Introduction
Gastric cancer (GC) is the fifth most common cancer
and the third most common cause of cancer-related death worldwide
(1,2). Although the incidence of GC has been
declining for several decades, GC is one of the most common cancers
in China, accompanied by a high incidence and mortality,
approximately accounting for 10% of all malignancies (3). The treatment strategies for gastric
cancer have made great progress, however, the prognosis of gastric
cancer is still poor; due to most cases being diagnosed in an
advanced stage, the 5-year survival rate is only 20–30% (4). Currently known major risk factors for
GC include Helicobacter pylori infection, living
environment, diet, genetic and immune factors and chronic stomach
diseases (5). There have been
advancements in the molecular biomarkers utilised in the cancer
detection, furthermore, in the development of therapeutic agents
based on the target genes for a few types of solid tumors including
GC (6). With the development of
biotechnology, the molecular mechanisms and alterations that lead
to initiation and progression of GC, including multiple genetic and
molecular alterations and mutations have been revealed (7). These biomarkers can help us make early
diagnosis and predict the prognosis of GC patients.
FTO also known as ALKBH9, it is localized on
chromosome 16q12.2, FTO belongs to the non-heme Fe
II/α-KG-dependent dioxygenase AlkB family proteins that also
includes ABH1 to ABH8 (8). After
identification of a fused-toe mutant mouse whose phenotype results
from a 1.6-Mb deletion of six genes, including FTO, the FTO gene
was first cloned (9). In 2007, FTO
was described as the first gene in association with the common
obesity. FTO is an AlkB-like 2-oxoglutarate-dependent nucleic acid
demethylase with a strong preference for 3-methylthymidine and
3-methyluracil single-stranded DNA and RNA (10). It has been reported that FTO can
oxidize demethylate m-3T and m-3U in single-stranded DNA (ssDNA)
and single-stranded RNA (ssRNA) in vitro (11). The expression of FTO mRNA is
extensive in different human tissues indicates that it may be
involved in important biological processes (11,12).
Although obesity is a basic biological mechanism of the risk of the
development of cancer it is not fully understood, recent studies
have shown that the FTO gene may associate with cancer risk
(13), such as breast (14), thyroid (15) and endometrial cancer (16).
To date, no research has been reported on whether
FTO is associated with GC. The aim of this study was to investigate
the FTO expression in GC patient specimens and to appraise the
clinicopathological implications of FTO expression in GC. Our
efforts are aimed at discovering the potential influence of FTO in
carcinogenesis and progression of GC.
Materials and methods
Tissue specimen
Gastric cancer (GC) specimens were collected from
128 patients with primary gastric cancer during surgery in Hospital
Affiliated of Nantong University from January 2010 to February
2011, and the patients were enrolled in this study. After surgical
resection, randomly selected carcinoma adjacent tissue specimens
and GC tissues were obtained from 128 patients. The carcinoma
adjacent tissues were assessed microscopically for the presence of
normal cells and absence of dysplastic cells, and taken >3 cm
distance from the tumor margin. Immediately the fresh sample was
divided into two parts, frozen in liquid nitrogen, one was
maintained at −80°C until use real-time PCR and western blot
analysis, the other was embedded in paraffin after fixation in 10%
formalin (24–48 h) fixed for immunohistochemical diagnosis.
Clinical data were also collected on sex, age, tumor size, TNM
stage, and lymph node metastasis. Distant metastasis was determined
by radiological examination. Staging and grading referred to the
classification of International Union Against Cancer criteria
(UICC). The patients who participated in the study did not receive
radiotherapy or chemotherapy before surgery. This study was
conducted with the approval of the institutional ethics board of
the Hospital Affiliated of Nantong University.
Western blot analysis
Paired cancer tissue and carcinoma adjacent tissue
of 24 cases were randomly chosen from 128 gastric cancer patients
for western blot analysis. The whole protein was extracted by lysis
buffer which contained protease inhibitors (Promega, Madison, WI,
USA). The 10% sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
was used to isolate equal amounts of protein, and these proteins
were then transferred into a polyvinylidene fluoride (PVDF)
membrane. Then the membranes were blocked for 2 h with 5% non-fat
milk in TBST (Tris-buffered saline containing 0.1% Tween-20) and
incubated with the primary antibodies overnight at 4°C; monoclonal
mouse anti-human FTO at 1:1,000 dilution (Abcam, Cambridge, UK) or
monoclonal mouse anti-β-actin as internal reference, at 1:2,000
dilution (Sigma-Aldrich, St. Louis, MO, USA). The membranes were
washed three times in TBST, for 5 min each time. Then, incubated
with IRDye 680 CS-conjugated goat anti-mouse secondary antibody
(1:1,000 dilution; Sigma-Aldrich) for 2 h at room temperature
according to the manufacturers instructions. Eventually signals
were scanned with an Odyssey Infrared Imaging System (LI-COR
Biosciences, Lincoln, NE, usa) and analyzed with PDQuest 7.2.0
software (Bio-Rad Laboratories, Hercules, CA, USA).
Immunohistochemical staining and
evaluation
A total of 128 GC tissues, 62 matched adjacent
non-cancerous tissues specimens were prepared and used in this
study. We used tissue microarray (TMA) system (Quick-Ray UT06;
Unitma Co., Ltd., Seoul, Korea) in the Department of Clinical
Pathology. Core tissue biopsies (2 mm in diameter) were taken from
individual paraffin-embedded sections and arranged in recipient
paraffin blocks. TMA specimens were cut into 4 µm sections and
placed on super frost-charged glass microscope slides. TMA analysis
was used as a quality control for hematoxylin and eosin staining.
Tissue sections were deparaffinized and rehydrated in graded
ethanol. Antigen retrieval was performed by boiling sections in
ethylene diaminetetra-acetic acid buffer, pH 6.0, for 3 min in a
pressure cooker. Endogenous peroxidase activity was quenched with
3% hydrogen peroxide for 30 min. TMA slides were stained using
monoclonal mouse anti-FTO antibody (Abcam) at 4°C overnight. Then,
by incubation with a horseradish-peroxidase-conjugated goat
anti-mouse secondary antibody (Sigma-Aldrich) at 37°C for 30 min.
