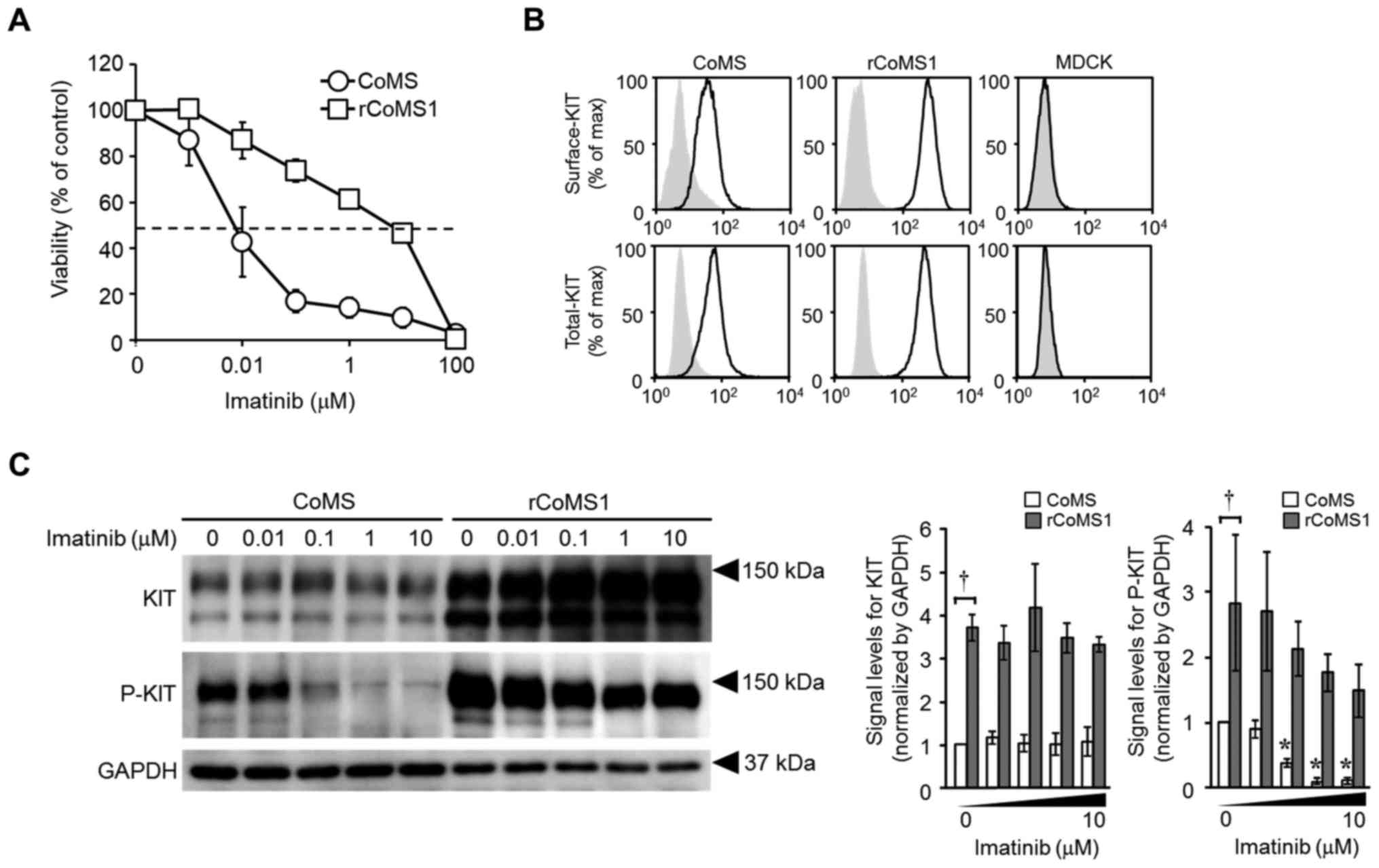
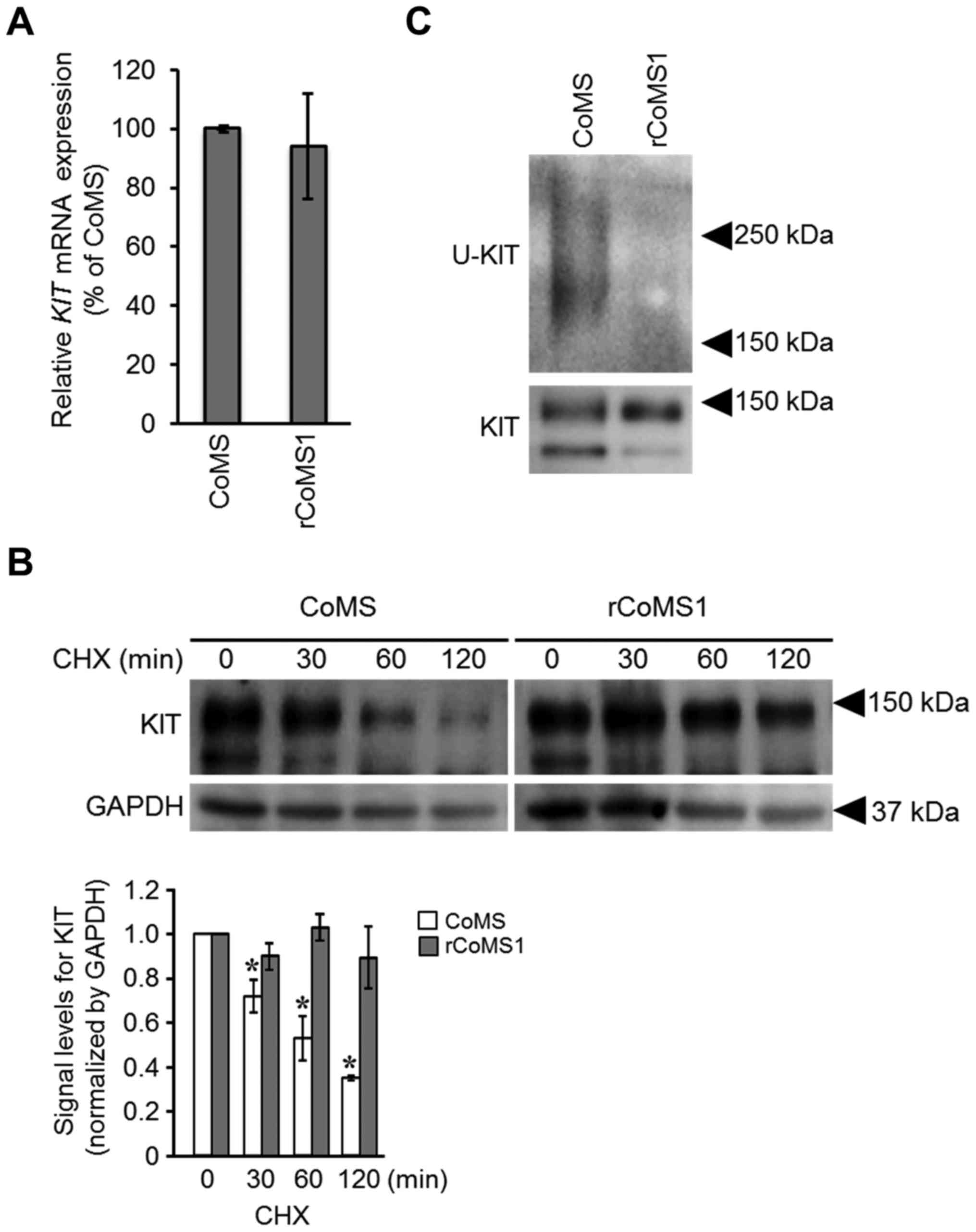
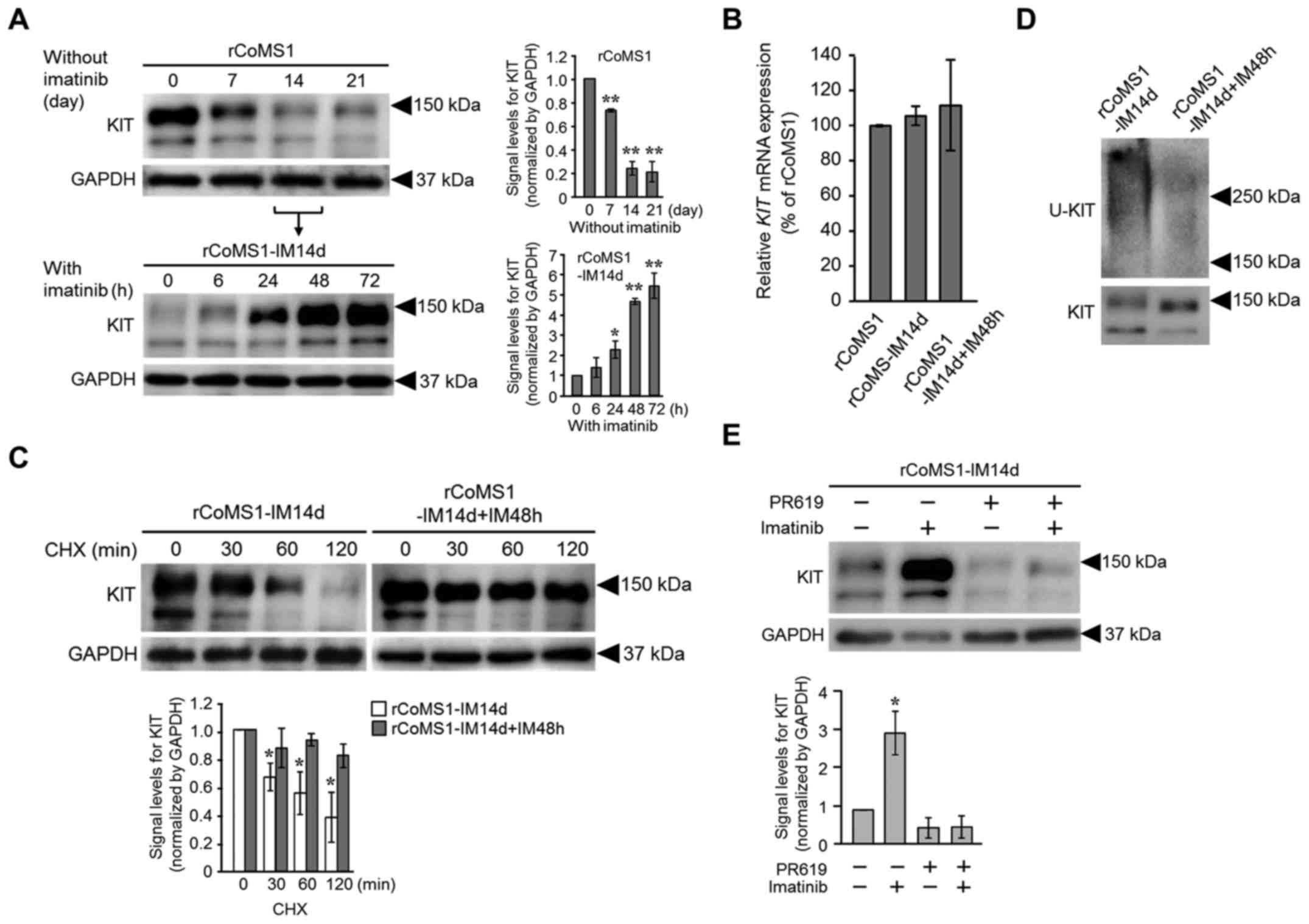
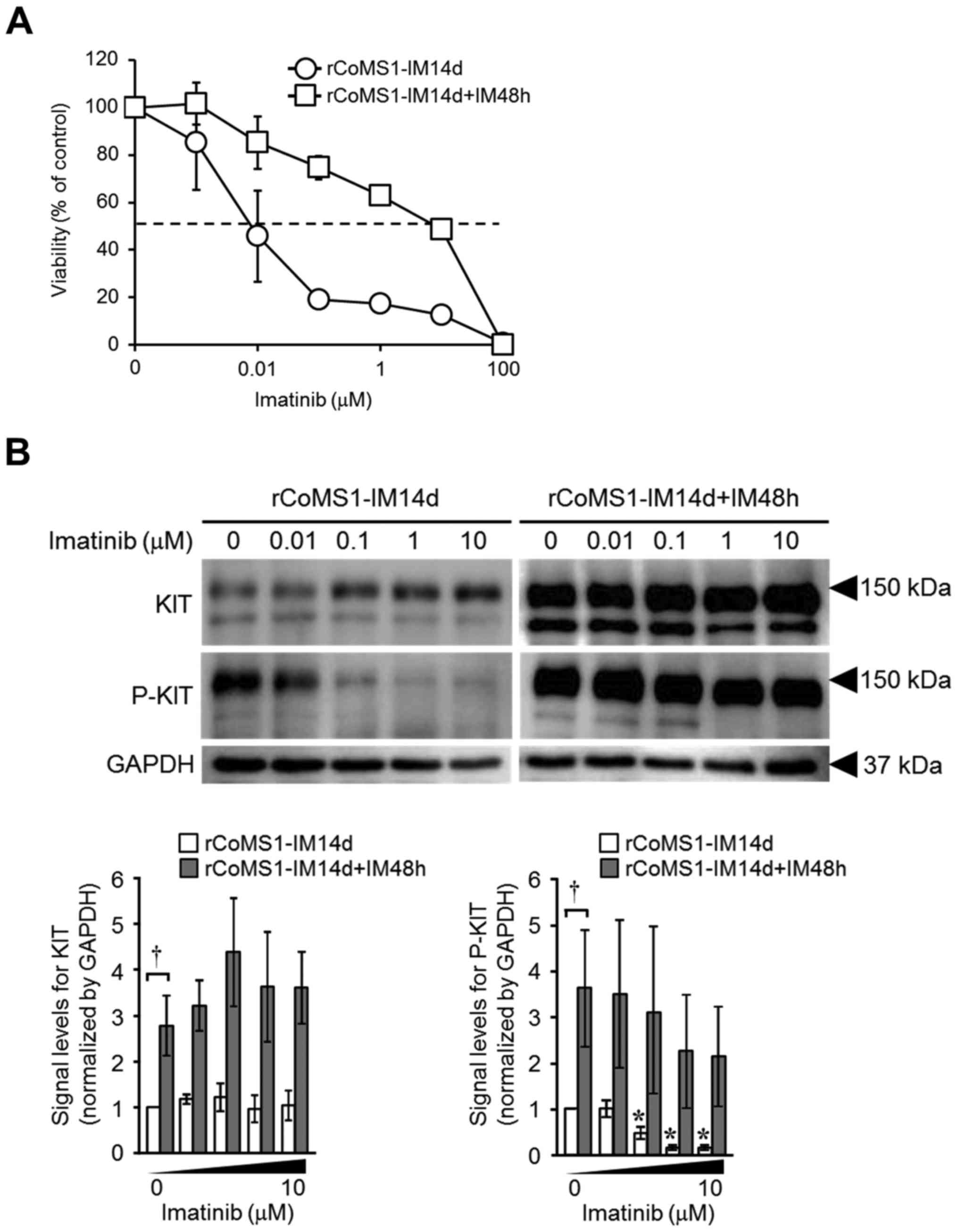
|
1
|
Blay JY, Casali PG, Tos Dei AP, Le Cesne A
and Reichardt P: Management of gastrointestinal stromal tumour:
Current practices and visions for the future. Oncology. 89:1–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alvarez-Twose I, Matito A, Morgado JM,
Sánchez-Muñoz L, Jara-Acevedo M, García-Montero A, Mayado A, Caldas
C, Teodósio C, Muñoz-González JI, et al: Imatinib in systemic
