Introduction
In the clinic, lung adenocarcinoma has the highest
mortality rate of all cancers due to its tendency to metastasize at
an early stage (1,2). Preventing metastasis is crucial for
improving the survival rate of these patients. During cancer
metastasis, angiogenesis and epithelial-mesenchymal transition
(EMT) are crucial events (3,4). Eph
receptors have been reported to be involved in many biological
processes, including angiogenesis and cell migration (5). In gastric cancer cells, EphA2 was
reported to promote EMT through the Wnt-β-catenin pathway (6). Recent genome-wide analyses revealed
that EphA2 is commonly overexpressed in non-small cell lung cancer
(NSCLC), which is correlated with poor prognosis (7–10).
Through binding to its ligand ephrinA1, EphA2 phosphorylates
multiple tyrosine residues, leading to activation of itself and its
downstream molecules. In certain carcinoma cells, Src and focal
adhesion kinase (FAK) were reported to be the downstream regulators
of the ephrinA1/EphA2 pathway, instructing cells to move and
facilitating tissue invasion of ephrin-sensitive carcinomas
(11). In addition, through
interacting with EGFR and HER2, Src also transduces survival
signals to downstream effectors, such as phosphoinositide 3-kinases
(PI3Ks), Akt and signal transducer and activator of transcription 3
(STAT3) (12).
Our previous study reported that Ophiopogonin B
(OP-B) inhibited the PI3K/Akt pathway in the NCI-H157, NCI-H460 and
A549 cell lines. However, the sensitivity of these cell lines to
OP-B differed greatly (13,14). Whether EphA2 is the key molecular
affecting the sensitivity of different cell lines to OP-B is still
unknown.
In the present study, we found that OP-B regulated
EMT in only A549 cells. Further detection of EphA2 in A549 and
NCI-H460 cells showed that OP-B inhibited the phosphorylation of
EphA2 in A549 cells but not in NCI-H460 cells.
Further experiments were carried out to verify the
effect of OP-B on A549 cell metastasis in vitro and in
vivo; meanwhile, the effects of OP-B on EphA2- and EMT-related
molecules such as E-cadherin, ZO-1, N-cadherin, vimentin, Snail,
Slug and Zeb1 were also detected to uncover their role in the
process of OP-B-regulated metastasis in A549 cells.
The present study may provide useful information for
the clinical application of Radix Ophiopogon Japonicus in NSCLC
treatment.
Materials and methods
Reagents
Ophiopogonin B (OP-B) (MW: 722.9, HPLC ≥98%) was
purchased from Nanjing Zelang Medical Technology Co. (Nanjing,
China). The compound was initially dissolved in dimethyl sulfoxide
(DMSO) (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as a stock
solution. For treatment of cells, OP-B was diluted in culture
medium to the appropriate concentrations, and the final
concentration of DMSO was <0.01%.
The primary antibodies against E-cadherin (cat.no.
sc-3195), ZO-1 (cat. no. sc-8193), vimentin (cat. no. sc-5741),
N-cadherin (cat. no. sc-13116), Snail (cat. no. sc-3879), Slug
(cat. no. sc-9585), TCF8/ZEB1 (cat. no. sc-3396), p-SRC (Tyr416)
(cat. no. sc-6943), p-FAK (Tyr397) (cat. no. sc-8556), p-Stat3
(Tyr705) (cat. no. sc-9145), Stat3 (cat. no. sc-12640), p-Stat5
(Tyr694) (cat. no. sc-9351), Stat5 (cat. no. sc-9363), VEGFR2 (cat.
no. sc-9698), Tie-2 (cat. no. sc-4224), p-Akt (Ser473) (cat. no.
sc-4060), p-PLCγ1 (Ser1248) (cat. no. sc-8713), EphA2 (cat. no.
sc-6997) and p-EphA2 (Ser897) (cat. no. sc-6347) were purchased
from Cell Signaling Technology (Danvers, USA). The secondary
antibody was also from Cell Signaling Technology.
Cell culture
The NSCLC cell lines A549, NCI-H460 and NCI-H596
were obtained from the Institute of Biochemistry and Cell Biology
(Shanghai, China). Cells were grown in Gibco® RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS) and 100 U/ml
penicillin-streptomycin mixed antibiotics and were cultured under
5% CO2 at 37°C. Total RNA was immediately extracted from
the cell lines treated with or without OP-B for 2 h.
Digital gene expression (DGE) library
preparation and sequencing
Total RNA was extracted using the Illumina Gene
Expression Sample Prep Kit (Illumina, Inc., San Diego, CA, USA)
according to the manufacturer's protocol. Quality and quantity
analyses of total RNA, DGE library preparation, and sequencing were
carried out at Huada Genomics Co., Ltd. (Shenzhen, China). We used
a false discovery rate (FDR) ≤0.05 and the absolute value of log2
ratio ≥1 as the thresholds to judge the significance of gene
expression differences (15).
Western blot analysis
After being treated with different concentrations of
OP-B, the cells were lysed in RIPA buffer. The protein
concentrations of the supernatants were determined by the BCA
protein assay. Equal amounts of protein (50 µg) were loaded onto
gels, separated by 12% SDS-PAGE and then transferred onto
nitrocellulose membranes (Millipore, Billerica, MA, USA). The
membranes were incubated with appropriate primary antibodies
(1:1,000) at 4°C overnight, followed by a 1-h incubation with
HRP-conjugated secondary antibodies (anti-rabbit or anti-mouse
immunoglobulin G, 1:2,000) at room temperature. Then, the protein
level was quantified by enhanced chemiluminescence (ECL; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and image acquisition was
performed by Image Lab™ Software (Bio-Rad Laboratories).
