated
fetal bovine serum (FBS; Excell Bio, Shanghai, China), penicillin
(100 µg/ml), streptomycin (100 mg/ml) solution (HyClone). For
miR-367 transient transfection, cells at 50% confluence were
transfected with micrON™ hsa-miR-367-3p mimic, micrOFF™
hsa-miR-367-3p inhibitor (RiboBio) or corresponding non-specific
ncRNA controls without homologous to any known human gene sequences
(both from RiboBio, Guangzhou, China), using X-tremeGENE siRNA
Transfection Reagent (Roche Diagnostics, Indianapolis, IN, USA).
FBXW7-specific siRNA and control siRNA were previously reported
(13).
Vector constructs
To construct the FBXW7 expression vector, the open
reading frame (ORF) of the human FBXW7 gene (without the 3′-UTR)
was amplified by PCR and subcloned into pcDNA3.0 (Invitrogen, Life
Technologies, Carlsbad, CA, USA). The fragment of FBXW7 3′-UTR
containing the predicted or mutated miR-367-3p binding sites was
subcloned into the XhoI site downstream of firefly
luciferase in pGL3 control vector (Promega, Madison, WI, USA). The
recombinant plasmids were renamed as FBXW7-3′-UTR-wt or
FBXW7-3′-UTR-mt. Lipofectamine 3000 (Invitrogen) was used for
transfection of plasmid alone or along with RNA oligonucleotides,
according to the manufacturer's instructions.
Quantitative real-time PCR (qRT-PCR)
and western blotting
Total RNA were extracted from NSCLC tissues or cell
lines using TRIzol reagent (Invitrogen). Total mRNA was
reverse-transcribed into cDNA using Mir-X miRNA qRT-PCR SYBR Kit or
Universal cDNA Synthesis Kit II (Takara, Dalian, China) according
to the manufacturer's instructions. With the use of a CFX96
Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA),
real-time quantitative PCR (RT-qPCR) was performed in triplicate
for each sample in a 25-µl reaction volume with SYBR®
Premix Ex Taq™ II (Takara). The expression of FBXW7 and miR-367
relative to endogenous control (GAPDH or U6) were calculated using
the 2−ΔΔCt method. The primers specific for miR-367-3p
were obtained from RiboBio, and other primer sequences are listed
in Table II and synthesized by
Sangon Biotech (Shanghai, China).
 | Table II.Human primer sequences used for
qRT-PCR. |
Table II.
Human primer sequences used for
qRT-PCR.
| Primers | Forward (5–3) | Reverse (5–3) |
|---|
| FBXW7 |
CACTCAAAGTGTGGAATGCAGAGAC |
GCATCTCGAGAACCGCTAACAA |
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
| GAPDH |
CGGAGTCAACGGATTTGGTCGTAT |
AGCCTTCTCCATGGTGAAGAC |
Protein extraction and western blot analysis were
performed as previously described (14). The primary antibodies used for
western blotting were as follows: FBXW7 (1:1,000; ab109617; Abcam,
Cambridge, MA, USA), c-Myc (1:500; 10828-1-AP; Proteintech, Wuhan,
China), c-Jun (1:500; ab32385; Abcam). β-actin (1:1,000;
20536-1-AP; Proteintech).
Cell proliferation assay
Cell proliferation of the NSCLC cell lines was
evaluated using a WST-8 assay as previously described (15). After being routinely incubated for
24 h in different treatments, cells were seeded into 96-well plates
at a density of 2×103 cells/well and cultured for
another 24, 48 and 72 h, separately. The cells were then washed
with phosphate-buffered saline (PBS), and incubated in complete
medium with 10% Cell Counting Kit-8 (CCK-8) solution (Beyotime,
Beijing, China) at 37°C for 4 h. The absorbance of each well was
measured using a mircoplate reader at 450 nm. These experiments
were carried out in triplicate.
Cell cycle assay
After transfection with the plasmids or RNA
oligonucleotides for 48 h, cells were washed twice with cold PBS
and fixed with 500 µl of 70% cold ethanol at 4°C overnight. The
cells were then incubated with 100 µl RNase at 37°C for 30 min and
stained with 400 mg/ml propidium iodide (PI) (KGA512; KeyGen,
Nanjing, China), and analyzed using FACSCalibur flow cytometry (BD
Biosciences, Franklin Lakes, NJ, USA).
Cell apoptosis assay
Cell apoptosis was assessed by flow cytometry with
Annexin V FITC/PI apoptosis detection kit (BD Biosciences).
Briefly, cells were collected and suspended in binding buffer, and
then stained using DNA staining solution (Annexin V-FITC and PI).
Subsequently, early and late apoptotic, or necrotic cells were
quantified and analyzed using FACSCalibur flow cytometry.
Luciferase reporter assays
For analysis of luciferase activity, HEK293 cells
were seeded at a density of 2×105/well of 24-well plates
for 24 h before transfection. The cells were co-transfected with
luciferase reporter vectors (FBXW7-3′-UTR-wt or FBXW7-3′-UTR-mt)
and miR-367-3p mimics/inhibitor or non-specific ncRNA controls,
using Lipofectamine 3000. Renilla luciferase plasmid (100
ng/well; Promega) was used as an internal control and
co-transfected with the described vectors. Luciferase activities
(firefly and Renilla) were determined 48 h after
transfection using the Dual-Luciferase® Reporter Assay
System (Promega). Each luciferase assay was performed in
triplicate.