mastocytosis: A phase IV clinical trial in patients lacking exon 17
KIT mutations and review of the literature. Oncotarget. Jul
19–2016.(Epub ahead of print). doi: 10.18632/oncotarget.10711.
|
|
3
|
Hodi FS, Corless CL, Giobbie-Hurder A,
Fletcher JA, Zhu M, Marino-Enriquez A, Friedlander P, Gonzalez R,
Weber JS, Gajewski TF, et al: Imatinib for melanomas harboring
mutationally activated or amplified KIT arising on mucosal, acral,
and chronically sun-damaged skin. J Clin Oncol. 31:3182–3190. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gramza AW, Corless CL and Heinrich MC:
Resistance to tyrosine kinase inhibitors in gastrointestinal
stromal tumors. Clin Cancer Res. 15:7510–7518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miranda C, Nucifora M, Molinari F, Conca
E, Anania MC, Bordoni A, Saletti P, Mazzucchelli L, Pilotti S,
Pierotti MA, et al: KRAS and BRAF mutations predict primary
resistance to imatinib in gastrointestinal stromal tumors. Clin
Cancer Res. 18:1769–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gounder MM and Maki RG: Molecular basis
for primary and secondary tyrosine kinase inhibitor resistance in
gastrointestinal stromal tumor. Cancer Chemother Pharmacol. 67
Suppl 1:S25–S43. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Debiec-Rychter M, Cools J, Dumez H, Sciot
R, Stul M, Mentens N, Vranckx H, Wasag B, Prenen H, Roesel J, et
al: Mechanisms of resistance to imatinib mesylate in
gastrointestinal stromal tumors and activity of the PKC412
inhibitor against imatinib-resistant mutants. Gastroenterology.
