Introduction
According to 2012 Global Cancer Statistics,
colorectal cancer has become the second leading cause of
cancer-related deaths in developed countries (1). Colorectal cancer also poses a
significant threat in developing countries. In China, colorectal
cancer was the fifth leading cause of cancer-related deaths in 2015
(2). Compared with other forms of
cancer, colorectal oncogenesis is highly correlated with diet
(3). Various experts believe that
phytate could be effective in preventing colon oncogenesis
(4–7).
Phytate, myto-inositol 1,2,3,4,5,6 hexaphosphate
(IP6), is ubiquitously distributed worldwide and exists in many
types of plant-derived foods (8–10).
When IP6 is ingested, it partially degraded by phytase into
hydrolysates. Therefore, it is likely that the epithelial cells of
the colon are exposed to the mixture of IP6 hydrolysates, but not
IP6. Ishizuka et al revealed that the partially degraded IP6
products were responsible for the suppression of colon oncogenesis.
It had been demonstrated that IP6 and IP6 hydrolysates were able to
suppress HCT116 colon carcinoma cells (11). Based on these findings, we attempted
to characterize the underlying antitumour mechanisms of IP6
hydrolysates.
According to our previous study, IP6 exerted
inhibitory effects on HT-29 cells via the phosphatidylinositol
3-kinase (PI3K)/protein kinase B (AKT) signalling axis (10). The PI3K/AKT signalling axis is a
major survival pathway. Abnormal activation of the PI3K/AKT pathway
is frequently involved in the development and progression of
various tumours, including colon cancer (12). In this pathway, AKT is a
serine/threonine kinase and plays an essential role. It is
activated by the 3′-phosphorylated phosphoinositides
3,4,5-trisphosphate (PIP3) protein and affects the activity of
downstream factors, including mTOR, BAD and GSK3β (13–15).
AKT contains the pleckstrin homology (PH) domain that has a high
affinity for PIP3. In addition, IP6 hydrolysates also possess a
similar PH domain as the PIP3 protein. Thus, based on the
presumption of a similar structure and evidence from our previous
study, we hypothesized that IP6 hydrolysates suppressed the
proliferation of colon carcinoma cells through the PI3K/AKT
pathway.
Materials and methods
Reagents
Inositol hexaphosphate (IP6) was purchased from
Muster Biological Science Technology Company (Sichuan, China).
Ascorbic acid, ammonium molybdate, antimony potassium tartrate,
monopotassium phosphate, potassium peroxydisulfate and sulfuric
acid were supplied by Sinopharm Chemical Reagent Co., Ltd.
(Shanghai, China). Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). RT-PCR was
performed by a Two-Step RT-PCR QuantiScript RT kit (KR103) and a
Real-Master/SYBR-Green kit (FR202) which were both purchased from
the Tiangen Biotech (Beijing) Co., Ltd. (Beijing, China). The
primers were designed using Primer Premier 5.0 (PREMIER Biosoft,
Inc., Palo Alto, CA, USA) and Oligo 6.0 (Molecular Biology
Insights, Inc., Colorado Springs, CO, USA) software and were
synthesized by Shanghai Biological Engineering Company (Shanghai,
China). The cell-based ELISA kits were supplied by ImmunoWay
Biotechnology Company (Plano, TX, USA).
Hydrolysis curve
The hydrolysis curve assays were performed using the
total phosphorus ammonium molybdate spectrophotometric method.
Different concentrations of IP6 (0.25, 1, 4, 16, 64, 250, 1,000,
4,000, 16,000 and 64,000 µg/ml) were suspended in water, and
potassium peroxydisulfate (50 g/l) was added for digestion. After
digestion, molybdate and ascorbic acid were added to the solution
and allowed to develop for 15 min. The absorbance of the solution
at 690 nm was determined using a microplate reader. Each experiment
was repeated three times. The optical density (OD) was recorded to
draw the hydrolysis curve.
Determination of the IP6 hydrolysis
rate
Forty milligrams IP6 (final concentration, 200
µg/ml) and 6 µg of phytase were suspended in 200 ml of 50 mM sodium
acetate (pH 5.5) and incubated at 37°C for 1–8 h. Two types of
phytase (EcAppA from Escherichia coli and phytase from
wheat) were assessed in the present study. The pretreated
hydrolysis solutions were then evaluated using the total phosphorus
ammonium molybdate spectrophotometric method aformentioned without
digestion. The degree of hydrolysis (DH) of IP6 was calculated as
follows:
DH=C1/C2C1–––––––Hydrolysis phosphorus,
m/lC2–––––––Total phosphorus, m/l
IP6 and its hydrolysates ranging from 10 to 90% DH
were concentrated via vacuum freeze dehydration and stored in a
cold dark place.
Cell culture
SW620, HCT116 and HT29 cells obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China) were
grown in RPMI-1640 medium supplemented with 10% fetal bovine serum
(FBS). The cells were cultured in the absence of antibiotics. The
cells were grown at 37°C in a 5% (v/v) CO2 atmosphere in
a humidified incubator. Decreased serum media [25 ml/l
phosphate-buffered saline (PBS)] was used for all experiments.
Cell proliferation assay
The cell proliferation assay was performed in order
to investigate the time- and concentration-dependent effects of IP6
on the growth of SW620, HCT116 and HT29 cells. Logarithmic phase
cells (1×104 cells/well) were seeded onto 96-well tissue
culture plates in 5% CO2 at 37°C. After 12 h, the medium
was replaced with fresh medium containing 0 (control group), 1, 10
or 100 µg/ml of IP6 hydrolysates, and the cells were incubated in
CO2 at 37°C for 48 h. The CCK-8 reagent was added to the
cells, and the plates were incubated at 37°C for 2 h. After
incubation, the absorbance was assessed at 490 nm using a
microplate reader. A decrease in absorbance was considered to
reflect a loss of cell viability. The cell viability rate was
calculated by comparison with the control group. Each experiment
was repeated three times.
Cell migration assays
To quantify the migratory potential of the
hydrolysates-treated SW620 cells; the cells were plated in culture,
placed on a 24-well plate at high density and grown with complete
culture medium until confluence. After carefully removing the
inserts, two cell monolayers separated by a cell-free gap of ~500
µm were created. The cells were washed with PBS and incubated with
the corresponding treatments: 10 µg/ml IP6, 20% DH hydrolysates,
30% DH hydrolysates and 90% DH hydrolysates in triplicate for 48 h.
After the treatment time, each well was captured with a digital
camera coupled to an inverted microscope. The distance of the gap
in each group was assessed using Image-Pro Plus (Media Cybernetics,
Inc., Rockville, MD, USA).
Real-time PCR
Using the TRIzol reagent kit, total RNA was
extracted from the SW620 cells treated with 1, 10 and 100 µg/ml of
IP6. The mRNA levels were determined using an Eppendorf protein
nucleic acid detector. Total RNA (2 µg) was reverse-transcribed
using the PrimeScript RT reagent kit, followed by reverse
transcription using the Bio-Rad MyCycler PCR system. The sense and
antisense primer sequences and the PCR product sizes are shown in
Table I. The PCR was performed in a
20 µl total reaction volume system. The cycling conditions were as
follows: for PI3K, 50 cycles at 95°C for 2 min, 95°C for 15 sec and
60°C for 20 sec. For AKT, 50 cycles at 95°C for 2 min, 95°C for 15
sec and 60°C for 20 sec. For BAD, 50 cycles of 95°C for 2 min, 95°C
for 15 sec and 60°C for 20 sec. The comparative Ct formula
2−ΔΔCt was used to calculate the relative gene
expression levels.
 | Table I.Real-time-PCR primer sequences and
product sizes. |
Table I.