Animals and in vivo treatment
Four-week-old BALB/c athymic nude mice (bisexual
each half) were obtained from the Center of Laboratory Animals of
Xi'an Jiaotong University. All experimental procedures involving
animals were approved by the Ethics Committee of the First
Affiliated Hospital of Xi'an Jiaotong University. Antagomir-367-
and antagomir-NC-treated H1299 cells (1×107) were
suspended in 150 µl PBS, and then subcutaneously inoculated into
the flank of nude mouse. After two weeks, tumor volume (V) was
determined by measuring tumor length (L) and width (W) once every
three days with a Vernier caliper, and then calculated in
accordance with the formula: V = (L × W2) × 0.5. At the
end of the experiment, all mice were sacrificed at 30 days after
cell injection, and xenograft tumors were removed and weighed. The
expression of miR-367 and FBXW7 in the isolated tumor tissues was
detected using qRT-PCR and western blotting.
Statistical analysis
All data were analyzed using SPSS 20 and GraphPad
Prism 5. All numerical data are shown as mean ± standard deviation
(SD). Comparison between different groups was carried out using
t-test or ANOVA, as appropriate. The associations between the level
of miR-367 and clinicopathological parameters were evaluated using
Chi-square (χ2) or Fisher's exact test. Univariate
survival analysis was performed using the Kaplan-Meier method and
log-rank test. Pearson correlation coefficient was used to examine
the correlation between miR-367 and FBXW7 mRNA. Each experiment was
repeated in triplicates. P<0.05 was considered statistically
significant.
Results
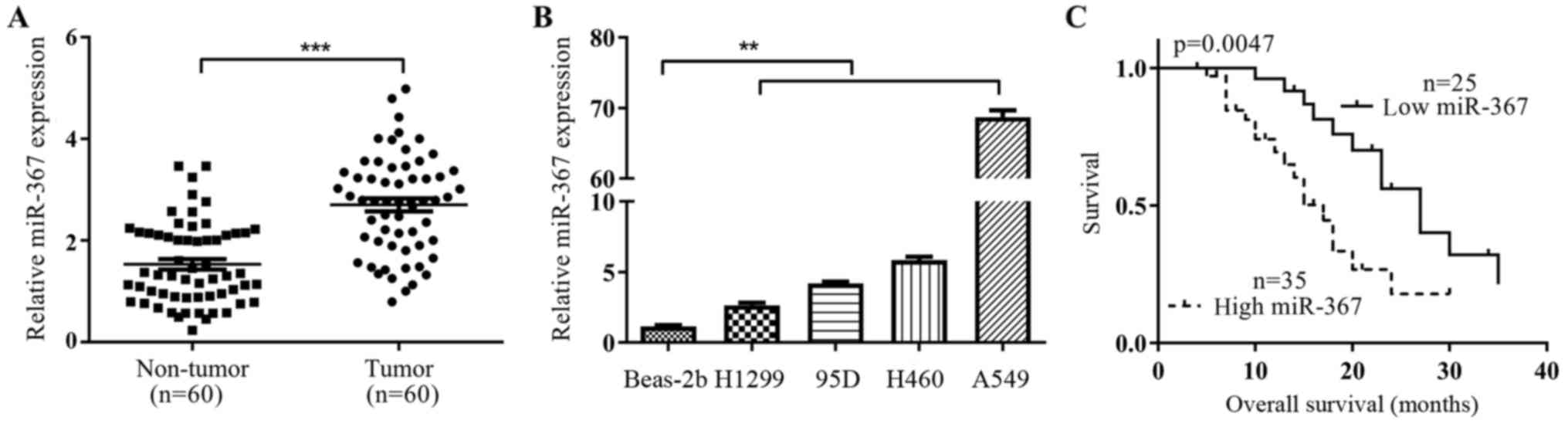
miR-367 is highy expressed in
NSCLC
To identify the level of miR-367 in NSCLC tissues
and cells, we examined the RNA level in 60 pairs of NSCLC tissues
and non-tumor adjacent tissues by qRT-PCR. The result revealed that
miR-367 RNA level was significantly higher in tumor tissues
compared with matched adjacent non-tumor tissues (p<0.001;
Fig. 1A). Similar to the
observations in clinical samples, miR-367 expression was also found
to be significantly higher in NSCLC cell lines (A549, H1299, H460
and 95D) as compared to the normal bronchial epithelial cells
Beas-2B (p<0.01; Fig. 1B). To
further identify the importance of miR-367 in NSCLC progression,
the correlation between miR-367 expression and clinicopathological
characteristics was analyzed. As shown in Table I, high expression of miR-367 was
positively associated with tumor-node-metastasis (TNM) (p=0.034),
degree of differentiation (p=0.001), tumor size (p=0.003).
Moreover, we investigated the prognostic value of miR-367. NSCLC
patients with high expression of miR-367 had shorter overall
survival (OS) (p=0.0047; Fig. 1C)
using Kaplen-Merier analysis. Therefore, miR-367 may be a very
important prognostic indicator in NSCLC.
