Introduction
According to the latest statistics, gastric cancer
(GC) remains to be the third most common malignant disease
globally, and its lethal malignant nature is responsible for the
high mortality and morbidity of patients (1). In China, statistical data confirmed
nearly 1 million GC cases and cancer-related deaths, making it the
second most lethal malignancy in the Chinese population (2). Although multiple factors contribute to
the morbidity and mortality of GC, tumor growth and metastasis are
key sources to the malignancy of GC (3). Methods that could alleviate or reverse
them are expected in the targeted treatment of GC and thus are
worthy of further investigation.
The PI3K/AKT/Stat3 signaling pathway is a classic,
multi-functional mechanism which is involved with the development
and progression of numerous cancers, such as human bladder, breast
and colorectal cancer (4–6). It has been thoroughly studied and is
believed to contribute to the malignancy of GC as well,
particularly to its metastatic characteristics (7).
Nuclear factors and their receptors are
indispensable in the development of tumor growth, metastasis and
epithelial-mesenchymal transition (EMT) in several types of cancers
(8). Nuclear factor I/B (NFIB) is a
recently discovered member of the nuclear factor family (which
includes NFI-A, B and X) and emerging evidence has shown that NFIB,
besides functioning during normal somatic development, is also
involved in the generation and progression of various types of
cancer, including small-cell lung cancer, melanoma and breast
cancer, through different molecular mechanisms such as forming the
oncogenic NFIB-MYB gene fusion or increasing the chromatin
accessibilities of its downstream targets (9–11).
Nevertheless, research on the functions and involvement of NFIB on
the development of GC has not been reported yet.
In the present study, we focused on the roles played
by NFIB on the malignant behavior of GC cells. Our research
demonstrated that NFIB is an oncogenic molecule which contributes
to the malignancy of GC by enhancing the proliferation, migration,
aggression and EMT processes of GC cells. We further discovered
that NFIB exerts its function by regulating CXCR4, AKT and
Jak2/Stat3 pathway members. Silencing of NFIB expression enhanced
AKT phosphorylation, in addition to the downregulation of CXCR4
expression as well as the phosphorylation of Jak2/Stat3 pathway
members. Our study for the first time revealed NFIB as a
developmental contributor of GC and a potential target for the
molecular treatment of GC.
Materials and methods
Quantitative real-time polymerase
chain reaction
Total RNA was extracted from tissues and cultured
cells by TRIzol reagent (Takara Biotechnology Co., Ltd., Dalian,
China). The quantity of RNA was assessed using an ultraviolet
spectrophotometer at absorbances of 260 and 280 nm. The ratio of
optical density (OD) 260/280 between 1.7 and 2.1 suggested an
adequate purity for subsequent experiments. The primers for
evaluating the NFIB expression in GC tissues and cell lines was: i)
NFIB forward, 5′-AAAAAGCATGAGAAGCGAATGTC-3′ and reverse,
5′-ACTCCTGGCGAATATCTTTGC-3′; ii) GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. The reverse transcription reactions were
carried out using the PrimeScript™ One Step qPCR kit (Takara
Biotechnology). The messenger RNA (mRNA) expression analysis of
NFIB was performed by real-time polymerase chain reaction (qPCR)
using a standard SYBR-Green PCR kit (Takara Biotechnology) protocol
on an Applied Biosystems 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's instructions. The reaction
conditions of qPCR were: 95°C for 30 sec, 60°C for 30 sec, 40
cycles at 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec.
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression was
regarded as the endogenous control in each sample. The relative
gene expression levels were calculated using the comparative Cq
(ΔΔCq) method, in which the relative expression is calculated as
2-ΔΔCq, and Cq represents the threshold cycle (12). Each experiment was conducted in
triplicate.
Cell procurement and maintenance
The normal human gastric normal epithelium cell line
GES1 and the GC cell lines AGS, MKN45, HGC27, MGC803 and SGC7901
were preserved and obtained from the Laboratory of General Surgery,
Union Hospital, Tongji Medical College. All cell lines were
cultured in the cell culture medium RPMI-1640 (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) mixed with 10% fetal
bovine serum (FBS; ScienCell Research Laboratories, Inc., San
Diego, CA, USA), streptomycin (100 µg/ml) and penicillin (100 U/ml;
both from Sigma-Aldrich; Merck, St. Louis, MO, USA) at 37°C and 5%
CO2. Antibodies against NFIB (dilution 1:500; cat. no.
ab186738) and GAPDH (dilution 1:1,000; cat. no. ab9485) were
purchased from Abcam (Cambridge, MA, USA), while E-cadherin
(dilution 1:1,000; cat. no. sc-71009), N-cadherin (dilution
1:1,000; cat. no. sc-59987) and vimentin (dilution 1:1,000; cat.
no. sc-80975) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Akt/p-Akt (dilution 1:1,000; cat. no.
9272S/4060S) and Stat3/p-Stat3 (dilution 1:1,000; cat. no.
12640S/9145S) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Cell viability assay
HGC27 and MKN45 cells in logarithmic growth were
washed twice with phosphate-buffered saline (PBS) and seeded on a
96-well plate with 6 duplicate wells at a final density of
5×103 cells/well at 37°C for 24 h. Then, the cells were
transfected with either NFIB siRNAs or NFIB controls under the same
concentration of 100 µM. At indicated time-points, 20 µl of 5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich; Merck KGaA) was added into each well and incubated
for another 4 h at 37°C and 5% CO2. Then, the culture
medium was removed and 150 µl dimethyl sulfoxide (DMSO) was added
to each MTT-treated well for 10 min to dissolve the crystals. The
absorbance at 490 nm was determined using a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA) to assess cell
viability. The experiment was repeated at least 3 times.
Colony formation assay
Transfected or non-transfected HGC27 and MKN45 cells
(500 cells/well) were evenly seeded on 6-well plates in triplicate
and were grown for 48 h in RPMI-1640 medium with 10% FBS at 37°C.
Then, both cell types were divided into 2 groups and transfected
with either NFIB siRNAs or NFIB controls under the same
concentration of 100 µM, separately. After 14 days, the cells were
fixed with 4% paraformaldehyde (Sigma-Aldrich; Merck) for 30 min,
stained with Giemsa (Sigma-Aldrich; Merck) for 20 min, and
photographed under an inverted fluorescence microscope (IX51;
Olympus Corp., Tokyo, Japan) equipped with an Olympus Qcolor 3
digital camera (Olympus Corp.). The number of colonies (>50
cells) were analyzed using an inverted microscope (Nikon, Inc.,
Garden City, NY, USA).
Cell migration and invasion assay
Cell migration and invasion were assessed with
Transwell chambers (Corning Incorporated, Corning, NY, USA)
containing 24-well inserts with 8-µm pores in the presence or
absence of Matrigel ECM (BD Biosciences, Franklin Lakes, NJ, USA),
according to the manufacturer's protocols. After 48 h of
transfection, both MKN45 (100,000/chamber) and HGC27
(100,000/chamber) cells were seeded to the upper chamber filled
with Gibco™ Opti-MEM™ (Thermo Fisher Scientific, Inc.) serum-free
medium and incubated for an additional 24 h for migration or 48 h
for invasion. The lower chamber was filled with 600 µl Gibco™
RMPI-1640 medium (Thermo Fisher Scientific, Inc.) mixed with 10%
FBS (ScienCell Research Laboratories, Inc., Carlsbad, CA, USA).