Real-time-PCR primer sequences and
product sizes.
| Gene | Primer sequence | Product size
(bp) |
|---|
| β-actin | F
5′-CCTGGCACCCAGCACAAT-3′ | 144 |
|
| R
5′-GGGCCGGACTCGTCATAC-3′ |
|
| PI3K | F
5′-CTTTTCCCCACAAATCCTCA-3′ | 117 |
|
| R
5′-CAGTTGCCCCTATCCCCTAT-3′ |
|
| AKT | F
5′-AGCGGAAGGAGGTGAAGAAT-3′ | 126 |
|
| R
5′-GGGAAAACGGAGACTTAGGG-3′ |
|
| BAD | F
5′-GGGTTCTGAGGGGAGACTGA-3′ | 211 |
|
| R
5′-CTCTGGGCTGTGAGGACAAG-3′ |
|
Cell-based ELISA
The cultured SW620 cells were seeded onto 96-well
plates at 5×104 cells/cm2. When appropriate,
the cells were serum-starved for 4 h and stimulated with IP6
hydrolysates for 10 min. After stimulation, the cells were fixed
with 4% formaldehyde in PBS for 20 min at room temperature and
washed three times with washing buffer. The cells were then
incubated in quench buffer for 20 min, washed three times in
washing buffer, blocked with 10% fetal calf serum buffer for 1 h
and incubated overnight with various dilutions of a primary
antibody at 4°C. The following day, the cells were washed three
times with washing buffer for 5 min and incubated with a secondary
antibody (peroxidase-conjugated goat anti-rabbit antibody; dilution
1:100) in PBS and Triton with 5% BSA for 1 h at room temperature
and then washed three times with washing buffer for 5 min and twice
with PBS. Subsequently, the cells were incubated with 50 µl of
substrate development solution for 15 min at room temperature in
the dark. The reaction was stopped with 50 µl of stop solution. The
absorbance was assessed, and the SD values were determined using a
microplate reader.
AKT-inhibited assay
Similar to the cell proliferation assay, logarithmic
phase cells (1×104 cells/well) were seeded onto 96-well
tissue culture plates in 5% CO2 at 37°C. After 12 h, the
medium was replaced with fresh medium containing 0.1 µmol/l of
MK2206 (IC50 concentration in SW620 cells) and 10 µg/ml
of IP6, and the cells were incubated in CO2 at 37°C for
48 h. The CCK-8 reagent was added to the cells, and the plates were
incubated at 37°C for 2 h. After incubation, the absorbance at 490
nm was determined using a microplate reader. A decrease in the
absorbance was considered to reflect a loss of cell viability. Each
experiment was repeated three times.
Molecular docking simulation
study
A docking study was performed to examine the
qualified binding positions of IP6 hydrolysates against AKT. The
crystallographic structure of AKT with its ligand was obtained from
the RCSB Protein Data Bank (PDB ID, 4EKL). The main protein
structure was obtained by removing the 4EKL ligand
(2S)-2-(4-chlorophenyl)-1-{4-[(5R,7R)-7-hydroxy-5-methyl-6,7-dihydro-5H-cyclopenta[d)pyrimidin-4-yl)piperazin-1-yl}-3-(propan-2-ylamino)propan-1-one
and the water molecules using AutoDock 4.2. After removing the
heteroatoms and adding the hydrogen atoms, the protein was suitable
for docking simulation with respect to obtaining the best ligand
binding results. Thirteen two-dimensional structures of the IP6
hydrolysate constituents were drawn using ChemDraw 8.0. All
two-dimensional IP6 hydrolysates were transformed into
three-dimensional structures using Avogadro 1.0.3 and converted
into PDB files with Open Babel 2.3.2. AutoDock 4.2, an open-source
program, was used for the docking simulation. Grid boxes 126 × 126
× 126 points in size with spacing of 0.375 A° between the points
were generated to cover almost the entire favourable protein
binding site. The X, Y and Z centres were 22.15, 2.53 and 15.74,
respectively. The binding aspects of the AKT residues and their
corresponding binding affinity scores were regarded as the best
molecular interactions. The results were analysed using UCSF
Chimera and LigPlot (v.1.4.5). The two-dimensional images of the
IP6 hydrolysate-AKT interactions were calculated using LigPlot
v.1.4.5 (European Bioinformatics Institute, London, England). All
docking simulations were performed using an Intel Core TM i5-2520 M
CPU @ 2.50 GHz with Windows 8.1 and a 64-bit operating system.
Statistical analysis
All reactions were performed for three times and
each independent experiment was carried out in duplicate or
triplicate according to the manufacturer's instructions. A
completely randomized design (CRD) was used for the statistical
analysis of the physical and chemical data. All data were subjected
to analysis of variance (ANOVA), and mean comparisons were carried
out using Duncan's multiple range test. Statistically analysed
using SPSS version 19.0 (SPSS for windows; SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered statistically significant.
Results
Comparison of the hydrolysis
efficiencies of different phytases
To determine the proper IP6 hydrolysis condition, a
series of IP6 concentrations ranging from 0.25 µg/ml to 64 mg/ml
were tested in the present study. All the free phosphoric acid was
released after IP6 was fully hydrolyzed by potassium
peroxydisulfate. The free phosphoric acid was detected with a
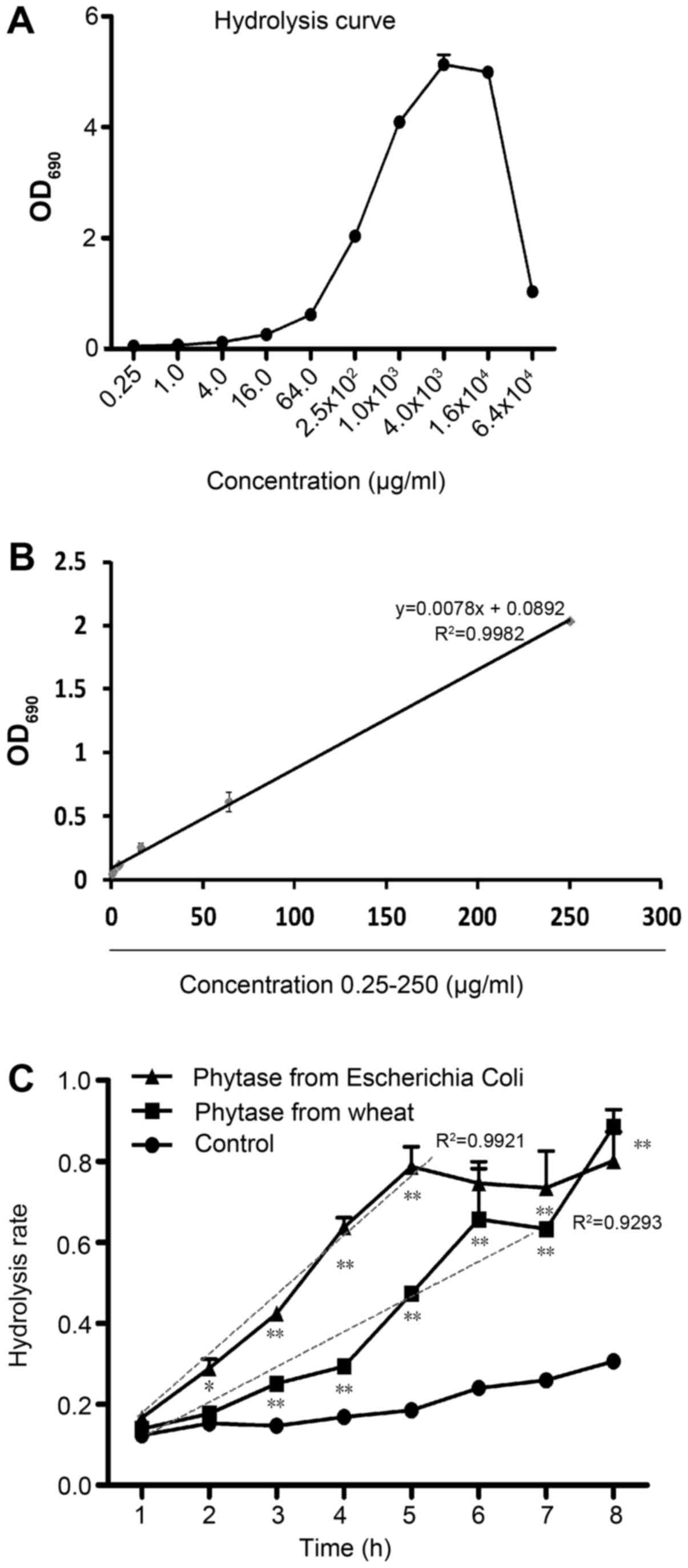
microplate reader. According to Fig.