Cell migration assays
For the invasion assays, after treatment with
different concentrations (0, 2.5, 5 and 10 µmol/l) of OP-B,
5×104 A549 cells in serum-free media were placed into
the upper chamber of an insert (8-µm pore size; BD Matrigel
Invasion Chamber; BD Biosciences, Franklin Lakes, NJ, USA), and
medium containing 10% FBS was added to the lower chamber. After
incubation for 24 h, the cells that had migrated through the
membrane were fixed with methanol, stained with Giemsa, imaged and
counted using a DMI 8 inverted microscope (Leica Microsystems,
Wetzlar, Germany).
For the scratch wound healing assay,
3×105 A549 cells in 500 µl of medium were seeded in a
culture dish. After 24 h, the confluent cell monolayer was scraped
with a pipette tip (10 µl) to generate 4 scratch wounds on each
slide and rinsed twice with PBS to remove the floating cells. Then,
fresh medium containing 0 (vehicle), 5 or 10 µmol/l OP-B was added;
after culture for 6 or 24 h, the images were captured immediately
under a phase contrast microscope (DMI 8; Leica Microsystems).
Microtubule formation assay
This experiment was performed in 96-well plates
coated with 50 µl of Matrigel (BD Biosciences, Bedford, MA, USA).
Then, 1.0×104 EA.hy926 cells were seeded per well.
Tubule formation was observed under a phase contrast microscope
(DMI 8; Leica Microsystems).
Migration assay in nude mice
The 5-week-old athymic BALB/c mice were maintained
under specific pathogen-free (SPF) conditions and manipulated
according to protocols approved by the Shanghai Medical
Experimental Animal Care Commission. A total of 2×107
A549 cells were injected into the tail vein of nude mice. After 7
days, when the lung metastasis model was successfully generated,
the mice were randomly divided into three groups (6 in each group),
including the OP-B groups (37.5 or 75 mg/kg p.o. daily; n=6) and
the control group (corn oil, 100 µl, p.o. daily; n=6). After 21
days of treatment, the mice were sacrificed by cervical
dislocation, and the tissues were isolated for subsequent
experiments.
Matrigel plug assay for angiogenesis
in nude mice
Athymic male mice were purchased from the Model
Animal Research Center of Nanjing University (Nanjing, China) and
maintained under SPF conditions. A549 cells were harvested, washed
with phosphate-buffered saline, and resuspended in serum-free
medium. Cell aliquots (0.2 ml) were mixed with 0.4 ml of
high-concentration Matrigel (BD Biosciences) and immediately
injected subcutaneously into the right flank of nude mice. After 7
days, tumor-bearing mice were randomly divided into three groups,
including those treated with OP-B (37.5 or 75 mg/kg p.o. daily;
n=6) or CMC-Na (control, 100 µl, p.o. daily; n=6) for 14 days.
Then, the tumors were isolated for subsequent experiments. The
hemoglobin content of the tumor was determined using Drabkin's
reagent kit (Sigma-Aldrich; Merck KGaA). All the above experiments
in mice were carried out in strict accordance with the Guide for
the Care and Use of Laboratory Animals of the National Institutes
of Health. Our protocol was approved by the Committee on the Ethics
of Animal Experiments of Nanjing University of Chinese
Medicine.
Statistical analysis
All the data are expressed as the mean ± SD, and the
results were analyzed by Student's t-test. P<0.05 indicated
statistical significance.
Results
Differentially expressed gene (DGE)
library sequencing of 3 NSCLC cell lines treated with OP-B
Six DGE libraries of NSCLC cells were sequenced:
A549 (vehicle), A549 (OP-B treatment), NCI-H460 (vehicle), NCI-H460
(OP-B treatment), NCI-H596 (vehicle), and NCI-H596 (OP-B
treatment).
After comparing the transcriptomes regulated by OP-B
in the 3 cell lines, we found that the sensitivity of different
NSCLC cell lines to OP-B differed greatly. In NCI-H460 cells, 530
genes had significantly different expression levels, with 102 and
428 upregulated and downregulated genes, respectively. In A549 or
NCI-H596 cells, only 62 or 85 significant differentially expressed
genes were detected, with 34 and 32 upregulated and 28 and 53
downregulated genes, respectively. The threshold for judging the
statistical significance of gene expression was FDR ≤0.05 and
absolute value of log2 ratio ≥1.
