Introduction
Primary bronchogenic carcinoma (also known as lung
cancer) refers to malignant tumors that originate in the bronchus,
mucous gland, bronchiolar epithelium or pulmonary alveolar
epithelium (1). Lung cancer is
among the most common malignant tumors in China. Its rate of
morbidity has markedly increased over recent years, and is now
ranked highest in a number of cities. Furthermore, its yearly
incidence now exceeds 500,000 cases (2), with a male:female ratio of ~7-7:1.
However, as the biological behaviors of tumors differ, lung cancer
is generally divided into small cell lung cancer (SCLC) and
non-small cell lung cancer (NSCLC) for convenient clinical
treatment (3). The latter includes
all epithelium-derived lung cancers excluding SCLCs. At present,
the first-choice therapeutic method for early-stage lung cancer is
surgical treatment (4), while the
main treatment methods for locally advanced lung cancer include
radiotherapy, chemotherapy, surgery and comprehensive treatment
involving a combination of these methods (3).
In glycogen synthase kinase (GSK)-3β,
phosphorylation of the ninth serine inactivates the kinase, which
can prevent the Wnt signal transduction pathway and cause
degradation of the protein, inhibiting apoptosis (5). Meanwhile, phospho(p)-GSK-3 prevents
GSK-3β from interacting with β-catenin and cyclin D1, enabling the
overexpression of β-catenin and cyclin D1, which favors the binding
of cyclin D1 to CDK4/6 to form a complex that promotes the G1/S
phase transition in cells (6). The
cells can then enter into the S phase of the cell cycle, and may
ultimately overproliferate and malignantly transform (6).
Research into the PI3K/Akt signaling pathways now
spans >10 years. After PI3K phosphorylation, the second
messenger phosphatidylinositol (PIP3) is generated on the
plasmalemma (7). PIP3 can then bind
to the N-terminal of Akt, which contains a PH structural domain for
such binding. This activates Akt and transfers it to the cytosolic
side of the plasmalemma (8), where
Akt itself activates or suppresses downstream target proteins
through phosphorylation, which ultimately stimulates the
proliferation, differentiation, apoptosis and migration of target
cells (9). As major kinases of
inositol and phosphatidylinositol, PI3K family members are
considered to be primary cancer genes, and are composed of the
regulatory subunit p85 and catalytic subunit p110. The catalytic
subunit is responsible for catalyzing phosphorylation of the third
hydroxyl of the inositol ring (10). Akt is a serine/threonine protein
kinase with a molecular weight of 57 ku, and is the mammalian
congener of the viral protooncogene Akt (11). Following activation of the PI3K/Akt
signaling pathway, activated Akt exerts its effects through the
activation of multiple downstream effector molecules, including
Bad, caspase-9, FKHR1 and NF-κB, enabling it to participate in the
suppression of apoptosis and regulation of the cell cycle (12). At present, a relatively
well-established effect of Akt is its upregulation of gene
expression through increased transcription of c-Myc. c-Myc is a
type of cell cycle-associated factor that enables cells to exit the
G0 phase and undergo proliferation (11), and may also promote tumor
angiogenesis. Similarly, Akt can activate nitric oxide synthase,
stimulate the growth and proliferation of endothelial cells and
increase vascular permeability (13). Furthermore, after hemangiectasis,
Akt can promote neovascularization, aiding with the provision of
sufficient nutrition for tumor cells, and can also promote cell
invasion and metastasis.
Previous evidence revealed that microRNAs (miRs)
serve important roles in the occurrence and development of human
tumors (14). Recently, it has been
demonstrated that miR-125b and its target gene play a critical role
in the invasion and metastasis of multiple tumor types (15). For example, miR-125b can directly
regulate c-Jun protein expression and directly suppress the
metastasis of melanoma cells on a transcriptional and translational
level (16). It has also been
reported that miR-125b can act as a tumor-inhibitory factor that
targets the PI3K/Akt signaling pathway, and prevents the invasion
and metastasis of cervical cancer cells (16). Therefore, the aim of the present
study was to evaluate the effects of miR-125b regulation on the
apoptosis of human NSCLC cells and the targeting of the PI3K/Akt
and Wnt/β-catenin signaling pathways in vitro.
Materials and methods
Patients and specimen selection
Approval for the present study was obtained from the
local Ethics Committee along with written informed consent from
each patient. From September to December of 2012, human NSCLC
specimens from patients (n=91) were obtained from the Department of
Oncology of Peking Union Medical College Hospital, Chinese Academy
of Medical Sciences. The main characteristics of all human NSCLC
specimens from patients are shown in Table I. Every 2 months, a follow-up of
NSCLC patients was conducted, over a 3-year period. We analyzed
overall survival (OS) and disease-free survival (DFS) in the
present study.
 | Table I.Association between miR-125b
expression and clinicopathological features. |
Table I.
Association between miR-125b
expression and clinicopathological features.
|
|
| miR-125b
expression |
|
|---|
|
|
|
|
|
|---|
| Variables | No. of pts. | Low (%) | High (%) | P-value |
|---|
| Sex |
|
|
| 0.437 |
|
Male | 79 | 42 (53.16) | 37 (46.84) |
|
|
Female | 12 | 4
(33.33) | 8
(66.67) |
|
| Age (years) |
|
|
| 0.014 |
|
≤60 | 57 | 21 (36.84) | 36 (63.16) |
|
|
>60 | 34 | 18 (52.94) | 16 (47.06) |
|
| Pathological
differentiation |
|
|
| 0.627 |
|
High | 7 | 5
(71.43) | 2
(28.57) |
|
|
Median | 61 | 23 (37.70) | 38 (62.30) |
|
|
Low | 23 | 13 (56.52) | 10 (43.48) |
|
| Clinical stage |
|
|
| 0.139 |
| I | 7 | 3
(42.86) | 4
(57.14) |
|
| II | 17 | 6
(35.29) | 11 (64.71) |
|
|
IIIA | 67 | 38 (56.71) | 29 (43.28) |
|
| Vascular
invasion |
|
|
| 0.074 |
|
Negative | 84 | 41 (48.81) | 43 (51.19) |
|
|
Positive | 7 | 2
(28.57) | 5
(71.43) |
|
| Pathological
type |
|
|
| 0.000 |
|
Squamous cancer | 39 | 25 (64.10) | 14 (35.90) |
|
|
Adenocarcinoma | 47 | 15 (31.91) | 32 (68.09) |
|
| Large
cell cancer | 5 | 2
(40.00) | 3
(60.00) |
|
Quantative real-time PCR analysis
Total RNA was extracted from NSCLC and
para-carcinoma tissues using TRIzol® (Invitrogen, Thermo
Fisher Scientific, Inc., Waltham, MA, USA). cDNA was synthesized
from total RNA (100–200 ng) using M-MLV reverse transcriptase
(Promega Corporation, Madison, WI, USA) with random primers (Takara
Bio, Inc., Otsu, Japan), according to the manufacturer's protocol.
Quantitative real-time PCR analysis was performed using the CFX
Connect™ Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and SYBR®-Green PCR Master Mix
kit (Applied Biosystems, Thermo Fisher Scientific, Inc.).
Quantative real-time PCR analysis was performed with 1 cycle at
95°C for 10 min, followed by 40 cycles of 95°C for 30 sec and 58°C
for 60 sec.
Cell culture
The human NSCLC A549 cell line was purchased from
the Shanghai Cell Bank (Shanghai, China), and maintained in
RPMI-1640 medium supplemented with 10% fetal bovine serum (both
from Gibco; Thermo Fisher Scientific Inc.) with 100 U/ml
streptomycin and 100 U/ml penicillin (both from Thermo Fisher
Scientific, Inc.) at 37°C in a humidified atmosphere containing 5%
CO2.
Transfection and lentiviral
transduction
Anti-miR-125b mimics and negative plasmids were
transfected into A549 cells using Lipofectamine 2000 transfection
reagent (Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. After transfection for 24 h, the cells
were maintained in new culture medium and used to execute
experimental research.
Cell proliferation assay
A549 cells transfected with anti-miR-125b mimics and
negative plasmids or treated with LY294002 (1 µM) for 48 h were
assayed 96 h later using an MTT assay kit (50 µg/ml; Sigma-Aldrich,
St. Louis, MO, USA) for 4 h. Subsequently, the culture medium was
removed and 150 µl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was
added into cells to dissolve. The absorbance was assessed using a
Genesys™ 15 ultraviolet spectrophotometer (Thermo Fisher
Scientific, Inc.) at 492 nm.
Cell apoptosis assay
A549 cell transfected with anti-miR-125b mimics and
negative plasmids or treated with LY294002 (1 µM) for 48 h was
assayed 6 h later using Annexin V-FITC/PI apoptosis assay. Annexin
V-FITC (BD Biosciences, San Jose, CA, USA) was added and incubated
for 20 min at room temperature in the dark. Then, PI (BD
Biosciences) was also added and incubated for 5 min at room
temperature in the dark. Then, cell apoptosis was examined using a
FACScan flow cytometer.