1A, when the IP6 concentration was increased to 16 mg/ml, the
total reaction system reached its limit and the OD 690 value
markedly decreased. According to Fig.
1B, the free phosphoric acid concentration released from IP6 at
concentrations ranging from 0.25 to 250 µg/ml exhibited suitable
reaction efficiencies (y=0.0078× + 0.0892, R2=0.9980).
To analyze the effects of different phytases on IP6 hydrolysis, we
assessed the two phytases, one extracted from wheat and one from
Escherichia coli. Free phosphorus of IP6 was determined per
hour for 8 h. Compared with 37°C control group, the two phytases
showed significantly enhanced hydrolysis effects. However, the
hydrolysis efficiency of the wheat phytase was low and unstable
(y=0.0805× + 0.0026, R2=0.9293), shown in Fig. 1C. In contrast, the hydrolysis
efficiency of EcAppA was high and stable (y=0.1507x-0.0027,
R2=0.9921). These results indicated that the EcAppA
phytase was more suitable for preparing IP6 hydrolysates. Using the
hydrolysis curve and the proper phytase, we were able to assess the
dynamic hydrolysis condition and calculate the IP6 hydrolysis rate.
We concentrated 10–90% DH IP6 hydrolysates via vacuum freeze
dehydration.
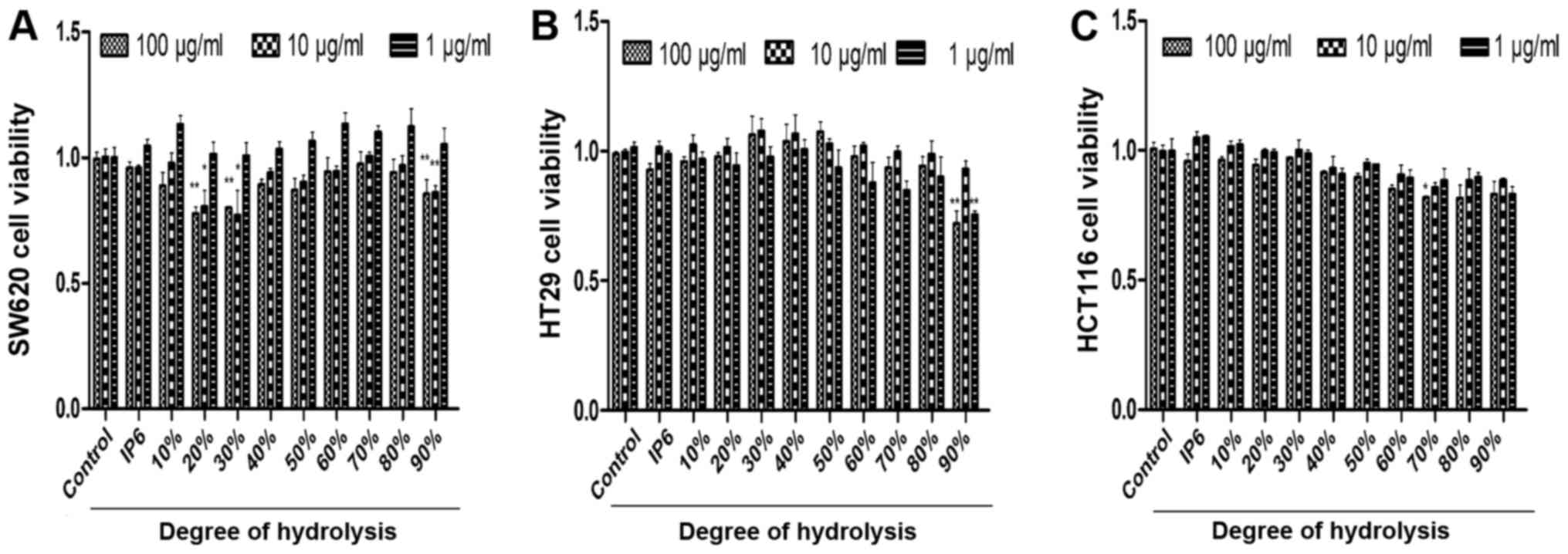
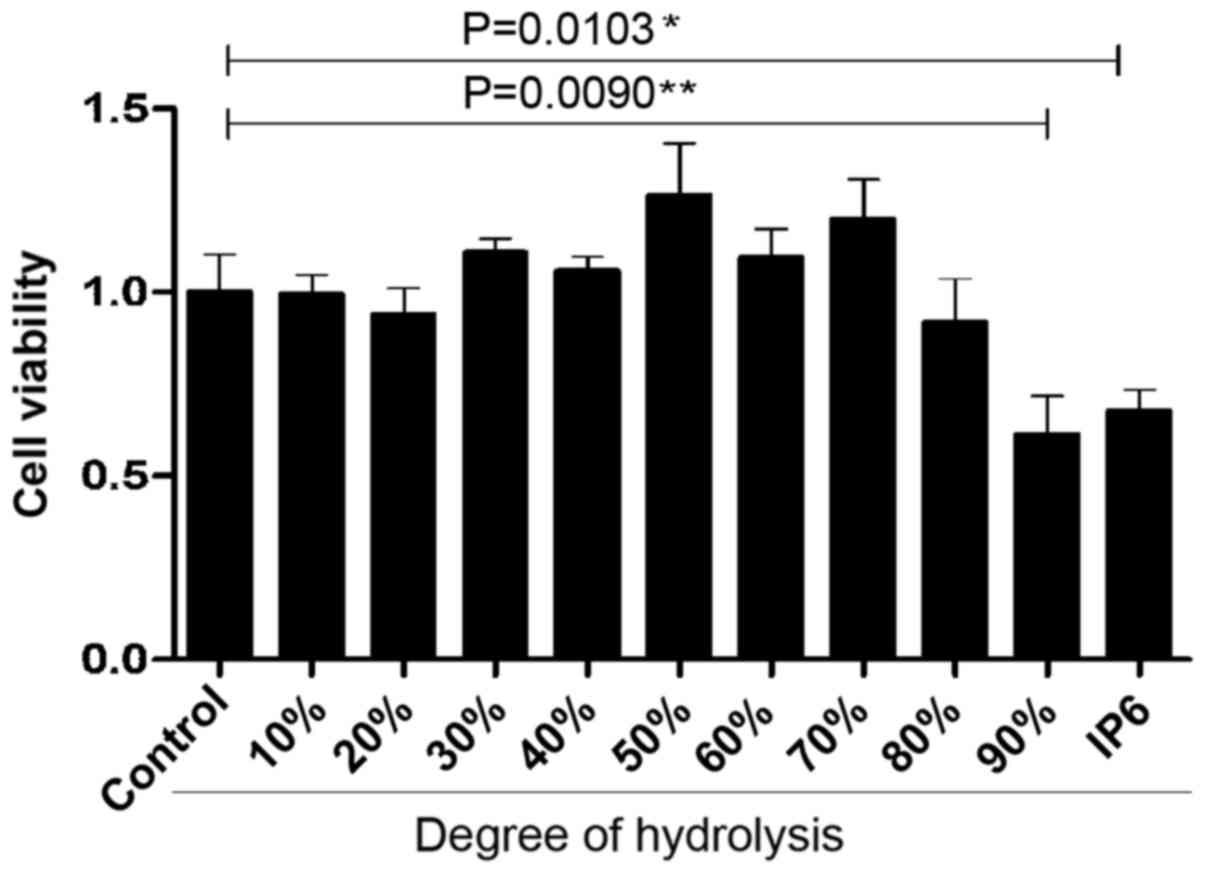
IP6 hydrolysates inhibits of SW620,
HCT116 and HT29 cell growth
After preparing the different DH hydrolysates, we