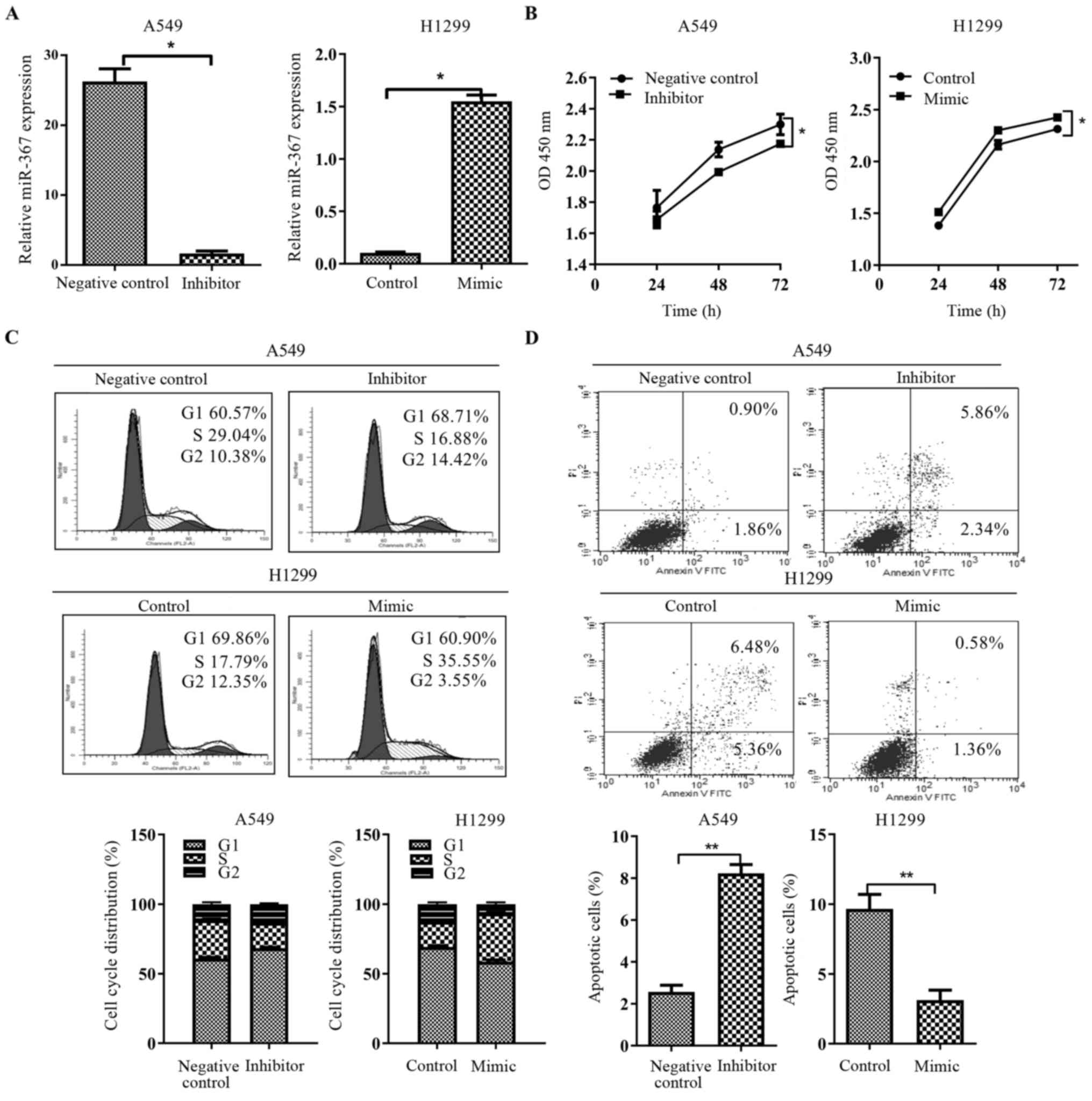
Effect of miR-367 on proliferation,
cell cycle distribution and apoptosis in NSCLC cells
Overexpression or loss of miRNA is normally
associated with the change in biological functions. We ectopically
expressed miR-367 using miR-367-3p mimics in the H1299 cell line
and inhibited endogenous miR-367 activity through miR-367-3p
inhibitors in the A549 cell line. After transfection, the
expression of miR-367 was significantly changed in the A549 and
H1299 cells (p<0.01; Fig. 2A).
Next, we examined the effects of miR-367 on cell proliferation,
cell cycle and apoptosis. CCK-8 assays demonstrated a statistically
significant increase in proliferation after miR-367 upregulation,
whereas downregulation of miR-367 in A549 cells showed a
significant decrease in cell proliferation as compared to the
control cells (p<0.01; Fig. 2B).
Furthermore, as determined by flow cytometric analysis, miR-367
overexpression significantly decreased the cellular population of
the G0/G1 phase and led to a significant increase in S phase cells
(p<0.05; Fig. 2C), while its
downregulation had the opposite result (p<0.05; Fig. 2C). Moreover, apoptosis assay
determined by flow cytometry, presented that miR-367 overexpression
prominently reduced the apoptosis ratio of H1299 cells (p<0.05;
Fig. 2D), but the inhibition of
miR-367 markedly increased the percentage of apoptotic A549 cells
(p<0.05; Fig. 2D). Taken
together, these data suggest an essential role for miR-367 as a
mediator of the biological effects of NSCLC cells.