Slides were then processed using horseradish peroxidase and
3,3-diaminobenzidine chromogen solution and counterstained with
hematoxylin. The semiquantitative H-score method was used to
convert the expression of FTO to continuous intensity values, based
on both the staining intensity and the percentage of cells at that
intensity. According to the traditional semi-quantitative pathology
scoring, staining intensity was scored as follows: 0 (−, no
staining), 1 (+, weak staining), 2 (++, moderate staining), or 3
(+++, intense staining). The percentage of cells stained at a
certain intensity was determined and multiplied by the intensity
score to generate an intensity percentage score. The final staining
score of each tissue sample was the sum of the four intensity
percentage scores, and these scores ranged from 0 (no staining) to
300 (100% of cells with +++ staining intensity).
Real-time PCR
Firstly, the TRIZol (Gibco, Grand Island, NY, USA)
was used to extract total RNAs from tumor tissue samples. Then
quantitative real-time PCR was performed using HotStart-IT
SYBR-Green qPCR Master Mix (2X; USB Corp., Cleveland, OH, USA). In
the light of the HotStart-IT protocol, 25 µl actions were run of
with 2 µl cDNA. We perform RT-PCR experiments in a LightCycler 480
system (Roche Applied Sciences). PCR steps: first hot start at 95°C
for 10 min; then 40 cycles of amplification/quantification at 95°C
for 10 sec, next 60°C for 30 sec and 72°C for 30 sec during the
time fluorescence was measured. Melting curve analysis was
implemented using continuous fluorescence acquisition from 65 to
97°C. These cycling parameters produced single amplicons for both
primer sets used on the basis of the presence of a single melt
peak. We use the β-actin as the internal reference. The primer
sequences are as follows: a 169-bp segment of the FTO gene
5-TGGTGTCCCAAGAAATCGTG-3 (sense) and 5-TGCAGGCCGTGAACCAC-3
(antisense), a 107-bp segment of the β-actin gene
5-AACTTCCGTTGCTGCCAT-3 (sense) and 5-TTTCTTCCACAGGGCTTTG-3
(antisense). All quantitative real-time PCRs were repeated 3 times
for each gene, and each sample was done in triplicate.
Cell culture
Four human GC cell lines (BGC823, MKN45, SGC7901 and
AGS) and the human normal stomach epithelial mucosa cell line (GES)
from the Cell Bank of the Committee on Type Culture Collection of
the Chinese Academy of Sciences (Shanghai, China). These cell lines
were grown in Dulbeccos modified Eagles medium (DMEM; Invitrogen,
Carlsbad, CA, USA) or RPMI-1640 medium (Gibco) containing with 10%
fetal bovine serum (FBS; HyClone Laboratories, Inc., Logan, UT,
USA) and were cultured in humidified incubator at 37°C with 5%
CO2.
Construction of plasmid and
transfection
The siRNA oligos for FTO were designed and purchased
from Shanghai GenePharma Co., Ltd., (Shanghai, China). The
targeting sequence was FTO-siRNA1: GCAGTGTATCTGAGGAGCTCCATAA,
FTO-siRNA2: CGGTATCTCGCATCCTCATTGGTAA and FTO-siRNA3:
TCAGCGGTGGCAGTGTACAGTTATA. A FTO overexpression plasmid
(pcDNA3.1-FTO) containing the coding sequence was constructed using
PCR-generated fragments and pcDNA 3.1(+) vector. Then the PCR
products were cloned into the mammalian expression vector pcDNA
3.1(+). All constructs were confirmed by DNA sequence analysis. We
use the stable transfectant of the pcDNA 3.1(+) vector as the
control group. During the course of transfection, the FTO siRNA and
pcDNA 3.1(+)-FTO expression plasmids were transfected into GC cells
using Lipofectamine 2000 (Invitrogen) according to the
manufacturers instructions. The western blot assay was used to
evaluate the level of FTO expression after transfection.
Cell viability assay and Transwell
assay
MTT assay was used to assess the cell viability.
Firstly, we use the 96-well plates at 5×103 cells/well
in complete medium for the cells. The cells were cultured for 24 h.
Next, the culture medium was replaced with a medium which contained
10% FBS. Then, 10 µl MTT was added to each corresponding test and
the cells were kept in culture for 4 h. Finally, all samples were
measured at 490 nm spectrophotometric absorbance.
Cell migratory capacity was confirmed by Transwell
assay (BD Biosciences, San Jose, CA, USA) according to the
manufacturers instructions. These transfected cells were harvested
24 h after transfection. Then 3.0×105 transfected cells
or untreated cells were added to each upper insert in serum-free
medium. To the matched lower chamber, 500 µl of medium containing
10% FBS was added. After incubation, non-migrated cells were
removed from the upper surface of the transwell membrane with a
cotton swab, and the migrated cells on the lower membrane surface
were fixed in methanol. Lastly, 0.1% crystal violet was used to
stain cells, then photographed and counted. The above experiments
were performed in triplicate and repeated three times.
Colony formation assay
Cells (5×104/well) were plated in a
24-well plate after transfection. After 24 h, the cells collected
and seeded (1,000–1,500/well) in a fresh 6-well plate for 12 days.
Surviving colonies (>50 cells/colony) were counted after fixed
with methanol/acetone (1:1) and stained with 5% gentian violet (ICM
Pharma, Singapore). After washing three times with
phosphate-buffered saline (PBS) to remove excess dye, the cells
were photographed and counted. The experiment was carried out in
triplicate wells three times.
Statistical analysis
All data were analyzed using statistical analyses
performed using the SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The
relationship between the FTO expression level and the
clinicopathological characteristics was subjected to χ2
analysis. Survival analysis was calculated using the Kaplan-Meier
method and curves were assessed using the log-rank test. Coxs
proportional hazards model was used to carry out the multivariate
analysis of several prognostic factors. The results are presented
as the mean ± SD of at least three independent experiments and
P<0.05 was considered to be statistically significant.