used the cell proliferation assay to identify the inhibitory
effects of three main colorectal cancer cell lines with various
concentrations. We were surprised to find that the SW620 cells were
very sensitive to 20, 30 and 90% DH hydrolysates. DH hydrolysates
(20, 30 and 90%) in 100 and 10 µg/ml concentration groups exhibited
better tumour-suppressor activity compared with the 1 µg/ml
concentration group (Fig. 2A). HT29
cells were not sensitive to the majority of the hydrolysates of
each concentration tested. Differences were observed in two groups
which were 90% DH hydrolysates in the 1 and 100 µg/ml concentration
groups (Fig. 2B). HCT116 cells were
not sensitive to the majority of the hydrolysates either. The 70%
DH hydrolysates (100 µg/ml) was the only group which suppressed
cell proliferation (Fig. 2C). The
results demonstrated that the inhibitory effects of DH hydrolysates
of IP6 were effective in SW620 cells.
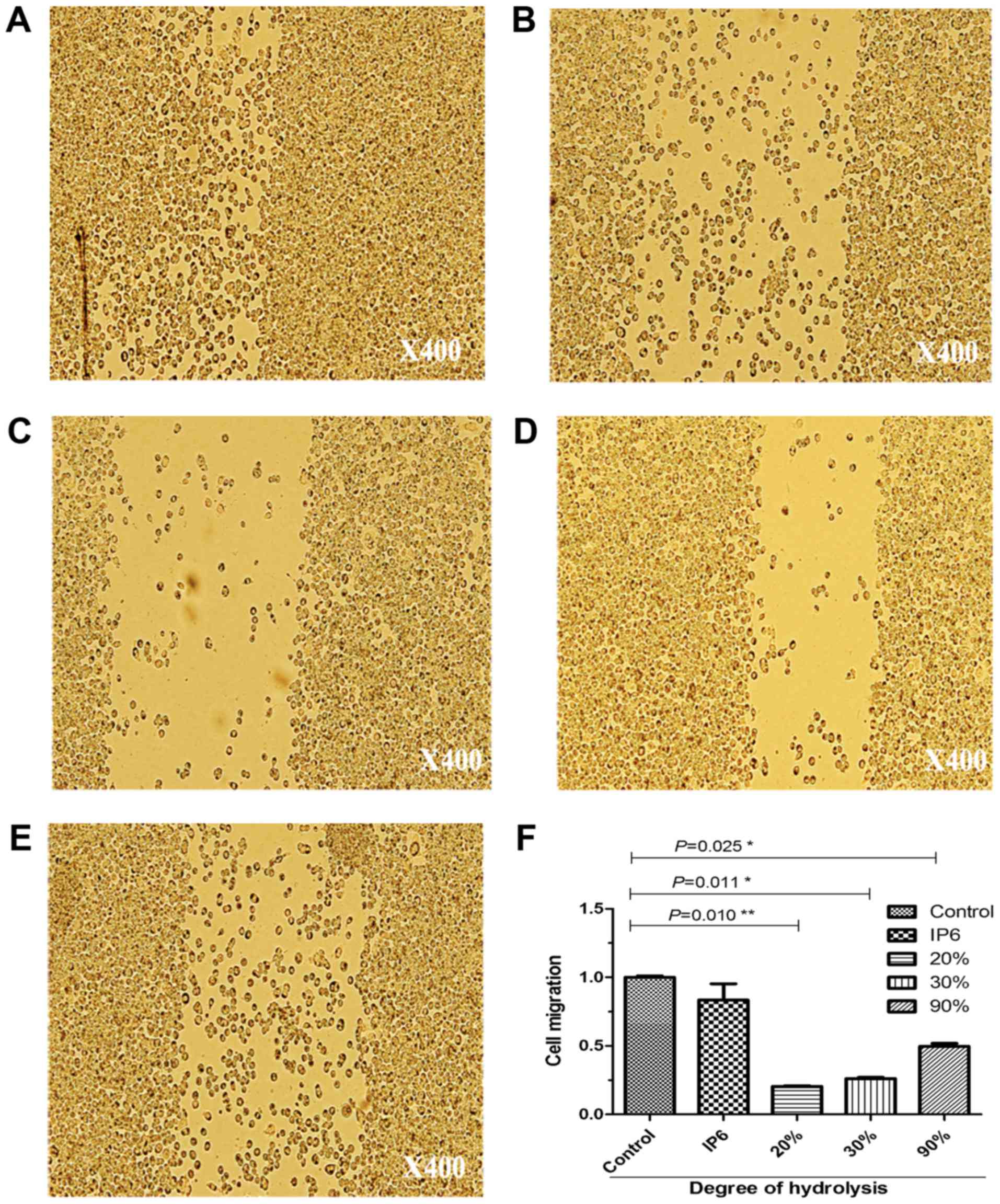
IP6 and its hydrolysates suppresses
the migration of SW620 cells
To ascertain the results of the cell proliferation
assay; we used a migration assay on SW620 cells. After being loaded
with 10 µg/ml of IP6, 20% DH, 30% DH and 90% DH IP6 hydrolysates,
the motility of the cells was markedly altered (Fig. 3). It should be noted that SW620 cell
proliferation and motility was inhibited after treatment with 20,
30 and 90% DH IP6 hydrolysates.