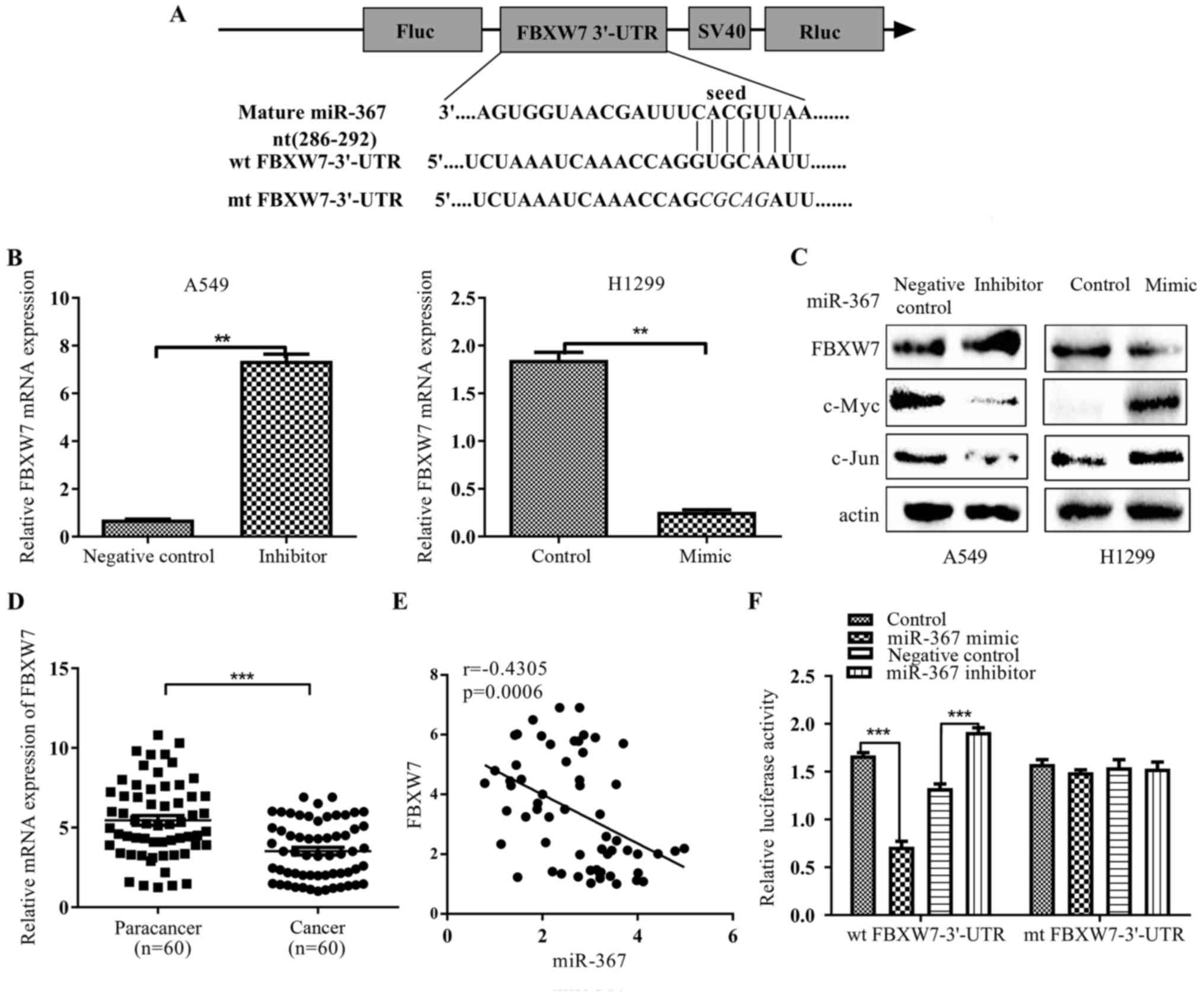
miR-367 negatively regulates FBXW7 in
NSCLC cells
To ascertain which potential targets are responsible
for the biological functions of miR-367 in NSCLC cells, we screened
for potential targets of miR-367-3p using three miRNA target
prediction programs (miRanda, TargetScan and PicTar). Eventually,
we predicted that FBXW7 may be a target of miR-367, as 3′-UTR of
FBXW7 mRNA harbors a highly conserved binding site that is
complementary to the miR-367 seed sequence (Fig. 3A). In order to validate the role of
miR-367 in FBXW7 expression regulation, we detected the mRNA
expression of FBXW7 in NSCLC cells transfected with miR-367 mimics
or inhibitors, respectively. miR-367 mimic significantly inhibited
the mRNA and protein levels of FBXW7 in H1299 cells (p<0.05;
respectively, Fig. 3B and C), while
inhibition of endogenous miR-367 using a synthetic miR-367
inhibitor significantly enhanced FBXW7 expression in A549 cells
(p<0.05; respectively, Fig. 3B and
C). In addition, we also detected the protein expression of
c-Myc and c-Jun, which could be directly downregulated by FBXW7. In
contrast to FBXW7, the protein expression of c-Myc and c-Jun were
reversely changed after miR-367 mimic or inhibitor treatment
(p<0.05; Fig. 3C). To further
analyze the correlation between miR-367 and FBXW7 in NSCLC tissues.
We examined mRNA levels of FBXW7 in 60 paired clinical NSCLC and
non-cancerous tissues. Our data revealed that the mRNA expression
levels of FBXW7 were decreased in NSCLC tissues compared with the
adjacent normal tissues (p<0.05; Fig. 3D). Markedly, The Pearson's
correlation analysis showed that miR-367 was inversely correlated
with FBXW7 expression in 60 samples (r=−0.4305, p=0.0006) (Fig. 3E). We then utilized a
dual-luciferase reporter system to determine whether miR-367-3p
effectively targets the 3′-UTR of FBXW7. Luciferase reporter assays
revealed that ectopic expression of miR-367-3p mimics inhibited the
luciferase activity of FBXW7-3′-UTR-wt reporter in HEK-293, whereas
miR-367 knockdown significantly increased the luciferase activity
of FBXW7-3′-UTR-wt reporter (p<0.001; Fig. 3F). In contrast, no significant
luciferase alteration was observed in cells transfected with mutant
vector. These results strongly indicated that FBXW7 is a direct
target of miR-367-3p.