Results
FTO expression in primary gastric
tumors
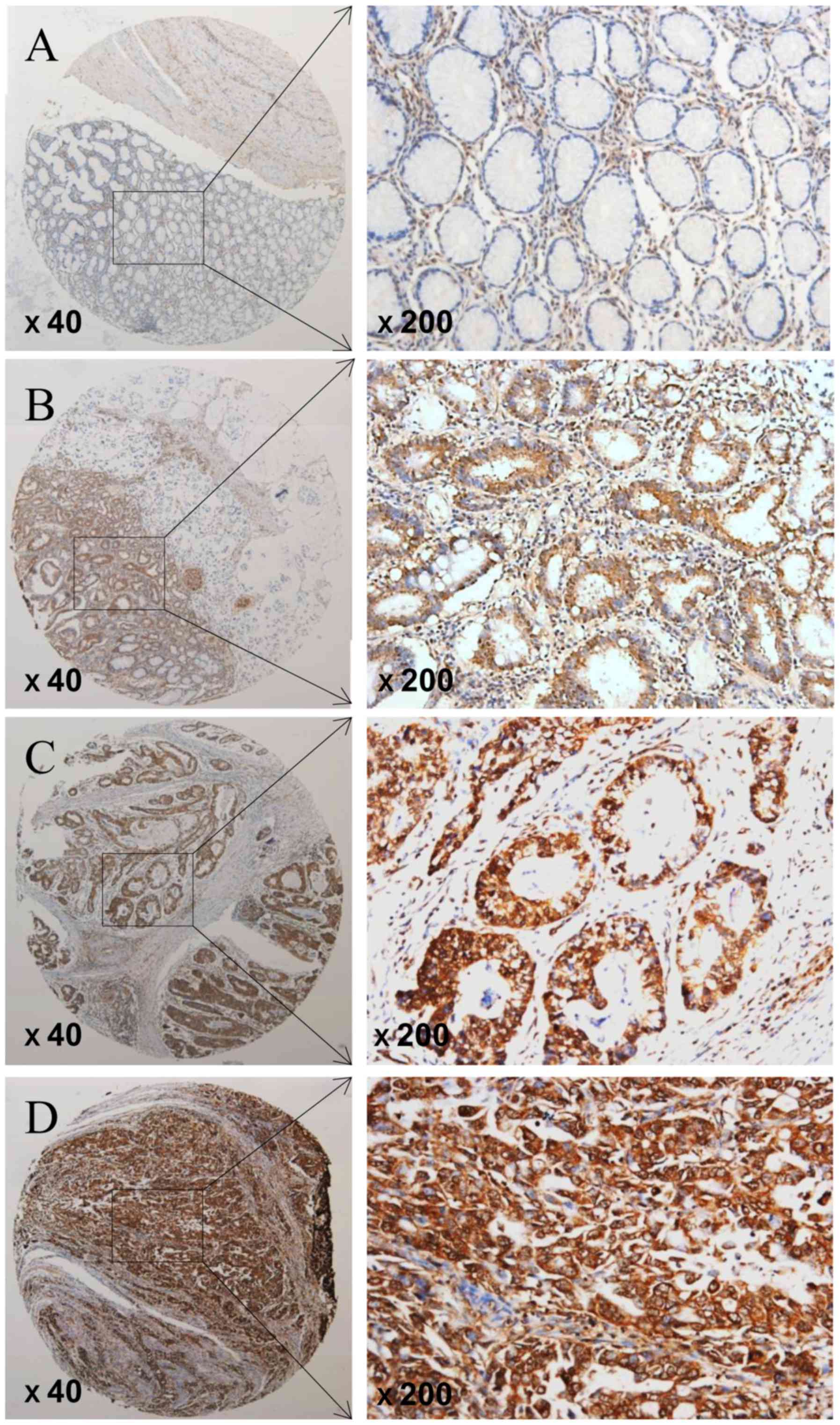
The expression of FTO was determined by TMA-based
immunohistochemistry (IHC) studies. We showed that the expression
level of FTO in GC tissues and adjacent tissues were different, FTO
expression was significantly elevated in GC patients but negative
or low in non-tumor tissues (Fig.
1). Positive staining was predominantly localized in the
nucleus of GC cells. Overall, FTO expression was positive (FTO ++
or FTO +++) in 72 of 128 GC tissues (56.3%), the carcinoma adjacent
tissues (38 of 62, 61.3%) were mostly negative of FTO expression
(FTO- or FTO+). Overall, GC tissues had high FTO expression as
compare with the adjacent non-cancerous tissues (P=0.023; Table I).
 | Table I.FTO expression compared in gastric
cancer and adjacent non-tumor tissues. |
Table I.
FTO expression compared in gastric
cancer and adjacent non-tumor tissues.
|
|
| FTO expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | N | Low level | High level | P-value |
|---|
| Gastric cancer
tissues | 128 | 56 | 72 | 0.023a |
| Adjacent non-tumor
tissues | 62 | 38 | 24 |
|
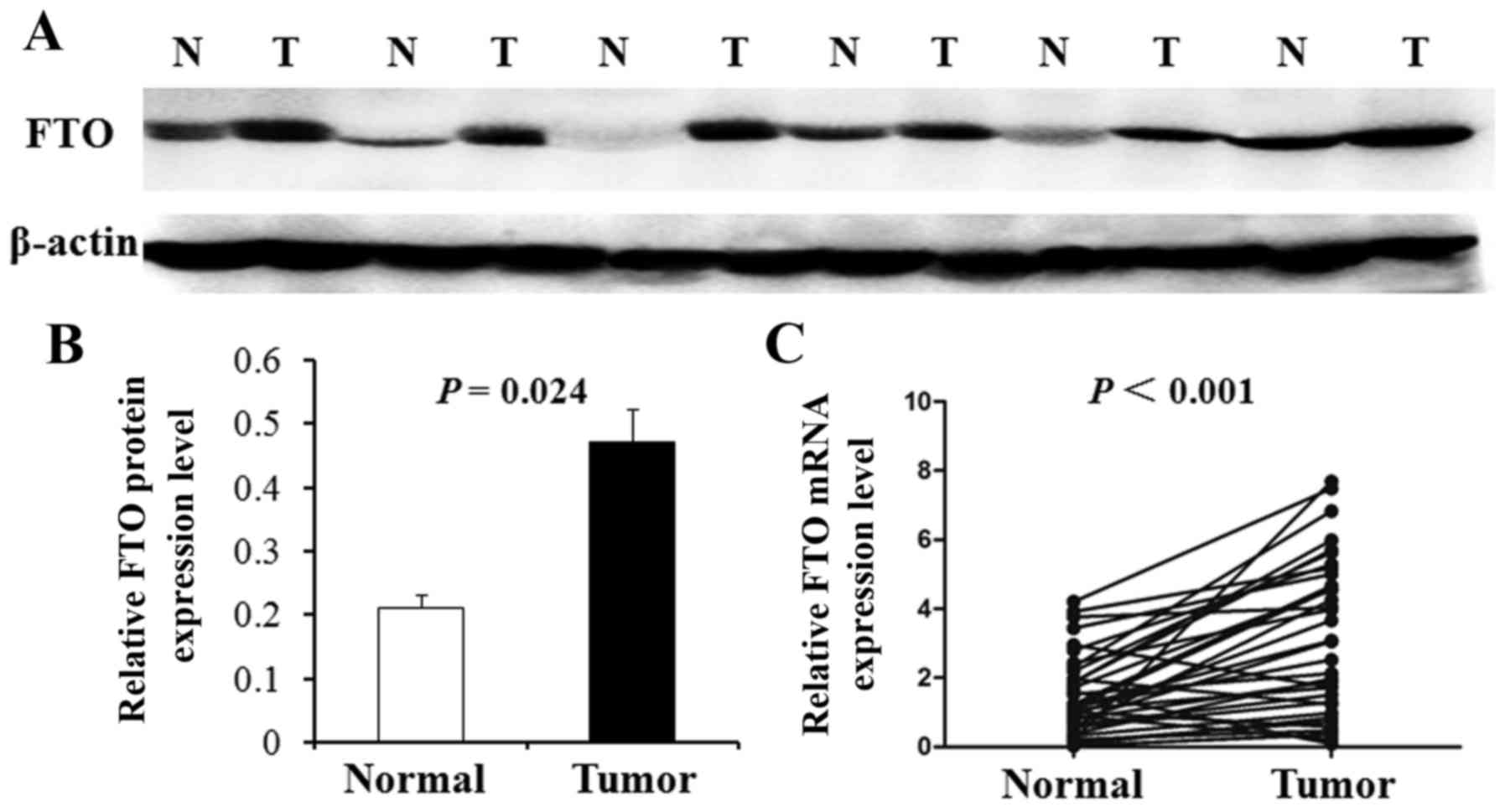
The above results were further tested by western
blot analysis in 24 random pairs of GC and corresponding carcinoma
adjacent tissues. Furthermore, the representative western blot
results in 6 cases are shown in Fig.