Caspase-3 activity assay
A549 cells transfected with anti-miR-125b mimics and
negative plasmids or treated with LY294002 (1 µM) for 48 h were
assayed 96 h later using a caspase-3 activity ELISA kit for 1 h at
37°C. Caspase-3 activity was assessed using a Genesys™ 15
ultraviolet spectrophotometer (Thermo Fisher Scientific, Inc.) at
405 nm.
Western blot analysis
A549 cells transfected with anti-miR-125b mimics and
negative plasmids or treated with LY294002 (1 µM) 48 h later. A549
cells were lysed with a hypotonic buffer containing a protease
inhibitor cocktail (both from Beyotime Institute of Biotechnology,
Shanghai, China). Protein concentration was determined by the BCA
method (Beyotime Institute of Biotechnology). Equal protein (50 µg)
was separated by 10% SDS-polyacrylamide gel and blotted onto a
polyvinylidene difluoride (PVDF) membranes (BD Biosciences). The
membranes were incubated with Akt (1:500), phosphorylated (p)-Akt
(1:500), p-GSK3β (1:500), Wnt (1:500), β-catenin (1:300), Bax
(1:300) and GAPDH (1:500) (all from Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) at 4°C overnight. Following washing with PBS
with Tween-20 (Sigma-Aldrich) twice, membranes were incubated with
horseradish peroxidase-conjugated secondary antibody IgG (Santa
Cruz Biotechnology, Inc.). Subsequently the membranes were revealed
with an Amersham ECL Western Blotting Detection kit (EMD Millipore,
Billerica, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparison between the means of two groups was performed using
Student's t-test and one-way analysis of variance followed by post
hoc pairwise comparisons using Tukey's test. p<0.05 was
considered to indicate a statistically significant difference.
Results
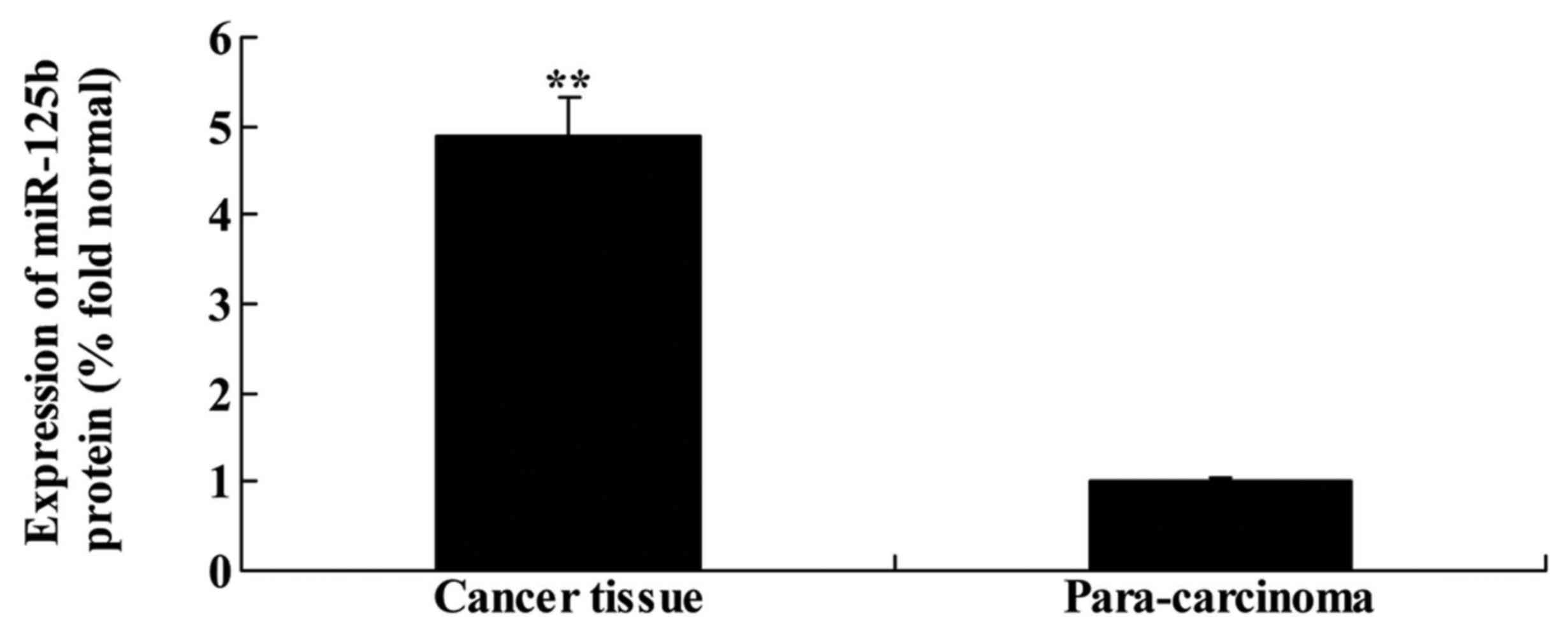
miR-125b expression in human non-small
cell lung cancer
Patients with NSCLC (n=91) were recruited from the
Oncology Department of the Peking Union Medical College Hospital,
Chinese Academy of Medical Sciences, and the expression of miR-125b
was assessed in all patients using quantitative PCR. As shown in
Fig. 1, the expression of miR-125b
in NSCLC tissue was higher than that in para-carcinoma tissue.
Association between miR-125b
expression and clinicopathological features
To determine the regulatory role of miR-125b in
human NSCLC, we examined the association between miR-125b
expression and the clinicopathological features of patients. As
shown in Table I, differences were
observed between miR-125b expression and the pathological type of
NSCLC, and between miR-125b expression and the age of patients with
NSCLC.
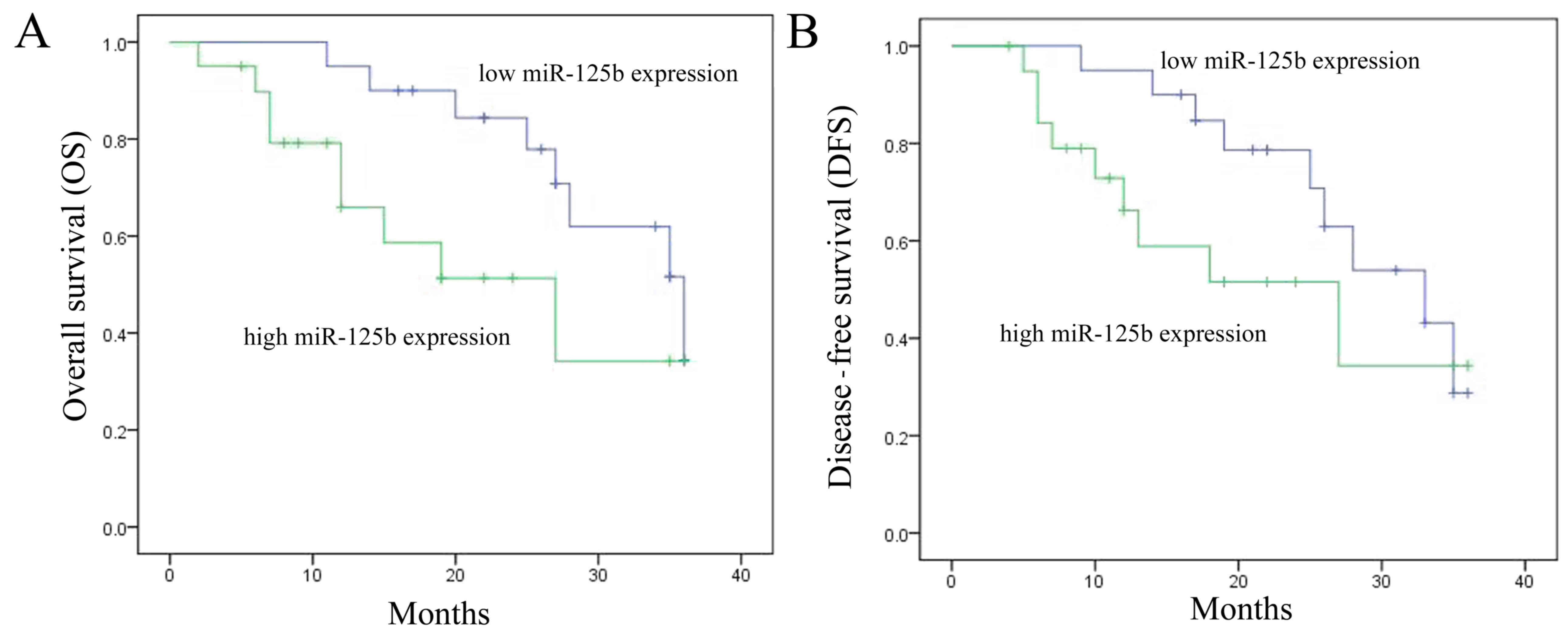
Association between miR-125b
expression and OS and DFS rates
A follow-up of NSCLC patients was conducted over a
3-year period, in which the OS and DFS rates of patients were
recorded. As shown in Fig. 2A and
B, the 3-year OS and DFS rates in patients with low miR-125b
expression were higher than those in patients with high miR-125b
expression.