Annotation of the molecular pathways
altered by OP-B in NSCLC cells
Over-represented Gene Ontology (GO) terms for all DE
genes identified in cells treated with OP-B for 24 h were analyzed
by Ingenuity pathway analysis, and the canonical pathways of the 3
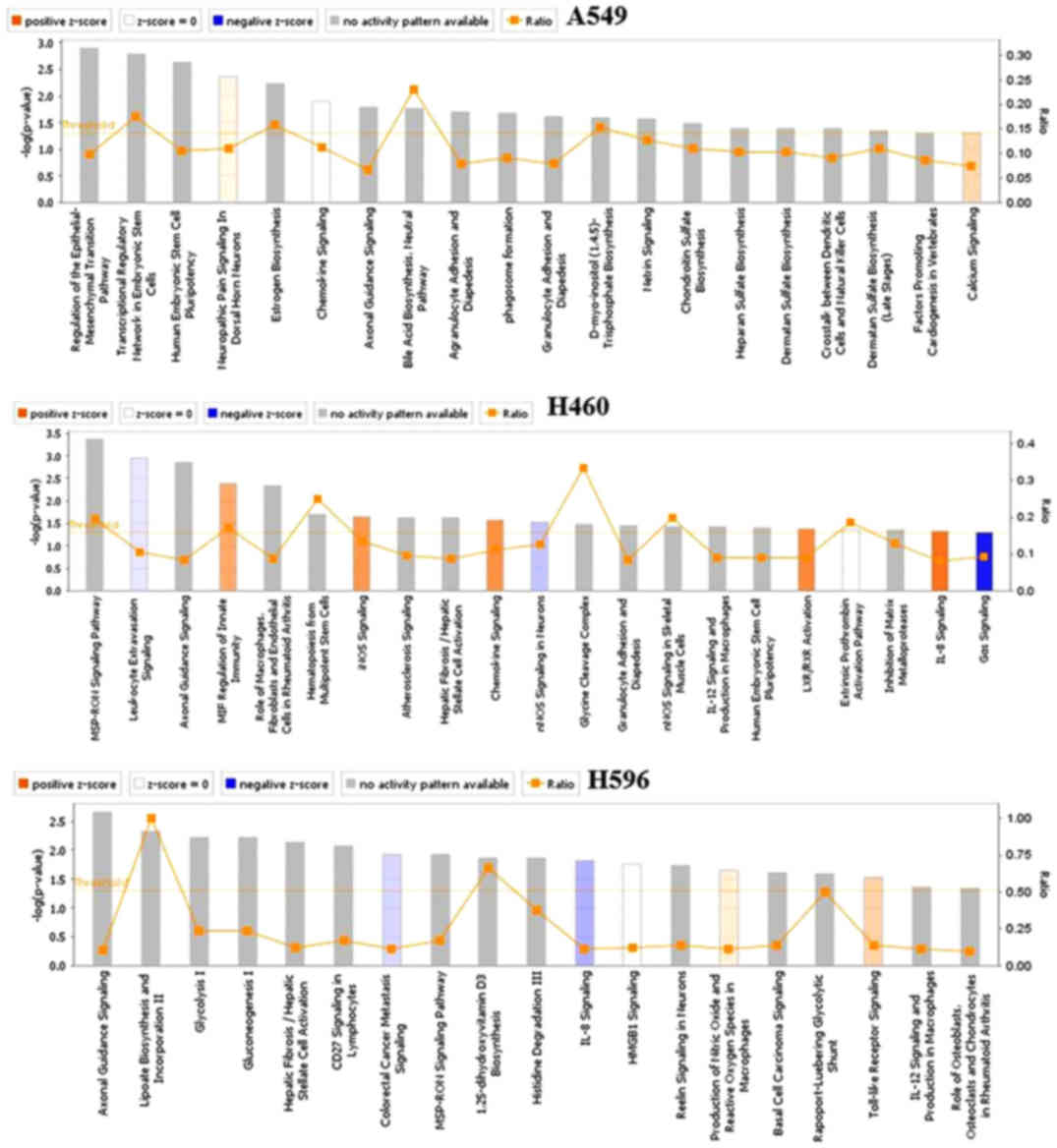
cell lines regulated by OP-B are shown in Fig. 1. Within these cell lines, we found
that the pathways regulated by OP-B were more meaningful in only
the A549 cell line, and the top canonical pathway regulated by OP-B
was the epithelial-mesenchymal transition (EMT) pathway (Table I).
 | Table I.Top canonical pathways in A549
cells. |
Table I.
Top canonical pathways in A549
cells.
| Name of pathway | P-value | Overlap |
|---|
| Regulation of the
epithelial-mesenchymal transition pathway | 1.25E-03 | 9.8%
18/84 |
| Transcriptional
regulatory network in embryonic stem cells | 1.59E-03 | 17.5% 7/40 |
| Human embryonic stem
cell pluripotency | 2.27E-03 | 10.4% 14/134 |
| Neuropathic pain
signaling in dorsal horn neurons | 4.36E-03 | 11.0% 11/100 |
| Estrogen
biosynthesis | 4.36E-03 | 15.8% 6/38 |
OP-B regulated EphA2/Akt pathway in
the A549 and H460 cell lines
Tight junctions (TJs) between cells promote the
binding of EphA2 and Ephrin-A1; then, the endocytosis and
degradation of EphA2 occur (16).
In contrast, lack of TJs between cells decreases the endocytosis
and degradation of EphA2. Crucially, overexpression of EphA2
promoted EMT and cell migration and invasion. From the results, we
found that 10 µmol/l OP-B induced the expression of Ephrin-A1 in
both A549 and NCI-H460 cells, while the level of EphA2 change
differed between them. In A549 cells, OP-B treatment somewhat
decreased the expression of EphA2, while in NCI-H460 cells, it
significantly increased the level of EphA2, which suggested that
TJs might be promoted by OP-B in A549 cells but not in NCI-H460
cells.
Otherwise, activation of EphA2 effectively inhibits
the Ras/Erk1/2 and PI3K/Akt pathways (17). The absence of ligand abrogates the
autophosphorylation of EphA2 and the subsequent activation of Ras
and Akt. Then, EphA2 would be phosphorylated at the cytoplasmic
S897 by Akt (18). In our previous
study, we reported that OP-B inhibited the PI3K/Akt pathway in
several NSCLC cell lines. Here, we found that the phosphorylation
of EphA2 (Ser897) was significantly inhibited by OP-B in A549 cells
but was stimulated by OP-B in NCI-H460 cells. Meanwhile, in A549
cells, the Raf/ERK pathway was unaffected, while in NCI-H460 cells,
the expression of A-Raf and c-Raf was significantly promoted, and
the phosphorylation of c-Raf and ERK1/2 was unaffected by OP-B
(Fig. 2).