2A. The results show that FTO protein level was upregulated in
the GC samples (75%, 18 of 24) compared with corresponding
carcinoma adjacent tissues. The average FTO protein level in 24
gastric carcinoma tissues was significantly higher than that in
corresponding carcinoma adjacent tissues (P=0.024; Fig. 2B). Real-time PCR also was used to
examine the mRNA expression of FTO in 36 primary GC tissues and
their corresponding adjacent normal tissues selected randomly. As
shown in Fig. 2C, the average
expression of FTO mRNA level was significantly higher in GC tissues
compared with adjacent non-cancerous tissues (P<0.001).
FTO expression is correlated to
clinicopathological factors in GC patient survival
We use the χ2 test to analyze the
correlation between the FTO expression in GC tissues and various
clinicopathological characteristics of GC patients are listed in
Table II. High expression of FTO
was significantly correlated with poor differentiation
(P<0.001), lymph node metastasis (P=0.029) and high TNM stage
(P<0.001). But the FTO expression was not significantly
correlated with sex, age, tumor size, location, distant metastasis
and H. pylori infection (P>0.05).
 | Table II.Clinicopathological correlation of FTO
expression in gastric cancer patients. |
Table II.
Clinicopathological correlation of FTO
expression in gastric cancer patients.
|
|
| FTO expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Total (n=128) | Low level (n=56) | High level
(n=72) | P-value |
|---|
| Sex |
|
|
| 0.929 |
| Male | 68 | 30 | 38 |
|
|
Female | 60 | 26 | 34 |
|
| Age (years) |
|
|
| 0.169 |
|
<50 | 42 | 22 | 20 |
|
| ≥50 | 86 | 34 | 52 |
|
| Tumor size (cm) |
|
|
| 0.052 |
|
<4 | 54 | 29 | 25 |
|
| ≥4 | 74 | 27 | 47 |
|
| Location |
|
|
| 0.681 |
|
Cardia | 50 | 23 | 27 |
|
|
Body/antrum | 78 | 33 | 45 |
|
|
Differentiation |
|
|
|
<0.001a |
|
Well/moderate | 52 | 13 | 39 |
|
|
Poor | 76 | 43 | 33 |
|
| Depth of
invasion |
|
|
| 0.907 |
|
T1/2 | 45 | 20 | 25 |
|
|
T3/4 | 83 | 36 | 47 |
|
| Lymph node
metastasis |
|
|
| 0.029a |
|
Negative | 46 | 26 | 20 |
|
|
Positive | 82 | 30 | 52 |
|
| TNM stage |
|
|
|
<0.001a |
|
I/II | 69 | 40 | 29 |
|
|
III/IV | 59 | 16 | 43 |
|
| Distant
metastasis |
|
|
| 0.270 |
|
Negative | 120 | 54 | 66 |
|
|
Positive | 8 | 2 | 6 |
|
| H. pylori
infection |
|
|
| 0.766 |
|
Negative | 36 | 15 | 21 |
|
|
Positive | 92 | 41 | 51 |
|
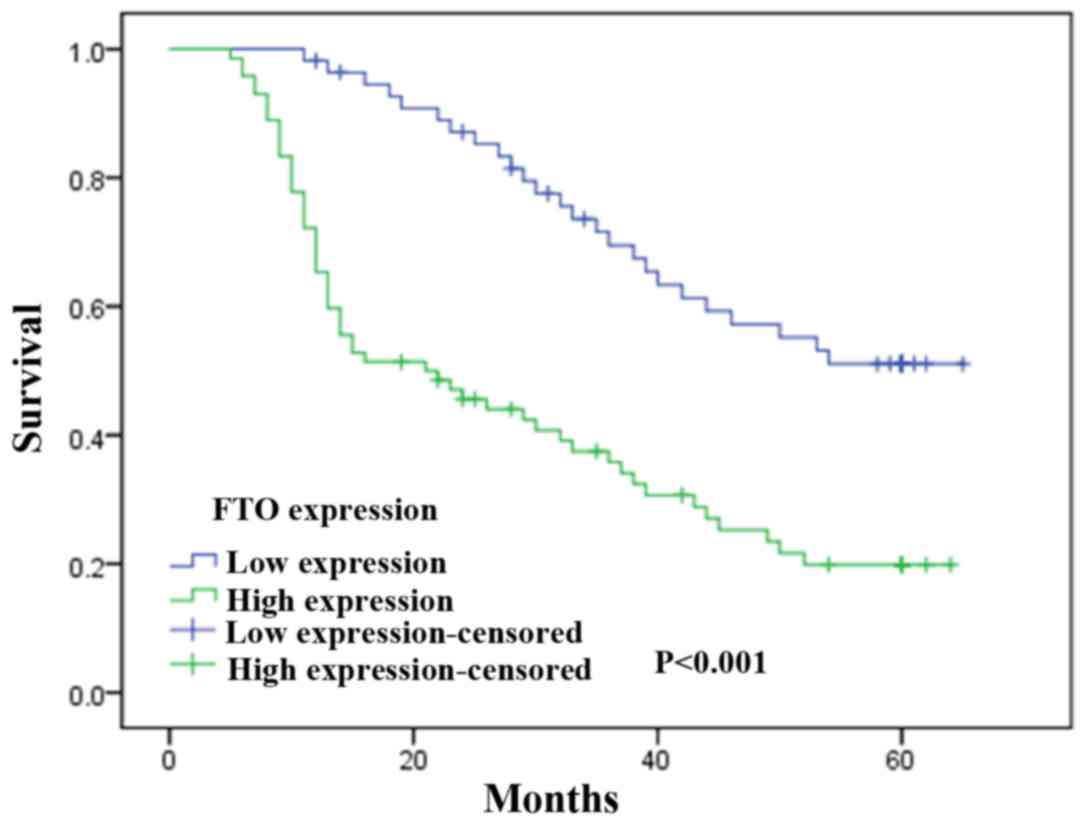
To investigate the prognostic effect of FTO
expression on overall survival rate of GC patients, Kaplan-Meier
survival curves and the log-rank test was used to compare the
5-year survival rate of patients with high or low FTO expression
level. According to the immunohistochemical results of FTO staining
in tumors cells, GC patients were divided into two groups including
FTO low expression group and high expression group. We found that
the group with the high FTO expression levels had a poorer
prognosis than the group with low levels of FTO expression
(χ2=8.415, P<0.001; Fig.