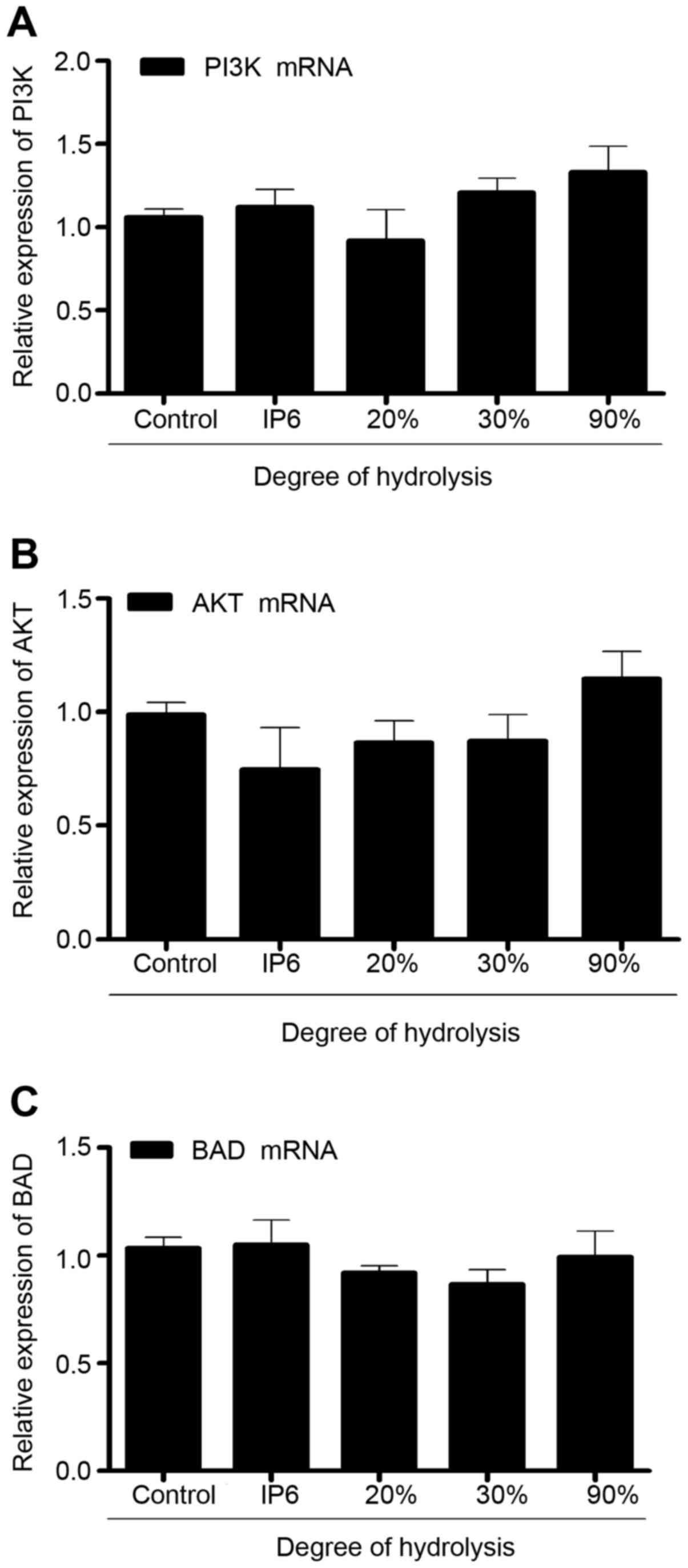
IP6 hydrolysates do not alter the mRNA
expression of PI3K, Akt, and BAD in the SW620 cells
To explore the effects of IP6 hydrolysates on SW620
cells, we measured the expression of PI3K, AKT and BAD mRNA using
real-time PCR. Compared with the control group, the expression of
PI3K, AKT and BAD mRNA in the SW620 cells did not exhibit any
differences. Results (Fig. 4)
indicated that IP6 hydrolysates did not affect the expression of
PI3K, AKT and BAD mRNA.
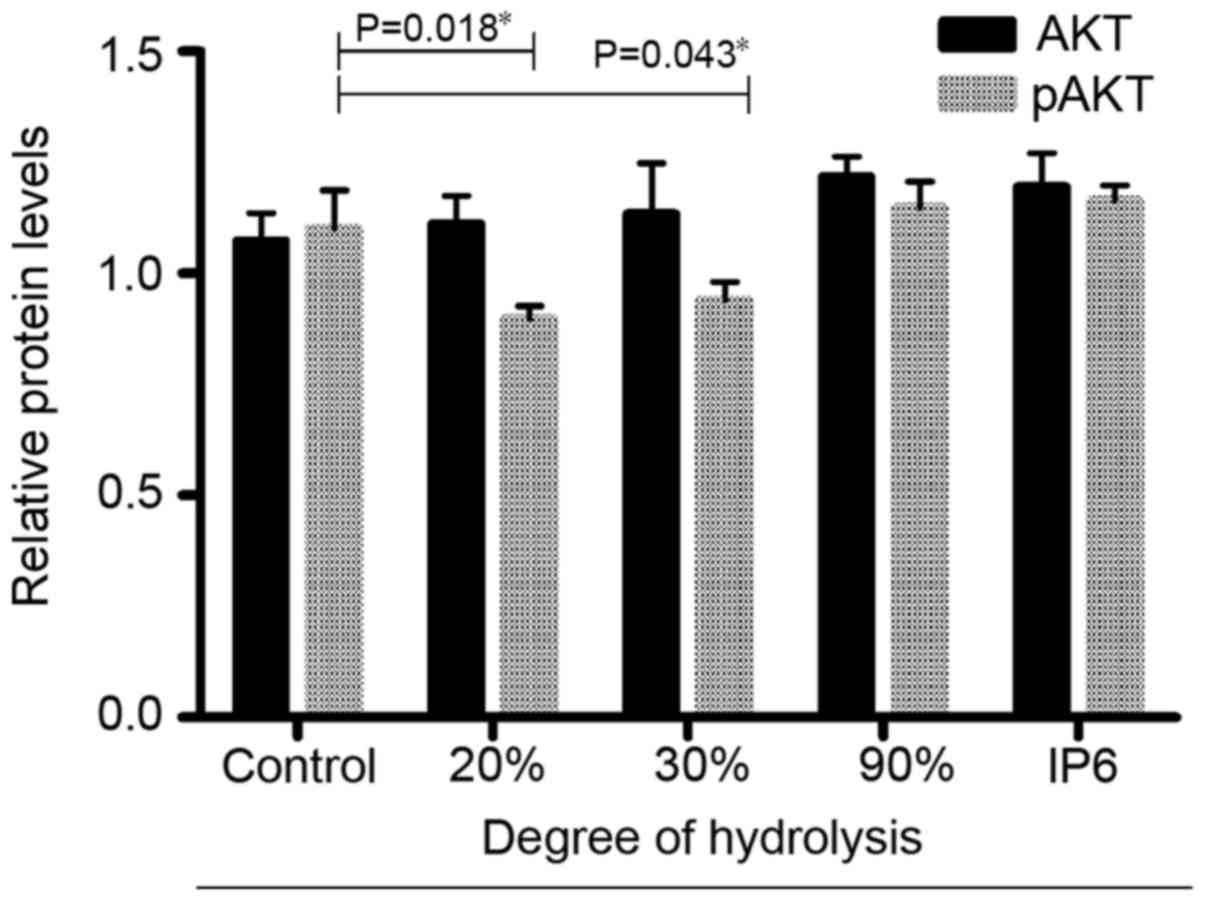
IP6 hydrolysates affect the expression
of Akt and pAkt in SW620 cells
Next, using cell-based ELISA, we investigated
whether the IP6 hydrolysate treatments affected the expression of
proteins related to the PI3K/AKT pathway. The results (Fig. 5) indicated that after hydrolysate
treatment (20, 30 and 90%), the protein expression of total Akt did
not change. In contrast, the protein expression of pAKT changed
significantly in the 20 and 30% DH groups. Based on these results,
IP6 hydrolysate treatment inhibited the expression of pAKT in the
SW620 cells.