Then, the cells in the upper chamber were wiped off, and the
penetrated cells were fixed in 4% paraformaldehyde and stained with
crystal violet staining solution (dilution rate: 1:3) at room
temperature for 15 min. Cells were quantified by counting in 5
randomly selected fields for each membrane under light microscope
with an ×100 magnification, and the average number of cells from 3
independent tests was regarded as the migration or invasion
value.
Western blot analysis
The total proteins from treated cells and biopsy
samples of gastric tumors were extracted with
radioimmunoprecipitation assay (RIPA) lysis buffer (Pierce; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The contents of the
extracted proteins were quantified using the BCA Protein Assay
Reagent kit (Pierce; Thermo Fisher Scientific, Inc.). For analysis
of protein expression, equal amounts of protein (40 µg) from each
sample were run on 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and electro-transferred to
polyvinylidene difluoride (PVDF) membranes (EMD Millipore,
Billerica, MA, USA). Following blocking with 5% bovine serum
albumin for 2 h at 37°C, the membranes were probed with the primary
antibody against phosphorylated Akt (cat. no. 4060S), Akt (cat. no.
9272S), phosphorylated Stat3 (cat. no. 9145S), Stat3 (cat. no.
12640S) and GAPDH (cat. no. ab9485) at a concentration of 1:1,000
overnight at 4°C. After being washed 3 times with Tris-buffered
saline with Tween-20 (TBST) buffer, the horseradish peroxidase-goat
anti-rabbit antibodies (cat. no. 10285–1-AP; ProteinTech, Co.,
Wuhan, China) were added at 1:3,000 dilutions and incubated with
the membranes at room temperature for 1 h. After being washed
again, the protein bands were visualized and quantified using
Electrochemiluminescence Plus Detection system (EMD Millipore).
GAPDH was used as an internal reference.
Immunohistochemistry (IHC)
analysis
Immunohistochemistry was performed with
polymer-based technology (EnVision; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) as described by previous studies
(13). The study was approved by
the Ethics Committee of Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology. The gathering and
use of the patient data and tissues were consented by all patients
involved. The tissue specimens of GC patients and adjacent normal
tissues (male:female, 2:17; age range, 37–83 years) collected by
the Department of Gastrointestinal Surgery of Union Hospital from
November 2013 to December 2016 were fixed with 4% paraformaldehyde
overnight at 4°C and then embedded in paraffin, sliced into 4-mm
sections with a microtome and then fixed onto slides. The tissue
sections were dewaxed with xylene and rehydrated with graded
alcohol concentrations as instructed by the standard procedures.
The sections were then treated in EDTA (pH 8.0) and autoclaved at
121°C for 5 min to retrieve the antigenicity. After being washed in
PBS (0.1 M, pH 7.4, 3 times for 5 min), the endogenous peroxidase
was blocked with 3% hydrogen peroxide for 15 min at room
temperature. After being washed again with PBS, the slides were
incubated with primary antibodies for 1 h at room temperature. The
primary antibody and dilution were: NFIB (cat. no. ab186738;
Abcam), 1:100 dilution. Immunostaining was performed using the
EnVision system with diaminobenzidine (Dako Cytomation, Glostrup,
Denmark). The relative intensity of NFIB expression was evaluated
with the Image-Pro Plus (IPP version 6.0; Media Cybernetics, Inc.,
Silver Spring, MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS 20.0
software package (SPSS, Inc., Chicago, IL, USA). All data were
compared and analyzed as the mean ± standard deviation (SD) from at
least 3 independent experiments. Statistical significance was
estimated with Student's t-test between 2 groups and one-way
analysis of variance (ANOVA) followed by the least significant
difference method for 3 and more groups. The association between
NFBI expression and clinical parameters was analyzed using a
Chi-squared test. P<0.05 was considered to indicate a
statistically significant difference.
Results
NFIB is highly expressed in GC tissues
and cell lines
For starters, we explored the functions of NFIB in
GC by examining its expression levels in GC tissues as well as GC
cell lines. NFIB expression was examined in 37 primary GC tissues
coupled with their corresponding normal adjacent mucosa, as well as
the human GC (AGS, MKN45, HGC27, MGC803 and SGC7901) cell lines. A
strong and widely-distributed cytonuclear staining pattern of NFIB
was detected in GC specimens by immunohistochemical (IHC) staining,
whereas the corresponding normal mucosa exhibited weak or no
staining of NFIB (Fig. 1A and B).
Western blotting, as shown in Fig.
1C, further confirmed that the expression of NFIB was
significantly higher in GC tissues than the corresponding adjacent
tissues. In addition, western blotting revealed that the expression
level of NFIB was also elevated in most human GC cell lines, among
which the MKN45 and HGC27 cell lines had the highest expression
(Fig. 1D). Consistent with the NFIB
expression comparisons at the protein levels, the mRNA levels of
NFIB in GC tissues were markedly enhanced, compared with those in
adjacent non-cancerous tissues (Fig.
1E).
