Introduction
Approximately one-third of lung cancer patients
develop bone metastasis in their disease course, and these patients
present with an extremely poor prognosis with a median survival of
approximately 7 months (1). Bone
metastasis is easy to be neglected in non-small cell lung cancer
(NSCLC) patients until pain and skeletal-related events (SREs)
occur. Most lung cancer bone metastatic diseases are osteolytic,
and spine, ribs, pelvis and proximal long bones are commonly
involved. Since the delayed demonstration of bone lesions seriously
affects the survival of lung cancer patients, the identification of
potentially useful and specific biomarkers is necessary for the
early diagnosis of bone metastasis and follow-up.
It is well known that bone remodeling can be
interrupted by tumor cells which leads to an inappropriate balance
of osteoblasts and osteoclasts. Mesenchymal stem cells (MSCs) are
capable of differentiating to osteoblast cells, and evidence has
demonstrated that MSCs are biologically abnormal in aspects of
differentiation potentials, gene expression and cytokine/chemokine
secretion in the tumor microenvironment of lung cancer (2). Aberrant expression of miR-139-5p has
been reported in various types of cancers (3–7). In
our previous unpublished work, using a high-throughput miRNA array,
we found that miR-139-5p was one of the downregulated miRNAs in
MSCs from multiple myeloma patients with bone lesions as compared
to those from normal donors. Therefore, we proposed that miR-139-5p
may be involved in the osteogenic differentiation of MSCs as well
as lung cancer bone lesions. This particular miRNA deserves further
study.
Materials and methods
Study population
The present study was conducted in accordance with
the Helsinki Declaration and was approved by the Ethics Committee
of Tianjin Medical University. Written informed consents were
obtained from all the NSCLC patients for blood sampling and healthy
volunteers for bone marrow (BM) sampling. To reduce the
discrepancy, only lung adenocarcinoma patients were recruited and
all of the recruited NSCLC patients with bone metastasis had only
lytic bone lesions. Positron emission tomography-computed
tomography (PET-CT) or emission computed tomography (ECT) was used
for the diagnosis of bone metastasis together with magnetic
resonance imaging (MRI) scan. No previous local or systemic
treatment had been conducted before the first-time serum sample
collection. Clinical data for the patients are summarized in
Table I.
 | Table I.Clinical data of the NSCLC
patients. |
Table I.
Clinical data of the NSCLC
patients.
|
| Stage IV patients
without bone metastasis (n=30) | Stage IV patients
with bone metastasis (n=25) |
|---|
|
|
|
|
|---|
| Median age (range) in
years | 54 (42–73) | 62 (35–76) |
| Sex |
|
|
| Male | 14 | 12 |
|
Female | 16 | 13 |
| Smoking status
(%) | 53 | 56 |
| Histology | Adenocarcinoma | Adenocarcinoma |
| Sites of
metastasis | Contralateral lobe,
8 | Skeleton (vertebrae,
ribs, long bones, or pelvis), 25 |
|
| Pleural, 12 |
|
|
| Brain, 8 |
|
|
| Liver, 10 |
|
|
| Adrenal gland, 2 |
|
Primary culture of the human MSCs
The primary culture of human MSCs was performed
according to our previously published protocol (8). Briefly, BM mononuclear cells were
isolated by density gradient centrifugation with Ficoll-Hypaque
(Nycomed; Lucron Bioproducts, De Pinte, Belgium) and seeded at a
density of 1×106/cm2 with MesenPro medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Medium was refreshed every 3–4 days until 80–90% confluence was
reached, and the cells were passaged into new flasks at 2,000
cells/cm2. MSCs were used at passage 2 in this study.
MSCs need to be characterized based on the morphology, specific
immunophenotype and tri-lineage differentiation potentials.
Osteogenic differentiation induction
and evaluation
To induce osteogenic differentiation in
vitro, MSCs were exposed to Osteogenic Induction Medium (Lonza
Group, Ltd., Basel, Switzerland) according to the manufacturer's
protocol. MSCs cultured in growth medium were used as negative
control. In order to examine MSC differentiation towards
osteoblasts, we performed alkaline phosphatase (ALP) staining
(early marker), qPCR analysis of osteoblast differentiation
markers, and Alizarin Red S staining (late marker) at day 3, 7 and
14, respectively, as shown in our previous publication (8). To quantify ALP activity, we used
BCIP/NBT liquid substrate and alkaline phosphatase yellow liquid
substrate system for ELISA (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Qualitative Alizarin Red S staining was used to evaluate
calcium deposits. After fixation with 10% paraformaldehyde, the
cells were stained with 40 mM fresh Alizarin Red solution (pH 4.2)
and destained using 10% cetylpyridinium chloride (CPC). The
concentration of Alizarin Red S was determined by measuring
absorbance at 562 nm with a multiplate reader (Thermo Labsystems,
Santa Rosa, CA, USA).
Western blot analysis
The procedures involved in cell lysis, protein
extraction, and immunoblotting were performed as previously
described (9), using Notch1
antibody (goat polyclonal IgG; 1:500; sc-23304; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), followed by a
horseradish peroxidase-conjugated secondary antibody (donkey
anti-goat IgG; 1:2,000; cat. no. sc-2033; Santa Cruz Biotechnology,
Inc.) and ECL western blotting reagents (Amersham Pharmacia
Biotech, Buckinghamshire, UK).
Preparation of conditioned medium
Conditioned medium was collected according to our
previous protocol (10). Briefly,
conditioned medium was prepared by exposure of lung cancer cells
(5×105) in 5 ml serum-free RPMI-1640 medium (Lonza
Group, Ltd.) for 48 h. The culture supernatant was then harvested
and centrifuged at 2,000 rpm to remove cell debris and was frozen
at −20°C and used within 24 h.