IP6 hydrolysates inhibit SW620 cell
proliferation when the AKT protein is inhibited
According to our results, certain IP6 hydrolysates
inhibited the proliferation of SW620 cells with MK2206. As shown in
Fig. 6, after treating SW620 cells
with MK2206, 10, 20, 30, 40, 50, 60 and 70% DH hydrolysates did not
inhibit cell proliferation. SW620 cells were treated with 80 and
90% DH hydrolysates and IP6 plus MK2206 exhibited a slight decrease
in the growth rate of SW620 cells.
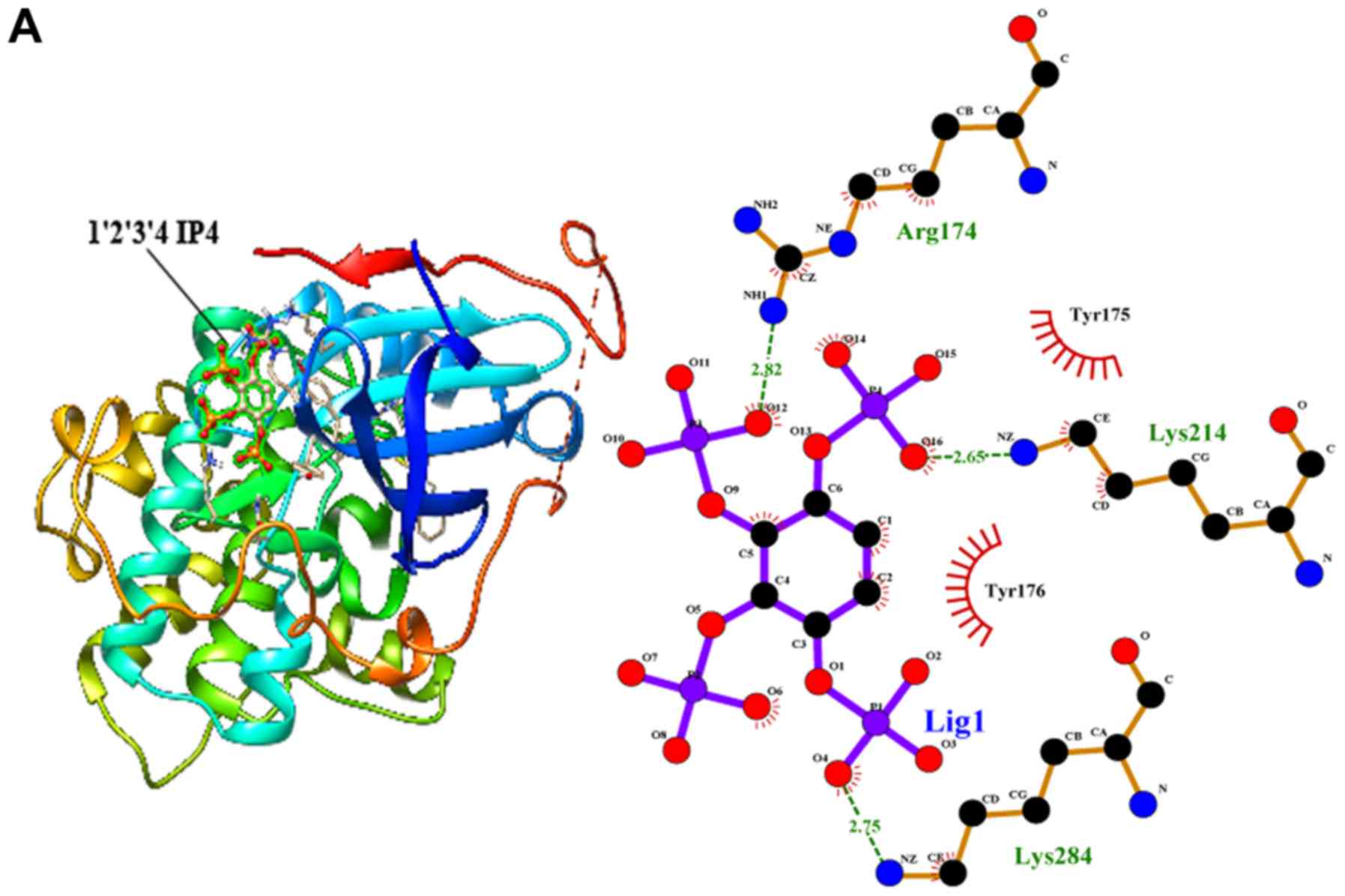
IP4 and IP5 bind to the AKT
protein
Next, we employed molecular docking studies to
obtain the predictions of the protein-ligand interaction geometries
of the IP6 hydrolysates and the AKT protein. Thirteen IP6
hydrolysates were used as control ligands for validation in
AutoDock 4.2. The docking scores of the IP6 hydrolysates with the
interacting residues, the number of hydrogen bonds formed between
the interacting residues and the residues exhibiting van der Waals
interacting force are shown in Table
II. The activity of the thirteen IP6 hydrolysates against AKT
was correlated with the binding energy, and the number of hydrogen
bonds formed at the active site. The thirteen hydrolysates were
arranged to dock with the AKT protein; however, only IP5 and 3 of
the IP4 isomers could bind to the AKT protein (Fig. 7). Inositol and the IP1, IP2 and IP3
isomers could not bind to the AKT protein.
 | Table II.Molecular interactions between the
IP4 isomers, IP5 and the AKT protein activator PIP3. |
Table II.
Molecular interactions between the
IP4 isomers, IP5 and the AKT protein activator PIP3.
| Compounds | Binding energy | No. of H-bonds | H-bond interacting
residues | Van der Waals
interacting residues |
|---|
| 1,2,3,4 IP4 | 6.85 | 4 | Lys284:HZ1
Lys284:HZ2 | Try175 Try176 |
|
|
|
| Lys214:HZ2
Arg174:HH11 |
|
| 1,2,3,5 IP5 | 6.56 | 5 | Lys284:HZ1
Lys284:HZ2 | Try175 Try176 |
|
|
|
| Tyr176:OH
Arg174:HH11 |
|
|
|
|
| Lys214:HZ2 |
|
| 1,2,4,5 IP6 | 6.02 | 3 | Tyr175:Hz1
Lys214:HZ2 | Ala212 Try176
Glu228 |
|
|
|
| Tyr175:HN | Leu213 Arg174 |
| IP5 | 5.88 | 4 | Lys214:HZ2
Lys214:HZ3 | Ala212 Ser478
Ala476 |
|
|
|
| Arg174:HH11
Lys284:HZ1 |
|
| PIP3 | 10 | 1 | Lys214:HZ2 | Arg174 Ser478
Ala476 Gln203 |
|
|
|
|
| Gln471 Asn204 Arg
200 |
Discussion
In the present study, IP6 hydrolysates exerted
inhibitory effects on SW620 cells, and certain hydrolysates
inhibited AKT activation as AKT protein inhibitors. We first
examined the effects of 10–90% DH hydrolysates on three colorectal
tumour cells (HT29, HCT116 and SW620) by cell proliferation assays.
DH hydrolysates (20, 30 and 90%) significantly inhibited the
proliferation of SW620 cells. The data from the wound healing assay
confirmed the results of the cell proliferation assay in SW620
cells. Thus, we cautiously speculated that metastatic SW620 tumour
cells may be susceptible to IP6 hydrolysates.
