Introduction
Oral leukoplakia (OL) is a white patch in the oral
cavity which cannot be classified as any other known disease
(1). It is the most common oral
precancerous lesion with high risk to transform into oral squamous
cell carcinoma (OSCC) (2–4). A number of etiological factors have
been implicated in the onset and transformation of OL, such as
tobacco, alcohol, areca nut chewing and chronic mechanical injuries
(5). However, no apparent
etiological factor could be found in some OL patients, suggesting
susceptibility plays a role in the development of OL and OSCC.
Clinically, severe dysplasia is an important indicator for OSCC
risk in patients with OL whereas OL with mild dysplasia or even no
dysplasia can also transform into OSCC (2,4).
Previously, we revealed that protein expression, such as podoplanin
expression, may serve as a biomarker to assess OSCC risk in
patients with OL (6,7). However, the roles of some proteins may
depend on cell types and stages during tumorigenesis. For example,
Smad4 may function as a tumor suppressor in OSCC development as
evidenced in mouse models (8).
However, we revealed that high Smad4 levels in OL were associated
with OSCC development, suggesting a complex role of Smad4 in oral
tumorigenesis (9).
The Notch signaling pathway is highly conserved
during evolution. In mammals, there are five Notch ligands
(Jagged1, Jagged2, DLL1, DLL3 and DLL4), and four Notch receptors
(Notch1-4). Upon ligand binding, the receptors are cleaved at the
transmembrane domain by the γ-secretase complex, which releases the
active Notch intracellular domain (NICD). NICD enters the nucleus
to form a transcription complex with CSL and mastermind-like
proteins, leading to transactivation of Notch target genes
(10–12).
The role of Notch1 signaling in cancer is, however,
cell context dependent (13).
Activated Notch1 signaling has been shown to inhibit the growth of
hepatocellular carcinoma, small-cell lung cancer, and prostate
cancer cells (14,15). In cervical cancers, Notch1 is
considered to be a tumor suppressor. It can activate p53, a tumor
suppressor, leading to cell cycle arrest and apoptosis (16). However, overexpression of Notch1 has
been observed in cutaneous SCC, suggesting Notch1 signaling may be
activated in some SCCs (17). In
OSCC, while overexpression of Notch1 has been reported in some
studies (18–21), downregulation of Notch1 has also
been observed (22). Notably,
inactivating mutations of Notch1 are detected in 10–15% of head and
neck squamous cell carcinoma (HNSCC) including OSCC samples of
Caucasian patients. However, Notch1 mutations are detected in ~40%
of OSCC samples from Chinese patients including a considerable
fraction of potentially activating mutations (23–25).
Recently, Izumchenko et al (26), reported a similar Notch1 mutation
rate in a Chinese OSCC cohort as well as a high Notch1 mutation
rate in a Chinese OL cohort. These results revealed that the Notch1
pathway plays a pivotal role in early oral tumorigenesis,
particularly in the Chinese population. To further explore the role
of Notch1 in OL and its progression to OSCC, we examined Notch1
expression patterns in OL and analyzed relationships between the
expression patterns and clinicopathological parameters as well as
OSCC progression in a Chinese OL cohort.
Materials and methods
Patients and tissue samples
Seventy-eight tissue samples from Chinese patients
with pathologically confirmed OL between January 1996 and December
2010 were obtained from the Department of Pathology, School of
Stomatology, Shanghai Jiao Tong University. Nineteen of the 78 OL
patients later developed OSCC. Tumor tissues from the 19 patients
were also available for the study. The diagnosis of all the samples
was verified by examining a hematoxylin and eosin section from each
tissue block used in the study. Clinicopathological parameters and
the follow-up information were obtained through chart review.
Informed consent was received from each enrolled patient, and the
research was carried out with the approval from the Ethics
Committee of Shanghai Jiao Tong University (Shanghai, China).
Cell line and reagents
The WSU-HN4 cell line previously described (27) was cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco-BRL, Grand Island, NY, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Gibco-BRL) at 37°C in a humidified 5% CO2 atmosphere.
For the EDTA (Sigma-Aldrich; Merck KGaA, Taufkirchen, Bayern,
Germany) activation assay, WSU-HN4 cells were washed twice in
phosphate-buffered saline (PBS) and incubated in PBS, 2.5 mM EDTA
or 2.5 mM CaCl2 (Sigma-Aldrich; Merck KGaA) for 15 min
at 37°C. The cells were then recovered in DMEM for 4 h and
subjected to western blot analysis or immunofluorescence
staining.
Western blot analysis and
immunofluorescence staining
Extracted proteins from cells were assessed using
the BCA protein assay reagent kit (Pierce; Thermo Fisher Scientific
Inc., Waltham, MA, USA). Protein samples were resolved by 10%
SDS-PAGE and immunoblotted with rabbit anti-Notch1 XP™ (1:1,000
dilution; clone D1E11; cat. no. 3608; Cell Signaling Technology,
Inc., Danvers, MA, USA) and appropriate HRP-conjugated antibodies
anti-rabbit and anti-mouse IgG HRP-linked secondary antibodies
(1:2,000 dilution; cat. no. 7074; cat. no. 7076; Cell Signaling
Technology, Inc). Mouse anti-β-actin (β-actin; clone AC-74;
Sigma-Aldrich; Merck KGaA) was used to normalize protein loading.
For immunofluorescence staining, the cells were washed twice in PBS
and fixed with 10% formalin in PBS for 10 min. The cells were then
treated with 0.25% Triton X-100 (Sigma-Aldrich; Merck KGaA) for 15
min and were blocked with 2.5% normal goat serum in PBS for 40 min.
The first antibody (1:500 dilution) was the same as that used
previously for the western blotting. The second antibody was goat
fluorescein-conjugated anti-rabbit IgG (1:500; DI-1488; Vector
Laboratories, Inc., Burlingame, CA, USA). Slides were mounted with
Vectashield containing DAPI (Vector Laboratories, Inc.).
Immunohistochemistry
Briefly, 4-µm formalin-fixed, paraffin-embedded
tissue sections were de-paraffinized and rehydrated. Heat-mediated
antigen retrieval using 0.01 M sodium citrate buffer (pH 6.0) was
performed and endogenous peroxidase was quenched with 3% hydrogen
peroxide for 20 min at room temperature. After incubation with 5%
normal goat serum to reduce nonspecific binding, the sections were
incubated with rabbit anti-Notch1 (D1E11) XP™ (1:500 dilution;
clone D1E11; cat. no. 3608; Cell Signaling Technology, Inc.)
according to the manufacturer's protocol.
Notch1 immunoreactivity in both animal and human
samples was semi-quantitatively evaluated according to staining
intensity and distribution using the immunoreactive score. The
grading for the percentage of positive cells was as follows: 0,
negative; 1, <10%; 2, 11–50%; 3, 51–80%; and 4, >80% positive
cells. For tinctorial strength, scores were distributed as: no
staining, 0 points; light yellow, 1 point; brownish yellow, 2
points; dark brownish yellow, 3 points. Finally, the result was
classified into four grades by multiplying the two scores
aforementioned: 0 points, negative (−); 1–4 points, weakly positive
(+); 5–8 points, positive (++); 9–12 points, strongly positive
(+++). Scores for >5 points were regarded as positive (28,29).