Plasmid construction and
dual-luciferase reporter assay
Wild-type sequences or mutant 3′ untranslated region
(3′UTR) of Notch1 was constructed, carrying mutated
sequences in the complementary sites for the seed region of
miR-139-5p, and inserted into the pmiR-RB-REPORT™ vector (Guangzhou
RiboBio Co., Ltd., Guangzhou, China). The procedure for the
dual-luciferase reporter assay was previously described (8).
miR-139-5p gain- and loss-of-function
analysis
Transfection of miR-139-5p inhibitor (MIN0000250;
Qiagen, Leusden, The Netherlands) or miR-139-5p mimic (MSY0000250;
Qiagen) was performed using Lipofectamine™ RNAiMAX reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and AllStars Negative
Control siRNA (1027280; Qiagen) and miScript Inhibitor Negative
Control (1027271; Qiagen) were used as negative controls according
to the manufacturer's protocol. Supplement of miR-139-5p mimic and
inhibitor (and their controls) into osteogenic medium was refreshed
every three days.
Notch1 knockdown by RNA
interference
The knockdown of Notch1 in MSCs was conducted by
transfection with FlexiTube GeneSolution which provides four
non-overlapping Notch1 RNAi duplexes (GS4851; Qiagen) together with
Lipofectamine RNAiMAX reagent according to the manufacturer's
protocol. AllStars Negative Control small interfering RNA (siRNA;
SI03650318; Qiagen) was used as a negative control. Real-time PCR
and western blot analysis were used to evaluate the knockdown
efficiency.
Quantitative real-time PCR (qPCR)
qPCR for miR-139-5p
For purification of microRNA from the cells, miRNA
extraction and reverse transcription were performed using
miReasy® Mini kit and miScript Reverse Transcription kit
(Qiagen) according to the manufacturer's protocol. qPCR for
miR-139-5p expression was performed using miScript SYBR-Green PCR
kit (Qiagen) with iCycler Thermal Cycler system (Bio-Rad
Laboratories, Nazareth, Belgium).
For purification of microRNA from human serum, blood
samples were collected from lung adenocarcinoma patients. Serum was
separated by centrifugation at 1,200 × g at 4°C for 15 min and then
frozen at −80°C prior to total RNA isolation. The miRNeasy
Serum/Plasma kit (Qiagen) was used to isolate RNA from the serum
samples according to the manufacturer instructions. Briefly, 20 µl
total RNA sample was converted into cDNA using miScript-II-RT-kit
(Qiagen). Real-time PCR was then performed with iCycler Thermal
Cycler system. Reactions were performed in triplicate, and miRNA
relative expression was calculated using a 2−∆∆Ct
method. U6 was used as an endogenous control.
qPCR for osteogenic markers
mRNA extraction and reverse transcription were
performed using Invitrogen™ TRIzol reagent (Thermo Fisher
Scientific, Inc.), Rneasy® Mini kit (Qiagen) and Thermo
Scientific Verso™ cDNA synthesis kit (Thermo Scientific,
Inc.) according to the manufacturer's protocol, and miScript
Reverse Transcription kit (Qiagen) according to the manufacturer's
protocol. qPCR of osteogenic markers was quantified by SYBR
GreenER™ qPCR for iCycler kit (Invitrogen; Thermo Fisher
Scientific, Inc.) using iCycler Thermal Cycler system (8). The primer sequences used are listed in
Table II.
 | Table II.Real-time PCR primers. |
Table II.
Real-time PCR primers.
| Gene | Primer | GenBank accession
no. | Annealing temp
(°C) |
|---|
| OPN |
5′-CTCCATTGACTCGAACGACTC-3′ | NM_000582 | 60 |
|
|
5′-CAGGTCTGCGAAACTTCTTAGAT-3′ |
|
|
| BSP |
5′-GAATGGCCTGTGCTTTCTCAA-3′ | NM_004967 |
|
|
|
5′-TCGGATGAGTCACTACTGCCC-3′ |
|
|
| COLA1 |
5′-AGACGAAGACATCCCACCAATC-3′ | NM_000088 |
|
|
|
5′-AGATCACGTCATCGCACAACA −3′ |
|
|
| Notch1 |
5′-CTTGTGTCAACGGCGGC-3′ | NM_017617 |
|
|
|
5′-TTGGGACCGCTGAAGCC-3′ |
|
|
| Hes1 |
5′-AGGCTGGAGAGGCGGCTAAG-3′ | NM_005524 |
|
|
|
5′-TGGAAGGTGACACTGCGTTGG-3′ |
|
|
| Hey1 |
5′-GGATCACCTGAAAATGCTGCATAC-3′ | NM_001040708 |
|
|
|
5′-CCGAAATCCCAAACTCCGATAG-3′ |
|
|
| Runx2 |
5′-GGAGTGGACGAGGCAAGAGTTT-3′ | NM_009820 |
|
|
|
5′-AGCTTCTGTCTGTGCCTTCTGG-3′ |
|
|
| β-actin |
5′-ATGTGGCCGAGGACTTTGATT-3′ | NM_001101 |
|
|
|
5′-AGTGGGGTGGCTTTTAGGATG-3′ |
|
|
Statistical analysis
Statistical analysis was performed by Mann-Whitney U
test or one-way analysis of variance (ANOVA) followed by Tukey's
post test with GraphPad Prism 7 software (GraphPad Software, Inc.,
La Jolla, CA, USA). P-values <0.05 were considered to indicate
statistically significant results. All experiments were conducted
at least three times.
Results
miR-139-5p and Notch1 exhibit an
inverse tendency in change during MSC osteogenesis in vitro
During MSC osteogenic differentiation in
vitro shown by ALP staining (Fig.
1A), we observed a considerable increase in miR-139-5p
expression (Fig. 1B), and inversely
a decreased expression of Notch1 (Fig. 1C). As expected, this demonstrated
that the expression of Notch signaling downstream genes Hes1
and Hey1 was widely suppressed in MSCs after culturing in
osteogenic induction medium (Fig.