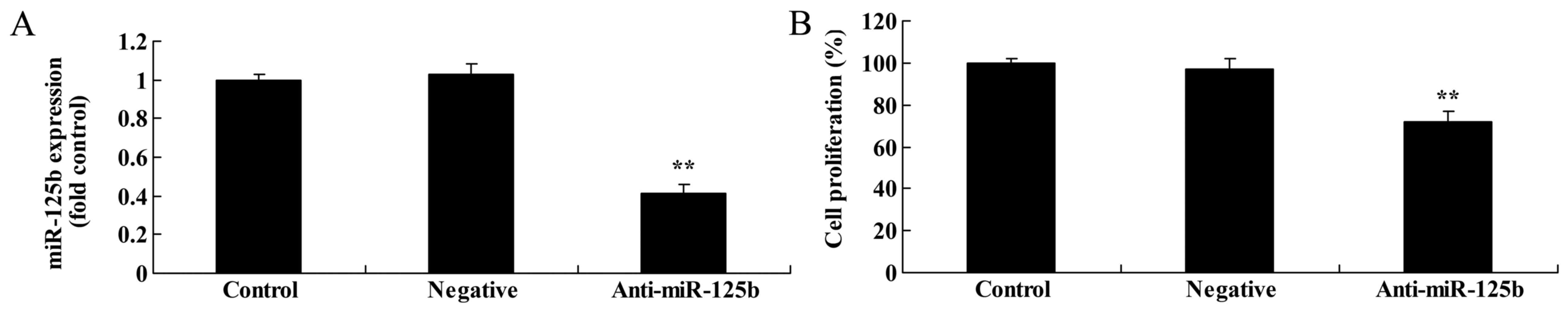
Downregulation of miR-125b inhibits
the proliferation of A549 cells
To assess the effects of miR-125b on the
proliferation of A549 cells, we investigated cell growth with an
MTT assay. Firstly, downregulation of miR-125b significantly
suppressed the expression of miR-125b in A549 cells when compared
with a negative control group (Fig.
3A). Notably, we found that miR-125b markedly suppressed A549
cell proliferation at 24 and 48 h when compared with the negative
control group (Fig. 3B).
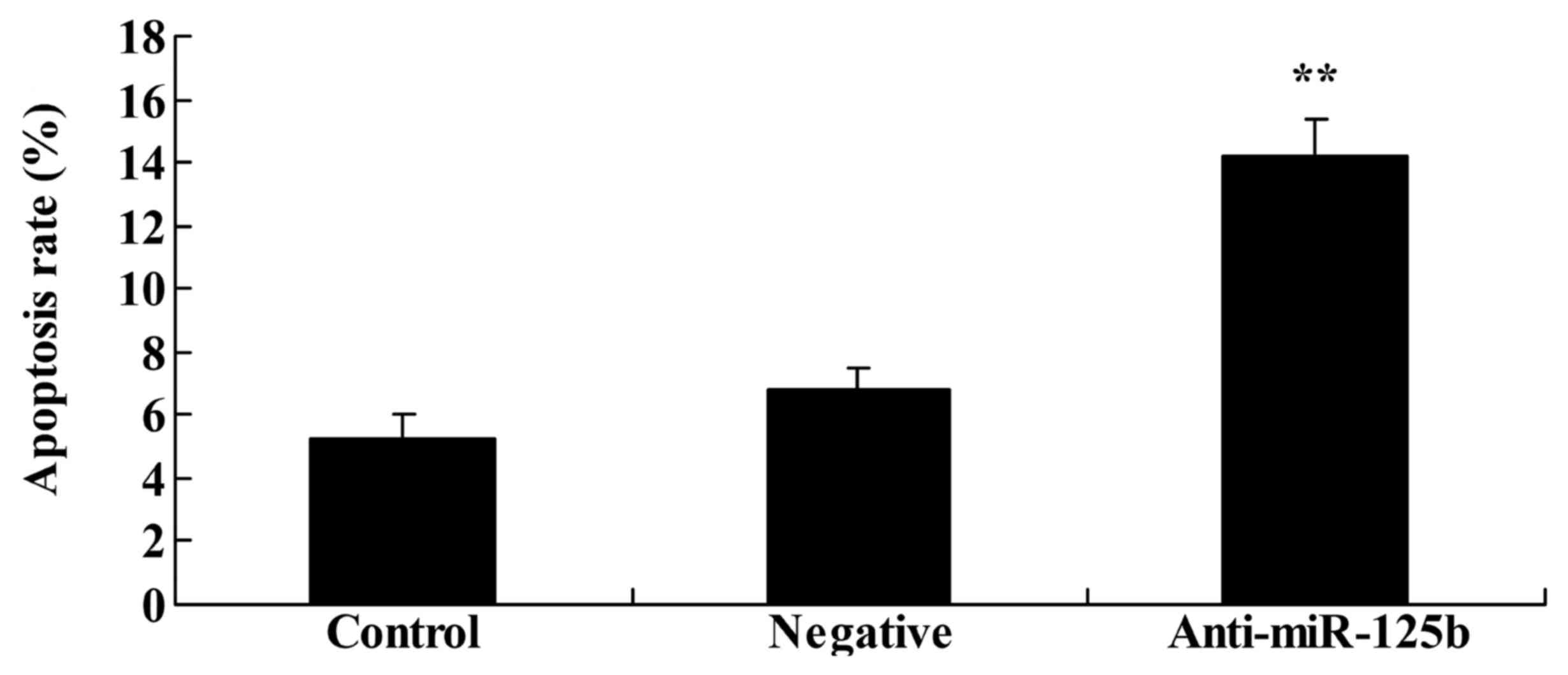
Downregulation of miR-125b induces
apoptosis in A549 cells
To examine whether downregulation of miR-125b
induced the apoptosis of A549 cells, as indicated by the
aforementioned results, an Annexin V-FITC/PI apoptosis assay was