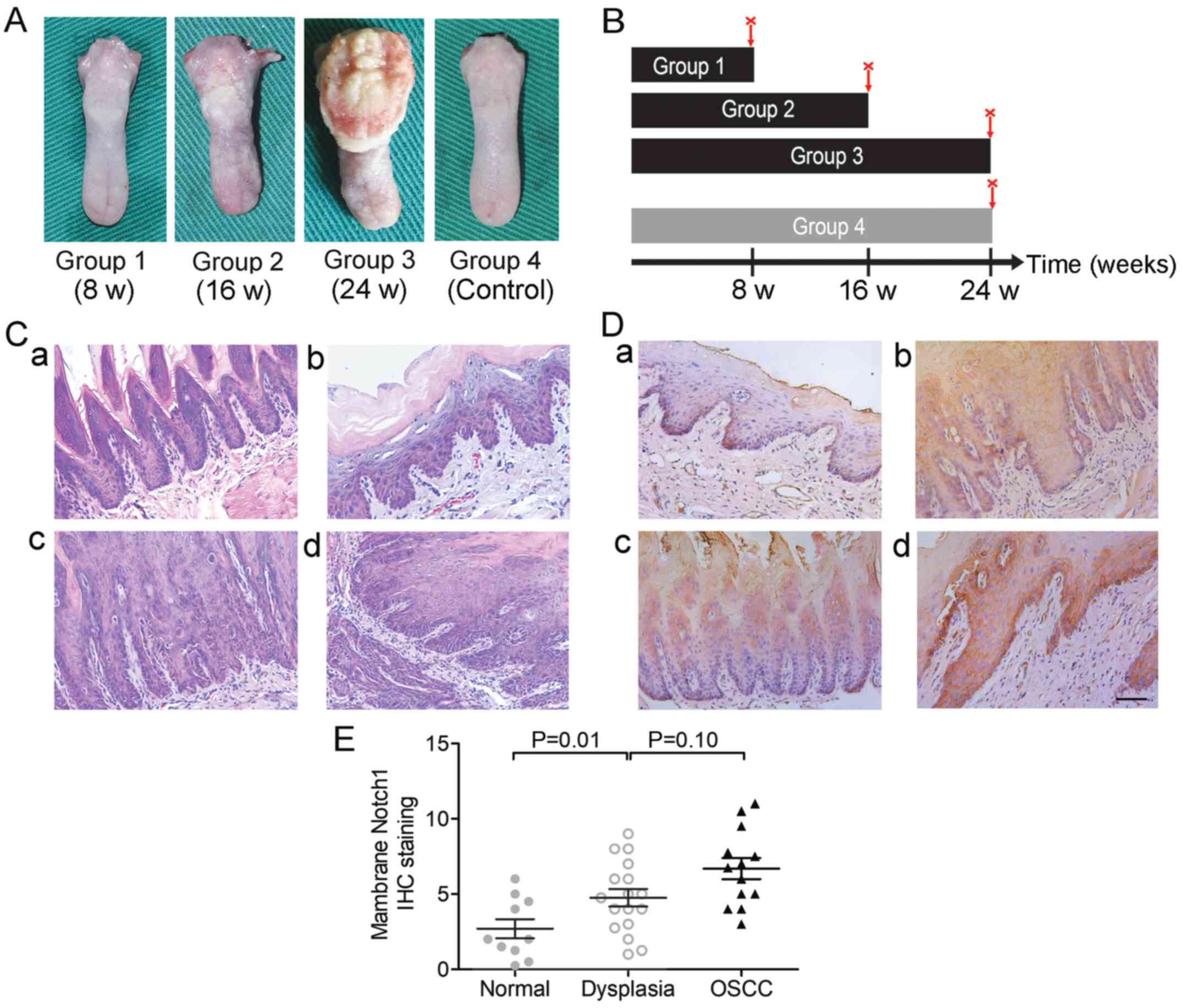
Animal model and treatments
A total of 48-week-old male SD rats were purchased
from the Nanjing Medical University Animal Research Center. The
animal experiments were approved by the Animal Ethics Committee and
all the procedures were performed following the institutional
animal welfare guidelines. After a week of acclimation, the rats
were separated into three experimental groups (Groups 1, 2 and 3,
n=10 for each group) and one group for control (Group 4, n=10)
randomly in separate cages. The rats in the experimental groups
were treated with 40 µg/ml 4-NQO (Sigma-Aldrich; Merck KGaA) in the
drinking water, while the rats in the control group were treated
with an equivalent volume of propylene glycol in water (30,31).
The treatment lasted for 8 weeks (Group 1), 16 weeks (Group 2) or
24 weeks (Groups 3 and 4). Then, the rats were euthanized, and the
tongues were resected and immediately fixed in 5% paraformaldehyde
solution for 12–24 h and paraffin-embedded for further
analysis.
Statistical analysis
Statistical analysis was performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). The Chi-square or Fisher's exact
tests were used to determine the relationship between the
expression of Notch1 and clinicopathological parameters. OSCC-free
survival (OFS) was determined as the outcome variable. The
association between Notch1 expression and OFS was estimated using
the log-rank test. All the variables were subjected to a univariate
and multivariate analysis using Cox proportional hazards regression
model. The hazard ratios (HRs) with their corresponding 95%
confidence intervals (CIs) and P-values were reported. All the
tests were two sided and a P-value <0.05 was considered as
statistically significant.
Results
Clinicopathological characteristics of
patients and ascertainment of Notch1 expression
Of the 78 patients with OL, 33 (42%) were male and
45 (58%) were female with age ranging from 27 to 85 years old
(mean, 56 years old). Forty-nine (63%) of the OL lesions were
located on the tongue and 29 (37%) at other anatomic locations in
the oral cavity. During the median 74.18 months follow-up period,
19 of the 78 (24%) patients developed OSCC. The clinicopathological
characteristics are listed in Table
I. No statistically significant difference was observed in age,
sex, OL location, smoking and alcohol consumption statuses between
those who developed OSCC and those who did not, except for a trend
towards more OL with severe dysplasia in the group that developed
OSCC (P=0.063, Chi-square test, Table
I).
 | Table I.Correlation between
clinicopathological features and malignant transformation
(OSCC). |
Table I.