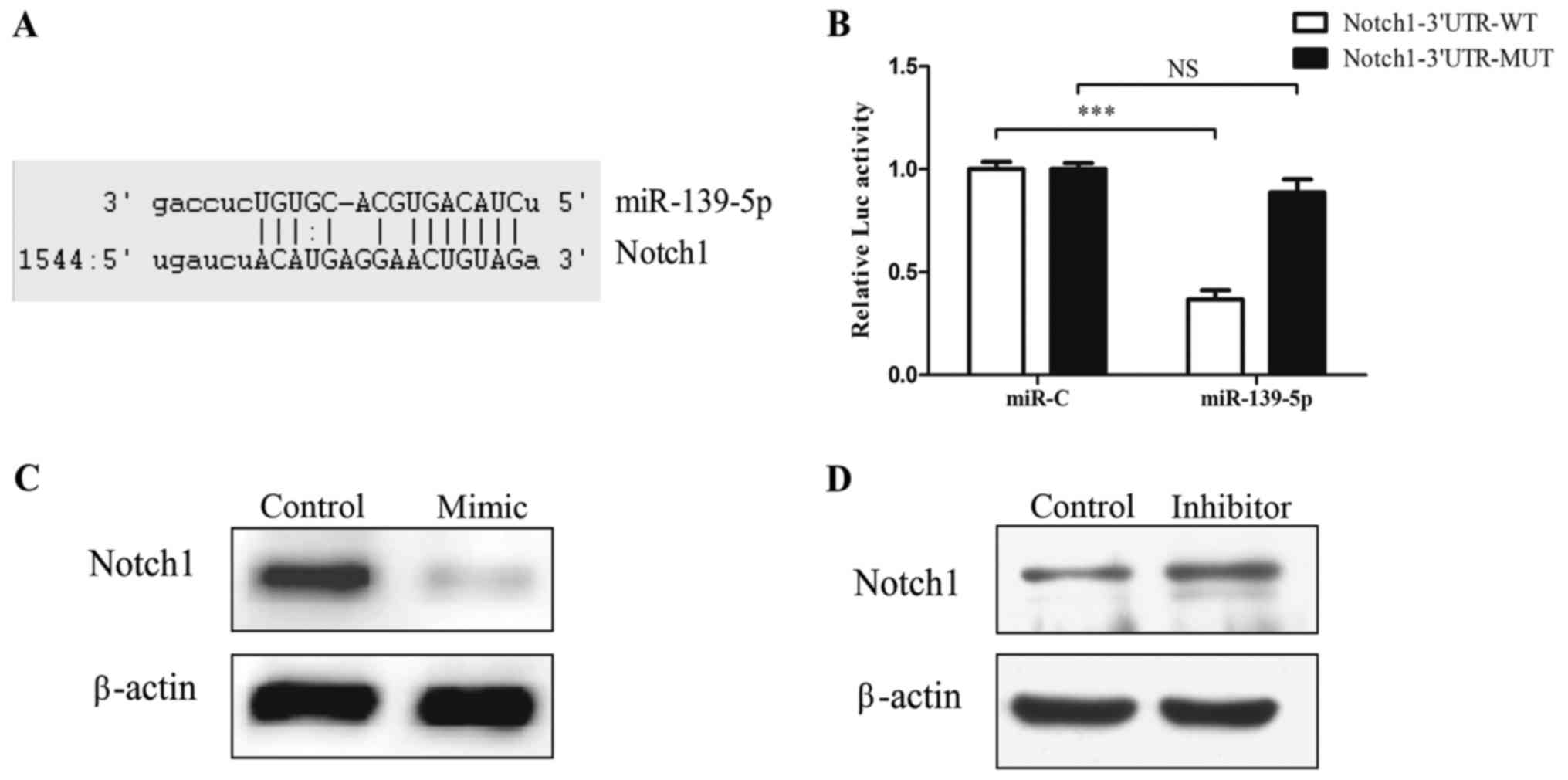
1D). Bioinformatic tools predicted that 3′UTR of Notch1
has binding sites for miR-139-5p (Fig.
2A). Functional luciferase activity assay and western blot
analysis confirmed that miR-139-5p binds directly to the predicted
binding sites in the Notch1 3′UTR and negatively regulates
Notch1 expression (Fig.
2B-D).
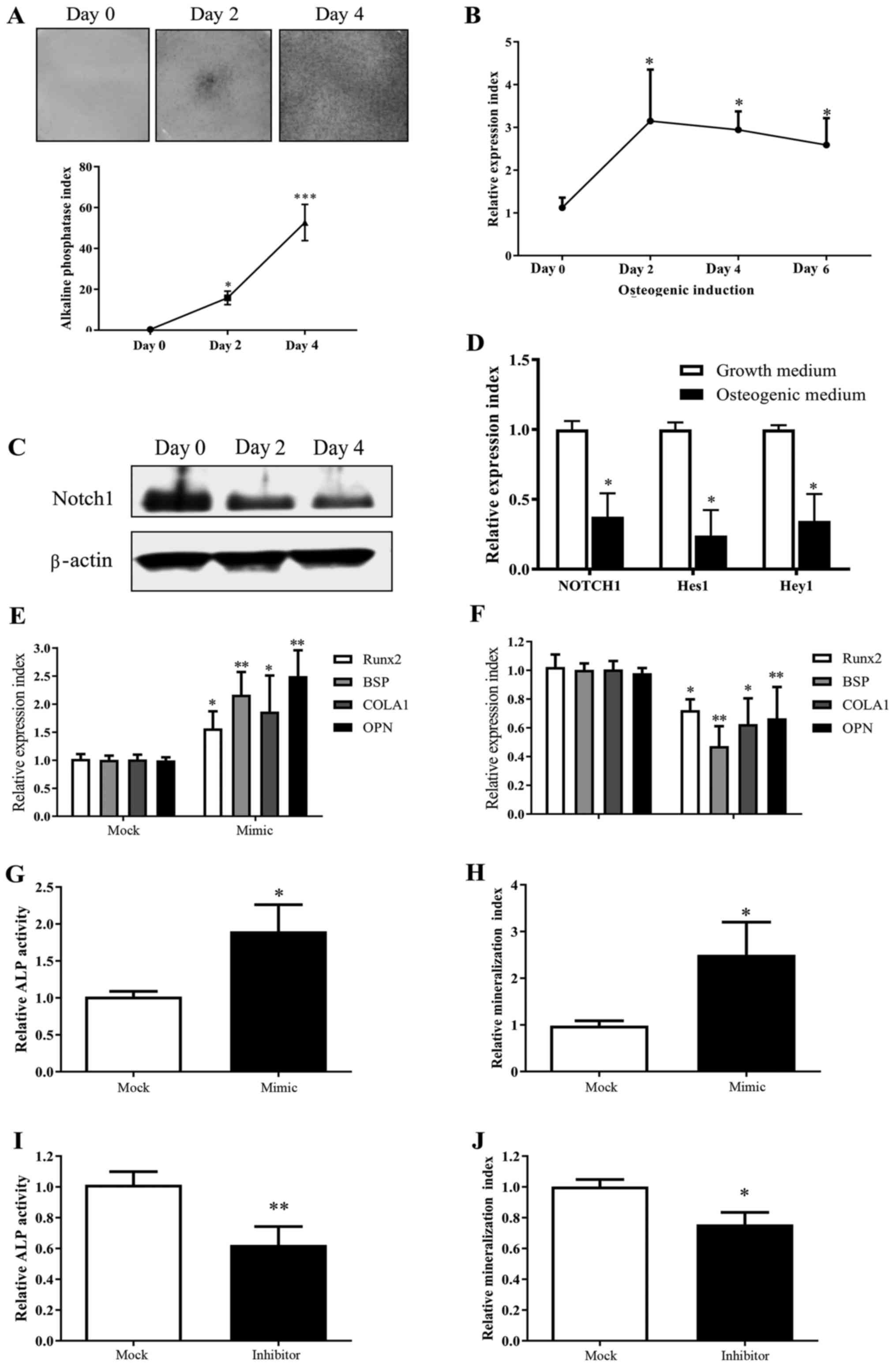 | Figure 1.miR-139-5p expression is increased
during MSC differentiation towards osteoblasts and positively
regulates osteogenic differentiation. (A) MSCs underwent osteogenic
differentiation when exposed to osteogenic induction medium in
vitro as shown by ALP staining. (B) Real-time PCR test showed
that miR-139-5p increased significantly during MSC osteogenic
differentiation. (C) Notch1 decreased significantly during
MSC osteogenic differentiation by western blot analysis. (D)
Notch downstream genes Hes1 and Hey1 decreased
during MSC osteogenic differentiation as well (day 4). (E)
Transfection with miR-139-5b mimic (50 nM) for 24 h led to
upregulation of Runx2, BSP, COLA1 and OPN expression.
(F) Transfection with miR-139-5b inhibitor (50 nM) for 24 h led to
downregulation of Runx2, BSP, COLA1 and OPN
expression. Transfection with miR-139-5b mimic (50 nM) led to (G)
upregulation of ALP activity (72 h) and (H) mineralization (14