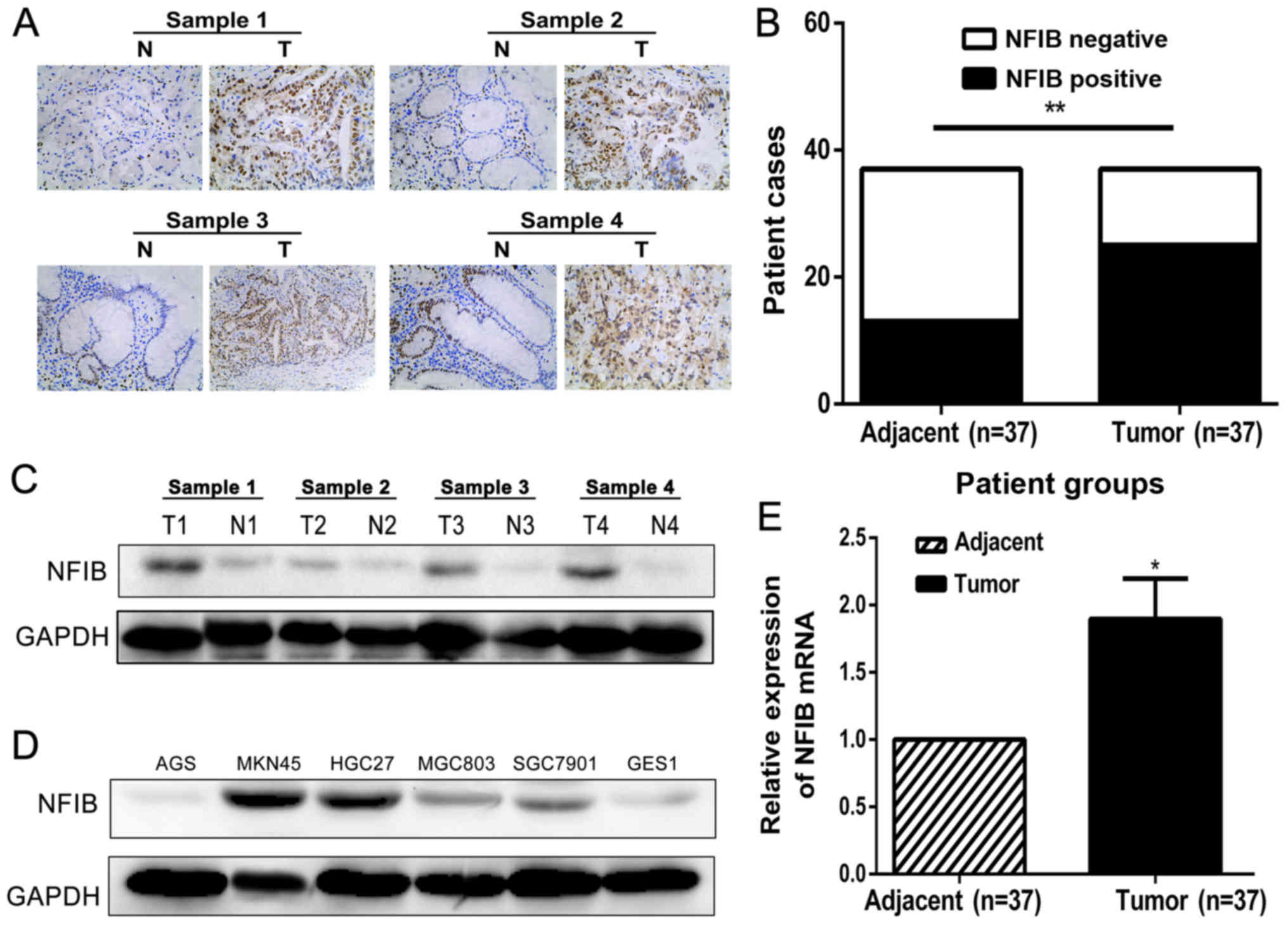 | Figure 1.NFIB is highly expressed in gastric
cancer (GC) tissues and cell lines. (A) The immunohistochemical
(IHC) staining detected strong NFIB expression in the cytonuclei of
GC tumor tissues, whereas in adjacent non-cancerous tissues, the
expression of NFIB was weak and majorly restricted to the gland
area. (B) The IHC detection of the mRNA samples of 37 GC tissue
pairs revealed an elevated NFIB expression ratio (64.8% positive,
24/37) in GC tissues, compared to the lower expression ratio of
NFIB (32.4% positive, 12/37) in adjacent non-cancerous tissues. (C)
Western blotting revealed a higher protein expression of NFIB in GC
tissue samples compared to the adjacent normal tissues. N, normal
tissue; T, GC tissue. (D) NFIB expression was examined by western
blotting in 5 human GC cell lines (AGS, MKN45, HGC27, MGC823 and
SGC7901) and the normal human gastric mucosal cell line GES1.
Higher NFIB expression was detected in all human GC cell lines
except for AGS, compared with the GES1 cell line. (E) Consistent
with the IHC and western blot analysis findings, the RT-PCR
detection of the mRNA samples of the 37 GC tissue pairs confirmed
significantly higher NFIB expression in GC tissues (1.7-fold
relative to the adjacent non-cancerous tissues). *P<0.05,
**P<0.01. NFIB, nuclear factor I/B. |
Elevated NFIB expression is associated
with poorer clinical prognosis of GC patients
To investigate the roles played by NFIB in the
clinical characteristics as well as the prognosis of GC patients,
we compared the relationships between the expression of NFIB in GC
tissues and the clinical features of 37 GC patients that we
collected (Table I).
 | Table I.Clinicopathological characteristics of
patients. |
Table I.
Clinicopathological characteristics of
patients.
|
|
| NFIB expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
factors | n | High | Low | P-value |
|---|
| Age (years) |
|
|
| 0.5766 |
|
>60 | 7 | 5 | 2 |
|
|
<60 | 30 | 19 | 11 |
|
| Sex |
|
|
| 0.1881 |
| Male | 20 | 15 | 5 |
|
|
Female | 17 | 9 | 8 |
|
| Pathological T
stage |
|
|
| 0.0011a |
|
T1+T2 | 10 | 2 | 8 |
|
|
T3+T4 | 27 | 22 | 5 |
|
| Pathological N
stage |
|
|
| 0.0353a |
| N0 | 22 | 11 | 11 |
|
|
N1+N2 | 15 | 13 | 2 |
|
| Tumor
differentiation |
|
|
| 0.0043a |
|
High | 5 | 0 | 5 |
|
|
Moderate | 26 | 19 | 7 |
|
|
Low | 6 | 5 | 1 |
|
Statistical analysis revealed that NFIB
overexpression in GC patients was significantly associated with
more advanced tumor-node-metastasis (TNM) stages and tumor
differentiation (P<0.05). Other clinical parameters, including
age and sex, were not statistically significantly associated to
NFIB overexpression. These statistical analysis data predicted NFIB
as an oncogenic molecule in GC and revealed it to be associated
with differentiation and metastasis, which prompted us to further
study its roles and functions with respect to the aggressiveness,
invasion, metastasis and proliferation of GC cells.
Elevated NFIB expression promotes the
migratory and invasive characteristics of GC cell lines
Since our data demonstrated that NFIB expression is
significantly elevated in GC tissue samples and cell lines, as well
as associated to poorer clinical prognosis, we next aimed to find
out whether NFIB overexpression, to some extent, is responsible for
the malignancy of GC. We chose the two cell lines, MKN45 and HGC27,
as the cell tools for our further research, since they expressed
the highest levels of NFIB protein among all the examined GC cell
lines.