3).
Univariate and multivariate analysis was used to
estimate the independent prognostic factors of FTO (Table III). The Coxs proportional hazards
model showed that that histological grade, invasive depth, TNM
stage, lymph node metastasis and FTO expression were significantly
associated with overall survival in GC patients. In addition, we
found that FTO expression and TNM stage were independent prognostic
indicators for overall survival of GC patients.
 | Table III.Cox proportional hazards model
analysis of prognostic factors. |
Table III.
Cox proportional hazards model
analysis of prognostic factors.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| FTO expression (low
vs. high) | 0.544 | 0.384–0.865 |
<0.001a | 0.627 | 0.476–0.926 |
<0.001a |
| Sex (male vs.
female) | 1.028 | 0.928–1.658 | 0.580 | – | – | – |
| Age (<50 vs. ≥50
years) | 1.016 | 0.930–1.806 | 0.925 | – | – | – |
| Tumor size (<4
vs. ≥4 cm) | 1.037 | 0.710–1.416 | 0.064 | – | – | – |
| Location (cardia
vs. body/antrum) | 1.325 | 1.085–2.140 | 0.904 | – | – | – |
| Differentiation
(poor vs. well/mod) | 0.863 | 0.526–1.376 | 0.007a | 0.830 | 0.516–1.526 | 0.353 |
| Distant metastasis
(+ vs. -) | 1.089 | 0.790–1.682 | 0.626 | – | – | – |
| Depth of invasion
(T3/T4 vs. T1/T2) | 1.140 | 0.850–1.783 | 0.072 | – | – | – |
| TNM stage (III + IV
vs. I + II) | 1.056 | 0.784–2.021 | 0.002a | 0.949 | 0.650–1.458 | 0.001a |
| Lymph node
metastasis (+ vs. -) | 1.956 | 1.264–2.560 | 0.030a | 1.418 | 0.850–1.813 | 0.552 |
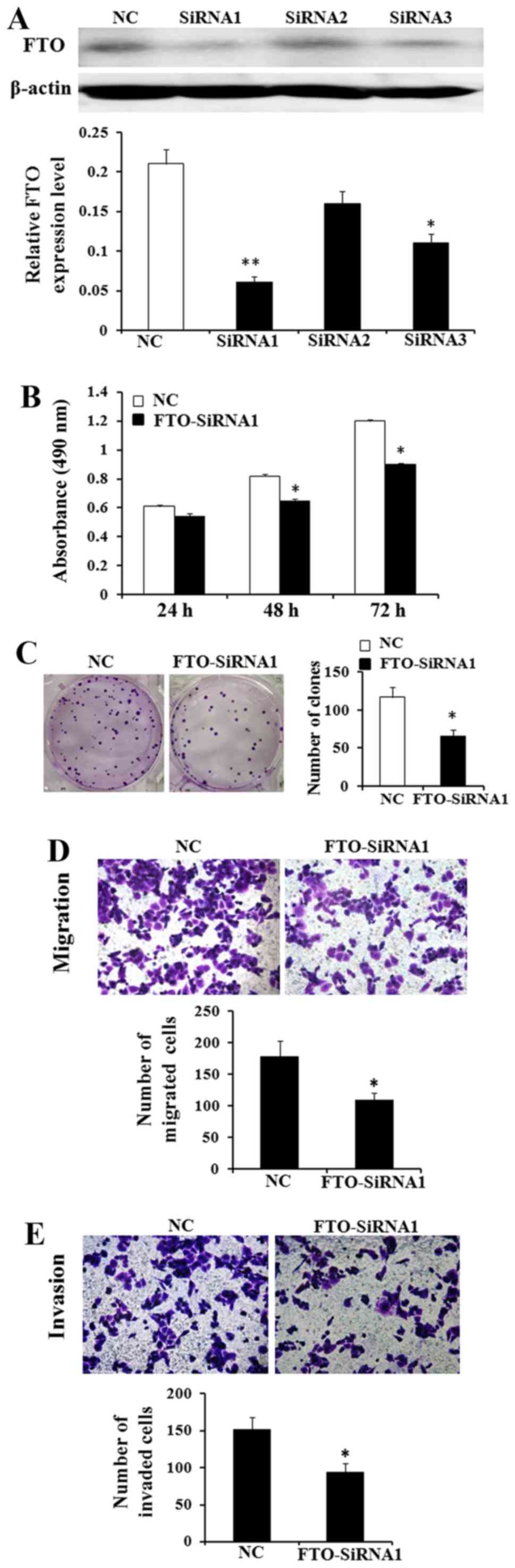
Downregulation of FTO expression
inhibits cell proliferation, migration and invasion abilities in
vitro
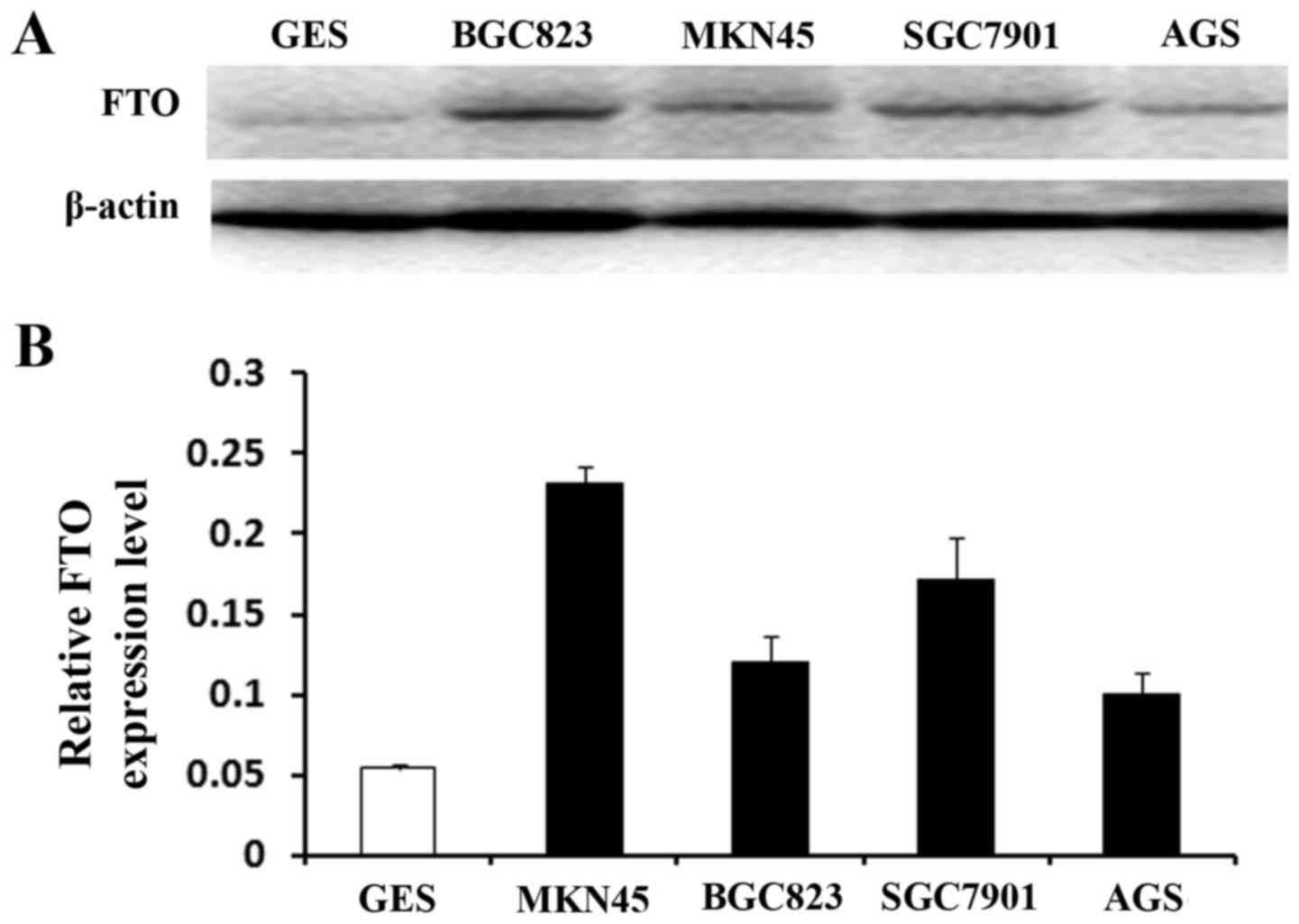
Thus, based on the above, we hypothesized that FTO
played the role as a cancer promoting gene. We used the MTT and
colony formation assays to identify the effect of FTO on cell
viability and proliferation. MKN45 cells have the highest FTO
expression in the four GC cells (Fig.
4). After transfected with FTO siRNA1, FTO siRNA2 and FTO
siNRA3, we found that siRNA1 reduced the level of endogenous FTO
expression more significantly than siRNA2 and siRNA3 by western
blot analysis (Fig. 5A). The
results demonstrated that the viability of MKN45 cells was lower
and the number of colonies of MKN45 cells in the siRNA treated
group was less than in the control group (Fig. 5B and C; P<0.05). Besides, the
Transwell assay shows that knockdown of FTO expression markedly
decreased the migrated and invaded cell number of MKN45 cell lines
(Fig. 5D and E; P<0.05).
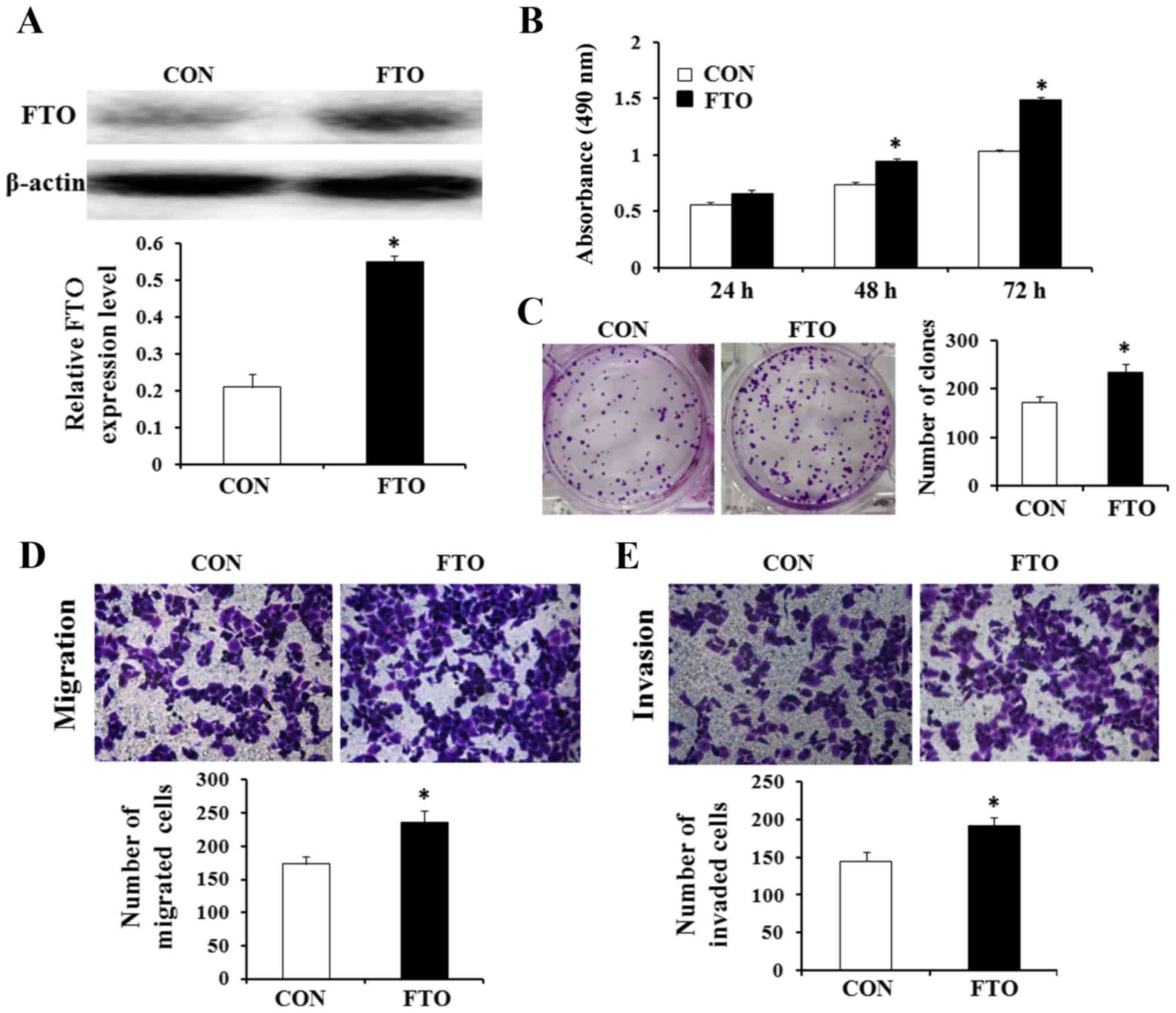
Overexpression of FTO promotes cell
viability, proliferation, migration and invasion in vitro
The effects of FTO on the viability, proliferation,