Correlation between
clinicopathological features and malignant transformation
(OSCC).
|
|
|
| MT | UT |
|
|---|
|
|
|
|
|
|
|
|---|
|
| No. | (%) | n | (%) | n | (%) | P-value |
|---|
| All patients | 78 | 100 | 19 | 24 | 59 | 76 |
|
| Age (years) |
|
|
|
|
|
|
|
|
≥60 | 24 | 31 | 7 | 9 | 17 | 22 | 0.51 |
|
<60 | 54 | 69 | 12 | 15 | 42 | 54 |
|
| Sex |
|
|
|
|
|
|
|
|
Male | 33 | 42 | 5 | 6 | 28 | 36 | 0.105 |
|
Female | 45 | 58 | 14 | 18 | 31 | 40 |
|
| Grade of
dysplasia |
|
|
|
|
|
|
|
|
Mild-moderate | 59 | 76 | 11 | 14 | 48 | 62 | 0.063 |
|
Severe | 19 | 24 | 8 | 10 | 11 | 14 |
|
| Lesion site |
|
|
|
|
|
|
|
|
Non-tongue | 49 | 63 | 10 | 13 | 39 | 50 | 0.297 |
|
Tongue | 29 | 37 | 9 | 11 | 20 | 26 |
|
| Smoking |
|
|
|
|
|
|
|
|
Never | 57 | 73 | 16 | 21 | 41 | 53 | 0.327 |
| Past
and present | 15 | 19 | 2 | 3 | 13 | 17 |
|
|
Unknown | 6 | 8 | 1 | 1 | 5 | 6 |
|
| Alcohol intake |
|
|
|
|
|
|
|
|
Never | 58 | 74 | 15 | 19 | 43 | 55 | 0.748 |
| Past
and present | 15 | 19 | 3 | 4 | 12 | 15 |
|
|
Unknown | 5 | 6 | 1 | 1 | 4 | 5 |
|
| Notch1
expression |
|
|
|
|
|
|
|
|
Strong | 10 | 13 | 4 | 5 | 6 | 8 | 0.246 |
| Weak to
moderate | 68 | 87 | 15 | 19 | 53 | 68 |
|
| MN |
|
|
|
|
|
|
|
|
Positive | 24 | 31 | 11 | 14 | 13 | 17 | 0.003 |
|
Negative | 54 | 69 | 8 | 10 | 46 | 59 |
|
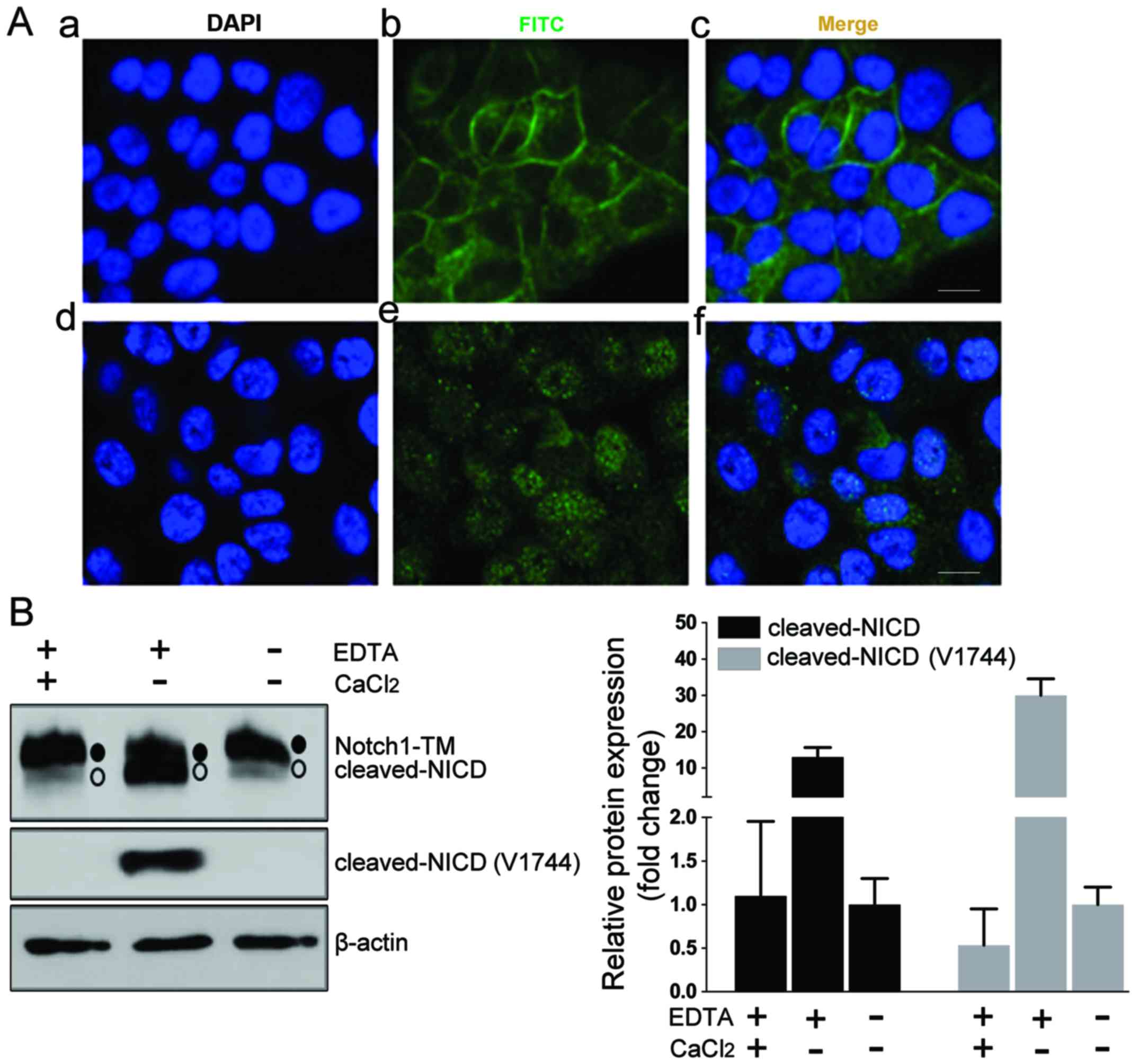
To ensure the quality of the Notch1 detection
method, we first assessed the anti-Notch1 antibody by
immunofluorescence in HN4 cells (Fig.
1A-a-c). Then, we artificially induced the dissociation of the
heterodimer by treating the cells with EDTA to activate Notch1
cleavage. After treatment with 2.5 mM EDTA, the immunofluorescence
staining for Notch1 was enriched in the nucleus of HN4 cells
(Fig. 1A-d-f). Notch1 cleaved at S2
and S3 sites could be detected by a Notch1-specific antibody (clone
D1E11) or Notch1 cleaved at S3 could be directly detected by Notch1
Val1744-specific antibody (clone D3B8). Western blot assays enabled
the detection of Notch1 as the expected transmembranous form (~120
kDa) in the HN4 cells (Fig. 1B). A
smaller size band expected to be cleaved-NICD was detected after
EDTA treatment, but not for the cells treated with
CaCl2, which neutralized the function of EDTA (Fig. 1B). These data ascertained the
usability of this Notch1 detection method.