To investigate the effects of NFIB silencing on the
migration and invasion aspects of GC cells, we first examined the
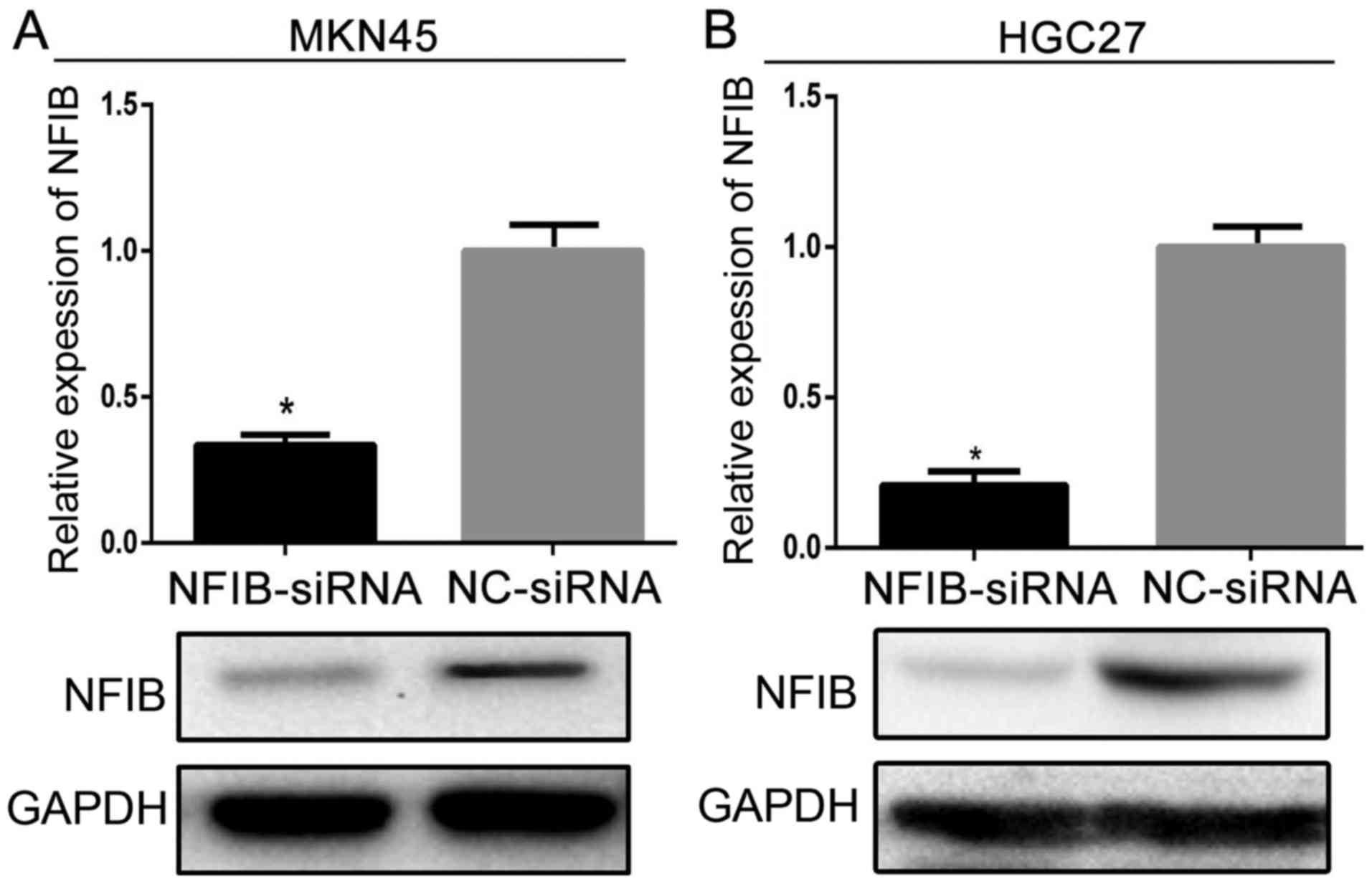
efficacy of NFIB-siRNA on decreasing NFIB expression. Both MKN45
and HGC27 cell lines were divided into two subgroups and designated
as siNFIB and siNC groups, which were transfected with either the
NFIB-siRNA (siNFIB) or the negative-control siRNA (siNC),
respectively. The western blotting revealed that siNFIB
significantly decreased the expression of NFIB in both cell lines,
compared with the siNC groups (Fig.
2).
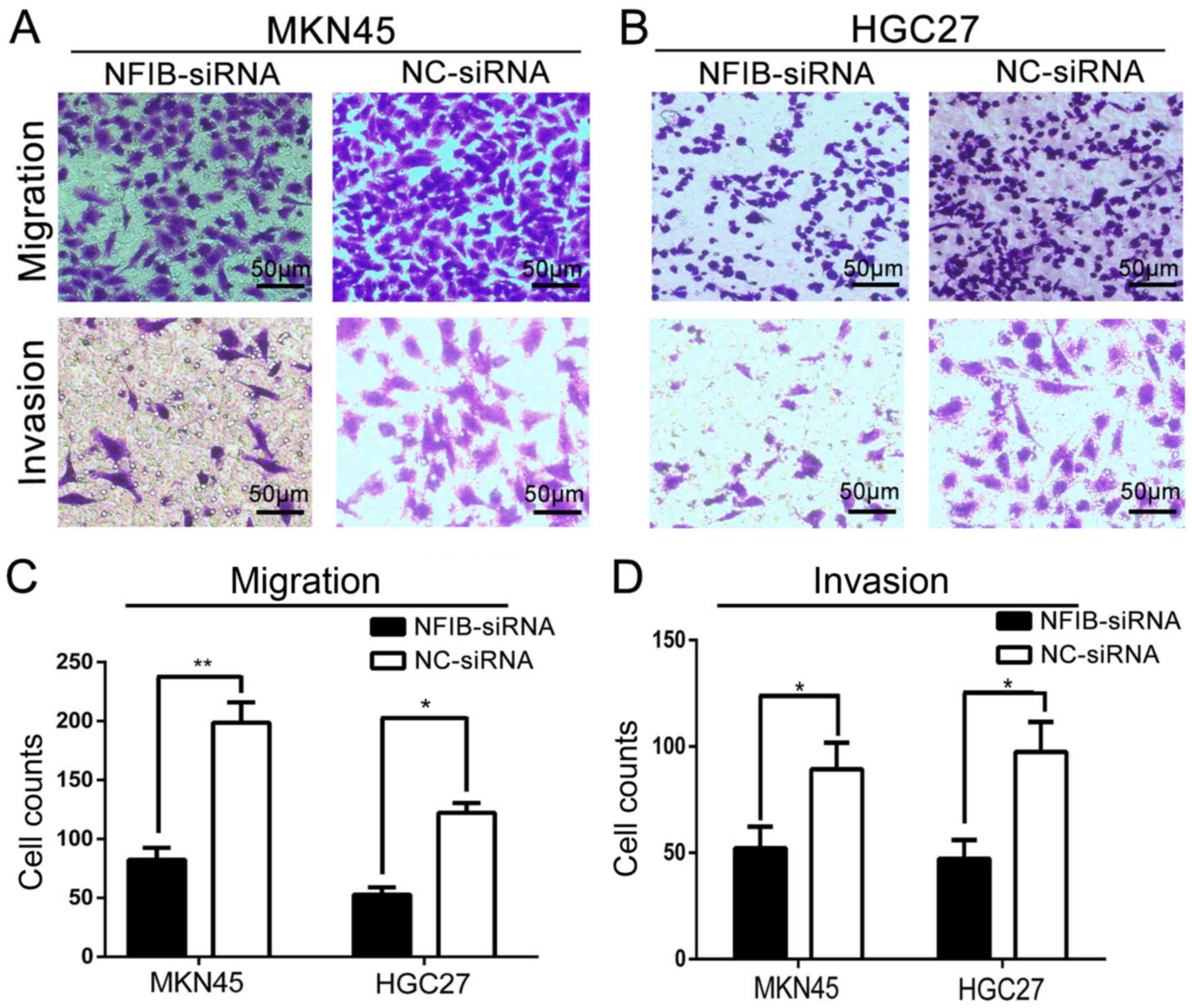
We further investigated the effects of NFIB
knockdown on the migratory and invasive capacities of the GS cell
lines with Transwell assays. The results revealed that the
downregulation of NFIB markedly inhibited the migration (Fig. 3C) as well as the invasion (Fig. 3B) abilities of GC cells. When
transfected with NFIB-siRNA, both MKN45 (Fig. 3A) and HGC27 (Fig. 3B) cells became less effective on
penetrating the Transwell chamber membranes and extracellular
matrix (ECM) coatings.