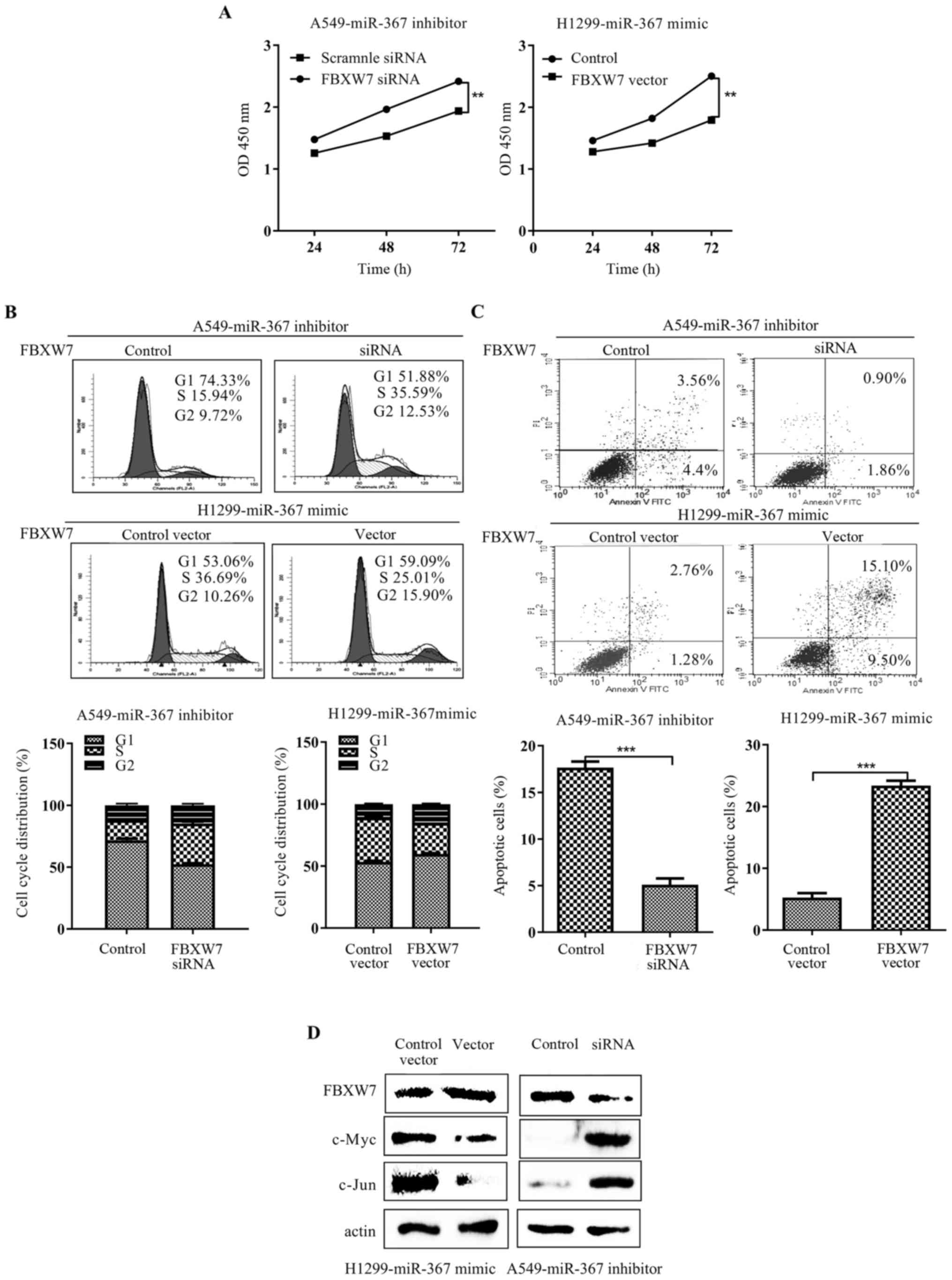
FBXW7 partly reverses the effect of
miR-367 on NSCLC cells
Most of the biological effects of miRNAs are altered
through regulation of their target genes. Thus, we assessed the
influence of FBXW7 alteration for the biological function of
miR-367. Then, we knocked down or upregulated the expression of
FBXW7 using siRNA or pcDNA3.0-FBXW7, respectively. As expected,
FBXW7 expression plasmid efficiently abrogated the cell
proliferation, cell cycle acceleration and apoptosis inhibition
induced by miR-367 mimic in H1299 cells (p<0.01; respectively,
Fig. 4A-C). Meanwhile, the effects
of miR-367 inhibitor on cell proliferation, cell cycle and
apoptosis were attenuated by siFBXW7 in the A549 cells (p<0.01;
respectively, Fig. 4A-C). Finally,
restoration of FBXW7 expression suppressed c-Myc and c-Jun
expression (Fig. 4D). Conversely,
FBXW7 silencing increased the expression of c-Myc and c-Jun
(Fig. 4D). Taken together, these
results demonstrated that FBXW7 mediated the carcinogenesis
properties of miR-367 in NSCLC cells.