days) in MSCs. In contrast, transfection with the miR-139-5b
inhibitor (50 nM) led to (I) downregulation of ALP activity (72 h)
and (J) mineralization (14 days). *P<0.05, **P<0.01,
***P<0.001. MSCs, mesenchymal stem cells; ALP, alkaline
phosphatase; Runx2, runt related transcription factor 2;
OPN, osteopontin; BSP, bone sialoprotein;
COLA1, collagen type І. |
miR-139-5p positively regulates the
MSC osteogenic differentiation
MSCs, which were transfected with miR-139-5p mimic,
exhibited a significant enhanced expression of osteogenic markers
(BSP, COLA1, OPN) and Runx2 expression by real-time
PCR, which is a key transcription factor for osteogenesis (Fig. 1E), as well as upregulated ALP and
mineralization activities (Fig. 1G and
H). In contrast, MSCs, which were transfected with miR-139-5p
inhibitor, showed a significant decreased osteogenic
differentiation compared to the controls (Fig. 1F, I and J). Moreover, we observed
that MSCs, which were knocked down for Notch1 expression (Fig. 3A and B), did not exhibit significant
alterations in ALP activity and bone formation marker expression
after treatment with miR-139-5p mimic or inhibitor (Fig. 3C-F).
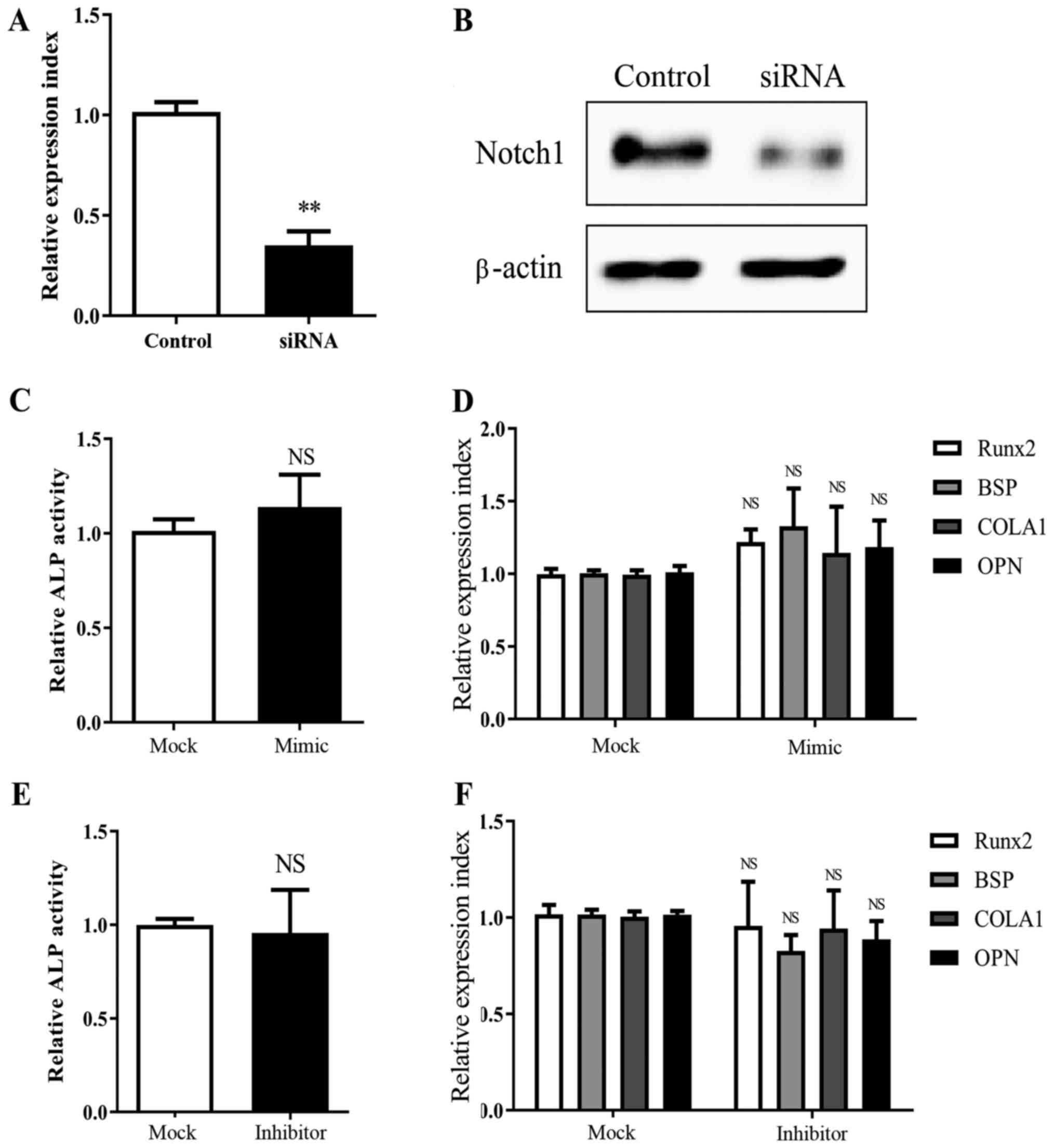 | Figure 3.Knockdown of Notch1 abrogates
miR-139-5p-induced MSC osteogenesis. Notch1 knockdown by