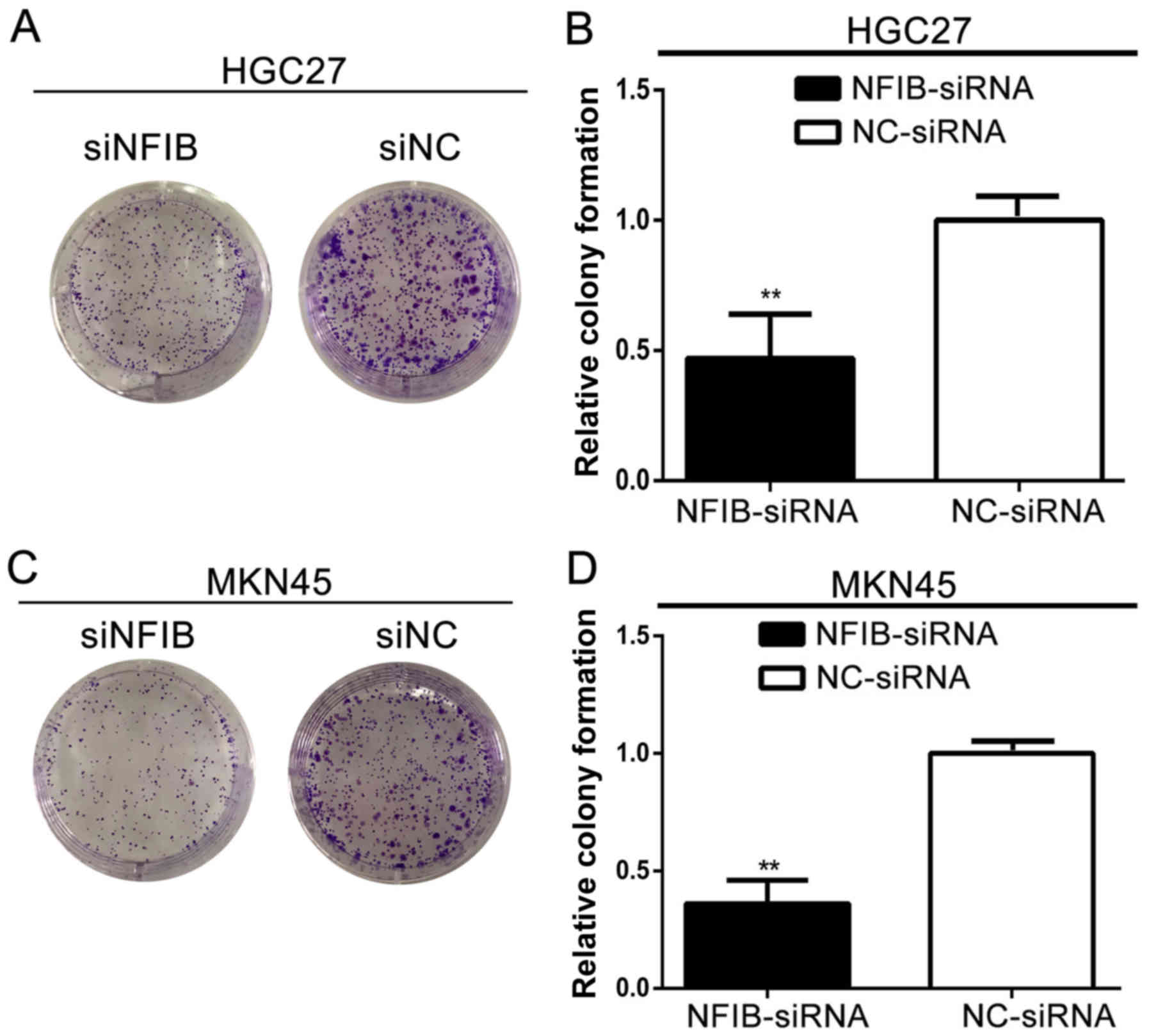
NFIB expression promotes the colony
formation in GC cell lines
Since the tumorigenicity of GC cells is one of the
most important factors for cancer malignancy, we also explored the
roles of NFIB in the tumorigenicity properties of GC with colony
formation assays. As shown in Fig.
4, the siNFIB groups of HGC27 (Fig.
4A) and MKN45 (Fig. 4C)
displayed inhibited ability to form colonies. The numbers and sizes
of the colonies were significantly limited for both cell lines
compared with the NC groups (Fig. 4B
and D).
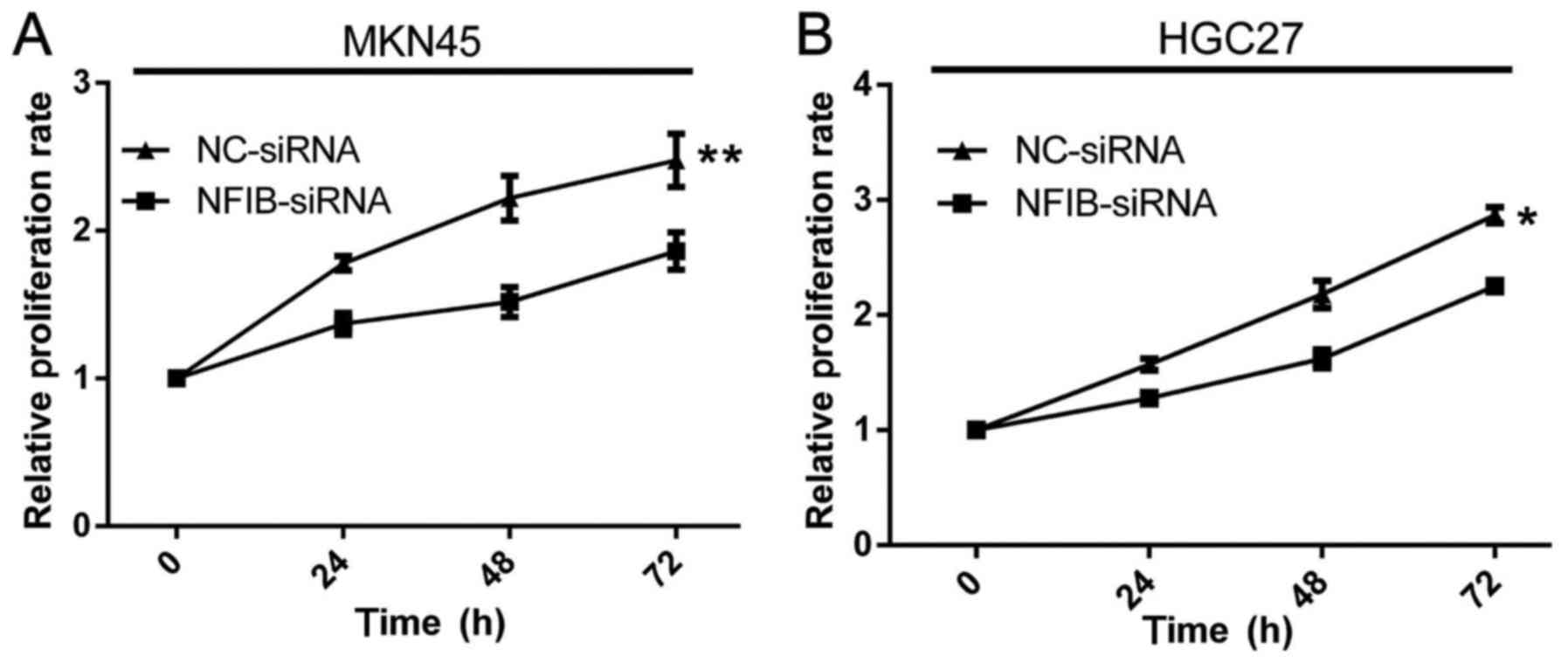
NFIB expression promotes the
proliferation and viability of GC cell lines
Another important factor of malignancy for GC is the
proliferation of GC cells. We studied the contribution of NFIB on
the proliferation and viability aspects of GC cells by MTT assays.