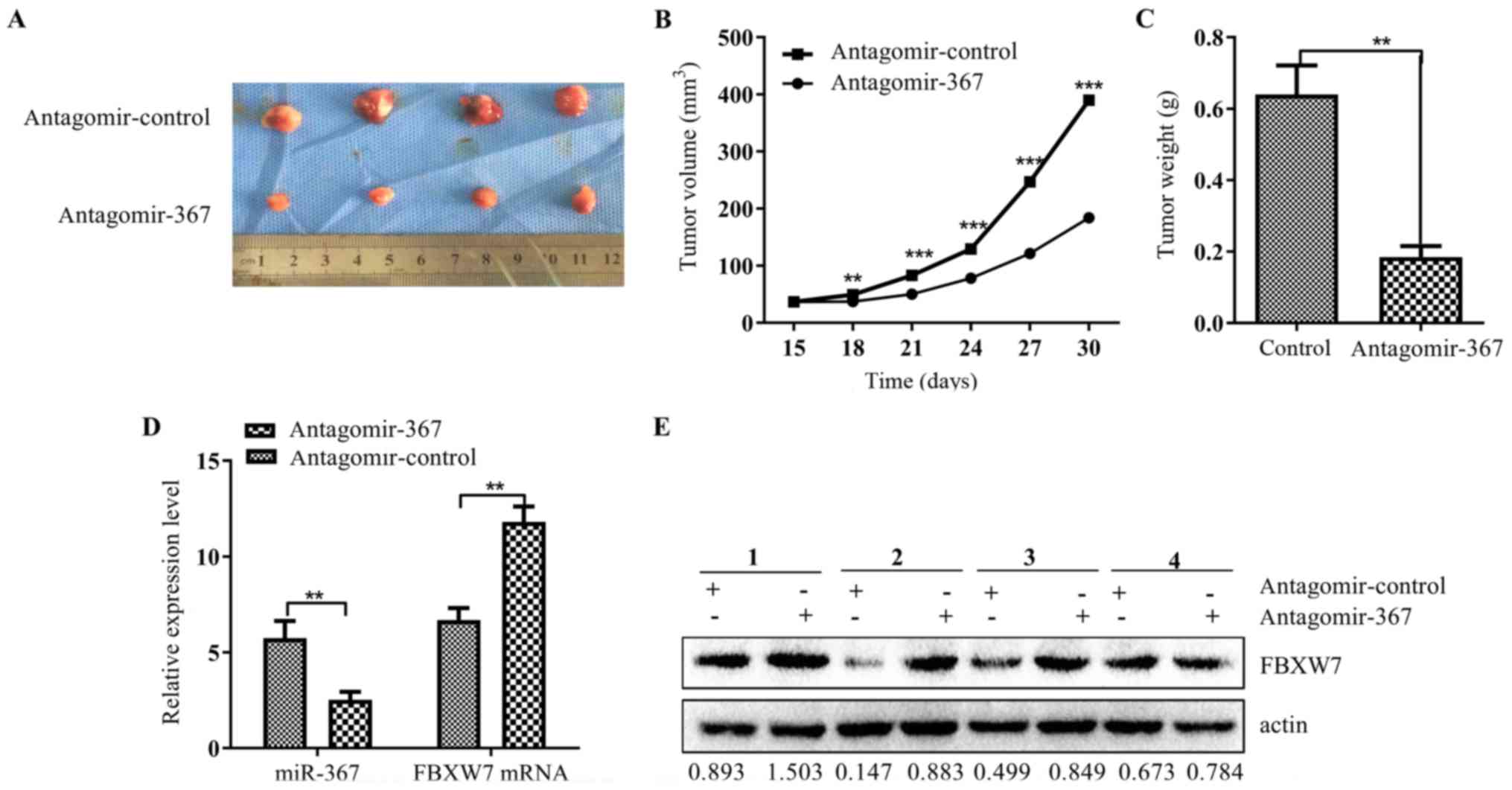
miR-367 promotes NSCLC tumor growth in
vivo
Considering the important roles of miR-367 in NSCLC,
the potential therapeutic effects of miR-367 attracted our
attention. Tumor growth of xenografts was significantly inhibited
in the group treated with antagomir-367 (p<0.001; Fig. 5A) compared to the negative control
group. This trend was also verified by the sizes (p<0.0001;
Fig. 5B) and weights (p<0.001;
Fig. 5C) of the tumors excised from
the nude mice. qRT-PCR and western blot analysis further revealed
that the mRNA and protein expression levels of FBXW7 were
significantly increased in the antagomir-367-treated xenograft
(p<0.05; respectively, Fig. 5D and
E) when the miR-367 level was decreased. These data indicated
that miR-367 was capable of promoting tumor growth and inhibiting
FBXW7 expression in vivo.
Discussion
Dysregulation of miR-367 has been found in several
types of human malignant tumors, including osteosarcoma (16), glioma (17), breast (18) and esophageal cancer (19). High expression of miR-367 in most of
these tumor tissues was closely related to the poor prognosis of
patients. A recent study also reported that the high expression of
miR-367 predicted poor prognosis in patients with NSCLC (9). However, the present study only
evaluated the clinical significance of miR-367 in NSCLC, but did
not explore the mechanisms of miR-367 in vitro and in
vivo. In the present study, similar results were observed in
clinical specimen analysis. However, we also found that high
miR-367 expression was positively correlated with TNM, degree of
differentiation, tumor size and poorer prognosis of NSCLC patients.
Consistent with the results in clinical NSCLC samples, miR-367
expression was significantly higher in several NSCLC cell lines
compared with human bronchial epithelial cells (BEAS-2B). These
results suggested that miR-367 is closely related to the malignant
progression of NSCLC.