migration and invasion of GC cells were evaluated. AGS cell line
was selected to investigate whether overexpression of FTO affected
cell viability, proliferation, migration and invasion in GC. In
addition, the FTO expression level of AGS cell line was the lowest
in the four GC cell lines. Thus, AGC cells were transfected with
recombinant plasmids containing the full ORF of the wild-type FTO.
Western blot analysis confirmed that the cells transfected with the
FTO recombinant plasmid showed higher expression levels of the FTO
protein compared with cells transfected with the empty vector
control pEGFP-N1 (Fig. 6A). The
results of MTT and colony formation assays showed that
overexpression of FTO promoted viability and the number of colonies
of AGS cells (Fig. 6B and C;
P<0.05). We also found that overexpression of FTO could increase
the transformation phenotype of GC cells in vitro. Then, to
investigate the potential affect of FTO on cellular migration and
invasion, FTO overexpressing plasmid or control plasmid were
transfected into AGS cells. We use Transwell assays to determine
their migratory and invasive abilities after 24 h. The result
reflects that overexpression of FTO enhanced the migratory and
invasive capacity of AGS cells (Fig. 6D
and E; P<0.05).
Discussion
Despite the incidence of gastric cancer is
declining, gastric cancer imposes a significant health burden
around the world. GC is often diagnosed in advanced stages and
carries a poor prognosis (17). At
present, gastric resection combined with chemotherapy and/or
radiotherapy is the rational treatment. But early diagnosis and
treatment are keys for better clinical outcome in with GC patients
(18). Owing to lack of effective
biomarkers for early gastric cancer, the outcome of gastric cancer
patients remains dismal (19).
Discovery of new biomarkers can help us find early and accurate
prediction of tumor behavior, and the survival of GC patients can
be improved. By taking advantage of serum protein antigens,
oncogenic genes or gene families through improving molecular
biological technologies, modern biomedical research has explored
many potential gastric cancer biomarker genes (19,20).
FTO is a new gene as a biological marker.
FTO is mapped on chromosome 16p12.2 and is widely
expressed in many tissues of human body, with highest levels are
detected in the brain, pancreatic islets, and the digestive organs
(12). It is proved that FTO gene
is link to obesity, dysmorphic facies and other diseases (21). The function of the FTO gene product
and the biological pathways involved are not completely known.
Furthermore, it has been shown that FTO localizes into the nucleus