used to analyze changes in the rate of apoptosis. The results
revealed that downregulation of miR-125b significantly increased
the rate of apoptosis and promoted cell death in A549 cells when
compared with the negative control group (Fig. 4).
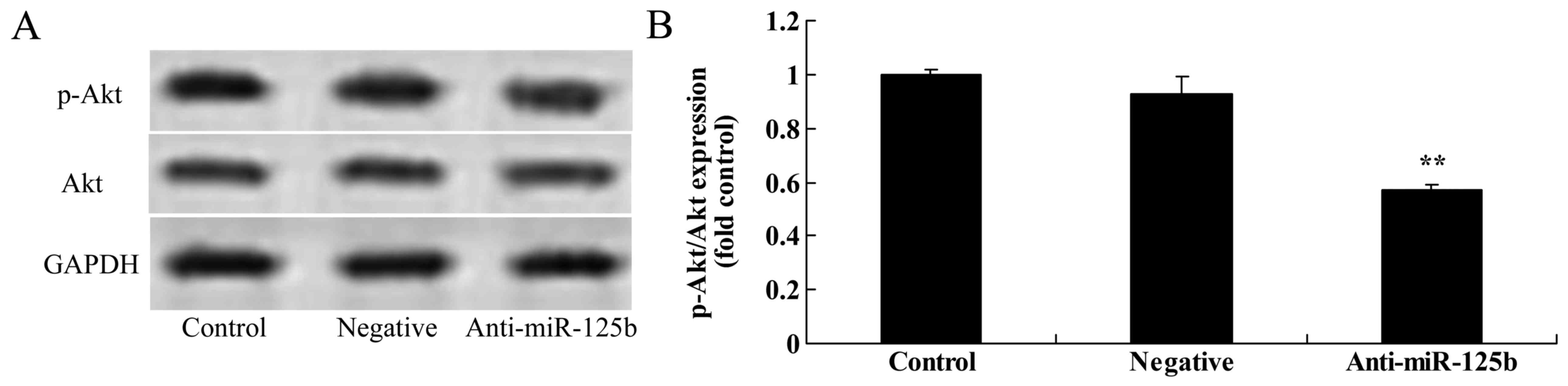
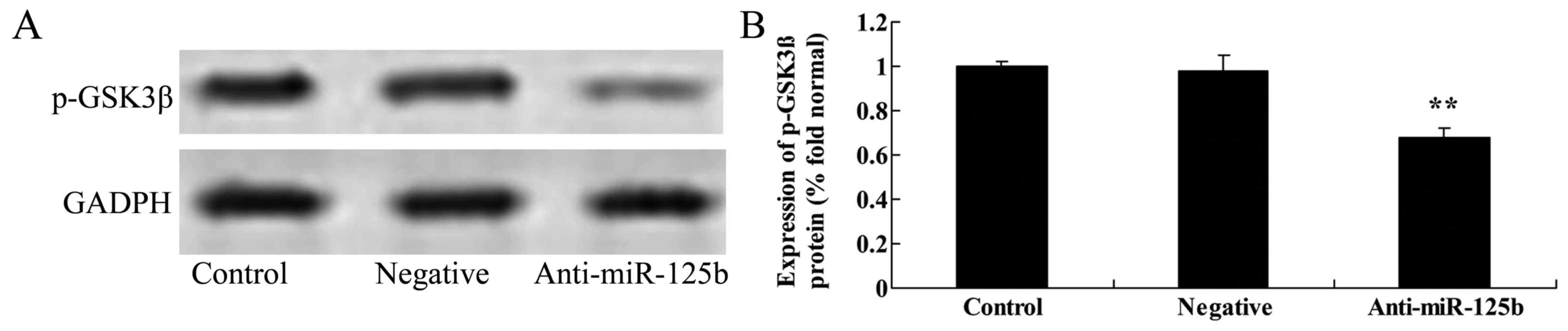
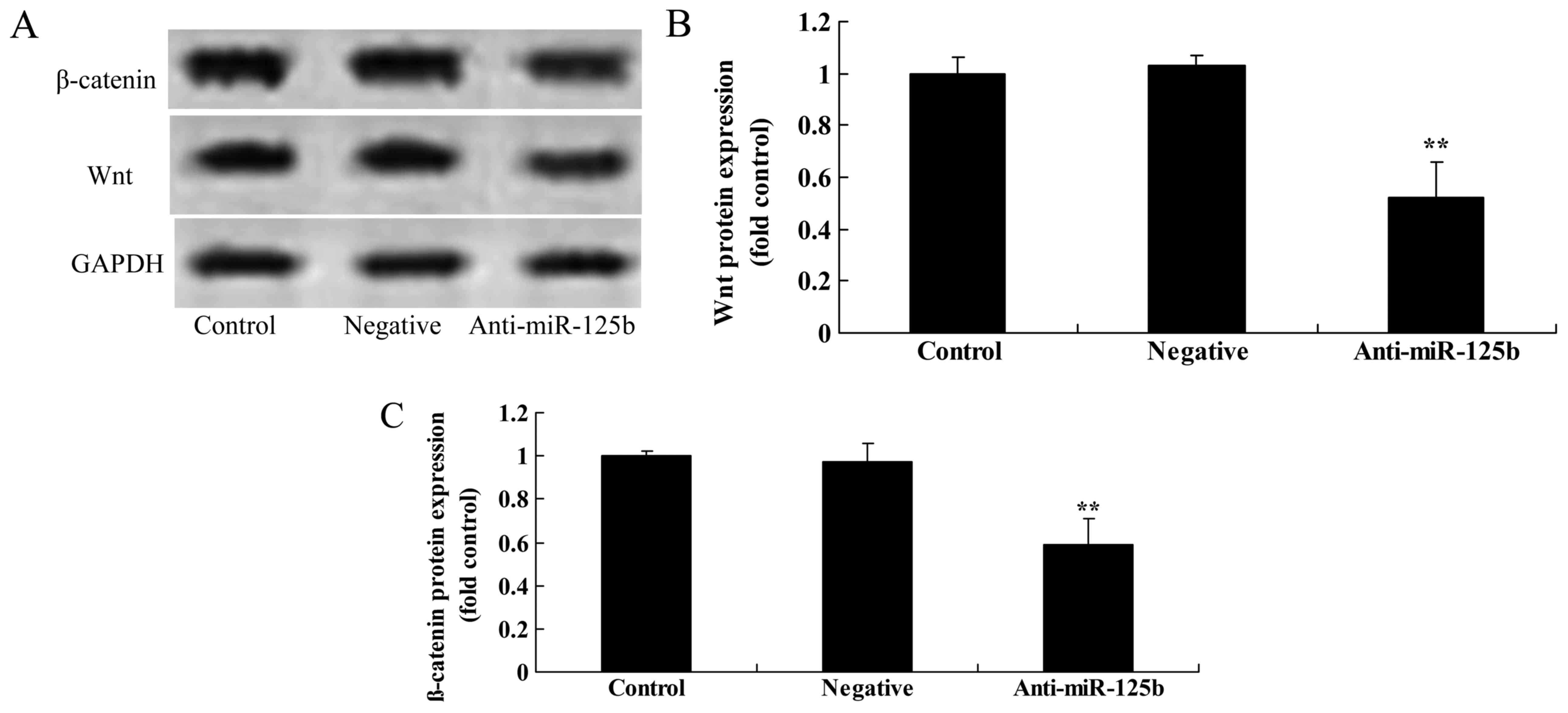
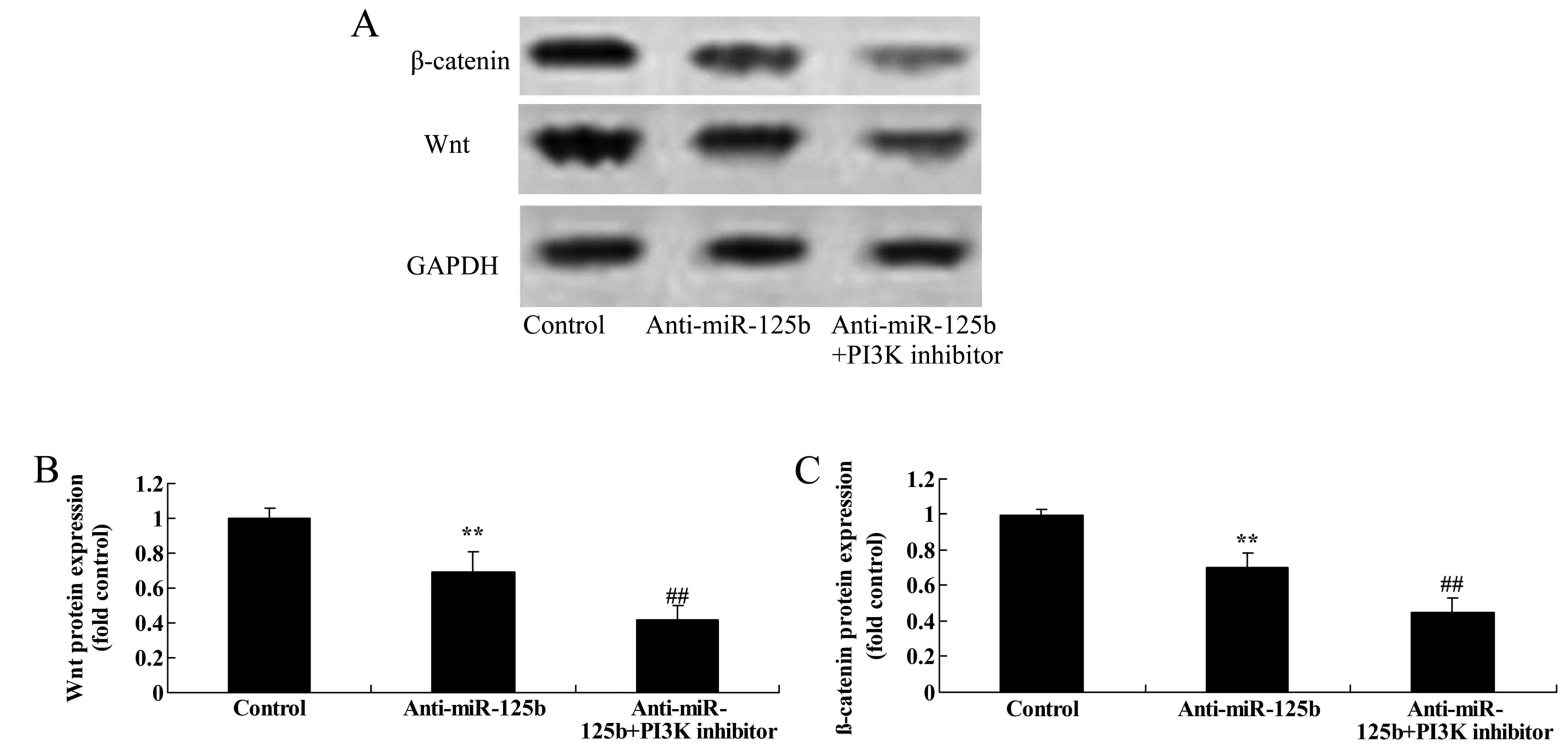
Downregulation of miR-125b suppresses
the protein expression of p-Akt, p-GSK3β, Wnt and β-catenin in A549
cells
We used western blot analysis to detect the protein
expression of p-Akt, p-GSK3β, Wnt and β-catenin in A549 cells. The
results revealed that the downregulation of miR-125b significantly
suppressed the expression of p-Akt (Fig. 5), p-GSK3β (Fig. 6), Wnt and β-catenin (Fig. 7) in A549 cells when compared with
the negative control group.