siRNA transfection was confirmed by (A) real-time PCR (24 h) and
(B) western blot analysis (72 h). Transfection with miR-139-5b
mimic (50 nM) did not lead to a significant change in (C) ALP
activity (72 h) and (D) Runx2, BSP, COLA1 and OPN
expression (24 h), in MSCs with Notch1 knockdown; Similarly,
miR-139-5b inhibitor (50 nM) had no significant effect on (E) ALP
activity (72 h) and (F) Runx2, BSP, COLA1 and OPN
expression (24 h), in MSCs with Notch1 knockdown.
**P<0.01; NS, not significant. MSCs, mesenchymal stem cells;
Runx2, runt related transcription factor 2; OPN,
osteopontin; BSP, bone sialoprotein; COLA1, collagen
type І. |
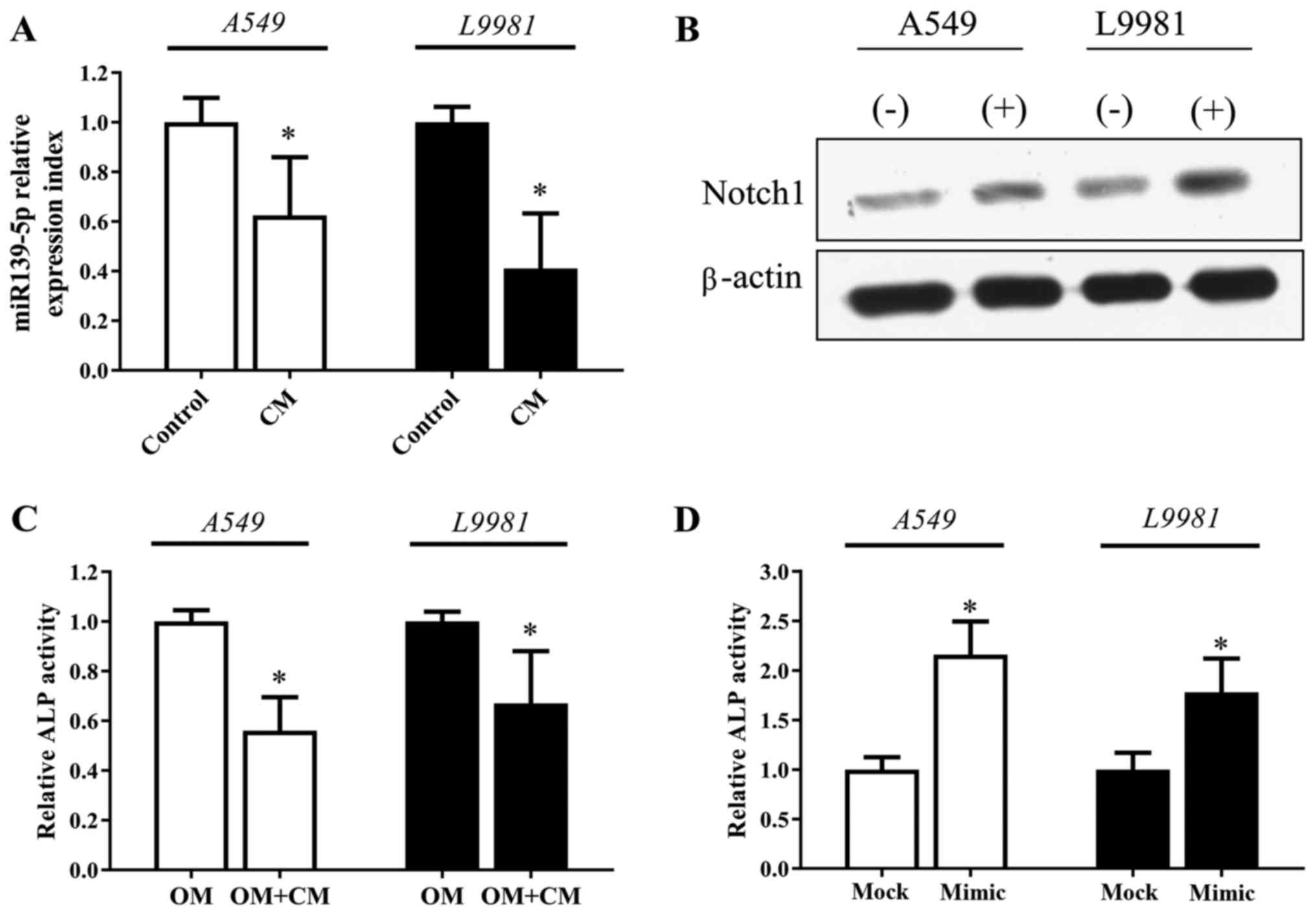
Lung cancer cell-derived factors
impair MSC osteogenic differentiation
After exposure to the conditioned medium of NSCLC
cell lines (A549 or L9981) for 72 h, MSCs exhibited a significant
downregulation in miR-139-5p expression and increased expression of
Notch1 expression (Fig. 4A and
B). Notably, we found that ALP activity of MSCs decreased in
the osteogenic induction medium mixed with NSCLC conditioned medium
(Fig. 4C). However, the decreased
ALP activity of MSCs induced by the NSCLC conditioned medium was
recovered significantly when the MSCs were transfected with the
miR-139-5p mimic (Fig. 4D).