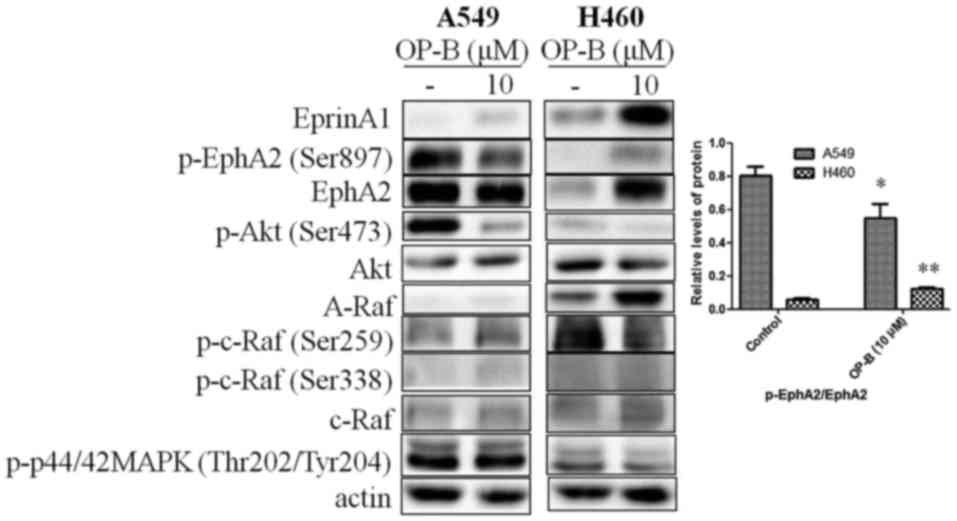 | Figure 2.Effects of OP-B on EphA2/Akt signaling
pathway in A549 and NCI-H460 cells. A549 or NCI-H460 cells were
treated with or without 10 µmol/l OP-B for 24 h, and then, the
expression levels of EphrinA1, EphA2, A-Raf, and c-Raf and the
phosphorylation levels of Akt (Ser473), EphA2 (Ser897), c-Raf
(Ser259), c-Raf (Ser338), and ERK1/2 were detected by western
blotting. β-actin was used as a loading control. The experiment was
repeated three times and yielded similar results. Densitometric
analysis of the western blots (right); n=3. *P<0.05 and
**P<0.01 represent significant differences compared with control
cells. OP-B, Ophiopogonin B. |
OP-B inhibits the motility and
invasiveness of A549 cells in vitro
Specifically, EMT starts by loss of cell junction
proteins, including E-cadherin, claudins, occludins and catenins,
associated with epithelial organization, followed by expression of
the mesenchymal markers N-cadherin and vimentin (19,20).
Slug is known to repress the transcription of E-cadherin by binding
to its promoter region during development (21). Additionally, binding of Slug to the
integrin promoter represses its expression and results in reduced
cell adhesion (22). ZEB family
proteins are also inhibitors of E-cadherin.
Next, the effects of OP-B on the migration and
invasion of A549 cells were investigated, and EMT-related molecules
were detected. Using Transwell migration and scratch wound healing
assays, we found that OP-B significantly reduced the invasion
ability (Fig. 3A and B) and
inhibited the wound closure of A549 cells at a concentration of 10
µmol/l (Fig. 3C).
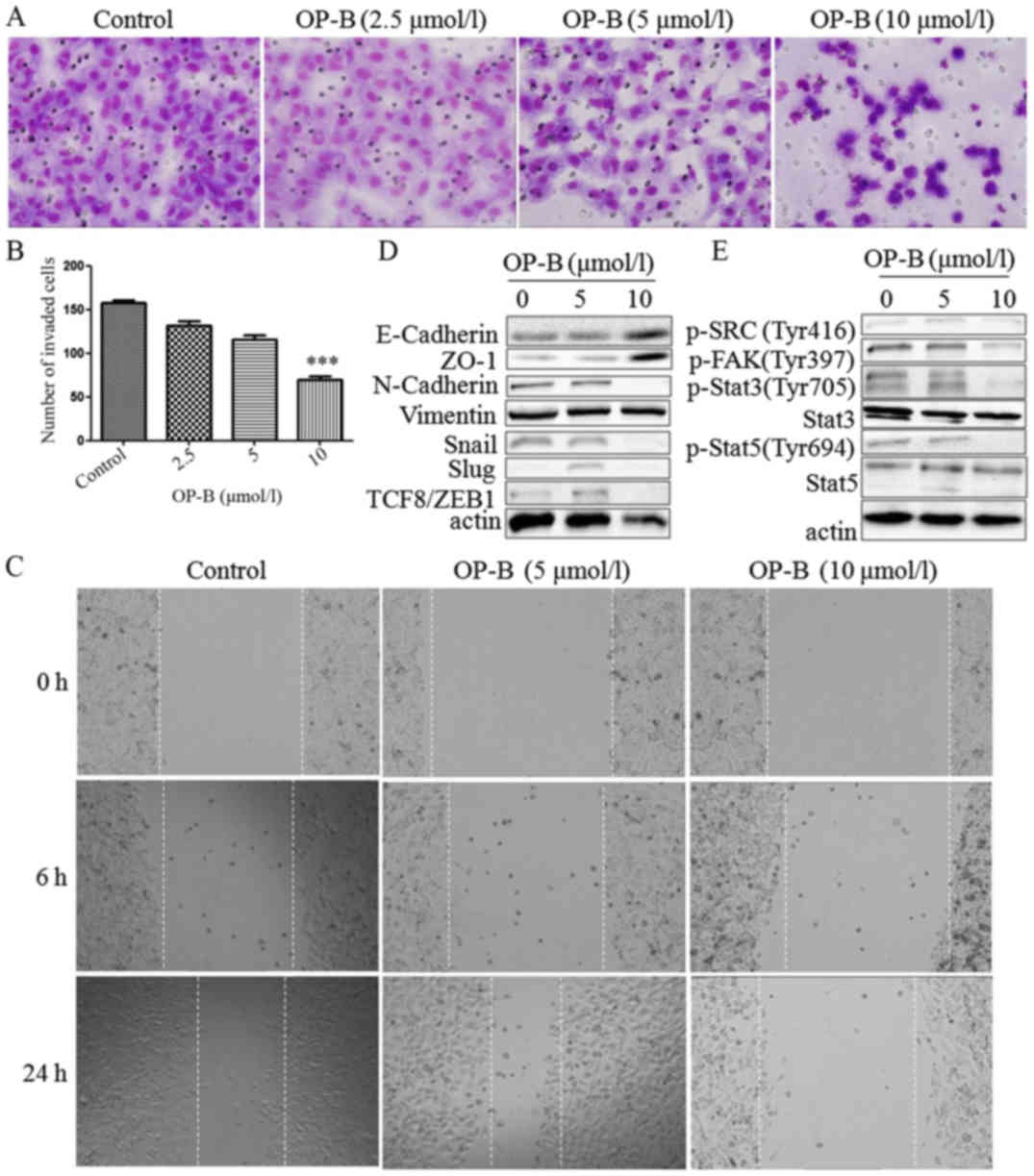 | Figure 3.OP-B inhibits the invasion and
migration of A549 cells in vitro and in vivo. (A)
Transwell migration and invasion assays were performed to examine
cell migration and invasion in A549 cells. Representative images of
migrated or invaded cells are displayed (magnification, ×200). (B)
Columns indicate the mean ± SD of triplicate experiments
(***P<0.001, independent Student's t-test). (C) Wound healing
assays were used to investigate the motility of A549 cells treated
with OP-B, and representative images are shown (magnification,