Notch1 expression in OL and the
corresponding OSCC specimens
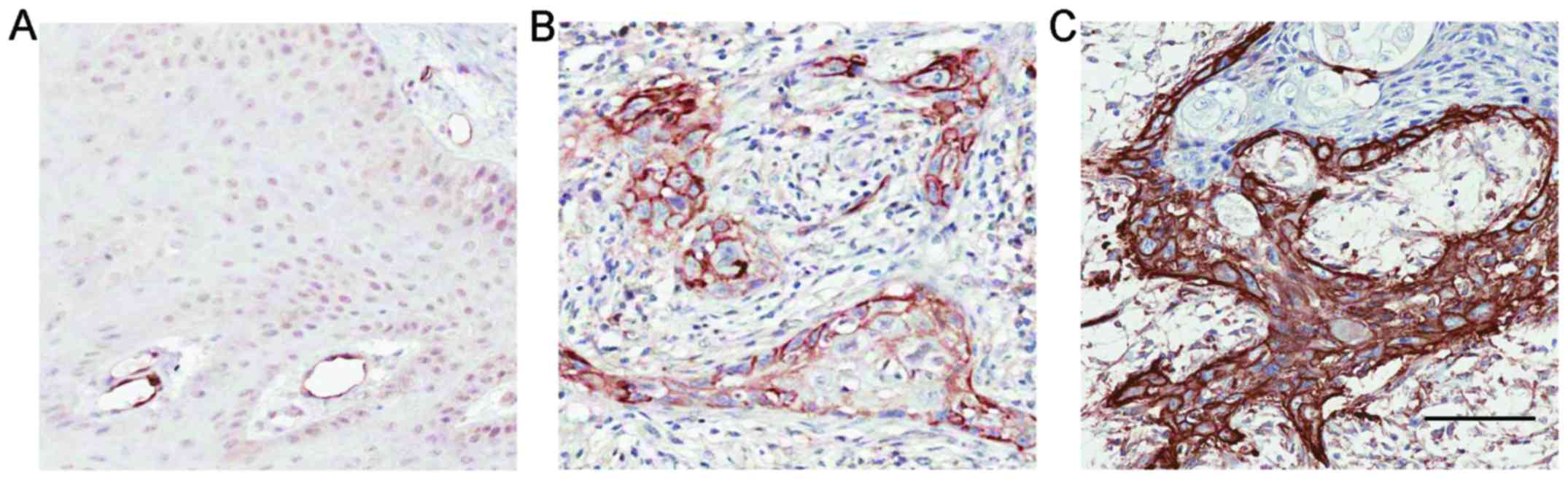
By performing IHC staining, we assessed the
expression of Notch1 in 78 OL specimens and 19 corresponding OSCC
specimens from patients who progressed from OL to OSCC. The
weak/moderate/strong levels of Notch1 staining were demonstrated in
Fig. 2A-C. In OL, Notch1 was
generally expressed in a diffused manner in the suprabasal layer of
the epithelium and mostly confined to the nucleus and cytoplasm
with variable intensities (Fig.
3A-C). Strong overall expression of Notch1 was observed in 10
(13%) cases (Table I). Notably, in
24 (31%) of the OL samples, membranous staining of Notch1 was
observed (Fig. 3D and E, Table I). We noted that lesions with severe
dysplasia (Fig. 3D) tended to have
stronger membrane staining and cytoplasmic staining of Notch1 than
in the lesions with low-to-medium grade dysplasia (Fig. 3E). This membrane staining revealed
variable intensity in the spinous layer, granular layer and
throughout the whole epithelium compared with the more strictly
suprabasal layer expression of Notch1 in the other OL samples.
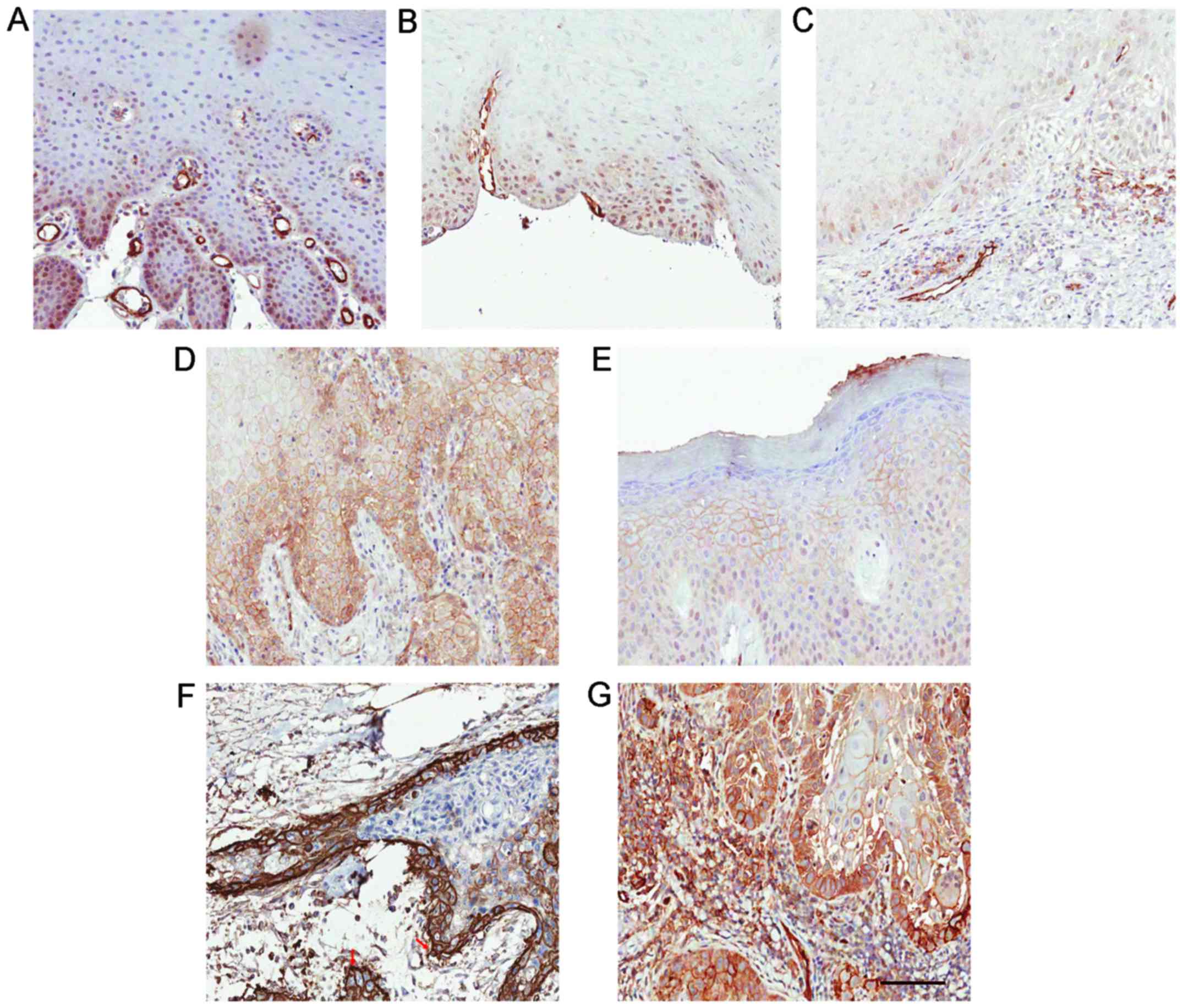
In the 19 malignant-transformed OL (or OSCC) samples
derived from OL cases, 11 (14%) exhibited positive membranous
Notch1 expression (Table I). To be
specific, in the 19 cases, the lesions in Fig. 3D and E turned into the malignancies
in Fig. 3F and G, respectively
during the follow-up studies. Moreover, Notch1 expression was more
diffused on the membrane and in the cytoplasm than in the nucleus
in these OSCC samples. Strong membranous Notch1 expression was
often observed at the invasive front or at the border of cancer
nests. Moreover, a significant decreased nuclear Notch1 expression
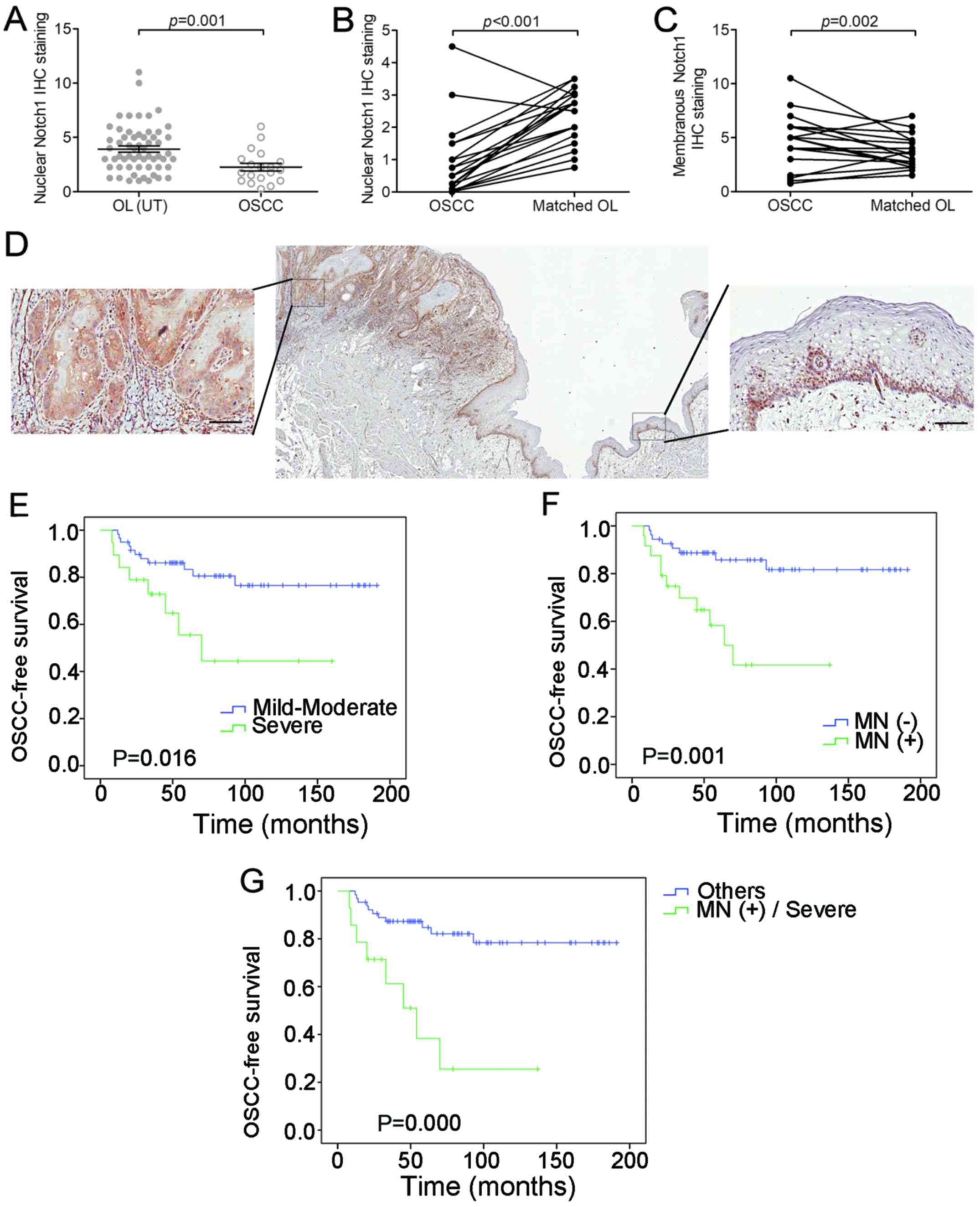
was detected in malignant-transformed (MT) OL samples, compared to
the untransformed (UT) OL samples (P=0.001, t-test; Fig. 4A). Meanwhile, a decrease of unclear
Notch1 expression (P<0.001, grouped t-test) and an increase of
membranous Notch1 expression (P=0.002, grouped t-test) were
observed in the 19 OSCC samples compared to their matched OL
samples (Fig. 4B and C). Even
within the same OSCC sample, we observed a decrease in nuclear
Notch1 and an increase in membranous Notch1 expression in cancer
nests compared to the adjacent non-cancerous epithelium (Fig. 4D).
Membranous Notch1 expression in OL is
associated with poor prognosis
The relationship between the membranous Notch1