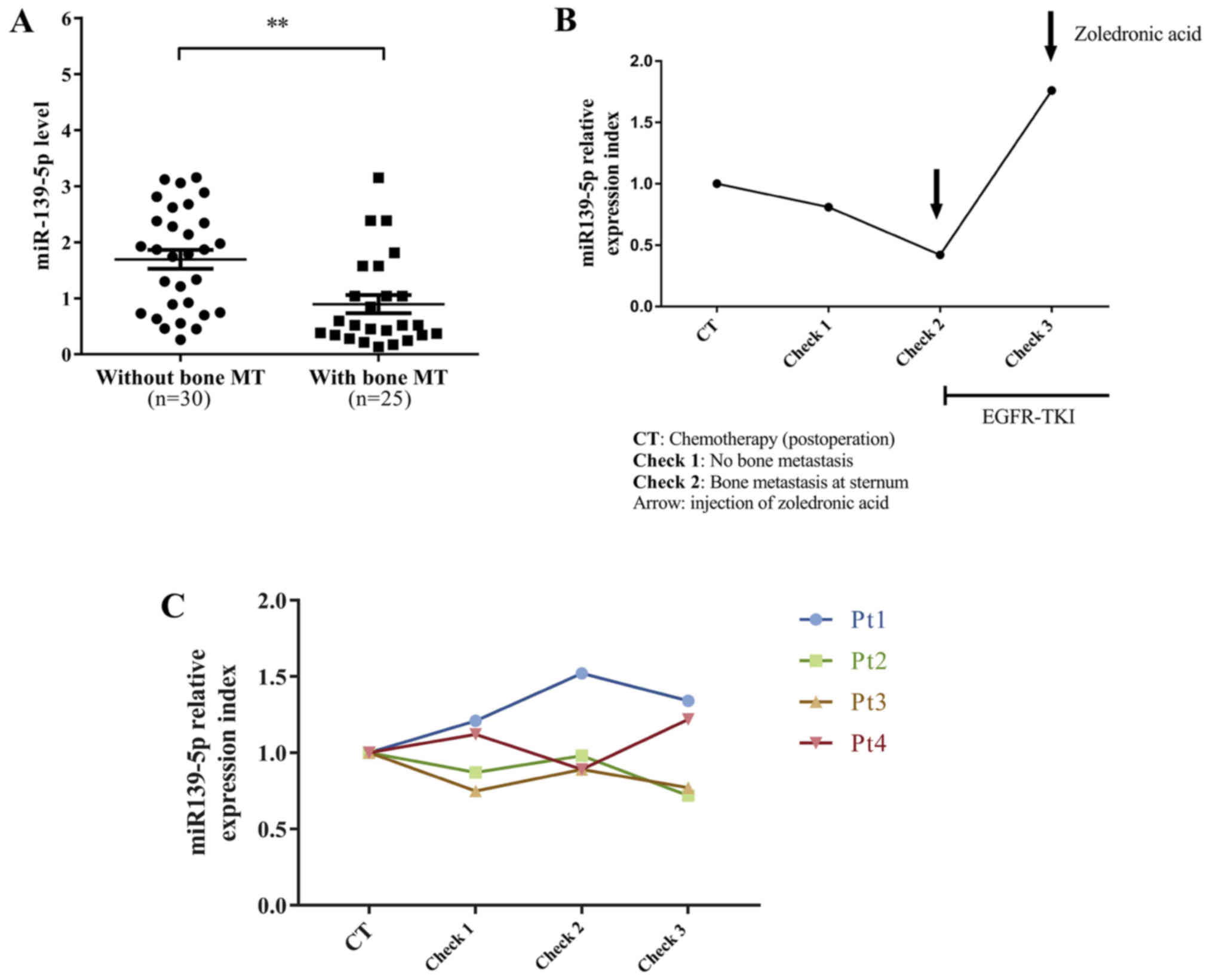
Serum miR-139-5p is significantly
lower in lung cancer patients with lytic bone metastasis
We collected blood from 55 untreated stage IV lung
adenocarcinoma patients and found that serum miR-139-5p was
significantly lower in lung cancer patients with bone metastasis
compared to the patients with metastasis at other sites (Fig. 5A). In addition, we recruited five
surgically resected lung adenocarcinoma patients (Table III) and examined their dynamic
levels of serum miR-139-5p. One of five patients eventually
developed an exclusive bone metastasis at the sternum. The patient
(female, 37 years of age) was admitted to our department due to a
persistent cough for approximately 6 months. Contrast-enhanced
chest computed tomography indicated a solitary mass located in the
left upper lobe which had invaded the main pulmonary artery and
bronchus. A left pneumonectomy was performed with systemic
lymphadenectomy, and the histology was adenocarcinoma with
mediastinal lymph node metastasis of station 5 (pT2aN2M0, IIIA,
AJCC 7th edition). Genetic analysis showed EGFR exon 21 L858R (+).