×40). (D and E) A549 cells were treated with or without 10 µmol/l
OP-B for 24 h, and then, the expression levels of vimentin,
N-cadherin, E-cadherin, ZO-1, Snail, Slug, TCF8/ZEB1, p-SRC
(Tyr416), p-FAK (Tyr397), p-Stat3 (Tyr705), Stat3, p-Stat5 (Tyr694)
and Stat5 were detected by western blotting. β-actin was used as a
loading control. The experiment was repeated three times and
yielded similar results. OP-B, Ophiopogonin B. OP-B inhibits the
invasion and migration of A549 cells in vitro and in
vivo. (F) OP-B inhibits the lung metastasis of A549 cells in
vivo. Representative images of H&E-stained metastatic lung
nodules. OP-B, Ophiopogonin B. |
Detection of EMT-associated proteins showed that
besides vimentin, which was unaffected by OP-B, N-cadherin, Snail,
Slug and TCF8/ZEB1 were all inhibited by 10 µmol/l OP-B, and
E-cadherin and ZO-1 were upregulated by OP-B (Fig. 3D).
In addition, Src is known to integrate and regulate
RTK signaling and to transduce survival signals to PI3K/Akt and
STAT3 (23). Src activation
dissociates cell junctions and facilities cell mobility. Src
activation also stabilizes focal adhesion complexes through FAK
phosphorylation (11).
Detection of this Src-associated pathway showed that
10 µmol/l OP-B inhibited the phosphorylation of Src, FAK, Stat3 and
Stat5 at the same time (Fig.
3E).
More importantly, 75 mg/kg OP-B significantly
reduced the number of metastatic nodules compared with the control
treatment group and this difference was further confirmed by
examination of hematoxylin and eosin (H&E)-stained lung
sections (Fig. 3F).
OP-B inhibits angiogenesis in vitro
and in vivo
To determine the anti-angiogenesis effect of OP-B on
endothelial cells, we performed a tubule formation assay in
EA.hy926 cells. As shown in Fig.
4A, 5 µmol/l OP-B obviously promoted tube formation from 2 to 4
h, while 10 µmol/l OP-B obviously inhibited tube formation in
EA.hy926 cells. Detection of angiogenesis-regulating proteins
showed that OP-B significantly inhibited the expression of VEGFR2
and Tie-2 at a concentration of 10 µmol/l (Fig. 4B). Meanwhile, phosphorylation of the
downstream proteins Akt (S473) and PLC (S1248) was also inhibited
by OP-B. In addition, the levels of EphA2 and phosphorylated EphA2
(S897) were also inhibited by OP-B (Fig. 4C).