expression in OL and clinicopathological parameters was also
analyzed. A statistically significant association was observed
between membranous Notch1 expression and a more severe dysplastic
status (P<0.001, Chi-square test, Table II). For further analysis, we
determined the role of membranous Notch1 expression in the
malignant transformation of OL. As expected, the grade of dysplasia
and expression levels of membranous Notch1 were associated with
OSCC-free survival (P=0.016 and 0.001, respectively, log-rank test,
Fig. 4E and F). After 5 years, only
7 (13%) of the 54 patients without membranous Notch1 expression
developed OSCC, whereas 9 (38%) of the 24 patients with membranous
Notch1 expression developed OSCC. When combined with grade of
dysplasia, the patients with membranous Notch1 expression and
severe dysplasia exhibited even shorter OSCC-free survival than the
other patients (P=0.000, log-rank test, Fig. 4G). As determined by univariate
analysis, both the expression level of membranous Notch1 and the
grade of dysplasia were associated with OSCC development (P=0.002
and P=0.022, respectively, Table
III). By multivariate analysis, however, only membrane Notch1
expression was an independent factor that was significantly
associated with OSCC development (P=0.019, Table III).
 | Table II.MN with clinicopathological
features. |
Table II.
MN with clinicopathological
features.
|
|
|
| MN (+) | MN (−) |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | No. of
patients | (%) | n | (%) | n | (%) | P-value |
|---|
| All patients | 78 | 100 | 24 | 31 | 54 | 69 |
|
| Age group
(years) |
|
|
|
|
|
| 0.462 |
|
<60 | 54 | 69 | 18 | 23 | 36 | 46 |
|
|
≥60 | 24 | 31 | 6 | 8 | 18 | 23 |
|
| Sex |
|
|
|
|
|
| 0.567 |
|
Female | 45 | 58 | 15 | 19 | 30 | 38 |
|
|
Male | 33 | 42 | 9 | 12 | 24 | 31 |
|
| Grade of
dysplasia |
|
|
|
|
|
|
<0.001 |
|
Mild-moderate | 59 | 76 | 11 | 14 | 48 | 61 |
|
|
Severe | 19 | 24 | 13 | 17 | 6 | 8 |
|
| Anatomic site |
|
|
|
|
|
| 0.585 |
|
Low-risk areas | 29 | 37 | 10 | 13 | 19 | 24 |
|
|
High-risk areas | 49 | 63 | 14 | 18 | 35 | 45 |
|
| Smoking |
|
|
|
|
|
| 0.95 |
|
Yes | 15 | 19 | 4 | 5 | 11 | 14 |
|
| No | 57 | 73 | 18 | 23 | 39 | 50 |
|
|
Unknown | 6 | 8 | 2 | 3 | 4 | 5 |
|
| Alcohol
drinking |
|
|
|
|
|
| 0.76 |
|
Yes | 15 | 19 | 5 | 6 | 10 | 12 |
|
| No | 58 | 75 | 17 | 22 | 41 | 53 |
|
|
Unknown | 5 | 6 | 2 | 3 | 3 | 4 |
|
 | Table III.Univariate and multivariate analysis
of clinicopathological features and MN with malignant
transformation of OL. |
Table III.