Relative to the higher proliferation ability of the siNC groups,
the proliferation rates of the siNFIB groups of MKN45 (Fig. 5A) and HGC27 (Fig. 5B) cells were significantly reduced.
The inhibition effects of NFIB knockdown on the proliferation of GC
cells were most evident during the first 48 h. Notably, after 48 h,
the siNFIB groups had almost restored the proliferation
viabilities. This may be explained by the effective duration of the
NFIB-siRNA which may have expired.
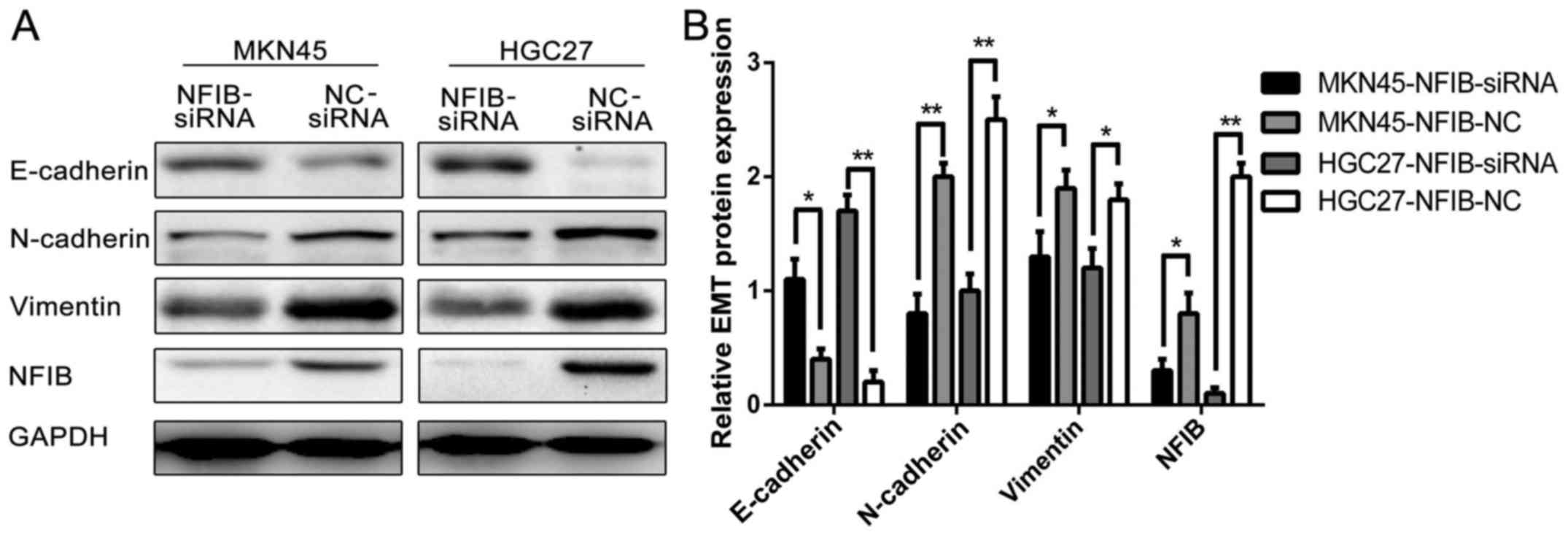
NFIB expression promotes the EMT of GC
cell lines
EMT has been studied and is believed to contribute
to the migration, transformation, invasion and malignant behavior
of numerous types of cancer, including GC. We thus investigated the
associations between NFIB expression and the EMT properties of GC
cells. The western blot results (Fig.
6) revealed that compared with the siNC groups, the siNFIB
groups of HGC27 and MKN45 cells expressed a higher level of
E-cadherin and lower levels of N-cadherin and vimentin, suggesting
that knockdown of NFIB expression in the GC cells did in fact
promote the EMT characteristics of them.
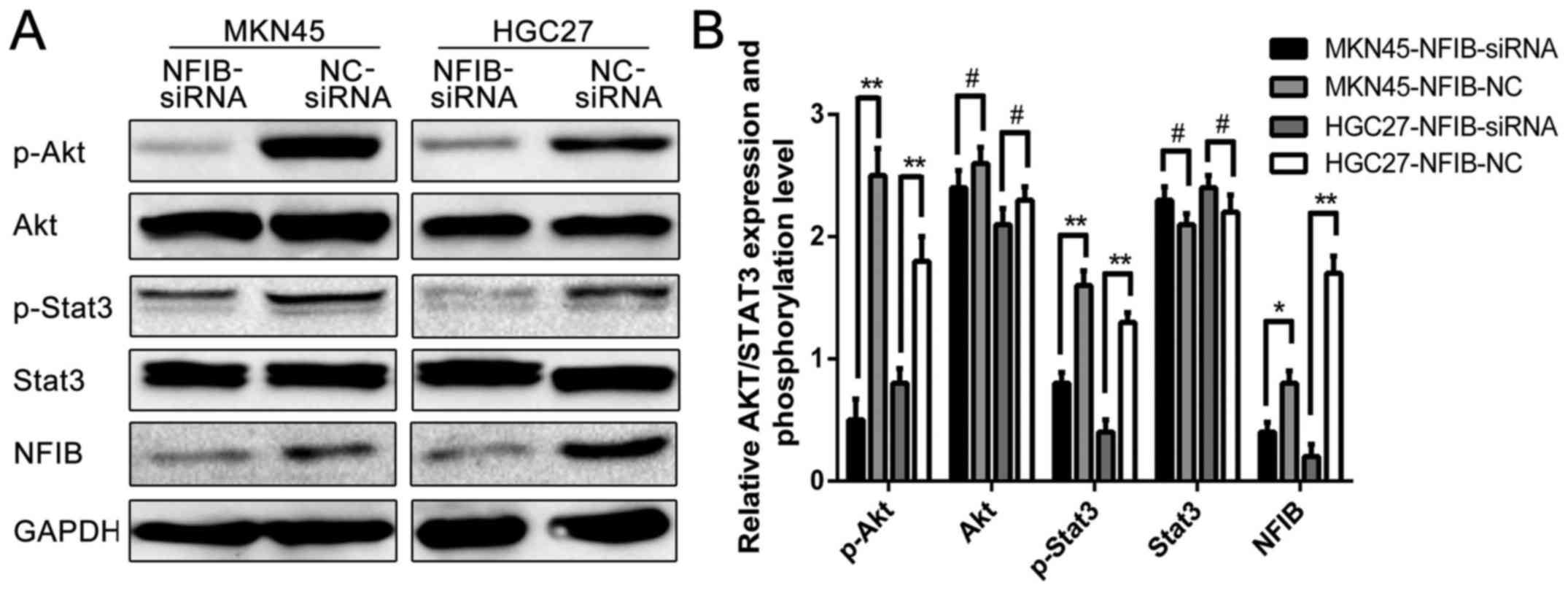
NFIB exhibits its functions in GC
cells by modulating the Akt/Stat3 signaling pathways
Since we demonstrated that NFIB is an important and
novel regulator for the malignancy of GC, we were interested in
determining the molecular mechanism by which NFIB functions. Since
NFIB is a member of the nuclear factor family, which regulates the
expression and phosphorylation of various molecules, we
hypothesized that overexpressed NFIB possessed its functions in GC
cells by regulating the phosphorylation of its downstream signaling
molecules. A number of studies have reported that the Akt/Stat3
signaling pathway is associated with the malignancy of cancer
cells, including lung (14),
bladder (15) and breast cancer
(16); however, no study has
investigated the relationships between NFIB and the Akt/Stat3
signaling pathways yet. Thus, we explored whether NFIB affected
this pathway in GC. Western blotting revealed that the expression
levels of p-Akt and p-Stat3 were suppressed when NFIB was inhibited
by NFIB-siRNA, while the expression levels of total protein of
these molecules exhibited no difference between the siNFIB and the
siNC group (Fig. 7). The results
clearly revealed that the Akt/Stat3 signaling pathway is
responsible for the NFIB-regulated malignancy in GC cells, which
may act as the downstream molecular signaling pathway of NFIB.