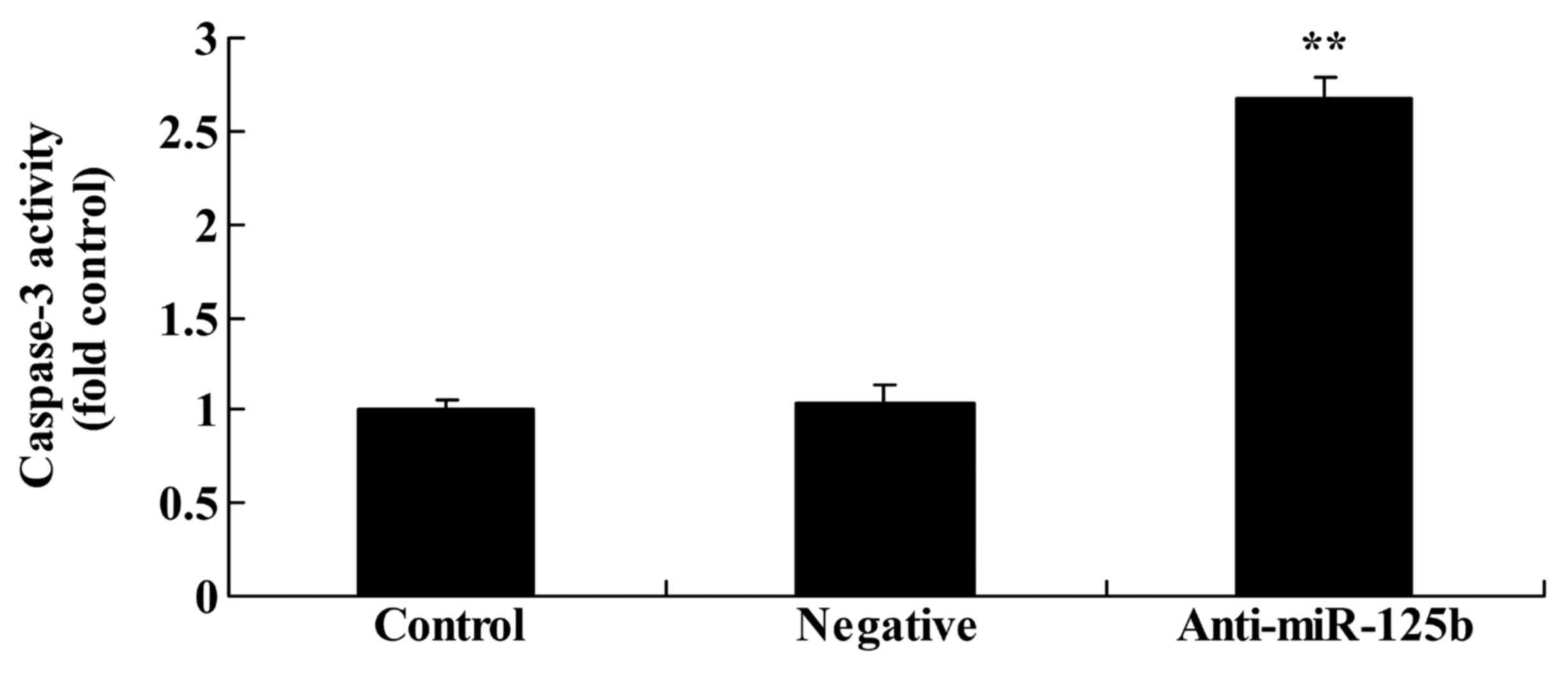
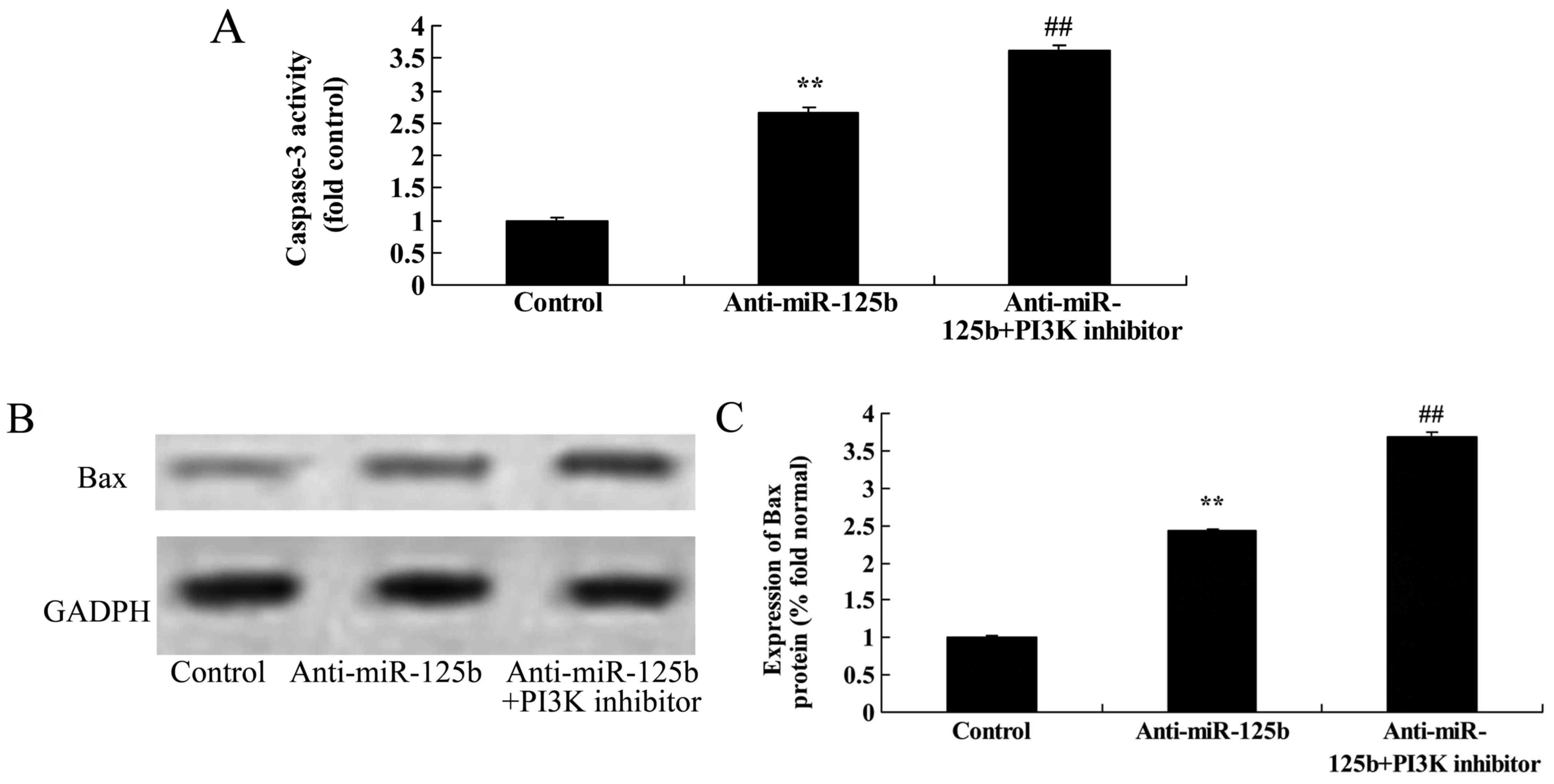
Downregulation of miR-125b increases
caspase-3 activity in A549 cells
To validate the effects of miR-125b on the apoptosis
of A549 cells, the effects of miR-125b downregulation on the
activity of caspase-3 were assessed using a caspase-3 activity
ELISA kit. The results revealed that the downregulation of miR-125b
significantly promoted caspase-3 activity in A549 cells when
compared with the negative control group (Fig. 8).
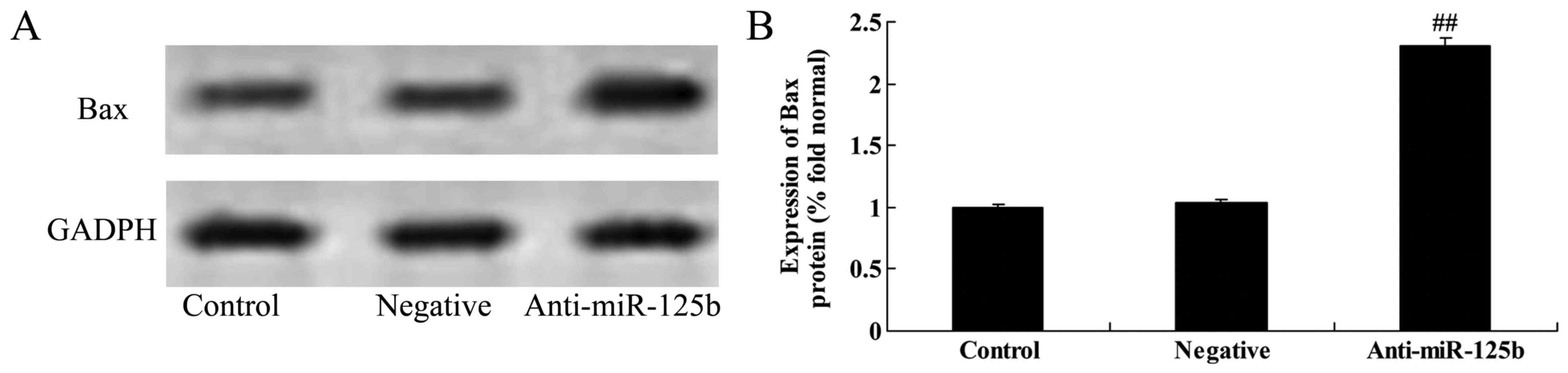
Downregulation of miR-125b increases
Bax protein expression in A549 cells
The effect of miR-125b on the apoptosis of A549
cells was also validated by assessing the protein expression of Bax
following miR-125b downregulation by western blot analysis. As
shown in Fig. 9, the downregulation
of miR-125b significantly increased Bax expression in A549 cells
when compared with the negative control group.