128:270–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miselli FC, Casieri P, Negri T, Orsenigo
M, Lagonigro MS, Gronchi A, Fiore M, Casali PG, Bertulli R, Carbone
A, et al: c-KIT/PDGFRA gene status alterations possibly related to
primary imatinib resistance in gastrointestinal stromal tumors.
Clin Cancer Res. 13:2369–2377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lai S, Wang G, Cao X, Luo X, Wang G, Xia
X, Hu J and Wang J: KIT over-expression by p55PIK-PI3K leads to
Imatinib-resistance in patients with gastrointestinal stromal
tumors. Oncotarget. 7:1367–1379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Irie M, Takeuchi Y, Ohtake Y, Suzuki H,
Nagata N, Miyoshi T, Kagawa Y and Yamagami T: Imatinib mesylate
treatment in a dog with gastrointestinal stromal tumors with a
c-KIT mutation. J Vet Med Sci. 77:1535–1539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi M, Kuroki S, Ito K, Yasuda A,
Sawada H, Ono K, Washizu T and Bonkobara M: Imatinib-associated
tumour response in a dog with a non-resectable gastrointestinal
stromal tumour harbouring a c-KIT exon 11 deletion mutation. Vet J.
198:271–274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
London CA, Galli SJ, Yuuki T, Hu ZQ,
Helfand SC and Geissler EN: Spontaneous canine mast cell tumors
express tandem duplications in the proto-oncogene c-KIT. Exp
Hematol. 27:689–697. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bostock DE: Neoplasms of the skin and
subcutaneous tissues in dogs and cats. Br Vet J. 142:1–19. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bonkobara M: Dysregulation of tyrosine
kinases and use of imatinib in small animal practice. Vet J.
205:180–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
London CA, Malpas PB, Wood-Follis SL,
Boucher JF, Rusk AW, Rosenberg MP, Henry CJ, Mitchener KL, Klein
MK, Hintermeister JG, et al: Multi-center, placebo-controlled,
double-blind, randomized study of oral toceranib phosphate
(SU11654), a receptor tyrosine kinase inhibitor, for the treatment
of dogs with recurrent (either local or distant) mast cell tumor
following surgical excision. Clin Cancer Res. 15:3856–3865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi M, Kuroki S, Tanaka Y, Moriya Y,
Kozutumi Y, Uehara Y, Ono K, Tamura K, Washizu T and Bonkobara M:
Molecular changes associated with the development of resistance to
imatinib in an imatinib-sensitive canine neoplastic mast cell line
carrying a KIT c.1523A>T mutation. Eur J Haematol. 95:524–531.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishiguro T, Kadosawa T, Mori K, Takagi S,
Okumura M and Fujinaga T: Establishment and characterization of a
new canine mast cell tumor cell line. J Vet Med Sci. 63:1031–1034.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin WL, Mao XY and Qiu GZ: Targeting
deubiquitinating enzymes in glioblastoma multiforme: Expectations
and challenges. Med Res Rev. 37:627–661. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reincke M, Sbiera S, Hayakawa A,
Theodoropoulou M, Osswald A, Beuschlein F, Meitinger T,
Mizuno-Yamasaki E, Kawaguchi K, Saeki Y, et al: Mutations in the
deubiquitinase gene USP8 cause Cushing's disease. Nat Genet.
47:31–38. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Masson K, Heiss E, Band H and Rönnstrand
L: Direct binding of Cbl to Tyr568 and Tyr936 of the stem cell
factor receptor/c-KIT is required for ligand-induced
ubiquitination, internalization and degradation. Biochem J.
399:59–67. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yee NS, Hsiau CW, Serve H, Vosseller K and
Besmer P: Mechanism of down-regulation of c-KIT receptor. Roles of
receptor tyrosine kinase, phosphatidylinositol 3′-kinase, and
protein kinase C. J Biol Chem. 269:31991–31998. 1994.PubMed/NCBI
|
|
22
|
Kim H, Frederick DT, Levesque MP, Cooper
ZA, Feng Y, Krepler C, Brill L, Samuels Y, Hayward NK, Perlina A,
et al: Downregulation of the ubiquitin ligase RNF125 underlies
resistance of melanoma cells to BRAF inhibitors via JAK1
deregulation. Cell Rep. 11:1458–1473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng S, Xu Z, Lipkowitz S and Longley JB:
Regulation of stem cell factor receptor signaling by Cbl family
proteins (Cbl-b/c-Cbl). Blood. 105:226–232. 2005. View Article : Google Scholar : PubMed/NCBI
|