Next, as aforementioned, IP6 hydrolysates possess an
analogous domain as PIP3. Thus, we wondered whether IP6
hydrolysates could affect the activation of the AKT protein. A
real-time PCR assay was utilized to clarify whether IP6
hydrolysates affected the mRNA expression of AKT. After treatment
with IP6 hydrolysates, AKT and its upstream factor, PI3K, and
downstream factors, BAD, did not exhibit any differences with the
control group in SW620 cells. Since IP6 hydrolysates could not
affect the expression of AKT mRNA, we investigated the AKT protein
expression using cell-based ELISA. Cell-based ELISA is a new
protein detection technique which permits direct detection of cell
proteins without extraction. This technique has significant
advantages in phosphoprotein analysis. Compared with traditional
western blot assay, cell-based ELISA detects the expression of
phosphoproteins quickly and decreases the unnecessary
phosphoproteins lost from extraction and phosphatise degradation.
Thus, we chose cell-based ELISA for pAKT and total protein
detection. In Fig. 5, the total AKT
protein did not exhibit any difference in each group, but the 20
and 30% DH hydrolysates inhibited the activation of the AKT protein
by inhibiting the expression of the pAKT protein. The results
indicated that certain IP6 hydrolysates at 20 and 30% DH may have a
close relationship with the activation of AKT protein.
The result of cell-based ELISA was insufficient to
conclude that hydrolysates may induce changes in the AKT protein.
Hence, the classical AKT inhibitor MK2206 was utilized in an AKT
inhibition assay to study the relationship between hydrolysates and
the AKT protein. In the present study, 20 and 30% DH hydrolysates
did not suppress the proliferation of SW620 cells when the AKT
protein was inhibited. It was proposed that 20–30% DH hydrolysates
inhibited tumour proliferation which was closely related to the AKT
protein. In contrast, 90% DH hydrolysates continually inhibited
cell growth. This implied that 90% DH hydrolysates inhibited SW620
cell growth through a different mechanism.
Lastly, the molecular docking simulation study was
performed to predict the binding energies and the specific binding
site of hydrolysates on AKT and to identify the interacting
residues using AutoDock software. According to our data, IP5 and
the isomers of IP4 exhibited a similar binding pattern as PIP3.
IP1, IP2, IP3 and IP6 could not bind to the AKT protein. According
to a study from Yuyinng, when the DH reached 16.67 and 33.36%, the
main contents of the hydrolysates of IP6 were IP5 and IP4 (16). Hence we carefully deduced that the
main contents of the 20–30% DH IP6 hydrolysates were IP4 isomers
and IP5. The results of the docking simulation study confirmed that
IP4 isomers and IP5 could attach to the binding area of AKT and
inhibit the activation of the AKT protein. This finding explained
why 20 and 30% DH hydrolysates inhibited the activation of the AKT
protein and slowed the tumour growth rate. Collectively, with the
data of the PCR analysis, the AKT inhibited assay and the molecular
docking simulation study we confirmed our hypothesis that IP6
hydrolysates, IP5 and IP4, contain a similar structural domain with
PIP3, and may bind to the PIP3 receptor of AKT. These hydrolysates
occupied the receptor, but could not exhibit any biological
functions. Therefore, we concluded that 20 and 30% DH of IP6
hydrolysates inhibited the activation of the AKT protein to
suppress the proliferation of SW620 cells, and these hydrolysates
inhibited AKT protein activation mainly through competitive
inhibition to the PIP3 receptor.
Although our results revealed that IP6 hydrolysates
act as an AKT protein inhibitor, there were a few limitations.
Separation of the unique hydrolysate was the first issue. With the
help of our laboratory colleagues, over a period of two years, we
attempted to separate the unique hydrolysate and its isomers by
several different methods; we utilized classic chromatography,
colorimetric, HPLC, paper chromatography, thin layer
chromatography, NMR, dialysis membrane and other methods (17–24).
Despite our best efforts, we were unsuccessful. However, we are not
discouraged. We are going to deal with this problem in the future
and we appreciate the researchers who motivated us. Although we
were unable to separate the unique hydrolysate, the mixture of IP6
hydrolysates was still worth being investigated. When IP6 was
obtained from the ingestion of food, it was hydrolysed into a group
of hydrolysates by intestinal bacteria phytases. These hydrolysate
mixtures may just be the exposure substances for colon epithelial
cells. Therefore, studying the IP6 hydrolysate mixtures is
biologically relevant. DH hydrolysates (90%) was the second issue.
This is a very special mixture of the present study. These
hydrolysates in 90% DH inhibited the proliferation of SW620 cells,
but did not inhibit AKT activation (shown in Fig. 2). According to the results reported
by Dinicola et al, inositol efficiently slowed the rate of
differentiation and the dissemination of breast cancer cells
(25–30). According to IP6 hydrolysis
progression, the more free phosphates of IP6 hydrolysed, the less
binding phosphates remained on the inositol molecular skeleton.
According to the study of Fu et al, when the DH reached to
83 and 100%, the main contents of the hydrolysates of IP6 were IP1
and inositol. We speculate that the main content of the 90% DH
hydrolysates was inositol. DH hydrolysate (90%) worked as an
antitumour agent in a different manner compared with the 20 and 30%
DH hydrolysates which may be investigated in our future study.
The present study, was greatly motivated by previous
studies which investigated the relationship between IP6 and its
hydrolysates in colon cancer. However, there were some differences
that should be mentioned. According to Ishizuka et al, IP6
and its hydrolysates were able to suppress HCT116 colon cells. They
also indicated that partially degraded IP6 products inhibited cell
proliferation via different mechanisms from those of intact IP6
(11). Suzuki et al believed
that IP6 hydrolysates induced F-actin ring formation, the key
factor in the phytate-mediated anticancer function in HT29 cells
(23). Their results were similar
to our findings; we all contended that partly hydrolysed IP6
inhibited the colon cell growth. However, there were still some
differences that remained. Their studies suggested that IP6 and IP6
hydrolysates inhibited HT29 and HCT116 cells, but we did not find
any differences in these two cell lines. The differences may be
derived from the different intervention concentrations. The
concentrations in the study of Ishizuka et al and Suzuki and
Hara, were 2.1–4.1 g/l and 66–3,000 mg/l, separately (11,23).
According to the study of Ishizuka et al, the hydrolysates
could not affect the protein phosphorylation when the concentration
did not reach 1 mM (300–500 mg/l) in HT29 cells. This concentration
was higher than the one we used (1–100 mg/l) in the present study.
In vitro, results may markedly change when the
concentrations are different. The concentrations were 3–40 times
different when comparing the present study to theirs, which
explained why the differences in the HCT116 and HT29 cells could
not be observed in the present study.
In addition to the lower hydrolysates and IP6
concentrations used in the present study, we also investigated the
whole DH hydrolysates of IP6 against colon cancer for the first
time. The mixture of IP6 hydrolysates is the absorbed form of IP6
in the colon. The intestine epithelial cells may not be exposed to
IP6 actually, however, they are commonly exposed to the IP6
hydrolysed mixture. We confirmed that the anticancer properties of
IP6 are primarily due to its hydrolysates which competitively
inhibited the activation of the AKT protein. The present study
first used the molecular docking simulation method to indicate that
IP4 and IP5 may be effective components of IP6 hydrolysates. In the
past few years, IP3 has been recognized as the main component of
all IP6 hydrolysates. However, according to our molecular docking
simulation data, IP4 and IP5 also play an important role in
preventing oncogenesis.