The patient received post-operative chemotherapy (pemetrex and
nedaplatin, 4 cycles) and then regular imaging and serological
evaluation. When bone metastasis of the sternum was detected at the
2nd postoperative check, she started to receive icotinib daily (125
mg t.i.d., oral) and zoledronic acid (0.4 mg, i.v.) each month. The
patient had a significantly partial response to the treatment and
the metastatic bone disease was controlled well. Since the first
postoperative chemotherapy, we started to examine the serum
miR-139-5p level and observed dynamic change. On the first
follow-up check, we already found that miR-139-5p started to
decrease but ECT and CT examinations did not report any
abnormality. On the second follow-up check, the level of serum
miR-139-5p continued to decrease. Meanwhile, ECT reported abnormal
metabolic accumulation in the sternum and CT scan showed a lytic
bone destruction in the sternum. After the patient was administered
EGFR-TKI and zoledronic acid infusion, the level of serum
miR-139-5p returned significantly and the ECT examination showed a
less metabolic accumulation in the sternum, indicating the
effectiveness of EGFR-TKI for this patient. We found that the
levels of serum miR-139-5p were well correlated with the disease
status and treatments for this patient (Fig. 5B). The dynamic serum miR-139-5p
expression of an additional 4 patients is shown in Fig. 5C.
 | Table III.Clinical features of 5 NSCLC patients
who underwent dynamic serum miR-139-5p evaluation. |
Table III.
Clinical features of 5 NSCLC patients
who underwent dynamic serum miR-139-5p evaluation.
|
|
|
|
|
|
| Metastasis |
|---|
|
|
|
|
|
|
|
|
|---|
| Patient no. | Age (years) | Sex | Surgery | Staging | Adjuvant
therapy | Check 1 | Check 2 | Check 3 | Check 4 |
|---|
| Pt1 | 71 | Male | RLL | IIA | Chemotherapy | No | No | No | Mediastinal LN
(station 7) |
| Pt2 | 53 | Female | RUL | IIB | Chemotherapy | No | No | No | No |
| Pt3 | 61 | Male | RUL | IIIA | Chemotherapy +
radiotherapy | No | No | No | Brain |
| Pt4 | 65 | Male | LUL | IIIA | Chemotherapy | No | No | Supraclavicular
LN | Supraclavicular
LN |
| Pt5 | 38 | Female | LLL | IIIA | Chemotherapy | No | Bone | Bone | Bone |
Discussion
Bone is among the most common sites of metastasis of
lung cancer and is associated with a poor patient quality of life
and survival. Most of lung cancer skeletal involvements appear
lytic with apparent cortical destruction. Radiological imaging is
essential in the diagnosis and monitoring of cancer-related bone
lesions. However, different radiological techniques have various
limitations, including lack of sensitivity for the detection of
early lesions, non-specificity and high cost. If bone involvement
can be predicted at its early stage, effective intervention could
be implemented and may result in an improvement in survival.
However, the studies of the biomarkers for the early detection of
skeletal metastasis and targeted therapies to reverse bone lesions
are both very limited.
Dysregulation of the bone remodeling process is
characterized by an imbalance between osteoblastic and osteoclastic
activity. An understanding of the mechanisms involved in bone
turnover and the crosstalk between bone marrow cells and lung
cancer cells contribute to the identification of novel biomarkers
and therapeutic targets for bone metastasis. In the present study,
we identified by gain- and loss-of-function experiments that
miR-139-5p positively regulated MSC differentiation towards
osteoblast cells. A previous study reported that miR-139-5p
targeted Notch1 expression directly, which is in accordance with
our findings (3). In addition,
previous studies from our and other groups have demonstrated that
the Notch pathway can maintain normal bone marrow mesenchymal
progenitors in a more undifferentiated state by suppressing
osteoblast differentiation (11–13).
We proposed that after exposure to osteogenic induction condition,
MSCs were forced to undergo epigenetic alterations, including the
upregulation of miR-139-5p, which inhibited Notch1-mediated
signaling activity and triggered osteogenic differentiation. This
conclusion was further confirmed by the finding that
miR-139-5p-induced MSC osteogenic differentiation was abrogated in
Notch1-knockdown MSCs.
A number of studies have demonstrated that the
crosstalk between tumor cells and MSCs through paracrine signaling
could increase metastatic potential and promote
epithelial-to-mesenchymal transition of tumor cells, and induce
MSCs to acquire abnormal genotypes and phenotypes (14,15).
In our previous studies, by coculture experiments with a Transwell
system, we found that MSCs favored the proliferation of lung cancer
and multiple myeloma cells. In turn, tumor cells could induce
epigenetic alterations and upregulate various growth factors in
MSCs which promote tumor cell growth, including prointerleukin-6
(IL-6), insulin growth factor-1 and vascular endothelial growth
factor (8,16). In the present study, after exposure
of the MSCs to the conditioned medium of lung cancer cells A549 and
L9981, we observed that MSCs exhibited downregulation of miR-139-5p
expression, upregulation of Notch1 expression, as well as impaired
ALP activity, which is an early osteogenic marker of MSCs. Our data
showed that lung cancer cell-derived soluble factors could
downregulate miR-139-5p expression and impair the osteogenesis in
MSCs. However, more research is necessary to identify these
molecules.
There are various non-invasive liquid biomarkers
used to monitor NSCLC patients with bone metastasis, including
N-telopeptide, bone-specific alkaline phosphatase, carboxy-terminal
telopeptide of type I collagen, amino-terminal propeptide of type I
collagen and IL-7 (17). However,
none of these candidates are very reliable and widely applied in
the current clinical settings due to the limited sensitivity and
specificity. In the present study, we observed that serum
miR-139-5p was significantly lower in lung cancer patients with
lytic bone lesions compared to those without bone metastasis.
Moreover, a representative case showed that the dynamic serum
miR-139-5p alterations were well correlated with disease
progression. The potential role of serum miR-139-5p as a
non-invasive biomarker for bone metastasis of lung cancer patients
warrants further investigation with larger samples.