Univariate and multivariate analysis
of clinicopathological features and MN with malignant
transformation of OL.
| Univariate | P-value | Risk ratio | 95% CI |
|---|
| Age (years) | 0.369 | 1.016 | 0.982–1.051 |
| Sex | 0.062 | 2.665 | 0.952–7.462 |
| Alcohol intake | 0.74 | 0.871 | 0.384–1.973 |
| Smoking | 0.301 | 0.611 | 0.241–1.552 |
| Pathology
grade | 0.022 | 2.921 | 1.168–7.302 |
| MN | 0.002 | 4.217 | 1.673–10.671 |
| Multivariate |
|
|
|
| Grade of
dysplasia | 0.317 | 1.68 | 0.608–4.643 |
| MN | 0.019 | 3.417 | 1.225–9.529 |
Notch1 expression patterns in a murine
OL/OSCC model
An OL/OSCC model was successfully established in
Sprague-Dawley rats by 4-nitroquinoline-1-oxide (4-NQO; Fig. 5A) induction as aforementioned to
study the expression of Notch1 in different stages of
carcinogenesis. Administration of 4-NQO was performed as outlined
in the general scheme in Fig. 5B.
We found that the experimental groups (Groups 1–3) treated with
4-NQO developed different pathological changes, including mild
dysplasia (Fig. 5C-b),
moderate-to-severe dysplasia (Fig.
5C-c) and OSCC (including in situ carcinoma and invasive
carcinoma; Fig. 5C-d) with the
increase in the duration of 4-NQO administration, and no visible
lesions were detected in the control group (Group 4, Fig. 5C-a). The pathological lesions of
samples were confirmed by two trained pathologists using H&E
staining in a double-blind fashion (Table IV). The rates of positive
expression of Notch1 in normal mucosa, dysplasia and carcinoma were
20% (2/10), 64.7% (11/17) and 84.6% (11/13), respectively
(P<0.01, Chi-square test, Table
V). Representative Notch1 IHC images are shown in Fig. 5D. We found that Notch1 was mainly
localized in the membrane and cytoplasm in this murine model.
Notch1 staining was negative in the normal tongue mucosa or was
mainly distributed in the stratum basale (Fig. 5D-a), whereas it extended from the
stratum basale to the stratum corneum during the
progression of cancer (Fig.
5D-a-d). The IHC scores of membranous Notch1 expression were
also increased during carcinogenesis (Fig. 5E).
 | Table IV.Pathological classification of tongue
tissues at different administration times. |
Table IV.
Pathological classification of tongue
tissues at different administration times.
|
|
| Pathological
lesions |
|---|
|
|
|
|
|---|
| Groups | No. | Normal
epithelial | Mild epithelial
dysplasia | Moderate-severe
epithelial dysplasia | In situ
carcinoma | Invasive
carcinoma |
|---|
| 1 (8 weeks) | 10 | 0 | 7 | 3 | 0 | 0 |
| 2 (16 weeks) | 10 | 0 | 2 | 4 | 3 | 1 |
| 3 (24 weeks) | 10 | 0 | 0 | 1 | 6 | 3 |
| 4 (Control) | 10 | 10 | 0 | 0 | 0 | 0 |
| Total | 40 | 10 | 9 | 8 | 9 | 4 |
 | Table V.Expression of Notch1 in normal mucosa
and different stages of carcinogenesis. |
Table V.
Expression of Notch1 in normal mucosa
and different stages of carcinogenesis.
| Pathological
lesions | No. | Negative | Positive |
|---|
| Normal | 10 | 8 | 2 |
| Dysplasia | 17 | 6 | 11 |
| OSCC | 13 | 2 | 11 |
Discussion
Notch1 signaling has been studied in various types
of malignancies. Although Notch1 signaling has been demonstrated to
play a significant oncogenic role in T-ALL (32), its role in several solid tumors,
including OSCC and HNSCC, remains controversial even within the
same tumor type (32,33). For example, both increased (18,34–37)
and decreased Notch1 expression or Notch1 signaling has been
discovered in OSCC and HNSCC samples, and improved (38) or worsened (37) survival has been revealed in HNSCC.
The reason behind these discrepancies may be due to the cellular
context, since the Notch signaling pathway can play opposing roles
in malignancies, depending on the cellular and tissue context, as
well as the level of its expression and potential crosstalk with
other signaling pathways (39).
However, the detection methods for Notch1 have not
been convincing, as very few studies have validated the
Notch1-specific antibodies before using them, and this could have
also contributed to the discrepancies. In the present study, we
first validated the Notch1-specific antibody using several
protocols to ensure that it could detect both the membrane-bound
and nuclear Notch1. First, membranous, cytoplasmic and nuclear
Notch1 staining was detected using the Notch1 primary antibody by
immunofluorescence. Calcium depletion by EDTA from the medium
triggered a ligand-independent activation of Notch signaling and
nuclear Notch1 could be detected by the antibody using both western
blotting and immunofluorescence; meanwhile, treatment with
CaCl2 neutralized the function of EDTA and reversed the
effect.
Subsequently, we provided an extensive evaluation of
Notch1 expression in human OL and OSCC tissue samples. We
determined that in our OL samples, Notch1 was mainly localized in
the suprabasal layer of the epithelium, indicating its role in cell
differentiation. This result was consistent with previous studies
which demonstrated that in normal oral epithelium, Notch was mainly
expressed in the suprabasal layer (36,40).
Although a previous study (36)
mentioned that nuclear Notch1 could hardly be detected in normal
tissues, we found strong nuclear expression of Notch1 in some OL or
OSCC tissues, and the nuclear expression of Notch1 was decreased
during the progression from OL to OSCC. Moreover, within the same
OSCC sample, we noticed a decrease in nuclear Notch1 from the
peri-cancerous epithelium to the cancer nests, revealing that
canonical Notch signaling plays a tumor-suppressive role in normal
oral epithelium and OL. In fact, there have been studies on nuclear
Notch1 expression in variable tissues during development and in
cancer, although the exact role of nuclear Notch1 is debatable
(41–43).
We also found diffused membranous Notch1 expression
in 24 OL samples, which had not been studied in detail before. In
the present study, membranous Notch1 expression was significantly
associated with advanced dysplasia and malignant transformation.