Moreover, our results indicated that IP6
hydrolysates could be a protective factor against colon carcinoma.
The protective functions may be significantly dependent on the
condition of colon bacteria. Intestinal flora disturbances may
decrease colon phytase secretion, which may hinder the IP6
hydrolysates to prevent oncogenesis. Hence, we hypothesize that the
enteric flora disturbances may be a new mechanism employed in colon
oncogenesis.
In conclusion, IP6 hydrolysates were able to
suppress SW620 colon carcinoma cells and inhibited the activation
of the AKT protein. The 20 and 30% DH IP6 hydrolysates suppressed
the proliferation and inhibited the AKT protein. IP6 has been known
to be an anti-nutrition phytochemical for decades. Our findings in
the present study suggest that IP6 and its hydrolysates may be a
valuable agent for cancer prevention and treatment, however further
investigation is required.
Acknowledgements
The present study was supported by grants from The
National Natural Science Foundation of China (no. 81373001).
Glossary
Abbreviations
Abbreviations:
|
IP6
|
myto-inositol 1,2,3,4,5,6
hexaphosphate
|
|
DH
|
degree of hydrolysis
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
AKT
|
protein kinase B
|
|
PH domain
|
pleckstrin homologydomain
|
|
PIP3
|
3′-phosphorylated phosphoinositides
3,4,5-trisphosphate
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Desantis C and Jemal A:
Colorectal cancer statistics, 2014. CA Cancer J Clin. 64:104–117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang GY and Shamsuddin AM: IP6-induced
growth inhibition and differentiation of HT-29 human colon cancer
cells: Involvement of intracellular inositol phosphates. Anticancer
Res. 15:2479–2487. 1995.PubMed/NCBI
|
|
5
|
Shamsuddin AM, Vucenik I and Cole KE:
IP6A novel anti-cancer agent. Life Sci. 61:343–354.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Somasundar P, Riggs DR, Jackson BJ,
Cunningham C, Vona-Davis L and McFadden DW: Inositol hexaphosphate
(IP6): A novel treatment for pancreatic cancer. J Surg Res.
126:199–203. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizvi I, Riggs DR, Jackson BJ, Ng A,
Cunningham C and McFadden DW: Inositol hexaphosphate (IP6) inhibits
cellular proliferation in melanoma. J Surg Res. 133:3–6. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shamsuddin AM: Metabolism and cellular
functions of IP6: A review. Anticancer Res. 19:3733–3736.
1999.PubMed/NCBI
|
|
9
|
Parfiniewicz B, Pendzich J, Kapral M,
Bednarek I and Weglarz L: The influence of TNF-alpha on
concentration of soluble adhesion molecules in cultures of HT-29
cells exposed to inositol hexaphosphate. Acta Pol Pharm.
69:1291–1297. 2012.PubMed/NCBI
|
|
10
|
Liu G, Song Y, Cui L, Wen Z and Lu X:
Inositol hexaphosphate suppresses growth and induces apoptosis in
HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a
potential target. Int J Clin Exp Pathol. 8:1402–1410.
2015.PubMed/NCBI
|
|
11
|
Ishizuka S, Saitoh K, Suzuki T, Lee JS and
Hara H: A partially degraded product of phytate suppresses the
proliferation of HCT116 colorectal cancer cells. Food Chem.
125:1219–1225. 2011. View Article : Google Scholar
|
|
12
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsushima M, Kikuchi E, Matsumoto K,
Hattori S, Takeda T, Kosaka T, Miyajima A and Oya M: Intravesical
dual PI3K/mTOR complex 1/2 inhibitor NVP-BEZ235 therapy in an
orthotopic bladder cancer model. Int J Oncol. 47:377–383. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tahir AA, Sani NF, Murad NA, Makpol S,
Ngah WZ and Yusof YA: Combined ginger extract & Gelam honey
modulate Ras/ERK and PI3K/AKT pathway genes in colon cancer HT29
cells. Nutr J. 14:312015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majewska E and Szeliga M: AKT/GSK3β
signaling in glioblastoma. Neurochem Res. 42:918–924. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuyinng F: Research on phytic acid
hydrolysis and the products of separation (D). 2010.
|
|
17
|
King EJ: The colorimetric determination of
phosphorus. Biochem J. 26:292–297. 1932. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rounds MA and Nielsen SS: Anion-exchange
high-performance liquid chromatography with post-column detection
for the analysis of phytic acid and other inositol phosphates. J
Chromatogr A. 653:148–152. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burbano C, Muzquiz M, Osagie A, Ayet G and
Cuadrado C: Determination of phytate and lower inositol phosphates
in Spanish legumes by HPLC methodology. Food Chem. 52:321–325.
1995. View Article : Google Scholar
|
|
20
|
Hong M, Li Z, Li SY and Yuan ZY:
Preparation of inositol tetrakisphosphate and its application in
modification of porcine hemoglobin. Sheng Wu Hua Xue Yu Sheng Wu Wu
Li Xue Bao. 32:31–34. 2000.PubMed/NCBI
|
|
21
|
Lehrfeld J: HPLC separation and
quantitation of phytic acid and some inositol phosphates in foods:
Problems and solutions. J Agric Food Chem. 42:2726–2731. 2002.
View Article : Google Scholar
|
|
22
|
Sandberg AS, Carlsson NG and Svanberg U:
Effects of inositol tri-, tetra-, penta-, and hexaphosphates on in
vitro estimation of iron availability. J Food Sci. 54:159–161.
2006. View Article : Google Scholar
|
|
23
|
Suzuki T and Hara H: Phytate hydrolysate
induces circumferential F-actin ring formation at cell-cell
contacts by a Rho-associated kinase-dependent mechanism in
colorectal cancer HT-29 cells. Mol Nutr Food Res. 54:1807–1818.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sandberg AS and Ahderinne R: HPLC method
for determination of inositol tri-, tetra-, penta-, and
hexaphosphates in foods and intestinal contents. J Food Sci.
51:547–550. 1986. View Article : Google Scholar
|
|
25
|
Dinicola S, Fabrizi G, Masiello MG,
Proietti S, Palombo A, Minini M, Harrath AH, Alwasel SH, Ricci G,
Catizone A, et al: Inositol induces mesenchymal-epithelial
reversion in breast cancer cells through cytoskeleton
rearrangement. Exp Cell Res. 345:37–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vucenik I and Shamsuddin AM: Cancer
inhibition by inositol hexaphosphate (IP6) and inositol:
From laboratory to clinic. J Nutr. 133 Suppl 1:3778S–3784S.
2003.PubMed/NCBI
|
|
27
|
Vucenik I and Shamsuddin AM: Protection
against cancer by dietary IP6 and inositol. Nutr Cancer.
55:109–125. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bacić I, Druzijanić N, Karlo R, Skifić I
and Jagić S: Efficacy of IP6 + inositol in the treatment
of breast cancer patients receiving chemotherapy: Prospective,
randomized, pilot clinical study. J Exp Clin Cancer Res. 29:122010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schröterová L, Hasková P, Rudolf E and
Cervinka M: Effect of phytic acid and inositol on the proliferation
and apoptosis of cells derived from colorectal carcinoma. Oncol
Rep. 23:787–793. 2010.PubMed/NCBI
|
|
30
|
Kim JN, Han SN and Kim HK: Phytic acid and
myo-inositol support adipocyte differentiation and improve insulin
sensitivity in 3T3-L1 cells. Nutr Res. 34:723–731. 2014. View Article : Google Scholar : PubMed/NCBI
|