and functional studies with bioinformatics analysis revealed that
the FTO gene encodes a 2-oxoglutarate-dependent nucleic acid
demethylase which may have a potential role in regulating the
transcription of genes related to in metabolism by nucleic acid
demethylation of DNA (22). FTO
gene is a nuclear protein of the AlkB related non-heme iron,
however, the precise physiological function of the gene is unclear.
What we do know is that other non-heme iron enzymes function to
reverse alkylated DNA and RNA damage by oxidative demethylation
(23). It has been suggested that
increased expression of FTO can promote the occurrence and
development of various types of cancer. Such as breast, thyroid,
endometrial cancer (14–16), and experiments suggest FTO
expression positively correlates with these cancers. Thus, we can
speculate that FTO acts as a tumor promoting gene. There are no
studies showing the expression profile of FTO in gastric cancer.
The above experiments were designed to predicate the role of FTO in
GC.
In the present study, the FTO protein expression was
detected by western blot analysis. We found that FTO protein was
upregulated in the GC tissues compared with the carcinoma adjacent
tissues. Moreover, we use quantitative PCR to analyze the mRNA
level of FTO. PCR results showed that the mRNA levels of FTO in the
GC tissues were obviously higher compared with the corresponding
carcinoma adjacent tissues. To further confirm the results of
western blot analysis and qPCR, we used immunohistochemical
staining to examine the FTO expression in GC tissues and to
estimate whether the FTO expression level has links with
clinicopathological parameters and the prognosis of GC patients. We
also found that FTO expression in GC tissues was significantly
higher than the adjacent non-cancerous tissues. In addition, FTO
expression was positively correlated with histological
differentiation, lymph node metastasis and TNM stage in GC.
Therefore, as well as in breast, thyroid and endometrial cancer,
FTO overexpression may promote the occurrence of GC and the
abnormal expression of FTO might be associated with GC tumor
progression and metastasis. Moreover, in vitro, the
viability, proliferation and migration and invasion of AGS cells
were markedly promoted by overexpression of FTO, however, knockdown
of FTO expression inhibited the viability, proliferation and
migration and invasion of MKN45 cells. It is generally known that
H. Pylori infection has been considered as a risk factor for
the occurrence of GC, 50% of the worlds population is infected with
H. Pylori (24).
Nevertheless, in the present study, no significant difference in
FTO expression was observed in patients with or without H.
pylori infection.