Moreover, membranous Notch1 expression was significantly higher in
the OSCC samples than in the OL samples. Notably, the elevated
membranous expression in OSCC was mainly observed at the border of
cancer nests or at invasive fronts. According to the Cox
proportional hazards regression model, the presence of membranous
Notch1 was even more specific and independent than the pathological
grade for predicting malignant transformation in OL. In our murine
model induced by 4-NQO gavages, similar Notch1 expression patterns
were also detected. Notch1 staining extended from the stratum
basale to the stratum corneum during the development of
cancer. The rate of membranous Notch1 expression also increased
during carcinogenesis.
It should be noted that NOTCH1 mutation frequency
was as high as 60% in OL, and almost 60% of leukoplakia patients
with mutations were identified in OSCC, although these OL and OSCC
samples were not from the same patient (44). Moreover, these mutations were mostly
confined to the EGF-like domain, which accounts for ligand-receptor
binding. An impaired ligand-receptor binding resulting from a
mutation in this domain of Notch1 may theoretically cause a surplus
of Notch1 proteins and thus passively result in its accumulation on
the membrane. Therefore, it is important to determine whether the
~30% membranous Notch1 expression revealed in our research is a
reflection of some of the common mutations found both in OL and in
OSCC. It should be noted, however, that the study described by
Izumchenko et al (44) and
the present study were based on Chinese populations. Potential
impact of etiology and genetic background for the particular
population should be considered in the data interpretation. As
Notch1 has been suggested as a tumor suppressor in several studies
(23,45,46),
it is possible that membranous Notch1 accumulation is simply an
alternative mechanism to inactivate the gene function. However, a
recent study revealed that the Notch signaling pathway was active
in about one third of Caucasian patients with HNSCC (34), suggesting an oncogenic role of Notch
signaling in a significant proportion of patients with HNSCC in
both Chinese and Caucasian populations, although the mechanisms of
tumorigenesis could be different (47). Further studies are warranted to
determine how membranous Notch1 accumulation leads to oral
tumorigenesis and OSCC progression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81402236), the National
Natural Science Foundation of China (no. 81772887), the Priority
Academic Program Development of Jiangsu Higher Education
Institutions (PAPD, 2014-37), the Jiangsu Provincial Medical
Innovation Team (CXTDA2017036), the Natural Science Foundation of
Jiangsu Province of China (BK20171488) and the Jiangsu Provincial
Medical Youth Talent (QNRC2016854).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XD, YZ and YW conceived and designed the study. XD,
YZ, ZW, YD and WZ performed the experiments. YZ, ZY, WZ and XS
wrote the manuscript. WC, JL, WC, LM and WZ reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The research was carried out with the approval from
the Ethics Committee of Shanghai Jiao Tong University (Shanghai,
China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Lodi G, Sardella A, Bez C, Demarosi F and
Carrassi A: Interventions for treating oral leukoplakia. Cochrane
Database Syst Rev. 18:CD0018292006.
|
|
2
|
Silverman S Jr, Gorsky M and Lozada F:
Oral leukoplakia and malignant transformation. A follow-up study of
257 patients. Cancer. 53:563–568. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van der Waal I: Potentially malignant
disorders of the oral and oropharyngeal mucosa; terminology,
classification and present concepts of management. Oral Oncol.
45:317–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papadimitrakopoulou VA, Hong WK, Lee JS,
Martin JW, Lee JJ, Batsakis JG and Lippman SM: Low-dose
isotretinoin versus beta-carotene to prevent oral carcinogenesis:
Long-term follow-up. J Natl Cancer Inst. 89:257–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schepman KP, van der Meij EH, Smeele LE
and van der Waal I: Malignant transformation of oral leukoplakia: A
follow-up study of a hospital-based population of 166 patients with
oral leukoplakia from The Netherlands. Oral Oncol. 34:270–275.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan P, Temam S, El-Naggar A, Zhou X, Liu
DD, Lee JJ and Mao L: Overexpression of podoplanin in oral cancer
and its association with poor clinical outcome. Cancer.
107:563–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawaguchi H, El-Naggar AK,
Papadimitrakopoulou V, Ren H, Fan YH, Feng L, Lee JJ, Kim E, Hong
WK, Lippman SM and Mao L: Podoplanin: A novel marker for oral
cancer risk in patients with oral premalignancy. J Clin Oncol.
26:354–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mitra D, Fernandez P, Bian L, Song N, Li
F, Han G and Wang XJ: Smad4 loss in mouse keratinocytes leads to
increased susceptibility to UV carcinogenesis with reduced
ercc1-mediated DNA repair. J Invest Dermatol. 133:2609–2616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia RH, Song XM, Wang XJ, Li J and Mao L:
The combination of SMAD4 expression and histological grade of
dysplasia is a better predictor for the malignant transformation of
oral leukoplakia. PLoS One. 8:e667942013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guruharsha KG, Kankel MW and
Artavanis-Tsakonas S: The Notch signalling system: Recent insights
into the complexity of a conserved pathway. Nat Rev Genet.
13:654–666. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Y, Wang Z, Ding X, Dong Y, Zhang W,
Zhang W, Zhong Y, Gu W, Wu Y and Song X: Combined Erlotinib and
PF-03084014 treatment contributes to synthetic lethality in head
and neck squamous cell carcinoma. Cell Prolif. Dec 12–2017.(Epub
ahead of print). View Article : Google Scholar
|
|
13
|
Ranganathan P, Weaver KL and Capobianco
AJ: Notch signalling in solid tumours: A little bit of everything
but not all the time. Nat Rev Cancer. 11:338–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patturajan M, Nomoto S, Sommer M, Fomenkov
A, Hibi K, Zangen R, Poliak N, Califano J, Trink B and Ratovitski
E: DeltaNp63 induces beta-catenin nuclear accumulation and
signaling. Cancer Cell. 1:369–379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sriuranpong V, Borges MW, Ravi RK, Arnold
DR, Nelkin BD, Baylin SB and Ball DW: Notch signaling induces cell
cycle arrest in small cell lung cancer cells. Cancer Res.
61:3200–3205. 2001.PubMed/NCBI
|
|
16
|
Yugawa T, Handa K, Narisawa-Saito M, Ohno
S, Fujita M and Kiyono T: Regulation of Notch1 gene expression by
p53 in epithelial cells. Mol Cell Biol. 27:3732–3742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Panelos J, Tarantini F, Paglierani M, Di
Serio C, Maio V, Pellerito S, Pimpinelli N, Santucci M and Massi D:
Photoexposition discriminates Notch 1 expression in human cutaneous
squamous cell carcinoma. Mod Pathol. 21:316–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang TH, Liu HC, Zhu LJ, Chu M, Liang YJ,
Liang LZ and Liao GQ: Activation of Notch signaling in human tongue
carcinoma. J Oral Pathol Med. 40:37–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao J, Duan L, Fan M and Wu X: Gamma
secretase inhibitors exerts antitumor activity via down-regulation
of Notch and Nuclear factor kappa B in human tongue carcinoma
cells. Oral Dis. 13:555–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin JT, Chen MK, Yeh KT, Chang CS, Chang
TH, Lin CY, Wu YC, Su BW, Lee KD and Chang PJ: Association of high
levels of Jagged-1 and Notch-1 expression with poor prognosis in
head and neck cancer. Ann Surg Oncol. 17:2976–2983. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hijioka H, Setoguchi T, Miyawaki A, Gao H,
Ishida T, Komiya S and Nakamura N: Upregulation of Notch pathway
molecules in oral squamous cell carcinoma. Int J Oncol. 36:817–822.