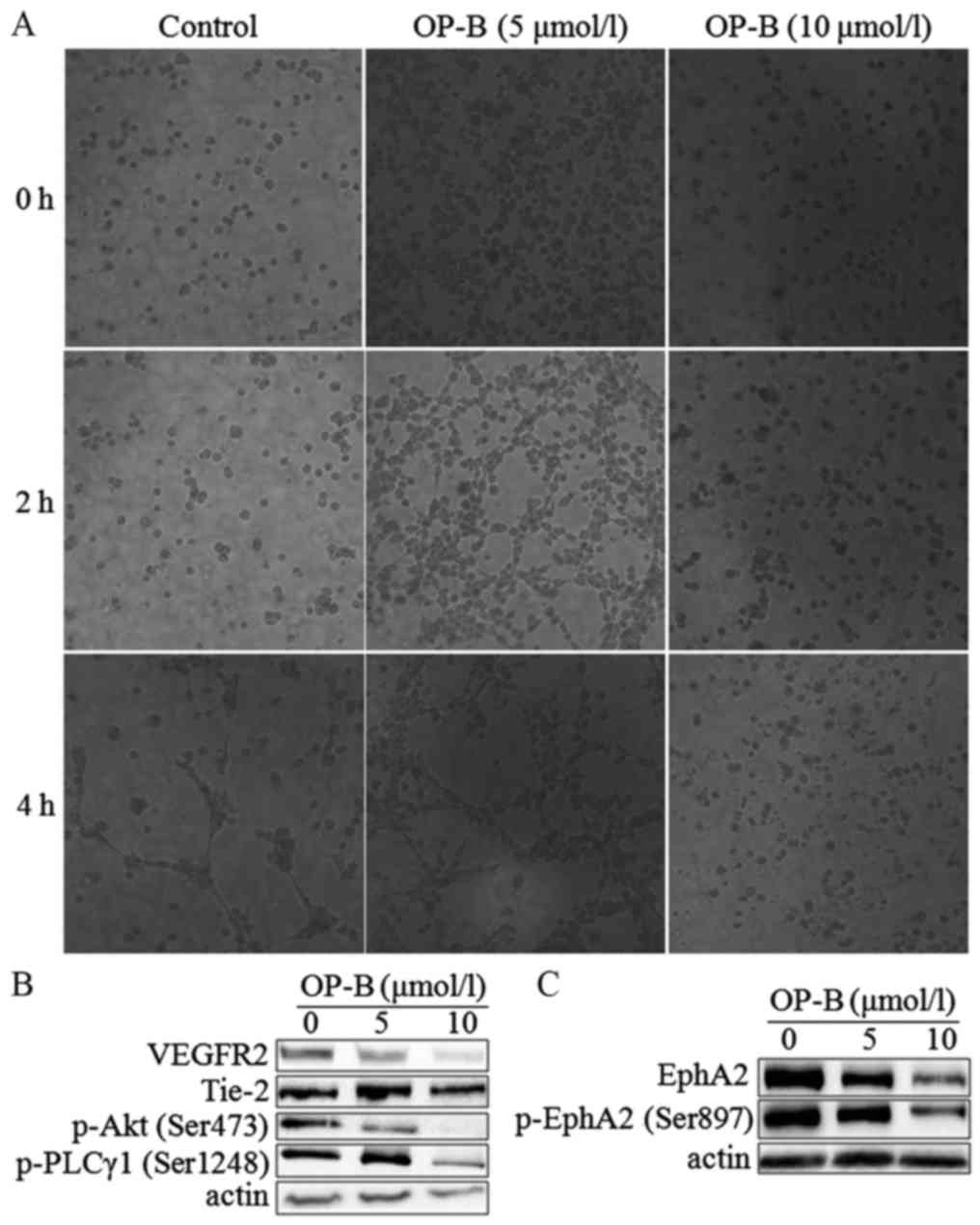 | Figure 4.OP-B inhibits tubule formation by
endothelial cells. (A) Matrigel assay analysis was used to detect
microtubule formation by EA.hy926 cells after treatment with 0, 5,
or 10 µmol/l OP-B for 0, 2, or 4 h. Representative images are
displayed (magnification, ×200). (B and C) A549 cells were treated
with 0, 5 or 10 µmol/l OP-B for 24 h, and the phosphorylation
levels of VEGFR2, Tie-2, p-Akt (Ser473), p-PLCγ1 (Ser1248), EphA2,
and p-EphA2 (Ser897) were detected by western blotting. β-actin was
used as a loading control. The experiment was repeated three times
and yielded similar results. OP-B, Ophiopogonin B. |
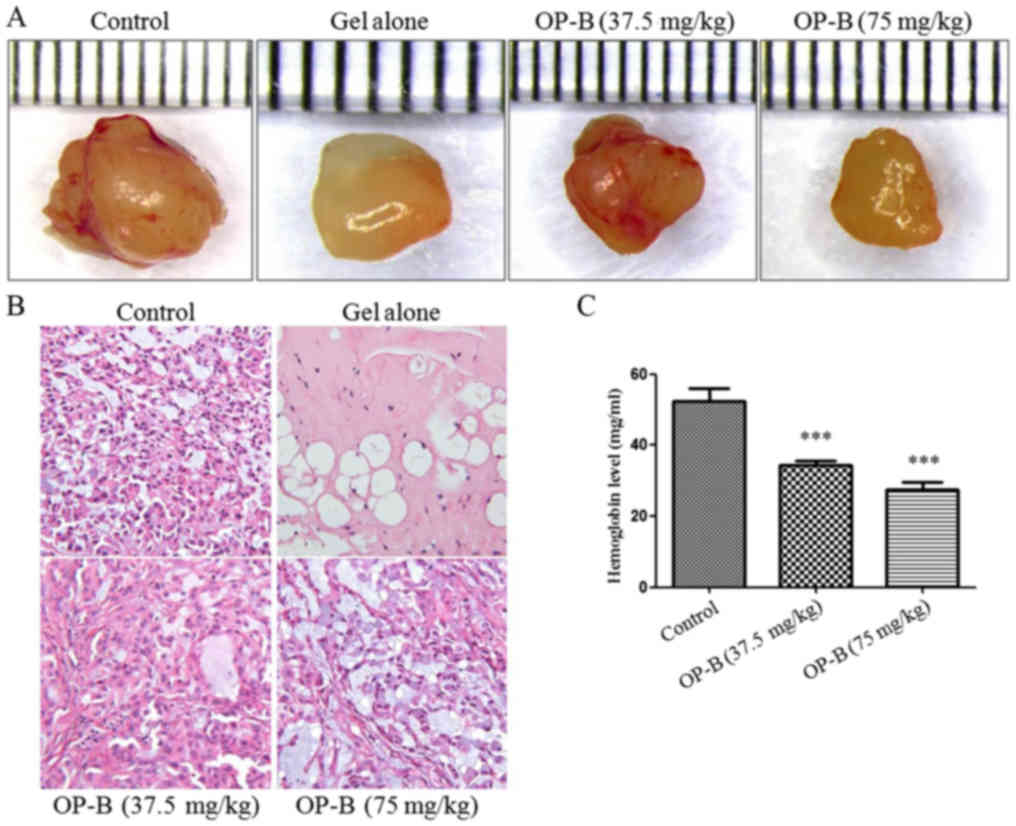
In vivo, we used a Matrigel plug assay to
determine the anti-angiogenesis effects of OP-B in nude mice. After
intragastric administration of 37.5 or 75 mg/kg OP-B on 14
consecutive days, the tumors were isolated and photographed, and
the hemoglobin content of the Matrigel plug was determined using
Drabkin's reagent kit according to the manufacturer's instructions.