According to the Kaplan-Meier analysis, the overall
5-year survival of GC patients and the level of FTO expression were
negatively correlated. High FTO expression had a significant
relationship with shorter survival time of GC patients. Based on
the above experimental results, we can conjecture that FTO may play
more vital role in advanced cancer of the stomach and the FTO
protein may have a direct effect on the invasion of GC.
Multivariate analysis indicated that in overall survival of GC
patients, FTO expression was an independent prognostic indicator,
and high expression of FTO gene may indicate poor prognosis.
Recently, FTO has been shown to contribute to the development of
several cancer types (14–16). Although in these reported studies,
the exact functions and the mechanistic actions of FTO have not
been defined completely so far.
In conclusion, our data indicate that the
overexpression of FTO is involved in cancer progression and
dedifferentiation in gastric carcinoma patients and the detection
of increased FTO expression might help confirm GC patients with a
poor prognosis. However, the exact mechanism of FTO gene in gastric
cancer and other tumors is not completely understood. Therefore,
further large studies are needed to figure out the relationship of
FTO expression with GC and to investigate the mechanisms underlying
the relationship.
Acknowledgements
The present study was funded by a grant from the
Nantong Science and Technology Project (MS22016033).
Glossary
Abbreviations
Abbreviations:
|
FTO
|
fat mass and obesity associated
|
|
GC
|
gastric carcinoma
|
|
TNM
|
tumor node metastasis
|
References
|
1
|
Mcguire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackson CB and Giraud AS: STAT3 as a
prognostic marker in human gastric cancer. J Gastroenterol Hepatol.
24:505–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tahara T, Shibata T, Nakamura M, Yamashita
H, Yoshioka D, Okubo M, Yonemura J, Maeda Y, Maruyama N, Kamano T,
et al: Association between IL-17A, −17F and MIF polymorphisms
predispose to CpG island hyper-methylation in gastric cancer. Int J
Mol Med. 25:36–54. 2010. View Article : Google Scholar
|
|
5
|
Compare D, Rocco A and Nardone G: Risk
factors in gastric cancer. Eur Rev Med Pharmacol Sci. 14:302–308.
2010.PubMed/NCBI
|
|
6
|
González CA and Agudo A: Carcinogenesis,
prevention and early detection of gastric cancer: Where we are and
where we should go. Int J Cancer. 130:745–753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Resende C, Ristimäki A and Machado JC:
Genetic and epigenetic alteration in gastric carcinogenesis.
Helicobacter. 15 Suppl 1:34–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurowski MA, Bhagwat AS, Papaj G and
Bujnicki JM: Phylogenomic identification of five new human homologs
of the DNA repair enzyme AlkB. BMC Genomics. 4:482003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peters T, Ausmeier K, Dildrop R and Rüther
U: The mouse Fused toes (Ft) mutation is the result of a 1.6-Mb
deletion including the entire Iroquois B gene cluster. Mamm Genome.
13:186–188. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerken T, Girard CA, Tung YC, Webby CJ,
Saudek V, Hewitson KS, Yeo GSH, McDonough MA, Cunliffe S, McNeill
LA, et al: The obesity-associated FTO gene encodes a
2-oxoglutarate-dependent nucleic acid demethylase. Science.
318:1469–1472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jia G, Yang CG, Yang S, Jian X, Yi C, Zhou
Z and He C: Oxidative demethylation of 3-methylthymine and
3-methyluracil in single-stranded DNA and RNA by mouse and human
FTO. FEBS Lett. 582:3313–3319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Frayling TM, Timpson NJ, Weedon MN,
Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H,
Rayner NW, et al: A common variant in the FTO gene is associated
with body mass index and predisposes to childhood and adult
obesity. Science. 316:889–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hernández-Caballero ME and Sierra-Ramírez
JA: Single nucleotide polymorphisms of the FTO gene and cancer
risk: An overview. Mol Biol Rep. 42:1–6. 2014.
|
|
14
|
Tan A, Dang Y, Chen G and Mo Z:
Overexpression of the fat mass and obesity associated gene (FTO) in
breast cancer and its clinical implications. Int J Clin Exp Pathol.
8:13405–13410. 2015.PubMed/NCBI
|
|
15
|
Sigurdson AJ, Brenner AV, Roach JA,
Goudeva L, Müller JA, Nerlich K, Reiners C, Schwab R, Pfeiffer L,
Waldenberger M, et al: Selected single-nucleotide polymorphisms in
FOXE1, SERPINA5, FTO, EVPL, TICAM1 and SCARB1 are associated with
papillary and follicular thyroid cancer risk: Replication study in
a German population. Carcinogenesis. 37:677–684. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu Y, Shen J, Gao L and Feng Y: Estrogen
promotes fat mass and obesity-associated protein nuclear
localization and enhances endometrial cancer cell proliferation via
the mTOR signaling pathway. Oncol Rep. 42:381–385. 2016.
|
|
17
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med. 44:192–201. 2013.PubMed/NCBI
|
|
19
|
Wu HH, Lin WC and Tsai KW: Advances in
molecular biomarkers for gastric cancer: miRNAs as emerging novel
cancer markers. Expert Rev Mol Med. 16:53–62. 2014. View Article : Google Scholar
|
|
20
|
Fan B, Zhang LH, Jia YN, Zhong XY, Liu YQ,
Cheng XJ, Wang XH, Xing XF, Hu Y, Li YA, et al: Presence of
S100A9-positive inflammatory cells in cancer tissues correlates
with an early stage cancer and a better prognosis in patients with
gastric cancer. BMC Cancer. 12:3162011. View Article : Google Scholar
|
|
21
|
Tschritter O, Preissl H, Yokoyama Y,
Machicao F, Häring HU and Fritsche A: Variation in the FTO gene
locus is associated with cerebrocortical insulin resistance in
humans. Diabetologia. 50:2602–2603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melnik BC: Milk: An epigenetic amplifier
of FTO-mediated transcription? Implications for Western diseases. J
Transl Med. 13:1–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raskandersen M, Almén MS and Schiöth HB:
Scrutinizing the FTO locus: Compelling evidence for a complex,
long-range regulatory context. Hum Genet. 134:1–11. 2016.
|
|
24
|
Brown LM: Helicobacter pylori:
Epidemiology and routes of transmission. Epidemiol Rev. 22:283–297.
2000. View Article : Google Scholar : PubMed/NCBI
|