Maintaining proliferation and resisting apoptosis
are two prominent hallmarks of cancer cells (20). Previous studies reported that
miR-367 plays a significant role in regulating tumor cell
proliferation and apoptosis. For example, miR-367 promoted the
proliferation of HCC cells by inhibiting PTEN expression (5), and suppressed adriamycin-induced
apoptosis of osteosarcoma cells by regulating KLF-4 (16). In addition, the downregulation of
miR-367 significantly inhibited cell proliferation and cell cycle
progression of esophageal cancer cells (19). However, how miR-367 regulates NSCLC
cell growth remains unclear. In the present study, we showed that
the reduced expression of miR-367 significantly inhibited the cell
proliferation, delayed cell cycle progression and promoted
apoptosis in NSCLC. To further elucidate the specific mechanism of
miR-367 in NSCLC, we discovered that FBXW7 is a potential target of
miR-367 using microRNA (miRNA) public databases. Several studies
have also demonstrated that various miRNAs play a role in promoting
proliferation and anti-apoptosis by targeting FBXW7. miR-92
promoted HCC cell proliferation and inhibited cell apoptosis by
targeting FBXW7 (21). miR-223
contributed to apoptosis reduction and increased proliferation and
invasion in gastric cancer cell lines by inhibiting FBXW7 (11). In the present study, our results
firstly confirmed that miR-367-3p directly bound to and regulated
the function of FBXW7. Supportively, a significantly inverse
correlation was found between miR-367 and FBXW7 mRNA expression in
NSCLC tissues. Moreover, we also investigated the effect of FBXW7
on the biological role of miR-367 and finally found that FBXW7
reversed miR-367-induced cell proliferation, cell cycle and
apoptosis inhibition. Our findings, therefore, provide a mechanism
that miR-367 may promote cell growth by regulating FBXW7 in
NSCLC.
Much evidence has indicated that the tumor
suppressor function of FBXW7 is mainly regulated by
ubiquitin-mediated degradation of several oncoproteins.
Downregulation of FBXW7 promoted the proliferation of colon cancer
cells by increasing the expression of c-Myc and cyclin E (22). Conversely, upregulation of FBXW7 in
renal carcinoma cell inhibited cell proliferation and promoted
apoptosis by suppressing c-Myc and c-Jun expression (23). c-Myc and c-Jun, as the direct
targets of FBXW7, are important oncoproteins related to
proliferation and apoptosis in many malignant tumors. c-Myc can
directly activate cyclin E-cdk2 complex, mediate phosphorylation of
Rb, and thereby activate E2F transcription regulation factor to
promote DNA replication (24). The
phosphorylation of c-Jun, another target protein of FBXW7,
participates in promoting the proliferation and cell cycle by
activating cyclin D (25). In the
present study, miR-367 mimic/inhibitor may decrease/increase the
expression of FBXW7 by changing the level of miR-367, and then
increase/decrease the expression of c-Jun and c-Myc in NSCLC cells.
In addition, overexpression/knockdown of FBXW7 may reverse the
function of miR-367 by decreasing/increasing the expression of
c-Myc and c-Jun under the treatment of miR-367 mimic/inhibitor.
Given that c-Myc and c-Jun are known as growth promoters whose
abnormal expression may induce tumorigenesis, we speculated that
the miR-367/FBXW7/c-Myc and miR-367/FBXW7/c-Jun signaling pathway
may play pivotal roles in the development of NSCLC
carcinogenesis.
Nevertheless, the present study focused on the
effect of miR-367 on the basic biological functions of NSCLC cells.
As an important regulatory factor involved in self-renewal and
pluripotency of embryonic stem cells, miR-367 also plays a vital
role in regulating the function of cancer stem cells (6). miR-367 overexpression significantly
promoted stem-like traits in medulloblastoma cell lines. Meanwhile,
stem cell-specific transcription factor Oct4 promoted the
expression of miR-367. Upregulation of miR-367 also increased the
expression of Oct4 or Sox2 in medulloblastoma cell lines. As is
known to all, the miR-302/367 family and special transcription
factors (Oct4, Sox2 and Nanog) have a positive feedback loop of
self-regulation (26,27). Notably, FBXW7 was found to
negatively regulated the protein expression of Sox2 and Nanog in
cholangiocarcinoma (28) and
colorectal cancer cells (29).
Thus, we may further explore the role of miR-367 and FBXW7 in the
regulation of NSCLC stem cells in future research.
In conclusion, we demonstrated that miR-367 was
expressed at a higher level in NSCLC tissues and cells than levels
in normal lung tissues and cells, and its high expression was
statistically correlated with the poor prognosis of NSCLC patients.
Moreover, miR-367 and FBXW7 mRNA expression in NSCLC tissues was
negatively correlated. The experiments in vivo and in
vitro showed that miR-367 promoted the cell proliferation, cell
cycle progression and inhibited cell apoptosis in NSCLC cells by
negatively regulating FBXW7 expression. Hence, miR-367 may serve as
a novel target for the diagnosis and treatment of NSCLC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81272418) (to
H.R).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor MD, Nagji AS, Bhamidipati CM,
Theodosakis N, Kozower BD, Lau CL and Jones DR: Tumor recurrence
after complete resection for non-small cell lung cancer. Ann Thorac
Surg. 93:1813–1821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao Z, Zhu X and Dou Y: The miR-302/367
cluster: A comprehensive update on its evolution and functions.
Open Biol. 5:1501382015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barroso-del Jesus A, Lucena-Aguilar G and
Menendez P: The miR-302-367 cluster as a potential stemness
regulator in ESCs. Cell Cycle. 8:394–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meng X, Lu P and Fan Q: miR-367 promotes
proliferation and invasion of hepatocellular carcinoma cells by
negatively regulating PTEN. Biochem Biophys Res Commun.
470:187–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaid C, Silva PBG, Cortez BA, Rodini CO,
Semedo-Kuriki P and Okamoto OK: miR-367 promotes proliferation and
stem-like traits in medulloblastoma cells. Cancer Sci.