There are some limitations to our study. Firstly, we
excluded the patients with sclerotic and sclerotic/lytic mixed bone
metastases in this study because we inferred that the underlying
mechanisms for lytic and sclerotic bone metastatic lesions may be
significantly different. The role of serum miR-139-5p in lung
cancer patients with sclerotic bone metastasis needs to be further
investigated. Furthermore, one previous study reported that
miRNA-139-5p was markedly downregulated in NSCLC tumor tissues and
cell lines, and tumor growth and invasion were inhibited by
overexpression of miRNA-139-5p (4).
We also observed that the level of serum miRNA-139-5p tended to be
gradually downregulated during the disease progression in several
NSCLC patients, but the decreased level was not as marked as that
in NSCLC patients with bone metastasis. Further studies must
explore an optimal cut-off value for serum miRNA-139-5p to
distinguish NSCLC patients with bone metastasis from other patients
at a late stage.
Taken together, for the first time, we demonstrated
that miR-139-5p plays a positive role in the regulation of MSC
osteogenic differentiation which is mediated by Notch1 and
its signaling pathway. Importantly, the expression of serum
miR-139-5p from lung adenocarcinoma patients with lytic bone
metastasis was significantly lower compared to the patients with
metastasis at other sites. Upregulation of miR-139-5p expression
may contribute to control lytic bone disease in lung cancer.
Therefore, the potential roles of miR-139-5p as a biomarker and
treatment target in monitoring and controlling bone metastasis in
lung cancer patients are worthy of further investigation.
Acknowledgements
Not applicable.
References
|
1
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu R, Wei S, Chen J and Xu S: Mesenchymal
stem cells in lung cancer tumor microenvironment: Their biological
properties, influence on tumor growth and therapeutic implications.
Cancer Lett. 353:145–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu
CW, Wang K, Zheng S, Ng SS, Chan FK, et al: microRNA-139-5p exerts
tumor suppressor function by targeting NOTCH1 in colorectal cancer.
Mol Cancer. 13:1242014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu W, Hang M, Yuan CY, Wu FL, Chen SB and
Xue K: MicroRNA-139-5p inhibits cell proliferation and invasion by
targeting insulin-like growth factor 1 receptor in human non-small
cell lung cancer. Int J Clin Exp Pathol. 8:3864–3870.
2015.PubMed/NCBI
|
|
5
|
Dai S, Wang X, Li X and Cao Y:
MicroRNA-139-5p acts as a tumor suppressor by targeting ELTD1 and
regulating cell cycle in glioblastoma multiforme. Biochem Biophys
Res Commun. 467:204–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yonemori M, Seki N, Yoshino H, Matsushita
R, Miyamoto K, Nakagawa M and Enokida H: Dual tumor-suppressors
miR-139-5p and miR-139-3p targeting matrix metalloprotease 11 in
bladder cancer. Cancer Sci. 107:1233–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dai H, Gallagher D, Schmitt S, Pessetto
ZY, Fan F, Godwin AK and Tawfik O: Role of miR-139 as a surrogate
marker for tumor aggression in breast cancer. Hum Pathol. 61:68–77.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu S, Santini Cecilia G, De Veirman K,
Vande Broek I, Leleu X, De Becker A, Van Camp B, Vanderkerken K and
Van Riet I: Upregulation of miR-135b is involved in the impaired
osteogenic differentiation of mesenchymal stem cells derived from
multiple myeloma patients. PLoS One. 8:e797522013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Menu E, Kooijman R, Van Valckenborgh E,
Asosingh K, Bakkus M, Van Camp B and Vanderkerken K: Specific roles
for the PI3K and the MEK-ERK pathway in IGF-1-stimulated
chemotaxis, VEGF secretion and proliferation of multiple myeloma
cells: Study in the 5T33MM model. Br J Cancer. 90:1076–1083. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu S, Menu E, De Becker A, Van Camp B,
Vanderkerken K and Van Riet I: Bone marrow-derived mesenchymal
stromal cells are attracted by multiple myeloma cell-produced
chemokine CCL25 and favor myeloma cell growth in vitro and in vivo.
Stem Cells. 30:266–279. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zanotti S, Smerdel-Ramoya A, Stadmeyer L,
Durant D, Radtke F and Canalis E: Notch inhibits osteoblast
differentiation and causes osteopenia. Endocrinology.
149:3890–3899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hilton MJ, Tu X, Wu X, Bai S, Zhao H,
Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R and
Long F: Notch signaling maintains bone marrow mesenchymal
progenitors by suppressing osteoblast differentiation. Nat Med.
14:306–314. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu S, Evans H, Buckle C, De Veirman K, Hu
J, Xu D, Menu E, De Becker A, Vande Broek I, Leleu X, et al:
Impaired osteogenic differentiation of mesenchymal stem cells
derived from multiple myeloma patients is associated with a
blockade in the deactivation of the Notch signaling pathway.
Leukemia. 26:2546–2549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gunn WG, Conley A, Deininger L, Olson SD,
Prockop DJ and Gregory CA: A crosstalk between myeloma cells and
marrow stromal cells stimulates production of DKK1 and
interleukin-6: A potential role in the development of lytic bone
disease and tumor progression in multiple myeloma. Stem Cells.
24:986–991. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yu PF, Huang Y, Xu CL, Lin LY, Han YY, Sun
WH, Hu GH, Rabson AB, Wang Y and Shi YF: Downregulation of CXCL12
in mesenchymal stromal cells by TGFβ promotes breast cancer
metastasis. Oncogene. 36:840–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Wu Y, Liu R, Guo L, Xu T, Chen J and
Xu S: Investigational study of mesenchymal stem cells on lung
cancer cell proliferation and invasion. Zhongguo Fei Ai Za Zhi.
18:674–679. 2015.(In Chinese). PubMed/NCBI
|
|
17
|
Roato I: Bone metastases: When and how
lung cancer interacts with bone. World J Clin Oncol. 5:149–155.
2014. View Article : Google Scholar : PubMed/NCBI
|