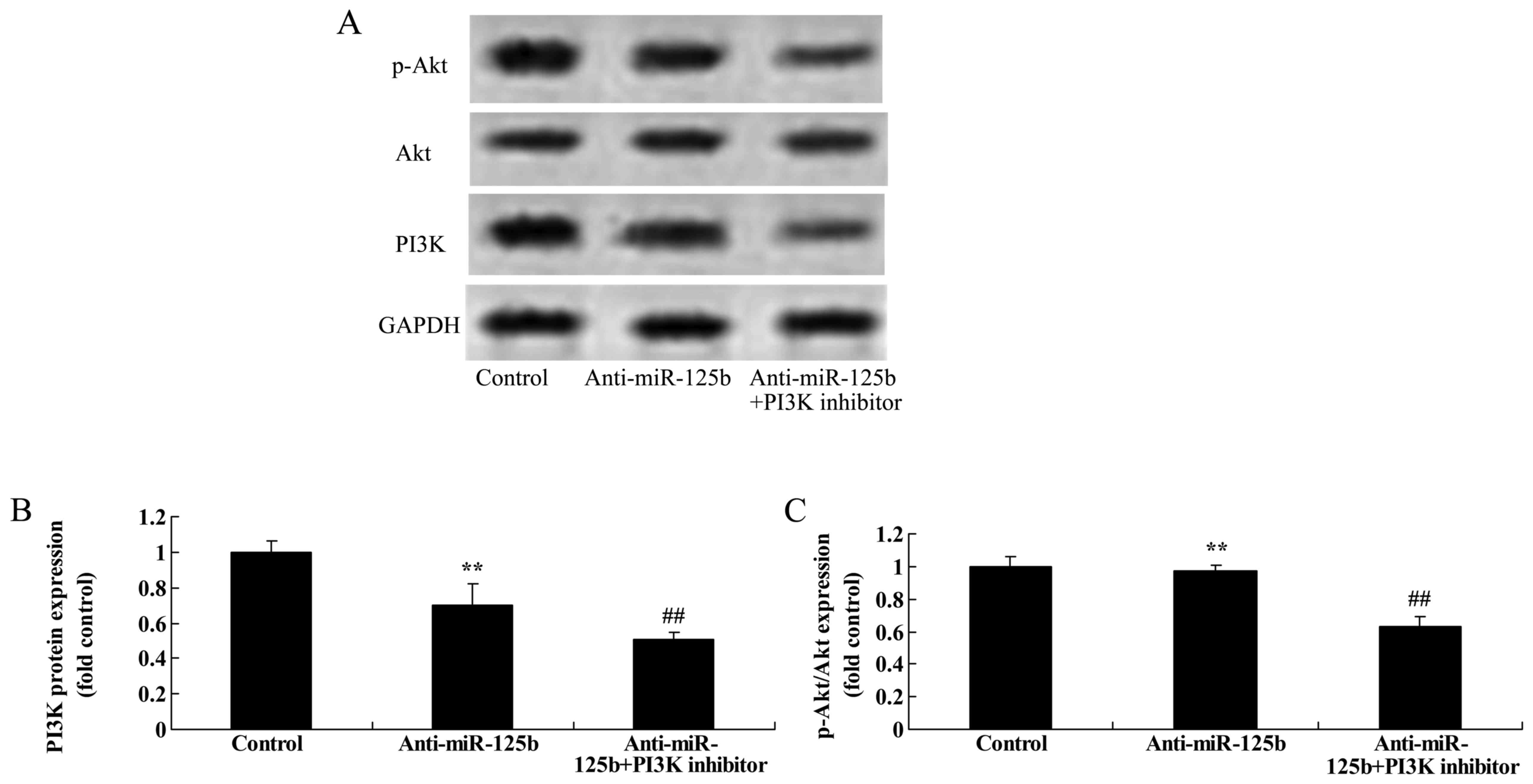
Downregulation of miR-125b combined
with a PI3K inhibitor suppresses PI3K and p-Akt protein expression
in A549 cells
Based on the aforementioned results, we explored the
effect of a PI3K inhibitor on the growth of A549 cells after the
downregulation of miR-125b. LY294002 was used as the PI3K inhibitor
to suppress the PI3K/Akt pathway in A549 cells following miR-125b
downregulation. As shown in Fig.
10, LY294002 markedly inhibited the expression of PI3K and
p-Akt in A549 cells following the downregulation of miR-125b at 48
h, relative to a control group with only miR-125b
downregulation.
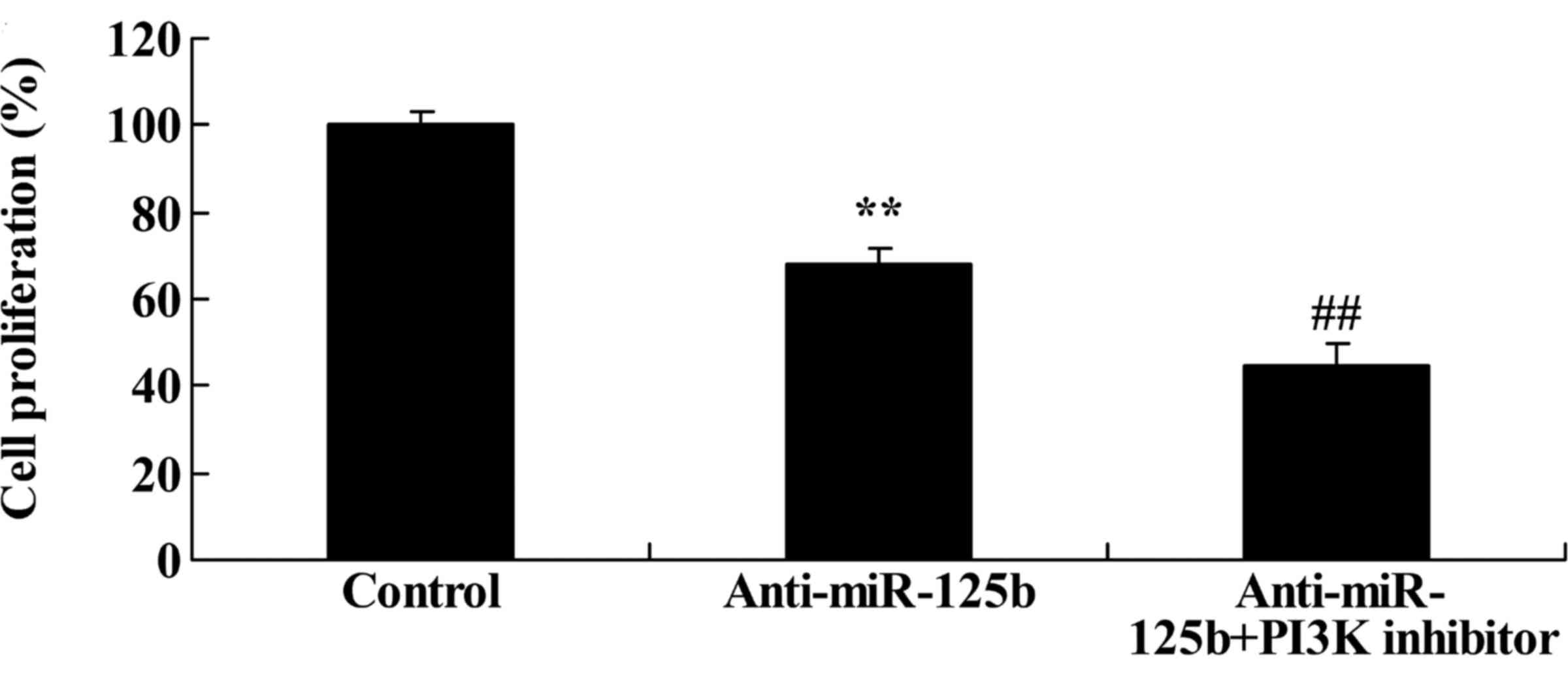
Downregulation of miR-125b combined
with a PI3K inhibitor enhances cell growth inhibition
We performed an MTT assay to assess the effects of
miR-125b on the proliferation of A549 cells following PI3K
inhibition. When compared with miR-125b downregulation alone, the
PI3K inhibitor markedly inhibited the proliferation of A549 cells
at 48 h following the downregulation of miR-125b (Fig. 11).