106:1188–1195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J
and Wang P: miR-367 promotes epithelial-to-mesenchymal transition
and invasion of pancreatic ductal adenocarcinoma cells by targeting
the Smad7-TGF-β signalling pathway. Br J Cancer. 112:1367–1375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bin Z, Dedong H, Xiangjie F, Hongwei X and
Qinghui Y: The microRNA-367 inhibits the invasion and metastasis of
gastric cancer by directly repressing Rab23. Genet Test Mol
Biomarkers. 19:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campayo M, Navarro A, Viñolas N, Diaz T,
Tejero R, Gimferrer JM, Molins L, Cabanas ML, Ramirez J, Monzo M,
et al: Low miR-145 and high miR-367 are associated with
unfavourable prognosis in resected nonsmall cell lung cancer. Eur
Respir J. 41:1172–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akhoondi S, Sun D, von der Lehr N,
Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D,
Marth C, et al: FBXW7/hCDC4 is a general tumor suppressor in
human cancer. Cancer Res. 67:9006–9012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Guo Y, Liang X, Sun M, Wang G, De W
and Wu W: MicroRNA-223 functions as an oncogene in human gastric
cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol.
138:763–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong J, Cui Z, Li L, Ma Q, Wang Q, Gao Y
and Sun H: MicroRNA-25 promotes gastric cancer proliferation,
invasion, and migration by directly targeting F-box and WD-40
Domain Protein 7, FBXW7. Tumour Biol. 36:7831–7840. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren H, Koo J, Guan B, Yue P, Deng X, Chen
M, Khuri FR and Sun SY: The E3 ubiquitin ligases β-TrCP and FBXW7
cooperatively mediates GSK3-dependent Mcl-1 degradation induced by
the Akt inhibitor API-1, resulting in apoptosis. Mol Cancer.
12:1462013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun X, Tang SC, Xu C, Wang C, Qin S, Du N,
Liu J, Zhang Y, Li X, Luo G, et al: DICER1 regulated let-7
expression levels in p53-induced cancer repression requires cyclin
D1. J Cell Mol Med. 19:1357–1365. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qiang BU and Jiang H: Chemosensitivity
testing for human prostatic cancer with primary culture cells and
purified culture cells using the CCK-8 assay. Pharmaceutical and
Clinical Research. 2013.
|
|
16
|
Wang GC, He QY, Tong DK, Wang CF, Liu K,
Ding C, Ji F and Zhang H: MiR-367 negatively regulates apoptosis
induced by adriamycin in osteosarcoma cells by targeting KLF4. J
Bone Oncol. 5:51–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guan Y, Chen L, Bao Y, Qiu B, Pang C, Cui
R and Wang Y: High miR-196a and low miR-367 cooperatively correlate
with unfavorable prognosis of high-grade glioma. Int J Clin Exp
Pathol. 8:6576–6588. 2015.PubMed/NCBI
|
|
18
|
Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu
H, Liu P, Hao X, Zhang W and Chen K: Functional SNP in the
microRNA-367 binding site in the 3′UTR of the calcium channel
ryanodine receptor gene 3 (RYR3) affects breast cancer risk
and calcification. Proc Natl Acad Sci USA. 108:13653–13658. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun J, Song K, Feng X and Gao S:
MicroRNA-367 is a potential diagnostic biomarker for patients with
esophageal squamous cell carcinoma. Biochem Biophys Res Commun.
473:363–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Awada A: The Hallmarks of
Cancer Review. Ann Oncol. 100:57–70. 2012.
|
|
21
|
Yang W, Dou C, Wang Y, Jia Y, Li C, Zheng
X and Tu K: MicroRNA-92a contributes to tumor growth of human
hepatocellular carcinoma by targeting FBXW7. Oncol Rep.
34:2576–2584. 2015.PubMed/NCBI
|
|
22
|
Guo Z, Zhou Y, Evers BM and Wang Q: Rictor
regulates FBXW7-dependent c-Myc and cyclin E degradation in
colorectal cancer cells. Biochem Biophys Res Commun. 418:426–432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu Y, Lin Y, Yang Z, Yang G, Li G, Liu Y,
Tan X, Huang Y, Wu X, Wang Y, et al: FBXW7 overexpression
suppresses renal cancer cell proliferation and induces apoptosis.
Med Oncol. 32:2152015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen J: Mechanism of molecular regulation
of cyclin E-CDK2. Journal of Medical Molecular Biology. 2006.
|
|
25
|
Schwabe RF, Bradham CA, Uehara T, Hatano
E, Bennett BL, Schoonhoven R and Brenner DA: c-Jun-N-terminal
kinase drives cyclin D1 expression and proliferation during liver
regeneration. Hepatology. 37:824–832. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Anokye-Danso F, Trivedi CM, Juhr D, Gupta
M, Cui Z, Tian Y, Zhang Y, Yang W, Gruber PJ, Epstein JA, et al:
Highly efficient miRNA-mediated reprogramming of mouse and human
somatic cells to pluripotency. Cell Stem Cell. 8:376–388. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin SL, Chang DC, Lin CH, Ying SY, Leu D
and Wu DT: Regulation of somatic cell reprogramming through
inducible mir-302 expression. Nucleic Acids Res. 39:1054–1065.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang H, Lu X, Liu Z, Chen L, Xu Y, Wang Y,
Wei G and Chen Y: FBXW7 suppresses epithelial-mesenchymal
transition, stemness and metastatic potential of cholangiocarcinoma
cells. Oncotarget. 6:6310–6325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Liu Y, Lu J, Zhang P, Wang Y, Xu
Y, Wang Z, Mao JH and Wei G: Rapamycin inhibits FBXW7 loss-induced
epithelial-mesenchymal transition and cancer stem cell-like
characteristics in colorectal cancer cells. Biochem Biophys Res
Commun. 434:352–356. 2013. View Article : Google Scholar : PubMed/NCBI
|