Discussion
To date, gastric cancer (GC) is still one of the
most lethal and common cancers worldwide, which is often associated
with a high mortality and morbidity (17–19).
The majority of GC patients are diagnosed at an advanced stage and
receive a poor prognosis (20). One
of the reasons for the poor outcomes of GC is the malignant
biological behaviors of GC cells, particularly for those with
low-to-intermediate differentiation stages, which often possess
strong abilities in proliferation, migration, invasion and
tumorigenesis (21,22).
Increasing evidence and studies have indicated that
nuclear factor I/B (NFIB), a member of the nuclear factor family
and a transcriptional factor necessary for the normal process of
body development as well as cell division and differentiation in
various types of tissues, is also critical in the formation and
development of cancers (23).
Previous studies have confirmed the oncogenic roles of NFIB in some
types of cancer, such as small-cell lung cancer (24), melanoma (25) and breast cancer (26). Unlike many other classic molecules
in tumor development, the roles played by NFIB are dependent of the
tissue origins and the source of cancer cells. In different types
of cancer, NFIB could play the part of an oncogene, a tumor
suppressor, or components of gene fusion (23). However, to the best of our
knowledge, the relationship between NFIB and GC has yet to be
reported. Thus, the present study demonstrated for the first time
that NFIB is associated to the malignancy of GC, which may be a
novel aspect for the investigation of GC and a new target for the
precise antitumor treatment of GC.
A recent study revealed that NFIB exerts its
functions by targeting various downstream signaling pathways and
molecules. For example, NFIB mediates the migration and invasion of
melanoma cells through the regulation of EZH2 and MITF (25). Since NFIB belongs to a family of
nuclear factors, it controls numerous cancer-related pathways, and
thus, it is usually enigmatic when it comes to which pathway it
controls for specific types of cancer. Finding the downstream
signaling pathway for NFIB functioning in the development of GC is
of critical importance.
It was reported that most GC patients experienced
mutations in the Akt/Stat3 pathway and that this pathway could
control the invasion, migration and proliferation of GC cells
(27). Since Akt is a protein
kinase and is responsible for the phosphorylation of different
types of functional proteins, finding an association between NFIB
and Akt would be helpful in explaining the oncogenic functions of
NIFB in GC (28). Stat3 is another
essential transcription factor that can translocate itself to the
cell nucleus to regulate the expression of various pro-invasive
factors, such as MMPs, HSP70 and HSP90 (29). No studies have depicted the
relationship among NFIB, Akt and Stat3 before. In the present
study, we detected the interplay among the NFIB/Akt/Stat3 pathways
in which NFIB served as the upstream regulator of Akt and Stat3.
Notably, according to our western blot examination results, after
knocking down the expression of NFIB, the phosphorylation of both
Akt and Stat3 were reduced, nevertheless, total protein expression
of Akt and Stat3 remained unchanged. This suggests that NFIB may
not be connected with the Akt/Stat3 signaling pathway by directly
controlling the protein expression levels of the signal molecules.
Given the fact that NFIB is a nuclear factor which is responsible
for the transcription of numerous nuclear proteins, which may
include the activators of the Akt/Stat3 signaling pathway we thus
hypothesized that NFIB, as a nuclear factor, could control the
activation of the Akt/Stat3 pathway by upregulating the expression
level of its activators. Further and more extensive research is
warranted to examine and ascertain our hypothesis.
Notably, our study has only initially identified the
functions and roles played by NFIB in GC, and the NFIB/Akt/Stat3
signaling pathway. Our research data is not suffcient to depict the
more detailed mechanisms of NFIB in the development of GC. Further
investigation is warranted to determine whether NFIB contributes to
other aspects of GC malignancy, such as apoptosis or the cell
cycle, or if other signaling pathways are also involved in the
function of NFIB in GC. Moreover, in vivo experiments are
also important to be conducted in the future in order to elucidate
the roles of NFIB.