2010.PubMed/NCBI
|
|
22
|
Duan L, Yao J, Wu X and Fan M: Growth
suppression induced by Notch1 activation involves Wnt-beta-catenin
down-regulation in human tongue carcinoma cells. Biol Cell.
98:479–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, et al: The mutational landscape of head and neck
squamous cell carcinoma. Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agrawal N, Frederick MJ, Pickering CR,
Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, et
al: Exome sequencing of head and neck squamous cell carcinoma
reveals inactivating mutations in Notch1. Science. 333:1154–1157.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song X, Xia R, Li J, Long Z, Ren H, Chen W
and Mao L: Common and complex Notch1 mutations in chinese
oral squamous cell carcinoma. Clin Cancer Res. 20:701–710. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Izumchenko E, Sun K, Jones S, Brait M,
Agrawal N, Koch WM, McCord C, Riley D, Angiuoli SV, Velculescu VE,
et al: Notch1 mutations are drivers of oral tumorigenesis. Cancer
Prev Res. 8:277–286. 2014. View Article : Google Scholar
|
|
27
|
Cao W, Feng Z, Cui Z, Zhang C, Sun Z, Mao
L and Chen W: Up-regulation of enhancer of zeste homolog 2 is
associated positively with cyclin D1 overexpression and poor
clinical outcome in head and neck squamous cell carcinoma. Cancer.
118:2858–2871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu W, Liang CG, Li YF, Ji YH, Qiu WJ and
Tang XZ: Involvement of Notch1/Hes signaling pathway in ankylosing
spondylitis. Int J Clin Exp Pathol. 8:2737–2745. 2015.PubMed/NCBI
|
|
29
|
Lakhani SR, Van De Vijver MJ, Jacquemier
J, Anderson TJ, Osin PP, Mcguffog L and Easton DF: The pathology of
familial breast cancer: Predictive value of immunohistochemical
markers estrogen receptor, progesterone receptor, HER-2, and p53 in
patients with mutations in BRCA1 and BRCA2. J Clin
Oncol. 20:2310–2328. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hasina R, Martin LE, Kasza K, Jones CL,
Jalil A and Lingen MW: ABT-510 is an effective chemopreventive
agent in the mouse 4-nitroquinoline 1-oxide model of oral
carcinogenesis. Cancer Prev Res. 2:385–393. 2009. View Article : Google Scholar
|
|
31
|
Chang NW, Pei RJ, Tseng HC, Yeh KT, Chan
HC, Lee MR, Lin C, Hsieh WT, Kao MC, Tsai MH and Lin CF:
Co-treating with arecoline and 4-nitroquinoline 1-oxide to
establish a mouse model mimicking oral tumorigenesis. Chem Biol
Int. 183:231–237. 2010. View Article : Google Scholar
|
|
32
|
South AP, Cho RJ and Aster JC: The
double-edged sword of Notch signaling in cancer. Semin Cell Dev
Biol. 23:458–464. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lefort K, Ostano P, Mello-Grand M, Calpini
V, Scatolini M, Farsetti A, Dotto GP and Chiorino G: Dual tumor
suppressing and promoting function of Notch1 signaling in human
prostate cancer. Oncotarget. 7:48011–48026. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun W, Gaykalova DA, Ochs MF, Mambo E,
Arnaoutakis D, Liu Y, Loyo M, Agrawal N, Howard J, Li R, et al:
Activation of the Notch pathway in head and neck cancer. Cancer
Rese. 74:1091–1104. 2014. View Article : Google Scholar
|
|
35
|
Upadhyay P, Nair S, Kaur E, Aich J, Dani
P, Sethunath V, Gardi N, Chandrani P, Godbole M, Sonawane K, et al:
Notch pathway activation is essential for maintenance of stem-like
cells in early tongue cancer. Oncotarget. 7:50437–504449. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoshida R, Nagata M, Nakayama H,
Niimori-Kita K, Hassan W, Tanaka T, Shinohara M and Ito T: The
pathological significance of Notch1 in oral squamous cell
carcinoma. Lab Invest. 93:1068–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SH, Do SI, Lee HJ, Kang HJ, Koo BS and
Lim YC: Notch1 signaling contributes to stemness in head and neck
squamous cell carcinoma. Lab Invest. 96:508–516. 2016.(In English).
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaka AS, Nowacki NB, Kumar B, Zhao S, Old
MO, Agrawal A, Ozer E, Carrau RL, Schuller DE, Kumar P and Teknos
TN: Notch1 overexpression correlates to improved survival in cancer
of the oropharynx. Otolaryngol Head Neck Surg. 156:652–659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sakamoto K, Fujii T, Kawachi H, Miki Y,
Omura K, Morita K, Kayamori K, Katsube K and Yamaguchi A: Reduction
of Notch1 expression pertains to maturation abnormalities of
keratinocytes in squamous neoplasms. Lab Invest. 92:688–702. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zagouras P, Stifani S, Blaumueller CM,
Carcangiu ML and Artavanis-Tsakonas S: Alterations in Notch
signaling in neoplastic lesions of the human cervix. Proc Natl Acad
Sci USA. 92:6414–6418. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmad I, Zaqouras P and Artavanis-Tsakonas
S: Involvement of Notch-1 in mammalian retinal neurogenesis:
Association of Notch-1 activity with both immature and terminally
differentiated cells. Mech Dev. 53:73–85. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Izumchenko E, Sun K, Jones S, Brait M,
Agrawal N, Koch W, McCord CL, Riley DR, Angiuoli SV, Velculescu VE,
et al: Notch1 mutations are drivers of oral tumorigenesis. Cancer
Prev Res. 8:277–286. 2015. View Article : Google Scholar
|
|
45
|
Zweidler-Mckay PA, He Y, Xu L, Rodriguez
CG, Karnell FG, Carpenter AC, Aster JC, Allman D and Pear WS: Notch
signaling is a potent inducer of growth arrest and apoptosis in a
wide range of B-cell malignancies. Blood. 106:3898–3906. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nicolas M, Wolfer A, Raj K, Kummer JA,
Mill P, van Noort M, Hui CC, Clevers H, Dotto GP and Radtke F:
Notch1 functions as a tumor suppressor in mouse skin. Nat Genet.
33:416–421. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng Y, Wang Z, Ding X, Zhang W, Li G,
Liu L, Wu H, Gu W, Wu Y and Song X: A novel Notch1 missense
mutation (C1133Y) in the Abruptex domain exhibits enhanced
proliferation and invasion in oral squamous cell carcinoma. Cancer
Cell Int. 18:62018. View Article : Google Scholar : PubMed/NCBI
|