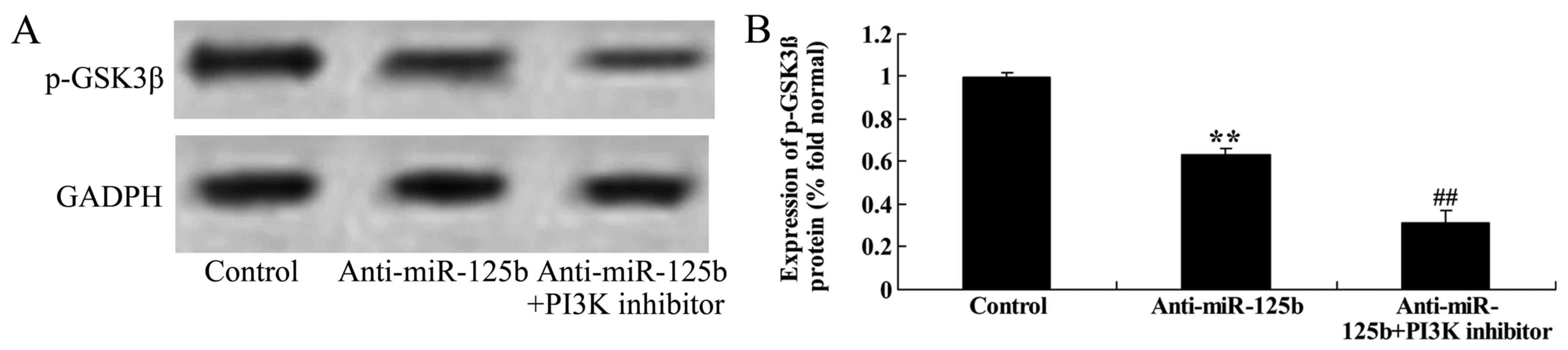
Downregulation of miR-125b combined
with a PI3K inhibitor suppresses the protein expression of p-GSK3β,
Wnt and β-catenin in A549 cells
To determine whether the downregulation of miR-125b
combined with a PI3K inhibitor affected the expression of p-GSK3β,
Wnt and β-catenin in A549 cells, western blot analysis was used to
assess the expression of the proteins. Suppression of PI3K
significantly decreased the expression levels of p-GSK3β (Fig. 12), Wnt and β-catenin (Fig. 13) in A549 cells following the
downregulation of miR-125b at 48 h, relative to a control group
with only miR-125b downregulation.
Downregulation of miR-125b combined
with a PI3K inhibitor promotes caspase-3 activity and Bax
expression in A549 cells
To determine whether the downregulation of miR-125b
combined with a PI3K inhibitor further promoted caspase-3 activity
in A549 cells, caspase-3 activity in A549 cells was assessed using
the caspase-3 activity ELISA kit. The protein expression of Bax was
also evaluated. The activation of caspase-3 and expression of Bax
in A549 cells following downregulation of miR-125b was
significantly increased by the PI3K inhibitor at 48 h, relative to
a control group with only miR-125b downregulation (Fig. 14).
Discussion
The treatment of cancer is a medical science
challenge that to date has had limited success. Although tumor
treatment has made progress in recent years, there is currently no
fundamental method of curing cancer. Lung cancer is among the most
common types of malignant tumors (17). A survey by the World Health
Organization revealed that the morbidity rate of lung cancer in
many countries and regions ranked the highest of all malignant
tumors (18). This is particularly
the case for China, where the morbidity rate of lung cancer is
higher than that in America and other Western countries. NSCLC
accounts for ~70% of all lung cancer cases (19). Despite the use of surgical treatment
for early-stage NSCLC, its 5-year survival rate remains relatively
low at ~30%, while late-stage NSCLC has a one-year survival rate of
~10% even after chemotherapy (20).
To the best of our knowledge, the present study is the first to
demonstrate that the downregulation of miR-125b could suppress the
proliferation and promote the apoptosis of A549 cells.
The activated substrates of PI3K serve as second
messengers on the plasmalemma and bind with the signaling proteins
Akt and PDK1 via interactions with their pH structure domains
(21). After Akt is recruited to
the cytomembrane, it undergoes Ser124 and Thr450 phosphorylation,
which promotes its catalytic activity (13). Akt can also be activated through
phosphorylation of Ser473 mediated by PDK2 enzymes, such as
integrin-linked kinase (11). The
PI3K/Akt pathway induces tumor occurrence via multiple mechanisms:
it downregulates the protein expression of p53 in the cell nucleus
by promoting the nuclear translocation of Mdm2 tumor proteins
(22); it promotes the abnormal
proliferation of cancer cells at a transcriptional and
translational level through excessive activation (23); and it can suppress the process of
apoptosis through multiple mechanisms, including via inhibitory
effects on the conformational changes of pre-apoptotic Bax, and via
the phosphorylation of other apoptotic structures at the
mitochondrial level (24). In the
present study, we found that the downregulation of miR-125b
significantly suppressed the protein expression of p-Akt in A549
cells. Furthermore, the present results revealed that a PI3K
inhibitor suppressed the proliferation of A549 cells following the
downregulation of miR-125b. Similarly, Shi et al reported
that a PI3K inhibitor combined with a miR-125b inhibitor suppressed
cell proliferation in glioma stem cell cancer through inactivation
of the Wnt/β-catenin signaling pathway (25). These data suggest that alterations
in p-Akt expression could be involved in the regulatory effect of
miR-125b downregulation on lung cancer progression.
Glycogen synthase kinase 3β (GSK3β) is a
serine-threonine protein kinase. GSK3β can phosphorylate a variety
of substrates, including metabolic and signaling proteins, cell
structural proteins and transcription factors, and plays an
important role in cell growth and development as well as tumor
occurrence (26). GSK3β is an
important kinase in the Wnt signaling pathway, where it acts as a
key inhibitory factor that blocks Wnt signaling to inhibit tumor
development (27). In the Wnt
signaling pathway, GSK3β also serves as a key regulatory kinase
that mediates the phosphorylation state of β-catenin in the
β-catenin/Axin/APC complex (27).
GSK3β is active in the absence of Wnt signaling, and catalyzes
phosphate addition to four sites within the amino terminus of
β-catenin, leading to its degradation and ultimately the inhibition
of Wnt signal transduction (28).
Our results revealed that the downregulation of miR-125b
significantly decreased the protein expression of p-GSK3β in A549
cells.
β-catenin is not only a component of the
cytomembrane epithelial cadherin/β-catenin complex, but is also a
critical molecule within the intracellular Wnt signal transduction
pathway (29). β-catenin acts as an
important link on the cytomembrane, where it aids to transduce
downstream signals of the Wnt pathway into the nucleus (30). The β-catenin gene CTNNB1 is
located on chromosome 3p22 and has a length of 23.2 kb, which
includes 16 exons. The third exon is regarded as a multi-functional
factor. β-catenin is expressed on the cytomembrane of normal
epithelial tissues (31). Its
characteristics in the cytomembrane are shown in the figure. There
are four ligands in the cytomembrane (32). Upon receipt of outside stimulation,
signals are conducted to the cytoplasm from the cytomembrane, which
involves phosphorylation of intracellular β-catenin.
Phosphorylation of β-catenin enables its dissociation from the
membrane and translocation to the nucleus, where it promotes gene
transcription (33). Ectopic
expression of β-catenin on the cytomembrane is generally rare,
although a previous study of NSCLC showed that the levels of
β-catenin in tumor tissues were significantly higher than those in
normal lung tissues (32). The
aberrant expression of β-catenin proteins has no obvious
correlation with different tumor types or stages. However, the rate
of aberrant expression of β-catenin has been reported to differ
between NSCLC tumors with different degrees of differentiation and
different lymphatic metastasis statuses (31). A codon can be coded into the
phosphorylated locus of GSK3β. The mutation of the locus can cause
abnormal accumulation of β-catenin, leading to cell proliferation
and potentially tumorigenesis (30). The present study revealed that the
downregulation of miR-125b suppressed the protein expression of Wnt
and β-catenin in A549 cells. In addition, PI3K inhibition combined
with miR-125b downregulation suppressed the expression of Wnt and
β-catenin in A549 cells to a greater extent than miR-125b
downregulation alone. Zang et al reported that the E6
protein of human papilloma virus type 16 promoted cell growth
through the downregulation of miR-125b and the activation of the
Wnt/β-catenin signaling pathway in esophageal cancer (34). Collectively these experiments
indicate that PI3K/Wnt/β-catenin signaling may play a role in the
suppression of NSCLC cell growth through the downregulation of
miR-125b.
In summary, this study demonstrates for the first
time that the downregulation of miR-125b can suppress the
proliferation and promote the apoptosis of A549 cells, potentially
through the suppression of PI3K/Wnt/β-catenin expression. These
findings may provide new insights into the mechanisms of miR-125
regarding its effects on PI3K/AKT and GSK3β/Wnt/β-catenin signaling
and the development of NSCLC, and may aid in the development of
potential diagnostic or therapeutic strategies for NSCLC.
References
|
1
|
Kelsey CR, Das S, Gu L, Dunphy FR III,
Ready NE and Marks LB: phase 1 dose escalation study of accelerated
radiation therapy with concurrent chemotherapy for locally advanced
lung cancer. Int J Radiat Oncol Biol Phys. 93:997–1004. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiappori AA, Kolevska T, Spigel DR, Hager
S, Rarick M, Gadgeel S, Blais N, Von Pawel J, Hart L, Reck M, et
al: A randomized phase II study of the telomerase inhibitor
imetelstat as maintenance therapy for advanced non-small-cell lung
cancer. Ann Oncol. 26:354–362. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machtay M, Duan F, Siegel BA, Snyder BS,
Gorelick JJ, Reddin JS, Munden R, Johnson DW, Wilf LH, DeNittis A,
et al: Prediction of survival by [18F]fluorodeoxyglucose
positron emission tomography in patients with locally advanced
non-small-cell lung cancer undergoing definitive chemoradiation
therapy: Results of the ACRIN 6668/RTOG 0235 trial. J Clin Oncol.