In summary, we demonstrated that NFIB plays an
oncogenic role in the formation, development, and metastasis of GC,
and it forms an axial relationship with Akt and Stat3 in which NFIB
inhibits the phosphorylation of both Akt and Stat3. Exploring the
molecular mechanism by which NFIB promotes GC malignancy through
the NFIB/Akt/Stat3 axis may be helpful for the understanding of the
formation and development of GC and may be the targets in GC
treatment which could be insightful to develop more effective and
targeted therapy for GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the The National
Natural Science Foundation of China, (ID: 81600401 to C.W.) and the
The Natural Science Foundation of Hubei Province, ID: 2017CFB474 to
C.W.).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
TR analyzed and interpreted the patient data. CW and
XZ performed western blotting and functional experiments, and were
major contributors in writing the manuscript. WL performed the
statistical analysis of the experimental data and proofread the
manuscript. WW performed the clinical data collection. KT
contributed to the conception of the study and designed the
research plan. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Union Hospital, Tongji Medical College, Huazhong University of
Science and Technology. The gathering and use of the patient data
and tissues were consented by all patients involved.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Uyama I, Suda K and Satoh S: Laparoscopic
surgery for advanced gastric cancer: Current status and future
perspectives. J Gastric Cancer. 13:19–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Ruan T, Liu W, Zhu X, Pan J, Lu W,
Yan C, Tao K, Zhang W and Zhang C: Effect and mechanism of curcumin
on EZH2-miR-101 regulatory feedback loop in multiple myeloma. Curr
Pharm Des. 24:564–575. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abbas M, Habib M, Naveed M, Karthik K,
Dhama K, Shi M and Dingding C: The relevance of gastric cancer
biomarkers in prognosis and pre- and post-chemotherapy in clinical
practice. Biomed Pharmacother. 95:1082–1090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Guo G, Song J, Cai Z, Yang J, Chen
Z, Wang Y, Huang Y and Gao Q: B7-H3 promotes the migration and
invasion of human bladder cancer cells via the PI3K/Akt/STAT3
signaling pathway. J Cancer. 8:816–824. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang LD, Chen L, Zhang M, Qi HJ, Chen L,
Chen HF, Zhong MK, Shi XJ and Li QY: Downregulation of ERRα
inhibits angiogenesis in human umbilical vein endothelial cells
through regulating VEGF production and PI3K/Akt/STAT3 signaling
pathway. Eur J Pharmacol. 769:167–76. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baba Y, Tamura T, Satoh Y, Gotou M, Sawada
H, Ebara S, Shibuya K, Soeda J and Nakamura K: Panitumumab
interaction with TAS-102 leads to combinational anticancer effects
via blocking of EGFR-mediated tumor response to trifluridine. Mol
Oncol. 11:1065–1077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu B, Lv X, Su L, Li J, Yu Y, Gu Q, Yan M,
Zhu Z and Liu B: MiR-148a functions as a tumor suppressor by
targeting CCK-BR via inactivating STAT3 and Akt in human gastric
cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen KS, Lim JWC, Richards LJ and Bunt J:
The convergent roles of the nuclear factor I transcription factors
in development and cancer. Cancer Lett. 410:124–138. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Geyer FC, Martelotto LG, Ng CK, Lim
RS, Selenica P, Li A, Pareja F, Fusco N, Edelweiss M, et al: MYBL1
rearrangements and MYB amplification in breast adenoid cystic
carcinomas lacking the MYB-NFIB fusion gene. J Pathol. 244:143–150.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matuzelski E, Bunt J, Harkins D, Lim JWC,
Gronostajski RM, Richards LJ, Harris L and Piper M: Transcriptional
regulation of Nfix by NFIB drives astrocytic maturation within the
developing spinal cord. Dev Biol. 432:286–297. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Y, Zhang J, Hou S, Cheng Z and Yuan M:
Non-small cell lung cancer: miR-30d suppresses tumor invasion and
migration by directly targeting NFIB. Biotechnol Lett.
39:1827–1834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Zhang X, Peng J, Zhai E, He Y, Wu
H, Chen C, Ma J, Wang Z and Cai S: VEGF promotes gastric cancer
development by upregulating CRMP4. Oncotarget. 7:17074–17086.
2016.PubMed/NCBI
|
|
14
|
Wei YL, Dong HM, Xie ZF and Cai SX: Role
of reactive oxygen species in hypoxia-induced non-small cell lung
cancer migration. Zhonghua Yi Xue Za Zhi. 97:3174–3178. 2017.(In
Chinese). PubMed/NCBI
|
|
15
|
Han MH, Lee DS, Jeong JW, Hong SH, Choi
IW, Cha HJ, Kim S, Kim HS, Park C, Kim GY, et al: Fucoidan induces
ROS-dependent apoptosis in 5637 human bladder cancer cells by
downregulating telomerase activity via inactivation of the PI3K/Akt
signaling pathway. Drug Dev Res. 78:37–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Rhun E, Bertrand N, Dumont A, Tresch E,
Le Deley MC, Mailliez A, Preusser M, Weller M, Revillion F and
Bonneterre J: Identification of single nucleotide polymorphisms of
the PI3K-AKT-mTOR pathway as a risk factor of central nervous
system metastasis in metastatic breast cancer. Eur J Cancer.
87:189–198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ina K and Furuta R: Complete response of
metastatic gastric cancer to chemoimmunotherapy. Indian J Med Res.
146:1412017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Z, Wang Q, Li B, Bai B and Zhao Q:
Influence of enhanced recovery after surgery programs on
laparoscopy-assisted gastrectomy for gastric cancer: A systematic
review and meta-analysis of randomized control trials. World J Surg
Oncol. 15:2072017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Q, Li H, Jiang H, Feng Y, Cui Y, Wang
Y, Ji Y, Yu Y, Li W, Xu C, et al: Predictive factors of
trastuzumab-based chemotherapy in HER2 positive advanced gastric
cancer: A single-center prospective observational study. Clin
Transl Oncol. 20:695–702. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawasaki K, Takeuchi D, Kaneko T, Miura S,
Kamiya J, Miyahara Y, Yoshimura K and Ogata A: A case of advanced
gastric cancer responding to neoadjuvant chemotherapy with
docetaxel, cisplatin, and 5-fluorouracil, leading to a pathological
complete response. Gan To Kagaku Ryoho. 44:1017–1020. 2017.(In
Japanese). PubMed/NCBI
|
|
21
|
He J, Wang X, Cai J, Wang W and Qin X:
High expression of eIF3d is associated with poor prognosis in
patients with gastric cancer. Cancer Manag Res. 9:539–544. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun GL, Li Z, Wang WZ, Chen Z, Zhang L, Li
Q, Wei S, Li BW, Xu JH, Chen L, et al: miR-324-3p promotes gastric
cancer development by activating Smad4-mediated Wnt/beta-catenin
signaling pathway. J Gastroenterol. 2017.
|
|
23
|
Becker-Santos DD, Lonergan KM,
Gronostajski RM and Lam WL: Nuclear factor I/B: A master regulator
of cell differentiation with paradoxical roles in cancer.
EBioMedicine. 22:2–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dooley AL, Winslow MM, Chiang DY, Banerji
S, Stransky N, Dayton TL, Snyder EL, Senna S, Whittaker CA, Bronson
RT, et al: Nuclear factor I/B is an oncogene in small cell lung
cancer. Genes Dev. 25:1470–1475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fane ME, Chhabra Y, Hollingsworth DE,
Simmons JL, Spoerri L, Oh TG, Chauhan J, Chin T, Harris L, Harvey
TJ, et al: NFIB mediates BRN2 driven melanoma cell migration and
invasion through regulation of EZH2 and MITF. EBioMedicine.
16:63–75. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fusco N, Geyer FC, De Filippo MR,
Martelotto LG, Ng CK, Piscuoglio S, Guerini-Rocco E, Schultheis AM,
Fuhrmann L, Wang L, et al: Genetic events in the progression of
adenoid cystic carcinoma of the breast to high-grade
triple-negative breast cancer. Mod Pathol. 29:1292–1305. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida R, Sasaki T, Minami Y, Hibino Y,
Okumura S, Sado M, Miyokawa N, Hayashi S, Kitada M and Ohsaki Y:
Activation of Src signaling mediates acquired resistance to ALK
inhibition in lung cancer. Int J Oncol. 51:1533–1540. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mo D, Fang H, Niu K, Liu J, Wu M, Li S,
Zhu T, Aleskandarany MA, Arora A, Lobo DN, et al: Human helicase
RECQL4 drives cisplatin resistance in gastric cancer by activating
an AKT-YB1-MDR1 signaling pathway. Cancer Res. 76:3057–66. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma DH, Li BS, Liu JJ, Xiao YF, Yong X,
Wang SM, Wu YY, Zhu HB, Wang DX and Yang SM: miR-93-5p/IFNAR1 axis
promotes gastric cancer metastasis through activating the STAT3
signaling pathway. Cancer Lett. 408:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|