As shown in Fig. 5A and B, tumor
angiogenesis was obviously inhibited by OP-B, and hemoglobin
content was significantly inhibited by OP-B (Fig. 5C).
Discussion
Local invasion and tumor metastasis occur with high
incidence in the clinic. Alterations in cell-cell and cell-matrix
adhesion molecules are associated with the progression of tumor
malignancy.
Whole transcriptome analyses are widely used to
characterize the underlying mechanisms based on global gene
expression changes in different cancers (24). Genomic information on Ophiopogonin B
(OP-B) in different NSCLC cell lines is currently unavailable, and
transcriptome and expression profiling data for OP-B-regulated
genes in different NSCLC cell lines are needed as an important
resource to better understand the regulatory mechanisms of OP-B in
NSCLC cell lines.
Histologically, lung adenocarcinoma is the most
common metastatic cancer, which is responsible for its high patient
mortality. Thus, the investigation of anti-metastatic agents for
lung adenocarcinoma is crucial for lung cancer treatment. In this
investigation, the obtained NSCLC cell line transcriptome and DGE
profiling data provide comprehensive information on gene expression
regulated by OP-B in different NSCLC cell lines, which facilitates
our understanding of the molecular mechanisms mediated by OP-B in
these cell lines and provides new insight into the regulation of
EMT and metastasis in A549 cells by OP-B.
To verify the results of the former transcriptome
analysis and to verify the role of the EphA2/Akt pathway in
OP-B-regulated EMT, we chose the A549 and NCI-H460 cell lines for
further experiments. From the results, we found that 10 µmol/l OP-B
promoted the expression of Ephrin-A1 in both A549 and NCI-H460
cells, while the level of EphA2 was altered differentially in these
two cell lines. In A549 cells, OP-B treatment decreased the
expression of EphA2, while in NCI-H460 cells, it significantly
increased the level of EphA2, which suggested that OP-B promoted
tight junctions (TJs) in A549 cells but not in NCI-H460 cells. In
addition, with the lack of ligand binding, the auto-tyrosine
phosphorylation of EphA2 and subsequent suppression of Ras and Akt
are abrogated; then, activated Akt can phosphorylate EphA2 at the
cytoplasmic S897 (17). Thus,
elevated levels of phosphorylated EphA2 (S897) predict the
increased ability for cell mobility. Here, we found that the
phosphorylation of EphA2 (Ser897) was significantly inhibited by
OP-B in A549 cells but stimulated by OP-B in NCI-H460 cells. The
Ras/ERK pathway was unaffected in A549 cells and enhanced in
NCI-H460 cells by OP-B (Fig. 2).
Therefore, the results indicated that OP-B may inhibit invasion and
mobility in A549 cells. In fact, through the Transwell migration
and scratch wound healing assays, we found that 10 µmol/l OP-B
significantly reduced the invasion and migration ability of A549
cells in vitro (Fig. 3A-C).
In vivo, we also found that 75 mg/kg OP-B i.g. inhibited
A549 cell metastasis in the lung metastasis nude mouse model.
Detection of the protein showed that the mechanism correlated with
the inhibition of EphA2. Further detection of EMT-related molecules
showed that OP-B treatment increased expression of the epithelial
markers ZO-1 and E-cadherin but decreased the expression of the
mesenchymal marker N-cadherin and the transcriptional repressors
Snail, Slug and ZEB1. In addition, the impact of OP-B on EMT also
correlated with inhibition of the activation of the non-receptor
tyrosine kinase Src and its downstream pathway.
In addition to EMT, tumor neoangiogenesis is also
involved in the development of metastasis from a primary tumor site
and spread of malignancy. In vitro, we found that 10 µmol/l
OP-B obviously inhibited tube formation in EA.hy926 cells (Fig. 4A). Detection of
angiogenesis-regulating proteins showed that OP-B obviously
inhibited the expression of VEGFR2 and Tie-2 at a concentration of
10 µmol/l and downregulated the phosphorylation of Akt (S473) and
PLC (S1248). More importantly, the levels of EphA2 and
phosphorylated EphA2 (S897) were also inhibited by OP-B (Fig. 4B and C). In vivo, through the
Matrigel plug assay, we found that tumor angiogenesis was inhibited
by OP-B (Fig. 5A and B); meanwhile,
hemoglobin content was significantly inhibited by OP-B (Fig. 5C).
Taken together, through improving TJs junctions
between A549 cells, OP-B inhibited EMT and then regulated the
EphA2/Akt/Raf/ERK and Src/FAK/Stat pathways in the cells. By these
mechanisms, OP-B inhibited the metastasis of A549 cells in
vitro and in vivo. Meanwhile, through inhibiting the
VEGFR2/Tie2/Akt/PLC and EphA2 pathways, OP-B also inhibited
angiogenesis of A549 tumors in vivo (Fig. 6).