31:3823–3830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu C, Li F and Jiao SC: Prognostic factors
for survival of patients with extensive stage small cell lung
cancer - a retrospective single institution analysis. Asian Pac J
Cancer Prev. 13:4959–4962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abhari K, Shekarforoush SS, Hosseinzadeh
S, Nazifi S, Sajedianfard J and Eskandari MH: The effects of orally
administered Bacillus coagulans and inulin on prevention and
progression of rheumatoid arthritis in rats. Food Nutr Res.
60:308762016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao K, Li J and Wang Z:
Dihydroartemisinin inhibits cell proliferation via
AKT/GSK3β/cyclinD1 pathway and induces apoptosis in A549 lung
cancer cells. Int J Clin Exp Pathol. 7:8684–8691. 2014.PubMed/NCBI
|
|
7
|
Zhang Q, Zhu H, Xu X, Li L, Tan H and Cai
X: Inactivated Sendai virus induces apoptosis and autophagy via the
PI3K/Akt/mTOR/p70S6K pathway in human non-small cell lung cancer
cells. Biochem Biophys Res Commun. 465:64–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li YC, He SM, He ZX, Li M, Yang Y, Pang
JX, Zhang X, Chow K, Zhou Q, Duan W, et al: Plumbagin induces
apoptotic and autophagic cell death through inhibition of the
PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells.
Cancer Lett. 344:239–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri
S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K,
et al: Caffeine induces apoptosis by enhancement of autophagy via
PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 7:176–187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong F, Jiang M, Huang Z, Chen M, Chen K,
Zhou J, Yin L, Tang Y, Wang M, Ye L, et al: A novel herbal formula
induces cell cycle arrest and apoptosis in association with
suppressing the PI3K/AKT pathway in human lung cancer A549 cells.
Integr Cancer Ther. 13:152–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trigka EA, Levidou G, Saetta AA,
Chatziandreou I, Tomos P, Thalassinos N, Anastasiou N, Spartalis E,
Kavantzas N, Patsouris E, et al: A detailed immunohistochemical
analysis of the PI3K/AKT/mTOR pathway in lung cancer: Correlation
with PIK3CA, AKT1, K-RAS or PTEN
mutational status and clinicopathological features. Oncol Rep.
30:623–636. 2013.PubMed/NCBI
|
|
12
|
Gadgeel SM and Wozniak A: Preclinical
rationale for PI3K/Akt/mTOR pathway inhibitors as therapy for
epidermal growth factor receptor inhibitor-resistant non-small-cell
lung cancer. Clin Lung Cancer. 14:322–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang C, Lan T, Hou J, Li J, Fang R, Yang
Z, Zhang M, Liu J and Liu B: NOX4 promotes non-small cell lung
cancer cell proliferation and metastasis through positive feedback
regulation of PI3K/Akt signaling. Oncotarget. 5:4392–4405. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin H, Sun Y, Wang X, Park J, Zhang Y, Li
M, Yin J, Liu Q and Wei M: Progress on the relationship between
miR-125 family and tumorigenesis. Exp Cell Res. 339:252–260. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duroux-Richard I, Pers YM, Fabre S, Ammari
M, Baeten D, Cartron G, Touitou I, Jorgensen C and Apparailly F:
Circulating miRNA-125b is a potential biomarker predicting response
to rituximab in rheumatoid arthritis. Mediators Inflamm.
2014:3425242014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cui F, Li X, Zhu X, Huang L, Huang Y, Mao
C, Yan Q, Zhu J, Zhao W and Shi H: MiR-125b inhibits tumor growth
and promotes apoptosis of cervical cancer cells by targeting
phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol
Biochem. 30:1310–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komaki R, Allen PK, Wei X, Blumenschein
GR, Tang X, Lee JJ, Welsh JW, Wistuba II, Liu DD and Hong WK:
Adding erlotinib to chemoradiation improves overall survival but
not progression-free survival in stage III non-small cell lung
cancer. Int J Radiat Oncol Biol Phys. 92:317–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edelman MJ, Tan MT, Fidler MJ, Sanborn RE,
Otterson G, Sequist LV, Evans TL, Schneider BJ, Keresztes R, Rogers
JS, et al: Randomized, double-blind, placebo-controlled,
multicenter phase II study of the efficacy and safety of apricoxib
in combination with either docetaxel or pemetrexed in patients with
biomarker-selected non-small-cell lung cancer. J Clin Oncol.
33:189–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su S, Li T, Lu B, Wang X, Li J, Chen M, Lu
Y, Bai Y, Hu Y, Ouyang W, et al: Three-dimensional radiation
therapy to the primary tumor with concurrent chemotherapy in
patients with stage IV non-small cell lung cancer: Results of a
multicenter phase 2 study from PPRA-RTOG, China. Int J Radiat Oncol
Biol Phys. 93:769–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Gool MH, Aukema TS, Schaake EE, Rijna
H, Codrington HE, Valdés Olmos RA, Teertstra HJ, van Pel R, Burgers
SA, van Tinteren H, et al: 18F-fluorodeoxyglucose
positron emission tomography versus computed tomography in
predicting histopathological response to epidermal growth factor
receptor-tyrosine kinase inhibitor treatment in resectable
non-small cell lung cancer. Ann Surg Oncol. 21:2831–2837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB
and Liu WC: Thymosin alpha 1 suppresses proliferation and induces
apoptosis in breast cancer cells through PTEN-mediated inhibition
of PI3K/Akt/mTOR signaling pathway. Apoptosis. 20:1109–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paul A, Bishayee K, Ghosh S, Mukherjee A,
Sikdar S, Chakraborty D, Boujedaini N and Khuda-Bukhsh AR:
Chelidonine isolated from ethanolic extract of Chelidonium majus
promotes apoptosis in HeLa cells through p38-p53 and PI3K/AKT
signalling pathways. Zhong Xi Yi Jie He Xue Bao. 10:1025–1038.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Chen L, Huang G, He D, He J, Xu W,
Zou C, Zong F, Li Y, Chen B, et al: Klotho sensitizes human lung
cancer cell line to cisplatin via PI3k/Akt pathway. PLoS One.
8:e573912013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu R, Shang C, Zhao J, Han Y, Liu J, Chen
K and Shi W: Activation of M3 muscarinic receptor by acetylcholine
promotes non-small cell lung cancer cell proliferation and invasion
via EGFR/PI3K/AKT pathway. Tumour Biol. 36:4091–4100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi L, Fei X, Wang Z and You Y: PI3K
inhibitor combined with miR-125b inhibitor sensitize TMZ-induced
anti-glioma stem cancer effects through inactivation of
Wnt/β-catenin signaling pathway. In Vitro Cell Dev Biol Anim.
51:1047–1055. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carotenuto M, De Antonellis P, Liguori L,
Benvenuto G, Magliulo D, Alonzi A, Turino C, Attanasio C, Damiani
V, Bello AM, et al: H-Prune through GSK-3β interaction sustains
canonical WNT/β-catenin signaling enhancing cancer progression in
NSCLC. Oncotarget. 5:5736–5749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katoh M: WNT/PCP signaling pathway and
human cancer (Review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
28
|
Zheng H, Saito H, Masuda S, Yang X and
Takano Y: Phosphorylated GSK3β-ser9 and EGFR are good
prognostic factors for lung carcinomas. Anticancer Res.
27:3561–3569. 2007.PubMed/NCBI
|
|
29
|
Jiang HL, Jiang LM and Han WD:
Wnt/β-catenin signaling pathway in lung cancer stem cells is a
potential target for the development of novel anticancer drugs. J
BUON. 20:1094–1100. 2015.PubMed/NCBI
|
|
30
|
Gao Y, Song C, Hui L, Li CY, Wang J, Tian
Y, Han X, Chen Y, Tian DL, Qiu X, et al: Overexpression of
RNF146 in non-small cell lung cancer enhances proliferation
and invasion of tumors through the Wnt/β-catenin signaling pathway.
PLoS One. 9:e853772014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li GH, Cui YS, Wu QY, Zhang XJ and Gao YF:
Clinicopathologic significance of β-catenin and matrix
metalloproteinase-2 expression in non-small cell lung cancer. Med
Oncol. 30:4372013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen CH, Chuang SM, Yang MF, Liao JW, Yu
SL and Chen JJ: A novel function of YWHAZ/β-catenin axis in
promoting epithelial-mesenchymal transition and lung cancer
metastasis. Mol Cancer Res. 10:1319–1331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee SB, Park YI, Dong MS and Gong YD:
Identification of 2,3,6-trisubstituted quinoxaline derivatives as a
Wnt2/β-catenin pathway inhibitor in non-small-cell lung cancer cell
lines. Bioorg Med Chem Lett. 20:5900–5904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zang B, Huang G, Wang X and Zheng S:
HPV-16 E6 promotes cell growth of esophageal cancer via
downregulation of miR-125b and activation of Wnt/β-catenin
signaling pathway. Int J Clin Exp Pathol. 8:13687–13694.
2015.PubMed/NCBI
|