Acknowledgements
The authors thank Professor Zhigang Tu (Jiangsu
University, China) for providing the EA.hy926 cell line.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81503374), the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD), the Natural Science Foundation of Jiangsu
Province (grant no. BK20151003) and the Research Foundation of
Education Bureau of Jiangsu Province (grant no. 16KJA360001).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XZ, HF and MW conceived and designed the study. MC,
CH, RJ, YG, HJ and YZ performed the experiments. MC wrote the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All the above experiments in mice were carried out
in strict accordance with the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. Our
protocol was approved by the Committee on the Ethics of Animal
Experiments of Nanjing University of Chinese Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Collins LG, Haines C, Perkel R and Enck
RE: Lung cancer: Diagnosis and management. Am Fam Physician.
75:56–63. 2007.PubMed/NCBI
|
|
2
|
Riihimaki M, Hemminki A, Fallah M, Thomsen
H, Sundquist K, Sundquist J and Hemminki K: Metastatic sites and
survival in lung cancer. Lung Cancer. 86:78–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang G, Liang Y, Zheng T, Song R, Wang J,
Shi H, Sun B, Xie C, Li Y, Han J, et al: FCN2 inhibits
epithelial-mesenchymal transition-induced metastasis of
hepatocellular carcinoma via TGF-β/Smad signaling. Cancer Lett.
378:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tandon M, Vemula SV and Mittal SK:
Emerging strategies for EphA2 receptor targeting for cancer
therapeutics. Expert Opin Ther Targets. 15:31–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang J, Xiao D, Li G, Ma J, Chen P, Yuan
W, Hou F, Ge J, Zhong M, Tang Y, et al: EphA2 promotes
epithelial-mesenchymal transition through the Wnt/β-catenin pathway
in gastric cancer cells. Oncogene. 33:2737–2747. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udayakumar D, Zhang G, Ji Z, Njauw CN,
Mroz P and Tsao H: EphA2 is a critical oncogene in melanoma.
Oncogene. 30:4921–4929. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dunne PD, Dasgupta S, Blayney JK, McArt
DG, Redmond KL, Weir JA, Bradley CA, Sasazuki T, Shirasawa S, Wang
T, et al: EphA2 expression is a key driver of migration and
invasion and a poor prognostic marker in colorectal cancer. Clin
Cancer Res. 22:230–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Lin P, Sun B, Zhang S, Cai W, Han
C, Li L, Lu H and Zhao X: Epithelial-mesenchymal transition
regulated by EphA2 contributes to vasculogenic mimicry formation of
head and neck squamous cell carcinoma. Biomed Res Int.
2014:8039142014.PubMed/NCBI
|
|
10
|
Song W, Ma Y, Wang J, Brantley-Sieders D
and Chen J: JNK signaling mediates EPHA2-dependent tumor cell
proliferation, motility, and cancer stem cell-like properties in
non-small cell lung cancer. Cancer Res. 74:2444–2454. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parri M, Buricchi F, Giannoni E, Grimaldi
G, Mello T, Raugei G, Ramponi G and Chiarugi P: EphrinA1 activates
a Src/focal adhesion kinase-mediated motility response leading to
rho-dependent actino/myosin contractility. J Biol Chem.
282:19619–19628. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang S and Yu D: Targeting Src family
kinases in anti-cancer therapies: Turning promise into triumph.
Trends Pharmacol Sci. 33:122–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen M, Du Y, Qui M, Wang M, Chen K, Huang
Z, Jiang M, Xiong F, Chen J, Zhou J, et al: Ophiopogonin B-induced
autophagy in non-small cell lung cancer cells via inhibition of the
PI3K/Akt signaling pathway. Oncol Rep. 29:430–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen M, Guo Y, Zhao R, Wang X, Jiang M, Fu
H and Zhang X: Ophiopogonin B induces apoptosis, mitotic
catastrophe and autophagy in A549 cells. Int J Oncol. 49:316–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naudin C, Sirvent A, Leroy C, Larive R,
Simon V, Pannequin J, Bourgaux JF, Pierre J, Robert B, Hollande F
and Roche S: SLAP displays tumour suppressor functions in
colorectal cancer via destabilization of the SRC substrate EPHA2.
Nat Commun. 5:31592014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wykosky J, Palma E, Gibo DM, Ringler S,
Turner CP and Debinski W: Soluble monomeric EphrinA1 is released
from tumor cells and is a functional ligand for the EphA2 receptor.
Oncogene. 27:7260–7273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang NY, Fernandez C, Richter M, Xiao Z,
Valencia F, Tice DA and Pasquale EB: Crosstalk of the EphA2
receptor with a serine/threonine phosphatase suppresses the
Akt-mTORC1 pathway in cancer cells. Cell Signal. 23:201–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miao H, Li DQ, Mukherjee A, Guo H, Petty
A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, et al: EphA2
mediates ligand-dependent inhibition and ligand-independent
promotion of cell migration and invasion via a reciprocal
regulatory loop with Akt. Cancer Cell. 16:9–20. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garg M: Epithelial-mesenchymal
transition-activating transcription factors-multifunctional
regulators in cancer. World J Stem Cells. 5:188–195. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alizadeh AM, Shiri S and Farsinejad S:
Metastasis review: From bench to bedside. Tumour Biol.
35:8483–8523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bolos V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turner FE, Broad S, Khanim FL, Jeanes A,
Talma S, Hughes S, Tselepis C and Hotchin NA: Slug regulates
integrin expression and cell proliferation in human epidermal
keratinocytes. J Biol Chem. 281:21321–21331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Playford MP and Schaller MD: The interplay
between Src and integrins in normal and tumor biology. Oncogene.
23:7928–7946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrett CL, DeBoever C, Jepsen K, Saenz
CC, Carson DA and Frazer KA: Systematic transcriptome analysis
reveals tumor-specific isoforms for ovarian cancer diagnosis and
therapy. Proc Natl Acad Sci USA. 112:E3050–E3057. 2015. View Article : Google Scholar : PubMed/NCBI
|